Abstract
FK506-binding protein-51 (FKBP51) is a molecular cochaperone recently shown to be a positive regulator of peroxisome proliferator-activated receptor (PPAR)γ, the master regulator of adipocyte differentiation and function. In cellular models of adipogenesis, loss of FKBP51 not only reduced PPARγ activity but also reduced lipid accumulation, suggesting that FKBP51 knock-out (KO) mice might have insufficient development of adipose tissue and lipid storage ability. This model was tested by examining wild-type (WT) and FKBP51-KO mice under regular and high-fat diet conditions. Under both diets, FKBP51-KO mice were resistant to weight gain, hepatic steatosis, and had greatly reduced white adipose tissue (WAT) but higher amounts of brown adipose tissue. Under high-fat diet, KO mice were highly resistant to adiposity and exhibited reduced plasma lipids and elevated glucose and insulin tolerance. Profiling of perigonadal and sc WAT revealed elevated expression of brown adipose tissue lineage genes in KO mice that correlated increased energy expenditure and a shift of substrate oxidation to carbohydrates, as measured by indirect calorimetry. To directly test PPARγ involvement, WT and KO mice were fed rosiglitazone agonist. In WT mice, rosiglitazone induced whole-body weight gain, increased WAT mass, a shift of substrate oxidation to lipids, and elevated expression of PPARγ-regulated lipogenic genes in WAT. In contrast, KO mice had reduced rosiglitazone responses for these parameters. Our results identify FKBP51 as an important regulator of PPARγ in WAT and as a potential new target in the treatment of obesity and diabetes.
FK506-binding protein-51 (FKBP51) is a cochaperone best known for its interaction with steroid receptors via the larger molecular chaperone heat shock protein-90 (1–4). Receptors known to interact with FKBP51 include the progesterone, androgen receptor and glucocorticoid receptor (GR). More recently, heat shock protein-90-independent roles for FKBP51 regulation of phosphorylation pathways have been uncovered, most notably nuclear factor κ-light-chain-enhancer of activated B cells (5–9) and protein kinase B (Akt) signaling (10–13). In the case of Akt, FKBP51 causes inhibition of the pathway through its activation of an Akt-specific phosphatase, PH domain leucine-rich repeat protein phosphatase. Thus, FKBP51 is now known to regulate 2 major cellular processes: nuclear receptors and phosphorylation cascades. Recently, we demonstrated that these functions can converge when it was found that FKBP51 not only interacts with peroxisome proliferator-activated receptor (PPAR)γ but also serves as a positive regulator of its transcriptional activity by preventing Akt phosphorylation at serine 112 of the receptor (13). In a follow-up study, the relevance of this mechanism was demonstrated by showing that FKBP51 is essential for PPARγ promotion of cellular adipogenesis and lipid accumulation (14). This is consistent with previous studies showing that a fraction of FKBP51 resides within mitochondria (15), perhaps to control mitochondrial-driven bioenergetics and metabolism, and that FKBP51 is a highly induced protein during adipocyte differentiation (14, 16–18).
Although the molecular and cellular actions of FKBP51 are becoming increasingly apparent, very little is known of its contributions to metabolism or other aspects of physiology. Global knock-out (KO) of FKBP51 (henceforth, FKBP51-KO mice) exerted no effect on male or female fertility, or in the development of sexual organs in either sex (19). Indeed, global FKBP51 deficiency appears to have no deleterious effect on longevity or any other physiological parameter. These results were surprising given that mice with global ablation of the closely related protein FKBP52 demonstrated infertility in both genders that correlated with dysfunctional and/or malformed reproductive organs (19–23). When patterns of FKBP51 expression are examined in human and mouse tissues, some interesting correlations have been uncovered. For example, elevated expression of FKBP51 was found in human prostate cancer tissues, suggesting a stimulatory role for this chaperone in androgen-induced prostate cancer progression and growth that was confirmed in cell-based studies (24). A growing body of evidence now suggests that elevated expression of FKBP51 is a contributing factor to cancer etiology and progression (25, 26). In several models of psychosocial stress, such as posttraumatic stress disorder, elevated levels or selective polymorphisms of FKBP51 have been identified as potential causes (27–29). The first direct test of this hypothesis was performed by Schmidt and coworkers who observed that both male and female FKBP51-KO mice were less vulnerable to the deleterious side effects of acute and chronic social stress (30, 31).
The first in vivo evidence for a role of FKBP51 in metabolism was provided by Mobbs and coworkers (32), who found that fasting elevates FKBP51 expression in the hypothalamus and that viral overexpression of FKBP51 in the hypothalamus made wild-type (WT) mice more susceptible to diet-induced obesity. In a similar study, high-fat (HF)-fed mice showed elevated levels of hypothalamic FKBP51 that correlated with the degree of weight gain (33). Finally, in a clinical study, administration of the glucocorticoid agonist dexamethasone to induce insulin resistance resulted in up-regulation of FKBP51 in sc and omental adipose tissue, with the degree of FKBP51 expression correlating with the magnitude of insulin resistance in each tissue (34). This study also reported the identification of single nucleotide polymorphisms of the FKBP51 gene (fkbp5) that associate with type 2 diabetes. The above studies point to a pivotal role for FKBP51 in promoting diet-induced obesity and adiposity. In this work, we directly address this hypothesis by analyzing FKBP51-KO mice in response to conditions inducing obesity and glucose intolerance. To directly test involvement of PPARγ in FKBP51 control of adiposity, treatment of mice with the antidiabetic PPARγ agonist rosiglitazone was also performed.
Materials and Methods
Animals
Generation of FKBP51 null mice has been previously described (19). The FKBP51-KO strain was established on a mixed C57BL/6J:129SvEv genetic background, followed by at least 6 generations of back-crossing to the C57BL/6J background. Mutant mice exhibit normal fertility. All experiments were performed on male mice, between 2 and 4 months of age. Animals were housed in a temperature-controlled environment with a 12-hour light, 12-hour dark cycle. All procedures were approved by the Institutional Animal Care and Utilization Committee of The University of Toledo and the Institutional Animal Care and Utilization Committee of the Institute of Laboratory Animal Science of Peking Union Medical College.
Metabolic analysis
The breeding colony was maintained on standard chow (regular diet [RD]) containing 12 kcal% fat ad libitum. In 1 experimental set, WT and FKBP51-KO mice were separated into 2 groups of 8-week old males. One group was fed (ad libitum) a RD for 4 weeks, and the second was fed (ad libitum) a HF diet containing 45 kcal% fat (catalog number D12451; Research Diets) for 4 weeks. This HF regimen is known to induce moderate obesity, steatosis, and insulin resistance in most strains of mice, without stimulating inflammation (35). In another set of experiments, WT and FKBP51-KO male mice, 3–4 months of age, were fed for 8 weeks either a nonsupplemented RD, or RD supplemented with rosiglitazone at a projected dose of 15 mg/kg · d (n = 5 per group). Food intake was monitored during the entire experiment to calculate an actual ingested dose of 17.6 mg/kg · d rosiglitazone. For all experimental groups, tissue and blood samples were collected from animals subjected to an overnight fast (12–16 h), unless otherwise noted. Whole venous blood was drawn from retro-orbital sinuses. Blood glucose levels were measured using a glucometer (Accu-check Aviva; Roche Diagnostics). Corticosterone (MP Biomedicals), plasma insulin, and C-peptide levels (Linco Research) were measured by RIA. Plasma free fatty acids (FFAs) (Wako) and triglycerides (TGs) (Pointe Scientific) were measured by colorimetric assay. To measure hepatic steatosis, frozen liver samples were sliced with a cryostat to 10-μm thickness and the sections stained with Oil Red O (Sigma) and counterstained with hematoxylin (Fisher Scientific).
