Abstract
Placental insufficiency is associated with reduced supply of amino acids to the fetus and leads to intrauterine growth restriction (IUGR). IUGR fetuses are characterized by lower glucose-stimulated insulin secretion, smaller pancreatic islets with less β-cells, and impaired pancreatic vascularity. To test whether supplemental amino acids infused into the IUGR fetus could improve these complications of IUGR we used acute (hours) and chronic (11 d) direct fetal amino acid infusions into a sheep model of placental insufficiency and IUGR near the end of gestation. IUGR fetuses had attenuated acute amino acid-stimulated insulin secretion compared with control fetuses. These results were confirmed in isolated IUGR pancreatic islets. After the chronic fetal amino acid infusion, fetal glucose-stimulated insulin secretion and islet size were restored to control values. These changes were associated with normalization of fetal pancreatic vascularity and higher fetal pancreatic vascular endothelial growth factor A protein concentrations. These results demonstrate that decreased fetal amino acid supply contributes to the pathogenesis of pancreatic islet defects in IUGR. Moreover, the results show that pancreatic islets in IUGR fetuses retain their ability to respond to increased amino acids near the end of gestation after chronic fetal growth restriction.
Placental insufficiency disrupts development of the fetal endocrine pancreas and results in islets that are smaller and contain fewer β-cells than normally developed islets (1). These structural defects are associated with functional deficits, including decreased fetal glucose-stimulated insulin secretion (GSIS) (2). Placental insufficiency is characterized by decreased nutrient and O2 transfer to the fetus (3). These nutrient transfer defects produce characteristic responses in intrauterine growth restriction (IUGR) fetuses, which include not only lower insulin concentrations and GSIS, but also lower concentrations of plasma glucose and IGF-1, lower blood O2 content, and higher plasma concentrations of norepinephrine, cortisol, and glucagon (2, 4–7). One of the most fundamental deficiencies in placental insufficiency is decreased placental-to-fetal transfer of amino acids, especially the essential and branched chain (leucine, isoleucine, and valine) amino acids (8–10). However, it is unknown to what extent decreased amino acid transfer from the placenta to the fetus, relative to other placental functional defects, is responsible for fetal pancreatic islet structural and functional defects seen in late gestation IUGR fetuses. This question is critical as insulin is a dominant fetal growth factor (11). Furthermore, fetal pancreatic defects may underlie the long term increase in risk that former IUGR individuals have of developing β-cell failure in the face of insulin resistance leading to type 2 diabetes (12).
Offspring from animal models of placental insufficiency and IUGR are characterized by lower insulin, GSIS, islet size, and β-cell numbers (13). In some models, pancreatic and islet vascularity also has been found to be lower, similar to human fetuses with severe IUGR (1, 14–16). In this study, we used direct fetal amino acid infusions into a sheep model of placental insufficiency and IUGR to test the role of amino acids in these pancreatic defects. These sheep develop progressive and severe placental insufficiency and share many characteristics with severe human placental insufficiency including lower rates of amino acid transfer to the fetus and lower fetal insulin, GSIS, islet size, and β-cell numbers (3, 13, 17, 18). Furthermore, defects in fetal islet structure and function are associated with lower pancreatic vascularity and impaired islet hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF)A paracrine signaling between the β-cell and vascular endothelial cell (16, 19).
Acute amino acid infusions (1.5 h) were used to test the responsiveness of amino acid-stimulated insulin secretion (AASIS) in IUGR fetuses in vivo. Confirmatory tests also were performed in isolated fetal pancreatic islets incubated in increasing amino acid concentrations in vitro. Chronic (∼11 d) amino acid infusions were used to determine if increasing the fetal amino acid supply, especially the supply of essential and branched chain amino acids (BCAAs), over the latter part of gestation could reverse structural and functional fetal islet defects in IUGR fetuses. At the end of the infusion we measured in vivo fetal GSIS and collected the pancreas for histological analysis and measurement of mRNA and proteins associated with insulin secretion, islet growth, vascularity, and paracrine islet signaling. Finally, we used isolated fetal pancreatic islets and fetal endothelial cells to test the relationship between amino acid supplementation and the paracrine regulation of islet function by HGF and vascular endothelial growth factor A (VEGFA). Our major findings show that, similar to acute glucose stimulation, acute amino acid stimulation of insulin secretion and insulin production is attenuated in IUGR fetuses and fetal islets. However, chronically increasing the amino acid supply for approximately 11 days to IUGR fetuses normalizes fetal pancreatic islet size and insulin secretion.
Research Design and Methods
Studies were conducted in pregnant Columbia-Rambouillet ewes carrying a singleton fetus in compliance with the Institutional Animal Care and Use Committee, University of Colorado Denver, National Institutes of Health, and United States Department of Agriculture. The laboratory is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Placental insufficiency was induced by exposing pregnant sheep to elevated ambient temperatures for 72.1 ± 0.8 days beginning on 39.4 ± 0.4 days gestation age (16, 20). Control (CON) ewes were maintained at normal ambient temperatures and fed to treated ewe average intake. IUGR sheep were placed in normal ambient temperatures before surgery for placement of maternal and fetal vascular catheters (21).
Acute in vivo amino acid stimulated insulin secretion
AASIS was measured with a fetal hyperaminoacidemic clamp using a 10% TrophAmine (wt/vol) bolus (100-mg amino acids per kg estimated fetal weight) at time 0 followed by a constant infusion (350-mg amino acids per kg estimated fetal weight per hour) (22). A continuous transfusion of maternal whole blood into the fetus compensated for sampled fetal blood. Plasma glucose, amino acids, insulin, glucagon, and IGF-1 concentrations were determined before and throughout the clamp.
Chronic in vivo amino acid infusion with GSIS
Some IUGR fetuses were used for a chronic fetal amino acid infusion group with 10% TrophAmine (wt/vol) (IUGR+amino acids [AA], n = 10) and a chronic saline infusion group (n = 8). Some CON fetuses also received a chronic saline infusion (n = 7). The TrophAmine infusion was adjusted daily to achieve a 25%–50% increase in BCAA concentrations. IUGR and CON fetuses received a saline infusion to match the sodium delivery from TrophAmine (21, 23). The duration of infusions was similar among groups (11.0 ± 0.9 d, CON; 10.1 ± 1.0, IUGR; and 10.6 ± 0.7, IUGR+AA).
On the final day of the chronic infusions, a fetal hyperglycemic clamp followed by a fetal arginine infusion was used to determine fetal GSIS and glucose-potentiated arginine-stimulated insulin secretion (GPASIS) (21, 24). We calculated GPASIS and arginine stimulated glucagon secretion as the difference between the hormone concentrations immediately before and 5 minutes after the arginine infusion. The chronic infusions continued through the insulin secretion studies until tissue collection the next day.
Biochemical analyses
Biochemical analysis was performed as previously described (21, 23, 25). Carbon dioxide, O2, pH, and hematocrit were measured with the ABL 520 analyzer (Radiometer). Plasma glucose and lactate concentrations were measured with the Yellow Springs Instruments model 2700 biochemistry analyzer. Plasma BCAA concentrations were measured with a spectrophotometric assay. Insulin, cortisol, and IGF-1 concentrations were measured by ELISA (ALPCO Immunoassays), glucagon by RIA (Millipore), and norepinephrine by HPLC. Amino acids were measured with a Dionex 300 model 4500 amino acid analyzer.
Organ isolation
At necropsy, some fetal pancreases from all groups were weighed and the splenic portion fixed in 4% paraformaldehyde (wt/vol) and frozen (16). The hepatic portion was snap frozen in liquid nitrogen and transferred to −80°C. Other pancreases from CON and IUGR fetuses were used for islet isolation (20). The placenta was dissected and weighed.
