Abstract
In individuals with compliant aortas, peripheral muscular artery stiffness exceeds central elastic artery stiffness. With ageing, central stiffness increases, with little change in peripheral stiffness, resulting in a reversal of the normal stiffness gradient. This reversal may reduce wave reflection amplitude, due to movement of the major “effective” reflection site further from the heart. To test this, we investigated the relationship among arterial stiffness gradients (normal and reversed), wave reflection amplitude and reflection site distance.
Subjects aged ≥50years were recruited from the Anglo-Cardiff Collaborative Trial. Central stiffness was assessed by carotid-femoral pulse wave velocity (cfPWV). In study 1, peripheral PWV was also measured in the arm (carotid-radial, crPWV), and in study 2 in the leg (femoral-dorsalis pedis, fpPWV). Reflection site distance was calculated from cfPWV and reflected wave travel time. Subjects were dichotomized into those with a normal stiffness gradient (peripheral>central PWV), or a reversed gradient (peripheral<central PWV).
In study 1, reflection site distance was greater in subjects with a reversed gradient (P<0.01), whereas time to reflection was lower (P<0.001). Both augmentation pressure (P<0.001) and augmentation index (P<0.05) were greater in subjects with a reversed gradient. In study 2, augmentation pressure, augmentation index and reflection site distance were greater in subjects with a reversed stiffness gradient (P<0.01, P<0.05 and P<0.01, respectively), and time to reflection was not different between groups.
A reversed arterial stiffness gradient is associated with increased reflection site distance and a paradoxical increase in reflected wave amplitude, and augmentation index.
Keywords: arterial stiffness gradient, augmentation index, pulse wave velocity, wave reflection, central pulse pressure
Introduction
Aortic stiffness[1, 2], wave reflection[3–7] and their surrogates[8–10], including central aortic pulse pressure (PP)[11, 12], are powerful independent predictors of cardiovascular risk and outcome. Wave reflections originate at arterial bifurcations and sites of impedance change or mismatch such as arterial taper and at conduit artery terminations[13]. These individual reflected waves then summate to form one apparent reflected wave, which summates with, and augments the initial, forward travelling pressure wave to form the measured pressure wave. Therefore, the central aortic pressure waveform is determined by the elastic properties and taper of the arterial tree, the amplitude of the reflected wave, and the time delay between the two waves (forward and reflected) which depends upon wave speed and the distance to the major “effective” reflection site[13, 14].
Central elastic arterial stiffness increases markedly with advancing age, whilst peripheral muscular arterial stiffness remains almost constant[15, 16]. As a result, central stiffness can equal peripheral stiffness (impedance matching), or even exceed peripheral stiffness (impedance mismatching), causing a reversal of the normal stiffness gradient[16, 17]. This has led to the suggestion that impedance matching (or mismatching) with central exceeding peripheral stiffness, reduces proximal wave reflection amplitude, by shifting the major effective reflection site more distally, resulting in a ‘flattening’ (followed by a decrease) effect of the age-related augmentation index (AIx) curve that occurs in individuals aged over 60 years[17, 18]. However, this hypothesis has not been tested directly.
The aim of this study was to compare aortic wave reflection amplitude and reflection site distance between individuals with a ‘normal’ forward arterial stiffness gradient (peripheral PWV>central PWV), and individuals where this gradient is reversed (peripheral PWV<central PWV). Earlier studies used PWV measured in the arm[17] or pressure waveforms measured in the carotid artery[18], however, we also chose to use the aortic pressure waveform and PWV measured in the leg where the major “effective” reflection site is located[13].
Methods
Subjects
All subjects and data were drawn from the Anglo-Cardiff Collaborative Trial (ACCT), a large, community-based investigation of the determinants of blood pressure and arterial stiffness across the adult age-span[16]. Participants with known peripheral vascular disease, or atrial fibrillation were excluded from the analyses. Data from 1,567 healthy subjects aged ≥ 50 years, who were free of diabetes, clinical cardiovascular disease and medication, were used for Study 1. In addition, a sub-set of 127 subjects from Study 1 were invited to undergo additional measurements, as described below (Study 2). Finally, data from 5117 subjects, aged between 18 and 90 years, were used to further examine the relationship between advancing age and wave reflection characteristics. Approval was obtained from the Local Research ethics committee, and written informed consent was obtained from all participants.
Measurements
Height and weight were assessed. Brachial cuff BP was measured in duplicate and averaged in the non-dominant arm, according to the British Hypertension Society Guidelines using a validated oscillometric device (HEM-711A-E, Omron Corp., Matsusaka, Japan). Following 15 minutes of supine rest, high quality pressure waveforms were acquired by applanation tonometry using the SphygmoCor device (Atcor Medical, Sydney, Australia) at the radial, carotid, femoral and, in a subset of 127 subjects, at the dorsalis-pedis arterial sites. Using the integrated software, pulse wave analysis was applied to the aortic pressure waveform synthesized from the radial artery waveform (Figure 1). The second derivative of the pressure wave was used by the system software to identify the inflection point on the upstroke of the aortic pressure wave to determine augmentation pressure (AP; the difference between the second and first systolic peaks of the aortic pressure waveform), augmentation index (AIx; AP/pulse pressure x 100), non-augmented pressure (pulse pressure – AP) and travel time (Tr) of the aortic pressure wave from the heart to the major reflection site and back using a validated transfer function[19]. Measurements were made on the non-dominant side throughout, in order to minimise variability[20]. Poor quality waveforms (according to quality criteria embedded within the system software), clearly erroneous values (i.e. AIx>50%) or where the system software was unable to calculate aortic characteristics, were not included in the analysis.
