Abstract
The expression of phase-I drug metabolizing enzymes in liver changes dramatically during postnatal liver maturation. Farnesoid X receptor (FXR) is critical for bile acid and lipid homeostasis in liver. However, the role of FXR in regulating ontogeny of phase-I drug metabolizing genes is not clear. Hence, we applied RNA-sequencing to quantify the developmental expression of phase-I genes in both Fxr-null and control (C57BL/6) mouse livers during development. Liver samples of male C57BL/6 and Fxr-null mice at 6 different ages from prenatal to adult were used. The Fxr-null showed an overall effect to diminish the “day-1 surge” of phase-I gene expression, including cytochrome P450s at neonatal ages. Among the 185 phase-I genes from 12 different families, 136 were expressed, and differential expression during development occurred in genes from all 12 phase-I families, including hydrolysis: carboxylesterase (Ces), paraoxonase (Pon), and epoxide hydrolase (Ephx); reduction: aldoketo reductase (Akr), quinone oxidoreductase (Nqo), and dihydropyrimidine dehydrogenase (Dpyd); and oxidation: alcohol dehydrogenase (Adh), aldehyde dehydrogenase (Aldh), flavin monooxygenases (Fmo), molybdenum hydroxylase (Aox and Xdh), cytochrome P450 (P450), and cytochrome P450 oxidoreductase (Por). The data also suggested new phase-I genes potentially targeted by FXR. These results revealed an important role of FXR in regulation of ontogeny of phase-I genes.
Abbreviations: ADH, alcohol dehydrogenase; AKR, aldoketo reductase; ALDH, aldehyde dehydrogenase; CES, carboxylesterase (Ces); DPYD, dihydropyrimidine dehydrogenase; EPHX, epoxide hydrolase; FMO, flavin monooxygenases, Farnesoid X receptor (FXR); NQO, quinone oxidoreductase; P450, cytochrome P450; PON, paraoxonase; POR, cytochrome P450 oxidoreductase
KEY WORDS: Drug metabolizing enzymes, Farnesoid X receptor, Liver, Ontogeny, Fxr-null mouse, Gene expression
Graphical abstract
The role of FXR in regulating ontogeny of phase-I drug metabolizing genes is still not clear. In his study, the RNA-sequencing was applied to quantify the developmental expression of phase-I genes in both Fxr-null and control (C57BL/6) mouse livers during development.
1. Introduction
Phase-I drug metabolizing enzymes catalyze the oxidation, reduction, and hydrolysis of xenobiotics. A functional transition occurs in liver after birth, and most of the phase-I drug-metabolizing enzymes mature during this period in mouse liver1, 2. Changes in expression of some phase-I enzymes during liver maturation in humans have also been reported, including cytochrome P450 (P450s)3, 4, 5, 6, carboxylesterase (CES)7, 8, paraoxonase (PON)9, 10, alcohol dehydrogenase (ADH)11, and flavin monooxygenase (FMO)12, 13, 14. The dynamic changes in the ontogenic expression of these genes are thought to be responsible for the substantial pharmacokinetic differences between newborns and adults, and this contributes to differences in therapeutic efficacy and adverse drug reactions in pediatric patients15, 16, 17, 18, 19. An in-depth understanding of the regulatory mechanisms of the ontogeny of phase-I enzymes is needed for safer and more effective drug therapy for pediatric patients.
Farnesoid X receptor (FXR) is a bile acid sensing nuclear receptor in maintenance of bile acid homeostasis by feedback inhibition of expression of important genes in bile acid synthesis, for example, CYP7A1, which is the rate-limiting enzyme in the classic pathway of bile acid synthesis, and CYP8B1, which is required for the synthesis of cholic acid20. FXR also plays a role in liver regeneration21. Dysfunction of FXR may lead to development of digestive system diseases22 and alcoholic liver disease23. The role of FXR in regulating certain drug metabolizing enzyme genes has also been established. Bile acids activate FXR in the intestine and liver, which induces the expression of PXR, facilitating the detoxification of lithocholate and xenobiotics by inducing CYP3A, phase-II enzymes (e.g., sulfotransferase 2A1), and transporters (e.g., MRP2 and MRP3) xenobiotics24, 25. A functional FXR polymorphism is common (2.5%–12%), and has been associated with changes in FXR-target gene expression in humans26, 27. Understanding the regulatory mechanism of FXR on hepatic expression of drug metabolizing enzyme genes in newborns will greatly help us to understand the natural and aberrant development of drug metabolizing enzyme genes in pediatric pharmacology.
In the current study, we used RNA-sequencing to quantify hepatic phase-I metabolizing enzyme gene expression during the developmental period from perinatal stage to adult in both wild-type and a well-established Fxr knockout mouse model20. FXR has been confirmed to be knocked out in whole body in this Fxr-null model. Our results revealed the significant differences in Fxr-null livers compared to control livers during postnatal development, and suggested novel roles of FXR in the regulation of phase-I gene developmental expression.
