Abstract
Charcoal-stripped bovine serum (CSS) is a critical reagent in the study of steroid hormones. However, CSS has high lot-to-lot variability, including residual growth factor and steroid hormone content. Assessing and reporting this variability is challenging but may affect experimental outcomes and data reproducibility. We hypothesized that CSS lot variability would affect endocrine response phenotypes in breast cancer cells, and we tested the effects of five individual CSS lots on endocrine response in MCF-7 and MDA MB 134VI (MM134) cells. Based on the effects of antiestrogens on MCF-7 cell proliferation, we defined CSS lots as having complete vs partial hormone deprivation. In partial deprivation CSS, the absolute effects of residual estrogens on cell proliferation were modest, but these effects masked the partial agonist activity of 4-hydroxytamoxifen in MM134 cells. Importantly, this effectively reversed the interpretation of tamoxifen-resistance in MM134 cells. Variable effects of CSS lots on endocrine resistance phenotypes were also observed in MCF-7 cells. In this context, we observed that partial vs complete deprivation CSS allowed for the development of unique early endocrine resistance phenotypes that correlated with the presence or absence of residual estrogenic hormones. We evaluated the methods of CSS preparation and identified factors contributing to the extent of hormone deprivation. Our observations suggest that CSS lot-to-lot variability has substantial effects on endocrine response phenotypes and that this ubiquitous factor in study methodology may confound reproducibility. Renewed vigilance in testing and reporting CSS phenotypes will greatly aid in interpreting and reproducing endocrine response and resistance data by the community.
Modeling the effects of steroid hormones in cell culture is subject to the effects of residual exogenous estrogens and estrogen-like compounds. The archetype of these effects is phenol red, which confounded the study of estrogens in breast cancer models until components of phenol red were found to act as estrogen receptor (ER) agonists (1). ER agonists are also pervasive in the cell culture plastics, from liquid handling plastics (2, 3) to dishes and plates used to culture cells (4), with substantial intermanufacturer variability. Another underlying source of exogenous estrogens is the hormone-depleted bovine serum used to supplement medium, ie, dextran-treated charcoal stripped serum (CSS). Charcoal treatment of serum depletes a wide range of peptides and small molecules, including lipophilic molecules like steroid hormones, eg, androgens and estrogens. After short-term maintenance of cells in medium containing CSS (ie, hormone deprivation), exogenous hormones can be applied, and phenotypes can be assessed after treatment. However, CSS is an agricultural product with the potential for high lot-to-lot variability, stemming from the source material and from charcoal-stripping methods. Fetal bovine serum (FBS) or calf serum typically serve as the source material, but these have broadly variable hormone and growth factor content (5) and may require different extents of charcoal stripping to adequately deplete steroid hormones. Variability in the source material is compounded by differences in charcoal preparation (eg, grade, washing), serum processing (eg, sulfatase treatment), and specific stripping protocols (eg, temperature, time) that vary across institutions and commercial sources, are not standardized, and are frequently proprietary. These factors lead to extensive variability in steroid hormone and growth factor content of CSS, and, coupled with challenges in reporting these factors in study methodology, this has the potential to effect experimental outcomes and reproducibility. We recently experienced problems with repeating well-established experiments while testing a new serum purchase and further explored the potential for lot variability to affect endocrine response phenotypes.
Materials and Methods
Cell culture
MCF-7 cells were obtained from the Georgetown University-Lombardi Comprehensive Cancer Center Shared Tissue Resource and were routinely maintained in DMEM (Gibco; 11965) + 10% FBS (Gibco; 26140). MDA MB 134VI (MM134; American Type Culture Collection) was maintained in equal parts DMEM/L-15 (Gibco; 11415) + 10% FBS. Cell lines are authenticated annually by PCR restriction fragment length polymorphism analyses and confirmed to be mycoplasma negative. Cells were hormone deprived in Improved minimum essential medium (Gibco; A10488) + 10% CSS (FBS derived CSS, referred to as CSS below) by washing twice daily with serum-free Improved minimum essential medium for 3 days prior to plating (6). Five individual CSS lots from two commercial sources were assessed (Gibco, 12676; Hyclone, SH30068); lot information is available on request but has been omitted to remove potential bias regarding the supplier.
