Abstract
Peptidylarginine deiminase (PAD) enzymes convert histone tail arginine residues to citrulline resulting in chromatin decondensation. Our previous work found that PAD isoforms are expressed in female reproductive tissues in an estrous cycle-dependent fashion, but their role in the anterior pituitary gland is unknown. Thus, we investigated PAD expression and function in gonadotrope cells. The gonadotrope-derived LβT2 cell line strongly expresses PAD2 at the protein level compared with other PAD isoforms. Consistent with this, PAD2 protein expression is highest during the estrous phase of the estrous cycle and colocalizes with the LH β-subunit in the mouse pituitary. Using the GnRH agonist buserelin (GnRHa), studies in LβT2 and mouse primary gonadotrope cells revealed that 30 minutes of stimulation caused distinct puncta of PAD2 to localize in the nucleus. Once in the nucleus, GnRHa stimulated PAD2 citrullinates histone H3 tail arginine residues at sites 2, 8, and 17 within 30 minutes; however, this effect and PAD2 nuclear localization was blunted by incubation of the cells with the pan-PAD inhibitor, biphenyl-benzimidazole-Cl-amidine. Given that PAD2 citrullinates histones in gonadotropes, we next analyzed the functional consequence of PAD2 inhibition on gene expression. Our results show that GnRHa stimulates an increase in LHβ and FSHβ mRNA and that this response is significantly reduced in the presence of the PAD inhibitor biphenyl-benzimidazole-Cl-amidine. Overall, our data suggest that GnRHa stimulates PAD2-catalyzed histone citrullination in gonadotropes to epigenetically regulate gonadotropin gene expression.
GnRH secreted from hypothalamic neurons binds to GnRH receptors (GnRHRs) on the plasma membrane of anterior pituitary gonadotropes. After GnRHR activation, increases in intracellular calcium from both the endoplasmic reticulum (ER) and voltage-gated calcium channels (VGCCs) initiate multiple MAPK signaling cascades, including ERK, c-Jun N-terminal kinase, and p38 MAPK (1, 2). Major downstream targets of MAPK pathways are transcription factors, which upon activation mediate the expression of the gonadotropins, LH and FSH (3, 4). Within the gonadotrope, LH and FSH are glycoproteins that consist of a common α-subunit and unique β-subunits (LHβ and FSHβ) that heterodimerize to form functional hormone. LH and FSH are critical for spermatogenesis, folliculogenesis, and ovulation in male and female gonads, respectively.
Given the essential role of gonadotropin synthesis to fertility, substantial work has investigated the mechanisms initiated by GnRH to regulate gonadotropin gene expression at the promoter/transcription factor level. However, a critical component of this mechanism requires that gonadotropin gene chromatin is decondensed so that it can access the transcriptional machinery. Modifications of histone tail amino acids are an important epigenetic pathway that gonadotropes utilize to accomplish this decondensation. For example, GnRH stimulation of the gonadotrope-derived αT3–1 cell line alters acetylation and methylation of histones, decondensing chromatin associated with gonadotropin subunit genes (5, 6). Despite the importance of these studies to our understanding of gonadotropin gene expression, other histone tail modifications have not yet been investigated in gonadotropes.
Peptidylarginine deiminases (PADs) are a family of calcium-dependent enzymes that convert positively charged arginine residues on target proteins to uncharged citrulline residues through a reaction termed deimination or citrullination. There are 5 PAD isoforms (PAD1–PAD4 and PAD6) organized in a highly conserved genomic arrangement on human chromosome 1 and on an orthologous region of mouse chromosome 4 (7). Some PAD enzymes display overlapping tissue expression patterns, but accumulating evidence suggests that each family member has distinct substrate specificities (8, 9). One exception is PAD6, which does not appear to possess catalytic activity but is associated with fibrous cytoplasmic lattices in mouse oocytes (10). A growing body of evidence also indicates that PAD expression is important to the function of female reproductive tissues. For example, studies by Horibata et al indicate that the expression of PAD2 and PAD4 change across the estrous cycle in mouse uterine and mammary tissue with highest expression during estrus (11). Despite this intriguing regulation of PADs, currently little is known about their expression or function in the anterior pituitary gland.
A well-characterized target of PAD enzymatic activity is arginine residues on histone tails. To date, only PAD2 and PAD4 have been shown to citrullinate histones, which results in chromatin decondensation and changes in gene expression. The first indication that PAD2 is an epigenetic regulator came from our studies in the canine mammary gland showing citrullinated histones (12). In addition, our work in MCF-7 breast cancer cells highlights that PAD2 localizes to punctate euchromatic regions in the nucleus and citrullinates histones resulting in changes in gene expression (13). Although PAD2 clearly has important functions in gene expression in MCF-7 cells, it is unclear whether a similar mechanism exists in gonadotropes.
Here, we report that PAD2 protein levels are higher than other PAD isoforms in LβT2 cells. In addition, PAD2 protein expression is sexually dimorphic in mouse gonadotropes, with low levels in the male and high expression during estrus in the female. GnRH agonist buserelin (GnRHa) stimulation of LβT2 or mouse primary pituitary cells for 30 minutes induces the localization of PAD2 to 4',6-diamidino-2-phenylindole (DAPI) poor, punctate regions in the nucleus. Once in the nucleus, PAD2 citrullinates histone H3 tail residues 2, 8, and 17, and this catalytic activity is inhibited by a pan-PAD inhibitor biphenyl-benzimidazole-Cl-amidine (BB-ClA). Finally, we show that stimulation of LβT2 cells with GnRHa increase LHβ and FSHβ mRNA expression; however, this response is inhibited by treatment with BB-ClA. Collectively, our results show that GnRHa stimulates a novel epigenetic mechanism to regulate gonadotropin gene expression.
Materials and Methods
Materials
Primary antibodies against PAD1 (ab24003), histone H3cit 2, 8, 17 (ab5103), and total histone H3 (ab1791) were purchased from Abcam. The anti-PAD2 antibody (12110–1-AP) was purchased from ProteinTech, and the anti-PAD3 antibody (ABIN347067) was purchased from Antibodies-Online. The anti-PAD4 (P4749) and anti-β-tubulin (T4026) antibodies and GnRHa (B3303) were purchased from Sigma-Aldrich. Horseradish peroxidase secondary antibodies were purchased from Cell Signaling Technology. The anti-LHβ antibody was obtained from the National Hormone and Peptide Program (National Institute of Diabetes and Digestive and Kidney Diseases). All fluorescently labeled Alexa Fluor secondary antibodies were purchased from Molecular Probes. Matrigel was purchased from BD Biosciences, and casein was from Vector Laboratories, Inc. The 35-mm glass bottom culture dishes were purchased from Mat-Tek Corp. The PAD inhibitor, BB-ClA, was synthesized by Dr Paul Thompson as previously described (14).
Cell culture
LβT2 cells, a generous gift from Dr Pamela Mellon (University of California, San Diego, CA), were maintained in high glucose DMEM containing 2mM glutamine, 100-U penicillin/mL, 100-μg streptomycin/mL, and 10% fetal bovine serum (FBS) (HyClone). All cells were grown in 5% CO2 at 37°C in a humidified environment.
