Abstract
BACKGROUND
Gastrointestinal stromal tumors (GISTs) are potentially malignant tumors; however their behavior and response to treatment is dependent on the type of mutation and immunohistochemical expression of antigens. It is recommended to perform routine molecular and immunohistochemical tests in KIT and platelet derived growth factor receptor alpha (PDGFRA) molecules for making decision regarding targeted therapy and prediction of the behavior of the tumor.
Objectives: There has been no study from Iran regarding the PDGFRA mutational analysis in GISTs. In this study, for the first time from Iran, we performed immunohistochemical and molecular analysis of PDGFRA molecule on GISTs.
METHODS
In a cross-sectional study during 7 years (2008-2014) on 50 untreated non-recurrent non-metastatic newly diagnosed GISTs, molecular analysis and immunohistochemical staining for PDGFRA were performed and findings were compared with different clinicopathological characteristics..
RESULTS
During the 7 years, 50 cases of GISTs according to the above mentioned criteria were found. 17 cases were negative for KIT mutation. Of them, 15 (30%) were positive for either exon 12 or 18 mutation of PDGFRA. These cases showed more epithelioid morphology and the number of mitotic figures were lower than PDFRA negative GISTs. Also according to the criteria for risk assessment, it seems that PDGFRA mutant GISTs are rarely in the high risk category.
CONCLUSION
PDGFRA mutant GISTs are common in Iranian population and it is recommended to perform mutation analysis for PDGFRA in every GIST with wild type KIT and epithelioid morphology.
Keywords: GIST, PDGFRA, Stromal Tumors, Iran
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) represent less than 1% of all malignancies; however they are the most common mesenchymal tumors of the gastrointestinal (GI) tract with the reported incidence of approximately 15 cases per million of population per year and a prevalence of approximately 129 cases per million.1 GISTs are believed to be originated from interstitial cells of Cajal or their precursors. Mutations in KIT or platelet derived growth factor receptor alpha (PDGFRA) receptor tyrosine kinase proteins observed in more than 80% of GISTs are central in the pathogenesis of GISTs.2
In the previous report we reported the frequency and types of KIT mutations.3 In this study, we will report (for the first time in Iranian patients) our experience with PDGFRA mutation analysis by molecular and immunohistochemical (IHC) methods in 50 GISTs from different parts of the GI tract.
MATERIAL AND METHODS
During 7 years (2008-2014), we selected 50 cases of confirmed GISTs from the archives of the pathology department of the affiliated hospitals. We excluded all the treated or recurrent cases so all the selected cases were newly diagnosed GISTs. Hematoxylin and Eosin (H&E) stained slides were reviewed and all of the histological characteristics including mitosis, atypia, and presence or absence of necrosis were recorded. The gross findings (especially size) were retrieved from the pathology reports.
Of these cases, there were 17 cases with wild type KIT. IHC for PDGFRA (SANTA CRUZ, dilution of 1/200) was performed on the best selected slide (by the pathologist), according to the routine protocol. Then the slides were evaluated and the cases with less than 10% staining were considered as negative.
For all the KIT negative cases (17 tumors), tissue from paraffin blocks was isolated and after DNA extraction, PCR amplification and then DNA sequencing, and mutation analysis was performed. Tumor DNA was extracted from the paraffin embedded formalin fixed GISTs according to the manufacturer’s instruction (QIAmp DNA FFPE tissue kit). Molecular analysis of PDGFRA mutation was performed by PCR and Sanger DNA sequencing very similar to the method previously described by Hostein and colleagues.3-5 The sequence of primers are listed in table 1.
Table 1 . Primers which have been used in this study (5’ to 3’) .
| Exon 12 | F TCCAGTCACTGTGCTGCTTC |
| Exon 12R | GCAAGGGAAAAGGGAGTCTT |
| Exon 18 | F ACCATGGATCAGCCAGTCTT |
| Exon 18R | TGA AGG AGG ATG AGC CTG ACC |
RESULTS:
The present study was conducted on 50 cases of GISTs. We had previously worked on them in regard of KIT mutation, which has already been published.3 29 patients were men and 21 patients were women, aged 58.7±14 years.