Body composition
Fat and lean mass were evaluated by nuclear magnetic resonance (NMR) using a Minispec MQ10 NMR analyzer (Bruker), or by magnetic resonance imaging (MRI), as follows. T1-weighted spin-echo anatomical images (repetition time = 200 ms; echo time = 11.7 ms; field of view = 10 × 10 cm; acquisition matrix = 256 × 256; average = 4; slice thickness = 1 mm) were acquired in axial orientation by the Varian 7.0T MRI system (7.0T micro MRI; Varian). IRW software (Inveon Research Workplace; Siemens) was used to calculate the fat content in each scan, thereby evaluating the whole-body fat content in WT and FKBP51-KO mice.
Intraperitoneal insulin tolerance test (ITT)
As previously described (56), mice were fasted for 7 hours starting at 7 am. Human insulin (Novolin, Novo Nordisk NDC 0169-1833-11) was administered by ip injection at 0.75-U/kg body weight to awake mice. Blood glucose levels (mg/dL) were measured from the tail vein at 0–180 minutes after insulin injection.
Intraperitoneal glucose tolerance test (GTT)
As previously described (56), mice were fasted overnight (from 5 pm to 8 am the next day), and glucose (50% dextrose solution) was administered by ip injection at 1.5-g/kg body weight to awake mice. Blood glucose levels (mg/dL) were measured from the tail vein at 0–120 minutes after glucose injection.
Indirect calorimetry
Mice were evaluated in calorimetric cages (CLAMS; Columbus Instruments) for 4 days and had free access to food and water. The mice were housed individually at thermoneutrality (30°C) under an alternating 12-hour light, 12-hour dark cycle. After adaptation for 1 day, O2 consumption (VO2), CO2 production, physical activity, and heat production were measured to determine energy expenditure. Respiratory exchange ratios (RERs) were calculated as the ratio of CO2 production to VO2. Food intake and water intake were determined continuously.
Immunohistochemistry
Formalin-fixed and paraffin-embedded visceral adipose tissue was deparaffinized at 60°C, rehydrated, placed in citrate buffer (1.8mM citric acid monohydrate and 8.2mM sodium citrate; pH 6) for antigen retrieval (15 min at 95°C), quenched in 3% hydrogen peroxide solution for 30 minutes at room temperature (RT) before blocking at RT for 1 hour with normal goat serum (VECTASTAIN Elite ABC peroxidase enzyme systems kit PK-6101; Vector Laboratories). Slides were then incubated with rabbit polyclonal uncoupling protein 1 antibody (1:1000 diluted in blocking buffer) (ab23841; Abcam) at 4°C overnight. After 3 washes with PBS-Tween for 10 minutes each, slides were incubated in biotinylated goat antirabbit IgG secondary antibody (ABC Vectastain kit) at RT for 1 hour, treated with ABC solution at RT for 30 minutes, and stained with DAB Vectastain kit (SK-4100) before being counterstained with hematoxylin and eosin, followed by dehydration. The examination of the slides was performed using Nikon NIS-Elements BR3.1 system. Images and measurements were collected under ×40 magnification from 6 representative fields per sample.
Quantitative real-time PCR analysis
Total RNA was extracted from mouse tissues using 5-Prime PerfectPure RNA Tissue kit (Fisher Scientific Co, LLC). Total RNA was read on a NanoDrop 2000 Spectrophotometer and cDNA was synthesized using iScript cDNA Synthesis kit (Bio-Rad). Polymerase chain reaction (PCR) amplification of the cDNA was performed by quantitative real-time PCR using quantitative PCR Core kit for SYBR Green I (Applied Biosystems). The thermocycling protocol consisted of 10 minutes at 95°C, 40 cycles of 15 seconds at 95°C, 30 seconds at 60°C, and 20 seconds at 72°C and finished with a melting curve ranging from 60°C–95°C to allow distinction of specific products. Primers were designed specific to each gene using Primer Express 3.0 software (Applied Biosystems). Normalization was performed in separate reactions with primers to 18S mRNA (TTCGAACGTCTGCCCTATCAA and ATGGTAGGCACGGCGACTA).
Statistical analysis
Data were analyzed with Prism 5 (GraphPad Software) using ANOVA combined with Tukey's post hoc test to compare pairs of group means, or unpaired t tests. P ≤ .05 was considered statistically significant.
Results
FKBP51-KO mice fed RD and HF diet are resistant to weight gain, adiposity, and hepatic steatosis
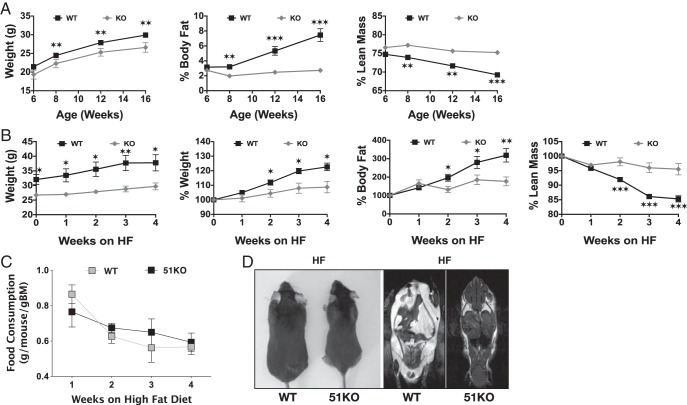
In previous studies assessing fertility in FKBP51-KO mice (19), a decrease in overall body weight was observed in mutant mice maintained on a regular chow diet (unpublished results). To more accurately characterize this phenotype, growth and body composition of WT and KO mice maintained on RD were measured by NMR (Figure 1A). Results show lower body weights for KO mice ages 6–16 weeks compared with WT littermates. More interesting was the near complete inability of KO mice to accumulate body fat while maintaining a normal lean mass composition. To further assess this propensity, WT and KO mice were analyzed by NMR during a 4-week HF diet regimen (Figure 1B). Once again, a dramatic resistance to weight gain and adiposity was observed beginning at week 2 of HF intake. Not surprisingly, lean mass showed only a small approximately 3% decline by the end of HF feeding. No significant decrease in HF food consumption was observed during this period (Figure 1C). To assess distribution of body fat, MRI was performed on WT and KO mice fed HF for 8 weeks (Figure 1D). In contrast to WT mice, KO animals showed almost no visceral fat and greatly reduced sc depots.
