Abstract
The previous investigation has proved that their existed pharmacokinetic difference between the different crystal forms of the polymorphic drugs after oral administration. However, no systemic investigations have been made on the change of this pharmacokinetic difference, resulted either from the physiological or from the pathological factors. In this paper, we used polymorphic nimodipine (Nim) as a model drug and investigated the effect of age difference (2- and 9-month old) on the pharmacokinetics after oral delivery in rats. As the results shown, for L-form of Nim (L-Nim), the AUC0–24 h in 2-month-old rats was 343.68±47.15 ng·h/mL, which is 23.36% higher than that in 9-month-old rats. For H-form of Nim (H-Nim), the AUC0–24 h in 2-month-old rats was 140.91±19.47 ng·h/mL, which is 54.64% higher than that in 9-month-old rats. The AUC0–24 h ratio between H-Nim and L-Nim was 2.44 in 2-month-old rats and 3.06 in 9-month-old rats. Since age difference could result in unparallelled change of the absorption and bioavailability of the polymorphic drugs, the results in this experiment are of value for further investigation of crystal form selection in clinical trials and rational clinical application of the polymorphic drugs.
KEY WORDS: Nimodipine, Age difference, Polymorphic drug, Pharmacokinetics, Crystal form
Graphical abstract
In this paper, polymorphic nimodipine (Nim) was used as a model drug to investigate the effect of age difference (2- and 9-month old) on the pharmacokinetics after oral delivery in rats. Since age difference could result in unparalleled change of the absorption and bioavailability of the polymorphic drugs, the results in this experiment are of value for further investigation of crystal form selection in clinical trials and rational clinical application of the polymorphic drugs.
1. Introduction
According to the previous investigation, the in vivo absorption and bioavailability of polymorphic drugs may be different due to the crystal form modification, which usually result in the change of the physical and chemical properties1, 2, 3. Wang et al.4 reported that after oral administration of A and B polymorphs of m-nisoldipine to rats, the area under the plasma concentration-time curve (AUC) of m-nisoldipine B was 1.17-fold higher than that of A, the maximum plasma concentration (Cmax) and the time to reach a Cmax (Tmax) values showed statistically significant differences between m-nisoldipine A and B. They concluded that the crystal form could influence the rate and extent of absorption and bioavailability of m-nisoldipine. Du et al.5 investigated the in vitro and in vivo correlation of Forms I, II and III of agomelatine, and found that the agomelatine polymorphic forms with faster dissolution rates in vitro would increase the rate and extent of oral absorption in vivo.
So far, numerous works have proved the physiological and pathological effects on the absorption and elimination of non-polymorphic drugs. These influencing factors generally refer to age, diabetes, nephropathy and hepatopathy6, 7, 8, 9. Considering the factor of age, possible reasons for these pharmacokinetic changes are diverse and complex. Natural developmental maturation and aging of gastrointestinal tract, liver and renal could lead to different changes in drug absorption, metabolism and clearance, and transport and elimination10, 11, 12, 13, 14. A recent population pharmacokinetic study showed statistically significant differences in carbamazepine pharmacokinetics between elderly and relatively young epileptic patients15. Tarral and Merdjan16 noted the prolonged elimination half-life of oral minodronic acid in elderly subjects and they concluded that it could be due to the reduced renal function. As Zhou et al.17 reported, the higher exposure of minodronic acid in the elderly subjects could be attributed to the reduction of renal blood flow, glomerular filtration rate and the bone uptake of the elderly.
According to the above investigations, the oral bioavailability of different crystal forms in healthy subjects might vary upon the physiological status. However, so far, little work has been done on the investigation as how the physiological factors influence the pharmacokinetics of the polymorphic drugs. Nim has high permeability and poor solubility, which belongs to Class II of the Biopharmaceutical Classification System18. Moreover, Nim is a kind of polymorphic drug presenting two different crystal forms, including H-Nim and L-Nim. As Grunenberg et al.18 reported, H-Nim, a meta-stable form, is a racemic compound that exhibits a characteristic melting point at 124 °C, while L-Nim, a stable form, is a conglomerate that melts at 116 °C.
