Abstract
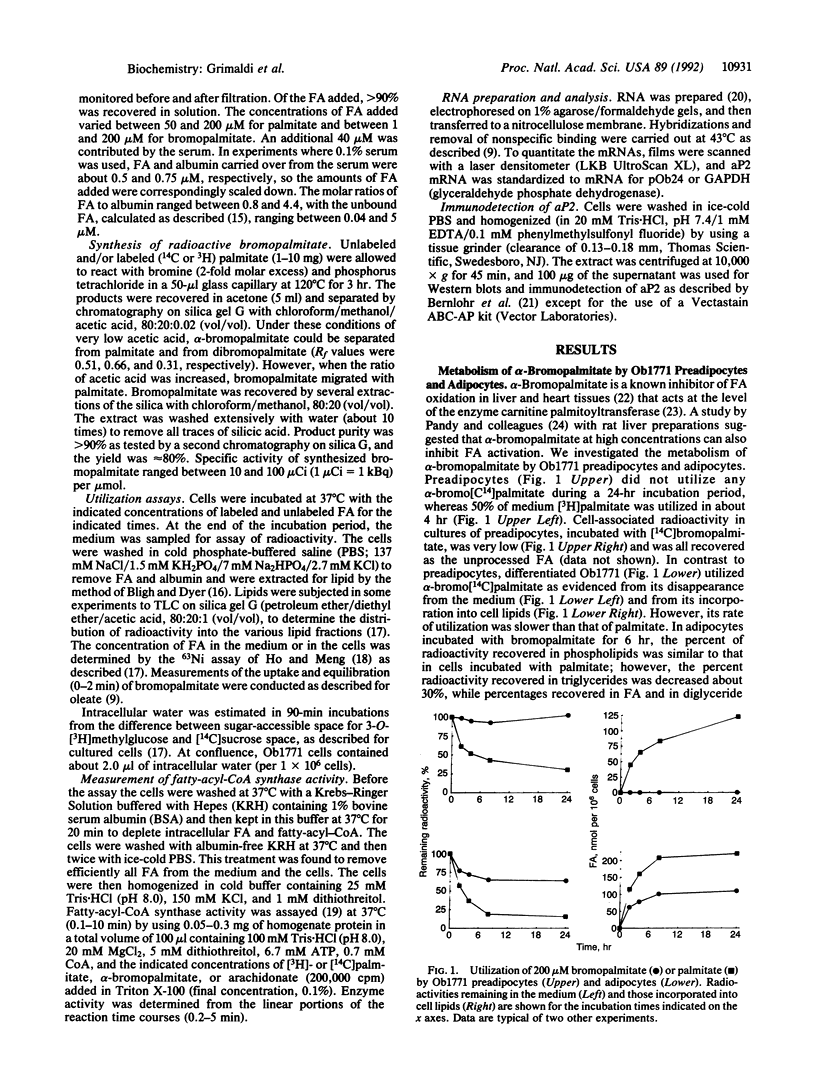
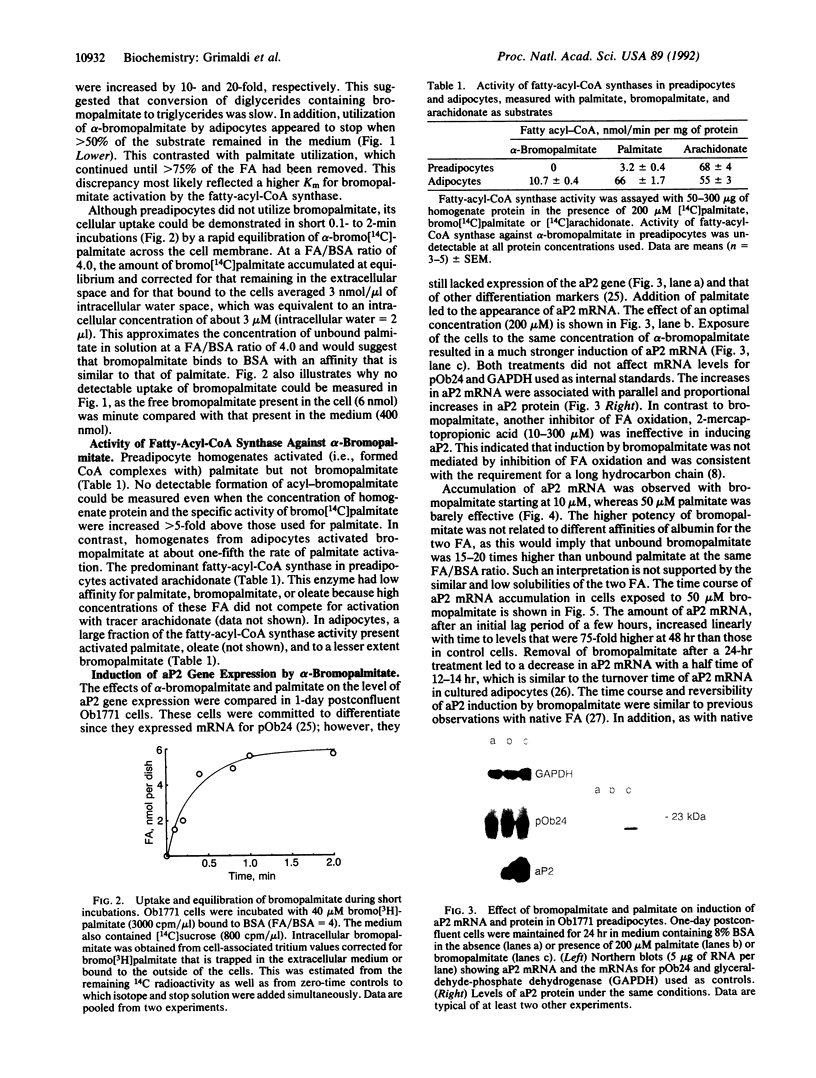
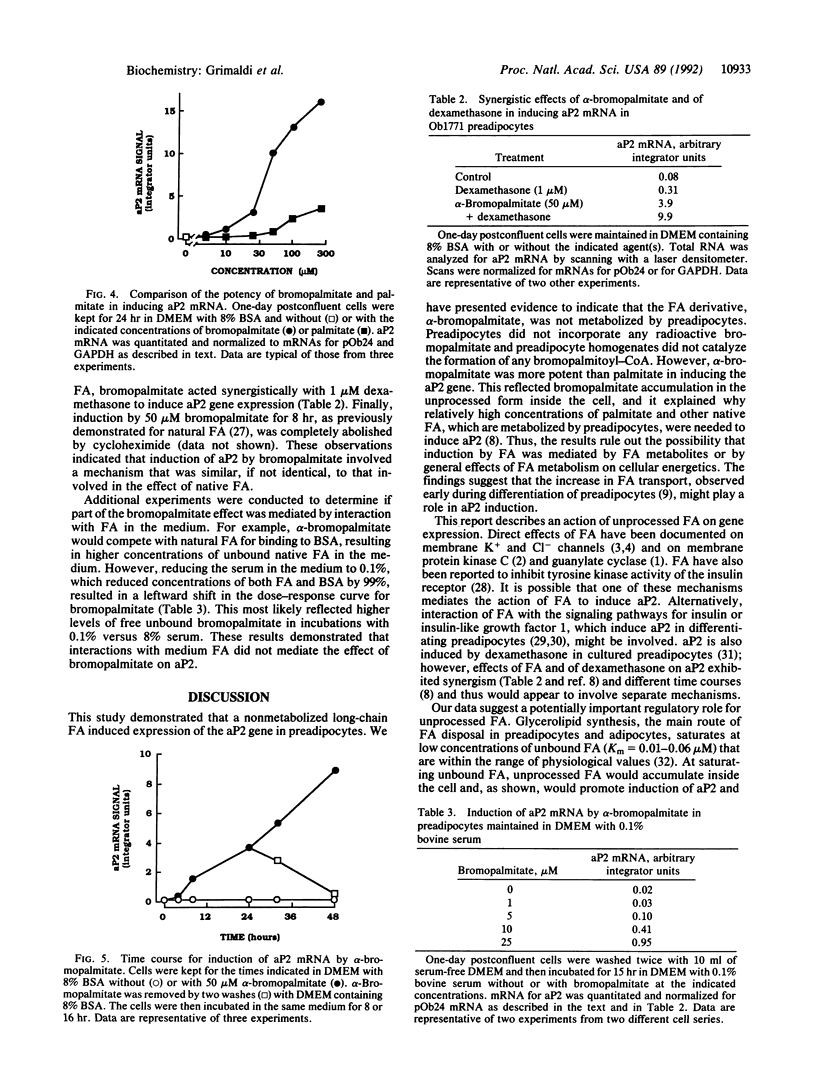
Long-chain fatty acids (FA) have been shown to regulate expression of the gene for the adipocyte FA-binding protein aP2. We examined whether this effect was exerted by FA themselves or by a FA metabolite. The alpha-bromo derivative of palmitate, an inhibitor of FA oxidation, was synthesized in the radioactive form, and its metabolism was investigated and correlated with its ability to induce aP2 in Ob1771 preadipocytes. alpha-Bromopalmitate was not utilized by preadipocytes. It was not cleared from the medium over a 24-hr period and was not incorporated into cellular lipids. Short incubations indicated that alpha-bromopalmitate exchanged across the preadipocyte membrane but remained in the free form inside the cell. In line with this, preadipocyte homogenates did not activate alpha-bromopalmitate to the acyl form. However, although it was not metabolized, bromopalmitate was much more potent than native FA in inducing aP2 gene expression. Induction exhibited the characteristics previously described for native FA, indicating that a similar if not identical mechanism was involved. The data indicated that induction of aP2 was exerted by unprocessed FA. Finally, in contrast to preadipocytes, adipocytes metabolized bromopalmitate. This reflected increased activity with cell differentiation of a palmitoyl-CoA synthase that could activate palmitate and bromopalmitate at about one-fifth the rate for palmitate. In preadipocytes, the predominant fatty-acyl-CoA synthase, arachidonyl-CoA synthase, had very low affinity for both FA. Increased activity of the palmitoyl-CoA synthase, which has a wider substrate range, is likely to be important for initiation of lipid deposition.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. A., Forest C. C., Regen D. M., Sanders S. Increase in membrane uptake of long-chain fatty acids early during preadipocyte differentiation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6008–6012. doi: 10.1073/pnas.88.14.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abumrad N. A., Forest C., Regen D. M., Barnella U. S., Melki S. A. Metabolism of oleic acid in differentiating BFC-1 preadipose cells. Am J Physiol. 1991 Jul;261(1 Pt 1):E76–E86. doi: 10.1152/ajpendo.1991.261.1.E76. [DOI] [PubMed] [Google Scholar]