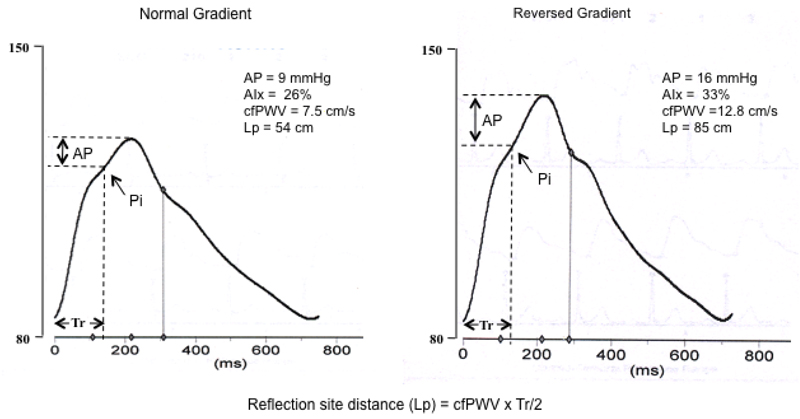
Figure 1.
Synthesized aortic pressure waves in a subject (age 62 years) with a “normal” arterial pressure gradient and one (age 79 years) with a “reversed” arterial pressure gradient. AP is augmentation pressure (reflected wave amplitude) and Tr is the round trip travel time of the forward wave from the ascending aorta to the major “effective” reflection site and back. Pi is the inflection point or upstroke of the reflected wave.
The SphygmoCor device was also used to determine cfPWV by sequentially recording ECG-gated carotid and femoral artery waveforms as previously described[21]. Carotid-radial PWV (crPWV) was determined from the carotid and radial waveforms. Similarly, femoral-dorsalis pedis PWV (fdPWV) was determined from the femoral and dorsalis-pedis waveforms in a subset of 127 subjects. Foot-to-foot transit time between the respective pressure waves was determined from the integrated software, and path length was measured on the body surface using a tape measure as previously described[22, 23]. For cfPWV the straight distance from the suprasternal notch to the carotid site was subtracted from the suprasternal notch to femoral site distance[23]. Distance to the major “effective” site (Lp) of wave reflection was calculated from cfPWV and the travel time of the reflected pressure wave from the reflection site to the heart (Tr/2; Figure 1) as previously described[24, 25].
Data Analysis
The study was conducted in two main parts, a large retrospective study, and a smaller prospective study. A further retrospective analysis (n=5117) provided additional information on wave reflection characteristics across the adult age-span.
Study 1 was a retrospective analysis utilising data from 1567 subjects, aged ≥ 50 years. Central stiffness was determined as cfPWV and peripheral stiffness was determined in the arm from the carotid and radial (crPWV) pressure waves.
Study 2 was a prospective analysis carried out in a subset of 127 subjects from Study 1. Since the reflected wave arrives mainly from the lower body[13], peripheral stiffness was measured in the leg, from the femoral and dorsalis-pedis pressure waves (fdPWV) and central stiffness was measured as cfPWV.
In both studies, subjects were dichotomized into two groups, those with a normal arterial stiffness gradient (peripheral PWV>central PWV) and those with a reversed stiffness gradient (peripheral PWV<central PWV). All data were analyzed using SPSS software (version 20.0, SPSS Inc., Chicago, Illinois). Student’s t-tests were applied to determine significance between stiffness gradient groups, with P<0.05 considered significant.
Results
Study 1
The subject characteristics for Study 1 are shown in Table 1. 1,567 subjects were included in the analysis (822 men and 745 women). Subjects with a reversed arterial stiffness gradient (crPWV<cfPWV) were older (69±7 vs 62 ± 6 years, P<0.001), more likely to have systolic hypertension (systolic pressure≥ 140mmHg, diastolic pressure< 90mmHg, 41% vs 14%, P<0.001), and higher triglycerides (1.4±0.8 mmol/L vs 1.6±0.9mmol/L, P<0.01) than those with a normal arterial stiffness gradient (crPWV>cfPWV). cfPWVand crPWV were 7.5±1.1m/s and 8.7±1.1m/s, respectively, in subjects with a normal stiffness gradient and 10.4±2.4m/s and 8.1±1.3m/s, respectively, in subjects with a reversed stiffness gradient. Central systolic blood pressure and central PP were greater in subjects with a reversed stiffness gradient (126±17mmHg vs 115±14mmHg and 49±13mmHg vs 40±10mmHg, respectively) as was the distance to the site of wave reflection (71.3±15.9cm vs 52.1±8.7cm, P<0.001), AP (15±7 mmHg vs 13±6 mmHg, P<0.001) and AIx (31±10% vs 30±9%, P<0.001). However, when AIx was adjusted for gender, height and heart rate, there was no difference between the normal and reversed stiffness gradient groups. Unaugmented central systolic and PP were also greater in the reversed stiffness gradient group (111±12mmHg vs 102±11 mmHg, and 34±8mmHg vs 27±6 mmHg, both P<0.001) compared to the normal stiffness gradient group. In multivariable regression models including age, gender, height, mean BP and heart rate, a reversed stiffness gradient was independently associated with reflection site distance, but not augmented pressure. Re-running the model using central (aortic) PWV instead of stiffness gradient per se, revealed that central PWV remained independently associated with reflection site distance and augmented pressure (Supplementary Table 1).