In this experiment, the effect of age on the pharmacokinetic properties of polymorphic drug was investigated in rats of 2- or 9-month old with Nim as a model polymorphic drug. The work may help us to choose the suitable crystal form of the polymorphic drugs in their dosage form design, clinical trial, as well as the rational clinical application.
2. Materials and methods
2.1. Materials
H-Nim (99.3%) was kindly supplied by Ruikang Pharmaceutical Co., Ltd. (Zhengzhou, China). L-Nim was prepared by recrystallization of H-Nim in diethyl ether in our laboratory. Methanol and acetonitrile of HPLC grade were purchased from Tianjin Jiangtian Chemical Technology Co., Ltd. (Tianjin, China). Diethyl ether was obtained from Real & Lead Chemical Co., Ltd. (Tianjin, China). All other reagents used were either of analytical or chromatographic grades. Nitrendipine as an internal standard was purchased from National Institutes for Food and Drug Control (Beijing, China).
2.2. Preparation of polymorphic Nim
H-Nim was sieved and the received powder with a particle size between 120 and 150 μm was used for further investigation.
L-Nim was prepared by recrystallization of H-Nim in ethyl ether. Generally, 1.0 g H-Nim was dissolved in 50 mL of ethyl ether at 30 °C to get a solution and then the solvent was evaporated by manually stirring with a glass rod in a draft cupboard in a water bath at 25 °C until a faint yellow powder was received. The obtained L-Nim was then stored in a desiccator for 24 h. The received L-Nim was sieved and the received powder with a particle size between 120 and 150 μm was used for further investigation.
2.3. Characterization of H-Nim and L-Nim
2.3.1. X-ray diffraction (XRD)
An X-ray diffractometer (X׳Pert Pro, PANalytical B.V., Netherlands) with Cu-Kα radiation of wavelength 1.5406 Å (40 kV, 40 mA) was employed to study the crystalline form of H-Nim and L-Nim. The samples were then analyzed over a 2θ range of 2–35° with a scanning rate of 8°/min and step size of 0.02.
2.3.2. Differential scanning calorimetry (DSC)
Thermal analysis was carried out using a differential scanning calorimeter (DSC 1/500, Mettler-Toledo, Switzerland) for H-Nim and L-Nim. Samples in sealed aluminum pans were scanned from 25 to 150 °C with a heating rate of 10 °C/min under a dry nitrogen purge.
2.4. In vitro dissolution study
The dissolution of H-Nim and L-Nim in the medium of simulated gastric fluid (SGF, pH=1.2 HCl) at 37±0.5 °C were investigated with the paddle method at the rotation speed of 50 rpm using a dissolution apparatus (Type RC806, Tianda Tianfa Technology Co., Ltd., Tianjin, China). Thirty mg of drugs were dispersed into 250 mL of the dissolution medium. At the determined time points, a 5 mL aliquot of sample was withdrawn and filtered through 0.22-μm membrane filter. The filtered samples were finally assayed by UV spectrophotometry at 238 nm (UV765, Shanghai Precision Scientific Instrument Co., Ltd., China). Each test was conducted in triplicates. The concentration of Nim was calculated relative to a Nim reference of y=0.0592x+0.0058 with r2 (correlation coefficient) value of 0.9998 (x is the concentration of Nim in μg/mL and y is the absorbance).
2.5. Pharmacokinetic study
Male Sprague–Dawley rats were obtained from the center for experimental animal reproduction, Academy of Military Medical Sciences, Tianjin, China. All animals received care in compliance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals and all procedures were approved by the Animal Care and Use Committee of Tianjin University. Ten 2-month-old rats weighted 250±25 g and ten 9-month-old rats with an average weight of 500±25 g were used in the experiment. The rats were maintained on a standard diet with free access to water. And all rats were fasted for 12 h before the experiment but had free access to drinking water. Each animal received a dose of 40 mg/kg H-Nim and L-Nim in the form of dry powder by intragastric administration through a special pipeline connected with an injector nozzle. Four hours after dosing, the rats were provided with standard food.
Approximately 250 μL blood samples were withdrawn from the oculi chorioideae vein before and 0.25, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 4.0, 6.0, 8.0, 10.0, 12.0 and 24.0 h after dosing. The blood samples were immediately transferred into a heparinized Eppendorf tube and centrifuged at 12,000×g for 5 min, and then the plasma was obtained. All the plasma samples were stored at −20 °C until analysis.