2. Materials and methods
2.1. Animals
We used C57BL/6 mice as the wild-type animals. The animal purchase, breeding and tissue collection for wild-type mice were performed as previously described1, 2. An Fxr-deficient mouse model28 was used to examine the role of FXR in determination of ontogeny of phase-I drug metabolizing enzyme genes in mouse liver. These knockout mice were bred under standard conditions in the Office of Animal Care Facility at the University of Connecticut (Storrs, CT, USA). The use of these mice was approved by the University of Connecticut׳s Institutional Animal Care and Use Committee. Liver samples (n=3) from wild-type and Fxr–/– mice were collected at the following 6 ages: day –2 (gestational day 17.5), day 1 (exactly 24 h after birth), and days 5, 20, 25, and 60 (collected at approximately 9:00 AM). These ages represent the periods of prenatal (day –2), neonatal (days 1 and 5), juvenile (days 20 and 25), and young adult (day 60). Due to potential variations caused by the estrous cycle in maturing female mice, only male livers were used in this study. The livers were immediately frozen in liquid nitrogen after removal and stored at –80 °C.
2.2. Total RNA extraction, sequencing library construction, and RNA-Seq
RNA extraction, library construction, RNA-Seq, and FASTQ data file collection were performed as previously described2.
2.3. RNA-Seq data analysis
For comparison of phase-I genes between wild-type and Fxr-knockout samples at the 6 ages of days –2, 1, 5, 20, 25 and 60, the RNA-Seq reads from the FASTQ files of these two types of mice were mapped to the mouse reference genome (GRCm38/mm10) by Tophat 2.0.8. The output files in BAM format were analyzed by Cufflinks 2.1.1 to estimate the transcript abundance.
2.4. Data visualization and statistics
In this study, we consider genes with FPKM>1 as expressed. ANOVA was used to test for significant difference in expression between wild-type and Fxr-null mice. P values were adjusted using Benjamini-Hochberg algorithm with a threshold of 0.05. To study the distance of phase-I gene ontogenic pattern between WT and Fxr–/– samples, the average FPKM values from three individual animals were used. For each gene, the FPKM values at the 12 ages (6 ages per genotype) were divided by the mean FPKM of the 12 ages before calculating distance in order to normalize the difference of expression level among genes.
3. Results
3.1. Phase-I gene ontogeny in Fxr–/– mice
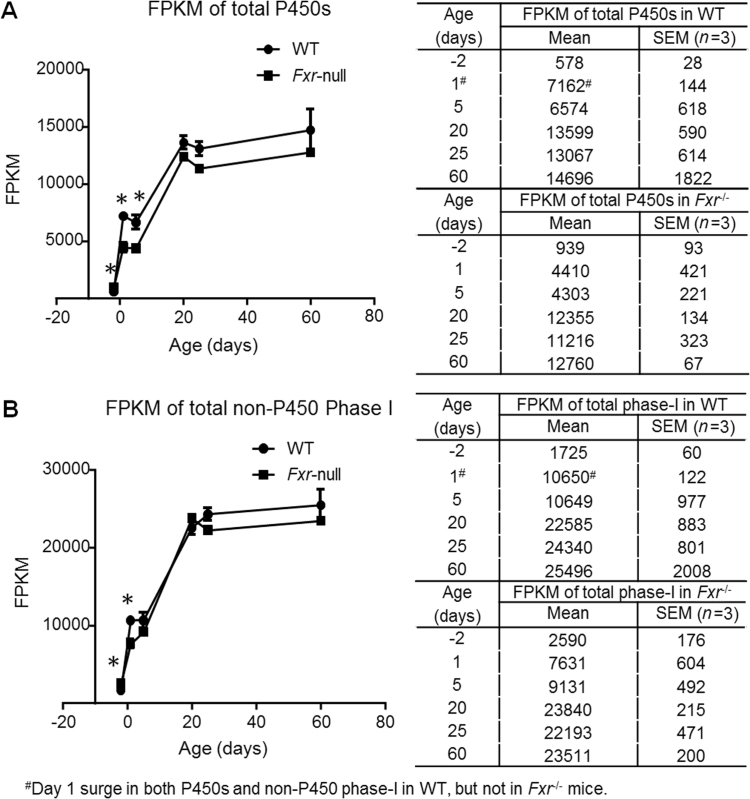
For all the P450 genes, significant differences in total mRNA expression between wild-type and Fxr–/– mice were found at ages of days –2, 1 and 5 (Fig. 1A). The expression of total P450s was higher before birth and lower after birth till day 5 in Fxr–/– mice than in wild-type. There was a surge in expression of P450 genes around birth. From days –2 to day 1, the total P450 mRNA level increased more than 12-fold in wild-type mice, yet this increase was less than 5-fold in Fxr–/– mice (Fig. 1A). The total P450 mRNAs reached similar levels from days 20 to 60 in both wild-type and Fxr-null mice. A similar change in total mRNA expression of non-P450 phase I genes between wild-type and Fxr–/– mice was found in Fig. 1B.
Figure 1.