Figure 1.
FKBP51-KO mice are resistant to weight gain and adiposity on RD and HF diet. A, NMR analysis of WT (n = 21) and KO (n = 10) mice ages 6–16 weeks fed a RD. B, NMR analysis of 16-week-old WT (n = 8) and KO (n = 8) mice fed a HF diet for 4 weeks. Both absolute and percent changes in animal weights are shown. C, Food consumption of mice in B. D, Coronal MRI analysis of WT and KO mice fed HF for 8 weeks. Images of representative mice are shown. For all experiments, data shown are mean ± SEM; *, WT vs KO; *, P < .05; **, P < .01; ***, P < .001; same statistical parameters apply to # symbol.
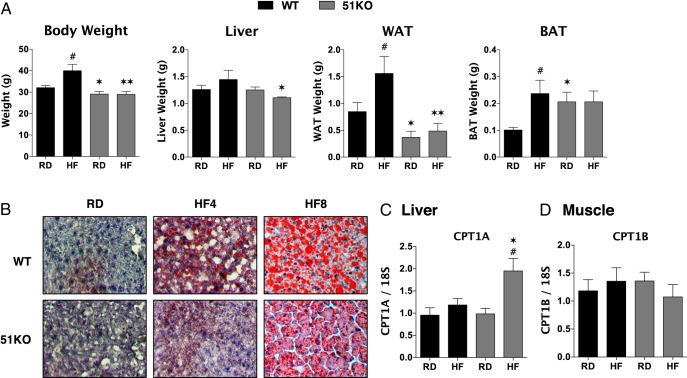
Dissectional analysis of WT and KO mice showed a dramatic reduction of perigonadal white adipose tissue (WAT) mass in KO mice fed both RD and HF (Figure 2A). Interestingly, interscapular brown adipose tissue (BAT) mass was higher in KO mice fed RD compared with WT and that mass did not increase in response to HF as seen in WT animals. Liver weights of HF-fed KO mice were significantly lower than WT counterparts. O Red O staining (Figure 2B) revealed reduced hepatic fat accumulation in KO mice at both 4 and 8 weeks of HF feeding. Because reduced fat load in the liver might reflect elevated β-oxidation of lipids, the liver-specific form of carnitine palmitoyltransferase (CPT)1A was measured (Figure 2C). Elevated CPT1A was observed in KO mice fed HF, suggesting that under these conditions, at least, enhanced β-oxidation of long-chain fatty acids may be occurring. Similarly, the high percentage of lean muscle in KO animals (Figure 1A) might also reflect increased β-oxidation. However, no change to the muscle-specific form CPT1B was observed in KO mice (Figure 2D).
Figure 2.
Reduced WAT but elevated BAT and resistance to hepatic steatosis in FKBP51-KO mice. A Whole body and organ weights for liver, visceral (perigonadal) WAT and interscapular BAT in WT and KO mice were fed a RD or a HF diet for 4 weeks (n = 5–8). B, Oil Red O staining of livers from WT and KO mice were fed RD or a HF diet for 4–8 weeks. Representative images are shown. C and D, Real-time PCR of the liver (n = 6) (C)- and muscle (n = 8) (D)-specific isoforms of CPT1. Data shown are mean ± SEM; #, RD vs HF; *, WT vs KO.
Low plasma lipids and improved insulin sensitivity in HF-fed FKBP51-KO mice
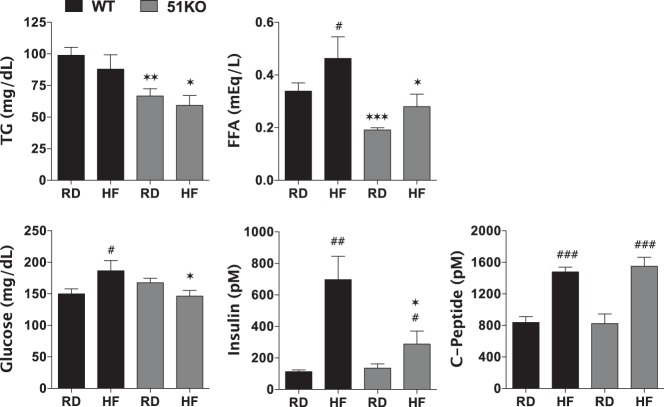
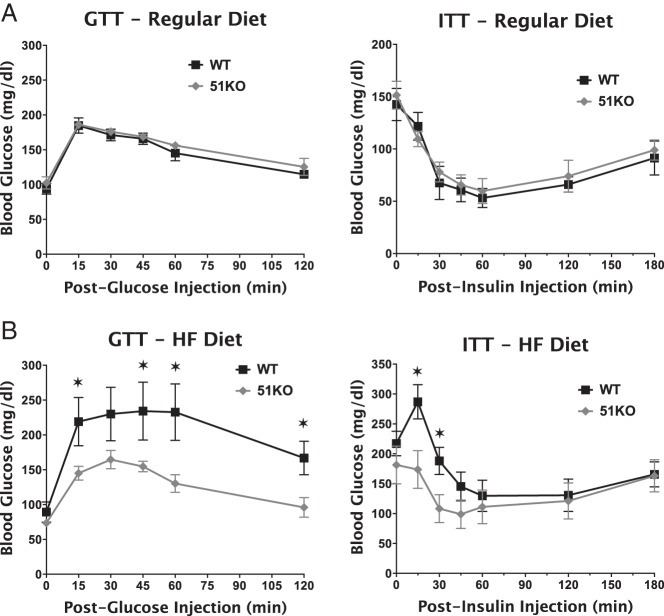
As Figure 3 reveals, plasma TGs and FFAs in KO mice were markedly reduced in comparison with WT mice when fed regular or HF diets. This is consistent with reduced hepatic steatosis of KO mice (Figure 2). As expected, HF feeding increased blood glucose and plasma insulin levels in WT mice. In contrast, KO mice showed no elevation of blood glucose and only moderately elevated plasma insulin levels. Fasting plasma C-peptide levels showed no difference between WT and KO, suggesting that the relatively low levels of insulin in HF-fed KO mice are not due to impaired secretion by β-cells. Both genotypes exhibited comparable glucose tolerance (GTT) and insulin tolerance (ITT) when fed a RD (Figure 4), indicating normal insulin sensitivity in the KO mice. When fed a HF diet, however, KO mice cleared glucose more efficiently in response to both ip glucose and insulin injections, indicating that KO mice are protected against diet-induced insulin resistance.
Figure 3.
FKBP51-KO mice are resistant to hyperglycemia with reduced plasma lipids. Plasma hormones and metabolites in WT and KO mice fed RD or HF diet for 4 weeks (n = 6–8). Data shown are mean ± SEM; #, RD vs HF; *, WT vs KO.
Figure 4.