Pancreatic histology
The proportion of the fetal pancreas comprised of β-cells and α-cells, islet size, pancreatic and islet vascularity, β-cell size, and β-cell mitosis (β-cell costaining with insulin and phosphorylated histone 3 [pHH3]) were measured by individuals blinded to treatment groups (16, 26, 27).
Pancreatic islet incubation
Pancreatic islets were isolated from 10 CON and 16 IUGR fetuses and incubated overnight in 95% O2 and 5% CO2 in RPMI 1640 with 2.8 mmol/L glucose and 1% (vol/vol) fetal bovine serum (20). Experimental additions included recombinant human HGF (100-ng/mL ProSpec) or amino acids to a final concentration of 2× or 6× based on calculations for leucine (MEM Amino Acids 50× solution, catalog number 25–030; Corning Cellgro). The next day islets were washed, and islet insulin secretion and content were measured (20). For each animal, insulin secretion was tested in 3–6 batches of islets (10 islets per batch) per condition.
Endothelial cell culture
Large vessel (pulmonary) endothelial cells were isolated from normally growing fetal sheep, propagated and purity verified as previously described (28). After cells (passage 4–6) reached 70% confluence, they were serum starved overnight and then incubated for 72 hours in DMEM with 10% fetal bovine serum and experimental additions, including amino acids (MEM Amino Acids 50× solution), leucine (2.4 mmol/L; Sigma), and recombinant human VEGFA (50 ng/mL; R&D Systems). Media were changed daily and mRNA was collected (29).
mRNA and protein analysis
Total RNA was extracted from pulverized pancreas or endothelial cells and reverse transcribed into complimentary DNA (21). INS (insulin), GCG (glucagon), SST (somatostatin), PPY (pancreatic polypepetide), GCK (glucokinase), IGF1 (IGF-1), and IGF2 (IGF-2) mRNA were measured as previously described (21, 30, 31). Real-time quantitative PCR assays were developed for VEGFA (for-TTGCCTTGCTGCTCTACCTT, rev-GGGCACACACTCCAGACTTT), HGF (for-AGGAGACGAGAAACGCAAACAGGT, rev-ATAGCAGGCCTAGCAAGCTTCAGT), MET (HGF receptor [cMET]; for-GAATGGCCTGGGCTGTGAACATTT, rev-TGTTGGCAGACAGGTCTCTTGAGT), and FLT1 (VEGF receptor 1 [VEGFR1]; for-TTGTCATGCTAATGGCGTCCCAGA, rev-GGTTGCTGGCTTTGCAGTGATACA). Pancreatic samples were analyzed in triplicate and endothelial cell samples in duplicate. The standard curve method of relative quantification was used (32). Genes of interest were normalized to reference genes RPS15 (ribosomal protein s15), ACTA1 (actin), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (21). Results are presented as fold change relative to the CON group for pancreatic mRNA and relative to no experimental additions for endothelial cell mRNA.
Total protein was extracted from pulverized pancreas, separated by gel electrophoresis, and transferred to a membrane for western blotting (25). The following primary antibodies were diluted in phosphate buffered saline with tween with 5% bovine serum albumin (wt/vol), except as noted: glucokinase (1:1000; Abcam), glucose transporter 2 (GLUT2) (1:1000; Santa Cruz Biotechnology), HGF (1:1000; diluted in 2% enhanced chemiluminescence Advance; R&D Systems), VEGFA (1:200 diluted in 2% enhanced chemiluminescence Advance; Santa Cruz Biotechnology), and actin (1:20,000; MP Biomedicals). Horseradish peroxidase-conjugated secondary antibodies were applied and detected. Densitometry results were normalized to β-actin and expressed relative to the CON group.
Statistical analysis
Analysis was performed using SAS 9.1 or GraphPad Prism 5 with a one-way or mixed models ANOVA which included terms for group (CON, IUGR, and IUGR+AA), time, and incubation condition. After determining that fetal sex did not impact outcomes in a preliminary mixed models ANOVA, we pooled data from male and female fetuses. Repeated measurements made in the same animal were accounted for. Individual means were compared using Fisher's least significant difference for mixed models ANOVA or Tukey's method for one-way ANOVA. Data were log transformed due to increased variability as indicated. P ≤ .05 was considered significant.
Results
Acute in vivo amino acid infusion
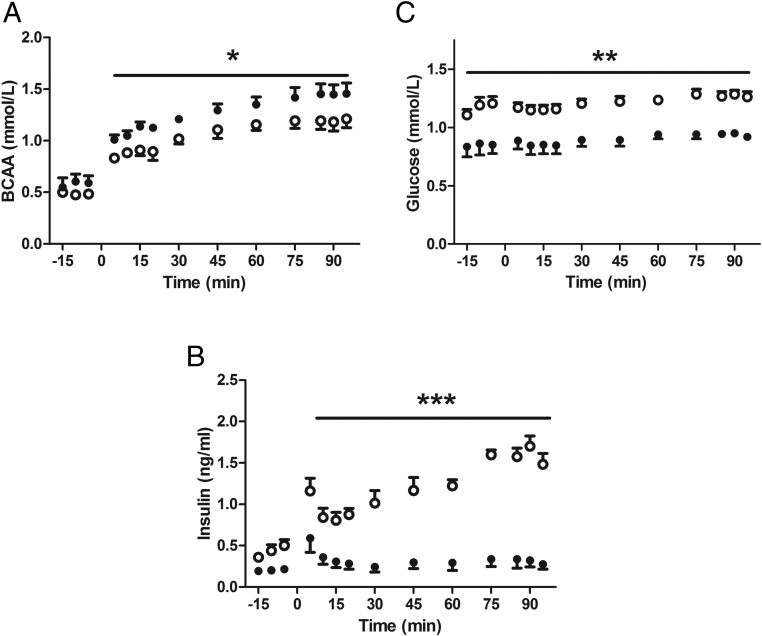
Acute AASIS was measured in CON (n = 5) and IUGR (n = 5) fetuses. The infusion rate was similar (377 ± 40 mg kg −1 per h−1 CON, 332 ± 38 mg kg−1 per h−1 IUGR). Despite higher BCAA concentrations (P < .05) (Figure 1A), IUGR fetuses had attenuated AASIS (P < .0001) (Figure 1B). Glucose concentrations increased by about 10% during the hyperaminoacidemic clamp in both groups (P < .0001) (Figure 1C). Concentrations of glucagon and almost all amino acids increased in both groups during the hyperaminoacidemic clamp (P < .05) (Supplemental Table 1). IGF-1 concentrations did not change during the clamp in either group (Supplemental Table 1).
Figure 1.
IUGR fetuses have attenuated acute in vivo AASIS. CON (open circles, n = 5) and IUGR (closed circles, n = 5) underwent a primed, continuous, constant-rate hyperaminoacidemic clamp with a direct fetal infusion of 10% TrophAmine (wt/vol) starting at time 0. A, BCAA concentrations increased in both groups and were higher in the IUGR fetuses compared with CONs (*, P < .05). B, IUGR fetuses had significantly lower fetal arterial plasma insulin concentrations during the clamp (***, P < .0001). C, Fetal arterial plasma glucose concentrations increased slightly in both groups (P < .005) but were consistently lower in the IUGR-SAL fetuses (**, P < .0001). Mean ± SE fetal arterial plasma concentrations are plotted and statistics are by mixed models ANOVA.