Table 2 shows the characteristics of the GISTs according to the site. 33 tumors originated from the stomach, four from the terminal ileum, two from the jejunum, two from the duodenum, one from the ascending colon, one from the rectum, one from the retroperitoneal area, and six tumors were multicentric from the intra-abdominal cavity.
Table 2 . Clinicopathological characteristics of the GISTs according to the site.
| Location | Number |
Size
(cm) |
Mitotic Rates
(/50HPF) |
IHC
(c-KIT) |
IHC
(PDFRA) |
Mutation in exon 9(KIT) | Mutation in exon 11(KIT) | Mutation in exon 12 (PDGFRA) | Mutation in exon 18 (PDGFRA) |
| Stomach | 33 | 1-20 | 1-50 | 32(94%) | 19(59.3%) | 5(15.2%) | 21(63.6%) | 8(32%) | 3(9.1%) |
| Duodenum | 2 | 5,18 | 0-8 | 2(100%) | 1(50%) | 1(50%) | 2(100%) | 0 | 0 |
| Jejunum | 2 | 3,7 | 1-2 | 2(100%) | 1(50%) | 0 | 0 | 2(100%) | 0 |
| Ileum | 4 | 2-8.5 | 0-10 | 4(100%) | 2(50%) | 0 | 1(50%) | 3 (75%) | 2(50%) |
| Ascending colon | 1 | 15 | 20 | 1(100%) | 0 | 0 | 0 | 1(100%) | 0 |
| Rectum | 1 | 1 | 11 | 1(100%) | 0 | 0 | 0 | 1(100%) | 0 |
| Intra-abdominal and retroperitoneal | 7 | 11-30 | 0-50 | 7(100%) | 6 (85.7%) | 5(71.4%) | 5 (71.4%) | 0 | 0 |
| Total | 50 | 1-30 | 1-50 | 49(98%) | 29 (56%) | 11 (22%) | 29 (58%) | 15 (28%) | 5 (10%) |
Macroscopic evaluation of the tumors showed 22 tumors larger than 10 cm, 13 tumors between 5 and 10 cm, 10 tumors were smaller than 5 cm, and only four tumors were smaller than 2 cm.
In microscopic evaluation, spindle cell morphology was detected in 29 cases (58%), seven (14%) cases showed pure epithelioid morphology, and 14 (28%) cases had mixed pattern (mixed epithelioid and spindle cell). Mitotic rate was less than 5/50 HPF in 31 cases while the rate was over 5/50 HPF for 19 cases. Regarding the most recent protocol of risk assessment in GISTs6, 20 cases were marked as high risk tumors while 13 were of moderate risk, and seven cases showed low risk. Out of the 50 cases, six tumors were marked as very low risk while only four tumors showed no risk.
In this study, stomach was the most common site for the tumors with 33 cases accounting for 66 % of all. Abdominal and retroperitoneal areas were together the second predominant location with seven (14%) cases.
IHC for PDGFRA was positive in 29 cases (figure 1). All the cases with negative IHC for PDGFRA, were reactive with c-KIT. 15 cases out of the 17 GISTs with wild type KIT, were positive for PDGFRA mutation. The mutation of exon 12 was detected in 15 cases (30%) and 5 (10%) cases were reported to have mutation of exon 18. In five (10%) cases both exon 12 and exon 18 mutations were detected at the same time.
Fig.1 .

Immunohistochemistry of PDGFRA
Of the PDFRA mutant cases, 11 tumors showed either epithelioid or mixed epithelioid and spindle cell morphology. It means that 66.7% of the PDGFRA mutant cases showed areas with epithelioid morphology.
Overall, of the 15 cases with PDGFRA mutations, six (40%) were women and nine (60%) were men.
