Abstract
Background
Inflammatory skin diseases such as atopic dermatitis (AD) and psoriasis often present in sensitive and thin-skinned areas that are at higher risk for topical treatment-related skin irritation (e.g., burning, stinging).
Objectives
Our objective was to address the need for topical treatments that can be safely applied to these areas. We assessed the local tolerability of crisaborole topical ointment when applied to sensitive and thin-skinned areas of healthy volunteers.
Methods
In this phase I, randomized, double-blind, vehicle-controlled, single-center study, 32 subjects were randomized 3:1 to twice-daily application of crisaborole topical ointment, 2 %, (n = 24) or vehicle ointment (n = 8) for 21 days to 13 anatomic skin areas, including the face/hairline, genitals, extensor, and intertriginous areas. The primary endpoint was assessment of the frequency and severity of local tolerability symptoms (burning/stinging, erythema, and pruritus) using the Local Tolerability Scale.
Results
Overall, 98.8 % of all tolerability assessments had a grade of 0 (no signs/symptoms of irritation) and 0.1 % had a grade >1 (mild); no differences were observed in the frequency of local tolerability scores between treatment groups. The total frequency of local tolerability assessments graded >0 (none) was lower across all application sites with crisaborole ointment (0.0–2.2 %) than with vehicle ointment (2.4–7.1 %). Local tolerability did not change over time, and was comparable between sensitive and nonsensitive skin areas.
Conclusions
Crisaborole ointment application to sensitive skin areas was well tolerated in healthy volunteers, supporting its potential role as a topical treatment alternative for patients with AD or psoriasis.
Key Points
| A well-tolerated topical treatment that can be safely applied to sensitive and thin-skinned areas, where atopic dermatitis and psoriasis lesions often present, is needed to minimize risk of skin irritation. |
| Crisaborole topical ointment, 2 %, application to sensitive skin areas of healthy volunteers was well tolerated throughout treatment, indicating it may represent a much-needed, well-tolerated topical treatment alternative for patients with atopic dermatitis or psoriasis. |
Introduction
Atopic dermatitis (AD) and psoriasis are two common chronic inflammatory skin diseases that have a negative impact on the quality of life of patients [1, 2]. Affecting both children and adults, AD is characterized by red, inflamed, eczematous lesions and pruritus [3–5], with up to 90 % of patients presenting with mild-to-moderate disease [6]. In infants and young children, the face, neck, and extensor skin areas are frequently involved, while older children and adults often present with flexural fold involvement of the extremities (i.e., antecubital and popliteal fossae) [4, 7]. In addition to disease involvement in sensitive skin areas, patients with AD have higher skin sensitivity (e.g., stinging, burning, itching, pain) to cosmetics and environmental factors [8]. Psoriasis can occur at any stage of an individual’s life, and is often characterized by red plaques of thickened skin covered in silver or white scales that may affect any area of the body, with the knees, elbows, lower back, and scalp being the most common sites [2]. While plaque psoriasis (also known as psoriasis vulgaris) is the most common type of psoriasis (approximately 90 % of all cases), other site-specific variants also exist, such as inverse psoriasis, which is often smooth, red, and shiny in appearance, affecting sensitive skin fold areas (e.g., groin, gluteal cleft, axillae, and genitals) [2, 9].
Although chronic inflammatory skin diseases such as AD and psoriasis can be effectively treated with topical treatments, a number of safety concerns warrant special consideration. In psoriasis, cutaneous irritation may develop following treatment with topical retinoids and vitamin D analogs [10–12]. Topical corticosteroids (TCSs) and topical calcineurin inhibitors (TCIs) are effective treatment options for AD, but use of TCS on thin-skinned areas such as the face, intertriginous areas, and genitals are limited by potential adverse effects. TCIs are associated with application-site reactions (e.g., burning or stinging sensations), particularly in sensitive skin areas, and contain a boxed warning citing cases of malignancy [13–15]. Additionally, the American Academy of Dermatology and the American Academy of Allergy, Asthma & Immunology has issued guidelines recommending that the application of TCSs on the face, eyelids, genitalia, and intertriginous areas be avoided or administered with caution to avoid increased risk of systemic absorption and subsequent systemic adverse effects [3, 16, 17].
