Abstract
Arsenic is a carcinogenic environmental factor found in food and drinking water around the world. The mechanisms in which arsenic alters homeostasis are not fully understood. Over the past few decades, light has been shed on varying mechanisms in which arsenic induces cancer. Such mechanisms include gut microbe perturbations, genotoxic effects, and epigenetic modification. Gut microbe perturbations have been shown to increase the level of pathogen-associated molecular patterns such as lipopolysaccharide (LPS) leading to uncontained inflammation. Increase in inflammation is the major factor in cirrhosis leading to hepatocellular carcinoma. Alterations in gut permeability and metabolites have also been observed as a fallout of arsenic induced gut microbe modification. The guts proximity and interaction through portal flow make the liver susceptible to gut perturbations and ensuing inflammatory responses. Genotoxic and epigenetic dysregulation induced by arsenic and its toxic metabolites present a more direct mechanism that works synergistically with gut microbe perturbations to induce the incidence of cancers. These pathways combined could be some of the main causes of arsenic-induced carcinogenesis.
KEY WORDS: Arsenic, Microbiome, Epigenetics, Liver cancer, Hepatocellular carcinoma, Gut microbiota, Lipopolysaccharide
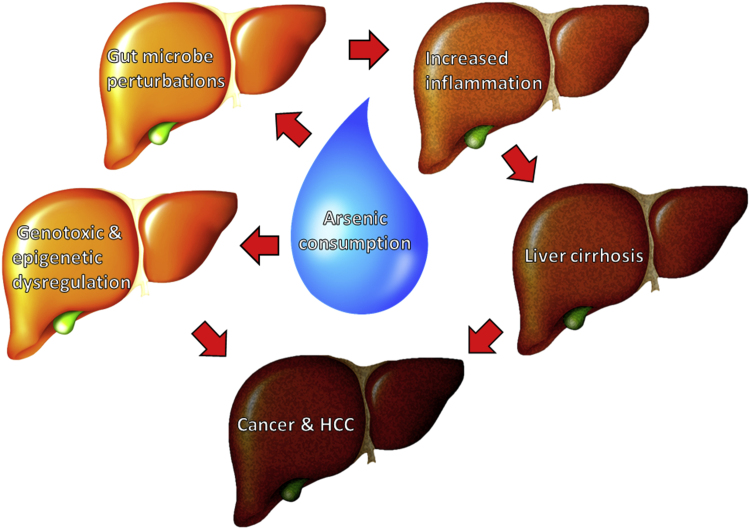
Graphical abstract
Arsenic is an environmental factor with known carcinogenic effects that make it a global health concern. Arsenic has shown the ability to perturb the gut microbe population leading to negative effects in mice and human. The ability of arsenic to increase pathogen-associated molecular patterns (PAMPs) in the perturbed gut can increase inflammatory response leading to adverse effects. The genotoxic and epigenetic effects of arsenic also pose a direct toxicity to tissues and organs, revealing multiple pathways in which it can induce cancer within skin, lung, kidney, urinary bladder, prostate, and liver.
1. Introduction
Arsenic is the 20th most common element in the earth׳s crust and is considered a group 1 carcinogen1. Arsenics abundance and known cariogenic properties make it a global health concern. Arsenic is an environmental factor that is known to contaminate drinking water and food supply if not adequately regulated. Over 100 million people worldwide rely on arsenic-contaminated drinking water on 5 of the earth׳s continents2. There are multiple forms of arsenic, but naturally occurring trivalent inorganic arsenic possess the highest toxicity. Trivalent arsenic can be directly methylated leading to volatile products while pentavalent arsenic is not readily taken up by cells and is often reduced to trivalent arsenic3. Once ingested arsenic is metabolized in the liver where hepatocytes uptake trivalent arsenic, within the hepatocytes, subsequent conjugations and methylations occur leading to volatile products4.
Arsenic has been found to increase the incidence of cancers including skin, lung, kidney, urinary bladder, prostate, and liver1. Hepatocellular carcinoma (HCC) is the leading form of liver cancer and will serve as the focus for this review. The mechanisms in which arsenic induces such cancers are continually investigated.
The carcinogenic effects of arsenic cannot be pinpointed to one simple mechanism, since arsenic perturbs physiological functions through multiple interworking pathways. Such proposed mechanisms leading to cancers include shifts in gut microbiota, genotoxic effects, and epigenetic dysregulation5, 6, 7. These mechanisms work synergistically to induce cancers, such as HCC.