Regarding the risk of malignancy6, in the 15 PDGFRA mutant GISTs, only two had very low risk while three had low risk. On the contrary four cases were high risk tumors and six cases showed moderate risk. Mitotic rate of less than 5/50 HPF was detected in 10 tumors, while five tumors had over 5/50 HPF mitotic rate.
The exon 12 mutated GISTs: (figure 2)
Fig .2 .
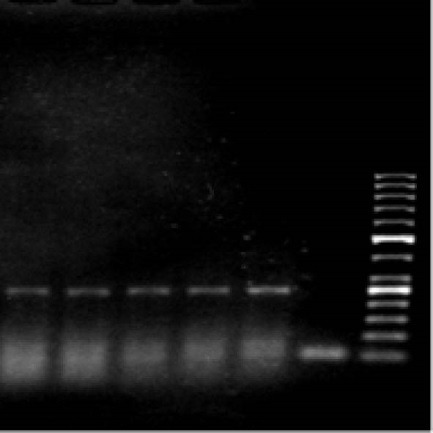
PCR from Exon 12 left to right: Sample 1, Sample 2, Sample 3, Sample 4, Sample 5, negative control, and 50 bps ladder.
Overall, six patients were women and nine individuals were men with the mean age of 60±10 years.
Spindle cell morphology was detected in five cases while seven individuals had tumors with mixed morphological pattern, and three had epithelioid morphology. Mitosis rate of less than 5/50 HPF was detected in 10 cases, while in the remaining five individuals the rate was over 5/50 HPF.
Eight tumors were in stomach, three in ileum, and two in jejunum while only two cases were originated from colon and rectum.
Six individuals had tumors larger than 10 cm, three patients had tumors between 5 and 10 cm, and six cases were less than 3 cm.
Four of the exon 12 mutated GISTs had high risk while those with moderate risk were six cases and five cases were marked as having low and very low risk.
The exon 18 mutated GISTs (figure 3)
Fig.3 .
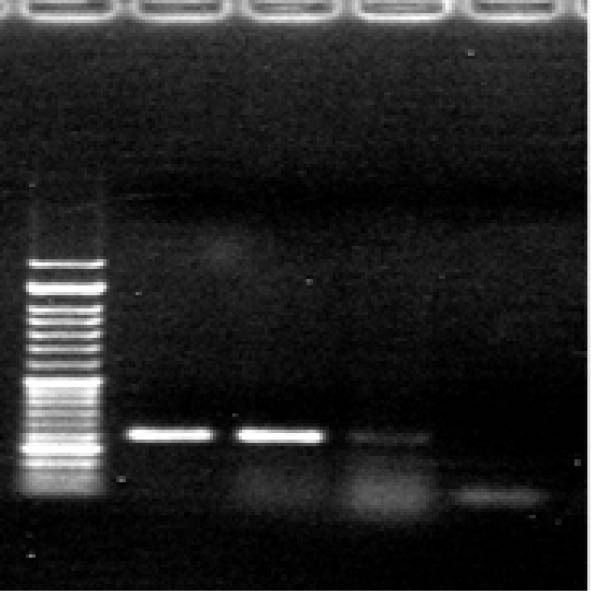
Exon 18 left to right: 50 bps ladder, Sample 1, Sample 2, Sample 3 and negative control.
Overall, one case was female patient and four individuals were male, aged 64±8 years.
Mixed patterns were detected in four cases while only one individual had tumor with spindle cell morphology. Mitosis rate less than 5/50 HPF was evident for four cases while in one case it was over 5/50 HPF.
Three of the tumors were detected in stomach and two in ileum. IHC for PDGFRA was positive in all exon 18 mutant GISTs.
Considering the size of the tumor, three individuals had tumors larger than 10 cm while two patients had tumors within 5 and10 cm.
One tumor of the exon 18 mutated GISTs had high risk while those with moderate risk accounted for three cases and a case was marked as having low risk.
