Abstract
Background
A recent systematic review suggested that drug registrations and onsite quality inspections may be effective in reducing the prevalence of counterfeit and substandard drugs. However, simply replicating the most effective interventions is problematic, as it denotes implementing the intervention without further adaptation.
Objective
The aim was to systematically review the evidence beyond effectiveness for systems-level interventions to combat or prevent drug counterfeiting.
Methods
We conducted an extensive search, including an electronic search of 14 databases. We included studies examining the efficiency, feasibility, reliability, and economic outcomes of the interventions, as well as barriers and facilitators to their implementation. Two reviewers selected eligible studies and abstracted data in duplicate and independently. We synthesized the results narratively, stratified by type of intervention.
Results
Of 10,220 captured citations, 19 met our inclusion criteria. The findings suggest that the following may strengthen regulatory measures (e.g., registration): minimizing drug diversion, enhancing lines of communications, ensuring feedback on drug quality, and promoting strict licensing criteria. There is evidence that onsite quality surveillance and inspection systems may be efficient and cost-effective for preliminary testing of large samples of drugs. Laws and legislation need to be specific to counterfeit drugs, include firm penalties, address online purchasing of drugs, and be complemented by education of judges and lawyers. Public awareness and education should rely on multiple platforms and comprehensive and dedicated content. While product authentication technologies may be efficient and reliable in detecting counterfeit drugs in the supply chain, they require a strong information system infrastructure. As for pharmacovigilance systems, it is critical to tackle the issue of underreporting, to enhance their chances of success.
Conclusion
Several factors are critical to the successful design and implementation of systems-level interventions to combat or prevent drug counterfeiting. Policymakers need to take these into consideration to ensure success of these interventions.
Electronic supplementary material
The online version of this article (doi:10.1007/s40290-016-0156-4) contains supplementary material, which is available to authorized users.
Key Points
| Drug counterfeiting has serious public health and safety implications; it is estimated that up to 15 % of drugs sold worldwide are counterfeit, with the percentage reaching up to 50 % in parts of Africa and Asia. |
| We examined a range of systems-level interventions to combat or prevent drug counterfeiting and found some evidence on their efficiency, reliability, ability to detect counterfeit drugs, cost-effectiveness, acceptability, and/or implementation considerations. |
| Contextual factors are critical in shaping health system decisions, and policymakers need to take these into consideration to ensure effective implementation and, ultimately, the success of systems-level interventions to combat or prevent drug counterfeiting. |
Introduction
Drug counterfeiting is a widespread public health problem that affects both developing and industrialized countries [1, 2]. It is estimated that up to 15 % of drugs sold worldwide are counterfeit [3]. In parts of Africa and Asia, this percentage can reach up to 50 % [4].
There is no standardized definition for counterfeit drugs [5]. The World Health Organization (WHO) defined a counterfeit medicine as “one which is deliberately and fraudulently mislabeled with respect to identity and/or source. Counterfeiting can apply to both branded and generic products and counterfeit products may include products with the correct ingredients or with the wrong ingredients, without active ingredients, with insufficient active ingredient or with fake packaging” [6]. The term “falsified” is increasingly being used in place of “counterfeit” to avoid debates over intellectual property rights [7].
The spread of counterfeit drugs is evident in countries that have weak legislative and enforcement bodies and where the manufacture, importation, distribution, supply, and sale of drugs are poorly regulated [1]. Internet purchasing of pharmaceuticals has further contributed to the explosive growth in counterfeit medications [8, 9]. A recent report by the National Association of Boards of Pharmacy (NABP) found that the majority of web sites offering prescription‐only medications for sale were not compliant with either federal or state laws, or with industry standards [10]. Similarly, the WHO suggests that drugs purchased online from sites that conceal their physical address are counterfeit over 50 % of the time [11].
Counterfeited medications can promote drug resistance and lead to treatment failures as well as contribute to morbidity and mortality [12]. They can also lead to loss of public confidence not only in medicine, but also in public health. In addition, they can undermine the reputation of drug companies, which is why some companies may be hesitant to announce incidents of counterfeiting of their products [13].
The need to identify effective anti-counterfeiting strategies has been raised as a main policy concern by policymakers from low- and middle-income countries, including those in the Eastern Mediterranean region [14]. A recent effectiveness review found positive effects of drug registrations, WHO prequalification of drugs, and onsite quality inspections and surveillance systems (which constituted key components of multi-faceted interventions) in reducing the prevalence of counterfeit and substandard drugs [15]. However, simply replicating the most efficacious and effective interventions is problematic, as it denotes disseminating the intervention without further adaptation, and is thus unlikely to succeed [16]. Understanding how systems-level interventions work and the contextual evidence is critical in shaping health system decisions, and failure to consider these factors can adversely affect the implementation and, ultimately, the success of interventions [17–19]. Thus, the objective of this study was to systematically review the evidence beyond effectiveness for systems-level interventions to combat or prevent drug counterfeiting. We specifically focused on studies that examined the efficiency, feasibility, reliability, and/or economic outcomes of the interventions, as well as barriers and facilitators to their implementation.
Methods
Protocol and Registration
We registered the protocol in PROSPERO, an international prospective register of systematic reviews (CRD42014009269) [20].
Eligibility Criteria
The inclusion and exclusion criteria were:
Types of studies: We included non-randomized studies (prospective, retrospective, pre-post, cross-sectional studies), descriptive case studies, qualitative studies, economic studies, and process evaluation studies on counterfeit drugs. We included both published and unpublished studies.
We excluded reviews, editorials, commentaries, letters to the editors, reflections, proposals, and studies published in abstract format only. We also excluded studies not published in English, Arabic, or French.
Problem: Counterfeit/spurious/falsely labeled/falsified/medicines. Counterfeit medicines were defined according to the WHO classification [21]. These include medicines with wrong active ingredients, no active ingredients, high levels of impurities, insufficient active ingredients, or fake packaging of drugs. Although the main focus of the review is on counterfeit drugs, we also included substandard drugs when a study failed to distinguish between the two, or if it was not clear whether the poor-quality drug was counterfeit or substandard.
We did not limit the review to any specific class of therapeutic drug. We excluded studies that focused on herbal medicines/dietary supplements/cosmetics/food products.
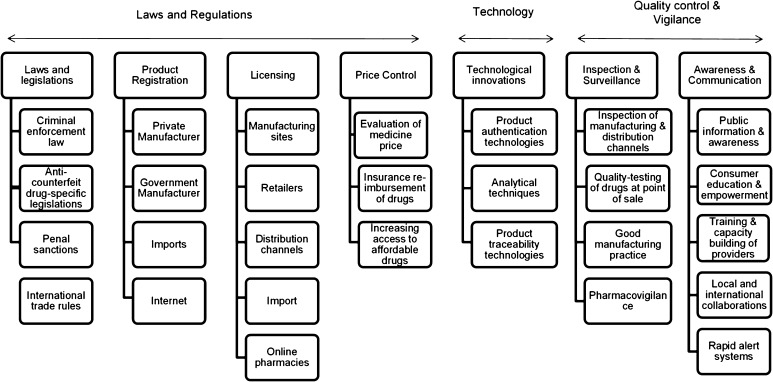
Types of interventions: We included any intervention at the health system level to combat or prevent drug counterfeiting (see framework in Fig. 1, developed by El-Jardali et al. [15]). The interventions included national anti-counterfeit drug laws and legislation, registration and licensing, inspection and quality control, training of personnel, price control, technological interventions (e.g., product authentication technology), national alert systems, and education and awareness campaigns.