2.6. HPLC analysis
The HPLC analysis was carried out on a LabAlliance Model 2000 HPLC system (Tianjin Lanbo Experimental Equipment Co., Ltd., Tianjin, China). The separation was performed on a Kromasil C18 column (250 mm×4.6 mm, 5 μm) preceded by a same type C18 pre-column (10 mm×4.6 mm, 5 μm). The flow rate was kept constant at 1.0 mL/min and the temperature of oven was maintained at ambient temperature. The mobile phase used in the separation of Nim was acetonitrile–water (70:30, v/v). The detection wavelength was set at 238 nm.
The method was validated in terms of specificity, linearity, precision, extract recovery and stability. The blank plasma had no interference with analysis of Nim or nitrendipine. The linear regression of the peak area ratios versus concentrations was fitted over the concentration range of 5–320 ng/mL for Nim in plasma. A typical regression equation for the calibration curve was: y=0.0084x+0.0089, r2=0.9999, where y is the peak area ratio of Nim to nitrendipine, and x is the concentration of Nim in plasma. The limit of quantification of Nim in plasma was 5 ng/mL. The intra-day and inter-day precisions were below 4.7%. The average extraction recovery of Nim and nitrendipine in plasma were 85.2% and 79.0%, respectively. The mean relative errors of Nim concentration measured in quality control samples being subjected to certain conditions ranged from −6.5% to 7.3%, which indicated the stability of Nim in plasma stored at room temperature for 24 h, at −20 °C for 30 days and during the three freeze–thaw cycles (−20 °C and room temperature, repectively).
2.7. Plasma sample preparation
A 10 μL aliquot of internal standard solution (nitrendipine, 800 ng/mL in methanol) and 500 μL methanol were added to 100 μL plasma sample, which was then vortex-mixed for 1 min, disposed in an ultrasonic bath for 5 min and centrifuged at 12,000×g for 10 min. The upper organic layer was evaporated to dryness under a gentle stream of nitrogen at 45 °C. The residue was reconstituted in 100 μL 30% methanol solution, vortex-mixed for 3 min and centrifuged at 12,000×g for 15 min. Aliquots (25 μL) of the supernate were injected into the HPLC system for analysis.
2.8. Statistical analysis
The main pharmacokinetic parameters were estimated by non-compartmental methods using the software of DAS 2.0, including the maximum plasma concentration (Cmax), the time to reach a Cmax (Tmax), elimination rate constant (Ke), half-life (t1/2), clearance rate (CL), apparent volume of distribution (Vd), mean residence time (MRT0–24 h), the area under the plasma concentration-time curve from time zero to infinity (AUC0–∞), the area under the plasma concentration-time curve from time zero to the measured time t in plasma (AUC0–t). Statistical analysis was carried out through independent sample t-test. The statistical evaluation of the results was performed in SPSS statistical software program (SPSS 17.0, SPSS Inc., USA). Numerical values in tables and figures were presented as mean±standard deviation (SD). Means were considered to be statistically significant when P<0.05.
3. Results
3.1. Characterization of H-Nim and L-Nim by XRD and DSC
As shown in Fig. 1A, the XRD patterns showed very different diffractograms for H-Nim and L-Nim, especially at low 2θ angles, which showed easy distinction between the two polymorphs. Major characteristic diffraction peaks of H-Nim were observed at 2θ of 6.4°, 12.3° and 12.8°, while L-Nim showed major characteristic diffraction peaks at 2θ of 10.3°, 19.4° and 20.7°. As shown in Fig. 1B, the DSC thermograms of H-Nim and L-Nim showed melting endothermic peaks at 125 °C and 114 °C, respectively.
Figure 1.
XRD patterns (A) and DSC thermograms (B) of H-Nim and L-Nim.
3.2. In vitro dissolution study
Dissolution profiles of H-Nim and L-Nim were shown in Fig. 2. Generally, the dissolution rate of L-Nim was much faster than that of H-Nim. For example, the accumulative percent drug released of L-Nim at 30 min was 4.15%, which showed 4.51-fold higher than that of H-Nim (0.92%, P<0.05). At the end of assay, the cumulative percent drug released of H-Nim and L-Nim were 5.27% and 2.22%, respectively, with the former 2.37-fold higher than that of the latter (P<0.01).