17β-Estradiol (E2) and 4-hydroxytamoxifen (4OHT) were obtained from Sigma; ICI 182780 (ICI; fulvestrant) was obtained from Tocris Biosciences. All compounds were dissolved in ethanol, and vehicle treatments are using 0.1% EtOH.
Cell proliferation assays
Cellular proliferation assays used the FluoReporter dsDNA quantitation kit (Thermo Fisher) as described (6). Points/bars represent the mean of five to six biological replicates ± SD.
Early endocrine resistance phenotypes assay
MCF-7 cells were hormone depleted as above in each of four CSS lots and plated at 75 k/well in a 12-well plate in triplicate; medium was changed every 3–4 days. After 2 weeks, cells were imaged using an inverted microscope. Twenty-one days after beginning hormone deprivation, cell phenotypes were assessed. Cellular metabolism was assessed using Alamar Blue (Thermo Scientific) per the manufacturer's instructions. From the same samples, DNA/RNA were harvested using the Allprep Mini kit (QIAGEN) according to the manufacturer's instructions; crude protein was collected by acetone precipitation of DNA/RNA harvest supernatants. Cell number was determined by DNA quantification (FluoReporter), relative to a standard curve of parental MCF-7 cells. Relative transcription and metabolism were determined by normalizing the relative cell number determined by total RNA yield or Alamar Blue signal (each vs parental MCF-7 standard curve) vs total cell number (from DNA quantification).
Preparation of charcoal-stripped FBS
Using a single lot of FBS, serum stripping methods based on a series of published protocols (7–11) were evaluated. Complete methodology is described in Supplemental Methods.
Statistical analyses
Curve-fitting and statistical analyses were performed using GraphPad Prism, version 5.04 (GraphPad Inc). Clustering (Manhattan complete) was performed with MeV software (TM4 Software Suite).
Methods supplement
Methods for immunoblotting and mRNA expression analyses are described in Supplemental Methods.
Results
Residual estrogenic compounds in CSS alter endocrine response phenotypes
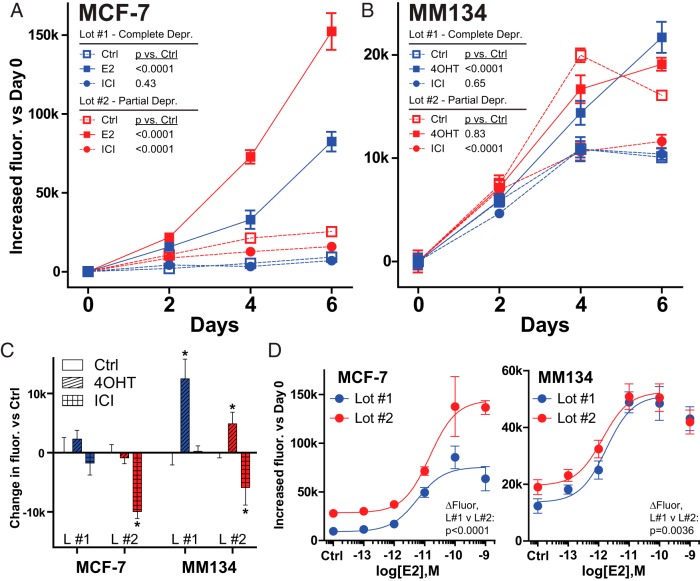
To manage variability in CSS, we routinely test new serum lots for residual estrogenic potential using MCF-7 breast cancer cells. MCF-7 cells are hormone depleted in test CSS lots, and proliferation is assessed after treatment with E2 or the selective ER degrader ICI vs vehicle. Although some variability in proliferation can be attributed to differences in other growth factors, a decrease in growth in ICI vs vehicle treatment is interpreted as being due to residual estrogenic compounds. Shown in Figure 1A are results from two representative CSS lots. With lot 1 (blue), E2 robustly induced proliferation, but ICI treatment caused no decrease in growth vs vehicle; we thus define CSS lot #1 as having complete steroid hormone deprivation. Conversely, with lot 2 (red), ICI decreased growth vs the vehicle (37% decrease at d 6), and we define CSS lot 2 as having partial hormone deprivation. These data are representative of five lots from two suppliers; based on the above definitions, two lots had complete deprivation, two had partial deprivation, and one had a mixed phenotype (discussed below).