We excluded studies that tackled proposed interventions or strategies. We also excluded studies that focused on interventions at the hospital level to improve medication administration processes or reduce medication errors. In addition, we excluded studies that focused on analytical techniques not implemented at the systems level (e.g., spectroscopic techniques).
Type of outcome measures: Outcomes included efficiency of intervention, feasibility of intervention, reliability of intervention, execution time, population coverage, regulatory visibility, detection of counterfeit drugs, economic outcome (cost and cost-effectiveness), implementation-related factors (barriers, facilitators, gaps, and loopholes, etc.), and acceptability by end users.
Fig. 1.
A framework for the different anti-counterfeit drug strategies
This framework was adopted from the study by El-Jardali et al. [15]
We did not limit our review to any specific type of setting or date of publication.
Literature Search
We searched the following electronic databases: MEDLINE, PubMed, EMBASE, Rx for Change, Cochrane Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Global Health Library, Health Systems Evidence, Cab Direct, and Academic Search Complete.
We also searched the grey literature using Google Scholar, Mednar, Greylit Network, and Opengrey. In addition, we searched relevant websites such as the WHO, the Food and Drug Administration (FDA), the Centers for Disease Control and Prevention (CDC), and the United States Pharmacopeia (USP). We screened the reference lists of the included papers and other relevant papers and reviews.
We developed and validated the search strategy with the information specialist at the American University of Beirut. The search combined various terms for counterfeit drugs and included both controlled vocabulary terms such as MeSH (Medical Subject Headings) and free-text words. We did not use any language, study design, or date restriction. The full search strategy is provided in Electronic Supplementary Material 1.
Selection Process
Two reviewers (RF and FA) independently screened the title and abstract of identified citations for potential eligibility. We retrieved the full text for studies judged as potentially eligible by at least one of the two reviewers. The two reviewers then screened the full texts in duplicate and independently for eligibility. They used a standardized and pilot-tested screening form. They resolved disagreements by discussion or with the help of a third reviewer.
We conducted a calibration exercise to ensure validity of the selection process.
Data Abstraction Process
Two reviewers (FA and HA) abstracted data from eligible studies in duplicate and independently. They used a standardized data abstraction form to collect data on funding, study design, context (country and timeframe), intervention, and reported outcomes and results. Disagreements were resolved by discussion or with the help of a third reviewer.
We did not encounter multiple reports of the same study or multiple studies in one report.
Data Analysis and Synthesis
Given the qualitative nature of the data, we synthesized and reported the results narratively. We stratified the results based on the type of interventions being considered. The stratification was guided by the conceptual framework developed by El-Jardali et al. for the different anti-counterfeit drug strategies (Fig. 1).
Results
Search Results
Figure 2 shows the study flowchart. Of the 10,220 citations identified through database and website searches, 19 met our inclusion criteria. No additional study was identified through screening the reference lists of the included studies. We excluded 41 studies at full-text screening for the following reasons: study not in English, French or Arabic (n = 3), not a primary research or country case study (n = 9), not about interventions to combat or prevent drug counterfeiting (n = 5), not a systems-level intervention (n = 7), does not assess outcome of interest (n = 9), does not assess “implemented” interventions (n = 7), and prevalence study (n = 1) (see Electronic Supplementary Material 2 for details).
Fig. 2.
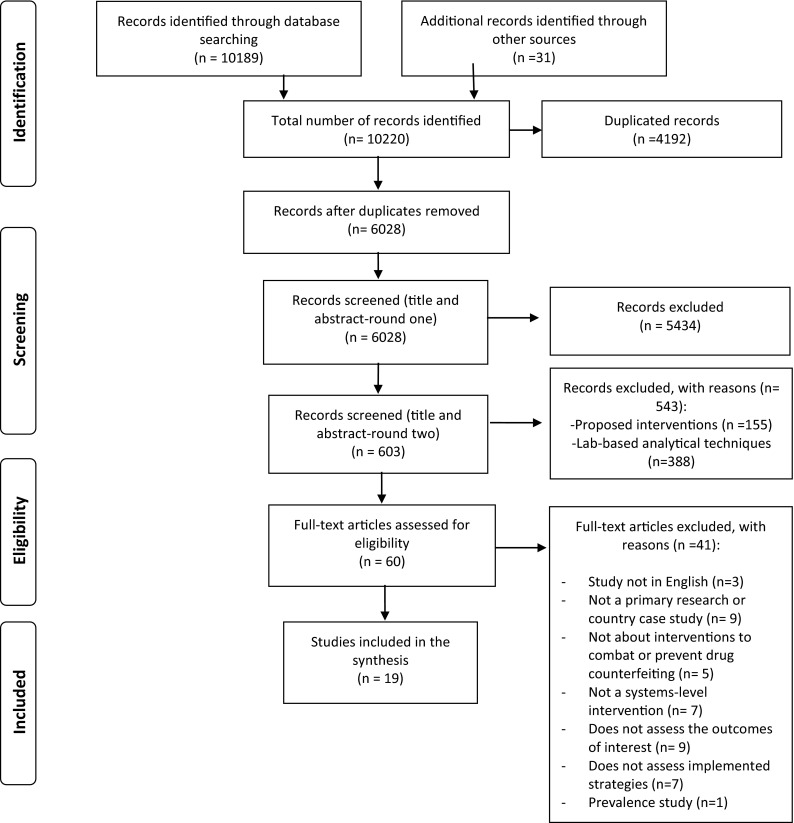
Flowchart for selection of the studies
The characteristics of the 19 included studies are shown in Electronic Supplementary Material 3. The majority of studies were country case studies (n = 9), followed by surveys (n = 4), mixed methods (n = 3), qualitative studies (n = 1), prospective audits (n = 1), and application of online algorithms (n = 1). With the exception of one French study [22], all other studies were reported in the English language. Three studies included multiple countries [10, 23, 24], one focused on online platforms [25], whereas the remaining 15 encompassed the following countries: Belgium and Greece (n = 1), Germany (n = 1), Mali and Mauritania (n = 1), South Africa (n = 1), Hong Kong (n = 1), China (n = 1), Tanzania (n = 1), Turkey (n = 1), Sweden (n = 1), the USA (n = 2), Burkina Faso (n = 1), the Philippines (n = 1), Nigeria (n = 1), and Kenya (n = 1). The multicountry reports aggregated the data from the different countries and analyzed them collectively to draw generic recommendations.
The included studies examined various types of interventions. These included drug regulatory measures (e.g., registration) and establishment of drug regulatory authorities (n = 3), onsite inspection and surveillance systems (n = 2), drug laws, legislation and decrees (n = 6), product authentication technology (n = 3), pharmacovigilance systems (n = 5), public awareness and education (n = 2), and recursive trust labeling for detecting fake medical web sites (n = 1). The types of outcomes assessed included efficiency, reliability, cost, cost-effectiveness, population coverage, regulatory visibility, acceptability by end user, ability to detect counterfeit drugs, and implementation-related factors (e.g., barriers, facilitators, gaps, etc.). All studies reported more than one type of outcome.
Findings by Intervention
We provide a summary of the findings of each included study in Electronic Supplementary Material 3. We also provide below a narrative synthesis of the findings, stratified by type of intervention.