The human body is host to many trillions of gut microbes and this microbial community works in symbiosis to aid in normal physiological functions, such as digestion and metabolism8. Perturbations in the gut microbiota have been associated with many diseases from obesity to various forms of cancer9. It has been shown that arsenic can alter the microbiome and metabolic profile in mice. It is proposed that alterations in the gut microbiota by arsenic can lead to many physiological imbalances aiding in HCC development.
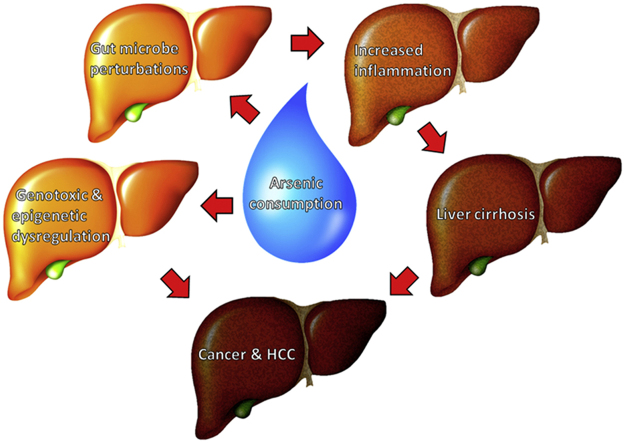
As mentioned previously, arsenic has the potential to induce genotoxic fallout and abnormal epigenetic modifications5. Arsenic is considered a genotoxic metalloid with the ability to cause DNA strand breaks, sister chromatid exchanges, and micronuclei10. Aberrant epigenetic modifications have also been related to arsenic exposure11. Arsenic has the potential to induce HCC through multiple mechanisms, therefore proving an elusive environmental carcinogen (Fig. 1).
Figure 1.
Schematic summary of arsenic-induced disease progression demonstrating synergistic mechanisms leading to liver cirrhosis and hepatocellular carcinoma (HCC). Arsenic consumption leads to both genotoxic/epigenetic dysregulation and gut perturbations. Gut perturbation increases the incidence of inflammation leading to cirrhosis and potentially HCC. Genotoxic and epigenetic dysregulation disrupts intracellular mechanisms, increasing the potential for HCC development.
2. Arsenic perturbs gut microbiota
When inorganic arsenic is ingested into the body through contaminated food and water, it is metabolized by the liver, where hepatocytes uptake trivalent arsenic via aquaglyceroporins and hexose permeases. Within the hepatocyte, arsenic is conjugated with glutathione, generating arsenic triglutathione. Methylation may also take place leading to dimethylarsenic glutathione which has been found to be excreted in bile and into the blood stream. Dimethylarsenic glutathione is unstable and has the potential to form volatile compounds, such as dimethylarsine. Red blood cells are also able to efficiently take up arsenic and store the arsenic as protein-bound trivalent dimethylarsenicals throughout the body4.
Whether arsenic is stored in the cells of the human body or within the gut microbe population, negative effects ensue. A metagenomics and metabolomics analysis conducted by Lu et al.7, in which mice were treated with arsenic through drinking water at a concentration of 10 ppm for four weeks, revealed that the control and treated animals were well separated with 19.95% and 10.66% variation explained by principal component analysis. In a second study, mice were treated with arsenic for 2, 5, and 10 weeks at low to moderate levels of arsenic (10–250 ppb) and microbial biofilms that lined the intestine of control mice were degraded in the treatment mice indicating that moderate levels of arsenic exposure (250 ppb) can alter the gut microbiota12. Degradation of symbiotic microbial biofilms in the intestine may alter normal physiological function and have the potential to open a niche for pathogenic infection. The destruction of microbial biofilms may also alter the permeability of the gut leading to abnormal absorption. Dheer and colleagues12 observed a significant increase in the number of bacteroidetes when mice were treated with 250 ppb arsenic in water for 2, 4, and 10 weeks. An increase in bacteroidetes is significant because bacteroidetes are Gram-negative bacteria with lipopolysaccharide (LPS) on their outer membrane. LPS is an important virulence factor which causes widespread inflammation disrupting normal biological functions13. An increase in pathogenic arginine metabolites in the mouse circulation was also observed12. Arginine is maintained in host cells and upon infection of intracellular pathogens, such as Salmonella typhimurium or Mycobacterium tuberculosis, and it has the ability to utilize the host arginine pool releasing increased levels of pathogenic arginine metabolites. The increase in arginine metabolites acts as an indicator of microbial perturbations and infection14. The combination of altered gut permeability and the increase in pathogen-associated molecular patterns (PAMPs), such as LPS, are both possible factors in the induction or progression of liver cirrhosis and HCC.