Increased glucose tolerance but normal insulin sensitivity in FKBP51-KO mice fed a HF diet. A, WT (n = 6) and KO (n = 5) 5-month-old mice fed a RD were fasted overnight (GTT) or 6 hours (ITT), followed by measurement of glucose tolerance (GTT) or insulin sensitivity (ITT), respectively. B, GTT and ITT assays performed on WT (n = 7) and KO (n = 8) 5-month-old mice at the end of a 4-week HF diet. Data shown are mean ± SEM. *, WT vs KO.
Altered substrate oxidation and elevated energy expenditure but reduced motor activity in FKBP51-KO mice
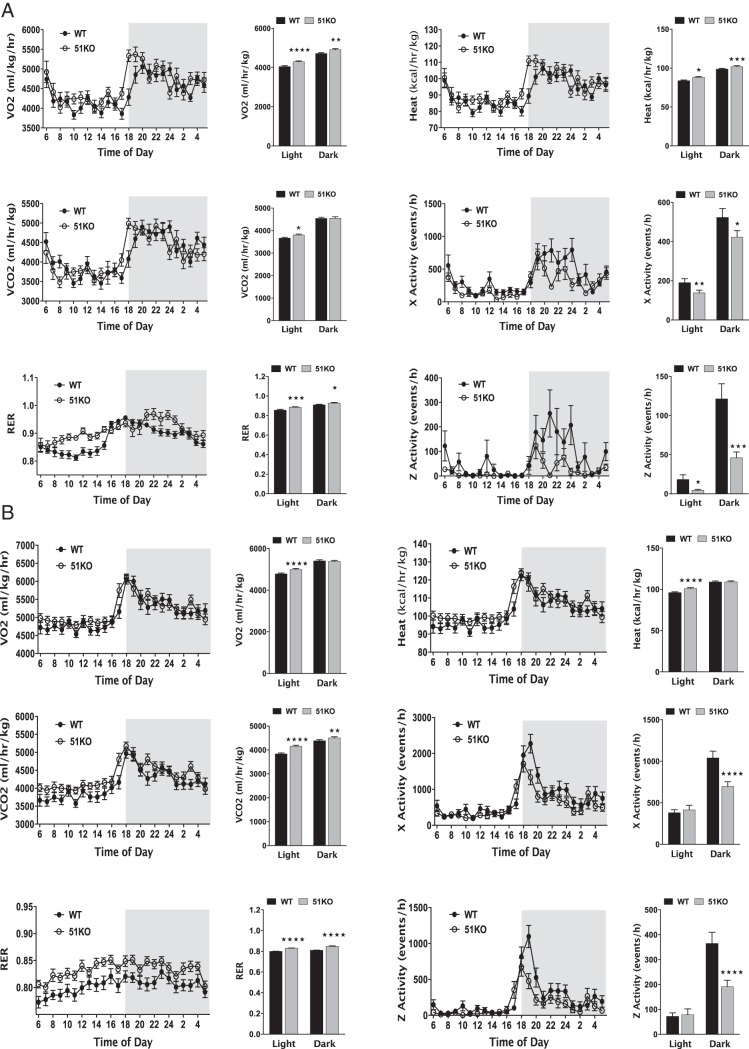
Low plasma TGs and FFAs in the KO mice (Figure 3) suggested that these animals must be relying on increased carbohydrate oxidation. This was tested by subjecting WT and KO mice fed RD to a 4-day screening by indirect calorimetry to assess substrate utilization and other factors (Figure 5A). In both light and dark phases, KO mice exhibited higher RERs compared with WT, indicating a preferential shift to carbohydrate oxidation. Energy expenditure (heat) and VO2 were also elevated in KO mice in both phases, properties that are consistent with mutant mice oxidizing rather than storing consumed calories. Interestingly, the KO mice had reduced spontaneous locomotor activity, showing that elevated caloric oxidation is occurring despite reduced exertional activity. An almost identical pattern of calorimetry was obtained in WT and KO mice fed HF (Figure 5B). Thus, deficiency of FKBP51 leads to an overall state of elevated thermogenesis with a shift to carbohydrate utilization independent of diet.
Figure 5.
FKBP51-KO mice fed RD and HF diet exhibit altered substrate oxidation and elevated energy expenditure with reduced motor activity. Five-month-old WT and KO mice were subjected to indirect calorimetry to assess RERs, energy expenditure (heat), and motor activity. A, WT (n = 6) and KO (n = 6) mice on RD. B, WT (n = 7) and KO (n = 8) mice fed a HF diet for 4 weeks. Data shown are mean ± SEM; *, WT vs KO.
“Browning” of visceral adipose tissue in FKBP51-KO mice
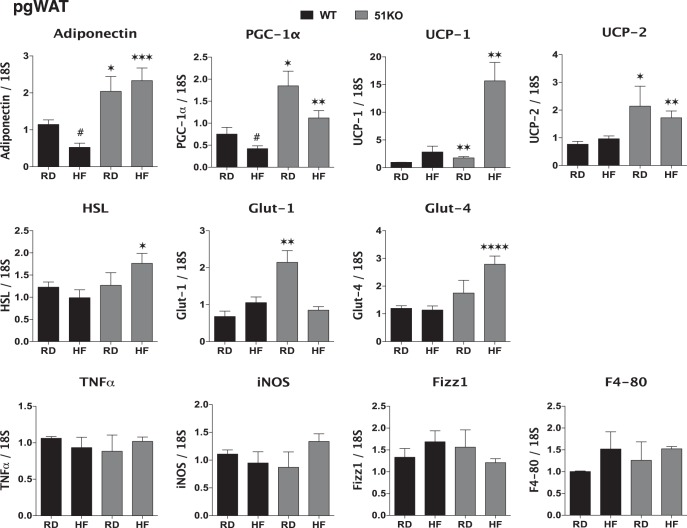
Given our recent observation that FKBP51 is an important regulator of PPARγ-mediated adipogenesis (14), we performed RT-PCR gene profiling of perigonadal fat in WT and KO mice fed RD and HF diet (Figure 6A). Well-characterized deleterious effects of most HF diets on perigonadal fat include reduced expression and secretion of adiponectin and increased inflammation. Analysis of macrophage markers showed no statistically significant differences between WT and KO perigonadal WAT for inflammatory M1 markers (TNF-α and inducible nitric oxide synthase), or an M2 repair marker (found in inflammatory zone 1), or for overall macrophage infiltration (F4/80). These results indicate that the short 4-week HF diet employed was not significantly increasing inflammation of this tissue. However, WT mice fed HF showed reduced expression of adiponectin, indicating that the HF diet regimen was compromising this function. In the KO mice, large increases in adiponectin were observed under RD and HF. This suggests that perigonadal fat of mutant mice is in a constitutively “healthy” state that resists the deleterious effects of HF. Mice overexpressing adiponectin are known to have elevated energy expenditure due to up-regulation of uncoupling proteins (36). Not surprisingly, KO mice on both diets showed elevated expression of the major uncoupling protein of WAT, UCP-2, but also showed high levels of UCP-1 and the master regulator of mitochondrial biogenesis and respiration, PPARγ coactivator-1α (37). Although glucose transporter-1 levels were elevated in KO mice under RD, the insulin-sensitive glucose transporter glucose transporter-4 was greatly increased in mutant mice fed HF, as was a key enzyme of lipolysis, hormone-sensitive lipase.