Figure 1.
Endocrine response phenotypes are altered between CSS lots. MCF-7 (A) or MM134 (B) cells were hormone deprived as described, prior to plating to 96-well plates. After attaching overnight, cells were treated with approximately EC95–100 of ER ligands (see panel D and reference 6): 100 pM E2, 10 nM ICI, or 10 nM 4OHT (these concentrations avoid oversaturation of ER vs potential serum estrogens). Proliferation was assessed at the indicated time, as described in Materials and Methods. Points represent average of six biological replicates ± SD; for points without visible error bars, SD < 2000 U. Treatments were compared by a two-way ANOVA; P values represent treatment effect. C, Hormone-deprived MCF-7 or MM134 cells in CSS lot 1 or 2 were treated with 10 nM 4OHT or ICI; growth was assessed at day 5 for MCF-7 and day 6 for MM134. *, One-way ANOVA vs respective control with multiple comparison correction (P < .05). D, Cells as in panel C were treated with increasing concentrations of E2. P values represent a comparison of the upper bound of the E2 dose-response curves for control-subtracted data (extra sum of squares F test). Ctrl, control.
Although the absolute magnitude of the effect of partial deprivation CSS on proliferation is modest in this setting, another model revealed more serious implications for the effects of CSS variability. We next assessed the proliferation of MM134 breast cancer cells because we previously reported that these cells recognize 4OHT as a partial ER agonist (6) and included this phenotype in our testing. MM134 cells were hormone deprived as above and treated with 4OHT or ICI vs vehicle (Figure 1B). Using lot 1 (blue), similar to MCF-7 cells, ICI did not reduce growth vs vehicle, confirming complete deprivation; 4OHT induced growth as expected. Using lot 2 (red), ICI decreased MM134 growth vs vehicle, consistent with partial hormone deprivation. However, 4OHT did not induce growth vs vehicle and appears to act instead as a pure antagonist. This reversal of the 4OHT response was observed in both of the CSS lots that were defined as having partial deprivation (data not shown for second partial deprivation lot). 4OHT-induced growth was not observed in MCF-7 in complete or partial deprivation (Figure 1C). Deprivation status did not shift E2 concentration-responses (Figure 1D). However, MCF-7 cells had greater growth induction in partial deprivation (1.76-fold vs complete); conversely, MM134 had decreased growth induction in partial deprivation (0.82-fold vs complete) because maximal growth was constant. These observations demonstrate that data interpretation and experimental reproducibility may differ based on the CSS lots used in individual experiments.
CSS variability drives unique modes of developing endocrine resistance
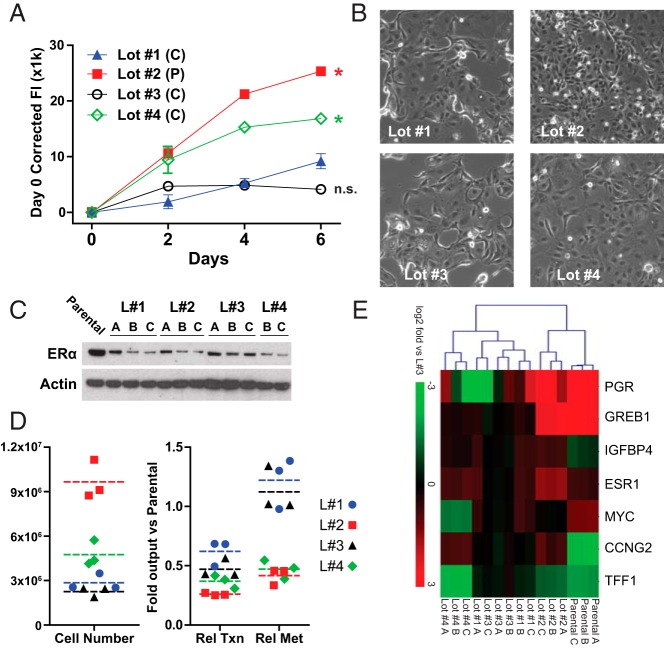
Maintenance of breast cancer cells in CSS-supplemented medium is also used to mimic estrogen-depleted conditions in patients receiving aromatase inhibitor therapy, so we next assessed MCF-7 proliferation during hormone depletion in test CSS lots. Shown in Figure 2A, short-term proliferation reflected the relative estrogenic content as defined in Figure 1; lots 1 and 3 (complete deprivation) showed minimal growth in CSS, whereas lot 2 (partial deprivation) maintained proliferation, consistent with exogenous estrogenic stimuli. Lot 4 had an intermediate growth phenotype, but because this lot was defined as complete deprivation (data not shown), nonestrogenic growth factors may be driving proliferation in this condition. This broad range of proliferation across lots prompted us to test for differential effects of individual CSS lots on the development of endocrine-resistance.