Therefore, effective and well-tolerated topical treatment alternatives that can be safely applied to the face and other thin and sensitive skin areas are needed for inflammatory skin diseases such as AD and psoriasis. Crisaborole topical ointment, 2 % (formerly AN2728; Anacor Pharmaceuticals, Palo Alto, CA, USA), is a nonsteroidal, anti-inflammatory, phosphodiesterase 4 inhibitor being investigated for the treatment of AD and psoriasis [18, 19]. In preclinical studies, topical administration of crisaborole in an in vivo mouse model demonstrated anti-inflammatory activity without dermal irritation [19, 20].
Phase II and III clinical studies evaluating crisaborole topical ointment have been conducted in patients with mild-to-moderate AD, providing evidence supporting the safety, efficacy, and tolerability of this novel topical treatment [21–24]. Additionally, phase II clinical studies have demonstrated preliminary evidence supporting safety, efficacy, and tolerability in patients with mild-to-moderate psoriasis [18, 20, 25]. Herein, we present the results of a phase I clinical study conducted to evaluate the local tolerability of crisaborole topical ointment, 2 %, when applied to anatomic skin areas, which included thin and sensitive skin areas, of healthy volunteers.
Methods
Study Design
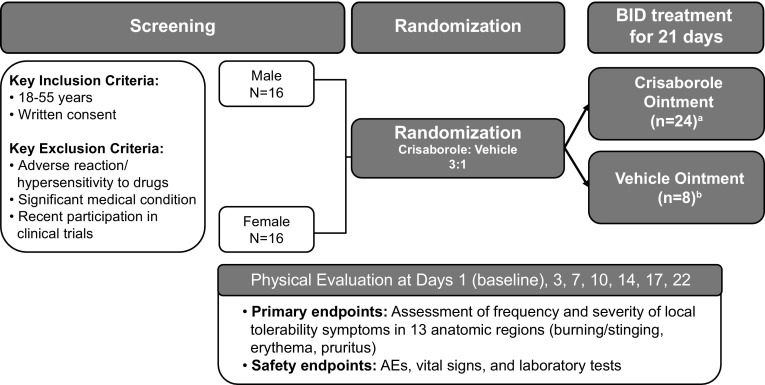
This randomized, double-blind, vehicle-controlled, single-center, phase I clinical study assessed the local tolerability of crisaborole ointment in sensitive and nonsensitive areas of healthy volunteers (Fig. 1). The study was conducted and monitored in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice, and all country-specific regulatory requirements. All subjects provided written informed consent.
Fig. 1.
Study design and treatment. Key screening criteria, subject enrollment, randomization, and assessments. AE adverse event, BID twice daily. aCrisaborole ointment-treated subjects included 12 male and 12 female subjects. bVehicle ointment-treated subjects included four male and four female subjects
Subjects
Subjects were stratified by sex (1:1) and randomized 3:1 to receive crisaborole topical ointment, 2 %, or vehicle ointment. Key inclusion criteria required subjects to be White males or females aged 18–55 years with the ability to understand and give written consent, who were willing and able to comply with study instructions and commit to all study visits. Subjects with a history of serious adverse reactions/hypersensitivity to any drug, or any clinically significant medical condition or abnormal laboratory value, were excluded from the study. Other key exclusion criteria included the chronic use of medications that would have interfered with study objectives (antihistamines, corticosteroids, analgesics, and anti-inflammatories), an unwillingness to refrain from shaving of the application sites and sexual activity, being uncircumcised (males), being pregnant or breastfeeding (females), and recent participation in other clinical studies.
Treatment
Treatment with crisaborole ointment or vehicle ointment was administered topically twice daily for 21 days to 13 anatomic skin areas, including the extensor areas, intertriginous areas, genitals, and face/hairline (Table 1). Subjects were required to attend the clinic for a supervised morning dose administration and study assessments on days 1, 3, 7, 10, 14, 17, and 22; all other doses were self-administered at home. Subjects were instructed to squeeze a bead of ointment from the distal skin crease to the tip of the index finger [one finger tip unit (FTU)] and rub it into the skin to encourage absorption, with one FTU covering approximately 2 % of body surface area (BSA) with a thin layer. Table 1 summarizes the approximate number of FTUs that were applied to each treatment area.