Chronic in vivo amino acid infusion
Maternal and fetal metabolites, blood gases, and hormones
IUGR+AA fetuses were infused with increasing rates of TrophAmine (Supplemental Figure 1) and compared with IUGR and CON. The final weight adjusted infusion rate was 2.40 ± 0.17 g kg−1 per d−1. There were no maternal biochemical differences among the groups (Table 1). Most fetal essential amino acid concentrations were higher in IUGR+AA compared with IUGR (Table 1). Fetal lactate in IUGR+AA was greater than the other 2 groups at the end of the infusion (P < .01) (Table 1). There were no differences among the groups for fetal hematocrit, pCO2, or pH (Table 1). Fetal pO2, hemoglobin-O2 saturation, total O2 content, and glucose were lower in both IUGR groups compared with CON (P < .005), but the IUGR groups were not different from each other (Table 1). Norepinephrine and cortisol concentrations were higher and insulin and IGF-1 concentrations lower in the both IUGR groups compared with CONs (P < .05), but the IUGR groups were not different from each other (Table 1). The amino acid infusion increased glucagon concentrations in the IUGR+AA group (P < .0001) (Table 1).
Table 1.
Maternal and Fetal Arterial Biochemistry
| CON | IUGR | IUGR+AA | |
|---|---|---|---|
| Maternal arterial biochemistry | |||
| Glucose (plasma, mmol/L) | 3.83 ± 0.13 | 3.91 ± 0.06 | 3.98 ± 0.20 |
| Lactate (plasma, mmol/L) | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.0 |
| pH | 7.44 ± 0.00 | 7.44 ± 0.01 | 7.44 ± 0.01 |
| pCO2 (mm Hg) | 34.3 ± 0.6 | 35.6 ± 0.8 | 35.3 ± 0.7 |
| pO2 (mm Hg) | 87.1 ± 1.7 | 87.7 ± 1.5 | 90.6 ± 2.4 |
| Hematocrit (%) | 32.1 ± 1.1 | 32.3 ± 1.0 | 30.7 ± 0.6 |
| sO2 (%) | 95.4 ± 0.6 | 95.8 ± 0.6 | 96.5 ± 0.5 |
| O2 content (mmol/L) | 5.9 ± 0.2 | 6.2 ± 0.2 | 5.7 ± 0.1 |
| Fetal arterial biochemistry | |||
| Glucose (plasma, mmol/L) | 1.04 ± 0.03 | 0.74 ± 0.04a | 0.72 ± 0.06a |
| Lactate (plasma, mmol/L) | 2.3 ± 0.1 | 2.6 ± 0.2 | 5.0 ± 1.4a,b |
| pH | 7.37 ± 0.00 | 7.36 ± 0.00 | 7.35 ± 0.01 |
| pCO2 (mm Hg) | 49.9 ± 0.9 | 51.4 ± 0.5 | 52.0 ± 1.2 |
| pO2 (mm Hg) | 19.6 ± 0.6 | 15.6 ± 0.5a | 14.2 ± 1.2a |
| Hematocrit (%) | 37.8 ± 1.4 | 37.5 ± 0.8 | 40.9 ± 1.7 |
| Hemoglobin-O2 saturation (%) | 49.3 ± 2.4 | 35.2 ± 2.0a | 30.5 ± 4.6a |
| O2 content (mmol/L) | 3.6 ± 0.2 | 2.6 ± 0.2a | 2.5 ± 0.4a |
| Insulin (plasma, ng/mL) | 0.37 ± 0.04 | 0.19 ± 0.02a | 0.24 ± 0.03a |
| Glucagon (plasma, pg/mL) | 37.8 ± 3.0 | 49.5 ± 5.1 | 216.5 ± 88.9a,b,c |
| Norepinephrine (plasma, pg/mL) | 641 ± 110 | 1901 ± 532a | 1694 ± 571a,c |
| Cortisol (plasma, ng/mL) | 8.1 ± 1.0 | 29.9 ± 10.0a | 40.7 ± 17.7a,c |
| IGF-1 (plasma, ng/mL) | 132.0 ± 7.9 | 56.4 ± 6.9a | 57.2 ± 13.2a,c |
| Fetal arterial plasma amino acid concentrations (μmol/L) | |||
| Alanine | 295.9 ± 13.3 | 330.2 ± 15.1 | 391.1 ± 91.2 |
| Arginine | 106.2 ± 11.2 | 63.9 ± 5.9a | 93.2 ± 10.1b |
| Asparagine | 44.0 ± 3.3 | 55.4 ± 6.3 | 47.4 ± 14.1 |
| Aspartate | 19.5 ± 1.9 | 16.4 ± 1.2 | 16.4 ± 1.5 |
| Citrulline | 182.4 ± 11.8 | 198.6 ± 13.6 | 204.4 ± 23.2 |
| Cystine | 12.6 ± 0.8 | 13.7 ± 1.4 | 11.1 ± 1.7 |
| Glutamate | 44.7 ± 4.1 | 38.5 ± 3.3 | 24.4 ± 3.5 |
| Glutamine | 394.8 ± 14.7 | 384.9 ± 12.8 | 360.6 ± 45.9 |
| Glycine | 341.4 ± 17.8 | 350.9 ± 17.5 | 387.9 ± 53.7 |
| Histidine | 60.7 ± 4.5 | 63.1 ± 3.5 | 91.6 ± 12.4a,b |
| Isoleucine | 80.8 ± 4.7 | 86.1 ± 5.5 | 129.3 ± 21.8a,b[r |
| Leucine | 126.0 ± 7.9 | 146.2 ± 7.4 | 242.4 ± 39.5a,b |
| Lysine | 87.6 ± 7.8 | 85.7 ± 5.2 | 192.2 ± 53.7a,b |
| Methionine | 86.8 ± 4.6 | 71.2 ± 4.2 | 86.0 ± 10.8 |
| Ornithine | 81.3 ± 6.3 | 76.0 ± 5.9 | 92.2 ± 9.5 |
| Phenylalanine | 95.7 ± 5.7 | 94.1 ± 4.3 | 129.3 ± 24.3a,b |
| Proline | 142.5 ± 8.2 | 171.7 ± 10.7 | 385.0 ± 95.2a,b |
| Serine | 569.9 ± 46.3 | 481.3 ± 46.9 | 317.3 ± 46.2 |
| Taurine | 91.9 ± 12.7 | 164.2 ± 23.4a | 252.5 ± 36.2a,b |
| Threonine | 339.9 ± 27.4 | 272.7 ± 18.0a | 258.8 ± 40.6a |
| Tryptophan | 41.6 ± 2.3 | 39.4 ± 2.6 | 58.4 ± 6.6b |
| Tyrosine | 105.9 ± 5.8 | 106.1 ± 7.8 | 125.6 ± 28.0 |
| Valine | 311.2 ± 18.3 | 392.5 ± 20.3b | 449.8 ± 48.9a,b |
Mean ± SE from measurements made immediately before the final acute physiological studies are presented in CON (n = 17), IUGR (n = 25), and IUGR-AA (n = 10). Groups were compared by one-way ANOVA.
Significant difference compared with CON.
Significant difference compared with IUGR (P < .05) with Tukey's correction for multiple comparisons.
Data were log transformed for analysis.
Fetal insulin secretion
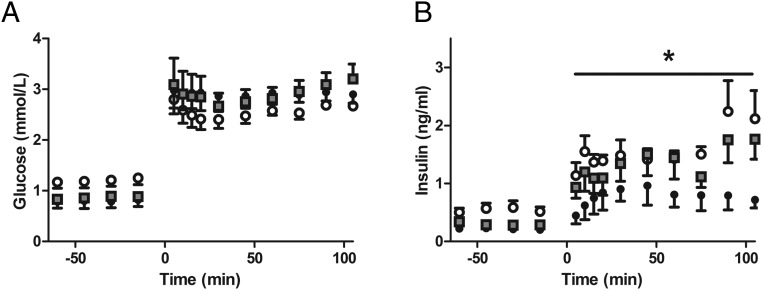
GSIS and GPASIS were measured in CON (n = 6), IUGR (n = 6), and IUGR+AA (n = 5) fetuses. After initiation of the hyperglycemic clamp, glucose concentrations were similar among all groups (Figure 2A). GSIS was restored to CON levels in IUGR+AA, both of which had more robust insulin secretion than IUGR (P < .05) (Figure 2B). There was no difference between the groups for GPASIS (1.87 ± 0.39-ng/mL CON, 1.32 ± 0.56-ng/mL IUGR, 1.28 ± 0.69-ng/mL IUGR+AA). Arginine stimulated glucagon secretion was greater in IUGR+AA (678 ± 408 pg/mL, P < .05, after log transformation) compared with the other 2 groups (82 ± 31-pg/mL CON, 89 ± 40-pg/mL IUGR).