Drug Regulatory Measures
We identified one study that focused on regulatory measures [26] and two that focused on the establishment of drug regulatory authorities at the national level [10, 27]. The studies aimed to examine the components of drug regulatory systems that constrained or facilitated the effectiveness of drug regulation. Some of the key highlighted issues hampering regulatory effectiveness related to drug diversion; communication gaps; a lack of adequate funds; shortages of qualified staff and equipment; the absence of a central drug regulatory authority; weak coordination, monitoring, and control; a lack of standardized regulatory tools and criteria; and the presence of regulatory double standards with respect to the different drug regulatory functions. None of the included studies highlighted any measures taken and their results in overcoming the factors hampering regulatory effectiveness.
The study conducted in South Africa included registration, licensing, and auditing of drugs and suppliers as key regulatory measures of the quality assurance system under the Medicines Control Council [26]. Semi-structured interviews with key stakeholders pointed to several factors that may hinder proper implementation of the abovementioned system. These included diversion of state medicines to the private sector, which affects distribution of medicines within the state sector; poor communication between manufacturers, regulatory authorities, and providers; the absence of feedback on complaints received on drug quality; and the ambiguity of criteria for licensing, which results in incompetent individuals operating as wholesalers. When asked about key strategies employed to protect drug quality, participants highlighted purchasing registered drugs from licensed suppliers, audits between manufacturer and distributor and/or provider, and use of standard operating procedures.
The multicountry study conducted by the WHO examined the experience of ten selected countries that have drug regulatory authorities [10]. Several phenomena impeding regulatory effectiveness were observed among the different countries. These included: (1) fragmentation of drug regulatory responsibilities, which could result in lapses in implementation with increased risk of duplication of efforts and wastage of resources; (2) delegation of drug regulatory powers in the form of either delegation with full authority but without coordination, or delegation without authority and accountability; (3) assignment of multiple functions to drug regulatory authorities with potential for conflicts of interest arising in respect to mandates and resource allocation; (4) regulatory double standards in which exemptions are sometimes made, depending on where the drug comes from, who manufactured it, or where it is distributed; (5) the lack of availability of regulatory tools such as documented guidelines and checklists for inspection among all drug regulatory authorities; (6) shortages of qualified personnel; (7) the absence of adequate and sustainable financing mechanisms; and (8) the lack of balance of priorities whereby different drug regulatory functions receive varying degrees of emphasis (e.g., formal vs. informal sector inspection and pre-marketing vs. post-marketing product assessment).
In Nigeria, the task forces on counterfeit drugs have been able to make a few seizures of some counterfeit medicines; however, they were rated as ineffective as a result of corruption, communication gaps, a lack of sufficient funding, shortages of human and material resources, and inadequate training of task force personnel. Furthermore, respondents regarded the coordination, monitoring, and control by the task forces as deficient [27]. Some of the key recommendations for improvement included control of the task forces by one agency, centralization of state and federal task forces, exclusion of military officers from joining task forces, exclusivity of membership to pharmacists, identification and dismissal of corrupt military officers, destruction of seized products (rather than allowing them to go into circulation), provision of sufficient funding, equipment and trained human resources, and adequate security for non-military members.
Onsite Quality Surveillance and Inspection Systems
We identified two country case studies that focused on this component [28, 29]. Both studies provided evidence that onsite quality surveillance and inspection systems may be efficient and cost-effective tools for preliminary field testing of large samples of drugs.
The first case study focused on the pilot project that took place in Tanzania for a year and a half on the use of Minilab kits, which are thin-layer chromatography (TLC)-based drug quality testing techniques [29]. The program intended to improve the testing capacity of inspectors stationed at key ports of entry to screen the quality of imported medicines as well as three non-ports-of-entry centers to screen drugs collected during post-marketing surveillance. The use of Minilab for quality screening was inexpensive, provided a high sample throughput, and required simple training and minimal resources for sustainability. The initial cost per kit was low, at US$5000 per kit. The cost of processing samples was also low at US$1.5 per sample, with no maintenance costs. Since the launch of the program, 1257 drug samples have been tested outside the central quality control laboratory, almost doubling the previous testing capacity. The large number of samples screened rendered the use of Minilab as cost-effective and contributed to the increased regulatory reach and visibility throughout the country. Despite the increased number of samples that have been tested, only five counterfeit products were detected. In addition, 46 batches (3.7 %) failed the USP dissolution test. The authors attributed these low numbers to the fact that the Minilab kit can only reliably detect “grossly” substandard or “wrong” drug samples, and therefore should be used in conjunction with full-service quality control. Also, reliability of detection depends on the operator’s visual perception, though the latter can be minimized by adopting approaches that improve inspectors’ testing competence and reliability in screening samples.
The second study focused on the mobile laboratory quality inspection system implemented by the Chinese National Institute for the Control of Pharmaceutical and Biological Products for onsite quality testing and extension of drug surveillance to remote countrysides [28]. The mobile laboratories offered in-time data collection, increased the efficiency of the drug surveillance program, expanded the monitoring area, and strengthened the capability of regulatory authorities to timely respond to evidence of adverse reactions related to drug products on the market. The near-infrared spectra (NIR)-based quick screening method demonstrated high reliability, where all 329 batches known to be counterfeit failed screening. In addition, following a toxic incident by diethylene glycol that caused 11 deaths, the mobile labs were sent out to screen all suspected drugs still on the market. All drugs screened positive for diethylene glycol by the mobile labs were later verified by gas chromatography. The mobile labs also reduced costs whereby only suspected batches (rather than the all batches) were sent to a district laboratory for further testing. By comparing the average analysis cost per batch of drugs using the traditional district lab versus the targeted mobile lab, the authors estimated that the system reduced the cost of analysis in the district laboratories by about 90 %. The authors highlighted some implementation considerations, such as the mobile laboratory requiring dedicated software to allow access to information and provide automatic analysis of the NIR, which may constitute a challenge to some developing countries that lack resources.
Drug Laws, Legislation, and Decrees
Six studies aimed to assess the strengths and weaknesses of laws and legislation addressing the problem of counterfeit and falsified drugs in different countries [10, 27, 30–32]. One of the studies focused specifically on laws and legislation pertaining to online pharmacies and online purchasing of drugs [24]. Key highlighted weaknesses across studies included the absence of counterfeit drug-specific laws and legislation, the lack of legal statutes for online sale of drugs, an insufficient legal and administrative framework to criminalize fraudulent falsification of medicines, and poor enforcement capacity for proper implementation and enforcement of laws. None of the included studies highlighted any measures taken and their results in overcoming the factors hampering legal effectiveness.
In Nigeria, the laws governing the manufacture, sale, distribution, importation, and exportation of drugs were not sufficient to control the illegal manufacture and sale of drugs. In addition, the implementation and enforcement of the various drug laws were highlighted as deficient [27].
In Hong Kong, the existing legislative system pertaining to falsified drugs has led to an increase in the quantity of counterfeit drugs seized at the retail level over recent years, amounting to US$98,625.45 in 2008 [30]; nonetheless, several weaknesses were identified by key stakeholders, including the absence of counterfeit drug-specific legislation, the presence of penalties that do not address the underlying public health impact of drug counterfeiting, light penalties (highest penalty was a fine of US$1290), and insufficient public health awareness and education of judges, lawyers, and prosecutors, who might feel bound by the existing commercial legislation. In Kenya, the anti-counterfeit act focused on intellectual property and failed to make a significant distinction between falsified medications and quality-assured generic medications, leading to its eventual suspension [32].