Histone purification and chromatin fractionation
LβT2 cells were incubated with phenol red-free medium containing charcoal-stripped FBS (DMEM/F-12 1:1 phenol red free, 2.5% charcoal-stripped FBS, and 1% Penicillin/Streptomycin) for 6 hours followed by treatment with vehicle or GnRHa (10nM) for 0, 30, 60, and 180 minutes. For PAD inhibition experiments, LβT2 cells were pretreated with Dimethyl Sulfoxide or 1μM BB-ClA for 12 hours in charcoal-stripped FBS medium followed by treatments described above. For calcium studies, LβT2 cells were serum starved for 6 hours, then treated with vehicle, FPL 64176 (500nM), Thapsigargin (50nM), or GnRHa (10nM) for 30 minutes. Histones were purified using an acid extraction method as previously described (15). Briefly, LβT2 cells were washed with 1× PBS and lysed with hypotonic lysis buffer (10mM Tris [pH 8], 1mM KCl, 1.5mM MgCl2,0.5% Nonidet-P40, 1mM Dithiothreitol, 1× protease inhibitor, 1× phenylmethylsulfonyl fluoride, and dH2O). The lysate was placed on ice for 30 minutes, then nuclei were isolated by centrifugation (10 000g for 10 min at 4°C). The nuclei pellet was dissolved in 0.4M H2SO4 and rotated overnight at 4°C. After centrifugation at 17 000g for 10 minutes at 4°C, the supernatant was collected and 100% Trichloroacetic acid was added (20% of total volume) and incubated on ice for 20 minutes. Centrifugation at 17 000g for 10 minutes at 4°C was again conducted, the supernatant was then discarded, and the pellet was washed with 300 μL of cold acetone. The purified histone pellets were air dried for approximately 10 minutes, resuspended in 50 μL of nanopure H2O, and quantified using a Pierce 660 nm Protein Assay. Chromatin fractionation was carried out as previously described (16). Protein concentrations for resulting fractions were determined by Pierce 660 nm Protein Assay and subject to Western blot analysis as described below.
Western blottings
Positive controls for PAD antibodies were generated by overexpressing human PAD 1–4 plasmids for 24 hours following a Mirus Bio TranIT-2020 transfection protocol. LβT2 cells were lysed with Radio-Immunoprecipitation Assay buffer containing 50mM Tris, 150mM NaCl, 0.1% Sodium Dodecyl Sulfate, 0.5% deoxycholate, 1% Triton X-100, 1mM phenylmethylsulfonyl fluoride, and 1× protease inhibitor (Thermo Scientific). The protein concentration of all cellular lysates and purified histones was measured by Pierce 660 nm Protein Assay before gel loading to ensure equal protein loading; 6× sample buffer consisting of 0.5M Tris-HCl (pH 6.8), 60% glycerol, 30mM Dithiothreitol, and 6% Sodium Dodecyl Sulfate was added into samples to yield a final concentration of 1× sample buffer and then boiled at 95°C for 5 minutes. The samples were subjected to SDS-PAGE using a 10% gel (acrylamide:bis-acrylamide ratio of 29:1) and subsequently transferred to Immobilin polyvinylidene difluoride membranes (EMD Millipore). Membranes were blocked in 2× casein (Vector Laboratories) diluted in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) overnight at 4°C. Primary antibodies were incubated overnight at 4°C: anti-PAD1 (1:1000), anti-PAD2 (1:2000), anti-PAD3 (1:800), anti-PAD4 (1:1000), or anti-H3cit 2, 8, 17 (1:1000) in 1× casein. The next morning, membranes were washed in TBS-T, followed by a 2-hour incubation at room temperature with 1:15 000 antirabbit horseradish peroxidase secondary antibody. All blots were washed for 50 minutes (5 × 10 min) with TBS-T after secondary antibody incubation and then visualized with a Bio-Rad Chemidoc XRS using SuperSignal West Pico or Femto chemiluminescence substrate (Pierce). To confirm equal protein loading, membranes were stripped and reprobed with anti-β-tubulin, anti-β-actin, or antitotal histone H3. Quantitative densitometry analysis was conducted with Bio-Rad Image Lab software.
Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
RNA was purified from LβT2 cells according to the Omega Bio-Tek Total RNA kit protocol (Omega Bio-Tek, Inc). One microgram of resulting RNA was reverse transcribed using iScript Reverse Transcription Supermix for RT-qPCR (Bio-Rad). TaqMan probe based qPCR was used to detect expression of LHβ, FSHβ, Common Glycoprotein alpha (CGA), GnRHR, and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Life Technologies). LβT2 cells were pretreated with DMSO or 1μM BB-ClA for 12 hours in phenol red-free media containing charcoal-stripped FBS. Cells were then treated with vehicle or GnRHa (10nM) for 0 or 180 minutes. RNA was purified and reverse transcribed as detailed above. Data were analyzed using the δ/δCt method, in which cycle threshold (Ct) values of all target genes are adjusted to corresponding Ct value of reference gene (GAPDH).
Mouse experiments
Male and female C57/Bl6 mice between 2 and 4 months of age were maintained on a 14-hour light, 10-hour dark cycle with ad libitum access to food and water. Euthanasia and tissue collection were performed in accordance with the guidelines outlined in the Report of the American Veterinary Medical Association on Euthanasia. All work in this study was approved by the University of Wyoming Institutional Animal Care and Use Committee (protocol number 20141009AN00125–02 and protocol 20160805AN00248–01).
Mouse vaginal cytology was conducted for 10 consecutive days on 8- to 16-week-old mice. After swabbing, vaginal cells were placed on slides with 30 μL of PBS and allowed to air dry. Slides were then stained with hematoxylin for 5 minutes, washed with dH2O, coversliped, and examined by light microscopy. Epithelial cells and leukocytes were differentiated based on cell morphology.
For pituitary lysates and primary culture studies, mice were euthanized on the morning of estrus. After euthanasia, pituitaries were explanted, rinsed free of blood in PBS, lysed in Radio-Immunoprecipitation Assay buffer, and sonicated. Pituitary lysates were then subjected to Western blot analysis using PAD isoform-specific antibodies. For primary culture, pituitaries (n = 10) were explanted and dispersed using collagenase, hyaluronidase, and deoxyribonuclease at 37°C for 30 minutes as previously described (17). Dissociated primary pituitary cultures were suspended in culture medium (DMEM supplemented with 10% FBS, 1% nonessential amino acids, 100-IU/mL penicillin, and 100-μg/mL streptomycin). Cells (1 × 106) were plated in 12-well dishes with 1 mL of medium and cultured overnight. The next morning, cells were serum starved for 6 hours then treated with vehicle or 10nM GnRHa for 0 or 30 minutes. Primary cultures were examined by immunofluorescence as described below.
LβT2 and mouse pituitary immunofluorescence
LβT2 cells were grown in 35-mm dish with a glass coverslip coated in diluted Matrigel (1:100). Cells were serum starved for 6 hours then treated with GnRHa (10nM) for 0, 30, and 60 minutes. The cells were fixed with 4% paraformaldehyde supplemented with 2% sucrose, then permeabilized with 0.05% Nonidet-P40 for 5 minutes at room temperature. The primary and secondary antibodies used in the Immunofluorescence protocol to detect PAD2 are described below. All samples were visualized on a Zeiss LSM 710 confocal microscope under a ×40 oil objective.
To image primary gonadotropes, pituitaries were explanted, rinsed free of blood in PBS, and fixed in 4% paraformaldehyde for 6 hours at 4°C before immersion in 20% sucrose. Pituitaries were then frozen in embedding medium (Tissue Tek, Sakura Finetech), sectioned (16 μm) on a cryostat, mounted on glass slides, and stored at −80°C. Slides were stained with anti-PAD2 (1:100) and anti-LHβ (1:50) antibodies diluted in 1× PBS overnight at 4°C. Tissues were washed 3 times for 5 minutes with 1× PBS and treated with the appropriate fluorescently labeled secondary antibodies for 2 hours at room temperature. Tissues were washed, stained with DAPI, coverslip mounted, and imaged.
Fluorescence quantification was performed using ImageJ and has been described previously (18, 19). Fluorescence intensity of PAD2 staining was measured in LHβ expressing gonadotropes using the region of interest feature in ImageJ and corrected for background fluorescence by highlighting pixels immediately adjacent to PAD2-immunoreactive gonadotropes. The following formula was used to calculate the corrected total cell fluorescence: integrated density − (area of selected cell × mean fluorescence of background readings).