Total mRNA levels of the 102 P450 genes (A) and 185 non-P450 phase-I genes (B) in the mouse liver during development. RNA-Seq was done for liver mRNAs of wild-type C57BL/6 and Fxr-null male mice at 6 ages from 2 days before birth to 60 days after birth. The FPKM values of all genes in the group at each age were added and plotted to show the developmental pattern of total mRNAs. Bars represent the mean FPKM and SEM of three individual animals. Star sign indicates significant difference of expression between wild-type and Fxr-null mice with adjusted P<0.05.
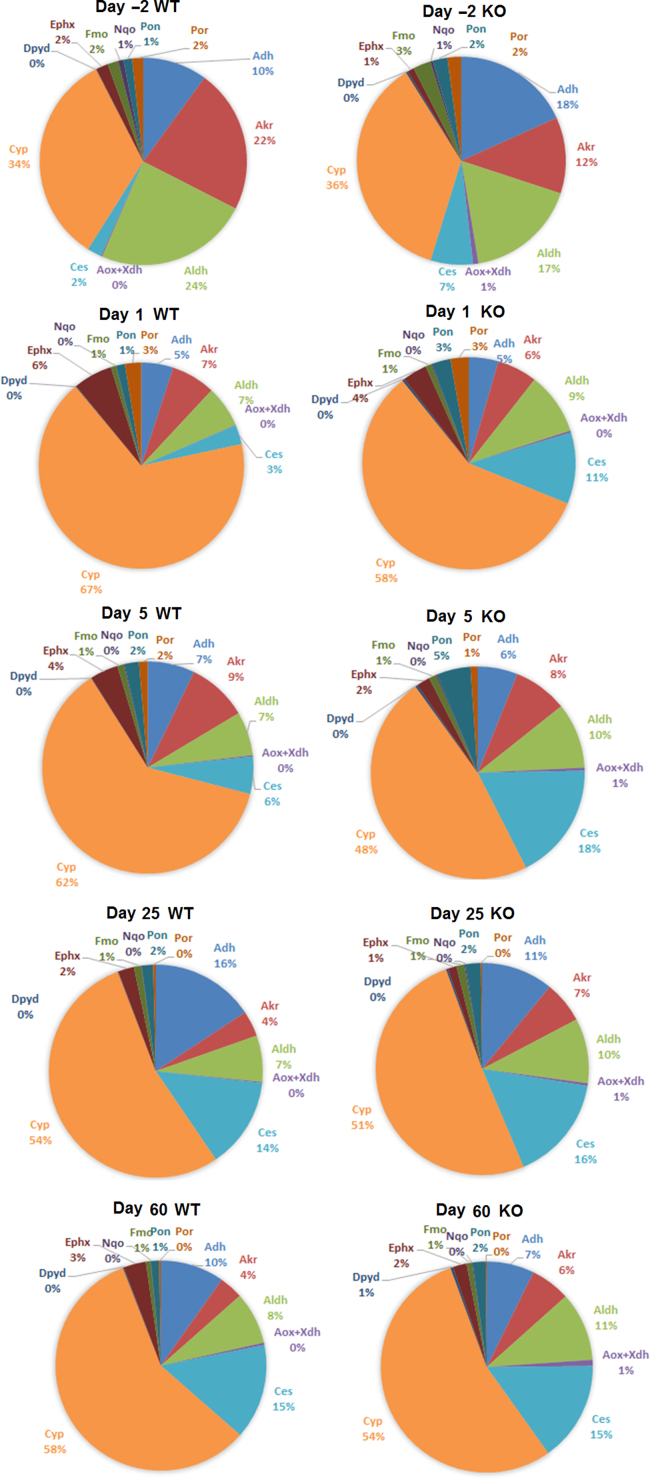
When examining the composition of different families of phase-I genes within this total expression, we also found dramatic changes around birth and significant differences between wild-type and Fxr-null mice (Fig. 2). At day –2, the mRNA percentage of Akr and Aldh were much lower and the percentage of Adh and Ces were much higher in Fxr-null mice compared with wild-type mice. The percentage of P450 mRNAs in all phase-I genes nearly doubled from days –2 to 1 in wild-type mice, but the P450 percentage was much lower in Fxr-null mice at days 1 and 5. Ces was a family of phase-I genes that showed dramatically higher percentage of expression in Fxr-null mice at perinatal stage of days –2, 1 and 5 (Fig. 2). It is possible that the loss of FXR activated other nuclear receptors or transcription factors in compensation that induced Ces and Aldh expression at neonatal ages. When the age got older, for example at days 25 and 60, both the total mRNA and the composition of different families of phase-I gene expression became similar in wild-type and Fxr-null mice.
Figure 2.
Percentages of FPKM values of each phase-I family at ages of days –2, 1, 5, 25, and 60 in both wild-type and Fxr-null mice. WT, wild-type and KO, Fxr-null.