The WHO multicountry study provided an overview of legislation pertaining to drugs in general without necessarily focusing on anti-counterfeit drug-specific legislation [10]. It was found that in many countries, drug legislation did not encompass all products with medicinal claims and all activities related to the manufacture, importation, distribution, dispensing, and promotion of drugs in both the public and the private sector. In addition, they did not clarify the roles, responsibilities, and relationships between the different entities involved in drug regulation, nor did they specify the regulatory tools, standards of operation, and consequences of poor performance. The abovementioned gaps could in turn undermine the effectiveness of drug legislation.
In Florida, the Prescription Drug Protection Act of 2003 had been billed as the toughest anti-counterfeit drug wholesale law in the country as it imposed considerable credentialing requirements and the use of pedigree papers for all prescription drugs subject to wholesaling or dispensing in Florida [31]. Nonetheless, several loopholes were identified, including the fact that wholesalers with “suspicious” backgrounds could still obtain a permit by listing another person on an application; administrative oversight that placed more emphasis on meeting timelines for processing applications and issuing permits than on closely scrutinizing the responses in the application forms; the restriction of pedigree paper use to only 34 selected drugs; and failure to verify or authenticate pedigree papers, or in some cases, circulation of inaccurate pedigree papers by wholesalers. Subsequent enactment of House Bill HB 371 in 2006 has resulted in the elimination of the pedigree paper trail in many types of drug transactions, further weakening the full scope of Florida’s drug pedigree laws.
A survey of 114 countries by the WHO Global Observatory for e-Health examined the challenges and advances of member states concerning safety and security on the Internet [24]. Specifically, 66 % of the countries responded that they lacked any legislation pertaining to online pharmacy operations. Where such legislation existed, it was more prominent in developed countries and prohibited Internet pharmacy operation more often than allowed it (19 vs. 7 %, respectively). In addition, the majority of countries (86 %) reported that they did not regulate, accredit, or certify online pharmacy sites. Regarding the regulation of online purchasing of drugs from abroad, 75 % of countries reported having no legislation permitting or prohibiting the practice. Almost 80 % said they “do not have, do not know, or did not respond” if there were consequences for breaching laws regulating the online purchasing of drugs. The findings highlighted the need for stronger governance mechanisms to promote the creation of political and legal frameworks for the online sale of drugs.
Product Authentication Systems
Three studies assessed the reliability, efficiency, usability, and/or cost-effectiveness of product authentication technologies in fighting drug counterfeiting [33–35].
Simoens examined the reliability, efficacy, and cost-effectiveness of a patient safety communication service that is based on mass serialization technology (a method of assigning a unique number to each drug package) at the level of dispensing in Greek and Belgian communities [33]. A prospective mystery shopping audit of hypothetical test codes conducted in a sample of 116 Belgian community pharmacies showed 100 % reliability, where actual responses provided by the service corresponded with the correct responses as derived from the authentication database. Of the 220,751 scans tested during June–August 2008 in Belgium, the service identified 212,205 scans relating to authentic products (96.13 % of scans), 1635 scans relating to recalled product (0.74 %), 6630 scans relating to products that may be recalled (3 %), and 281 scans relating to expired products (0.13 %). No scans relating to suspicious products were identified. Similar results were observed in Greece (see Electronic Supplementary Material 3). The authors conducted a modeling exercise for a hypothetical country using the assumptions of five pharmacy software providers covering 10,000 pharmacies that dispense 400 million packs per year. Costs included start-up costs and annual running costs whereas benefits originated from identifying recalled, expired, or suspicious products. The modeling exercise showed that an authentication service would become cost-neutral in a scenario where 0.47 % of products per year are identified as recalled or expired. The total benefit of an authentication service was estimated at US$8,535,749 for Belgium, US$4,896,476 for Greece, and US$6,753,608 for the cost-neutral scenario per year. The pre-requisites for such a system are the availability of the necessary information systems in pharmacies to run the service and the display of unique serialized numbers on reimbursable drugs.
Another pilot project implemented in Sweden focused on the reliability and user friendliness of a 2-D matrix product verification system at the level of dispensing [34]. Feedback provided by the pharmacists participating in the survey showed that a large majority (more than 90 %) found the system easy or very easy to use. About 85 % rated the system’s response time as “generally fast” or “consistently fast”. On the other hand, it was reported that the presence of more than one code on the package caused confusion for the user and minimized its acceptance. Although the system was capable of identifying an illicit product before being dispensed to the patient, it could not be used for automatic identification of the entry point of a suspect product into the legitimate supply chain. Also, reliability was dependent on the quality of the existing information system and the sustained readability of the data carrier used.
Similarly, a case study conducted in Turkey by Altunkan et al. discussed the track-and-trace system implemented in the pharmacy sector [35]. The system was reported to ensure reliable supply of drugs to patients and prevent the sale of spurious/counterfeit drugs and barcode scams. However, empirical data were not provided to support this claim. The system was also reported to detect harmful drugs within a very short period of time (seconds) compared with the older procedure, as well as to promote smooth communication among stakeholders. The authors highlighted the importance of the Data Matrix (which acted as a data carrier) in ensuring that every single step and action of a particular unit in the supply chain can be traced via web services, thus allowing single identification at a time and ensuring high accuracy and security at a relatively lower price compared with radio frequency identification (RFID) tags.
Pharmacovigilance Systems
Five case studies focusing on pharmacovigilance systems defined these as a reporting system that seeks to detect cases of adverse drug reactions (ADRs) and investigate their causes, be it a counterfeit drug or a medication error, retroactively [23, 32, 36–38]. The systems have been successful in detecting a host of drug-related problems, including a lack of active ingredients, an absence of clinical effects, adulterated content, and contaminations. Nonetheless, a number of barriers and facilitators to their implementation have been highlighted.
In Burkina Faso, Kabore et al. indicated that the formal pharmacovigilance system launched by the Ministry of Health enhanced the reporting of ADRs and accounted for most of the reports sent to the National Drug Authority in 2010 [37]. While the data collected have not led to the identification of local drug-related risks, relevant drug safety alerts from external sources have been monitored and acted upon. In 2010, 31 marketing authorizations were modified to include new safety information, while seven others were suspended and the corresponding drugs withdrawn from the national market. The study also stated some advantages to implementing such a system, such as its ease of operation, relatively low cost, high coverage, lifecycle follow-up of medicines, non-interference with prescribing habits, and ability to allow follow-up studies.
Olsson et al. provided an overview of the status of pharmacovigilance systems in 55 low- and middle-income countries [23]. The authors found that in 40 countries, the system captured events related to an unexpected lack of efficacy, due to counterfeiting, quality defects, antibiotic resistance, irrational drug use, and/or inadequate quality of patient care [23]. Seven countries also had sentinel sites to monitor HIV/AIDS patients and other special groups. The number of individual case reports received by the pharmacovigilance programs in 2007 was less than 1000 for the majority of countries (72 %), whereas it exceeded 10,000 in Mexico, Singapore, and Thailand. The information gathered through pharmacovigilance activities were mostly used to assist regulatory functions (88 %, n = 42 countries) and have resulted in changes in product information (n = 21 countries), safety warnings (n = 24), and market withdrawals (n = 20).
In the Philippines, the pharmacovigilance system provided a way to detect substandard and counterfeit drugs should they pass regulatory inspections, with subsequent identification of a range of problems [38]. These included Chinese “DeWitts’ Kidney and Bladder Pills” for patients with renal diseases that were found to contain wrong and harmful ingredients, health supplements claiming to be natural in origin, hazardous weight-reducing products imported illegally into the country, and steroid compound and phenylbutazone adulterating Chinese herbal products. The system has also resulted in warnings and health advisories to health professionals and the public.