Figure 6.
Elevated expression of BAT lineage genes in adipose of FKBP51-KO mice fed RD and HF diet. Real-time PCR analysis of the indicated genes in perigonadal WAT of WT and KO mice fed RD or HF diets (n = 6–9); mean ± SEM; *, WT vs KO. All genes were normalized to 18S RNA.
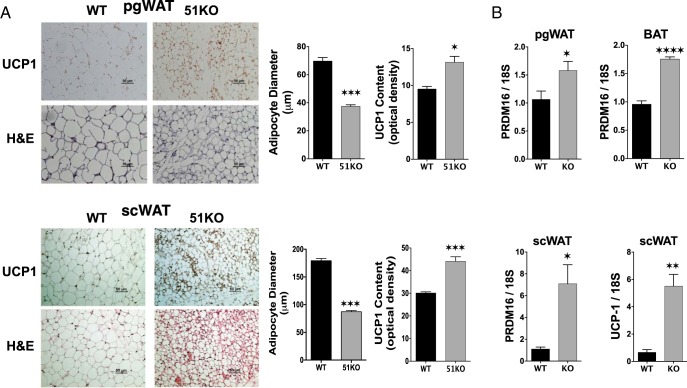
Because UCP-1 has been implicated in the browning of WAT to an elevated thermogenic state (38), direct measurement of UCP-1 protein levels in the perigonadal WAT of WT and KO mice was performed. The results of Figure 7A show higher levels of UCP-1 protein compared with WT in KO mice fed a RD. In addition, average adipocyte area (data not shown) and adipocyte diameter were reduced in KO WAT compared with WT, suggesting reduced lipid storage. PCR analysis revealed elevated levels of the BAT determination factor PR domain containing 16 (PRDM16) (39) in KO compared with WT in both WAT and BAT depots (Figure 7B). Because sc WAT is a major contributor to energy expenditure, a similar analysis was performed in this tissue. Results show reduced adipocyte size of sc WAT in KO mice, along with elevated expression of UCP-1 and PRDM16 (Figure 7, C and D).
Figure 7.
Evidence for browning of WAT in FKBP51-KO mice A, Perigonadal (pgWAT) and sc (scWAT) adipose tissues from WT and KO mice fed RD were subjected to indirect immunohistochemical and H&E staining to assess UCP-1 content and adipocyte diameters, respectively. B, Real-time PCR analysis of PRDM16 and UCP-1 in pgWAT, scWAT and BAT adipose tissues from WT and KO mice fed a RD (n = 4–5). Data shown are mean ± SEM; #, RD vs HF; *, WT vs KO. All genes were normalized to 18S RNA.
FKBP51-KO mice are resistant to the PPARγ agonist rosiglitazone
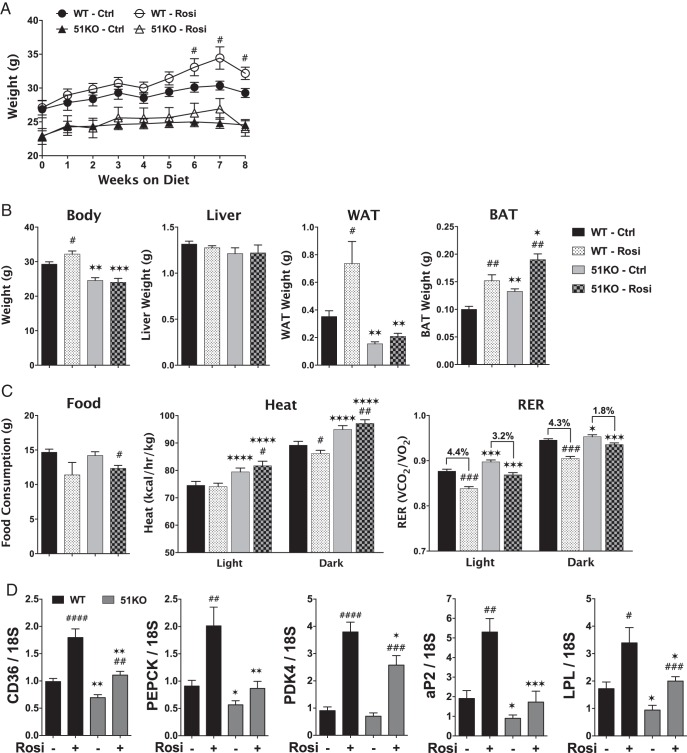
Our previous work establishing FKBP51 as a positive modulator of PPARγ (13, 14) suggests that FKBP51-KO mice may have impaired PPARγ activity. To test this hypothesis, WT and KO mice were fed a diet containing the PPARγ agonist rosiglitazone for 8 weeks, using a regimen that induces PPARγ-mediated metabolic responses (40). As Figure 8A reveals, WT mice exhibited elevated body weight by the sixth week of rosiglitazone feeding. In contrast, body weights of KO mice were not altered by PPARγ activation. At the end of the eighth week of feeding, dissectional analysis of WT mice revealed no effect of rosiglitazone on liver weight, but greater mass of both perigonadal WAT and interscapular BAT (Figure 8B). KO mice had lower basal WAT mass, as expected, and showed no increase of WAT with rosiglitazone treatment. Interestingly, BAT mass was increased by rosiglitazone in KO mice. Indirect calorimetry analysis (Figure 8C) revealed reduced food consumption in response to rosiglitazone for both genotypes. Energy expenditure (heat) was higher in KO mice, as previously shown (Figure 5), and it increased even more in response to rosiglitazone. In contrast, heat was reduced by rosiglitazone in WT mice, at least in the dark phase. In WT mice, RERs were reduced by rosiglitazone under both light (4.4%) and dark (4.3%) phases, indicating a shift to lipid utilization in response to the drug. Although the RERs of KO mice also dropped in response to rosiglitazone, the magnitude of the drop was not as great as in WT mice (3.2% and 1.8%, respectively). Lastly, analysis of perigonadal WAT by real-time PCR was performed (Figure 8D). In WT mice, there was robust rosiglitazone-induced up-regulation of 5 PPARγ-induced genes: cluster of differentiation 36 (lipid importer), phosphoenolpyruvate carboxykinase and pyruvate dehydrogenase kinase 4 (promoters of glyceroneogenesis), the adipocyte marker aP2, and lipoprotein lipase. In contrast, rosiglitazone-induced expression of all 5 genes was greatly to moderately reduced in KO mice.
Figure 8.
FKBP51-KO mice are resistant to the PPARγ agonist rosiglitazone. A, Growth curves for 3- to 4-month-old WT and KO mice placed on control and rosiglitazone-containing diets for 8 weeks (n = 5). B, Organ and tissue weights of control and rosiglitazone-fed WT and KO mice on the eighth week of diet (n = 5). C, Indirect calorimetry of control and rosiglitazone-fed WT and KO mice on the eighth week of diet (n = 5). D, Real-time PCR analysis of the indicated genes in perigonadal WAT of control and rosiglitazone-fed WT and KO mice (n = 5). Data shown are mean ± SEM; #, Ctrl vs Rosi at same genotype; *, WT vs KO at same treatment.