Table 1.
Application regions and instructions
| Treatment region | Treatment area | Approximate FTUs to be applied |
|---|---|---|
| Extensor areas | Elbows | 0.5 FTU to each elbow |
| Knees | 1 FTU to each knee | |
| Intertriginous areas | Groins | 0.5 FTU to each side |
| Axillae | 0.5 FTU to each side | |
| Gluteal cleft | 0.5 FTU | |
| Retroauricular area | 0.5 FTU to each side | |
| Genitals | Proximal glans (avoiding the urethra), corona, and distal shaft of the penis; labia majora | 0.5 FTU |
| Face/hairline | A fingertip-width strip below the hairline from one ear to the other side | 0.5 FTU |
FTU finger tip unit
Chronic use of medications such as antihistamines, corticosteroids, analgesics, and anti-inflammatories, which may have interfered with the study objectives, were prohibited 1 week prior to enrollment and during the study. Concomitant inhaled or nasal corticosteroids were allowed throughout the study, along with systemic antibiotics (if required) or medications for other chronic medical conditions that were not expected to interfere with the objectives of the study.
Outcome Evaluations
The primary endpoint for this study was assessment of the frequency and severity of local tolerability symptoms using the Local Tolerability Scale for burning/stinging, erythema, and pruritus, which was evaluated at each of the 13 anatomic skin areas during each clinic visit (i.e., days 1, 3, 7, 10, 14, 17, and 22). Using the Local Tolerability Scale, signs/symptoms were measured on a 4-point grading scale ranging from 0 (none) to 3 (severe); grades of 0.5, 1.5, and 2.5 were allowed as midpoints between the defined grades of 0, 1, 2, and 3 (Table 2).
Table 2.
Grading of local tolerability symptoms
| Gradea | Burning/stinging | Pruritus | Erythema |
|---|---|---|---|
| 0 (none) | No stinging/burning | No pruritus | No detectable erythema; skin of normal color |
| 1 (mild) | Slight warm, tingling sensation; not really bothersome | Occasional, slight itching/scratching | Slight pinkness present |
| 2 (moderate) | Definite warm, tingling sensation that is somewhat bothersome | Constant or intermittent itching/scratching that is not disturbing sleep | Definite redness, easily recognized |
| 3 (severe) | Hot, tingling/stinging sensation that has caused definite discomfort | Bothersome itching/scratching that is disturbing sleep | Intense redness |
aGrades of 0.5, 1.5, and 2.5 were allowed as midpoints between the definite grades of 0, 1, 2, and 3
Additional safety endpoints included the incidence and severity of adverse events (AEs) and treatment-emergent AEs (TEAEs) other than local tolerability symptoms, as well as changes in vital signs and laboratory tests.
Statistical Analysis
Safety analyses were performed using the safety analysis population, which included all subjects who received at least one dose of crisaborole ointment or vehicle ointment and had at least one post-baseline assessment. Safety and tolerability were assessed during each clinic visit, and local tolerability was evaluated at each of the 13 anatomic skin areas at each visit. Local tolerability signs and symptoms were evaluated with frequency tables for each treatment group and visit, and summarized by descriptive statistics [mean, standard deviation (SD), median, and minimum and maximum]. The incidence and severity of AEs and TEAEs were summarized using descriptive statistics, and comparisons between treatment groups were made by tabulating the frequency of subjects with one or more AEs or TEAEs.
Results
Baseline Characteristics
A total of 32 subjects were enrolled and randomized to treatment with crisaborole ointment (n = 24) and vehicle ointment (n = 8), and all subjects completed the study. The mean (SD) age was 29.9 (11.6) years. Baseline characteristics of the study population were generally balanced across treatment groups (Table 3).
Table 3.