Figure 2.
In vivo GSIS is normalized in IUGR+AA fetuses at the end of the chronic amino acid infusion period. Mean ± SE fetal arterial plasma glucose (A) and insulin (B) concentrations are shown relative to the start of a primed continuous variable-rate fetal hyperglycemic clamp (time = 0 min) in CONs (open circles, n = 6), IUGR (closed circles, n = 6), and IUGR+AA (gray squares, n = 5); *, significantly lower insulin concentrations in IUGR compared with both CON and IUGR+AA (P < .05) by mixed models ANOVA.
Characteristics of the fetal pancreas (Table 2)
Table 2.
Characteristics of the Fetal Pancreas
| CON | IUGR | IUGR+AA | |
|---|---|---|---|
| Pancreatic morphology | |||
| Islet size (μm2) | 2876 ± 295 | 1706 ± 230a | 2878 ± 280a |
| Insulin+ area (%) | 3.52 ± 0.21 | 2.07 ± 0.07a | 2.98 ± 0.25a,d |
| β-Cell mass (mg) | 108.1 ± 7.5 | 43.3 ± 5.5a | 77.6 ± 12.4a,d |
| β-Cell size (μm2) | 73.4 ± 2.9 | 79.8 ± 5.3 | 82.6 ± 5.8 |
| β-Cell mitosis (%) | 0.39 ± 0.02 | 0.24 ± 0.04a | 0.27 ± 0.03a |
| Glucagon+ area (%) | 0.90 ± 0.06 | 0.68 ± 0.10 | 0.78 ± 0.06 |
| α-Cell mass (mg) | 27.6 ± 2.2 | 15.4 ± 3.0a | 20.4 ± 2.8 |
| Islet vessel density (%) | 15.03 ± 1.63 | 16.61 ± 1.64 | 14.11 ± 0.88 |
| Pancreatic vessel density (%) | 3.29 ± 0.32 | 2.25 ± 0.19c | 3.63 ± 0.43b,d |
| Pancreatic protein and mRNA expression | |||
| Endocrine hormones | |||
| Insulin (mRNA) | 1.00 ± 0.11 | 0.49 ± 0.08c | 0.96 ± 0.28d |
| Gucagon (mRNA) | 1.00 ± 0.05 | 0.60 ± 0.09 | 1.70 ± 0.46b,d |
| Somatostatin (mRNA) | 1.00 ± 0.12 | 0.67 ± 0.13 | 0.96 ± 0.19 |
| Pancreatic polypeptide (mRNA) | 1.00 ± 0.34 | 0.81 ± 0.54 | 1.63 ± 0.61 |
| Glucose transport and metabolism | |||
| GLUT2 (protein) | 1.00 ± 0.28 | 4.01 ± 0.84a | 2.13 ± 0.49d |
| Glucokinase (protein) | 1.00 ± 0.07 | 0.90 ± 0.18 | 1.00 ± 0.06 |
| Glucokinase (mRNA) | 1.00 ± 0.22 | 0.91 ± 0.15 | 0.77 ± 0.22 |
| Paracrine hormones and receptors | |||
| VEGFA (protein) | 1.00 ± 0.25 | 2.41 ± 0.37 | 4.14 ± 0.54a,b |
| VEGFA (mRNA) | 1.00 ± 0.23 | 1.41 ± 0.20 | 1.63 ± 0.59d |
| HGF (protein) | 1.00 ± 0.13 | 1.35 ± 0.27 | 0.91 ± 0.22 |
| HGF (mRNA) | 1.00 ± 0.26 | 1.05 ± 0.27 | 0.78 ± 0.24 |
| cMET (mRNA) | 1.00 ± 0.41 | 1.12 ± 0.42 | 1.09 ± 0.40 |
| VEGFR1 (mRNA) | 1.00 ± 0.29 | 1.22 ± 0.32 | 1.51 ± 0.63d |
| IGF-1 (mRNA) | 1.00 ± 0.25 | 0.67 ± 0.26 | 0.55 ± 0.21 |
| IGF-2 (mRNA) | 1.00 ± 0.07 | 0.80 ± 0.25 | 0.92 ± 0.09 |
Data are presented as mean ± SE for CON (n = 7), IUGR (n = 6–8), and IUGR-AA (n = 9).
Significant difference compare with CON.
Significant difference compared with IUGR (P < .05) by one-way ANOVA with Tukey's correction for multiple comparisons.
Significant difference between CON and IUGR on post hoc Student's t test (P < .05).
Data were log transformed for analysis.
Histology of the fetal pancreas
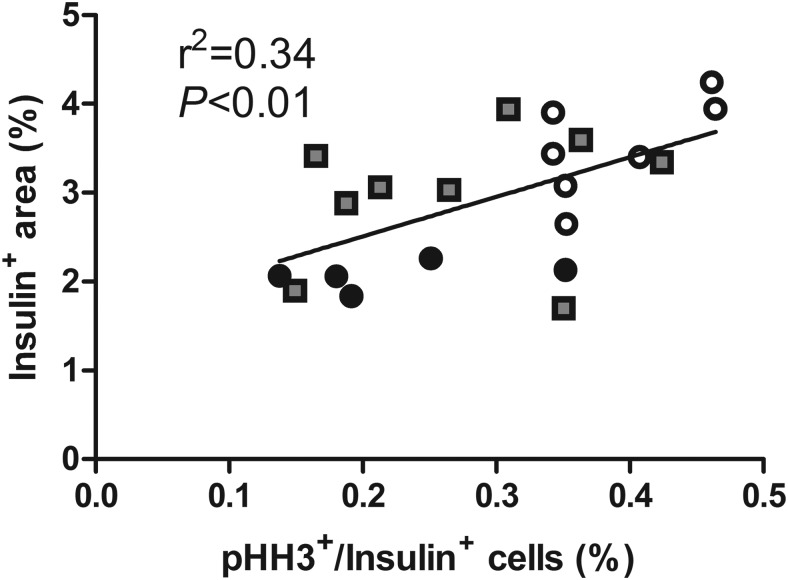
Islet size, the percentage of the pancreatic sections staining for insulin (β-cell area), and β-cell mass were all greater in IUGR+AA (n = 9) than IUGR (n = 6) (P < .05). They were equivalent in IUGR+AA to CON (n = 7). β-Cell size was not different among the groups. The percentage of β-cells staining for pHH3 was lower in both IUGR groups compared with CON. There was a positive correlation between β-cell pHH3 staining and the pancreatic β-cell area (P < .01) (Figure 3).
Figure 3.
Pancreatic insulin+ area is correlated with the percentage of β-cells in mitosis. Fetal pancreatic sections from CONs (open circles, n = 7), IUGR (closed circles, n = 6), and IUGR+AA (gray squares, n = 9) were immunostained for insulin to define β-cells and pHH3 to define cells in mitosis. A linear association was found between the insulin+ area (y-axis) and the percentage of β-cells in mitosis (x-axis) by linear regression for all fetuses combined (P < .01).
The percentage of the pancreatic sections staining for glucagon (α-cell area) was the same among groups. α-cell mass was lower in IUGR compared with CON (P < .01). The α-cell mass in IUGR+AA fetuses was intermediate. IUGR+AA pancreatic vessel density was higher than the IUGR group (P < .05) and equivalent to CON. Islet vessel density was not different among the groups.