Statistics
All statistical analysis was done using GraphPad Prism 6.0. Data are expressed as mean ± SEM of at least 3 independent experiments. Results were analyzed for significance using one-way ANOVA. Post hoc group comparisons were made using Tukey's honest significant difference (HSD) as appropriate with the critical value *, P < .05 and **, P < .01 for declaring significance.
Results
PAD2 is expressed in the gonadotrope-derived LβT2 cell line
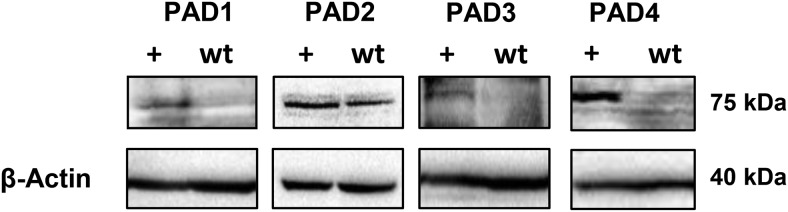
To investigate the role of PADs in gonadotropes, we first examined the protein expression levels of PAD1–PAD4 protein in LβT2 cells. PAD6 was excluded in our analysis due to its exclusive localization in oocytes and lack of catalytic activity (10). In order to validate the specificity and efficacy of the PAD antibodies, a subset of cells were transiently transfected with individual PAD1–PAD4 plasmids for 24 hours. Positive control samples (+) were compared against untransfected (wt) LβT2 cells. All antibodies detected their specific PAD isoform in overexpressing cells. Importantly, PAD2 protein levels are higher than the other PAD isoforms in untransfected (wt) LβT2 cells (Figure 1). This is the first data to show PAD expression in a gonadotrope-derived cell line.
Figure 1. PAD2 is expressed in the gonadotrope-derived LβT2 cell line.
Protein concentration of LβT2 cell lysates was determined and equal amounts examined by Western blotting. Membranes were probed with anti-PAD1, anti-PAD2, anti-PAD3, and anti-PAD4 antibodies or anti-β-actin for a loading control. Positive controls (+) were generated by transient overexpression of PAD1–PAD4 plasmids in LβT2 cells.
PAD2 expression in mouse gonadotropes is highest during estrus
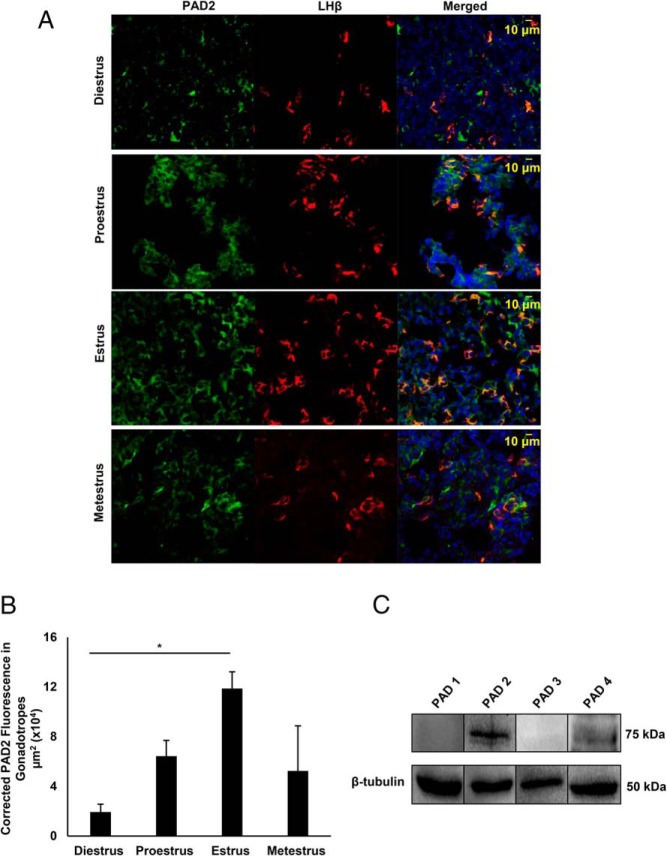
To validate our findings from LβT2 cells, we examined PAD2 expression in mouse primary gonadotrope cells from 2- to 4-month-old animals. Mice were estrous cycle staged by vaginal cytology, and pituitaries were collected during diestrus, proestrus, estrus, and metestrus. Pituitary sections were immunofluorescently stained using anti-PAD2 and anti-LHβ antibodies followed by imaging with confocal laser scanning microscopy (CLSM). Images revealed that PAD2 is expressed throughout the female anterior pituitary gland and, importantly, colocalizes with LHβ in gonadotrope cells (Figure 2A). In contrast, anterior pituitaries showed very low PAD2 staining in both sham and castrated males, suggesting sexual dimorphic regulation of PAD expression (Supplemental Figure 1A). Quantification of PAD2 fluorescence in LHβ-labeled gonadotropes in sham vs castrate males revealed no significant difference (Supplemental Figure 1B). In females, quantification of PAD2 fluorescence in LHβ-labeled gonadotropes revealed that PAD2 expression is highest during the estrus stage of the estrous cycle (P < .05) (Figure 2B). High PAD2 expression in the pituitary during estrus is consistent with previous work examining the uterus and mammary gland (11). Pituitaries collected during estrus were also examined by Western blotting for PAD protein expression. Similar to LβT2 cells, PAD2 protein expression is highest compared with other PADs, although low levels of PAD4 are also detected (Figure 2C). Our results highlight that PAD2 is the predominate PAD isoform expressed in the female anterior pituitary gland and its levels appear regulated over the estrous cycle.
Figure 2. PAD2 expression in mouse gonadotrope cells is highest during estrus.
A, Female mice were estrous cycle staged by vaginal cytology and pituitary glands were collected on the morning of diestrus, proestrus, estrus, and metestrus. Pituitaries were fixed, sectioned frozen (16 μm) and probed with appropriate anti-PAD2 (green) and anti-LHβ (red) antibodies and stained with DAPI (blue) to label nuclei. Tissues were imaged with a Zeiss LSM710 confocal microscope at ×40 resolution. B, Three independent pituitary tissue sections from each stage of the estrous cycle were examined for corrected total fluorescence micrometer square (μm2) of PAD2 in gonadotropes using the region of interest (ROI) feature in ImageJ software. Means were separated using Tukey's HSD, asterisks indicate significant differences (*, P < .05), and error bars are SEM. C. Mice were estrous cycle staged by vaginal cytology and pituitary glands collected during estrus. Protein concentrations of female mouse pituitary lysates were determined and equal concentrations loaded and examined by Western blotting. Membranes were probed with anti-PAD1, anti-PAD2, anti-PAD3, and anti-PAD4 antibodies or with anti-β-tubulin as a loading control.