Table 1. Study 1: Subject Characteristics.
| Normal Gradient crPWV>cfPWV N=528 | Reverse Gradient crPWV<cfPWV N=1039 | Significance | |
|---|---|---|---|
| Age (years) | 62 ± 6 | 69 ± 7 | P<0.001 |
| Gender (m/f) | 583/456 | 239/289 | P<0.001 |
| Height (m) | 1.67 ± 0.08 | 1.68 ± 0.09 | NS |
| Weight (kg) | 71.4 ± 13.7 | 75.3 ± 13.7 | P<0.001 |
| Brachial Systolic BP (mmHg) | 122 ± 17 | 135 ± 21 | P<0.001 |
| Brachial Diastolic BP (mmHg) | 74 ± 7 | 76 ± 8 | NS |
| Pulse Pressure (mmHg) | 48 ± 14 | 58 ± 18 | P<0.001 |
| Central Systolic BP (mmHg) | 115 ± 14 | 126 ± 17 | P<0.001 |
| Central Diastolic BP (mmHg) | 75 ± 7 | 77 ± 8 | P<0.001 |
| Central Pulse Pressure (mmHg) | 40 ± 10 | 49 ± 13 | P<0.001 |
| Mean Arterial Pressure (mmHg) | 88 ± 9 | 93 ± 10 | P<0.001 |
| Unaugmented Central Systolic BP (mmHg) | 102 ± 11 | 111 ± 12 | P<0.001 |
| Unaugmented Central Pulse Pressure (mmHg) | 27 ± 6 | 34 ± 8 | P<0.001 |
| Heart Rate (beats/min) | 63 ± 9 | 66 ± 10 | P<0.001 |
| Augmentation Index (%) | 30 ± 9 | 31 ± 10 | P<0.05 |
| Augmentation Pressure (mmHg) | 13 ± 6 | 15 ± 7 | P<0.001 |
| Carotid-Femoral PWV (m/s) | 7.5 ± 1.1 | 10.4 ± 2.4 | P<0.001 |
| Carotid-Radial PWV (m/s) | 8.7 ± 1.1 | 8.1 ± 1.3 | P<0.001 |
| Time to Reflection (ms) | 78.3 ± 3.6 | 72.2 ± 4.3 | P<0.001 |
| Distance to Reflection Site (cm) | 52.1 ± 8.7 | 71.3 ± 1.6 | P<0.001 |
| Total Cholesterol (mmol/L) | 5.4 ± 1.0 | 5.4 ± 1.1 | NS |
| Triglycerides (mmol/L) | 1.4 ± 0.8 | 1.6 ± 0.9 | P<0.01 |
Data are means ± SD. BP, blood pressure; NS, not-significant; PWV, pulse wave velocity
Study 2
The subject characteristics for Study 2 are shown in Table 2. Forty-three men and 84 women were included in the study analysis. Subjects with a reversed arterial stiffness gradient were older (69±7 vs 62 ± 6 years, P<0.001), more likely to have systolic hypertension (52% vs 29%, P=0.014) and higher triglycerides (1.3±0.8mmol/L vs 1.7±0.9mmol/L, P<0.01) than those with a normal stiffness gradient. cfPWV and fdPWV were 7.9±1.0m/s and 9.9±1.0m/s, respectively, in subjects with a normal stiffness gradient and 10.9±2.0m/s and 9.0±1.5m/s, respectively, in subjects with a reversed stiffness gradient. Of those subjects with a reversed stiffness gradient based on fdPWV, the majority (n=41; 93%) also had a reversed gradient based on crPWV. Central systolic and PP, and AP were higher in subjects with a reversed stiffness gradient (126±12mmHg vs 116±15 mmHg and 51±10mmHg vs 40±13 mmHg and 16±6mmHg vs 13±7 mmHg, respectively, P<0.01 for all), as was AIx (32±8% vs 28±11%, P<0.05) and reflection site distance (74.6±14.9cm vs 55.9±9.9cm, P<0.001). An example of aortic pressure waves recorded in these two groups (normal gradient and reversed gradient) is shown in Figure 1. AIx remained significantly higher in the reversed stiffness gradient group when adjusted for gender, height and heart rate. Time to reflection did not differ between groups (70.7±6.6ms vs. 68.6±7.0ms). Additionally, unaugmented central systolic and PP were also greater in those with a reversed stiffness gradient (115±13mmHg vs 107±13 mmHg and 35±7mmHg vs 30±7 mmHg, respectively, P<0.001 for both). Multivariable regression analyses revealed that central PWV, rather than stiffness gradient per se, remained independently associated with augmented pressure (Supplementary Table 2). Composite data including augmented pressure and reflection site distance from Study 1 and Study 2 are shown in Figure 2.