Mutation types
Exon 12: All exon 12 mutated GISTs had nucleotide substitution of G instead of A that leads to P567P. All cases had only one mutation. (figure 4)
Fig.4 .
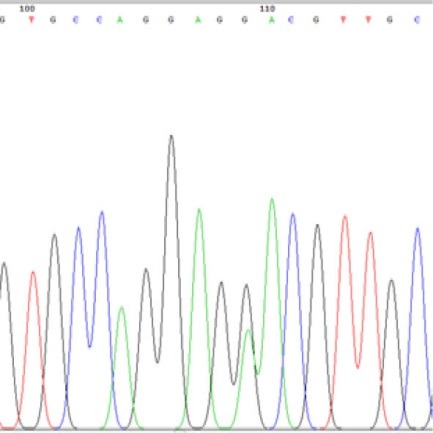
DNA sequencing of one of the GISTs, mutant for PDGFR
In this study, stomach was the most common site for the tumors with 33 cases accounting for 66 % of all. Abdominal and retroperitoneal areas were together the second predominant location with seven (14%) cases.
Exon 18: Four individuals had nucleotide substitution of T>A, T>C, A>T, and C>T while in one patient 12 nucleotides (CTGTGACTTTGG) deletion was detected. Two patients had more than single mutations. The following were different types of point mutations of the exon 18 detected in our study: A820AA, I831II, I834IF, V815VD, D818DE, A821AA, V824VV and D842DV.
DISCUSSION
For the first time, GIST has been reported by Hirotaet and colleagues in 1998.7-9 Now after more than 17 years, GISTs are well documented and well known specially KIT expressing tumors of the GI tract, which are characterized by mutations in either KIT or PDGFRA in more than 80–85% of cases.6
First reports of GIST from Iran have been from our center.3,10,11 Since then, many types of KIT and PDGFRA mutations have been described in GISTs. PDGFRA-mutated GISTs have typically been reported to be characterized by a low mitotic count and low malignant potential, and in most cases may represent clinically benign tumors.7
In our previous report, we performed IHC and molecular study accompanied by DNA sequencing for KIT in GIST, and it turned out that in our population about 34% of the cases are negative for KIT mutation in exon 9 and 11.3 We performed IHC and molecular studies to find the frequency of PDGFRA mutation in GISTs from our center. IHC for PDGFRA was positive in 29 cases of GISTs, the frequency of which is very similar to previous studies.12-16 Therefore according to our experience it seems that PDGFRA IHC should be just done on c-KIT negative cases, because all of the PDGFRA IHC negative cases have been c-KIT positive and there has been one case with negative c-KIT, which showed reactive PDGFRA by IHC. The results of previous reports have been much the same, with no definite recommendation for performing this test.15,16
In all of the cases with positive IHC for PDGFRA, c-kit and DOG-1(Discovered on GIST-1) were positive, however just one case with spindle cell morphology, showed negative c-kit, with positive DOG-1 and it was also mutant for exon-11, however IHC for PDGFRA was also negative. According to the size (3cm) and low mitosis (2/50HPF), this case was classified as a very low risk of malignancy. This case showed no PDGFRA mutation by molecular studies.
According to some of the previous studies, PDGFRA mutant cases frequently show epithelioid morphology.12,13 In our experience, of the 21 GISTs with pure epithelioid or mixed pattern, 10 cases have been PDGFRA mutant. On the other hand among 15 PDGFRA mutant GISTs, 11 cases showed at least some areas of epithelioid morphology.