3. Perturbed gut microbiota has potential to induce HCC
Altering the gut microbe population has shown to stimulate inflammatory pathways that in turn interrupts liver function leading to cirrhosis and HCC. A recent study by Seki and colleagues15 reported that inflammation triggered through toll like receptor 4 (TLR4) activation induced hepatic fibrogenesis. TLR4 is a receptor responsible for recognizing certain PAMPs. TLR4 specifically recognizes the LPS layer found on gram negative bacteria. Seki et al.16 challenged mice with LPS and observed TLR4 activation in quiescent hepatic stellate cells (HSCs) which resulted in chemokine secretion and an increase in chemotaxis of Kupffer cells. The over active inflammatory response in the liver due to LPS leads to damaged hepatic tissue and function. Seki and colleagues16 also showed that gut sterilization and bile duct ligation in mice lead to a decrease in hepatic fibrosis. Isolating the liver from the gut microbe population decreases the livers exposure to PAMPs, which in turn decreases the inflammatory response and hepatic damage.
Increased levels of PAMPs and inflammation have been associated with viral hepatitis in clinical study. Viral hepatitis is a leading cause of liver cirrhosis. Sandler and colleagues17 reported through clinical studies that compared with uninfected individuals, HCV- and HBV-infected individuals had higher plasma levels of LPS, I-FABP (indicating enterocyte death), sCD14 (produced upon LPS activation of monocytes), and IL-6. Levels of CD14 correlated with markers of hepatic inflammation and fibrosis. CD14 is a co-receptor with TLR4 that detects LPS and other PAMPs, such as lipoteichoic acid.
Inflammation and cirrhosis are major causative factors in the development of hepatocellular carcinoma. There are many mechanisms that lead to HCC, but all of them start with some form of cirrhosis of the liver18. Inflammation which was induced via the mechanisms mentioned above leads to cirrhosis and potentially HCC. Yu et al.19 reported in a laboratory study that depletion of Gram-negative bacteria reduces toxic models of HCC. Yu et al.19 treated rats with bactericidal drugs against enteric Gram-negative bacteria days prior to diethylnitrosamine (DEN) administration. DEN is a cancer inducing model commonly used in rodents. Twenty-one days after DEN injection, bactericidal treated rats showed a reduced number and size of HCC nodules. The ability to reduce the occurrence of HCC nodules by simply altering the gut microbiome shows the direct and significant impact the microbes of the body have on homeostasis. Since arsenic has been shown to alter the gut microbe population, it should be considered as a serious environmental factor with the potential to cause disease via multiple mechanisms.
4. Arsenic has genotoxic and epigenetic altering effects which can induce HCC
When arsenic enters the body through water consumption, it is metabolized in the liver. Arsenic has been shown to induce genotoxic effects and epigenetic dysregulation which has the potential to aid in HCC progression5. Some genotoxic mechanisms of arsenic include DNA repair inhibition and development of micronuclei. Sinha et al.20 reported that oxidative damage induced by arsenic interferes with DNA repair mechanisms through downregulation of DNA repair enzymes like β-polymerase and inhibition of ligation. The exact mechanism in which arsenic deploys its genotoxic effect is elusive because there are more than 5 metabolites that are all known to have toxic properties21. The methylated arsenic metabolite methylarsonous acid (MMAIII) is known to have carcinogenic potential. Tokar et al.22 chronically exposed TRL1215 rat liver cells to MMAIII (0.25–1.0 μmol/L) and were tested for oxidative DNA damage and acquired malignant phenotype. The liver cells acquired a cancer phenotype with MMAIII exposure at about 20 weeks, based on increased matrix metalloproteinase secretion, colony formation and invasion. Kojima et al.23 reported that arsenic biomethylation seems to be obligatory for arsenic-induced oxidative DNA damage and appears linked in TRL1215 rat liver cells with the accelerated transition to an in vitro cancer phenotype.