In Kenya, Cohn et al. described an incident related to the identification of falsified antiretroviral drugs in the supplies of the Médecins Sans Frontières (MSF) projects [32]. Nurses working in an MSF-supported HIV/AIDS treatment program found two discolored and molding batches of Zidolam-N. The affected batches were immediately isolated and sent back to the distributor. At the same time, the pharmacovigilance department of the Kenya Pharmacy and Poisons Board (KPPB), the drug regulatory agency for Kenya, and the WHO Pre-Qualification Program were notified of the quality problem, leading to a series of investigations. Within 3 months of detecting the falsified drugs in the MSF supply chain, around 95 % of patients who had taken the drug had returned to an MSF clinic for medical review and medication replacement. Some of the highlighted factors that may have hindered the responsiveness and process of investigation related to the fact that the KPPB did not immediately issue any public information on the incident, with no official public communication from the government until several weeks after the incident had occurred. In addition, the KPPB communication did not include information beyond the initial WHO alert or issue a clear product recall.
In the USA, the “forensic” pharmacovigilance system resulted in the discovery and investigation of ADRs caused by contaminated heparin in 2008 [36]. Specifically, a case study of a severe allergic-type reaction to heparin occurred in patients treated with hemodialysis at a single pediatric hospital and was identified by both the manufacturer and the National Regulatory Authority. The series of rapid investigations that ensued revealed the presence of a contaminant in samples of heparin crude materials, heparin active pharmaceutical ingredients, and final heparin drug products. This led to national alerts and calls for immediate withdrawal of the product from the US market. The issue was contained in the USA within a period of 4 months. However, a total of 785 adverse reaction reports, including 81 deaths, were associated with the contamination.
Several barriers to proper functioning of pharmacovigilance systems have been highlighted. All included studies pinpointed underreporting as a major obstacle. Hindrances to underreporting included a low level of awareness and recognition among patients and healthcare providers regarding counterfeit drugs and adverse drug events, adverse events being misinterpreted as part of the healing process, ignorance of the reporting requirements, the availability and accessibility of reporting forms, and fear of litigation and being held accountable for the ADR [23, 37]. Other factors limiting the development of pharmacovigilance in low- and middle-income countries included the lack of national guidelines and standardized operating procedures on pharmacovigilance, insufficient coordination and networking of pharmacovigilance stakeholders throughout the country, no specific legislation on pharmacovigilance, a lack of staff trained in pharmacovigilance, and insufficient funding to ensure sustainability of newly developed systems [23, 37]. In Burkina Faso, the system achieved a performance score of 70 %; some of the main weaknesses identified pertained to the absence of specific laws pertaining to pharmacovigilance, the lack of national guidelines and standard operating procedures on pharmacovigilance, and inadequate coordination among pharmacovigilance stakeholders. In addition, the reporting form designed by the national drug authority takes ADRs and medication errors into account, but does not include treatment failure or pharmaceutical product quality [37].
Several suggestions have been provided to facilitate the implementation of such systems. These included raising the level of awareness among healthcare providers; integration of pharmacovigilance into undergraduate, postgraduate, and health professional curricula; formalization of pharmacovigilance structures and activities within hospitals and public health programs; and establishment of a “reporting culture” in healthcare practitioners [37]. Methods to encourage reporting include the use of continuing medical education credits for medical professionals, active dissemination of reporting forms to all facilities where potential reporters are practicing, and provision of individual acknowledgement letters to reporters whenever a report has been received [23]. Additional facilitators included pooling of adverse event data from countries into one large database, such as VigiBase, to help increase sensitivity and specificity of detection as well as support analysis strategies [36]. The extension of regulatory authorities’ communication activities, development of newsletters and web sites, and active engagement with media to provide the public with updated safety information have also been highlighted as relevant [23]. Importantly, governments should take a leadership role to “propel” pharmacovigilance towards the goal of ensuring drug safety as well as provide the needed policy and regulatory framework and funding [23].
Public Awareness and Education on Counterfeit Drugs
We identified two eligible studies that focused on educating and raising awareness on counterfeit drugs from illicit drug outlets.
The French study by Cuchet-Chosseler et al. examined a public awareness campaign (mainly poster based) on counterfeit street medicines in Mali and Mauritania [22]. A survey was used to measure the exposure of school students to the posters as well as assess the overall efficiency and efficacy of the campaign. Eighty-four percent of students reported hearing about the dangers of drugs on the streets. In addition, 61 % reported seeing the posters in pharmacies; however, only 41 % recalled what it was about. Those who had seen the posters and heard about the dangers were more likely to indicate a need for better control of illicit drug outlets. Overall, the campaign partially increased the knowledge of participants regarding the danger of illicit drug outlets, with inconsistent results observed for opinions and behaviors. Some highlighted areas for improvement included the utilization of broader sets of media channels (e.g., TV), the distribution of posters in more public places, better elaboration on prices of drugs as part of the key messages (to contest the commonly held belief that street drugs are cheaper, thus more appealing), and the integration of courses on this topic in the school curriculum. Future campaigns could also recruit trainers to design and monitor the contents of key messages to ensure proper conveyance of the messages to target audience without distortions.
Thomson et al. examined whether online awareness and education about counterfeit drugs could reach the target audience and influence counterfeit medicine purchasing behaviors [39]. The authors developed an imitation online pharmacy that aimed at directly engaging and warning those at risk from fake drugs once accessed. The online pharmacy was heavily promoted for 9 months. Eighty-five percent of individuals searching for online pharmacies visited the website within this period, yielding a total of 360,532 visitors. These included 182,602 unique visits to the landing page, which displayed a warning about the danger of illegal online pharmacies, 142,676 visits to the key warning page, and 16,378 visits to extra advice and information. In addition, 12,227 unique visitors clicked through the link directing them to the German Institute of Medical Documentation and Information (DIMDI) that listed legitimate online/high street pharmacies. The findings indicated that the key messages reached the targeted audience as well as supported the potential for change in consumer behaviors once awareness on how to buy safely is raised.
Recursive Trust Labeling
One study examined the efficacy of an adaptive learning algorithm called recursive trust labeling (RTL), which makes use of underlying graph-based and content classifiers, combined with a recursive labeling mechanism, for better detection of fake medical web sites [25]. The method was evaluated on a test bed covering approximately 100 million links between 930,000 web sites, including 1000 known legitimate and fake medical sites. Analysis of the performance results showed that RTL attained over 90 % accuracy in its detection capabilities on all three test bed subsets (online pharmacy, health information, and medical institution web sites). The findings demonstrated the viability of RTL in detecting fake medical web sites. Moreover, robustness analysis showed that it was able to attain high performance levels even when the dataset consisted of as little as 30 web sites.
Discussion
Summary and Interpretation of Findings
Overall, we identified 19 studies providing evidence beyond effectiveness for systems-level interventions to prevent or combat drug counterfeiting. The findings highlighted several factors that are critical to the successful design and implementation of systems-level interventions to combat or prevent drug counterfeiting.