Discussion
Before initiation of this study, the unexpected good health of FKBP51-KO mice had already been documented. Unlike mice with global ablation of the closely related FKBP52 chaperone, loss of FKBP51 did not result in discernible developmental, reproductive or viability defects (19). Here, we demonstrate that FKBP51-KO mice maintained on a RD exhibit many characteristics of a beneficial metabolic profile, including reduced WAT mass and plasma lipids, as well as elevated energy expenditure. More importantly, FKBP51-KO animals were remarkably resistant to the deleterious effects of a HF diet, such as weight gain, visceral and sc adiposity, hepatic steatosis, and insulin resistance. Under HF, a notable feature of FKBP51-KO mice was not only a resistance to WAT expansion, but also maintenance of their already high levels of BAT mass. Indeed, gene analysis of both visceral and sc WAT from KO mice fed RD and HF diet showed elevated levels of several thermogenic genes, such as the master regulator of mitochondrial energetics PPARγ coactivator-1α. In addition, both WAT depots of KO mice also expressed high levels of 2 genes that are classical markers of BAT, UCP-1, and PRDM16. The latter protein is a transcription factor that is essential to determination of the BAT lineage (39, 41). Thus, FKBP51-KO mice under both RD and HF diet appear to undergo a browning of whole-body WAT that is probably a major contributor to the elevated energy expenditure and insulin sensitivity observed in these mice.
In previous work, we uncovered a potential molecular basis for the above results when it was found that FKBP51 serves to negatively regulate the Akt/p38 MAPK pathway that targets both PPARγ and the GR (13). In this role, FKBP51 acts as a chaperone to the Akt-specific phosphatase PH domain leucine-rich repeat protein phosphatase (10, 42), leading to inactivation of Akt by dephosphorylation and to reduced targeting of PPARγ and GR by downstream p38 MAPK. Because serine-specific phosphorylation of both receptors by p38 results in GR activation but inhibition of PPARγ, lack of FKBP51 activity in KO cells increased GR transcriptional activity, but reduced the activity of PPARγ. The relevance of this mechanism to adipogenesis was demonstrated when it was found that FKBP51 loss completely prevents adipocytic differentiation and accumulation of lipid (14). These results thus serve as functional validation of the long-standing observation that FKBP51 is one of the most highly induced proteins during WAT adipocyte differentiation (14, 16–18). In our adipogenic studies, it was demonstrated that reduced PPARγ activity due to elevated phosphorylation at serine 112 of the receptor was the major contributor to the inability of FKBP51-KO cells to differentiate into functional adipocytes. In this study, we therefore used the PPARγ agonist rosiglitazone to measure in vivo PPARγ activity in FKBP51-KO mice. KO mice were resistant to rosiglitazone effects on heat expenditure, weight gain, increased WAT mass and PPARγ activity at WAT genes. Thus, it is reasonable to conclude that some of the metabolic phenotypes observed in the KO mice, such as reduced WAT mass, are at least partially due to decreased PPARγ activity.
In an effort to put our results in context, we have analyzed 3 reports of mice with adipose-specific KO of PPARγ (43–45), under the assumption that our results should at least partially mimic these models. Although this comparison proved somewhat difficult due to some controversial differences between the findings, key commonalities do exist between the PPARγ KO models. Two of the 3 reports showed various degrees of insulin resistance in the mutant mice (43, 45), whereas all 3 showed greatly reduced whole-body adiposity. Because adipogenesis and insulin sensitivity are controlled by 2 distinct domains of PPARγ involving phosphorylation of serines 112 and 273, respectively (46, 47), it is not surprising that complete loss of PPARγ from adipose would produce mice with reduced adiposity that are insulin resistant. In our previous studies, loss of FKBP51 resulted in elevated phosphorylation of PPARγ at S112, along with diminished adipocyte differentiation and lipid accumulation. However, we and others have found no evidence that the p38 MAPK pathway controlled by FKBP51 has any effect on S273 PPARγ phosphorylation (our unpublished results). Thus, our model of FKBP51 action on PPARγ is in good agreement with the observed FKBP51-KO mouse phenotypes of reduced WAT mass and normal insulin sensitivity.
Because FKBP51 is a chaperone to several nuclear receptors, and due to its newly found role in Akt signaling, it is not likely that the metabolic phenotypes uncovered in the FKBP51-KO mice are solely due to altered PPARγ activity in adipose tissue. In a cellular adipogenesis model, we showed elevated GR-mediated lipolytic activity in FKBP51-KO cells (14), suggesting that increased lipolysis may contribute to the reduced WAT mass observed in KO animals. However, plasma fatty acids levels were low, not high, in the KO mice, indicating that lipolysis may not be a big factor or that compensatory fatty acid oxidation may be involved. Indeed, our observation of elevated levels of CPT1, an essential enzyme in the β-oxidation of long-chain fatty acids (48), in liver, but not muscle, of KO mice suggests that this potential effect of FKBP51 is worth future investigation. Clearly, to fully understand the effects of FKBP51 loss on GR, a separate systematic investigation will be required, especially because GR is more widely expressed than PPARγ, with many metabolic functions besides lipolysis, such as gluconeogenesis in liver and proteolysis in skeletal muscle (49). Besides adipose, it is possible that FKBP51 also regulates PPARγ in liver, muscle or BAT. It should be noted that the BioGPS mRNA tissue profile for the mouse (http://biogps.org/#goto=genereport&id=2289) shows that WAT expresses at least 10 times more FKBP51 than liver, skeletal muscle, or BAT. Thus, loss of FKBP51 from these organs and tissues is not likely to have as major an impact on PPARγ or other chaperone clients compared with WAT. Of particular interest are the results obtained at BAT. Unlike WAT, BAT mass remained responsive to rosiglitazone in KO mice, which might simply reflect low levels of FKBP51 expression in this tissue, or that the Akt/p38 kinase pathway regulated by FKBP51 may not be operative. Yet, we do not have an explanation for the elevated levels of BAT mass in untreated KO mice. Possibilities include compensatory signaling from WAT or other tissues, or the existence of distinct FKBP51 clients in BAT. With all of the above in mind, an important and informative next step will be generation of WAT- and BAT-specific FKBP51-KO strains.
In summary, it appears that FKBP51 null mice exhibit a constellation of metabolic phenotypes that can be partially explained by reduced PPARγ activity at WAT. Observations supporting this claim include: 1) previous molecular and cellular studies demonstrating decreased PPARγ activity in FKBP51-KO cells, including resistance to adipogenesis; 2) reduced WAT adiposity of KO mice under RD and HF diet, especially with respect to sc and perigonadal fat; and 3) resistance of the KO mice to the proadipogenic WAT effects of the PPARγ agonist rosiglitazone. Moving forward, it will be important to determine the molecular and cellular underpinnings responsible for other metabolic phenotypes in the KO mice, such as elevated BAT mass, browning of WAT, and increased insulin sensitivity and energy expenditure.