Figure 2.
Dissolution profiles of H-Nim and L-Nim in the dissolution medium of SGF. Data are expressed as Mean±SD, n=3. *P<0.05, **P<0.01 compared to H-Nim.
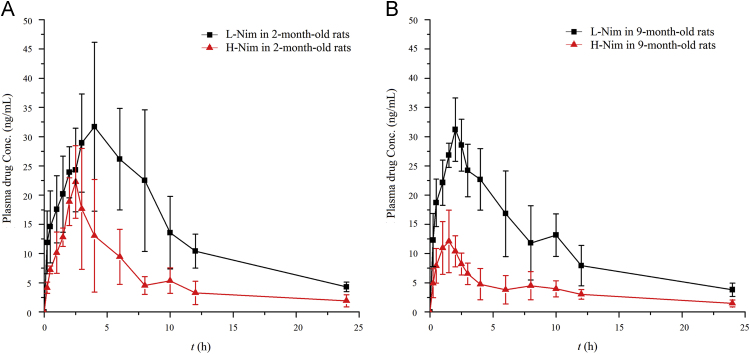
3.3. The effect of polymorphism on the Pharmacokinetics of Nim
Mean plasma concentration-time profiles of H-Nim and L-Nim in 2-month-old and 9-month-old rats were shown in Fig. 3. The pharmacokinetic parameters of H-Nim and L-Nim in 2-month-old and 9-month-old rats were listed in Table 1. As shown in Fig. 3A, the plasma concentration of H-Nim and L-Nim in 2-month-old rats gradually increased up to the maximum value at 2.5 and 4 h, respectively, then both went down gradually. As shown in Fig. 3B, the plasma concentration of H-Nim and L-Nim in 9-month-old rats reached peaks at 1.5 and 2 h, respectively, and then declined gradually. In Table 1, compared with the pharmacokinetic parameters of H-Nim in the two rat groups, AUC0–24 h, Cmax and Tmax of L-Nim were significantly increased (P<0.05), but the CL value of L-Nim was remarkably decreased (P<0.05). Although the Vd value had no significant difference between H-Nim and L-Nim in 2-month-old rats, the Vd value of L-Nim was significantly lower than that of H-Nim in 9-month-old rats (P<0.05). Meanwhile, the other parameters, MRT0–24 h and t1/2 were not significantly different between H-Nim and L-Nim both in 2-month-old and 9-month-old rats.
Figure 3.
Mean plasma concentration-time profiles of H-Nim and L-Nim in 2-month-old rats (A) and 9-month-old rats (B). Data are expressed as Mean±SD, n=5.
Table 1.
Pharmacokinetic parameters of H-Nim and L-Nim after oral administration at the dose of 40 mg/kg to 2-month-old rats and 9-month-old rats.
| Pharmacokinetic parameter | 2-month-old rats |
9-month-old rats |
||
|---|---|---|---|---|
| H-Nim | L-Nim | H-Nim | L-Nim | |
| AUC0—24 h (ng·h/mL) | 140.91±19.47∆ | 343.68±47.15⁎ | 91.12±22.83 | 278.58±55.74* |
| AUC0-∞ (ng·h/mL) | 159.27±44.00 | 400.61±37.32* | 123.00±43.14 | 321.81±50.91* |
| MRT0—24 h (h) | 7.243±1.62 | 8.06±0.63 | 8.16±0.84 | 7.743±0.66 |
| t1/2 (h) | 6.03±0.22 | 9.06±2.90 | 6.85±1.76 | 8.00±4.15 |
| CL (L/h/kg) | 263.73±57.70 | 100.47±8.40* | 362.28±142.06 | 127.15±22.69* |
| Vd (L/kg) | 2007.39±746.75 | 1324.10±480.44 | 3923.73±1529.70 | 1465.05±768.83* |
| Cmax (ng/mL) | 26.29±5.00∆ | 41.93±6.34*,∆ | 15.16±1.62 | 32.88±3.59* |
| Tmax (h) | 2.40±0.42∆ | 5.00±2.00∆ | 1.50±0.35 | 2.10±0.42* |
⁎P<0.05 compared to H-Nim; ∆P<0.05 compared to 9-month-old rats.