Figure 2.
Hormone depletion in different CSS lots yields unique proliferative and signaling phenotypes. A, MCF-7 proliferation was assessed as in Figure 1. *, Two-way ANOVA vs lot 1 (P < .05 for cell line effect.) B–E, Methodology for assessing short-term endocrine resistance phenotypes are described in Materials and Methods. B, Representative phase-contrast images at magnification ×100 after 2 weeks of hormone deprivation. C, Immunoblotting from hormone-deprived MCF-7 cells was performed using crude protein lysates after DNA/RNA extraction as described in Materials and Methods. Panels A–C represent biological triplicate samples; protein could not be extracted from lot 4-A. D, Left panel, Cell number was determined by DNA quantification relative to a standard curve of parental MCF-7 cells. D, Right panel, Relative transcription (total RNA) and metabolism (Alamar Blue) vs parental MCF-7 cells were determined as described in Materials and Methods. E, Quantitative PCR for target genes was normalized to ribosomal protein lateral stalk subunit P0. Data are normalized to lot 3 because this lot was used by us previously (6). A–C represent biological triplicate samples.
MCF-7 cells were hormone depleted in each of four CSS lots and maintained for 3 weeks (biological triplicates A, B, and C; see Materials and Methods). During hormone depletion, cell morphology was consistent with growth observed in Figure 2A; cells in CSS lots 1 and 3 appear to have ceased proliferation, whereas lots 4 and 2 appear increasingly proliferative. After 3 weeks, we assessed proliferation and viability phenotypes, using parental MCF-7 in hormone replete conditions (medium + 10% FBS) as a calibrator. All samples maintained ER expression, although at lower levels than parental cells (Figure 2C). Total cell number reflected the cellular morphology and initial short-term growth observed (Figure 2D, left panel). Surprisingly, increased total RNA content (transcriptional activity) and Alamar Blue conversion (mitochondrial activity/metabolism) suggested that cells in lots 1 and 3 were more metabolically active (Figure 2D, right panel) despite lower proliferation. We also assessed the expression of a series of canonical ER target genes, and examined whether their expression was consistent with hormone replete MCF-7 cells (Figure 2E). Cells maintained in CSS lot 2 most closely matched parental MCF-7 because they maintained high progesterone receptor and growth regulation by estrogen in breast cancer 1 expression, consistent with active ER. Expression data from other cells were consistent with a lack of canonical ER-driven gene expression. These data show that individual CSS lots may differentially activate survival, growth, or metabolic pathways to maintain viability during hormone depletion. Individual CSS lots likely promote the acquisition of unique mechanisms of endocrine resistance.