Table 2. Study 2: Subject Characteristics.
| Normal Gradient fdPWV>cfPWV N=83 | Reverse Gradient fdPWV<cfPWV N=44 | Significance | |
|---|---|---|---|
| Age (years) | 62 ± 6 | 69 ± 7 | P<0.001 |
| Gender (m/f) | 32/51 | 11/33 | NS |
| Height (m) | 1.66 ±0.09 | 1.65 ± 0.08 | NS |
| Weight (kg) | 70 ± 11 | 72 ± 13 | NS |
| Brachial Systolic BP (mmHg) | 126 ± 17 | 138 ± 16 | P<0.001 |
| Brachial Diastolic BP (mmHg) | 74 ± 8 | 76 ± 7 | NS |
| Pulse Pressure (mmHg) | 53 ± 12 | 62 ± 13 | P<0.001 |
| Central Systolic BP (mmHg) | 116 ± 15 | 126 ± 12 | P<0.001 |
| Central Diastolic BP (mmHg) | 75 ± 8 | 77 ± 7 | NS |
| Central Pulse Pressure (mmHg) | 40 ± 13 | 51 ± 10 | P<0.001 |
| Mean Arterial Pressure (mmHg) | 93 ± 11 | 99 ± 10 | P<0.01 |
| Unaugmented Central Systolic BP (mmHg) | 107 ± 13 | 115 ± 13 | P<0.01 |
| Unaugmented Central Pulse Pressure (mmHg) | 30 ± 7 | 35 ± 7 | P<0.001 |
| Heart Rate (beats/min) | 66 ± 10 | 68 ± 7 | NS |
| Augmentation Index (%) | 28 ± 11 | 32 ± 8 | P<0.05 |
| Augmentation Pressure (mmHg) | 13 ± 7 | 16 ± 6 | P<0.01 |
| Carotid-Femoral PWV (m/s) | 7.9 ± 1.0 | 10.9 ± 2.0 | P<0.001 |
| Carotid-Radial PWV (m/s) | 8.3 ± 1.0 | 8.1 ± 1.1 | NS |
| Femoral-Dorsalis Pedis PWV (m/s) | 9.9 ± 1.1 | 9.2 ± 1.6 | P<0.01 |
| Time to Reflection (ms) | 70.7 ± 6.6 | 68.6 ± 7.0 | NS |
| Distance to Reflection Site (cm) | 55.9 ± 9.9 | 74.6 ± 14.9 | P<0.001 |
| Total Cholesterol (mmol/L) | 5.4 ± 1.0 | 5.7 ± 1.0 | NS |
| Triglycerides (mmol/L) | 1.3 ± 0.8 | 1.7 ± 0.9 | P<0.01 |
Data are means ± SD. BP, blood pressure; NS, not-significant; PWV, pulse wave velocity.
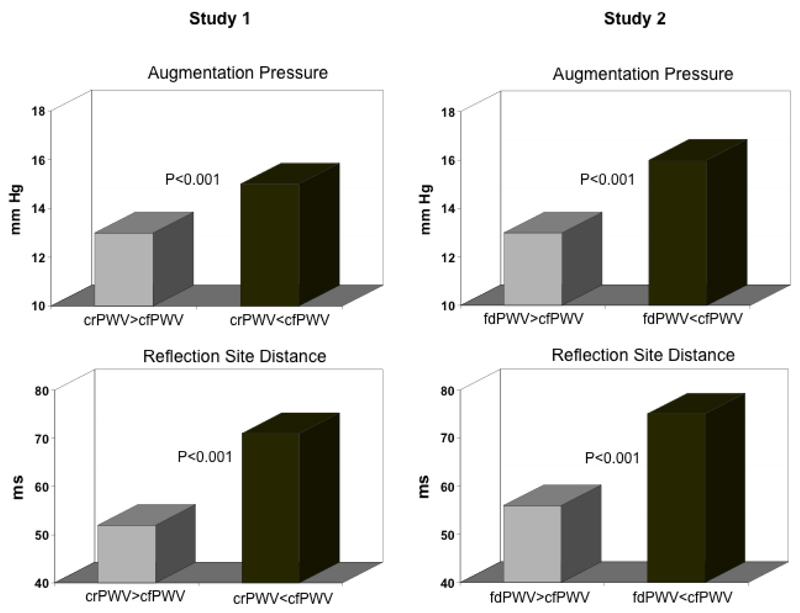
Figure 2.
Composite data (augmentation pressure and reflection site distance) from all subjects in Study 1 and Study 2. Gray bars represent data from subjects with a “normal” arterial stiffness gradient and black bars represent data from subjects with a “reversed” arterial stiffness gradient.
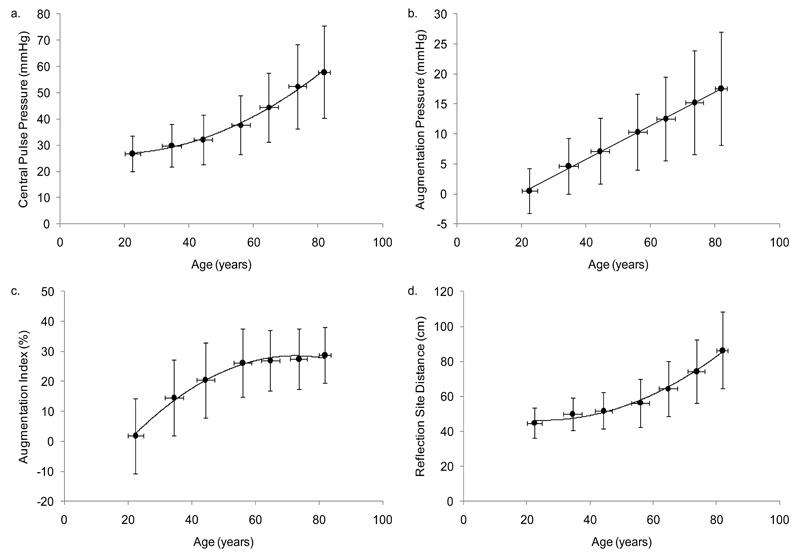
Age-related changes in central hemodynamics and reflection site distance were calculated from 5,117 individuals and the results are illustrated in Figure 3. With advancing age, central PP and distance to the major reflection site increased exponentially (R2=0.47, and R2=0.41, respectively, both P<0.001) and paralleled each other, whereas AP increased linearly (R2=0.45, P<0.001). As expected, AIx increased linearly with age until 55-60 years, after which a flattening effect was observed (R2=0.49, P<0.001) with no decrease, even in the very old.