Lastly, this work is further evidence that FKBP51 may be an excellent inhibitory drug target for the treatment of obesity or diabetes. As stated above, FKBP51 null mice are unusually healthy, with no apparent defects in immunity, fertility, or longevity. This may be attributable to a unique tissue expression profile for FKBP51, in which high-level expression is restricted to only a few organs, including WAT. Moreover, evidence to-date suggests that FKBP51 correlation with disease states, such as cancer, posttraumatic stress disorder, and depression, is linked to overexpression of the protein. Last but not least, inhibitory drug development against related “immunophilins” like FKBP12 is already well advanced, as evidenced by the wide-spread use of FK506 (tacrolimus) in organ transplantation (50). Analogues of FK506 selective for the large molecular mass FKBPs are under investigation (51–53), one such being VX-853 (timcodar), which may exhibit specificity for FKBP51 (54), and which also controls PPARγ activity (55). Future development of an FKBP51-selective drug may therefore be warranted and attainable.
Acknowledgments
This work was supported in part by Department of Physiology and Pharmacology (University of Toledo College of Medicine) funds (E.R.S.) and by National Institutes of Health Grants DK70127 (to E.R.S.) and DK054254, DK083850, and HL112248 (to S.M.N.). W.Y. was supported by the National Basic Research Program of China Grant 2013CB945001 and the Natural Science Foundation of China Grant 81272273. B.L.-C. was supported by the American Diabetes Association Award 7-13-BS-089. L.A.S. was supported in part by the Center for Diabetes and Endocrine Research (University of Toledo College of Medicine) Hiss Foundation Fellowship. T.D.H. was supported by National Institutes of Health PRIDE Grant HL106365. L.R. was supported by a fellowship from the American Heart Association.
Disclosure Summary: The authors have nothing to disclose.
Appendix
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| UCP-1 | ab23841, Abcam | Rabbit; polyclonal | 0.736111111 |
Footnotes
- Akt
- protein kinase B
- BAT
- brown adipose tissue
- CPT
- carnitine palmitoyltransferase
- FFA
- free fatty acid
- FKBP51
- FK506-binding protein-51
- GR
- glucocorticoid receptor
- GTT
- glucose tolerance test
- HF
- high fat
- ITT
- insulin tolerance test
- KO
- knock-out
- MRI
- magnetic resonance imaging
- NMR
- nuclear magnetic resonance
- PPAR
- peroxisome proliferator-activated receptor
- PRDM16
- PR domain containing 16
- RD
- regular diet
- RER
- respiratory exchange ratio
- RT
- room temperature
- TG
- triglyceride
- VO2
- O2 consumption
- UCP-1
- uncoupling protein 1
- WAT
- white adipose tissue
- WT
- wild type.
References
- 1. Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. [DOI] [PubMed] [Google Scholar]
- 2. Storer CL, Dickey CA, Galigniana MD, Rein T, Cox MB. FKBP51 and FKBP52 in signaling and disease. Trends Endocrinol Metab. 2011;22:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stechschulte LA, Sanchez ER. FKBP51-a selective modulator of glucocorticoid and androgen sensitivity. Curr Opin Pharmacol. 2011;11:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sanchez ER. Chaperoning steroidal physiology: lessons from mouse genetic models of Hsp90 and its cochaperones. Biochim Biophys Acta. 2012;1823:722–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Romano MF, Avellino R, Petrella A, Bisogni R, Romano S, Venuta S. Rapamycin inhibits doxorubicin-induced NF-κB/Rel nuclear activity and enhances the apoptosis of melanoma cells. Eur J Cancer. 2004;40:2829–2836. [DOI] [PubMed] [Google Scholar]
- 6. Komura E, Tonetti C, Penard-Lacronique V, et al. Role for the nuclear factor κB pathway in transforming growth factor-β1 production in idiopathic myelofibrosis: possible relationship with FK506 binding protein 51 overexpression. Cancer Res. 2005;65:3281–3289. [DOI] [PubMed] [Google Scholar]
- 7. Park J, Kim M, Na G, et al. Glucocorticoids modulate NF-κB-dependent gene expression by up-regulating FKBP51 expression in Newcastle disease virus-infected chickens. Mol Cell Endocrinol. 2007;278:7–17. [DOI] [PubMed] [Google Scholar]
- 8. Jiang W, Cazacu S, Xiang C, et al. FK506 binding protein mediates glioma cell growth and sensitivity to rapamycin treatment by regulating NF-κB signaling pathway. Neoplasia. 2008;10:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Erlejman AG, De Leo SA, Mazaira GI, et al. NF-κB transcriptional activity is modulated by FK506-binding proteins FKBP51 and FKBP52: a role for peptidyl-prolyl isomerase activity. J Biol Chem. 2014;289:26263–26276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pei H, Li L, Fridley BL, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fabian AK, Marz A, Neimanis S, Biondi RM, Kozany C, Hausch F. InterAKTions with FKBPs–mutational and pharmacological exploration. PLoS One. 2013;8:e57508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei XE, Zhang FY, Wang K, Zhang QX, Rong LQ. Assembly of the FKBP51-PHLPP2-AKT signaling complex in cerebral ischemia/reperfusion injury in rats. Brain Res. 2014;1566:60–68. [DOI] [PubMed] [Google Scholar]
- 13. Stechschulte LA, Hinds TD, Jr, Ghanem SS, Shou W, Najjar SM, Sanchez ER. FKBP51 reciprocally regulates GRα and PPARγ activation via the Akt-p38 pathway. Mol Endocrinol. 2014;28:1254–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stechschulte LA, Hinds TD, Jr, Khuder SS, Shou W, Najjar SM, Sanchez ER. FKBP51 controls cellular adipogenesis through p38 kinase-mediated phosphorylation of GRα and PPARγ. Mol Endocrinol. 2014;28:1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gallo LI, Lagadari M, Piwien-Pilipuk G, Galigniana MD. The Hsp90-binding immunophilin FKBP51 is a mitochondrial protein that translocates to the nucleus to protect cells against oxidative stress. J Biol Chem. 2011;286:30152–30160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yeh WC, Li TK, Bierer BE, McKnight SL. Identification and characterization of an immunophilin expressed during the clonal expansion phase of adipocyte differentiation. Proc Natl Acad Sci USA. 1995;92:11081–11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yeh WC, Bierer BE, McKnight SL. Rapamycin inhibits clonal expansion and adipogenic differentiation of 3T3-L1 cells. Proc Natl Acad Sci USA. 1995;92:11086–11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Toneatto J, Guber S, Charó NL, et al. Dynamic mitochondrial-nuclear redistribution of the immunophilin FKBP51 is regulated by the PKA signaling pathway to control gene expression during adipocyte differentiation. J Cell Sci. 2013;126:5357–5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yong W, Yang Z, Periyasamy S, et al. Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J Biol Chem. 2007;282:5026–5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheung-Flynn J, Prapapanich V, Cox MB, Riggs DL, Suarez-Quian C, Smith DF. Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol. 2005;19:1654–1666. [DOI] [PubMed] [Google Scholar]