In the previous effectiveness review, regulatory measures (e.g., registration and WHO prequalification of drugs), and onsite quality inspections (which constituted key components of multifaceted interventions) were suggested as effective measures in reducing the prevalence of counterfeit and substandard drugs [15]. In this review, we identified three studies that focused on regulatory measures and two that focused on onsite quality inspections and surveillance systems. The findings suggest that the implementation of regulatory measures should be complemented by efforts to minimize drug diversion; strengthen communication between manufacturers, providers, and regulatory authorities; ensure feedback on drug quality complaints post-registration; and promote unambiguity and strict criteria for licensing of wholesalers. Importantly, a central drug regulatory authority should be accountable for the overall effectiveness of drug regulation. The action taken by the regulatory authority should cover all drug regulatory functions in a balanced fashion and encompass both the formal and informal sector (given the prominence of drug counterfeiting in the latter sector). In addition, adequate and sustainable funding should be made available, personnel of integrity who are appropriately trained and qualified should be recruited, and appropriate standards and guidelines should be developed and used as tools for application of all regulatory processes. Finally, the regulatory process should be monitored and evaluated to identify emerging problems and ensure that the actual activities are meeting the established objectives.
Similarly, we found some evidence that the use of onsite quality surveillance and inspection systems could offer regulators in limited-resource settings with an efficient and cost-effective tool for preliminary testing of large samples of drugs as well as increase regulatory reach and visibility throughout the country. Two systems have been implemented; the Minilab kit, which relies on TLC [29], and the mobile lab that uses near-infrared spectroscopy as the main screening tool in addition to other equipment such as TLC, visible microscopy, and test kits for specific chemical reactions [28]. Although TLC is relatively less expensive (costing between US$5000–10,000 for a fully equipped kit) [29, 40], a drawback is that it can only reliably detect highly manipulated drugs, which might result in missing slightly substandard drugs. Nonetheless, it is recommended that both Minilab kits and mobile labs be used in conjunction with a full-service quality control laboratory capable of auditing and verifying reported substandard and counterfeit results.
With regard to drug laws and legislation, the findings from one study suggest that these could contribute to an increased amount of counterfeit drugs being detected at the retail level [30]. However, to enhance their success, laws and legislation need to be specific to counterfeit drugs, focus on public health rather than the intellectual property perspective, address the entire illicit online pharmacy ecosystem, include a sufficient legal and administrative framework to criminalize fraudulent falsification, and be complemented by strong enforcement capacity as well as education of judges, lawyers, and the public.
Although product authentication systems such as track and trace and RFID are increasingly being promoted as preventive measures against drug counterfeiting [41, 42], there is no evidence on their effectiveness in reducing the prevalence of counterfeit drugs. The studies we identified suggested that product authentication technologies at the point of dispensing and based on 2D Data Matrix codes may be efficient and reliable in detecting counterfeit drugs in the supply chain. However, the establishment of a strong infrastructure linking all pharmacies to an information system requires time and effort in addition to high costs and resources, which would pose a huge challenge for low- and middle-income countries. Despite the high start-up costs of such a system, one modeling study suggested it could be cost-effective in the long run. Nonetheless, all included studies on product authentication systems were executed as part of pilot tests, thus their operation in real settings and their effects in the long run could not be established.
Pharmacovigilance systems seem to be growing at fast pace in low- and middle- income countries to promote drug safety and allow routine post-marketing surveillance of pharmaceuticals at the national level. These systems have been able to detect a host of counterfeited and substandard drugs at the national level. To enhance their chances of success, it is critical to tackle the issue of underreporting that would arise, as well as ensure ongoing training, monitoring, and feedback. Importantly, interventions to strengthen the legal framework and structures for pharmacovigilance activities as well as improve the coordination of stakeholders countrywide should be undertaken before the national pharmacovigilance system is capable of collecting its own data, generating indicators, evaluating drug-related risks, and eventually serving as a genuine tool for public health [37].
The findings on raising awareness and educating the general public on the danger of counterfeit drugs from illicit drug outlets supported their potential to promote changes in purchasing behaviors and reach the target audiences. Nonetheless, better results could have been achieved by utilizing broader sets of media channels, including effective social media communications, as well as ensuring comprehensive and dedicated content on the issue. One of the authors also recommended recruitment of trainers to design and regularly monitor the content of the messages to avoid distortion, as well as better elaboration on the prices of drugs in the key messages given the commonly held belief that drugs from illicit outlets are cheaper, and thus more appealing to the public [22].
Although the previous systematic review did not address online drug counterfeiting, it is widely acknowledged that illicit online drug outlets have fueled the global spread of counterfeit and substandard medicine [8, 24]. Indeed, previous reviews on this subject have only focused on the quality of medicines purchased online, characteristics of online web sites, online supply issues, consumer profiles, and challenges of illicit online pharmacies [24, 43–45]. This current systematic review identified three eligible studies that pointed to deficiencies in laws and regulations addressing online pharmacies and online purchasing of drugs as well as highlighted the potential of online educational platforms to reach individuals at risk and inform them about purchasing drugs online. Despite the increased recognition of the global challenges posed by the Internet and illicit online pharmacies [5, 24], few solutions have emerged to confront this problem. For instance, the USA, passed the Ryan Haight Online Pharmacy Consumer Protection Act of 2008, named after an 18-year-old boy who died from an overdose of drugs purchased online. The law mandates prescriptions for online purchases, but is limited to controlled substances [46]. However, commentaries have been critical about its effectiveness [24]. A few studies have also found that verification schemes, seals, and certifications of online pharmacies can be an important step towards achieving online patient safety [47, 48].
Strengths and Limitations
Our systematic review complements the findings of the review of effectiveness and provides the contextual evidence for interpreting the findings. Such synthesis would make an important contribution to understanding how systems-level interventions work and the challenges that may arise, taking into account the context of application. Other strengths include pre-publishing a protocol, searching multiple databases, and including both published and unpublished studies to ensure the comprehensiveness of our search. In addition, our systematic review responds to a policy-relevant priority as identified by policymakers and stakeholders.
A main limitation of this systematic review is that we did not formally assess the risk of bias in each of the included studies. Indeed, the majority of included studies were descriptive country case studies. In addition, two of the included studies on product authentication technology were respectively designed [18] and funded [33] by pharmaceutical industries, thus we could not eliminate the possibility of reporting bias in favor of the technology. Also, for some interventions, only a single study was retrieved, limiting our ability to draw any reliable conclusions. Another limitation relates to the fact that we only included studies conducted in English, Arabic, or French, thus we may have missed out on relevant articles written in other languages.
Implications for Policy
Contextual factors are critical in shaping health system decisions, and policymakers need to take these into consideration to ensure the effective implementation and, ultimately, the success of systems-level interventions to combat or prevent drug counterfeiting.
Policymakers and stakeholders may consider strengthening the drug registration procedure and complementing it with stringent post-marketing surveillance using “standard pharmacovigilance methods of registration, analysis and investigation” [36] to help identify counterfeit drugs as well as sustain the quality of drugs circulating the market. Such regulatory measures could be further synergized by legal measures such as the establishment of counterfeit drug-specific laws with a public health perspective, tough sanctions and penalties, and non-legal measures such as the education of judges, lawyers, and the public, as well as emphasizing the important role of pharmacists in ensuring drug quality. Importantly, they should ensure that legal and regulatory measures also address the entire illicit online pharmacy ecosystem for enhanced effectiveness. Indeed, without suitable harmonized legislation and cooperative agreements between countries, “rogue” online pharmacies can bypass stringent regulation by operating their web sites within jurisdictions that have the least restrictive regulatory framework [24].