Baseline characteristics of study population
| Characteristic | Crisaborole ointment (n = 24) | Vehicle ointment (n = 8) | Total (N = 32) |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 30.2 (11.4) | 29.1 (13.1) | 29.9 (11.6) |
| Range | 18–52 | 19–53 | 18–53 |
| Gender, n (%) | |||
| Male | 12 (50) | 4 (50) | 16 (50) |
| Female | 12 (50) | 4 (50) | 16 (50) |
| Height, cm | |||
| Mean (SD) | 173.7 (10.3) | 170.6 (6.7) | 172.9 (9.5) |
| Range | 155–190 | 162–181 | 155–190 |
| Weight, kg | |||
| Mean (SD) | 81.17 (17.84) | 77.13 (18.62) | 80.16 (17.82) |
| Range | 57.6–132.2 | 53.5–103.8 | 53.5–132.2 |
| White race, n (%) | 24 (100) | 8 (100) | 32 (100) |
SD standard deviation
Extent of Drug Exposure
All subjects were scheduled to apply 42 doses of study drug, and the total mean weight of ointment administered per subject was 184.00 g crisaborole ointment and 171.08 g vehicle ointment. Overall, the majority of subjects applied all doses of the crisaborole ointment (23/24) or vehicle ointment (7/8). One crisaborole ointment-treated subject missed a single dose, and one vehicle-treated subject had only 36 confirmed doses because this patient did not return one study diary.
Assessment of Local Tolerability
Overall, 98.8 % of all assessments for crisaborole ointment and vehicle ointment had a local tolerability grade of 0 (i.e., no evidence of signs/symptoms of irritation), 0.85 % were grade 1, and 0.1 % (10 of 8697 total assessments) had a grade >1, the majority of which were resolved back to 0 by the next study visit. One crisaborole-treated patient exhibited grade 1.5 erythema on both the left and right retroauricular sites, which reduced to 1 on the left and 0.5 on the right, respectively, by the next study visit, and resolved back to 0 for both by the following visit. Similar frequency of grade 0 local tolerability scores were seen in both the crisaborole ointment and vehicle ointment treatment groups (99.0 vs. 98.2 %, respectively). Three of 24 subjects (12.5 %) treated with crisaborole ointment reported grade 2 symptoms (moderate) on the elbows (one subject exhibited erythema and one reported application-site pain) and the retroauricular area (one subject exhibited erythema).
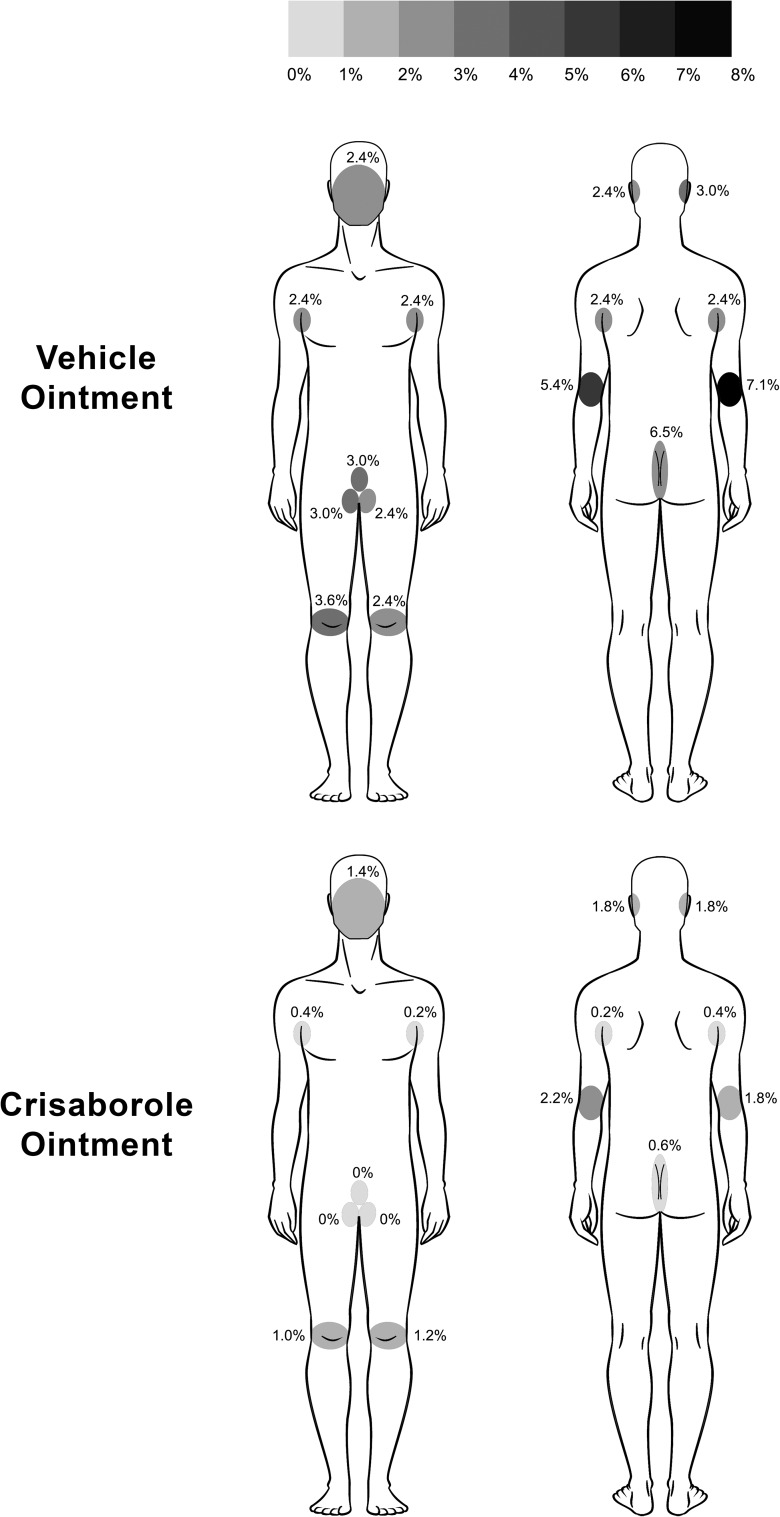
Figure 2 summarizes the total frequency of local tolerability assessments graded >0 for each of the 13 application sites. The frequency of non-zero scores in the crisaborole ointment treatment group tended to be lower across all application sites (0.2–2.2 %) than in the vehicle ointment group (2.4–7.1 %). Mean local tolerability symptom scores were similar across all the assessed application sites (both the sensitive and nonsensitive areas) and between treatment groups. During the time course of the study, overall local tolerability was similar between the two treatment groups.