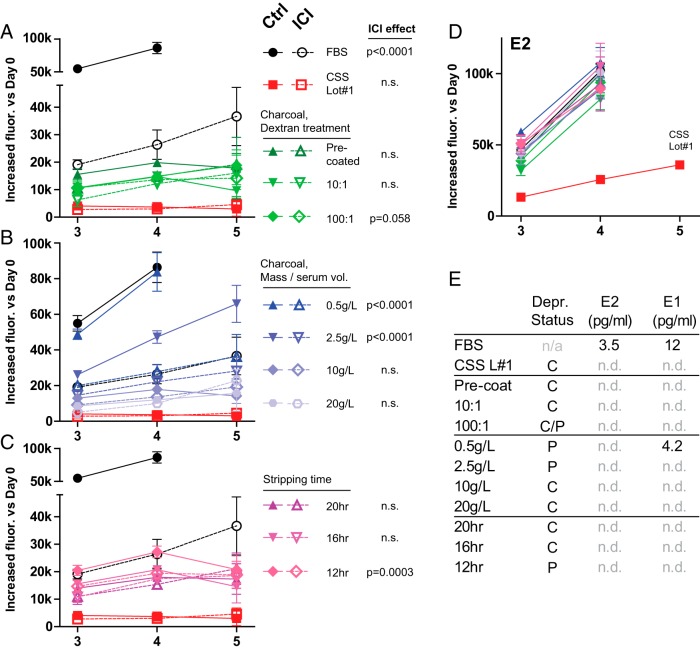
Methods of charcoal stripping modify serum hormone deprivation
The effects of differing serum-stripping protocols on hormone deprivation are not well characterized. We tested CSS generated from a single FBS lot after varying charcoal type/preparation, charcoal mass per serum volume, and stripping time. MCF-7 cells in each test CSS were treated with E2 or ICI to assess relative growth induction or decrease vs control, respectively. Shown in Figure 3, each stripping condition variable contributed to hormone deprivation. Charcoal preparation had a modest effect (Figure 3A), whereas mass/volume and stripping time had stronger effects; less than 10 g/L charcoal or less than 16 hours stripping time were insufficient for complete deprivation (Figure 3, B and C). Strikingly, no condition produced the severity of deprivation observed in commercial lot 1 because E2-induced growth was comparable in all test conditions and greatly surpassed that in commercial CSS (Figure 3D). In parallel, we measured estrogen content by mass spectrometry (Figure 3E). Although several test lots were defined as having partial deprivation, estradiol was undetectable, and estrone was detected in only one sample. These observations demonstrate that stripping conditions have widely variable effects on relative hormone deprivation of serum and highlight the importance of phenotypic endocrine response assays in characterizing CSS.
Figure 3.
Serum-stripping conditions modify the extent of hormone deprivation. CSS preparation is described in Supplemental Methods. A–D, MCF-7 cells were hormone deprived as described above in each test serum and treated with 100 pM E2 or 100 nM ICI vs vehicle control; proliferation was assessed at the indicated time point. Cells in hormone-replete conditions reached total confluence by day 5; these points are omitted. CSS preparation was varied by charcoal preparation (A), charcoal mass per serum volume (B), and stripping time (C). P value represents ICI effect by a two-way ANOVA for vs control for each serum type. n.s., not significant. P > .1. D, See sample keys for panels A–C. E, Estradiol and estrone were assessed by liquid chromatography/tandem mass spectrometry. n.d., not detected (<1 pg/mL). Complete vs partial deprivation (C v P) was defined by a significant ICI effect in panels A-C.
Discussion
CSS is a ubiquitous reagent in the study of steroid hormones, but lot-to-lot variability in CSS can have substantial effects on endocrine response and resistance phenotypes; these effects have important implications for model development and data interpretation. Based on our data, these concerns may be managed by characterization of CSS lots used in individual studies or experiments. Importantly, neither the complete nor partial deprivation CSS represents a better option for all research. The improved dynamic range in cell growth in partial deprivation CSS may be a boon for experiments on ER function (eg, signaling, transcription, chromatin immunoprecipitation); in Figure 1A, using lot 2, E2 had a greater induction of growth than lot 1, perhaps owing to the lack of total hormone starvation initially and presence of residual estrogens (12, 13). Conversely, complete deprivation CSS is required to assess the effects of weak or partial ER agonists (14, 15) or potentially nonestrogenic hormones and growth factors. Notably, the low level proliferation caused by residual estrogens is consistent with the equivalent of subpicomolar/high-femtomolar concentrations of E2 (ref. 6 and Figure 1D), at or below the limits of detection of current mass spectrometry-based assays (16). Phenotypic assays using cell line models as described above will likely be necessary to assess the presence and contributions of residual estrogenic compounds from individual CSS lots.