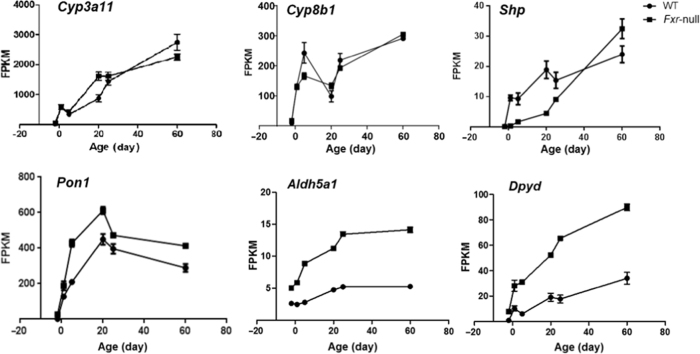
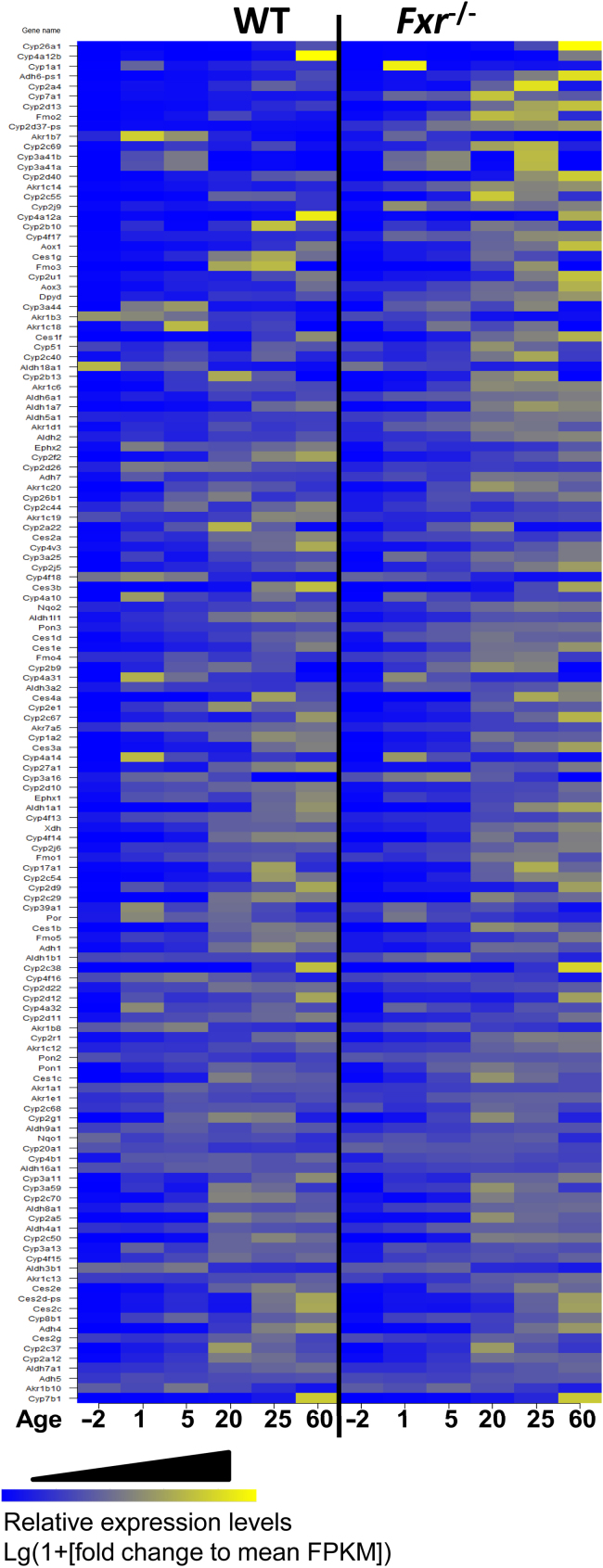
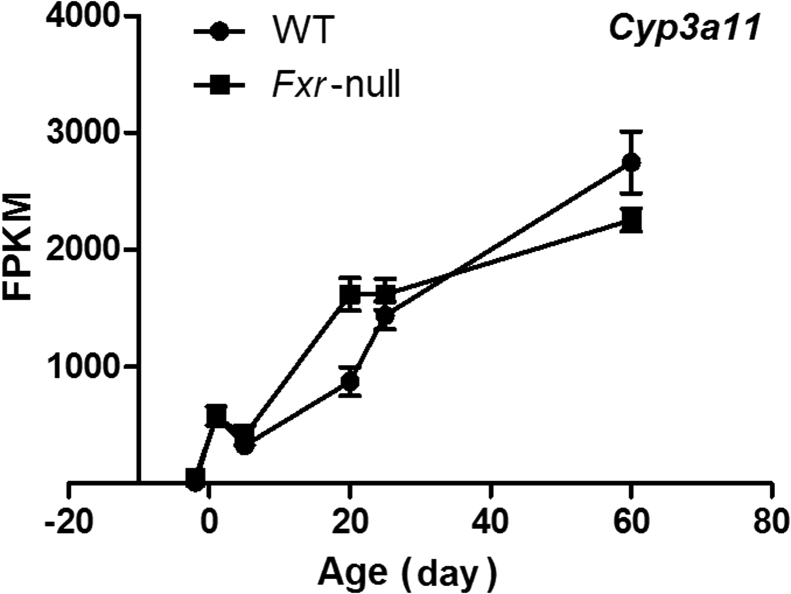
After examining the overall effect of Fxr knockout on phase-I gene expression during liver development, we took a further step to assess the ontogeny of individual phase-I genes. Based on UCSC Genes Track mm10, there were 185 phase-I genes in the 12 major families of phase-I genes, and our data showed 136 of them were expressed during liver maturation in wild-type (WT) and Fxr–/– mice (average FPKM>1). Differential expression between wild-type and knockout mice occurred in genes from all 12 phase-I gene families for at least one age during development, suggesting an extensive role of FXR in gene regulation. The distances of developmental pattern between WT and Fxr–/– samples were calculated and phase-I genes were listed in the order from large to small distance in Fig. 3. The genes at the upper part showed more difference in their developmental expression patterns caused by the knockout of Fxr, and the top 10 most altered genes were Cyp26a1, Cyp4a12b, Cyp1a1, Adh6-ps1, Cyp2a4, Cyp7a1, Cyp2d13, Fmo2, Cyp2d37-ps, and Akr1b7. Changes in expression of these genes showed developmental stage-specific effects, for example, Cyp26a1 and Cyp4a12b were only strongly induced or repressed at day 60, Cyp2a4 was induced at adolescent stage, and Akr1b7 was markedly repressed at neonatal stage in the knockout mice compared with wild-type. Cyp3a11 is one of the most important genes in drug metabolism. Research has shown that CYP3A11 expression was induced in the absence of FXR29. But in our results, induction of Cyp3a11 mRNA only happened at day 20 after birth, and Fxr–/– does not significantly alter CYP3A11 ontogenic trend (Fig. 4).
Figure 3.
Developmental expression patterns of phase-I genes in livers of wild-type and Fxr–/– mice. Heat map of expression profiles are drawn for all expressed phase-I genes (FPKM>1). The genes are listed in an order based on the distance of ontogenic pattern between WT and Fxr–/– samples, with the ones at the top showing patterns most altered in Fxr-knockout mice.
Figure 4.
Expression patterns of CYP3A11 during liver development. Data are expressed as mean FPKM and SEM of three individual animals.