Figure 3.
Age-related changes in a) central pulse pressure (R2=0.47, P<0.001), b) augmentation pressure (R2=0.45, P<0.001), c) augmentation index (R2=0.49, P<0.001), and d) reflection site distance (R2=0.41, P<0.001)
Discussion
This study tested the hypothesis that the greater increase in central than peripheral artery stiffness with advancing age, attenuates impedance mismatches between the central and peripheral arteries, leading to a shift of the major effective reflection site more distally in the lower body, and an attenuation of the age-related increase in wave reflection amplitude and AIx. We tested this hypothesis by dichotomizing a large number of healthy subjects into those with a normal arterial stiffness gradient (peripheral PWV>central PWV) and those where the stiffness gradient has been reversed (peripheral PWV<central PWV), and comparing the reflection site distance and amplitude of the global reflected pressure wave.
When PWV in either the arm (Study 1) or leg (Study 2) was used as a measure of peripheral arterial stiffness, a reversed stiffness gradient was not associated with reduced proximal wave reflection or a lower AIx, despite a greater distance to the major effective reflection site as previously suggested by Mitchell et al[17] and Vyas et al[18]. The time (Tr/2) to the return of the reflected wave was lower in those with a reversed arterial stiffness gradient in Study 1 but not statistically different between groups in Study 2, suggesting that the greater travel distance is offset by increased aortic stiffness. Moreover, reversal of the stiffness gradient was associated with a greater AP and a greater AIx in Study 2, indicating increased wave reflection amplitude, left ventricular afterload and myocardial oxygen demand in these individuals. It is highly likely that the increased reflected wave amplitude observed in individuals with a reversed stiffness gradient was driven, predominantly, by significantly higher central artery stiffness in these individuals. Unaugmented central systolic and PP were also increased in this group, indicating greater ascending aortic stiffness and incident wave amplitude. Moreover, central artery stiffness, rather than a reversed stiffness gradient per se, remained independently associated with reflected wave amplitude in multivariable regression models. Nevertheless, it is highly unlikely that a reversal of the normal stiffness gradient in older subjects results in reduced proximal wave reflection, as suggested by a report from the Framingham Heart Study[17]. The Framingham Heart Study investigators suggested that a reversal of the arterial stiffness gradient results from attenuated impedance mismatching between peripheral arteries and the central aorta, and causes a distal shift of the major reflection site in the lower part of the body, attenuating proximal wave reflection intensity which improves cardiovascular function. However, strictly speaking, frequency dependent impedance of the central aorta and peripheral arteries is always mismatched because of the influence of wave reflection at lower frequencies[13]. Impedance matching can only occur at higher frequencies where the spectrum is influenced only minimally by wave reflection (i.e characteristic impedance). Although the current data are inconclusive, previous studies have suggested that the majority of wave reflections originate either in the descending aorta, at a single primary site occurring at the aortic bifurcation or between the aortic bifurcation and femoral artery site[25–27].
The contrast between the results of the current study and those of The Framingham Heart Study investigators[17, 18] may be due to erroneous estimation of the inflection point used to calculate wave reflection time, as suggested by Nichols et al[13]. Mitchell et al[17] and Vyas et al[18] obtained the inflection point by visual inspection, while in the current study it was obtained automatically by the SphygmoCor device, which uses the second derivative of the aortic pressure wave, although analyses based on the fourth derivative have also been used[28]. Individuals greater than 60-65 years of age often exhibit ‘Type D’ aortic pressure waves which have no inflection point[13], and therefore it cannot be detected visually. This is a clear limitation to the findings reported by Mitchell et al[17] and Vyas et al[18]. The inflection point does, however, occur at peak aortic blood flow velocity in Type D pressure waveforms[13].
In order to further examine the relationship between advancing age and wave reflection characteristics, the age-related changes in central hemodynamics and reflection site distance were calculated from a large number of subjects across the adult age-span, from the Anglo-Cardiff Collaborative Trial. These data showed a linear rise in augmentation pressure but an attenuation of wave reflection amplitude with advancing age, and an exponential increase in central aortic PP, which parallels the exponential increase in reflection site distance. Because AIx is directly proportional to AP, and indirectly proportional to PP, the observed increase of the age-AIx relationship results from the incident pressure wave amplitude (which is directly related to ascending aortic stiffness) increasing to a lesser degree than the reflected wave amplitude (16% vs 23%). However, another hypothesis has been proposed, suggesting that the reduced rate of rise of AIx beyond 60 years of age is attributable, at least in part, to a mathematical principle. Namasivayam et al[29] suggested that the ratio of two linear relationships, such as AP vs age, and PP vs age (this group considered PP vs age to be a linear relationship), results in a curvilinear trend (AIx vs age). This mathematical phenomenon should, therefore, be considered for its potential contribution to the ‘flattening’ of the age-AIx curve.