It seems that PDFRA mutant GISTs have lower mitotic figures and lower risk of malignancy.13 In our patients mitotic figures were significantly lower in PDGFRA mutant GISTs, in comparison with wild type cases (table 3). However there has been no significant difference in tumor size. Overall risk assessment showed that of the 20 cases in high risk group, only four showed PDGFRA mutation. This finding is similar to the findings by Miettinen and co-workers.6 Incidence of PDGFRA mutation that have been reported from different parts of the world i.e. Korea 12, China 14, USA 15, Peru 16, India 17, Slovenia 18, Panama 19, and Czech republic 20 have been from 5.7% to 25%, which is very versatile. Also the types of mutations in PDGFRA are very different in various reports, which can be either ethnical or dependent on the method of analysis.14 More multicentric studies are necessary to find the best method of PDGFRA analysis along with the true incidence and type of mutation in these rare tumors.15
Table 3 . Difference of characteristics between PDGFRA mutant and wild type GISTs.
| Characteristics | PDGFR mutant cases | PDGFRA wild type cases | |
| Number | 15 (30%) | 35 (70%) | |
| Stomach | 8 | 25 | |
| Small intestine | 5 | 3 | |
| Large intestine | 2 | 0 | |
| Intra-abdominal and retroperitoneal | 0 | 7 | |
| Morphology | Spindle | 4 | 25 |
| Epithelioid | 3 | 4 | |
| Mixed | 7 | 7 | |
| Mitotic numbers/50HPF | 0-20 (4.8±5.4) | 0-50 (8.9±12) | |
| Size | 1-30 (8.9±7.68) | 1-30 (10.8±7.6) |
According to our results, PDGFRA mutation is common in GISTs of Iranian population. In the labs that are working on GISTs, PDGFRA mutation analysis should be part of routine examination especially in KIT negative GISTs. PDGFRA mutants GISTs have lower mitosis, and lower risk of malignancy. GISTs are not very common therefore more cases with multicentre studies and long follow-up are recommended for more definite results.
CONFLICT OF INTEREST
The author declares no conflict of interest related to this work.
Please cite this paper as:
Geramizadeh B, Jowkar Z, Mousavi SJ. Molecular and Immunohistochemical Study of Platelet Derived Growth Factor Receptor Alpha in KIT Negative Gastrointestinal Stromal Tumors; the First Report from Iran. Middle East J Dig Dis 2016;8:226-231. DOI: 10.15171/mejdd.2016.25
References
- 1.Palmirotta R, De Marchis ML, Ludovic G, Leone B, Covello R, Conti S. et al. Mutational analysis of gastrointestinal stromal tumors (GISTs): Procedural approach for diagnostic purposes. Cancer Genomics Proteomics. 2013;10:115–24. [PubMed] [Google Scholar]
- 2.Miettinen M, Lasota J. Gastrointestinal Stromal Tumors Review on Morphology, Molecular Pathology, Prognosis, and Differential Diagnosis. Arch Pathol Lab Med. 2006;130:1466–78. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 3.Geramizadeh B, Joekar Z, Ranjbar Z. Frequency of KIT Mutation in Gastrointestinal Stromal Tumors According to Histologic and Immunohistochemical Findings, the First Report from Iran. Iran J Med Sci. 2015;40:316–21. [PMC free article] [PubMed] [Google Scholar]
- 4.Merkelbach-Bruse S, Dietmaier W, Fuzesia L, Gaumann A, Hallera F, Kitz J. et al. Pitfalls in mutational testing and reporting of common KIT and PDGFRA mutations in gastrointestinal stromal tumors. BMC Med Genet. 2010;11:106–20. doi: 10.1186/1471-2350-11-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hostein I, Debiec-Rychter M, Olschwang S, Bringuier PP, Toffolati L, Gonzalez D. et al. A quality control program for mutation detection in KIT and PDGFRA in gastrointestinal stromal tumours. J Gastroenterol. 2011;46:586–94. doi: 10.1007/s00535-011-0375-0. [DOI] [PubMed] [Google Scholar]