Protein and mRNA expression (Table 2)
Expression of specific pancreatic protein and mRNAs were measured (7 CON, 8 IUGR, and 9 IUGR+AA). Pancreatic insulin mRNA expression was similar between IUGR+AA and CON. Pancreatic glucagon mRNA was higher in IUGR+AA compared with IUGR (P < .05). There were no differences among the groups for pancreatic somatostatin or pancreatic polypeptide mRNA. Pancreatic GLUT2 protein expression was higher in IUGR compared with CON (P < .05). Expression in IUGR+AA was intermediate. Pancreatic glucokinase protein and mRNA were not different among groups. Pancreatic VEGFA protein expression was higher in IUGR compared with the CON (P ≤ .05) and was even higher in IUGR+AA compared with IUGR (P < .05). Pancreatic HGF protein and mRNA, and cMET, VEGFR1, IGF-1, and IGF-2 mRNA were not different among groups.
Necropsy measurements
Placental weight, fetal weight, and fetal lengths were lower in both IUGR groups compared with CON. Relative to fetal weight, pancreases in IUGR+AA were heavier than the pancreases from IUGR or CON (P < .05) (Table 3).
Table 3.
Necropsy Measurements
| CON | IUGR | IUGR+AA | |
|---|---|---|---|
| Gestational age (d) | 133.6 ± 0.7 | 134.4 ± 0.5 | 134.6 ± 0.5 |
| Sex (% male) | 59 | 68 | 60 |
| Fetal weight (gm) | 3127 ± 141 | 2059 ± 120a | 2128 ± 225a |
| Crown rump length (cm) | 46.9 ± 0.8 | 42.5 ± 0.9a | 41.2 ± 1.8a |
| Lower limb length (cm) | 34.1 ± 0.8 | 29.9 ± 0.7a | 29.1 ± 1.2a |
| Placentome number | 74.2 ± 2.9 | 72.4 ± 2.7 | 76.6 ± 3.4 |
| Placental weight (gm) | 345.7 ± 22.0 | 190.8 ± 13.3a | 183.1 ± 23.4a |
| Pancreas to fetal weight ratio (%) | 0.095 ± 0.005 | 0.100 ± 0.006 | 0.127 ± 0.016a,b |
Data are presented as mean ± SE for CON (n = 17), IUGR (n = 25), and IUGR-AA (n = 10). For the pancreas, n = 7, 6, and 9 in CON, IUGR, and IUGR-AA, respectively.
Significant difference compared with CON.
Significant difference compared with IUGR (P < .05) by one-way ANOVA with Tukey's correction for multiple comparisons.
In vitro islet insulin secretion
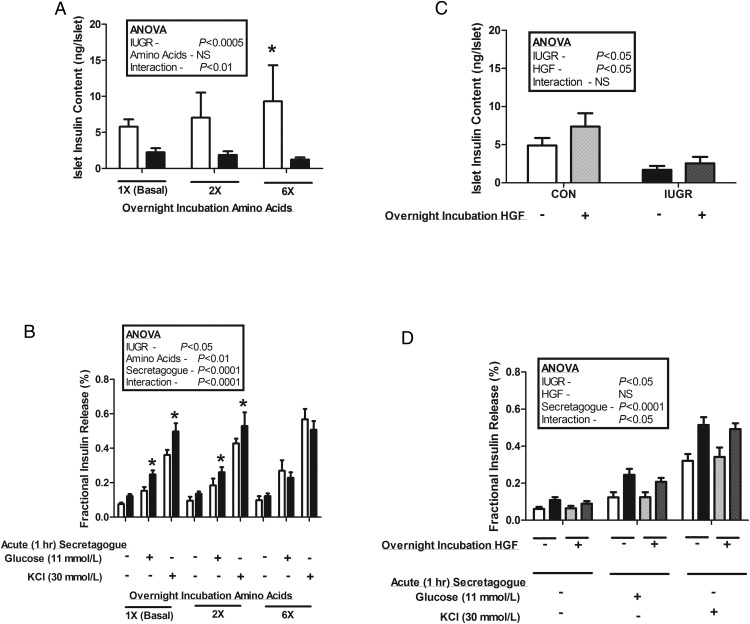
Pancreatic islets were isolated from CON (n = 10) and IUGR (n = 16) fetuses. Islets were not obtained from IUGR+AA fetuses. To determine if the attenuated response to acute AASIS in the IUGR fetus in vivo was independent of other in vivo abnormalities, we incubated isolated islets in increasing amino acid concentrations overnight and then measured islet insulin concentration and secretion. Insulin content was lower in IUGR islets compared with CON regardless of supplemental amino acids (P < .0005). A dose-dependent increase in islet insulin content and glucose- and potassium-stimulated insulin secretion after incubation with supplemental amino acids was observed in CON islets but not in IUGR islets (P < .05) (Figure 4A). As we have previously reported, the fractional insulin release was higher in IUGR islets compared with CON after overnight incubations in normal media (P < .05) (20). However, there were no differences between IUGR and CON islet fractional insulin release after overnight incubation in 6× amino acids (Figure 4B).
Figure 4.
In vitro pancreatic islet insulin secretion. Pancreatic islets were isolated from CON (white bars, n = 10) and IUGR (black bars, n = 16) fetuses and incubated overnight with or without additional amino acids (2× or 6×) or HGF (100 ng/mL). A, Insulin content was lower in IUGR islets than CON islets (P < .0005). CON islet insulin content increased with increasing overnight amino acid supplementation (*, relative to basal conditions, P < .005), an effect not seen in IUGR islets. B, Overnight amino acid supplementation had no effect on IUGR islet glucose and potassium-stimulated insulin secretion. However, CON islets had a dose-response increase in glucose and potassium-stimulated insulin secretion after overnight incubation with supplemental amino acids (P < .05); *, significantly more fractional insulin release from IUGR islets compared with CON islets incubated in the same conditions (P < .05). C, Overnight incubation with HGF resulted in higher insulin contents of both CON and IUGR islets (P < .05). D, Overnight incubation in HGF had no effect on CON or IUGR islet glucose or potassium-stimulated insulin secretion. Data are presented as mean ± SE, and statistics are by mixed models ANOVA.
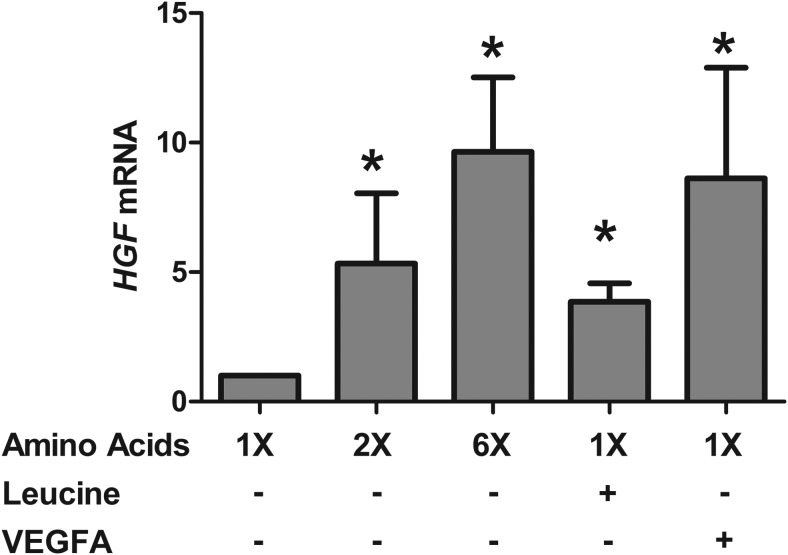
Due to the increase in pancreatic VEGFA protein and pancreatic vessel density in IUGR+AA fetuses, we sought to further determine the relationship between amino acids, VEGFA, and the paracrine regulation of islet function and growth by endothelial cells. We incubated large vessel fetal sheep endothelial cells in supplemental amino acids and VEGFA. Amino acids dose dependently increased HGF mRNA (P < .0001) (Figure 5). Supplemental leucine and VEGFA also increased HGF mRNA (P < .0005) (Figure 5). Given these results and the role that HGF plays in endothelial cell stimulation of β-cell insulin production and secretion (16, 19), we incubated islets overnight in supplemental HGF. HGF increased the insulin content of both CON and IUGR islets (P < .05) (Figure 4C). Fractional insulin release, however, did not increase in either group (Figure 4D).