GnRHa activation of L-type calcium channels induces PAD-catalyzed citrullination of arginine residues on histone H3 tails
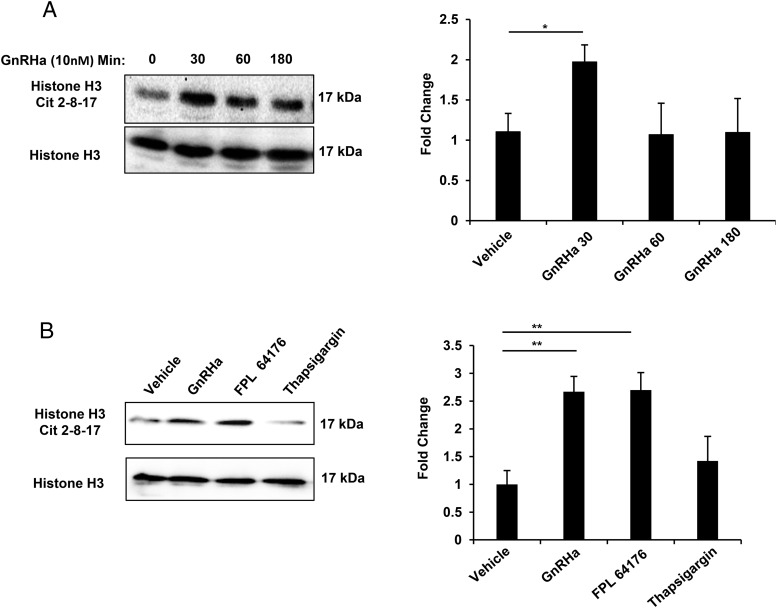
Because gonadotrope cells robustly express PAD2, we next hypothesized that GnRHa stimulation of LβT2 cells would increase PAD-catalyzed citrullination. To assess this, we examined histones, a well-characterized target of PAD activity. LβT2 cells were serum starved then treated with vehicle or 10nM GnRHa for 0, 30, 60, and 180 minutes. LβT2 histones were purified by acid extraction, quantified, and examined by Western blotting. Membranes were probed with primary antibodies that detect either citrullinated histone H3 arginine residues 2, 8, and 17 or total histone H3 as a loading control. The representative Western blotting on the left shows that levels of citrullinated histone H3 arginine residues 2, 8, and 17 are elevated after 30 minutes of GnRHa stimulation compared with vehicle control (Figure 3A). Quantification of Western blottings (n = 3) illustrated on the right indicates that 30 minutes of GnRHa stimulation significantly increases citrullinated histones (P < .05) (Figure 3A). After 60 and 180 minutes of treatment, levels of citrullinated histones were not significantly different than those from vehicle-treated cells.
Figure 3. GnRHa stimulation of L-type calcium channel activity induces citrullination of arginine residues on histone H3 tails.
A, Cells were serum starved for 6 hours, then treated with either vehicle or 10nM GnRHa for 0, 30, 60, or 180 minutes. After treatment, histones were purified by acid extraction and quantified, and equal concentrations were examined by Western blotting. Membranes were probed with an antihistone H3 arginine residue 2, 8, and 17 antibody or antihistone H3 total antibody as a loading control. Bar graphs on the right show quantitative analysis of the Western blottings (n = 3) conducted using Bio-Rad ImageLab software and normalized to total histone H3 levels. Means were separated using Tukey's HSD, asterisks indicate significant differences (P < .05), whereas error bars are SEM. B, Cells were serum starved for 6 hours, then treated with either vehicle, GnRHa (10nM), FPL 64176 (500nM), or Thapsigargin (50nM) for 30 minutes followed by stimulation with vehicle or 10nM GnRHa. After treatment, histones were purified and equal concentrations examined by Western blotting as described above. Bar graphs on the right show quantitative analysis of the Western blottings (n = 3) conducted using Bio-Rad ImageLab software and normalized to total histone H3 levels. Means were separated using Tukey's HSD, asterisks indicate significant differences (**, P < .01), and error bars are SEM.
It is well documented that PAD activity is calcium dependent (20, 21). Therefore, we next investigated whether GnRHa-induced intra- or extracellular calcium release is required for histone citrullination. To assess the role of extracellular calcium, we used the nondihydropyridine L-type Ca2+ channel agonist FPL 64176, which previously was shown to induce calcium influx in gonadotrope cells (22). To assess the role of intracellular calcium, we raised cytosolic calcium concentrations by using Thapsigargin, an inhibitor of the ER Ca2+-ATPase. LβT2 cells were treated with vehicle, GnRHa (10nM), FPL 64176 (500nM), or Thapsigargin (50nM) for 30 minutes and subjected to histone purification and Western blot analysis. The representative Western blotting on the left and bar graph quantifying multiple blots (n = 3) on the right indicate that L-type VGCCs are the main calcium source necessary to induce histone citrullination (Figure 3B). Thus, GnRHa-induced calcium influx from L-type VGCCs rapidly induces PAD activation and subsequent downstream histone citrullination in LβT2 cells.
GnRHa induces PAD2 nuclear localization in LβT2 cells
If GnRHa induces histone citrullination, then PAD2 should localize to the nucleus of gonadotropes. Thus, we next examined the subcellular localization of PAD2 in LβT2 cells after GnRHa treatment. Cells were treated with vehicle or 10nM GnRHa for 0, 30, and 60 minutes. Using an IF protocol, cells were probed with an anti-PAD2 antibody and an Alexa Fluor 488-conjugated secondary antibody followed by imaging with CLSM. Although vehicle-treated cells showed diffuse PAD2 expression, 30 minutes of GnRHa treatment results in a redistribution of PAD2 to distinct puncta inside the nucleus (Figure 4A). After 60 minutes of GnRHa treatment, PAD2 levels in the cytoplasm also appear to increase (Figure 4A). To confirm our results, we next used small scale chromatin fractionation to isolate chromatin-associated proteins from LβT2 cell nuclei following the same GnRHa treatment paradigm. Our results indicate that 30 minutes of GnRHa stimulation increases the association of PAD2 with chromatin in LβT2 cells (Figure 4B).
Figure 4. GnRHa induces PAD2 nuclear localization in LβT2 cells.
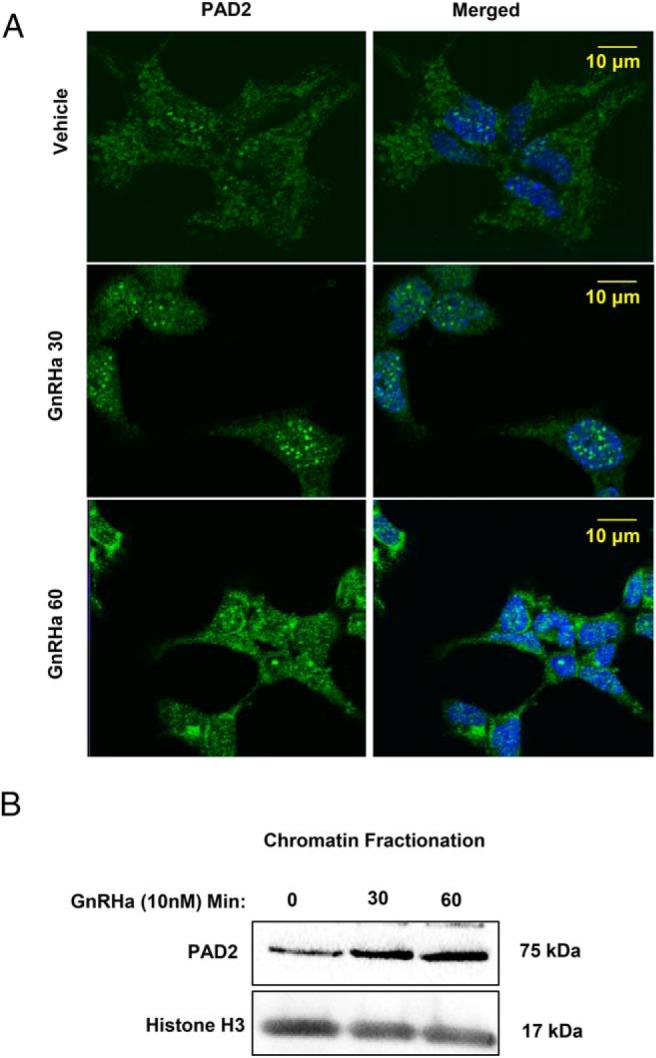
A, LβT2 cells were plated on glass bottom confocal dishes, serum starved for 6 hours, and then treated with either vehicle or 10nM GnRHa for 0, 30, or 60 minutes. Cells were then fixed in 4% PFA and probed with an anti-PAD2 antibody with the appropriate fluorescently labeled secondary antibody and stained with DAPI. Cells were imaged with a confocal microscope using a ×40 objective. B, LβT2 cells were serum starved for 6 hours, then treated with either vehicle or 10nM GnRHa for 0, 30, or 60 minutes. The chromatin-associated protein fraction was isolated and quantified, and equal concentrations were examined by Western blotting. Membranes were probed with an anti-PAD2 and with antihistone H3 total as a loading control.