- Abumrad N. A., Perkins R. C., Park J. H., Park C. R. Mechanism of long chain fatty acid permeation in the isolated adipocyte. J Biol Chem. 1981 Sep 10;256(17):9183–9191. [PubMed] [Google Scholar]
- Amri E. Z., Ailhaud G., Grimaldi P. Regulation of adipose cell differentiation. II. Kinetics of induction of the aP2 gene by fatty acids and modulation by dexamethasone. J Lipid Res. 1991 Sep;32(9):1457–1463. [PubMed] [Google Scholar]
- Amri E. Z., Bertrand B., Ailhaud G., Grimaldi P. Regulation of adipose cell differentiation. I. Fatty acids are inducers of the aP2 gene expression. J Lipid Res. 1991 Sep;32(9):1449–1456. [PubMed] [Google Scholar]
- Anderson M. P., Welsh M. J. Fatty acids inhibit apical membrane chloride channels in airway epithelia. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7334–7338. doi: 10.1073/pnas.87.18.7334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bernier M., Laird D. M., Lane M. D. Insulin-activated tyrosine phosphorylation of a 15-kilodalton protein in intact 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1844–1848. doi: 10.1073/pnas.84.7.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr D. A., Doering T. L., Kelly T. J., Jr, Lane M. D. Tissue specific expression of p422 protein, a putative lipid carrier, in mouse adipocytes. Biochem Biophys Res Commun. 1985 Oct 30;132(2):850–855. doi: 10.1016/0006-291x(85)91209-4. [DOI] [PubMed] [Google Scholar]
- Blake W. L., Clarke S. D. Induction of adipose fatty acid binding protein (a-FABP) by insulin-like growth factor-1 (IGF-1) in 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 1990 Nov 30;173(1):87–91. doi: 10.1016/s0006-291x(05)81025-3. [DOI] [PubMed] [Google Scholar]
- Bloch K., Vance D. Control mechanisms in the synthesis of saturated fatty acids. Annu Rev Biochem. 1977;46:263–298. doi: 10.1146/annurev.bi.46.070177.001403. [DOI] [PubMed] [Google Scholar]
- Braughler J. M., Mittal C. K., Murad F. Purification of soluble guanylate cyclase from rat liver. Proc Natl Acad Sci U S A. 1979 Jan;76(1):219–222. doi: 10.1073/pnas.76.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cook J. S., Lucas J. J., Sibley E., Bolanowski M. A., Christy R. J., Kelly T. J., Lane M. D. Expression of the differentiation-induced gene for fatty acid-binding protein is activated by glucocorticoid and cAMP. Proc Natl Acad Sci U S A. 1988 May;85(9):2949–2953. doi: 10.1073/pnas.85.9.