Fig. 2.
Body map summarizing the percentage of total tolerability assessments graded as >0 (none) by application site
Other Safety Evaluations
A total of 38 TEAEs were reported in 22 of 32 (69 %) subjects, with a similar proportion of subjects reporting TEAEs in the crisaborole ointment (71 %) and vehicle ointment treatment groups (63 %). The most frequently reported TEAEs were headache (25 % of subjects), nasopharyngitis (19 %), and sunburn (16 %). The majority of TEAEs were classified as mild (34% of subjects) or moderate (25 %) in severity. Four subjects (13 %) reported severe TEAEs during treatment with crisaborole ointment (dental caries, upper respiratory tract infection, and seasonal allergy) or vehicle ointment (oropharyngeal pain), which were deemed not related to treatment. Treatment-related TEAEs were reported in only one subject (3 %); this subject had three mild application-site pain TEAEs in the retroauricular areas (stinging and burning) and across the hairline (stinging) that were considered definitely related to crisaborole ointment. All of the treatment-related TEAEs lasted less than a day.
No deaths, serious AEs, or discontinuations due to AEs were reported during the study. Similarly, no clinically significant changes in clinical laboratory test parameters or vital signs were observed during the study.
Discussion
In this randomized, vehicle-controlled, phase I study, crisaborole topical ointment, 2 %, was well tolerated when applied to sensitive skin areas in healthy volunteers, including the intertriginous areas, genitals, and face/hairline. Overall, tolerability signs/symptoms (burning/stinging, erythema, and pruritus) were graded as 0 (none) in 98.8 % of all assessments; only 0.1 % were graded >1 (mild) and were transient in nature, often resolving before the next in-clinic visit. Furthermore, similar scores in local tolerability were observed between the crisaborole ointment and the vehicle ointment treatment arms during the time course of the study. The frequency of total tolerability assessments graded >0 was lower with crisaborole ointment than vehicle ointment across all 13 anatomic skin areas. Additionally, the extent of drug exposure and patient adherence indicated that crisaborole ointment and vehicle ointment were well-tolerated on all application sites. Finally, the majority of TEAEs were mild to moderate in severity and not related to study treatment, with similar frequency between the two treatment groups.