PAD inhibition decreases GnRHa-induced histone citrullination in LβT2 cells
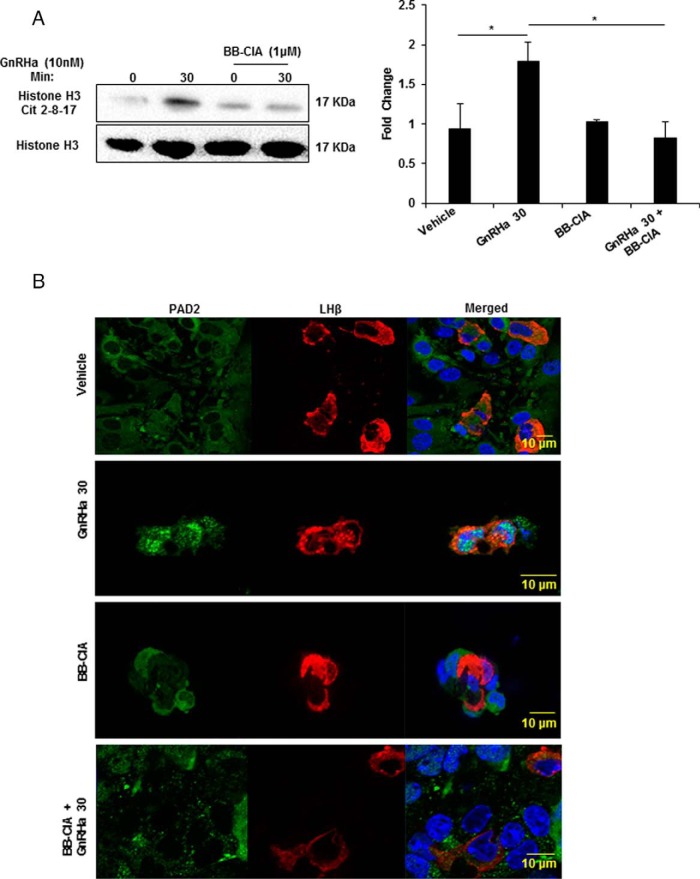
To confirm PAD-catalyzed histone citrullination in gonadotropes, we used BB-ClA, a pan-PAD inhibitor that strongly inhibits PAD catalytic activity by covalently blocking the active site (23). LβT2 cells were pretreated with DMSO or 1μM BB-ClA in media containing 2.5% charcoal-stripped FBS for 12 hours. Next, cells were treated with vehicle or 10nM GnRHa for 0 or 30 minutes. After treatment, histones were isolated, quantified, and examined by Western blotting. Consistent with previous results (Figure 3A), stimulation of LβT2 cells with 10nM GnRHa for 30 minutes increased the level of citrullinated histone H3 arginine residues 2, 8, and 17. In contrast, pretreatment of LβT2 cells with 1μM with BB-ClA decreased GnRHa-induced citrullination of histone H3 arginine residues 2, 8, and 17 (Figure 5A). Quantification of blots (n = 3) revealed that BB-ClA significantly inhibits the increase in citrullinated histones after 30 minutes of 10nM GnRHa treatment (P < .05) (Figure 5A). Thus, GnRHa-induced histone citrullination is significantly inhibited when PAD activity is blocked.
Figure 5. PAD inhibition decreases GnRHa-induced histone citrullination in LβT2 cells.
A, LβT2 cells were pretreated for 12 hours with DMSO or 1μM BB-ClA, then treated with either vehicle or 10nM GnRHa for 0 and 30 minutes. After treatment, histones were purified by acid extraction and quantified, and equal concentrations were examined by Western blotting. Membranes were probed with an antihistone H3 antibody that detects citrullination at arginine residues 2, 8, and 17 and antihistone H3 as a loading control. Bar graphs on the right show quantitative analysis of the Western blottings (n = 3) conducted using Bio-Rad ImageLab software and normalized to total histone H3 levels. Means were separated using Tukey's HSD, asterisks indicate significant differences (P < .05), and error bars are SEM. B, Mice were estrous cycle staged by vaginal cytology and pituitary glands collected during estrus. Pituitaries were explanted, dispersed, and cultured for 12 hours with DMSO or 1μM BB-ClA. Cells were next treated with vehicle or 10nM GnRHa for 30 minutes, then fixed and probed with anti-PAD2 (green) and anti-LHβ (red) antibodies and stained with DAPI (blue). Tissues were imaged with a Zeiss LSM710 confocal microscope using a ×40 objective.
Following PAD inhibition, it is possible that the reduction in GnRHa-induced histone citrullination is associated with a loss of PAD2 mobilization to the nucleus. To address this, female murine pituitaries were collected during estrus, dispersed, and plated. Pituitary cells were treated for 12 hours with DMSO or 1μM BB-ClA and the next morning received vehicle or 10nM GnRHa for 30 minutes. Cells were then subjected to IF using anti-PAD2 and anti-LHβ antibodies followed by imaging with CLSM. In LHβ-labeled gonadotropes, PAD2 expression shows diffuse staining with vehicle treatment, but 30 minutes of GnRHa simulation induces PAD2 localization to distinct puncta in the nucleus (Figure 5B). This result is consistent with data from LβT2 cells in Figure 4A. Interestingly, pretreatment of murine pituitary cells with 1μM BB-ClA prevents nuclear localization of PAD2 after GnRHa stimulation. In LβT2 cells, the same experimental paradigm also blocks PAD2 nuclear localization (Supplemental Figure 2). These results suggest that PAD activity may be necessary for PAD2 mobilization to the nucleus.
Inhibition of PAD-catalyzed citrullination reduces LHβ and FSHβ mRNA expression
It is well established that GnRH stimulates the synthesis of specific gene products that are critical for reproduction function. In order to investigate the functional role of GnRHa-induced histone citrullination in gonadotropes, we examined the expression of known target genes: Lhb, Fshb, Gnrhr, and Cga. LβT2 cells were pretreated with DMSO or 1μM BB-ClA for 12 hours, then stimulated with 10nM GnRHa for 0 or 180 minutes. After treatment, RNA was purified, reverse transcribed, and resulting cDNA analyzed by TaqMan probe-based qPCR for LHβ, FSHβ, GnRHR, CGA, and GAPDH as a control. Our results show an increase in LHβ and FSHβ mRNA following GnRHa treatment; however, this increase is significantly attenuated by BB-ClA pretreatment (Figure 6). In contrast, the same treatment paradigm did not significantly alter the expression of CGA mRNA (Figure 6) or GnRHR mRNA (data not shown). This novel finding suggests that GnRHa induces PAD-catalyzed citrullination in gonadotropes to regulate gonadotropin β-subunit gene expression.
Figure 6. Inhibition of PAD-catalyzed citrullination reduces LHβ and FSHβ mRNA expression.