3.2. Developmental expression of known FXR target phase-I genes
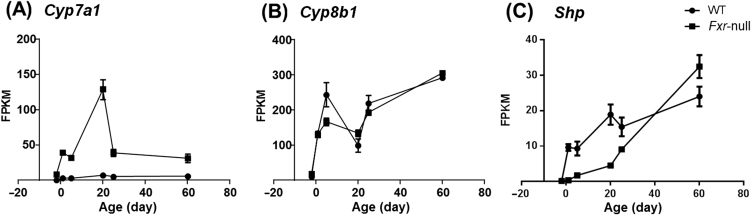
As we have found out widespread changes of gene expression in Fxr-null mice, especially in those hepatic nuclear receptors and core transcription factors, it would be difficult for us to conclude on the direct role of FXR in regulation of gene transcription. Therefore, we examined the developmental expression pattern of several known FXR target genes. CYP7A1 is the rate-limiting enzyme in the classic bile acid synthesis pathway, and CYP8B1 is required for synthesis of cholic acid. The known mechanisms of FXR in regulating CYP7A1 and CYP8B1 are as follows. The activation of FXR by bile acids in the liver induces nuclear receptor SHP (Nr0b2), which interacts with HNF4α and LRH-1 and blocks their activation of Cyp7a1 and Cyp8b1 transcription. On the other hand, activated FXR in the intestine can induce intestinal FGF-15 (FGF-19 in human), which circulates to liver and bind FGFR4 to activate JNK phosphorylation pathway and suppress Cyp7a1 and Cyp8b1 expression30. Our results demonstrated that Cyp7a1 mRNA level was significantly induced at all 6 ages in Fxr knockout mice, and the induction fold change was largest at day 20, indicating the repression of CYP7A1 by FXR was more critical at day 20 (Fig. 5A). Although it was proposed that CYP7A1 and CYP8B1 were under the same FXR regulatory pathway, Cyp8b1 mRNA level was not significantly altered in Fxr-null mice (Fig. 5B). So the regulation of FXR on Cyp8b1 gene was relatively weak, and there might be other critical regulatory factors that maintain the expression of CYP8B1 in the absence of FXR. The expression of SHP, the direct target gene of FXR in the liver pathway for regulation of CYP7A1 and CYP8B1, was only reduced at young ages in Fxr-null mice, also suggesting developmental stage-specific effect of FXR (Fig. 5C). And the result indicated that the induction of CYP7A1 at day 60 was mainly due to failed suppression from the FXR intestinal pathway. Taken together, these data provided new insights for the role of FXR in regulation of its target gene expression.
Figure 5.
Expression patterns of (A) Cyp7a1, (B) Cyp8b1 and (C) Shp during liver development. Data are expressed as mean FPKM and SEM of three individual animals.