Paradoxically, and in agreement with Mitchell et al[17], in the current study reflection site distance increased with advancing age, and, in agreement with Sugawara et al[26], did so at a greater rate beyond 60 years. In addition, recent studies have reported a small increase in length and diameter of the aorta[30] and femoral arteries[31] with advancing age, which may increase the travel distance of the forward and reflected wave as the central arteries stiffen, shifting the reflection site distally. However, these reports contradict a number of earlier studies that suggested a shift of wave reflections sites closer to the heart[28, 32], rather than further away. Although wave separation analysis is a more robust and accurate method for determining wave reflections, the distance to the major “effective” reflection site in the current study was estimated from the wave speed and the measured time delay between incident and reflected waves. This method was proposed and validated in animals by Van Den Bos et al[24] and used in humans by Murgo et al[25] and Mitchell et al[17]. Recently, a report by Westerhof et al[33] suggested that reflection site distance cannot be accurately determined from the given formula due to a phase shift in the waveform at the site of reflection. The phase shift would induce a time delay, resulting in a later return of the reflected wave, and a falsely increased calculated distance to this site. However, reflection site distance estimated using the method in the current study is comparable to reflection site distance calculated using the classical method that uses the first minimum of the input impedance moduli spectra commonly known as the quarter-wavelength method[13, 25]. The values of reflected wave travel time and PWV measured in the different arterial segments of the present study are similar to those previously reported for this age group using a variety of methods[15, 17, 34–37].
Limitations
The current study has a number of limitations, including several methodological considerations. Firstly, wave reflection is often considered to arise from a single point along the arterial tree (ie the aortic bifurcation). However, the global reflected wave is a summation of numerous individuals reflected waves, making the precise site of wave reflection theoretical. In the current study, the formula from Van den Bos et al[24] and Murgo et al[25] was used to calculate reflection site distance, and, although reliable, it lacks the precision of wave separation analyses as used by Wang et al[5] and Segers et al[32]. Moreover, PWV varies by vascular territory, and, in general, is greater in the muscular arteries compared to the more elastic central arteries[16, 38], although studies in animals[39] and humans[40] demonstrate regional variations in PWV with the aorta itself. As such, the use of proximal aortic PWV in the calculation may underestimate the distance to the site of wave reflection, since this occurs in the lower limbs, beyond the approximate 50cm length of the aorta[30]. Since aortic flow velocity was not assessed in the current study, we were unable to determine whether the reflection point occurred at the same time as peak aortic blood flow velocity. In addition, we were unable to determine precisely whether readings not calculated by the SphygmoCor software or excluded on the basis of erroneous values arose from Type D pressure waveforms, where there is no identifiable inflection point. Finally, the measurements of PWV used in this study to dichotomize subjects into normal or reverse arterial stiffness gradients may have been influenced, somewhat, by mean arterial pressure, which was higher in subjects with a reverse stiffness gradient. Therefore, a measure of arterial stiffness independent of (or adjusted for) MAP may have been preferable.
Conclusions
Overall, the findings of this study in subjects aged ≥ 50 years, dispute the hypothesis that preferential stiffening of the central arteries attenuates the rise in aortic augmentation pressure (and AIx) with age by shifting the site of wave reflection more distally. However, reversal of the central to peripheral stiffness gradient seen in older individuals remains important clinically, as it is associated with a greater central systolic pressure, PP, and aortic stiffening and not a reduction in these parameters. These alterations in arterial properties and wave reflection characteristics place an additional load on the heart and increases cardiac work and myocardial oxygen demand, which lead to increased cardiovascular risk. Moreover, reversal of the normal stiffness gradient, and an increased distance to the site of wave reflection, may adversely affect the microvascular circulation due to increased pulsatile stress on these small vessels, leading to potential tissue and organ damage. Therefore, further studies are desired to investigate this question.
Supplementary Material
Acknowledgements
The ACCT Study Investigators: John Cockcroft, Zahid Dhakam, Stacey Hickson, Julia Howard, Kaisa Maki-Petaja, Barry McDonnell, Carmel McEniery, Karen Miles, Maggie Munnery, Pawan Pusalkar, Christopher Retallick, Jane Smith, Edna Thomas, Sharon Wallace, Ian Wilkinson, Susannah Williams, Jean Woodcock-Smith, Yasmin.
Sources of Funding:
S.S.H is supported by the Cambridge Commonwealth Trust and the Cambridge Overseas Trust, I.B.W. by a British Heart Foundation Senior Clinical Fellowship. This work was funded by the British Heart Foundation and in part through the National Institute for Health Research Cambridge Biomedical Research Centre.