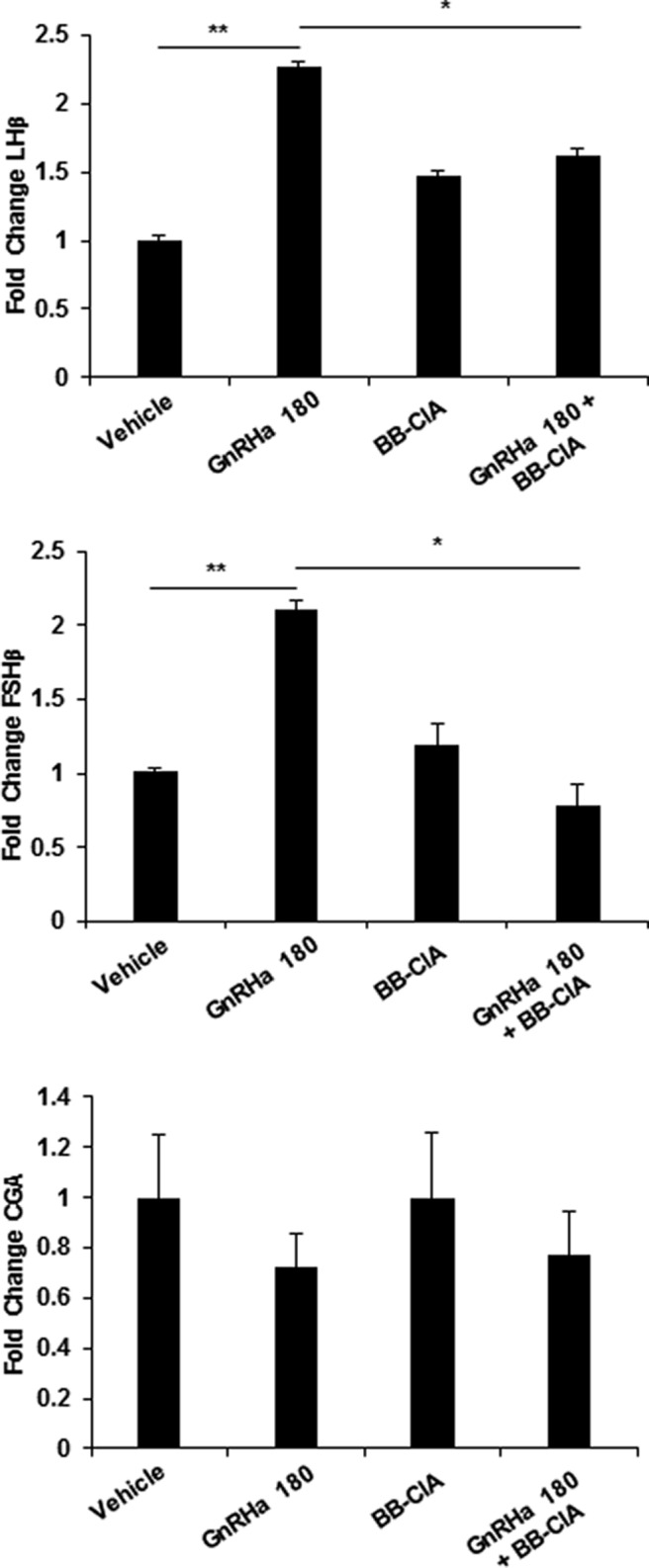
LβT2 cells were pretreated for 12 hours with DMSO or 1μM BB-ClA, then treated with either vehicle or 10nM GnRHa for 0 and 180 minutes. Total RNA was purified from cells, reverse transcribed, and resulting cDNA examined by TaqMan probe qPCR for LHβ, FSHβ, CGA, and GAPDH as the reference gene control. All data values were normalized to vehicle treated to yield fold change, and data are expressed as mean ± SEM. Means were separated using Tukey's HSD, and asterisks indicate significant differences (*, P < .05 and **, P < .01).
Discussion
Here, we examined PAD expression and function in anterior pituitary gland gonadotropes to further define the role of PADs in female reproduction. Our studies clearly show that PAD2 is present in multiple cells types in the female mouse anterior pituitary, including LHβ containing gonadotropes. In addition, our Western blot analysis indicates that PAD2 is the predominate PAD isoform expressed in the female anterior pituitary. This finding is supported by RNA-sequencing (RNA-seq) data, which show that PAD2 is the most highly expressed isoform in purified mouse gonadotropes (24). It should be noted that there is weak, but detectable, levels of PAD4 protein present, suggesting the possibility of functional overlap between the 2 PAD isoforms. This may explain why PAD2 knockout and PAD4 knockout mice have not proven to be independently useful models for reproductive studies due to the compensatory role that PADs appear to play for one another (25).
Our work highlights that PAD2 expression is largely absent in the anterior pituitary gland of intact and castrated males. These results suggest that neither GnRH nor testosterone regulate PAD2 expression in the male. Interestingly, previous studies in rats show that PAD expression increases as males age (26). In 3- and 9-month-old males, there is negligible PAD expression or activity in pituitaries; however, this increases by 18 and 24 months of age. The factors regulating this increase are currently unknown. Our male PAD2 protein expression results contrast with RNA-seq data showing that PAD2 mRNA in 14-week-old male murine gonadotropes is similar to that of cycling females (24). The reason for this discrepancy is unclear, although there is strong evidence for translational regulation of PAD2. For example, monocytes contain the same level of PAD2 mRNA as macrophages, but little PAD2 protein compared with macrophages (27). Collectively, the regulation of expression and activity of PAD2 in the male pituitary appears unique from that of the female and this interesting discrepancy is a focus of our ongoing work.
Previous studies have also demonstrated that PAD activity in the rat anterior pituitary gland is estrous cycle dependent and sexually dimorphic (28, 29). The work describing rat pituitary PAD activity occurred before the characterization of the multiple PAD isoforms but, based on our data, is most likely PAD2. Additional studies also concluded that the PAD enzymes are also expressed in lactotrope cells and that levels increase during pregnancy (30, 31). This work, however, did not examine PAD expression over the course of the estrous cycle. Thus, it is likely that PAD2 expression in female pituitaries is also localized to lactotropes, although the functional significance of this is currently unclear.
PAD2 has 6 calcium-binding sites that are critical for its enzymatic activity (21). Given this dependence, at issue is the source of GnRH-induced calcium release that is involved in PAD activation. It is well established that GnRH stimulation of gonadotropes induces an initial rise in cytosolic calcium that reflects release from intracellular ER stores (32, 33). Following this, a more sustained phase of calcium elevation occurs, due primarily to influx of extracellular calcium via L-type voltage-gated channels (34, 35). Our results suggest that calcium influx through VGCC using the calcium channel agonist FPL 64176 is sufficient to stimulate an increase in histone H3 citrullination by PADs similar to GnRHa treatment. Recent work shows that the VGCC signal within the gonadotrope is spatially restricted to microdomains that can induce localized ERK activation at the sites of Ca2+ influx (22). It is possible that localization of PAD2 near activated VGCCs could promote discrete sites of elevated calcium concentrations that are sufficiently high enough to induce PAD activity and subsequent histone citrullination (36).
Once activated by GnRH-induced calcium influx, PAD2 translocates to the nucleus where it forms distinct puncta in both primary gonadotropes and LβT2 cells. A similar observation was made in MCF-7 nuclei, where PAD2 forms puncta in DAPI poor regions and colocalizes with acetylated histone H3 lysine 9, a euchromatic marker (13). Interestingly, in gonadotropes, PAD2 nuclear localization is blocked by BB-ClA. Thus, it would appear that PAD-catalyzed citrullination is a necessary signal for PAD2 nuclear localization. Whether this localization requires autocitrullination of PAD2 or citrullination of an associated protein remains unknown, and studies are underway to test these intriguing possibilities. Once in the nucleus, PADs hydrolyze the positive guanidinium group of arginine residues on histone H2A, H3, and H4 tails to alter the charge association between DNA and histone octomers to decondense chromatin and subsequently alter gene expression (37, 38). Effectively, PADs antagonize histone arginine methylation by coactivator-associated arginine methyltransferase 1 and protein arginine methyltransferase 1 (37).