Arsenic has also been shown to induce abnormal epigenetic modifications, such as DNA hypermethylation of tumor suppressor genes. In the past few decades, epigenetic regulation has been identified as a major factor in post-transcriptional regulation. Many studies have indicated that arsenic exposure leads to globally altered DNA methylation24, 25, 26. Tsang et al.27 showed that arsenic exposure in utero of mice in tandem with high folate administration caused a hugely significant change in CpG island methylations. CpG island methylation is known to modify chromatin structure and potentially inhibit transcription. Cui and colleagues28 demonstrated that mice fed orally with arsenic water (100 ppm) showed an abnormal P16 and RASSF1A methylation pattern in lung tissues, and an increased incidence in tumor formation. A meta-analysis conducted by Wang et al.29 indicated that long-term inorganic arsenic exposure through drinking water increases the risk of liver cancer mortality. Overall, arsenic has shown to alter the gut microbe population and also cause direct genotoxic and epigenetic effects leading to various diseased states, including cirrhosis and HCC.
5. Concluding remarks
Arsenic is an environmental factor with known carcinogenic effects that make it a global health concern. Arsenic has shown the ability to perturb the gut microbe population leading to negative effects in mice and human. The ability of arsenic to increase PAMPs in the perturbed gut can increase inflammatory response leading to adverse effects. The genotoxic and epigenetic effects of arsenic also pose a direct toxicity to tissues and organs, revealing multiple pathways in which it can induce cancer within above-mentioned organs.
Acknowledgments
This work is supported by the U.S. National Institutes of Health (Nos. R01DK080440, R01DK104656, R01ES025909, R21AA022482, R21AA024935), the VA Merit Award (Nos. 1I01BX002634), Natural Scientific Foundation of China (No. 81572443), and Yale Liver Center, USA (No. P30 DK34989).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Aitio A, Cantor KP, Attfield MD, Demers PA, Fowler BA, Grandjean P, et al. Arsenic and arsenic compounds. In: Elvers E, Hamean AS, Moutinho S, Russell D, editors. A review of human carcinogens. Vol. 100C. Arsenic, metals, fibres, and dusts. IARC monographs on the evaluation of carcinogenic risks to humans. Lyon: IARC Working Group; 2012. p. 41–93.
- 2.Polya D., Charlet L. Environmental science: rising arsenic risk. Nat Geosci. 2009;2:383–384. [Google Scholar]
- 3.Jomova K., Jenisova Z., Feszterova M., Baros S., Liska J., Hudecova D. Arsenic: toxicity, oxidative stress and human disease. J Appl Toxicol. 2011;31:95–107. doi: 10.1002/jat.1649. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe T., Hirano S. Metabolism of arsenic and its toxicological relevance. Arch Toxicol. 2013;87:969–979. doi: 10.1007/s00204-012-0904-5. [DOI] [PubMed] [Google Scholar]
- 5.Bustaffa E., Stoccoro A., Bianchi F., Migliore L. Genotoxic and epigenetic mechanisms in arsenic carcinogenicity. Arch Toxicol. 2014;88:1043–1067. doi: 10.1007/s00204-014-1233-7. [DOI] [PubMed] [Google Scholar]
- 6.Tao X., Wang N., Qin W. Gut microbiota and hepatocellular carcinoma. Gastrointest Tumors. 2015;2:33–40. doi: 10.1159/000380895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu K., Abo R.P., Schlieper K.A., Graffam M.E., Levine S., Wishnok J.S. Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: an integrated metagenomics and metabolomics analysis. Environ Health Perspect. 2014;122:284–291. doi: 10.1289/ehp.1307429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 9.Roderburg C., Luedde T. The role of the gut microbiome in the development and progression of liver cirrhosis and hepatocellular carcinoma. Gut Microbes. 2014;5:441–445. doi: 10.4161/gmic.29599. [DOI] [PubMed] [Google Scholar]
- 10.Gebel T.W. Genotoxicity of arsenical compounds. Int J Hyg Environ Health. 2001;203:249–262. doi: 10.1078/S1438-4639(04)70036-X. [DOI] [PubMed] [Google Scholar]