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook K. S., Hunt C. R., Spiegelman B. M. Developmentally regulated mRNAs in 3T3-adipocytes: analysis of transcriptional control. J Cell Biol. 1985 Feb;100(2):514–520. doi: 10.1083/jcb.100.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani C., Doglio A., Amri E. Z., Bardon S., Fort P., Bertrand B., Grimaldi P., Ailhaud G. Cloning and regulation of a mRNA specifically expressed in the preadipose state. J Biol Chem. 1989 Jun 15;264(17):10119–10125. [PubMed] [Google Scholar]
- Ho R. J., Meng H. C. A simple and ultrasensitive method for determination of free fatty acid by radiochemical assay. Anal Biochem. 1969 Oct 1;31(1):426–436. doi: 10.1016/0003-2697(69)90284-x. [DOI] [PubMed] [Google Scholar]
- Kim D., Clapham D. E. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 1989 Jun 9;244(4909):1174–1176. doi: 10.1126/science.2727703. [DOI] [PubMed] [Google Scholar]
- Lane M. D., Flores-Riveros J. R., Hresko R. C., Kaestner K. H., Liao K., Janicot M., Hoffman R. D., McLenithan J. C., Kastelic T., Christy R. J. Insulin-receptor tyrosine kinase and glucose transport. Diabetes Care. 1990 Jun;13(6):565–575. doi: 10.2337/diacare.13.6.565. [DOI] [PubMed] [Google Scholar]
- Laposata M., Reich E. L., Majerus P. W. Arachidonoyl-CoA synthetase. Separation from nonspecific acyl-CoA synthetase and distribution in various cells and tissues. J Biol Chem. 1985 Sep 15;260(20):11016–11020. [PubMed] [Google Scholar]
- Melki S. A., Abumrad N. A. Glycerolipid synthesis in isolated adipocytes: substrate dependence and influence of norepinephrine. J Lipid Res. 1992 May;33(5):669–678. [PubMed] [Google Scholar]
- Murakami K., Chan S. Y., Routtenberg A. Protein kinase C activation by cis-fatty acid in the absence of Ca2+ and phospholipids. J Biol Chem. 1986 Nov 25;261(33):15424–15429. [PubMed] [Google Scholar]
- Ntambi J. M., Buhrow S. A., Kaestner K. H., Christy R. J., Sibley E., Kelly T. J., Jr, Lane M. D. Differentiation-induced gene expression in 3T3-L1 preadipocytes. Characterization of a differentially expressed gene encoding stearoyl-CoA desaturase. J Biol Chem. 1988 Nov 25;263(33):17291–17300. [PubMed] [Google Scholar]