Onsite quality inspections at different levels of the supply chain could offer regulators in limited-resource settings with an efficient and cost-effective intervention for preliminary testing of large drug samples and increased regulatory visibility. This can be critical especially for countries with no adequate national labs, since only the suspicious samples are sent to the national lab for further testing (rather than the whole batches). Nevertheless, establishing national labs should be considered in the long run.
While the evidence for product authentication technologies suggests they may be efficient, reliable, and cost-effective in the long run, the very high start-up costs that these systems entail in terms of infrastructure and information technology may serve as a barrier for implementation, particularly in low- and middle-income countries where resources are scarce.
Finally, policymakers and stakeholders may consider investing in national public awareness and education initiatives using multiple platforms and disseminating comprehensive and dedicated content about the risks of purchasing drugs from illicit outlets and the warning signs to look for.
Implications for Research
Despite the serious public health implications of drug counterfeiting, there is still a dearth of methodologically rigorous studies to assess interventions to combat or prevent drug counterfeiting. Future research should address the methodological limitations of existing studies, including clear explanations of the sampling and recruitment methods, as well as the use of reliable and valid data collection tools. Also, more rigorous and objective studies assessing the cost-effectiveness of the different systems-level interventions should be considered, including product authentication systems, which are increasingly being promoted by international agencies for combating counterfeit drugs. Furthermore, given the growing phenomenon of online sales of drugs, it is critical to assess the effectiveness of interventions aimed at regulating or preventing online drug counterfeiting.
Finally, there is a need to standardize the definition of what constitutes counterfeit drugs in order to establish consistency when implementing interventions and comparing findings across different studies and settings.
Conclusion
Several factors are critical to the successful design and implementation of systems-level interventions to combat or prevent drug counterfeiting. We found some evidence on the efficiency, reliability, cost-effectiveness, regulatory visibility, acceptability, ability to detect counterfeit drugs, and/or implementation considerations of a range of systems-level interventions. Policymakers need to take these into consideration to ensure effective implementation and, ultimately, the success of the interventions. Based on the findings, strong regulatory measures, onsite quality control and surveillance of drugs, national pharmacovigilance systems, and educational and awareness initiatives on the danger of illicit drug outlets seem promising. Regulatory measures can be strengthened by minimizing drug diversion, ensuring stringent post-marketing surveillance, and placing equal emphasis on the different drug regulatory functions. Deficiencies in laws and legislation include the lack of counterfeit drug-specific laws, the lack of legal statutes for online sale of drugs, insufficient legal and administrative frameworks to criminalize fraudulent falsification of drugs, and poor enforcement capacity. Future research should address the methodological limitations of existing studies in terms of study design and data collection tools. Also, more rigorous studies are needed to assess the cost-effectiveness of the different systems-level interventions, including product authentication systems and interventions targeting online drug counterfeiting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We would like to thank Ms. Aida Farha for her valuable help in designing the search strategy.
Authors’ contributions
RF, FE, and EA were involved in the concept and design. RF and FE performed the searches. RF and FA conducted the title and abstract screening and the full-text screening. FA and HA performed the data extraction. RF, FE, and EA performed the analyses and prepared the discussion. All of the authors contributed in preparing the draft of the manuscript and revising it. All of the authors have read and approved the edited manuscript.
Compliance with Ethical Standards
Funding
This work was supported by the Alliance for Health Policy and Systems Research at the WHO, Grant Number 102716. The funding body was not involved in the design of the study, data collection, analysis, interpretation of findings, or in writing the manuscript. Open access was funded by the Alliance for Health Policy and Systems Research.
Conflict of interest
Racha Fadlallah, Fadi El-Jardali, Farah Annan, Hayat Azzam, and Elie Akl declare that they have no conflict of interest.
Contributor Information
Racha Fadlallah, Email: rsf07@mail.aub.edu.
Fadi El-Jardali, Email: fe08@aub.edu.lb.
Farah Annan, Email: fra07@mail.aub.edu.
Hayat Azzam, Email: hna06@aub.edu.lb.
Elie A. Akl, Email: ea32@aub.edu.lb
References
- 1.Buowari OV. Fake and counterfeit drug: a review. Afrimedic J. 2013;3(2):1–4. [Google Scholar]
- 2.Chika A, Bello SO, Jimoh AO, Umar MT. The menace of fake drugs: consequences, causes and possible solutions. Res J Med Sci. 2006;1(5):257–261. [Google Scholar]
- 3.Lancet. Fighting fake drugs: the role of WHO and pharma. Lancet. 2011;377(9778):1626. [DOI] [PubMed]
- 4.Newton PN, Green MD, Fernandez FM, Day NP, White NJ. Counterfeit anti-infective drugs. Lancet Infect Dis. 2006;6(9):602–613. doi: 10.1016/S1473-3099(06)70581-3. [DOI] [PubMed] [Google Scholar]
- 5.Mackey TK, Liang BA. Improving global health governance to combat counterfeit medicines: a proposal for a UNODC-WHO-Interpol trilateral mechanism. BMC Med. 2013;11:233. doi: 10.1186/1741-7015-11-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Report of the situation of counterfeit medicines bases on data collection tool: WHO Regions for Africa and Eastern Mediterranean. 2010. Available at http://www.who.int/medicines/services/expertcommittees/pharmprep/WHO-ACM-3IMPACTSurveyDataCollectionToolReport.pdf. Accessed 7 July 2016.
- 7.Newton PN, Amin AA, Bird C, Passmore P, Dukes G, Tomson G, et al. The primacy of public health considerations in defining poor quality medicines. PLoS Med. 2011;8(12):e1001139. doi: 10.1371/journal.pmed.1001139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark F. Rise in online pharmacies sees counterfeit drugs go global. Lancet. 2015;386(10001):1327–1328. doi: 10.1016/S0140-6736(15)00394-3. [DOI] [PubMed] [Google Scholar]
- 9.MacKey TK, Liang BA. Promoting online drug safety: using public-private partnerships to deter illicit online drug sales. J Commer Biotechnol. 2011;17(3):266–271. doi: 10.1057/jcb.2011.19. [DOI] [Google Scholar]
- 10.Ratanawijitrasin S, Wondemagegnehu, E. Effective drug regulation. A multicountry study. Geneva: World Health Organization; 2002.
- 11.World Health Organization. International Medical Products Anti-Counterfeiting Taskforce. Counterfeit drugs kill! 2008. Available at: http://www.who.int/impact/FinalBrochureWHA2008a.pdf. Accessed 8 July 2016.