The primary goals of treatment for AD are to improve skin barrier function, suppress inflammation, and ultimately relieve the signs and symptoms associated with the disease [5]. Given the prominence of pruritus in AD, which decreases quality of life and often results in exacerbation of symptoms [26, 27], relief from this distressing symptom is also an important treatment goal [28]. Topical formulations of AD medications should also emolliate and improve skin barrier function [3]. In addition, topical medications offer the ability to locally target skin inflammation with a lower risk of systemic adverse events [7, 28].
Effective topical therapies are available for the treatment of AD and psoriasis, but the treatment of these conditions in sensitive skin areas, such as the face, neck, genitals, and flexural folds of the extremities, can be challenging [2, 17]. Application of TCS to these thin skin sites is often avoided due to the increased risk of systemic absorption and subsequent development of systemic side effects [e.g., hypothalamic–pituitary–adrenal axis suppression] [3, 16, 17]. TCSs should also be used with caution in pediatric patients who have a greater BSA-to-weight ratio, which increases the degree of TCS absorption [3]. Although TCIs do not carry the same risks as TCSs with sensitive skin areas, their use is often associated with application-site reactions (stinging/burning/itching), with 8.5–10.4 % of Elidel™-treated (Novartis Pharmaceuticals Corporation, Whippany, NJ, USA) patients and 43–58 % of Protopic™-treated (Astellas Pharma US, Inc., Deerfield, IL, USA) patients experiencing application-site burning in vehicle-controlled phase III studies [31, 32]. For psoriasis, topical vitamin D analogs and retinoids are associated with cutaneous irritation [10–12]. Furthermore, currently available topical medications for the treatment of psoriasis subtypes, such as inverse and genital psoriasis, should be used with care to minimize the risk of cutaneous irritation and toxicity [10]. Patient concern for these potential adverse effects of topical therapies for both AD and psoriasis may reduce treatment adherence or compliance [10, 29–32]. There is a need for new alternative topical treatment options that can be applied safely with minimal tolerability issues, particularly in sensitive skin areas.
Current evidence from clinical studies has demonstrated that crisaborole topical ointment, 2 %, may be an efficacious and safe treatment for mild-to-moderate AD and psoriasis [18, 20, 21, 23, 25]. Phase I and II studies in patients with psoriasis demonstrated that crisaborole ointment was well tolerated for 12 days or 4 weeks of treatment [20]. In addition, phase II studies in patients with AD demonstrated that crisaborole treatment for 28 days, without application-site restrictions for sensitive skin areas, was well tolerated by the majority of patients [21–23]. The results of this phase I study in healthy volunteers indicate that crisaborole ointment has a promising safety and tolerability profile when applied to thin and sensitive skin areas, further supporting its use as a potential topical treatment option in inflammatory skin diseases such as AD and psoriasis. Future studies are needed to allow for direct comparison of drug efficacy and tolerability of crisaborole to TCS and TCI.
The current study is associated with limitations that should be considered. First, the study was conducted at a single center in Australia in a small racially homogenous (all White) sample of healthy adult subjects whose skin sensitivity may not be representative of pediatric subjects, subjects with chronic inflammatory skin disease, or subjects of different races/ethnicities. Second, the study did not examine all possible anatomic areas that may be affected by AD or psoriasis.
Conclusion
Findings from this study demonstrate that crisaborole ointment was well tolerated when applied to thin and sensitive skin areas in healthy volunteers and may represent a desirable topical treatment option for patients with AD or psoriasis. Future clinical studies are needed to confirm these findings in both AD and psoriasis patient populations.
Acknowledgments
The authors would like to thank the patients, investigators, and investigational site, whose participation made this study possible.