Figure 5.
Large vessel endothelial cell HGF mRNA is increased with in vitro supplemental amino acids, leucine, and VEGFA. Large vessel endothelial cells from normal fetal sheep (n = 3) were incubated for 72 hours in basal conditions or with indicated supplementation (amino acids [2× or 6×], leucine [2.4 mmol/L], VEGFA [50 ng/mL]). HGF mRNA was then measured and normalized to basal conditions. For each incubation condition, cells derived from all 3 animals were tested in replicates of 1–3; *, significant difference from basal conditions (P < .005). Data are presented as mean ± SE, and statistics are by mixed models ANOVA after log transformation.
Discussion
In this study, we have shown that acute AASIS is decreased in IUGR fetuses compared with CON, but chronically (∼11 d) increasing IUGR fetal amino acid supply and concentrations restores fetal GSIS, pancreatic vascularity, and islet size, as well as pancreatic β-cell mass. The specific ability of amino acids to restore GSIS, islet size, and β-cell mass is not a general property of all fetal nutrients, as we have previously demonstrated that chronically increasing the fetal glucose supply in IUGR has no impact on β-cell mass and actually lowers fetal GSIS (33). These results advance our understanding of the pathogenesis of β-cell dysfunction and impaired islet development in IUGR. Our novel findings point to amino acid insufficiency as fundamental in regulation of fetal islet development and insulin secretion in IUGR.
Previously published evidence of a role for decreased fetal amino acid supply in the pathogenesis of islet dysfunction in IUGR comes from pregnant rats fed a low-protein diet. The fetuses from these dams are characterized by smaller islets, less islet insulin secretion, and lower β-cell mass (14, 34–36). However, amino acid delivery has not been measured directly in the low-protein diet rat model of IUGR. This is unlike the situation for human IUGR and the sheep model of placental insufficiency and IUGR used in this study, in which decreased amino acid supply to the fetus from the placenta has been directly measured and shown to be decreased (9, 10, 18, 31). Therefore, we undertook a comprehensive investigation of the role of amino acids in the pathogenesis of islet dysfunction using this sheep model of IUGR. Similar to the low-protein diet model of IUGR and to severe human IUGR, the placental insufficiency model of IUGR used in the current study is characterized by decreased fetal insulin concentrations, decreased insulin secretion, decreased islet size, decreased β-cell mass, and decreased islet vascularity (13, 16, 20, 27).
We first measured acute fetal AASIS with frequent blood sampling during an amino acid clamp, which has never been specifically tested in a model of placental insufficiency and IUGR. The significant attenuation of the acute insulin secretory response to an infused amino acid mixture in the late gestation IUGR fetuses is consistent with previous reports of their β-cell dysfunction, as demonstrated by decreased in vivo GSIS and smaller islets with lower β-cell mass and less islet insulin content (20, 27). These functional and structural defects certainly point to a possible fixed β-cell deficit by the end of gestation. However, recent studies have shown that acute GSIS in IUGR fetuses also is regulated by catecholamines and acute adrenergic signaling, implicating a more complex situation than might be expected based on structural analysis of the IUGR pancreas alone (37, 38). Therefore, we confirmed the attenuated in vivo AASIS using isolated fetal sheep islets incubated in different concentrations of supplemental amino acids, which eliminated confounding by adrenergic signaling. Consistent with our in vivo results, only the islets isolated from CON fetuses showed an increase in islet insulin concentrations or fractional insulin release in response to acute (overnight) exposure to supplemental amino acids. In contrast, the islets isolated from IUGR fetuses were not responsive to amino acids.
Contrary to the lack of responsiveness to acute amino acid supplementation in IUGR fetuses or isolated IUGR islets, an 11-day amino acid infusion completely normalized in vivo GSIS in the IUGR fetus. This was independent of changes in glucose, O2, IGF-1, cortisol, and norepinephrine, demonstrating specificity of the amino acid supplementation. Of the nutrients we measured, only lactate and amino acid concentrations increased. Glucagon concentrations also were notably increased in the IUGR+AA fetuses. This is consistent with previous studies in isolated α-cells, adult animals, and fetal animals that have demonstrated amino acid stimulated glucagon secretion (21, 39–41). Increased fetal plasma glucagon concentrations and fetal pancreatic glucagon mRNA in our IUGR+AA fetuses is especially noteworthy as glucagon can potentiate GSIS by binding to specific receptors on the β-cell and augmenting insulin secretion by increasing cAMP concentrations (21, 40, 42–46).
A major and unique observation in the current studies is that islet size in the IUGR+AA fetuses was equivalent to CON fetuses, providing another possible explanation for restored GSIS. The increase in islet size represented an expansion of all cells measured but with a greater expansion of β-cells compared with the other cell types. β-Cell mass in the IUGR+AA fetuses was almost completely restored to levels found in CON fetuses. We could not explain the higher proportion of β-cells by identifying larger β-cells or increased rates of β-cell mitosis based on costaining of insulin and pHH3. However, the proportion of β-cells in mitosis was directly associated with the percent of the pancreas staining for insulin, indicating that increased rates of β-cell mitosis might have played a role in the improved structure and function of the IUGR+AA fetuses.
The increase in islet size with a proportional increase in islet vascularity, as well as increased overall pancreatic vascularity in the IUGR+AA fetuses, was associated with more pancreatic VEGFA protein compared with saline infused IUGR fetuses. We did not identify any associated changes in pancreatic HGF protein or mRNA. Given the stimulatory effect that VEGFA has on islet endothelial cell HGF production and the ability of endothelial cell-produced HGF to stimulate islet growth and insulin secretion (47–49); however, we tested the effect of these growth factors and amino acids in vitro on HGF production and insulin secretion using isolated fetal islets and large vessel fetal sheep endothelial cells. Both amino acids and VEGFA significantly increased HGF mRNA in fetal sheep endothelial cells. These results are consistent with experiments in isolated adult rodent islet endothelial cells and other cell types (49, 50). Together, they show the potential for either amino acids directly or via increased pancreatic concentrations of VEGFA to increase local islet concentrations of HGF. Given these results, we tested the impact of HGF on in vitro function in isolated fetal pancreatic islets and found that HGF could increase the insulin concentrations of both CON and IUGR islets after an overnight incubation, thereby further supporting a link between amino acids, VEGFA, and the paracrine regulation of islet insulin production in both normal and IUGR islets by HGF (16, 19).
Our results pose questions for future studies. Here, we tested GSIS while the amino acids were being infused. It remains to be determined if increased GSIS will persist after the amino acid infusion is discontinued. Future studies also will be required to determine which amino acid, or combination of amino acids, was most critical for our outcomes. Given the major defects in IUGR islet morphology (16, 27), we chose to focus our studies here on the islet structure of the IUGR+AA group. However, this precluded isolation of pancreatic islets and pancreatic islet endothelial cells from these same fetuses. Thus, a new cohort of animals will be required to measure islet- and islet endothelial cell- specific proteins and mRNA as opposed to pancreatic proteins and mRNAs measured in the current study. We also will need to replicate our findings from isolated fetal sheep large vessel endothelial cells in fetal islet-derived endothelial cells as there may be differences in the response of different types of endothelial cells, again requiring a new cohort of animals.