- 12.Kelesidis T, Kelesidis I, Rafailidis PI, Falagas ME. Counterfeit or substandard antimicrobial drugs: a review of the scientific evidence. J Antimicrob Chemother. 2007;60(2):214–236. doi: 10.1093/jac/dkm109. [DOI] [PubMed] [Google Scholar]
- 13.Swaminath G. Faking it-I The menace of counterfeit drugs. Indian J Psychiatry. 2008;50(4):238–240. doi: 10.4103/0019-5545.44743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bigdeli M, Javadi D, Hoebert J, Laing R, Ranson K, Alliance for Health Policy and Systems Research Network of Researchers on Access to Medicines Health policy and systems research in access to medicines: a prioritized agenda for low- and middle-income countries. Health Res Policy Syst. 2013;11:37. doi: 10.1186/1478-4505-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Jardali F, Akl EA, Fadlallah R, Oliver S, Saleh N, El-Bawab L, et al. Interventions to combat or prevent drug counterfeiting: a systematic review. BMJ Open. 2015;5(3):e006290. doi: 10.1136/bmjopen-2014-006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edwards N, Barker PM. The importance of context in implementation research. J Acquir Immune Defic Syndr. 2014;67(Suppl 2):S157–S162. doi: 10.1097/QAI.0000000000000322. [DOI] [PubMed] [Google Scholar]
- 17.Lavis JN, Rottingen JA, Bosch-Capblanch X, Atun R, El-Jardali F, Gilson L, et al. Guidance for evidence-informed policies about health systems: linking guidance development to policy development. PLoS Med. 2012;9(3):e1001186. doi: 10.1371/journal.pmed.1001186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewin S, Bosch-Capblanch X, Oliver S, Akl EA, Vist GE, Lavis JN, et al. Guidance for evidence-informed policies about health systems: assessing how much confidence to place in the research evidence. PLoS Med. 2012;9(3):e1001187. doi: 10.1371/journal.pmed.1001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells S, Bullen C. A near miss: the importance of context in a public health informatics project in a New Zealand case study. J Am Med Inform Assoc: JAMIA. 2008;15(5):701–704. doi: 10.1197/jamia.M2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anonymous. Supreme Court won’t review invasion-of-privacy case. AIDS Policy Law. 1996;11(21):10. [PubMed]
- 21.World Health Organization. Medicines: spurious/falsely-labelled/falsified/counterfeit (SFFC) medicines. Fact sheet N 275. 2012. http://www.who.int/mediacentre/factsheets/fs275/en/. Accessed 7 Apr 2014.
- 22.Cuchet-Chosseler M, Bocoum O, Camara M, Abad B, Yamani E, Ordre des Pharmaciens du M. Results of a survey to evaluate the efficacy of a regional awareness campaign on counterfeit street medicines in Bamako, Mali and Nouakchott, Mauritania. Med Trop (Mars). 2011;71(2):152–6. [PubMed]
- 23.Olsson S, Pal SN, Stergachis A, Couper M. Pharmacovigilance activities in 55 low- and middle-income countries: a questionnaire-based analysis. Drug Saf. 2010;33(8):689–703. doi: 10.2165/11536390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Safety and security on the internet: challenges and advances in member states: based on the findings of the second global survey on eHealth. World Health Organization; 2011.
- 25.Abbasi A, Zahedi FM, Kaza S. Detecting fake medical web sites using recursive trust labeling. ACM trans Inf Syst. 2012;30(4):22–36. doi: 10.1145/2382438.2382441. [DOI] [Google Scholar]
- 26.Patel A, Norris P, Gauld R, Rades T. Drug quality in South Africa: perceptions of key players involved in medicines distribution. Int J Health Care Qual Assur. 2009;22(5):547–560. doi: 10.1108/09526860910975643. [DOI] [PubMed] [Google Scholar]
- 27.Erhun WO, OO Babalola, MO Erhun. Drug regulation and control in Nigeria: the challenge of counterfeit drugs. J Health Popul Dev Ctries 2001;4(2): 23–4.
- 28.Jin S. The mobile laboratory: a new concept in medicines surveillance. WHO Drug Inf. 2009;23(1):16–20. [Google Scholar]
- 29.Risha PG, Msuya Z, Clark M, Johnson K, Ndomondo-Sigonda M, Layloff T. The use of minilabs to improve the testing capacity of regulatory authorities in resource limited settings: Tanzanian experience. Health Policy. 2008;87(2):217–222. doi: 10.1016/j.healthpol.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Lai CW, Chan WK. Legislations combating counterfeit drugs in Hong Kong. Hong Kong Med J. 2013;19(4):286–293. doi: 10.12809/hkmj133841. [DOI] [PubMed] [Google Scholar]
- 31.Laven DL. Prescription drug wholesalers: drug distribution and the inspection process (a Florida perspective) J Pharm Pract. 2006;19(4):196–214. doi: 10.1177/0897190006293518. [DOI] [Google Scholar]
- 32.Cohn J, von Schoen-Angerer T, Jambert E, Arreghini G, Childs M. When falsified medicines enter the supply chain: description of an incident in Kenya and lessons learned for rapid response. J Public Health Policy. 2013;34(1):22–30. doi: 10.1057/jphp.2012.53. [DOI] [PubMed] [Google Scholar]
- 33.Simoens S. Analysis of drug authentication at the point of dispensing in Belgian and Greek community pharmacies. Ann Pharmacother. 2009;43(10):1701–1706. doi: 10.1345/aph.1M215. [DOI] [PubMed] [Google Scholar]
- 34.Astra Zeneca. EFPIA product verification project-joint final report. European Federation of Pharmaceutical Industries and Associations; 2010. p. 1–32.
- 35.Altunkan SM, Yasemin A, Aykac IT, Akpinar E. Turkish pharmaceuticals track and trace system. Health Inform Bioinform (HIBIT) 2012:24–30.
- 36.Labadie J. Forensic pharmacovigilance and substandard or counterfeit drugs. Int J Risk Saf Med. 2012;24(1):37–39. doi: 10.3233/JRS-2012-0551. [DOI] [PubMed] [Google Scholar]
- 37.Kabore L, Millet P, Fofana S, Berdai D, Adam C, Haramburu F. Pharmacovigilance systems in developing countries: an evaluative case study in Burkina Faso. Drug Saf. 2013;36(5):349–358. doi: 10.1007/s40264-013-0043-9. [DOI] [PubMed] [Google Scholar]
- 38.Hartigan-Go K. Developing a pharmacovigilance system in the Philippines, a country of diverse culture and strong traditional medicine background. Toxicology. 2002;181–182:103–107. doi: 10.1016/S0300-483X(02)00263-9. [DOI] [PubMed] [Google Scholar]
- 39.Thomson J, Reid WS, Pullen H. Evaluation of counterfeit medicines available via the internet in Germany; implications for patient safety across the globe. J Sex Med. 2013;10:254–255. [Google Scholar]
- 40.Bate R, Tren R, Hess K, Mooney L, Porter K. Pilot study comparing technologies to test for substandard drugs in field settings. Afr J Pharm Pharmacol. 2009;3(4):165–170. [Google Scholar]
- 41.Growing threat from counterfeit medicines. Bull World Health Organ. 2010;88(4):247–8. [DOI] [PMC free article] [PubMed]
- 42.Williams A. Europe prepares to battle the counterfeiters. Pharm Technol Eur 2011;23:9–11.
- 43.Orizio G, Merla A, Schulz PJ, Gelatti U. Quality of online pharmacies and websites selling prescription drugs: a systematic review. J Med Internet Res. 2011;13(3):e74. doi: 10.2196/jmir.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fung CH, Woo HE, Asch SM. Controversies and legal issues of prescribing and dispensing medications using the Internet. Mayo Clin Proc. 2004;79(2):188–194. doi: 10.4065/79.2.188. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen S, Barratt MJ. Prescription drug misuse: is technology friend or foe? Drug Alcohol Rev. 2009;28(1):81–86. doi: 10.1111/j.1465-3362.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- 46.Department of Justice, Drug Enforcement Administration. Implementation of the Ryan Haight Online Pharmacy Consumer Protection Act of 2008; Final Rule. Fed Regist. 2009;74(64):15596–625. [PubMed]
- 47.Bate R, Hess K. Assessing website pharmacy drug quality: safer than you think? PLoS One. 2010;5(8):e12199. doi: 10.1371/journal.pone.0012199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arrunada B. Quality safeguards and regulation of online pharmacies. Health Econ. 2004;13(4):329–344. doi: 10.1002/hec.827. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.