Compliance with Ethical Standards
Lee T. Zane and Matilda H. Hughes are employees of Anacor Pharmaceuticals, Inc., own Anacor stock and/or stock options, and received travel support from Anacor Pharmaceuticals, Inc. to the study site. Independent of trial outcomes, Sepehr Shakib received contract payment for principal investigator-related activities on behalf of the CMAX clinical trial unit, where the study was conducted. The study was conducted and monitored in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice, and all country-specific regulatory requirements. All subjects provided written informed consent.
Writing assistance
Editorial and medical writing support was provided by ApotheCom and funded by Anacor Pharmaceuticals, Inc. The authors had full access to and control of the data and analyses, and are fully responsible for the opinions expressed, content, and editorial decisions in the current article. The first author takes responsibility for the integrity and accuracy of the reported results.
Funding
This study was sponsored by Anacor Pharmaceuticals, Inc.
References
- 1.Lapidus CS, Kerr PE. Social impact of atopic dermatitis. Med Health R I. 2001;84(9):294–295. [PubMed] [Google Scholar]
- 2.Griffiths CEM, Barker JNWN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263–271. doi: 10.1016/S0140-6736(07)61128-3. [DOI] [PubMed] [Google Scholar]
- 3.Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–132. doi: 10.1016/j.jaad.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358(14):1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 5.Bieber T. Atopic dermatitis. Ann Dermatol. 2010;22(2):125–137. doi: 10.5021/ad.2010.22.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arkwright PD, Motala C, Subramanian H, Spergel J, Schneider LC, Wollenberg A. Management of difficult-to-treat atopic dermatitis. J Allergy Clin Immunol Pract. 2013;1(2):142–151. doi: 10.1016/j.jaip.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Thomsen SF. Atopic dermatitis: natural history, diagnosis, and treatment. ISRN Allergy. 2014;2014:354250. doi: 10.1155/2014/354250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darlenski R, Kazandjieva J, Fluhr JW, Maurer M, Tsankov N. Lactic acid sting test does not differentiate between facial and generalized skin functional impairment in sensitive skin in atopic dermatitis and rosacea. J Dermatol Sci. 2014;76(2):151–153. doi: 10.1016/j.jdermsci.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Syed ZU, Khachemoune A. Inverse psoriasis: case presentation and review. Am J Clin Dermatol. 2011;12(2):143–146. doi: 10.2165/11532060-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions. J Am Acad Dermatol. 2011;65(1):137–174. doi: 10.1016/j.jaad.2010.11.055. [DOI] [PubMed] [Google Scholar]
- 11.Lebwohl M, Ting PT, Koo JY. Psoriasis treatment: traditional therapy. Ann Rheum Dis. 2005;64(Suppl II):ii83–6. [DOI] [PMC free article] [PubMed]
- 12.Trémezaygues L, Reichrath J. Vitamin D analogs in the treatment of psoriasis: where are we standing and where will we be going? Dermatoendocrinol. 2011;3(3):180–186. doi: 10.4161/derm.17534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Draelos ZD. Use of topical corticosteroids and topical calcineurin inhibitors for the treatment of atopic dermatitis in thin and sensitive skin areas. Curr Med Res Opin. 2008;24(4):985–994. doi: 10.1185/030079908X280419. [DOI] [PubMed] [Google Scholar]
- 14.Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15(4):303–310. doi: 10.1007/s40272-013-0013-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol. 2013;14(3):163–178. doi: 10.1007/s40257-013-0020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pariser D. Topical corticosteroids and topical calcineurin inhibitors in the treatment of atopic dermatitis: focus on percutaneous absorption. Am J Ther. 2009;16(3):264–273. doi: 10.1097/MJT.0b013e31818a975c. [DOI] [PubMed] [Google Scholar]