Overall, our studies identified a novel role for AA to improve GSIS in the placental insufficiency induced IUGR fetus. An 11-day chronic infusion of a complete amino acid mixture restored fetal GSIS and islet size in IUGR fetuses. However, acute in vivo AASIS and in vitro responsiveness to supplemental amino acids were attenuated in the IUGR fetal sheep. These results demonstrate that chronically decreased fetal amino acid supply contributes directly to the pathogenesis of islet dysfunction in fetuses that are growth restricted from placental insufficiency. The results also point to the need for testing the exciting potential role of glucagon for promoting islet size and β-cell function, as well as the roles that VEGFA and HGF may play in this process. Late gestation IUGR fetuses respond to chronically increased amino acid supply with increased fetal insulin secretion. Furthermore, chronically increased amino acid supply to late gestation IUGR fetuses not only increases insulin secretion, but also increases islet size. This has important implications when considering the timing of interventions that might be used to prevent or even reverse the complications of IUGR.
Acknowledgments
This work was supported by a Pilot and Feasibility Award from the Center for Women's Health Research, University of Colorado as well as by National Institutes of Health (NIH) Grants R01 DK088139 and K08 HD060688 (to P.J.R.). L.D.B. was supported by NIH Grants K12 HD057022 Building Interdisciplinary Careers in Women's Health Scholar Award and R01 HD079404–01A1. S.R.W. was supported by NIH Grants K01 DK090199 and R03 DK102972. W.W.H. was supported by NIH Grants T32007186–32 (PI and PD), K12HD068372 (PD); NIH-National Center for Advancing Translational Sciences Grants UL1TR001082, TL1TR001081, and KL2TR001080 (Co-Director); and a Grand Challenges Exploration Grant from the Bill and Melinda Gates Foundation (OPP1061082). S.W.L. was supported by the NIH Grant R01 DK084842.
Disclosure Summary: The authors have nothing to disclose.
Appendix
See Table 4.
Table 4.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | |
|---|---|---|---|---|---|---|
| Glucagon | Glucagon | Sigma-Aldrich | G2654 | Mouse; monoclonal | 1:500 | |
| Insulin | Insulin | Dako | A0564 | Guinea pig; polyclonal | 1:250 | |
| Pancreatic polypeptide | Pancreatic polypeptide | Millipore | AB939 | Rabbit; polyclonal | 1:500 | |
| Somatostatin | Somatostatin | Dako | A0566 | Rabbit; polyclonal | 1:500 | |
| Phosphorylated histone 3 | pHH3 | Upstate (now Millipore) | 06-775 | Rabbit; polyclonal | 7.5 μg/mL | |
| Mouse IgG | Mouse IgG | Jackson ImmunoResearch | 715-545-150 | Donkey; polyclonal | 1:500 | |
| Rabbit IgG | Rabbit IgG | Jackson ImmunoResearch | 711-585-152 | Donkey; polyclonal | 1:500 | |
| Guinea pig IgG | Guinea pig IgG | Jackson ImmunoResearch | 706-155-148 | Donkey; polyclonal | 1:500 | |
| Glucokinase | Glucokinase | Abcam | ab88056 | Rabbit; polyclonal | 1:1000 | |
| GLUT2 | GLUT2 | Santa Cruz Biotechnology | sc-7580 | Goat; polyclonal | 1:1000 | |
| VEGFA | VEGFA | Santa Cruz Biotechnology | sc-507 | Rabbit; polyclonal | 1 μg/mL | |
| Actin | Actin | MP Biomedicals | 869 133 | Mouse; monoclonal | 1:20 000 | |
Footnotes
- AA
- amino acid
- AASIS
- amino acid-stimulated insulin secretion
- BCAA
- branched chain amino acid
- CON
- control
- GLUT2
- glucose transporter 2
- GPASIS
- glucose-potentiated arginine-stimulated insulin secretion
- GSIS
- glucose-stimulated insulin secretion
- HGF
- hepatocyte growth factor
- IUGR
- intrauterine growth restriction
- pHH3
- phosphorylated histone 3
- VEGF
- vascular endothelial growth factor.
References
- 1. Van Assche FA, De Prins F, Aerts L, Verjans M. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol. 1977;84:751–753. [DOI] [PubMed] [Google Scholar]
- 2. Nicolini U, Hubinont C, Santolaya J, Fisk NM, Rodeck CH. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm Metab Res. 1990;22:426–430. [DOI] [PubMed] [Google Scholar]
- 3. Thorn SR, Rozance PJ, Brown LD, Hay WW., Jr The intrauterine growth restriction phenotype: fetal adaptations and potential implications for later life insulin resistance and diabetes. Semin Reprod Med. 2011;29:225–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Economides DL, Nicolaides KH, Linton EA, Perry LA, Chard T. Plasma cortisol and adrenocorticotropin in appropriate and small for gestational age fetuses. Fetal Ther. 1988;3:158–164. [DOI] [PubMed] [Google Scholar]
- 5. Greenough A, Nicolaides KH, Lagercrantz H. Human fetal sympathoadrenal responsiveness. Early Hum Dev. 1990;23:9–13. [DOI] [PubMed] [Google Scholar]
- 6. Hubinont C, Nicolini U, Fisk NM, Tannirandorn Y, Rodeck CH. Endocrine pancreatic function in growth-retarded fetuses. Obstet Gynecol. 1991;77:541–544. [PubMed] [Google Scholar]
- 7. Lassarre C, Hardouin S, Daffos F, Forestier F, Frankenne F, Binoux M. Serum insulin-like growth factors and insulin-like growth factor binding proteins in the human fetus. Relationships with growth in normal subjects and in subjects with intrauterine growth retardation. Pediatr Res. 1991;29:219–225. [DOI] [PubMed] [Google Scholar]
- 8. Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr Res. 1998;44:532–537. [DOI] [PubMed] [Google Scholar]
- 9. Marconi AM, Paolini CL, Stramare L, et al. Steady state maternal-fetal leucine enrichments in normal and intrauterine growth-restricted pregnancies. Pediatr Res. 1999;46:114–119. [DOI] [PubMed] [Google Scholar]
- 10. Paolini CL, Marconi AM, Ronzoni S, et al. Placental transport of leucine, phenylalanine, glycine, and proline in intrauterine growth-restricted pregnancies. J Clin Endocrinol Metab. 2001;86:5427–5432. [DOI] [PubMed] [Google Scholar]
- 11. Fowden AL. The role of insulin in fetal growth. Early Hum Dev. 1992;29:177–181. [DOI] [PubMed] [Google Scholar]
- 12. Hales CN, Barker DJ. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. [DOI] [PubMed] [Google Scholar]
- 13. Green AS, Rozance PJ, Limesand SW. Consequences of a compromised intrauterine environment on islet function. J Endocrinol. 2010;205:211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boujendar S, Arany E, Hill D, Remacle C, Reusens B. Taurine supplementation of a low protein diet fed to rat dams normalizes the vascularization of the fetal endocrine pancreas. J Nutr. 2003;133:2820–2825. [DOI] [PubMed] [Google Scholar]
- 15. Ham JN, Crutchlow MF, Desai BM, Simmons RA, Stoffers DA. Exendin-4 normalizes islet vascularity in intrauterine growth restricted rats: potential role of VEGF. Pediatr Res. 2009;66:42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rozance PJ, Anderson M, Martinez M, et al. Placental insufficiency decreases pancreatic vascularity and disrupts hepatocyte growth factor signaling in the pancreatic islet endothelial cell in fetal sheep. Diabetes. 2015;64:555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barry JS, Rozance PJ, Anthony RV. An animal model of placental insufficiency-induced intrauterine growth restriction. Semin Perinatol. 2008;32:225–230. [DOI] [PubMed] [Google Scholar]