3.4. The effect of age on the Pharmacokinetics of Nim
As shown in Fig. 3, the plasma concentration of H-Nim in 2-month-old and 9-month-old rats presented peaks at 2.5 and 1.5 h, respectively. The plasma concentration of L-Nim in 2-month-old and 9-month-old rats reached peaks at 4 and 2 h, respectively. In Table 1, the Cmax of H-Nim and L-Nim in 2-month-old rats were 26.29±5.00 ng/mL and 41.93±6.34 ng/mL, respectively, which showed 1.73- and 1.27-fold higher than that of Cmax (15.16±1.62 ng/mL and 32.88±3.59 ng/mL) in 9-month-old rats (P<0.05). Furthermore, the Tmax of H-Nim and L-Nim in 2-month-old rats were significantly longer than that in 9-month-old rats (P<0.05). However, although the AUC0–24 h of H-Nim in 2-month-old rats was significantly higher than that in 9-month-old rats (P<0.05), the AUC0–24 h of L-Nim in 2-month-old rats was just slightly higher than that in 9-month-old rats. Similarly, there were no significant differences of CL values of H-Nim and L-Nim between 2-month-old and 9-month-old rats. Furthermore, statistically insignificant were still revealed for t1/2, MRT0–24 h and Vd.
3.5. The AUC ratios between H-Nim and L-Nim in the two rat groups
The AUC ratios between H-Nim and L-Nim at different time intervals in 2-month-old and 9-month-old rats were shown in Fig. 4. In general, except that the AUC0–4 h ratio between H-Nim and L-Nim in 9-month-old rats was significantly higher than that in 2-month-old rats (P<0.05), the ratios of AUC0–8 h, AUC0–12 h and AUC0–24 h between H-Nim and L-Nim were not significantly different between the two rat groups. For instance, the AUC0–4 h ratio between H-Nim and L-Nim in 2-month-old rats (1.72±0.66) was 1.80-fold lower than that in 9-month-old rats (3.09±0.95), while the AUC0–24 h ratio between H-Nim and L-Nim in 2-month-old rats (2.50±0.44) just showed 1.31-fold lower than that in 9-month-old rats (3.28±1.34).
Figure 4.
The AUC ratios between L-Nim and H-Nim at different time intervals in 2-month-old rats and 9-month-old rats. Data are expressed as Mean±SD, n=5. *P<0.05 compared to 2-month-old rats.
4. Discussion
4.1. Characterization of H-Nim and L-Nim by XRD and DSC
According to the results of XRD and DSC, both of H-Nim and L-Nim showed well-resolved diffraction patterns with distinct peaks and thermograms characteristic of each specific form, which were in agreement with previously reported literatures19, 20, 21. It was therefore confirmed that H-Nim and L-Nim were properly formed, which could be used for further studies.
4.2. The effect of polymorphism on the pharmacokinetics of Nim
According to the current data, the AUC0–24 h and Cmax of L-Nim were significantly higher than that of H-Nim in the two rat groups. These results indicated that the absorption of L-Nim was higher than that of H-Nim. As shown in the results of dissolution study, the percentage of drug released of L-Nim was remarkably higher than that of H-Nim. Furthermore, it was reported that differences in solubility or dissolution kinetics usually caused differences in bioavailability of polymorphs of the same compound22, 23. Therefore, the higher absorption of L-Nim was attributed to the higher dissolution rate of it. It was speculated that these phenomena could be explained by the different crystal structure of H-Nim and L-Nim. As Grunenberg et al.18 reported, the H-Nim was the racemic compound which contained equimolar ratio of two enantiotrophs in the crystal lattice, and the L-Nim was the conglomerate compound which was the conglomerate of equimolar physical mixture of pure enantiomers. It was presumed that the crystal lattice of H-Nim could lead to lower dissolution rate due to its high bond energy, and the L-Nim could be easy to be hydrated in the dissolution medium and then presented a higher dissolution rate. Additionally, the higher CL of H-Nim revealed that its elimination was faster than that of L-Nim. It was reported that Nim was a substrate for cytochrome P450 (CYP) enzymes24, 25. Moreover, as Markel et al.26 reported, γ-glycine had higher biological activity than α-glycine in ameliorating the behavioral disorders in the genetic catalepsy strain after oral administration of two glycine crystals. They concluded that this result may be related to differences in the supramolecular complexes formed during the interaction of the two polymorphs with biological liquids and the drug receptors. Therefore, it was speculated that the binding capacity of H-Nim and L-Nim to the active site of CYP enzymes was different due to the different ability of forming supramolecular complexes between receptors and clusters of Nim molecules caused by the different crystal structure of H-Nim and L-Nim, which then led to different elimination rate.