Footnotes
Conflicts of Interest: None
References
- 1.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–27. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, et al. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta-analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63(7):636–46. doi: 10.1016/j.jacc.2013.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chirinos JA, Zambrano JP, Chakko S, Veerani A, Schob A, Willens HJ, Perez G, Mendez AJ. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension. 2005;45(5):980–5. doi: 10.1161/01.HYP.0000165025.16381.44. [DOI] [PubMed] [Google Scholar]
- 4.Nurnberger J, Keflioglu-Scheiber A, Opazo Saez AM, Wenzel RR, Philipp T, Schafers RF. Augmentation index is associated with cardiovascular risk. J Hypertens. 2002;20(12):2407–14. doi: 10.1097/00004872-200212000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon HA, Ting CT, Najjar SS, Lakatta EG, Yin FC, Chou P, et al. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension. 2010;55(3):799–805. doi: 10.1161/HYPERTENSIONAHA.109.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber T, Auer J, O'Rourke MF, Kvas E, Lassnig E, Lamm G, Stark N, Rammer M, Eber B. Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur Heart J. 2005;26(24):2657–63. doi: 10.1093/eurheartj/ehi504. [DOI] [PubMed] [Google Scholar]
- 7.Mizuno A, Miyauchi K, Nishizaki Y, Yamazoe M, Komatsu I, Asano T, Mitsuhashi H, Nishi Y, Niwa K, Daida H. Impact of the augmentation time ratio on direct measurement of central aortic pressure in the presence of coronary artery disease. Hypertens Res. 2015;38(10):684–9. doi: 10.1038/hr.2015.53. [DOI] [PubMed] [Google Scholar]
- 8.Kollias A, Rarra V, Karpettas N, Roussias L, O'Brien E, Stergiou GS. Treatment-induced changes in ambulatory arterial stiffness index: one-year prospective study and meta-analysis of evidence. Hypertens Res. 2015;38(9):627–31. doi: 10.1038/hr.2015.44. [DOI] [PubMed] [Google Scholar]
- 9.Saji N, Kimura K, Yagita Y, Kawarai T, Shimizu H, Kita Y. Comparison of arteriosclerotic indicators in patients with ischemic stroke: ankle-brachial index, brachial-ankle pulse wave velocity and cardio-ankle vascular index. Hypertens Res. 2015;38(5):323–8. doi: 10.1038/hr.2015.8. [DOI] [PubMed] [Google Scholar]
- 10.Schillaci G, Battista F, Settimi L, Anastasio F, Pucci G. Cardio-ankle vascular index and subclinical heart disease. Hypertens Res. 2015;38(1):68–73. doi: 10.1038/hr.2014.138. [DOI] [PubMed] [Google Scholar]
- 11.Safar ME, Blacher J, Pannier B, Guerin AP, Marchais SJ, Guyonvarc'h PM, London GM. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39(3):735–8. doi: 10.1161/hy0202.098325. [DOI] [PubMed] [Google Scholar]
- 12.Roman MJ, Devereux RB, Kizer JR, Okin PM, Lee ET, Wang W, Umans JG, Calhoun D, Howard BV. High central pulse pressure is independently associated with adverse cardiovascular outcome the strong heart study. J Am Coll Cardiol. 2009;54(18):1730–4. doi: 10.1016/j.jacc.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols WW, O'Rourke MF, Vlachopoulos C. McDonald's Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. Sixth Edition. London: Hodder Arnold; 2011. [Google Scholar]
- 14.Westerhof N, Sipkema P, van den Bos GC, Elzinga G. Forward and backward waves in the arterial system. Cardiovasc Res. 1972;6(6):648–56. doi: 10.1093/cvr/6.6.648. [DOI] [PubMed] [Google Scholar]
- 15.Choi CU, Kim EJ, Kim SH, Shin SY, Choi UJ, Kim JW, Lim HE, Rha SW, Park CG, Seo HS, Oh DJ. Differing effects of aging on central and peripheral blood pressures and pulse wave velocity: a direct intraarterial study. J Hypertens. 2010;28(6):1252–60. doi: 10.1097/HJH.0b013e328337dad6. [DOI] [PubMed] [Google Scholar]
- 16.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal Vascular Aging: Differential Effects on Wave Reflection and Aortic Pulse Wave Velocity The Anglo-Cardiff Collaborative Trial (ACCT) J Am Coll Cardiol. 2005;46(9):1753–1760. doi: 10.1016/j.jacc.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43(6):1239–45. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- 18.Vyas M, Izzo JL, Jr, Lacourciere Y, Arnold JM, Dunlap ME, Amato JL, Pfeffer MA, Mitchell GF. Augmentation index and central aortic stiffness in middle-aged to elderly individuals. Am J Hypertens. 2007;20(6):642–7. doi: 10.1016/j.amjhyper.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Karamanoglu M, O'Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14(2):160–7. doi: 10.1093/eurheartj/14.2.160. [DOI] [PubMed] [Google Scholar]