- 11.Han Z.J., Song G., Cui Y., Xia H.F., Ma X. Oxidative stress is implicated in arsenic-induced neural tube defects in chick embryos. Int J Dev Neurosci. 2011;29:673–680. doi: 10.1016/j.ijdevneu.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Dheer R., Patterson J., Dudash M., Stachler E.N., Bibby K.J., Stolz D.B. Arsenic induces structural and compositional colonic microbiome change and promotes host nitrogen and amino acid metabolism. Toxicol Appl Pharmacol. 2015;289:397–408. doi: 10.1016/j.taap.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allcock G.H., Allegra M., Flower R.J., Perretti M. Neutrophil accumulation induced by bacterial lipopolysaccharide: effects of dexamethasone and annexin 1. Clin Exp Immunol. 2001;123:62–67. doi: 10.1046/j.1365-2249.2001.01370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gogoi M., Datey A., Wilson K.T., Chakravortty D. Dual role of arginine metabolism in establishing pathogenesis. Curr Opin Microbiol. 2016;29:43–48. doi: 10.1016/j.mib.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwabe R.F., Seki E., Brenner D.A. Toll-like receptor signaling in the liver. Gastroenterology. 2006;130:1886–1900. doi: 10.1053/j.gastro.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 16.Seki E., De Minicis S., Österreicher C.H., Kluwe J., Osawa Y., Brenner D.A. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 17.Sandler N.G., Koh C., Roque A., Eccleston J.L., Siegel R.B., Demino M. Host response to translocated microbial products predicts outcomes of patients with HBV or HCV infection. Gastroenterology. 2011;141:1220–1230. doi: 10.1053/j.gastro.2011.06.063. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fattovich G., Stroffolini T., Zagni I., Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 19.Yu L.X., Yan H.X., Liu Q., Yang W., Wu H.P., Dong W. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology. 2010;52:1322–1333. doi: 10.1002/hep.23845. [DOI] [PubMed] [Google Scholar]
- 20.Sinha D., Roy M. Antagonistic role of tea against sodium arsenite–induced oxidative DNA damage and inhibition of DNA repair in Swiss albino mice. J Environ Pathol Toxicol Oncol. 2011;30:311–322. doi: 10.1615/jenvironpatholtoxicoloncol.v30.i4.40. [DOI] [PubMed] [Google Scholar]
- 21.Pinyayev T.S., Kohan M.J., Herbin-Davis K., Creed J.T., Thomas D.J. Preabsorptive metabolism of sodium arsenate by anaerobic microbiota of mouse cecum forms a variety of methylated and thiolated arsenicals. Chem Res Toxicol. 2011;24:475–477. doi: 10.1021/tx200040w. [DOI] [PubMed] [Google Scholar]
- 22.Tokar E.J., Kojima C., Waalkes M.P. Methylarsonous acid causes oxidative DNA damage in cells independent of the ability to biomethylate inorganic arsenic. Arch Toxicol. 2014;88:249–261. doi: 10.1007/s00204-013-1141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kojima C., Ramirez D.C., Tokar E.J., Himeno S., Drobná Z., Stýblo M. Requirement of arsenic biomethylation for oxidative DNA damage. JNCI J Natl Cancer Inst. 2009;101:1670–1681. doi: 10.1093/jnci/djp414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagnyukova T.V., Luzhna L.I., Pogribny I.P., Lushchak V.I. Oxidative stress and antioxidant defenses in goldfish liver in response to short-term exposure to arsenite. Environ Mol Mutagen. 2007;48:658–665. doi: 10.1002/em.20328. [DOI] [PubMed] [Google Scholar]
- 25.Xie Y.X., Trouba K.J., Liu J., Waalkes M.P., Germolec D.R. Biokinetics and subchronic toxic effects of oral arsenite, arsenate, monomethylarsonic acid, and dimethylarsinic acid in v-Ha-ras transgenic (Tg.AC) mice. Environ Health Perspect. 2004;112:1255–1263. doi: 10.1289/txg.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelch K.E., Tokar E.J., Merrick B.A., Waalkes M.P. Differential DNA methylation profile of key genes in malignant prostate epithelial cells transformed by inorganic arsenic or cadmium. Toxicol Appl Pharmacol. 2015;286:159–167. doi: 10.1016/j.taap.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsang V., Fry R.C., Niculescu M.D., Rager J.E., Saunders J., Paul D.S. The epigenetic effects of a high prenatal folate intake in male mouse fetuses exposed in utero to arsenic. Toxicol Appl Pharmacol. 2012;264:439–450. doi: 10.1016/j.taap.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui X., Wakai T., Shirai Y., Hatakeyama K., Hirano S. Chronic oral exposure to inorganic arsenate interferes with methylation status of p16INK4a and RASSF1A and induces lung cancer in A/J mice. Toxicol Sci. 2006;91:372–381. doi: 10.1093/toxsci/kfj159. [DOI] [PubMed] [Google Scholar]
- 29.Wang W.J., Cheng S., Zhang D.F. Association of inorganic arsenic exposure with liver cancer mortality: a meta-analysis. Environ Res. 2014;135:120–125. doi: 10.1016/j.envres.2014.08.034. [DOI] [PubMed] [Google Scholar]