- Négrel R., Grimaldi P., Ailhaud G. Establishment of preadipocyte clonal line from epididymal fat pad of ob/ob mouse that responds to insulin and to lipolytic hormones. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6054–6058. doi: 10.1073/pnas.75.12.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordway R. W., Singer J. J., Walsh J. V., Jr Direct regulation of ion channels by fatty acids. Trends Neurosci. 1991 Mar;14(3):96–100. doi: 10.1016/0166-2236(91)90069-7. [DOI] [PubMed] [Google Scholar]
- Ordway R. W., Walsh J. V., Jr, Singer J. J. Arachidonic acid and other fatty acids directly activate potassium channels in smooth muscle cells. Science. 1989 Jun 9;244(4909):1176–1179. doi: 10.1126/science.2471269. [DOI] [PubMed] [Google Scholar]
- Pande S. V., Siddiqui A. W., Gattereau A. Inhibition of long-chain fatty acid activation by -bromopalmitate and phytanate. Biochim Biophys Acta. 1971 Nov 5;248(2):156–166. doi: 10.1016/0005-2760(71)90002-6. [DOI] [PubMed] [Google Scholar]
- Samuel D., Ailhaud G. Comparative aspects of fatty acid activation in Escherichia coli and Clostridium butyricum. FEBS Lett. 1969 Feb;2(4):213–216. doi: 10.1016/0014-5793(69)80022-0. [DOI] [PubMed] [Google Scholar]
- Smith P. J., Wise L. S., Berkowitz R., Wan C., Rubin C. S. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J Biol Chem. 1988 Jul 5;263(19):9402–9408. [PubMed] [Google Scholar]
- Spiegelman B. M., Frank M., Green H. Molecular cloning of mRNA from 3T3 adipocytes. Regulation of mRNA content for glycerophosphate dehydrogenase and other differentiation-dependent proteins during adipocyte development. J Biol Chem. 1983 Aug 25;258(16):10083–10089. [PubMed] [Google Scholar]
- Storch J., Shulman S. L., Kleinfeld A. M. Plasma membrane lipid order and composition during adipocyte differentiation of 3T3F442A cells. Studies in intact cells with 1-[4-(trimethylamino)phenyl]-6-phenylhexatriene. J Biol Chem. 1989 Jun 25;264(18):10527–10533. [PubMed] [Google Scholar]
- Waggoner D. W., Bernlohr D. A. In situ labeling of the adipocyte lipid binding protein with 3-[125I]iodo-4-azido-N-hexadecylsalicylamide. Evidence for a role of fatty acid binding proteins in lipid uptake. J Biol Chem. 1990 Jul 15;265(20):11417–11420. [PubMed] [Google Scholar]
- Wilson D. B., Prescott S. M., Majerus P. W. Discovery of an arachidonoyl coenzyme A synthetase in human platelets. J Biol Chem. 1982 Apr 10;257(7):3510–3515. [PubMed] [Google Scholar]