- 20.Martin JS, Borges AR, Christy JBt, Beck DT. Considerations for SphygmoCor radial artery pulse wave analysis: side selection and peripheral arterial blood pressure calibration. Hypertens Res. 2015;38(10):675–83. doi: 10.1038/hr.2015.36. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, Webb DJ. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16(12 Pt 2):2079–84. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 22.Laurent S, Cockcroft JR, van Bortel LM, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson IB, Struijker Boudier HA. Abridged version of the expert consensus document. Artery Research. 2007;1(1):2–12. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 23.Weber T, Ammer M, Rammer M, Adji A, O'Rourke MF, Wassertheurer S, Rosenkranz S, Eber B. Noninvasive determination of carotid-femoral pulse wave velocity depends critically on assessment of travel distance: a comparison with invasive measurement. J Hypertens. 2009;27(8):1624–30. doi: 10.1097/HJH.0b013e32832cb04e. [DOI] [PubMed] [Google Scholar]
- 24.Van Den Bos GC, Westerhof N, Elzinga G, Sipkema P. Reflection in the systemic arterial system: effects of aortic and carotid occlusion. Cardiovasc Res. 1976;10(5):565–73. doi: 10.1093/cvr/10.5.565. [DOI] [PubMed] [Google Scholar]
- 25.Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: relationship to pressure wave forms. Circulation. 1980;62(1):105–16. doi: 10.1161/01.cir.62.1.105. [DOI] [PubMed] [Google Scholar]
- 26.Sugawara J, Hayashi K, Tanaka H. Distal shift of arterial pressure wave reflection sites with aging. Hypertension. 2010;56(5):920–5. doi: 10.1161/HYPERTENSIONAHA.110.160549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latham RD, Westerhof N, Sipkema P, Rubal BJ, Reuderink P, Murgo JP. Regional wave travel and reflections along the human aorta: a study with six simultaneous micromanometric pressures. Circulation. 1985;72(6):1257–69. doi: 10.1161/01.cir.72.6.1257. [DOI] [PubMed] [Google Scholar]
- 28.Kelly R, Hayward C, Avolio A, O'Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation. 1989;80(6):1652–9. doi: 10.1161/01.cir.80.6.1652. [DOI] [PubMed] [Google Scholar]
- 29.Namasivayam M, McDonnell BJ, McEniery CM, O'Rourke MF. Does wave reflection dominate age-related change in aortic blood pressure across the human life span? Hypertension. 2009;53(6):979–85. doi: 10.1161/HYPERTENSIONAHA.108.125179. [DOI] [PubMed] [Google Scholar]
- 30.Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-associated elongation of the ascending aorta in adults. JACC Cardiovasc Imaging. 2008;1(6):739–48. doi: 10.1016/j.jcmg.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Vermeersch SJ, Rietzschel ER, De Buyzere ML, De Bacquer D, De Backer G, Van Bortel LM, Gillebert TC, Verdonck PR, Segers P. Age and gender related patterns in carotid-femoral PWV and carotid and femoral stiffness in a large healthy, middle-aged population. J Hypertens. 2008;26(7):1411–9. doi: 10.1097/HJH.0b013e3282ffac00. [DOI] [PubMed] [Google Scholar]
- 32.Segers P, Rietzschel ER, De Buyzere ML, De Bacquer D, Van Bortel LM, De Backer G, Gillebert TC, Verdonck PR. Assessment of pressure wave reflection: getting the timing right! Physiol Meas. 2007;28(9):1045–56. doi: 10.1088/0967-3334/28/9/006. [DOI] [PubMed] [Google Scholar]
- 33.Westerhof BE, van den Wijngaard JP, Murgo JP, Westerhof N. Location of a reflection site is elusive: consequences for the calculation of aortic pulse wave velocity. Hypertension. 2008;52(3):478–83. doi: 10.1161/HYPERTENSIONAHA.108.116525. [DOI] [PubMed] [Google Scholar]
- 34.van der Heijden-Spek JJ, Staessen JA, Fagard RH, Hoeks AP, Boudier HA, van Bortel LM. Effect of age on brachial artery wall properties differs from the aorta and is gender dependent: a population study. Hypertension. 2000;35(2):637–42. doi: 10.1161/01.hyp.35.2.637. [DOI] [PubMed] [Google Scholar]
- 35.Di Iorio BR, Cucciniello E, Alinei P, Torraca S. Reproducibility of regional pulse-wave velocity in uremic subjects. Hemodial Int. 2010;14(4):441–6. doi: 10.1111/j.1542-4758.2010.00471.x. [DOI] [PubMed] [Google Scholar]
- 36.Ring M, Eriksson MJ, Zierath JR, Caidahl K. Arterial stiffness estimation in healthy subjects: a validation of oscillometric (Arteriograph) and tonometric (SphygmoCor) techniques. Hypertens Res. 2014;37(11):999–1007. doi: 10.1038/hr.2014.115. [DOI] [PubMed] [Google Scholar]
- 37.Seidlerova J, Filipovsky J, Mayer O, Wohlfahrt P, Cifkova R. Positive effects of antihypertensive treatment on aortic stiffness in the general population. Hypertens Res. 2014;37(1):64–8. doi: 10.1038/hr.2013.113. [DOI] [PubMed] [Google Scholar]
- 38.Nichols WW, Denardo SJ, Wilkinson IB, McEniery CM, Cockcroft J, O'Rourke MF. Effects of arterial stiffness, pulse wave velocity, and wave reflections on the central aortic pressure waveform. J Clin Hypertens (Greenwich) 2008;10(4):295–303. doi: 10.1111/j.1751-7176.2008.04746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katsuda S, Takazawa K, Miyake M, Kobayashi D, Kusanagi M, Hazama A. Local pulse wave velocity directly reflects increased arterial stiffness in a restricted aortic region with progression of atherosclerotic lesions. Hypertens Res. 2014;37(10):892–900. doi: 10.1038/hr.2014.96. [DOI] [PubMed] [Google Scholar]
- 40.Hickson SS, Butlin M, Graves M, Taviani V, Avolio AP, McEniery CM, Wilkinson IB. The relationship of age with regional aortic stiffness and diameter. JACC Cardiovasc Imaging. 2010;3(12):1247–55. doi: 10.1016/j.jcmg.2010.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.