4.3. The effect of age on the pharmacokinetics of Nim
The Cmax and AUC0–24 h of H-Nim and L-Nim in 9-month-old rats were both lower than that in 2-month-old rats, which suggested that the absorption of polymorphic Nim were decreased with aging. As previous reported, Nim was well absorbed in the gastrointestinal tract after oral administration27, while the basal gastric blood flow and the intestinal peristalsis were decreased in elder individuals28, 29, 30. This could be the explanation for the decline in the absorption of H-Nim and L-Nim in 9-month-old rats. Nevertheless, the t1/2, CL, Vd and MRT0–24 h value of H-Nim and L-Nim were not significantly different between 2-month-old and 9-month-old rats, which indicated the age-related changes in the physiological state of kidney and liver have no significant effect on their elimination. It has been reported that activities of CYP enzymes were preserved in normal aging31 and there were no significant differences in CYP3A4 activity between young and old subjects32. Since CYP3A4, the major isozyme of CYP enzymes, is responsible for the Nim metabolism24, 25, this may be the reason why the elimination of Nim was not significantly different between 2- and 9-month old rats.
4.4. The differences of AUC ratios between H-Nim and L-Nim in the two rat groups
The AUC ratios between H-Nim and L-Nim represented the relative bioavailability of H-Nim and L-Nim after oral administration. The AUC ratios between H-Nim and L-Nim presented in Fig. 4 showed that this ratio was always larger than 1.0 in any cases, which meant that L-Nim showed higher bioavailability if compared to the H-Nim.
Compared to 2-month-old rats, the AUC ratios between H-Nim and L-Nim at different time intervals in 9-month-old rats were slightly increased especially for the ratio of AUC0–4 h, which meant that the relative bioavailability of H-Nim and L-Nim between 2-month-old and 9-month-old rats were not significantly different but unparalleled. It was known that the impairment of gastrointestinal tract could result in the decreased absorption of polymorphic Nim in 9-month-old rats28, 29, 30 and the degree of decreased absorption of H-Nim was larger than that of L-Nim, while the activity of CYP3A4 was not significantly changed with aging31, 32. Therefore, although the lower concentration of polymorphic Nim in plasma could be effectively metabolized by the hepatic CYP3A4, the degree and rate of metabolism of H-Nim and L-Nim in 9-month-old rats were not the same as in 2-month-old rats. As a result, the relative bioavailability of H-Nim and L-Nim between 2-month-old and 9-month-old rats was unparalleled.
5. Conclusions
This study showed that the pharmacokinetics of the polymorphic Nim could be affected by the factor of age. An increased drug absorption and bioavailability was found in younger rats than in the elder rats for both H-Nim and L-Nim. The decreased drug absorption in the elder rats may be attributed to the reduction of basal gastric blood flow and slower intestinal peristalsis. Since the activities of CYP3A4 which is responsible for the elimination of Nim rarely changed with aging, the elimination of both H-Nim and L-Nim in rats of all ages kept nearly unchanged. Though an increase in drug absorption of polymorphic Nim was found for younger rats when compared to the elder rats, the unparalleled change of the absorption and bioavailability of two different crystal forms of polymorphic Nim caused by age differences means that a further investigation is needed to understand the deeper mechanism as how the drug absorption and elimination were affected by aging. Anyhow, the results in this work are valuable for the selection of suitable crystal form of polymorphic drugs in dosage form design, clinical trial as well as the rational clinical application.