Although the residual estrogens from partial deprivation CSS lots tested caused relatively modest absolute changes in cell proliferation, this was sufficient to mask the partial agonist effect of 4OHT in MM134 cells. In the presence of a low concentration of residual estrogenic compounds, 4OHT likely competes for ER binding but because it acts as a weak agonist, the net effect on growth is zero. This has a drastic effect on the interpretation of these data. In Figure 1B, with lot 1, MM134 are tamoxifen resistant (because growth is induced by the antiestrogen), but using lot 2, MM134 would not be considered tamoxifen resistant (no apparent effect on growth vs control). These observations demonstrate that in some scenarios, variability in CSS lots may not only alter endocrine response phenotypes but that this variability in fact has the potential to undermine study reproducibility.
The presence of residual estrogenic compounds in partial deprivation CSS may also alter the development of endocrine resistance. We previously reported that endocrine resistance phenotypes were modified by low concentrations of estrogenic steroids (17) and hypothesized that partial vs complete deprivation CSS represented an analogous scenario. Although the 3-week window used herein falls short of the 3–12 months typically used for acquired resistance models, our data suggest three unique programs, driven by CSS lot variability, during long-term hormone depletion 1) for lots 1 and 3, complete deprivation causes initial growth arrest, but cells increase metabolic activity independent of canonical ER activity; 2) for lot 2, low concentrations of estrogens maintain ER activity and low growth/metabolism; and 3) for lot 4, nonestrogenic factors maintain cells at low growth/metabolism, independent of ER. It is likely that each program will yield unique endocrine resistance phenotypes. Importantly, as above, either partial or complete deprivation CSS may be considered appropriate for the development of endocrine resistance models; the former may reflect residual aromatization, incomplete aromatase inhibition, or the contribution of alternative estrogens, and the latter may enrich for ER-independent modes of resistance, as suggested by our observations.
A potential solution to commercial CSS variability is to optimize laboratory FBS stripping. Selection of stripping parameters, as in Figure 3, would allow control over the extent of hormone deprivation. However, the conditions necessary to adequately deprive individual FBS lots will likely vary, as will the resulting endocrine response phenotypes. Similar endocrine response phenotyping of FBS lots prior to stripping may be needed to provide a baseline from which to apply selected stripping protocols.
Based on our observations, increased vigilance in manuscripts is necessary to properly provide context for endocrine response and resistance data. This includes reporting CSS source, supplier, and lot information with catalog numbers for medium components, thorough CSS testing and reporting of CSS phenotypes, and the use of single lots when possible for individual studies. Reporting these details routinely will greatly aid in interpreting and reproducing endocrine response and resistance data by the community.
Acknowledgments
This work was supported by the Breast Cancer Research Foundation (to S.O.), Nicole Meloche Memorial Breast Cancer Fund, Shear Family Fund, and Pathway to Independence Award K99 CA193734 (to M.J.S.) from the National Institutes of Health. This project used the University of Pittsburgh Cancer Institute Tissue and Research Pathology Services Facility that is supported in part by award P30CA047904.
Disclosure Summary: The authors have nothing to disclose.
Appendix
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|---|
| ER-α | Full length recombinant | ER 6F11 | Leica, ORG-8871 | Mouse monoclonal | 1:1000 |
| β-Actin | Slightly modified β-cytoplasmic actin N-terminal peptide, Ac-Asp-Asp-Asp-Ile-Ala-Ala-Leu-Val-Ile-Asp-Asn-Gly-Ser-Gly-Lys, conjugated to KLH | Clone AC-15, ascites fluid | Sigma, A5441 | Mouse monoclonal | 1:10 000 |
| Mouse IgG | n/a | Mouse IgG, HRP-linked whole Ab | GE/Amersham, NA931 | Sheep | 1:10 000 |
Abbreviation: Ab, antibody; HRP, horseradish peroxidase.