Epigenetic mechanisms are known to regulate expression of gonadotropin genes. For example, expression of the gonadotropin β-subunit genes is regulated by both histone acetyltransferases and histone deacetylases (5, 39). In addition, GnRH stimulation increases levels of histone H3 lysine 4 trimethylation associated with the β-subunit gene promoters (6). Our data show that GnRH stimulation also induces PAD2-catalyzed citrullination of histone H3 arginine residues 2, 8, and 17 but that the PAD inhibitor BB-ClA attenuates this effect. BB-ClA is currently the most potent and selective PAD2 inhibitor available for both in vitro and in vivo studies (14, 40). The attenuation rather than complete elimination of PAD-catalyzed histone citrullination may indicate that newly synthesized PAD2 is less affected by inhibition as BB-ClA reaches its half-life.
Our results suggest that PAD-catalyzed histone citrullination regulates gonadotropin β-subunit gene expression; however, Chromatin immunoprecipitation (ChIP) studies with PAD2 and citrullinated histone antibodies will be needed to test whether this is a direct affect. More globally, histone citrullination may regulate the expression of a distinct subset of gonadotrope genes after GnRH stimulation, and RNA-seq studies are currently underway to identify the full cohort of genes. Moving forward, it will be important to evaluate genes regulated by citrullination, along with acetylation and methylation, to determine the critical set of histone modifications that regulate expression of gonadotropin genes.
At issue is how these histone modifications work in concert after GnRH treatment to stimulate the high levels of gonadotropin synthesis necessary for the preovulatory LH surge. GnRH and estrogen levels peak just before estrus and are critical to prime gonadotropes for the LH surge (41–44). In mouse uteri and mammary glands, estrogen strongly drives PAD2 expression, and in MCF-7 cells, PAD2 functions as an estrogen receptor coactivator via the citrullination of histone tail arginine residues at estrogen receptor-binding sites (11, 45). Because males do not undergo a similar LH surge, the epigenetic mechanisms controlling gene expression in gonadotropes would appear to be different. As such, GnRH stimulation in males may activate distinct epigenetic mechanisms from those occurring in female gonadotropes during the LH surge. In the female, GnRH and estrogen may synergistically promote PAD2 expression and activity as a mechanism to stimulate high levels of gonadotropin gene expression necessary for ovulation.
Collectively, our studies are the first to demonstrate that PADs are expressed in gonadotrope cells in vitro and in vivo. We propose a model where following GnRHa binding, influx of calcium through VGCC contributes to PAD activation, which facilitates the enzymes translocation to the nucleus. Once in the nucleus, PADs citrullinate arginine residues on histone tails to alter protein-DNA interactions effectively decondensing chromatin of key gonadotrope genes, LHβ and FSHβ. Given that PAD2 expression is highest at estrus, we suggest that the epigenetic events mediated by PADs potentially underlie regulation required for the enhanced gonadotrope synthesis necessary for ovulatory events in the female. If true, targeting PAD-catalyzed citrullination in gonadotropes may represent a novel approach to regulate fertility.
Acknowledgments
This work was supported by the National Institutes of Health National Institute of General Medical Sciences Grant P20GM103432 (to A.M.N. and B.D.C.) and by the National Institute of General Medical Sciences Grant GM079357 (to P.R.T.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BB-ClA
- biphenyl-benzimidazole-Cl-amidine
- CGA
- common glycoprotein alpha
- CLSM
- confocal laser scanning microscopy
- Ct
- cycle threshold
- DAPI
- 4',6-diamidino-2-phenylindole
- DMSO
- dimethyl sulfoxide
- ER
- endoplasmic reticulum
- FBS
- fetal bovine serum
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- GnRHa
- GnRH agonist buserelin
- GnRHR
- GnRH receptor
- HSD
- honest significant difference
- IF
- Immunofluorescence
- PAD
- peptidylarginine deiminase
- qRT-PCR
- quantitative real time polymerase chain reaction
- RNA-seq
- RNA sequencing
- TBS-T
- Tris-buffered saline containing 0.1% Tween 20
- VGCC
- voltage-gated calcium channel.
References
- 1. Naor Z. Signaling by G-protein-coupled receptor (GPCR): studies on the GnRH receptor. Front Neuroendocrinol. 2009;30:10–29. [DOI] [PubMed] [Google Scholar]
- 2. Thompson IR, Kaiser UB. GnRH pulse frequency-dependent differential regulation of LH and FSH gene expression. Mol Cell Endocrinol. 2014;385:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pernasetti F, Vasilyev VV, Rosenberg SB, et al. Cell-specific transcriptional regulation of FSHb by activin and GnRH in the LbT2 pituitary gonadotrope cell model. Endocrinology. 2001;142:2284–2295. [DOI] [PubMed] [Google Scholar]
- 4. Ferris HA, Walsh HE, Stevens J, Fallest PC, Shupnik MA. Luteinizing hormone β promoter stimulation by adenylyl cyclase and cooperation with gonadotropin-releasing hormone 1 in transgenic mice and LβT2 Cells. Biol Reprod. 2007;77:1073–1080. [DOI] [PubMed] [Google Scholar]
- 5. Mouillet JF, Sonnenberg-Hirche C, Yan X, Sadovsky Y. p300 regulates the synergy of steroidogenic factor-1 and early growth response-1 in activating luteinizing hormone-β subunit gene. J Biol Chem. 2004;279:7832–7839. [DOI] [PubMed] [Google Scholar]
- 6. Wijeweera A, Haj M, Feldman A, Pnueli L, Luo Z, Melamed P. Gonadotropin gene transcription is activated by menin-mediated effects on the chromatin. Biochim Biophys Acta. 2015;1849:328–341. [DOI] [PubMed] [Google Scholar]
- 7. Vossenaar ER, Zendman AJ, van Venrooij WJ, Pruijn GJ. PAD, a growing family of citrullinating enzymes: genes, features and involvement in disease. Bioessays. 2003;25:1106–1118. [DOI] [PubMed] [Google Scholar]
- 8. Assohou-Luty C, Raijmakers R, Benckhuijsen WE, et al. The human peptidylarginine deiminases type 2 and type 4 have distinct substrate specificities. Biochim Biophys Acta. 1844:829–836. [DOI] [PubMed] [Google Scholar]
- 9. Darrah E, Rosen A, Giles JT, Andrade F. Peptidylarginine deiminase 2, 3 and 4 have distinct specificities against cellular substrates: novel insights into autoantigen selection in rheumatoid arthritis. Ann Rheum Dis. 2012;71:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wright PW, Bolling LC, Calvert ME, et al. ePAD, an oocyte and early embryo-abundant peptidylarginine deiminase-like protein that localizes to egg cytoplasmic sheets. Dev Biol. 2003;256:73–88. [DOI] [PubMed] [Google Scholar]
- 11. Horibata S, Coonrod SA, Cherrington BD. Role for peptidylarginine deiminase enzymes in disease and female reproduction. J Reprod Dev. 2012;58:274–282. [DOI] [PubMed] [Google Scholar]