Acknowledgments
This work was partially supported by the National Natural Science Foundation of China, China (No. 21176173) and Tianjin Natural Science Foundation, China (No. 14JCYBJC29100).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Contributor Information
Junbo Gong, Email: junbo_gong@tju.edu.cn.
Zhenping Wei, Email: zpwei2000@sina.com.
References
- 1.Xu C.H., Zou M.J., Liu Y., Ren J.G., Tian Y., Yan J. Pharmacokinetics of carbamazepine polymorphs and dihydrate in rats, related to dogs and humans. Arch Pharm Res. 2011;34:1973–1982. doi: 10.1007/s12272-011-1118-8. [DOI] [PubMed] [Google Scholar]
- 2.Sun Z.L., Yao D., Yuan J.P., Yuan G.Y., Bu F.L., Jiang Z.M. Human pharmacokinetic variations of different nitrendipine crystalline polymorphs. Lat Am J Pharm. 2014;33:1182–1187. [Google Scholar]
- 3.Chinsangaram J., Honeychurch K.M., Tyavanagimatt S.R., Bolken T.C., Jordan R., Jones K.F. Pharmacokinetic comparison of a single oral dose of polymorph form I versus form V capsules of the antiorthopoxvirus compound ST-246 in human volunteers. Antimicrob Agents Chemother. 2012;56:3582–3586. doi: 10.1128/AAC.06090-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H.R., Zhang L.T., Wang Q., Yuan Z.F., Li M. Validated LC–MS–MS method for determination of m-nisoldipine polymorphs in rat plasma and its application to pharmacokinetic studies. J Chromatogr B. 2006;835:71–76. doi: 10.1016/j.jchromb.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 5.Du W., Zhou Y.F., Gong Y.F., Zhao C.S. Investigation of physicochemical properties and in-vitro in-vivo evaluation of agomelatine polymorphs. Asian J Pharm Sci. 2013;8:181–190. [Google Scholar]
- 6.Huang J., Pei Q., Tan H., Yuan H., Lu Y., Liu G. Pharmacokinetics of trantinterol and its metabolite in healthy elderly and young subjects. Int J Clin Pharmacol Ther. 2015;53:875–882. doi: 10.5414/CP202315. [DOI] [PubMed] [Google Scholar]
- 7.Zeng X.Y., Dong S., He N.N., Jiang C.J., Dai Y., Xia Y.F. Comparative pharmacokinetics of arctigenin in normal and type 2 diabetic rats after oral and intravenous administration. Fitoterapia. 2015;105:119–126. doi: 10.1016/j.fitote.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Zhao M., Qian D.W., Shang E.X., Jiang S., Guo J.M., Liu P. Comparative pharmacokinetics of the main compounds of Shanzhuyu extract after oral administration in normal and chronic kidney disease rats. J Ethnopharmacol. 2015;173:280–286. doi: 10.1016/j.jep.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Yang T., Liu S., Zheng T.H., Tao Y.Y., Liu C.H. Comparative pharmacokinetics and tissue distribution profiles of lignan components in normal and hepatic fibrosis rats after oral administration of Fuzheng Huayu recipe. J Ethnopharmacol. 2015;166:305–312. doi: 10.1016/j.jep.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Gidal B.E. Drug absorption in the elderly: biopharmaceutical considerations for the antiepileptic drugs. Epilepsy Res. 2006;68 Suppl 1:S65–S69. doi: 10.1016/j.eplepsyres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 11.Wynne H.A., Cope L.H., Mutch E., Rawlins M.D., Woodhouse K.W., James O.F.W. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology. 1989;9:297–301. doi: 10.1002/hep.1840090222. [DOI] [PubMed] [Google Scholar]
- 12.Schmucker D.L. Liver function and phase I drug metabolism in the elderly: a paradox. Drugs Aging. 2001;18:837–851. doi: 10.2165/00002512-200118110-00005. [DOI] [PubMed] [Google Scholar]
- 13.Gong I.H., Hwang J., Choi D.K., Lee S.R., Hong Y.K., Hong J.Y. Relationship among total kidney volume, renal function and age. J Urol. 2012;187:344–349. doi: 10.1016/j.juro.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 14.White W., Cove-Smith A. Kidney disease in the elderly. Medicine. 2015;43:489–492. [Google Scholar]