- 17.Schneider L, Tilles S, Lio P, Boguniewicz M, Beck L, LeBovidge J, et al. Atopic dermatitis: a practice parameter update 2012. J Allergy Clin Immunol. 2013;131(2):295–299. doi: 10.1016/j.jaci.2012.12.672. [DOI] [PubMed] [Google Scholar]
- 18.Freund YR, Akama T, Alley MR, Antunes J, Dong C, Jarnagin K, et al. Boron-based phosphodiesterase inhibitors show novel binding of boron to PDE4 bimetal center. FEBS Lett. 2012;586(19):3410–3414. doi: 10.1016/j.febslet.2012.07.058. [DOI] [PubMed] [Google Scholar]
- 19.Akama T, Baker SJ, Zhang YK, Hernandez V, Zhou H, Sanders V, et al. Discovery and structure-activity study of a novel benzoxaborole anti-inflammatory agent (AN2728) for the potential topical treatment of psoriasis and atopic dermatitis. Bioorg Med Chem Lett. 2009;19(8):2129–2132. doi: 10.1016/j.bmcl.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Nazarian R, Weinberg JM. AN-2728, a PDE4 inhibitor for the potential topical treatment of psoriasis and atopic dermatitis. Curr Opin Investig Drugs. 2009;10(11):1236–1242. [PubMed] [Google Scholar]
- 21.Stein Gold LF, Spelman L, Spellman MC, Hughes MH, Zane LT. A phase 2, randomized, controlled, dose-ranging study evaluating crisaborole topical ointment, 0.5 % and 2 % in adolescents with mild to moderate atopic dermatitis. J Drugs Dermatol. 2015;14(12):1394–1399. [PubMed] [Google Scholar]
- 22.Tom WL, Van SM, Chanda S, Zane LT. Pharmacokinetic profile, safety, and tolerability of crisaborole topical ointment, 2 % in adolescents with atopic dermatitis: an open-label phase 2a study. Pediatr Dermatol. 2016;33(2):150–159. doi: 10.1111/pde.12780. [DOI] [PubMed] [Google Scholar]
- 23.Murrell DF, Gebauer K, Spelman L, Zane LT. Crisaborole topical ointment, 2 % in adults with atopic dermatitis: a phase 2a, vehicle-controlled, proof-of-concept study. J Drugs Dermatol. 2015;14(10):1108–1112. [PubMed] [Google Scholar]
- 24.Draelos ZD, Stein Gold LF, Murrell DF, Hughes MH, Zane LT. Post hoc analyses of the effect of crisaborole topical ointment, 2 % on atopic dermatitis: associated pruritus from phase 1 and 2 clinical studies. J Drugs Dermatol. 2016;15(2):172–176. [PubMed] [Google Scholar]
- 25.Moustafa F, Feldman SR. A review of phosphodiesterase-inhibition and the potential role for phosphodiesterase 4-inhibitors in clinical dermatology. Dermatol Online J. 2014;20(5):22608. [PubMed] [Google Scholar]
- 26.Blume-Peytavi U, Metz M. Atopic dermatitis in children: management of pruritus. J Eur Acad Dermatol Venereol. 2012;26(Suppl 6):2–8. doi: 10.1111/j.1468-3083.2012.04710.x. [DOI] [PubMed] [Google Scholar]
- 27.Yosipovitch G, Papoiu AD. What causes itch in atopic dermatitis? Curr Allergy Asthma Rep. 2008;8(4):306–311. doi: 10.1007/s11882-008-0049-z. [DOI] [PubMed] [Google Scholar]
- 28.Hong J, Buddenkotte J, Berger TG, Steinhoff M. Management of itch in atopic dermatitis. Semin Cutan Med Surg. 2011;30(2):71–86. doi: 10.1016/j.sder.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charman CR, Morris AD, Williams HC. Topical corticosteroid phobia in patients with atopic eczema. Br J Dermatol. 2000;142(5):931–936. doi: 10.1046/j.1365-2133.2000.03473.x. [DOI] [PubMed] [Google Scholar]
- 30.Gelbard CM, Hebert AA. New and emerging trends in the treatment of atopic dermatitis. Patient Prefer Adherence. 2008;2:387–392. [PMC free article] [PubMed] [Google Scholar]
- 31.Feldman SR. Disease burden and treatment adherence in psoriasis patients. Cutis. 2013;92(5):258–263. [PubMed] [Google Scholar]
- 32.Zschocke I, Mrowietz U, Karakasili E, Reich K. Non-adherence and measures to improve adherence in the topical treatment of psoriasis. J Eur Acad Dermatol Venereol. 2014;28(Suppl 2):4–9. doi: 10.1111/jdv.12445. [DOI] [PubMed] [Google Scholar]