- 21. Tranguch S, Cheung-Flynn J, Daikoku T, et al. Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci USA. 2005;102:14326–14331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang Z, Wolf IM, Chen H, et al. FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor a isoform. Mol Endocrinol. 2006;20:2682–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hong J, Kim ST, Tranguch S, Smith DF, Dey SK. Deficiency of co-chaperone immunophilin FKBP52 compromises sperm fertilizing capacity. Reproduction. 2007;133:395–403. [DOI] [PubMed] [Google Scholar]
- 24. Periyasamy S, Hinds T, Jr, Shemshedini L, Shou W, Sanchez ER. FKBP51 and Cyp40 are positive regulators of androgen-dependent prostate cancer cell growth and the targets of FK506 and cyclosporin A. Oncogene. 2010;29:1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Romano S, Di Pace A, Sorrentino A, Bisogni R, Sivero L, Romano MF. FK506 binding proteins as targets in anticancer therapy. Anticancer Agents Med Chem. 2010;10:651–656. [DOI] [PubMed] [Google Scholar]
- 26. Li L, Lou Z, Wang L. The role of FKBP5 in cancer aetiology and chemoresistance. Br J Cancer. 2010;104:19–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Binder EB, Salyakina D, Lichtner P, et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. [DOI] [PubMed] [Google Scholar]
- 28. Ising M, Depping AM, Siebertz A, et al. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur J Neurosci. 2008;28:389–398. [DOI] [PubMed] [Google Scholar]
- 29. Kirchheiner J, Lorch R, Lebedeva E, et al. Genetic variants in FKBP5 affecting response to antidepressant drug treatment. Pharmacogenomics. 2008;9:841–846. [DOI] [PubMed] [Google Scholar]
- 30. Hartmann J, Wagner KV, Liebl C, et al. The involvement of FK506-binding protein 51 (FKBP5) in the behavioral and neuroendocrine effects of chronic social defeat stress. Neuropharmacology. 2012;62:332–339. [DOI] [PubMed] [Google Scholar]
- 31. Hoeijmakers L, Harbich D, Schmid B, et al. Depletion of FKBP51 in female mice shapes HPA axis activity. PLoS One. 2014;9:e95796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang L, Isoda F, Yen K, et al. Hypothalamic Fkbp51 is induced by fasting and elevated hypothalamic expression promotes obese phenotypes. Am J Physiol Endocrinol Metab. 2012;302:E987–E991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Balsevich G, Uribe A, Wagner KV, et al. Interplay between diet-induced obesity and chronic stress in mice: potential role of FKBP51. J Endocrinol. 2014;222:15–26. [DOI] [PubMed] [Google Scholar]
- 34. Pereira MJ, Palming J, Svensson MK, et al. FKBP5 expression in human adipose tissue increases following dexamethasone exposure and is associated with insulin resistance. Metabolism. 2014;63:1198–1208. [DOI] [PubMed] [Google Scholar]
- 35. Al-Share QY, DeAngelis AM, Lester SG, et al. Forced hepatic overexpression of CEACAM1 curtails diet-induced insulin resistance. Diabetes. 2015;64:2780–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bauche IB, El Mkadem SA, Pottier AM, et al. Overexpression of adiponectin targeted to adipose tissue in transgenic mice: impaired adipocyte differentiation. Endocrinology. 2007;148:1539–1549. [DOI] [PubMed] [Google Scholar]
- 37. Arany Z, He H, Lin J, et al. Transcriptional coactivator PGC-1 α controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. [DOI] [PubMed] [Google Scholar]
- 38. Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. 2010;11:257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seale P, Kajimura S, Yang W, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2011;50:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cohen P, Levy JD, Zhang Y, et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell. 2014;156:304–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sierecki E, Sinko W, McCammon JA, Newton AC. Discovery of small molecule inhibitors of the PH domain leucine-rich repeat protein phosphatase (PHLPP) by chemical and virtual screening. J Med Chem. 2010;53:6899–6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. He W, Barak Y, Hevener A, et al. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA. 2003;100:15712–15717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jones JR, Barrick C, Kim KA, et al. Deletion of PPARγ in adipose tissues of mice protects against high fat diet-induced obesity and insulin resistance. Proc Natl Acad Sci USA. 2005;102:6207–6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang F, Mullican SE, DiSpirito JR, Peed LC, Lazar MA. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARγ. Proc Natl Acad Sci USA. 2013;110:18656–18661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Choi JH, Banks AS, Estall JL, et al. Anti-diabetic drugs inhibit obesity-linked phosphorylation of PPARγ by Cdk5. Nature. 2010;466:451–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Choi JH, Banks AS, Kamenecka TM, et al. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature. 2011;477:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bonnefont JP, Djouadi F, Prip-Buus C, Gobin S, Munnich A, Bastin J. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Aspects Med. 2004;25:495–520. [DOI] [PubMed] [Google Scholar]
- 49. Vegiopoulos A, Herzig S. Glucocorticoids, metabolism and metabolic diseases. Mol Cell Endocrinol. 2007;275:43–61. [DOI] [PubMed] [Google Scholar]
- 50. Gaali S, Gopalakrishnan R, Wang Y, Kozany C, Hausch F. The chemical biology of immunophilin ligands. Curr Med Chem. 2011;18:5355–5379. [DOI] [PubMed] [Google Scholar]
- 51. Schmidt MV, Paez-Pereda M, Holsboer F, Hausch F. The prospect of FKBP51 as a drug target. ChemMedChem. 2012;7:1351–1359. [DOI] [PubMed] [Google Scholar]
- 52. Gaali S, Kirschner A, Cuboni S, et al. Selective inhibitors of the FK506-binding protein 51 by induced fit. Nat Chem Biol. 2015;11:33–37. [DOI] [PubMed] [Google Scholar]
- 53. LeMaster DM, Hernandez G. Conformational dynamics in FKBP domains: relevance to molecular signaling and drug design. Curr Mol Pharmacol. 2015;9:5–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hinds TD, Stechschulte LA, Elkhairi F, Sanchez ER. Analysis of FK506, timcodar (VX-853) and FKBP51 and FKBP52 chaperones in control of glucocorticoid receptor activity and phosphorylation. Pharmacol Res Perspect. 2014;2:e00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hinds TD, Jr, John K, McBeth L, Trabbic CJ, Sanchez ER. Timcodar (VX-853) is a non-FKBP12 binding drug that inhibits PPARγ and suppresses adipogenesis [published online ahead of print April 14, 2016]. PPAR Res. 10.1155/2016/6218637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ghosh S, Kaw M, Patel PR, et al. Mice with null mutation of Ceacam I develop nonalcoholic steatohepatitis. Hepat Med. 2010;2:69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]