3.3. Phase-I genes potentially targeted by FXR
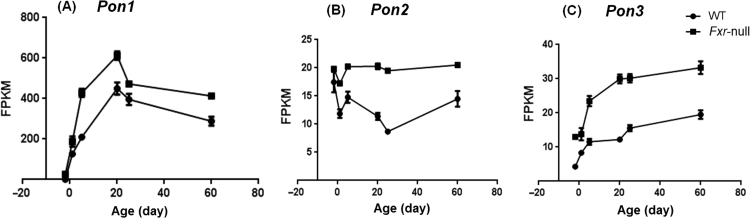
Our data also suggested new phase-I genes potentially targeted by FXR. Previous study has shown that bile acid and FGF19 treatment decreased PON1 mRNA level31. And in our Fxr-null mice, the Pon1 mRNA level was induced during development (Fig. 6A), which further supported the role of FXR in repression of PON1 expression, probably through intestinal FXR function. In addition, the induction of Pon2 and Pon3 mRNA in Fxr-null mice was also observed (Fig. 6B and C). Pon1/2/3 genes are located next to each other in the genome and it is highly likely that they are co-regulated by FXR.
Figure 6.
Expression patterns of (A) Pon1, (B) Pon2, and (C) Pon3 during liver development. Data are expressed as mean FPKM and SEM of three individual animals.
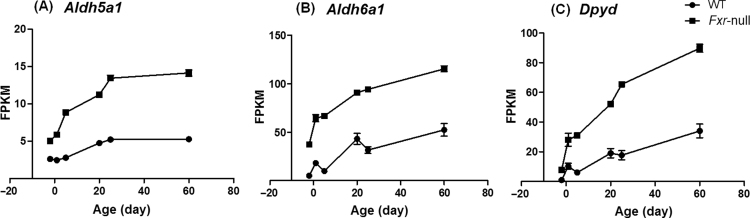
Besides Cyp7a1, only three other genes, Aldh5a1, Aldh6a1 and Dpyd, showed significant changes of expression at all 6 ages in Fxr-null mice (Fig. 7). ChIP-Seq study identified FXR binding on Aldh5a1 gene32. So the mRNA basal expression of these phase-I genes during development may be repressed by FXR. However, further study is needed to validate the direct involvement of FXR in control of the expression of these genes.
Figure 7.
Expression patterns of (A) Aldh5a1, (B) Aldh6a1, and (C) Dpyd during liver development. Data are expressed as mean FPKM and SEM of three individual animals.
4. Discussion
In this study, comparison of ontogeny of hepatic phase-I genes in wild-type and Fxr-knockout mice by RNA-Seq revealed important roles of FXR in regulating developmental expression of phase-I genes. The loss of FXR resulted in significant changes of phase-I gene expression from all 12 major families, and suggested new phase-I genes potentially targeted by FXR.
The baby mice take milk from their mother, which has a high fat content. Bile acids are necessary for the absorption of fat, and they are mainly synthesized and secreted at the immediate perinatal period to facilitate milk absorption. As the endogenous ligands for FXR, bile acids activated FXR signaling may be important for regulating perinatal gene expression in liver. Studies have shown that FXR functions to initiate the expression of major bile acid transporters in newborn mice33. FXR is critical for the “day 1 surge” pattern of Ntcp, Bsep, and Mdr2 in newborn mouse liver. And here in our results, FXR is also critical for the “day 1 surge” of total phase-I expression (Fig. 1). The total mRNA of P450s was expressed significantly lower at newborn ages (days 1 and 5) in Fxr-null mice.
For the phase-I gene expressions most altered by the loss of FXR, Cyp26a1 mRNA was highly induce at day 60. CYP26A1 functions in the catabolism of retinoic acid, and is normally induced by increased retinol or retinoic acid levels. It is possible that this gene induction is a result of altered metabolism in retinoic acid pathway. The second gene in the distance list, Cyp4a12, was markedly suppressed at day 60 (Fig. 3). Cyp4 gene family is known to be regulated by PPAR receptors, and FXR can influence PPAR expressions34. So this may also be an indirect effect of Fxr knockout. Many genes identified in this project may be indirectly regulated by FXR. With a complicated regulatory network, it is difficult for us to conclude on the role of FXR in some of the changes of expression patterns. ChIP-Seq technology using specific anti-FXR antibody is a powerful tool to identify genome-wide FXR binding sites35. Future studies combining the current data with ChIP-Seq experiments during liver development would provide important information to understand the regulatory mechanism of FXR on ontogeny of phase-I genes.
Quantifying the developmental expression is an initial step to study ontogeny of phase-I genes. It utilized advanced technology to generate an overall picture of gene expression, and revealed potential fields of interest that await further researches. For example, future studies to over-express FXR or treat the animals with FXR agonists during development may complement the loss-of-function study and help differentiate the direct and indirect effects of FXR in regulating development gene expression. Characterization of functional FXR polymorphisms in human and their roles in bile acid homeostasis will help to understand the important roles of FXR. Altogether, these studies would enable a more profound understanding of liver development and ontogeny of phase-I genes, and provide a foundation to assist researchers in understanding developmental susceptibility and improving the safety and effectiveness of pharmacotherapy in children.