- 12. Cherrington BD, Morency E, Struble AM, Coonrod SA, Wakshlag JJ. Potential role for peptidylarginine deiminase 2 (PAD2) in citrullination of canine mammary epithelial cell histones. PLoS One. 2010;5:e11768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cherrington BD, Zhang X, McElwee JL, Morency E, Anguish LJ, Coonrod SA. Potential role for PAD2 in gene regulation in breast cancer cells. PLoS One. 2012;7:e41242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Knight JS, Subramanian V, O'Dell AA, et al. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann Rheum Dis. 2015;74:2199–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shechter D, Dormann HL, Allis CD, Hake SB. Extraction, purification and analysis of histones. Nat Protoc. 2007;2:1445–1457. [DOI] [PubMed] [Google Scholar]
- 16. Bezy O, Vernochet C, Gesta S, Farmer SR, Kahn CR. TRB3 blocks adipocyte differentiation through the inhibition of C/EBPβ transcriptional activity. Mol Cell Biol. 2007;27:6818–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Navratil AM, Knoll JG, Whitesell JD, Tobet SA, Clay CM. Neuroendocrine plasticity in the anterior pituitary: gonadotropin-releasing hormone-mediated movement in vitro and in vivo. Endocrinology. 2007;148:1736–1744. [DOI] [PubMed] [Google Scholar]
- 18. Rahman MM, Rosu S, Joseph-Strauss D, Cohen-Fix O. Down-regulation of tricarboxylic acid (TCA) cycle genes blocks progression through the first mitotic division in Caenorhabditis elegans embryos. Proc Natl Acad Sci USA. 2014;111:2602–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moore JK, Li J, Cooper JA. Dynactin function in mitotic spindle positioning. Traffic. 2008;9:510–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arita K, Hashimoto H, Shimizu T, Nakashima K, Yamada M, Sato M. Structural basis for Ca(2+)-induced activation of human PAD4. Nat Struct Mol Biol. 2004;11:777–783. [DOI] [PubMed] [Google Scholar]
- 21. Slade DJ, Fang P, Dreyton CJ, et al. Protein arginine deiminase 2 binds calcium in an ordered fashion: implications for inhibitor design. ACS Chem Biol. 2015;10:1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dang AK, Murtazina DA, Magee C, Navratil AM, Clay CM, Amberg GC. GnRH evokes localized subplasmalemmal calcium signaling in gonadotropes. Mol Endocrinol. 2014;28:2049–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Subramanian V, Knight JS, Parelkar S, et al. Design, synthesis, and biological evaluation of tetrazole analogs of Cl-amidine as protein arginine deiminase inhibitors. J Med Chem. 2015;58:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qiao S, Nordström K, Muijs L, et al. Molecular plasticity of male and female murine gonadotropes revealed by mRNA sequencing. Endocrinology. 2016;157:1082–1093. [DOI] [PubMed] [Google Scholar]
- 25. van Beers JJ, Zendman AJ, Raijmakers R, Stammen-Vogelzangs J, Pruijn GJ. Peptidylarginine deiminase expression and activity in PAD2 knock-out and PAD4-low mice. Biochimie. 2013;95:299–308. [DOI] [PubMed] [Google Scholar]
- 26. Akiyama K, Nagata S, Watanabe K, et al. Age-related increase in peptidylarginine deiminase in the male rat pituitary. Mech Ageing Dev. 1995;81:119–129. [DOI] [PubMed] [Google Scholar]
- 27. Vossenaar ER, Radstake TR, van der Heijden A, et al. Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Ann Rheum Dis. 2004;63:373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Senshu T, Akiyama K, Nagata S, Watanabe K, Hikichi K. Peptidylarginine deiminase in rat pituitary: sex difference, estrous cycle-related changes, and estrogen dependence. Endocrinology. 1989;124:2666–2670. [DOI] [PubMed] [Google Scholar]
- 29. Watanabe K, Hikichi K, Nagata S, Senshu T. Estrous cycle dependent regulation of peptidylarginine deiminase transcripts in female rat pituitary. Biochem Biophys Res Commun. 1990;172:28–34. [DOI] [PubMed] [Google Scholar]
- 30. Akiyama K, Inoue K, Senshu T. Immunocytochemical study of peptidylarginine deiminase: localization of its immunoreactivity in prolactin cells of female rat pituitaries. Endocrinology. 1989;125:1121–1127. [DOI] [PubMed] [Google Scholar]
- 31. Akiyama K, Nagata S, Tanaka S, Inoue K, Watanabe K, Senshu T. Search for functional significance of peptidylarginine deiminase in rat pituitaries: variation during pregnancy and ultrastructural localization in prolactin cells. Cell Biol Int. 1993;17:487–494. [DOI] [PubMed] [Google Scholar]
- 32. Merelli F, Stojilković SS, Iida T, et al. Gonadotropin-releasing hormone-induced calcium signaling in clonal pituitary gonadotrophs. Endocrinology. 1992;131:925–932. [DOI] [PubMed] [Google Scholar]
- 33. McArdle CA, Forrest-Owen W, Davidson JS, et al. Ca2+ entry in gonadotrophs and α T3–1 cells: does store-dependent Ca2+ influx mediate gonadotrophin-releasing hormone action? J Endocrinol. 1996;149:155–169. [DOI] [PubMed] [Google Scholar]
- 34. Iida T, Stojilković SS, Izumi S, Catt KJ. Spontaneous and agonist-induced calcium oscillations in pituitary gonadotrophs. Mol Endocrinol. 1991;5:949–958. [DOI] [PubMed] [Google Scholar]
- 35. Mulvaney JM, Zhang T, Fewtrell C, Roberson MS. Calcium influx through L-type channels is required for selective activation of extracellular signal-regulated kinase by gonadotropin-releasing hormone. J Biol Chem. 1999;274:29796–29804. [DOI] [PubMed] [Google Scholar]
- 36. Takahara H, Okamoto H, Sugawara K. Calcium-dependent properties of peptidylarginine deiminase from rabbit skeletal muscle. Agric Biol Chem. 1986;50:2899–2904. [Google Scholar]
- 37. Wang Y, Wysocka J, Sayegh J, et al. Human PAD4 regulates histone arginine methylation levels via demethylimination. Science. 2004;306:279–283. [DOI] [PubMed] [Google Scholar]
- 38. Hagiwara T, Hidaka Y, Yamada M. Deimination of histone H2A and H4 at arginine 3 in HL-60 granulocytes. Biochemistry. 2005;44:5827–5834. [DOI] [PubMed] [Google Scholar]
- 39. Lim S, Luo M, Koh M, et al. Distinct mechanisms involving diverse histone deacetylases repress expression of the two gonadotropin β-subunit genes in immature gonadotropes, and their actions are overcome by gonadotropin-releasing hormone. Mol Cell Biol. 2007;27:4105–4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Horibata S, Vo TV, Subramanian V, Thompson PR, Coonrod SA. Utilization of the soft agar colony formation assay to identify inhibitors of tumorigenicity in breast cancer cells. J Vis Exp. 2015:e52727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clarke IJ, Cummins JT. Increased gonadotropin-releasing hormone pulse frequency associated with estrogen-induced luteinizing hormone surges in ovariectomized ewes. Endocrinology. 1985;116:2376–2383. [DOI] [PubMed] [Google Scholar]
- 42. Clarke IJ, Cummins JT, Jenkin M, Phillips DJ. The oestrogen-induced surge of LH requires a 'signal' pattern of gonadotrophin-releasing hormone input to the pituitary gland in the ewe. J Endocrinol. 1989;122:127–134. [DOI] [PubMed] [Google Scholar]
- 43. Moenter SM, Caraty A, Karsch FJ. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology. 1990;127:1375–1384. [DOI] [PubMed] [Google Scholar]
- 44. Moenter SM, Caraty A, Locatelli A, Karsch FJ. Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology. 1991;129:1175–1182. [DOI] [PubMed] [Google Scholar]
- 45. Zhang X, Bolt M, Guertin MJ, et al. Peptidylarginine deiminase 2-catalyzed histone H3 arginine 26 citrullination facilitates estrogen receptor α target gene activation. Proc Natl Acad Sci USA. 2012;109:13331–13336. [DOI] [PMC free article] [PubMed] [Google Scholar]