- 15.Bondareva I.B., Jelliffe R.W., Gusev E.I., Guekht A.B., Melikyan E.G., Belousov Y.B. Population pharmacokinetic modelling of carbamazepine in epileptic elderly patients: implications for dosage. J Clin Pharm Ther. 2006;31:211–221. doi: 10.1111/j.1365-2710.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 16.Tarral A., Merdjan H. Effect of age and sex on the pharmacokinetics and safety of avibactam in healthy volunteers. Clin Ther. 2015;37:878–886. doi: 10.1016/j.clinthera.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Zhou Y., He X.M., Li H.Q., Ni Y., Xu M.Z., Sattar H. Pharmacokinetics and tolerability of minodronic acid tablets in healthy Chinese subjects and food and age effects on the pharmacokinetics. Clin Ther. 2015;37:869–876. doi: 10.1016/j.clinthera.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Grunenberg A., Keil B., Henck J.O., Siesler H.W. Polymorphism in binary mixtures, as exemplified by nimodipine. Int J Pharm. 1995;118:11–21. [Google Scholar]
- 19.Riekes M.K., Pereira R.N., Rauber G.S., Cuffini S.L., Maduro de Campos C.E., Silva M.A.S. Polymorphism in nimodipine raw materials: development and validation of a quantitative method through differential scanning calorimetry. J Pharm Biomed Anal. 2012;70:188–193. doi: 10.1016/j.jpba.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Rahman Z., Siddiqui A., Khan M.A. Assessing the impact of nimodipine devitrification in the ternary cosolvent system through quality by design approach. Int J Pharm. 2013;455:113–123. doi: 10.1016/j.ijpharm.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 21.Zu Y.G., Li N., Zhao X.H., Li Y., Ge Y.L., Wang W.G. In vitro dissolution enhancement of micronized L-nimodipine by antisolvent re-crystallization from its crystal form H. Int J Pharm. 2014;464:1–9. doi: 10.1016/j.ijpharm.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 22.Bernstein J. Polymorphism in Molecular Crystals. Clarendon Press; Oxford: 2002. [Google Scholar]
- 23.Hilfiker R. Polymorphism: in the pharmaceutical industry. Wiley-VCH; Weinheim: 2006. [Google Scholar]
- 24.Liu X.Q., Ren Y.L., Qian Z.Y., Wang G.J. Enzyme kinetics and inhibition of nimodipine metabolism in human liver microsomes. Acta Pharmacol Sin. 2000;21:690–694. [PubMed] [Google Scholar]
- 25.Wacher V.J., Salphati L., Benet L.Z. Active secretion and enterocytic drug metabolism barriers to drug absorption. Adv Drug Deliv Rev. 2001;46:89–102. doi: 10.1016/s0169-409x(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 26.Markel A.L., Achkasov A.F., Alekhina T.A., Prokudina O.I., Ryazanova M.A., Ukolova T.N. Effects of the alpha- and gamma-polymorphs of glycine on the behavior of catalepsy prone rats. Pharmacol Biochem Behav. 2011;98:234–240. doi: 10.1016/j.pbb.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Langley M.S., Sorkin E.M. Nimodipine: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in cerebrovascular disease. Drugs. 1989;37:669–699. doi: 10.2165/00003495-198937050-00004. [DOI] [PubMed] [Google Scholar]
- 28.Newton J.L. Changes in upper gastrointestinal physiology with age. Mech Ageing Dev. 2004;125:867–870. doi: 10.1016/j.mad.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Navaratnarajah A., Jackson S.H.D. The physiology of ageing. Medicine. 2013;41:5–8. [Google Scholar]
- 30.Merchant H.A., Rabbie S.C., Varum F.J.O., Afonso-Pereira F., Basit A.W. Influence of ageing on the gastrointestinal environment of the rat and its implications for drug delivery. Eur J Pharm Sci. 2014;62:76–85. doi: 10.1016/j.ejps.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Shi S.J., Klotz U. Age-related changes in pharmacokinetics. Curr Drug Metab. 2011;12:601–610. doi: 10.2174/138920011796504527. [DOI] [PubMed] [Google Scholar]
- 32.Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41:67–76. doi: 10.1080/03602530902722679. [DOI] [PubMed] [Google Scholar]