Acknowledgments
This study was supported in the part by the U. S. National Institutes of Health National Institute for Environmental Health Sciences [Grant R01ES-019487 to Xiao-bo Zhong] and U. S. National Institutes of Health National Institute of General Medical Sciences [Grants R01GM-087376 and R01GM118367 to Xiao-bo Zhong].
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
References
- 1.Peng L., Cui J.Y., Yoo B., Gunewardena S.S., Lu H., Klaassen C.D. RNA-sequencing quantification of hepatic ontogeny of phase-I enzymes in mice. Drug Metab Dispos. 2013;41:2175–2186. doi: 10.1124/dmd.113.054635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peng L., Yoo B., Gunewardena S.S., Lu H., Klaassen C.D., Zhong X.B. RNA sequencing reveals dynamic changes of mRNA abundance of cytochromes P450 and their alternative transcripts during mouse liver development. Drug Metab Dispos. 2012;40:1198–1209. doi: 10.1124/dmd.112.045088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koukouritaki S.B., Manro J.R., Marsh S.A., Stevens J.C., Rettie A.E., McCarver D.G. Developmental expression of human hepatic CYP2C9 and CYP2C19. J Pharmacol Exp Ther. 2004;308:965–974. doi: 10.1124/jpet.103.060137. [DOI] [PubMed] [Google Scholar]
- 4.Stevens J.C., Hines R.N., Gu C., Koukouritaki S.B., Manro J.R., Tandler P.J. Developmental expression of the major human hepatic CYP3A enzymes. J Pharmacol Exp Ther. 2003;307:573–582. doi: 10.1124/jpet.103.054841. [DOI] [PubMed] [Google Scholar]
- 5.Stevens J.C., Marsh S.A., Zaya M.J., Regina K.J., Divakaran K., Le M. Developmental changes in human liver CYP2D6 expression. Drug Metab Dispos. 2008;36:1587–1593. doi: 10.1124/dmd.108.021873. [DOI] [PubMed] [Google Scholar]
- 6.Croom E.L., Stevens J.C., Hines R.N., Wallace A.D., Hodgson E. Human hepatic CYP2B6 developmental expression: the impact of age and genotype. Biochem Pharmacol. 2009;78:184–190. doi: 10.1016/j.bcp.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 7.Yang D., Pearce R.E., Wang X., Gaedigk R., Wan Y.J.Y., Yan B. Human carboxylesterases HCE1 and HCE2: ontogenic expression, inter-individual variability and differential hydrolysis of oseltamivir, aspirin, deltamethrin and permethrin. Biochem Pharmacol. 2009;77:238–247. doi: 10.1016/j.bcp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu H.J., Appel D.I., Jiang Y., Markowitz J.S. Age- and sex-related expression and activity of carboxylesterase 1 and 2 in mouse and human liver. Drug Metab Dispos. 2009;37:1819–1825. doi: 10.1124/dmd.109.028209. [DOI] [PubMed] [Google Scholar]
- 9.Cole T.B., Jampsa R.L., Walter B.J., Arndt T.L., Richter R.J., Shih D.M. Expression of human paraoxonase (PON1) during development. Pharmacogenetics. 2003;13:357–364. doi: 10.1097/00008571-200306000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Huen K., Harley K., Brooks J., Hubbard A., Bradman A., Eskenazi B. Developmental changes in PON1 enzyme activity in young children and effects of PON1 polymorphisms. Environ Health Perspect. 2009;117:1632–1638. doi: 10.1289/ehp.0900870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith M., Hopkinson D.A., Harris H. Developmental changes and polymorphism in human alcohol dehydrogenase. Ann Hum Genet. 1971;34:251–271. doi: 10.1111/j.1469-1809.1971.tb00238.x. [DOI] [PubMed] [Google Scholar]
- 12.Koukouritaki S.B., Simpson P., Yeung C.K., Rettie A.E., Hines R.N. Human hepatic flavin-containing monooxygenases 1 (FMO1) and 3 (FMO3) developmental expression. Pediatr Res. 2002;51:236–243. doi: 10.1203/00006450-200202000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Cherrington N.J., Cao Y., Cherrington J.W., Rose R.L., Hodgson E. Physiological factors affecting protein expression of flavin-containing monooxygenases 1, 3 and 5. Xenobiotica. 1998;28:673–682. doi: 10.1080/004982598239254. [DOI] [PubMed] [Google Scholar]
- 14.Hines R.N. Developmental and tissue-specific expression of human flavin-containing monooxygenases 1 and 3. Expert Opin Drug Metab Toxicol. 2006;2:41–49. doi: 10.1517/17425255.2.1.41. [DOI] [PubMed] [Google Scholar]
- 15.Kearns G.L., Abdel-Rahman S.M., Alander S.W., Blowey D.L., Leeder J.S., Kauffman R.E. Developmental pharmacology-drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 16.Hines R.N. Developmental expression of drug metabolizing enzymes: impact on disposition in neonates and young children. Int J Pharm. 2013;452:3–7. doi: 10.1016/j.ijpharm.2012.05.079. [DOI] [PubMed] [Google Scholar]