Footnotes
- CSS
- charcoal-stripped bovine serum
- E2
- estradiol
- ER
- estrogen receptor
- FBS
- fetal bovine serum
- ICI
- ICI 182,780 or fulvestrant
- MM134
- MDA MB 134VI
- 4OHT
- 4-hydroxytamoxifen;
References
- 1. Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci USA 1986;83(8):2496–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McDonald GR, Hudson AL, Dunn SMJ, et al. Bioactive contaminants leach from disposable laboratory plasticware. Science. 2008;322(5903):917. [DOI] [PubMed] [Google Scholar]
- 3. Olivieri A, Degenhardt OS, McDonald GR, et al. On the disruption of biochemical and biological assays by chemicals leaching from disposable laboratory plasticware. Can J Physiol Pharmacol. 2012;90(6):697–703. [DOI] [PubMed] [Google Scholar]
- 4. Ishikawa T, Takano K, Yasufuku-Takano J, et al. Estrogenic impurities in labware. Nat Biotechnol. 2001;19(9):812. [DOI] [PubMed] [Google Scholar]
- 5. Esber HJ, Payne IJ, Bogden A. Variability of hormone concentrations and ratios in commercial sera used for tissue culture. J Natl Cancer Inst. 1973;50(2):559–562. [DOI] [PubMed] [Google Scholar]
- 6. Sikora MJ, Cooper KL, Bahreini A, et al. Invasive lobular carcinoma cell lines are characterized by unique estrogen-mediated gene expression patterns and altered tamoxifen response. Cancer Res. 2014;74(5):1463–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lippman M, Bolan G, Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976;36(12):4595–4601. [PubMed] [Google Scholar]
- 8. Leake RE, Green B, eds. Steroid Hormones: A Practical Approach. Oxford, UK: IRL Press; 1987. [Google Scholar]
- 9. Eckert RL, Katzenellenbogen BS. Effects of estrogens and antiestrogens on estrogen receptor dynamics and the induction of progesterone receptor in MCF-7 human breast cancer cells. Cancer Res. 1982;42(1):139–144. [PubMed] [Google Scholar]
- 10. Dembinski TC, Leung CK, Shiu RP. Evidence for a novel pituitary factor that potentiates the mitogenic effect of estrogen in human breast cancer cells. Cancer Res. 1985;45(7):3083–3089. [PubMed] [Google Scholar]
- 11. Herbert V, Lau KS, Gottlieb CW, Bleicher SJ. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965;25(10):1375–1384. [DOI] [PubMed] [Google Scholar]
- 12. Stewart AJ, Johnson MD, May FE, Westley BR. Role of insulin-like growth factors and the type I insulin-like growth factor receptor in the estrogen-stimulated proliferation of human breast cancer cells. J Biol Chem. 1990;265(34):21172–21178. [PubMed] [Google Scholar]
- 13. Lee AV, Cui X, Oesterreich S. Cross-talk among estrogen receptor, epidermal growth factor, and insulin-like growth factor signaling in breast cancer. Clin Cancer Res. 2001;7(suppl 12):4429s–4435s; discussion 4411s–4412s. [PubMed] [Google Scholar]
- 14. Sikora MJ, Cordero KE, Larios JM, Johnson MD, Lippman ME, Rae JM. The androgen metabolite 5α-androstane-3β,17β-diol (3βAdiol) induces breast cancer growth via estrogen receptor: implications for aromatase inhibitor resistance. Breast Cancer Res Treat. 2009;115(2):289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DuSell CD, Umetani M, Shaul PW, Mangelsdorf DJ, McDonnell DP. 27-Hydroxycholesterol is an endogenous selective estrogen receptor modulator. Mol Endocrinol. 2008;22(1):65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Q, Bottalico L, Mesaros C, Blair IA. Analysis of estrogens and androgens in postmenopausal serum and plasma by liquid chromatography-mass spectrometry. Steroids. 2015;99(Pt A):76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sikora MJ, Strumba V, Lippman ME, Johnson MD, Rae JM. Mechanisms of estrogen-independent breast cancer growth driven by low estrogen concentrations are unique versus complete estrogen deprivation. Breast Cancer Res Treat. 2012;134(3):1027–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]