- 6.Miettinen M, Lasota J. Gastrointestinal stromal tumors. Gastroenterol Clin North Am. 2013;42:399–415. doi: 10.1016/j.gtc.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gheorghe M, Predescu D, Iosif C, Ardeleanu C, Băcanu F, Constantinoiu S. Clinical and therapeutic considerations of GIST. J Med Life. 2014;7:139–49. [PMC free article] [PubMed] [Google Scholar]
- 8.Nishida T, Blay JY, hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016;19:3–14. doi: 10.1007/s10120-015-0526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin BP, Heinrich MC. Genotyping and immunohistochemistryof gastrointestinal stromal tumors:Anupdate. Semin Diagn Pathol. 2015;32:392–9. doi: 10.1053/j.semdp.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Geramizadeh B, Nosrati A. Histological and immunological evaluation of gastrointestinal stromal tumors. Iran J Med Sci. 2006;31:14–7. [Google Scholar]
- 11.Geramizadeh B, Bahador A, Ganjei-Azar P, Asadi A. Neonatal gastrointestinal stromal tumor Report of a case and review of literature. J Ped Surg. 2005;40:572–4. doi: 10.1016/j.jpedsurg.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 12.Yoo C, Tyu MH, Jo J, Park I, Ryoo BY, Kang YK. Efficacy of Imatinib in patients with platelet derived growth factor receptor-alpha mutated gastrointestinal stromal factors. Cancer res Treat. 2015:22. doi: 10.4143/crt.2015.015. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeh CN, Chen TW, Lee HL, Liu YY, Cha TC, Hwang TL, Jan YY, Chen MF. et al. Kinase mutatins and Imatinib mesylate response for 64 Taiwanese with advanced GISR: preliminary experience from Chang Guang Memorial hospital. Ann Surg Oncol. 2006;14:1123–8. doi: 10.1245/s10434-006-9288-1. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Yunfei HZ, Chen Z, Su K. Presence of PDGFRA and DOG-1 mutations in gastrointestinal stromal tumors among Chinese population. Int J Clin Exp Pathol. 2015;8:5721–26. [PMC free article] [PubMed] [Google Scholar]
- 15.Heinrich MC, Maki RG, Coreless CL, Antonesco CR, Harlow A, Griffith D. et al. Primary and secondary kinase genotypes correlates with the biological and clinical activity of Sunitinib in Imatinib-activity gastrointestinal stromal tumors. J Clin Oncol. 2008;26:5352–9. doi: 10.1200/JCO.2007.15.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buleje J, Acosta O, Guevara-Fujita M, Enriquez Y, Taxa L, Machicado E. et al. Mutational profile of KIT and PDGFRA genes in gastrointestinal stromal tumors in Pruvian samples. Rev Esp Enferm Dig. 2015;107:72–8. [PubMed] [Google Scholar]
- 17.Ahmad F, Lad P, Bhatia S, Das BR. Molecular spectrum of c-KIT and PDGFRA gene mutations in gastro intestinal stromal tumor: determination of frequency, distribution pattern and identification of novel mutations in Indian patients. Med Oncol. 2015;32:413–24. doi: 10.1007/s12032-014-0424-7. [DOI] [PubMed] [Google Scholar]
- 18.Minarika G, Plank L, Losabova Z, Szemes T, Burjaninova T, Szebe P. et al. Spectrum of mutations in gastrointestinal patients-a population-based study from Slovakia. APMIS. 2012;121:539–48. doi: 10.1111/apm.12019. [DOI] [PubMed] [Google Scholar]
- 19.Mendoza Y, Singh C, Mewa JC, Fonseca E, Smith R, Pascale JM. Beginning of personalized medicine in Panama: Molecular and pathological characteristics of gastrointestinal stromal tumors from archival paraffin-embedded tissue. Oncology letters. 2011;2:941–7. doi: 10.3892/ol.2011.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostka R, Gurlich R, Koldova L. Gastrointestinal stromal tumors (GIST): a single center experience. Acta Chir Belg. 2012;112:33–9. doi: 10.1080/00015458.2012.11680792. [DOI] [PubMed] [Google Scholar]