- 17.Hines R.N. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacol Ther. 2008;118:250–267. doi: 10.1016/j.pharmthera.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Blake M.J., Castro L., Leeder J.S., Kearns G.L. Ontogeny of drug metabolizing enzymes in the neonate. Semin Fetal Neonatal Med. 2005;10:123–138. doi: 10.1016/j.siny.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Hines R.N. Ontogeny of human hepatic cytochromes P450. J Biochem Mol Toxicol. 2007;21:169–175. doi: 10.1002/jbt.20179. [DOI] [PubMed] [Google Scholar]
- 20.Sinal C.J., Tohkin M., Miyata M., Ward J.M., Lambert G., Gonzalez F.J. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 21.Li G., Guo G.L. Farnesoid X receptor, the bile acid sensing nuclear receptor, in liver regeneration. Acta Pharm Sin B. 2015;5:93–98. doi: 10.1016/j.apsb.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding L., Yang L., Wang Z., Huang W. Bile acid nuclear receptor FXR and digestive system diseases. Acta Pharm Sin B. 2015;5:135–144. doi: 10.1016/j.apsb.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manley S., Ding W. Role of farnesoid X receptor and bile acids in alcoholic liver disease. Acta Pharm Sin B. 2015;5:158–167. doi: 10.1016/j.apsb.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kliewer S.A., Goodwin B., Willson T.M. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- 25.Jung D., Mangelsdorf D.J., Meyer U.A. Pregnane X receptor is a target of farnesoid X receptor. J Biol Chem. 2006;281:19081–19091. doi: 10.1074/jbc.M600116200. [DOI] [PubMed] [Google Scholar]
- 26.Marzolini C., Tirona R.G., Gervasini G., Poonkuzhali B., Assem M., Lee W. A common polymorphism in the bile acid receptor farnesoid X receptor is associated with decreased hepatic target gene expression. Mol Endocrinol. 2007;21:1769–1780. doi: 10.1210/me.2007-0025. [DOI] [PubMed] [Google Scholar]
- 27.Kovacs P., Kress R., Rocha J., Kurtz U., Miquel J.F., Nervi F. Variation of the gene encoding the nuclear bile salt receptor FXR and gallstone susceptibility in mice and humans. J Hepatol. 2008;48:116–124. doi: 10.1016/j.jhep.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 28.Lambert G., Amar M.J., Guo G., Brewer H.B., Jr, Gonzalez F.J., Sinal C.J. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 2003;278:2563–2570. doi: 10.1074/jbc.M209525200. [DOI] [PubMed] [Google Scholar]
- 29.Schuetz E.G., Strom S., Yasuda K., Lecureur V., Assem M., Brimer C. Disrupted bile acid homeostasis reveals an unexpected interaction among nuclear hormone receptors, transporters, and cytochrome P450. J Biol Chem. 2001;276:39411–39418. doi: 10.1074/jbc.M106340200. [DOI] [PubMed] [Google Scholar]
- 30.Claudel T., Staels B., Kuipers F. The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005;25:2020–2030. doi: 10.1161/01.ATV.0000178994.21828.a7. [DOI] [PubMed] [Google Scholar]
- 31.Shih D.M., Kast-Woelbern H.R., Wong J., Xia Y.R., Edwards P.A., Lusis A.J. A role for FXR and human FGF-19 in the repression of paraoxonase-1 gene expression by bile acids. J Lipid Res. 2006;47:384–392. doi: 10.1194/jlr.M500378-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Lee J., Seok S., Yu P., Kim K., Smith Z., Rivas-Astroza M. Genomic analysis of hepatic farnesoid X receptor binding sites reveals altered binding in obesity and direct gene repression by farnesoid X receptor in mice. Hepatology. 2012;56:108–117. doi: 10.1002/hep.25609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui J.Y., Aleksunes L.M., Tanaka Y., Fu Z.D., Guo Y., Guo G.L. Bile acids via FXR initiate the expression of major transporters involved in the enterohepatic circulation of bile acids in newborn mice. Am J Physiol Gastrointest Liver Physiol. 2012;302:G979–G996. doi: 10.1152/ajpgi.00370.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Renga B., Mencarelli A., Migliorati M., Cipriani S., D’Amore C., Distrutti E. SHP-dependent and -independent induction of peroxisome proliferator-activated receptor-γ by the bile acid sensor farnesoid X receptor counter-regulates the pro-inflammatory phenotype of liver myofibroblasts. Inflamm Res. 2011;60:577–587. doi: 10.1007/s00011-010-0306-1. [DOI] [PubMed] [Google Scholar]
- 35.Thomas A.M., Hart S.N., Kong B., Fang J., Zhong X.B., Guo G.L. Genome-wide tissue-specific farnesoid X receptor binding in mouse liver and intestine. Hepatology. 2010;51:1410–1419. doi: 10.1002/hep.23450. [DOI] [PMC free article] [PubMed] [Google Scholar]