- 18. Brown LD, Rozance PJ, Thorn SR, Friedman JE, Hay WW., Jr Acute supplementation of amino acids increases net protein accretion in IUGR fetal sheep. Am J Physiol Endocrinol Metab. 2012;303:E352–E364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rozance PJ, Hay WW., Jr Pancreatic islet hepatocyte growth factor and vascular endothelial growth factor A signaling in growth restricted fetuses [published online ahead of print November 5, 2016]. Mol Cell Endocrinol. 10.1016/j.mce.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW., Jr Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology. 2006;147:1488–1497. [DOI] [PubMed] [Google Scholar]
- 21. Gadhia MM, Maliszewski AM, O'Meara MC, et al. Increased amino acid supply potentiates glucose-stimulated insulin secretion but does not increase β-cell mass in fetal sheep. Am J Physiol Endocrinol Metab. 2013;304:E352–E362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brown LD, Rozance PJ, Barry JS, Friedman JE, Hay WW., Jr Insulin is required for amino acid stimulation of dual pathways for translational control in skeletal muscle in the late-gestation ovine fetus. Am J Physiol Endocrinol Metab. 2009;296:E56–E63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maliszewski AM, Gadhia MM, O'Meara MC, Thorn SR, Rozance PJ, Brown LD. Prolonged infusion of amino acids increases leucine oxidation in fetal sheep. Am J Physiol Endocrinol Metab. 2012;302:E1483–E1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Green AS, Macko AR, Rozance PJ, et al. Characterization of glucose-insulin responsiveness and impact of fetal number and sex difference on insulin response in the sheep fetus. Am J Physiol Endocrinol Metab. 2011;300:E817–E823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Andrews SE, Brown LD, Thorn SR, et al. Increased adrenergic signaling is responsible for decreased glucose-stimulated insulin secretion in the chronically hyperinsulinemic ovine fetus. Endocrinology. 2015;1:367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frost MS, Zehri AH, Limesand SW, Hay WW, Jr, Rozance PJ. Differential effects of chronic pulsatile versus chronic constant maternal hyperglycemia on fetal pancreatic β-cells. J Pregnancy. 2012;2012:812094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Limesand SW, Jensen J, Hutton JC, Hay WW., Jr Diminished β-cell replication contributes to reduced β-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1297–R1305. [DOI] [PubMed] [Google Scholar]
- 28. Rozance PJ, Seedorf GJ, Brown A, et al. Intrauterine growth restriction decreases pulmonary alveolar and vessel growth and causes pulmonary artery endothelial cell dysfunction in vitro in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2011;301:L860–L871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rozance PJ, Limesand SW, Zerbe GO, Hay WW., Jr Chronic fetal hypoglycemia inhibits the later steps of stimulus-secretion coupling in pancreatic β-cells. Am J Physiol Endocrinol Metab. 2007;292:E1256–E1264. [DOI] [PubMed] [Google Scholar]
- 30. Chen X, Rozance PJ, Hay WW, Jr, Limesand SW. Insulin-like growth factor and fibroblast growth factor expression profiles in growth-restricted fetal sheep pancreas. Exp Biol Med (Maywood). 2012;237:524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Thorn SR, Regnault TR, Brown LD, et al. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology. 2009;150:3021–3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. Biotechniques. 2005;39:75–85. [DOI] [PubMed] [Google Scholar]
- 33. Rozance PJ, Limesand SW, Barry JS, Brown LD, Hay WW., Jr Glucose replacement to euglycemia causes hypoxia, acidosis, and decreased insulin secretion in fetal sheep with intrauterine growth restriction. Pediatr Res. 2009;65:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Boujendar S, Reusens B, Merezak S, et al. Taurine supplementation to a low protein diet during foetal and early postnatal life restores a normal proliferation and apoptosis of rat pancreatic islets. Diabetologia. 2002;45:856–866. [DOI] [PubMed] [Google Scholar]
- 35. Dahri S, Snoeck A, Reusens-Billen B, Remacle C, Hoet JJ. Islet function in offspring of mothers on low-protein diet during gestation. Diabetes. 1991;40(suppl 2):115–120. [DOI] [PubMed] [Google Scholar]
- 36. Snoeck A, Remacle C, Reusens B, Hoet JJ. Effect of a low protein diet during pregnancy on the fetal rat endocrine pancreas. Biol Neonate. 1990;57:107–118. [DOI] [PubMed] [Google Scholar]
- 37. Macko AR, Yates DT, Chen X, et al. Adrenal demedullation and oxygen supplementation independently increase glucose-stimulated insulin concentrations in fetal sheep with intrauterine growth restriction. Endocrinology. 2016;157:2104–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leos RA, Anderson MJ, Chen X, Pugmire J, Anderson KA, Limesand SW. Chronic exposure to elevated norepinephrine suppresses insulin secretion in fetal sheep with placental insufficiency and intrauterine growth restriction. Am J Physiol Endocrinol Metab. 2010;298:E770–E778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuhara T, Ikeda S, Ohneda A, Sasaki Y. Effects of intravenous infusion of 17 amino acids on the secretion of GH, glucagon, and insulin in sheep. Am J Physiol. 1991;260:E21–E26. [DOI] [PubMed] [Google Scholar]
- 40. Pipeleers DG, Schuit FC, in't Veld PA, et al. Interplay of nutrients and hormones in the regulation of insulin release. Endocrinology. 1985;117:824–833. [DOI] [PubMed] [Google Scholar]
- 41. Quesada I, Tudurí E, Ripoll C, Nadal A. Physiology of the pancreatic α-cell and glucagon secretion: role in glucose homeostasis and diabetes. J Endocrinol. 2008;199:5–19. [DOI] [PubMed] [Google Scholar]
- 42. Huypens P, Ling Z, Pipeleers D, Schuit F. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia. 2000;43:1012–1019. [DOI] [PubMed] [Google Scholar]
- 43. Moens K, Flamez D, Van Schravendijk C, Ling Z, Pipeleers D, Schuit F. Dual glucagon recognition by pancreatic β-cells via glucagon and glucagon-like peptide 1 receptors. Diabetes. 1998;47:66–72. [DOI] [PubMed] [Google Scholar]
- 44. Samols E, Marri G, Marks V. Promotion of insulin secretion by glucagon. Lancet. 1965;2:415–416. [DOI] [PubMed] [Google Scholar]
- 45. Schuit FC, Pipeleers DG. Regulation of adenosine 3′,5′-monophosphate levels in the pancreatic B cell. Endocrinology. 1985;117:834–840. [DOI] [PubMed] [Google Scholar]
- 46. Wang JL, Corbett JA, Marshall CA, McDaniel ML. Glucose-induced insulin secretion from purified β-cells. A role for modulation of Ca2+ influx by cAMP- and protein kinase C-dependent signal transduction pathways. J Biol Chem. 1993;268:7785–7791. [PubMed] [Google Scholar]
- 47. Dai C, Li Y, Yang J, Liu Y. Hepatocyte growth factor preserves β cell mass and mitigates hyperglycemia in streptozotocin-induced diabetic mice. J Biol Chem. 2003;278:27080–27087. [DOI] [PubMed] [Google Scholar]
- 48. Garcia-Ocaña A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases β cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem. 2000;275:1226–1232. [DOI] [PubMed] [Google Scholar]
- 49. Johansson M, Mattsson G, Andersson A, Jansson L, Carlsson PO. Islet endothelial cells and pancreatic β-cell proliferation: studies in vitro and during pregnancy in adult rats. Endocrinology. 2006;147:2315–2324. [DOI] [PubMed] [Google Scholar]
- 50. Tomiya T, Nishikawa T, Inoue Y, et al. Leucine stimulates HGF production by hepatic stellate cells through mTOR pathway. Biochem Biophys Res Commun. 2007;358:176–180. [DOI] [PubMed] [Google Scholar]