Abstract
Alcohol use disorder (AUD) is debilitating and costly. Identification and better understanding of risk factors influencing the development of AUD remain a research priority. Although early life exposure to trauma increases the risk of adulthood psychiatric disorders, including AUD, many individuals exposed to early life trauma do not develop psychopathology. Underlying genetic factors may contribute to differential sensitivity to trauma experienced in childhood. The hypothalamic-pituitary-adrenal (HPA) axis is susceptible to long-lasting changes in function following childhood trauma. Functional genetic variation within FKBP5, a gene encoding a modulator of HPA axis function, is associated with the development of psychiatric symptoms in adulthood, particularly among individuals exposed to trauma early in life. In the current study, we examined interactions between self-reported early life trauma, past-year life stress, past year trauma, and a single nucleotide polymorphism (rs1360780) in FKBP5 on heavy alcohol consumption in a sample of 1845 college students from two university settings. Although we found no effect of early life trauma on heavy drinking in rs1360780*T-allele carriers, rs1360780*C homozygotes exposed to early life trauma had a lower probability of heavy drinking compared to rs1360780*C homozygotes not exposed to early life trauma (p < 0.01). The absence of an interaction between either current life stress or past year trauma, and FKBP5 genotype on heavy drinking suggests that there exists a developmental period of susceptibility to stress that is moderated by FKBP5 genotype. These findings implicate interactive effects of early life trauma and FKBP5 genetic variation on heavy drinking.
Keywords: polymorphism, early life stress, gene-environment interaction, alcohol use disorder, alcohol
Introduction
Alcohol use disorder (AUD) is a complex psychiatric disorder affecting 14% of the U.S. population during a one-year period (Grant et al. 2015). Identifying factors that influence the development of AUD would be beneficial in the identification of at-risk individuals and potentially in the treatment of those with AUDs. A meta-analysis of 124 studies showed that abuse and neglect experienced during childhood are significantly associated with depressive disorders, substance use, suicide attempts, and risky sexual behavior (Norman et al. 2012). Early life stress is also associated with greater alcohol intake and abuse (Enoch 2011), which predispose to alcohol-related health problems and the development of AUD (Kranzler et al. 1990, Dawson et al. 1993).
Data from the National Comorbidity Survey indicate that childhood adversity including childhood maltreatment, trauma, and stressful life events such as divorce or death of a family member are reported by 45% of subjects with childhood-onset and 26–32% of individuals with adult-onset psychiatric illness (Green et al. 2010). Yet many individuals exposed to early trauma/stress do not develop psychopathology. Genetic variation is likely one contributor to between-person sensitivity or resilience to adverse outcomes following trauma. Exposure to childhood stressful events can influence reactivity of the hypothalamic-pituitary-adrenal (HPA) axis, effects that can persist into adulthood, as shown by dysregulated release of the glucocorticoid cortisol to acute stress in adults with a history of physical or emotional adversity (Lovallo et al. 2012).
Termination of the HPA axis stress response is in part regulated via the glucocorticoid receptor (GR), a ligand-activated transcription factor located in the cytosol with a high affinity for glucocorticoids (de Kloet et al. 2005, Schatzberg et al. 2014). The FK506 binding protein (FKBP5), a glucocorticoid receptor chaperone encoded by FKBP5, has been implicated in the pathophysiology of HPA axis abnormalities associated with traumatic events. FKBP5 protein functions to down regulate GR activity by reducing GR binding affinity for cortisol and inhibiting GR translocation into the nucleus (Grad et al. 2007, Stechschulte et al. 2011, Sinclair et al. 2013). Hormone-activated GR binding at regulatory sites in the FKBP5 gene leads to an upregulation of FKBP5 mRNA expression, creating an ultra-short feedback loop reducing GR signaling and prolonging the HPA axis response (Binder 2009). Polymorphisms in a 100-kb haplotype block in FKBP5 have been associated with risk of several stress-related conditions, including post-traumatic stress disorder (PTSD) (Binder et al. 2008), anxiety, depression (Binder et al. 2004, Lavebratt et al. 2010), addictive disorders (Huang et al. 2014, Levran et al. 2014, Jensen et al. 2015) and aggressive or violent behavior (Bevilacqua et al. 2012). The most widely studied tag SNP for this haplotype block, rs1360780 is located in an enhancer region adjacent to a GR binding site and has been shown to have functional effects using reporter gene assays (Klengel et al. 2013). The minor T-allele of the rs1360780 polymorphism has been associated with increased psychiatric symptoms in trauma exposed subjects (Zannas and Binder 2014) and greater FKBP5 mRNA and protein levels (Binder et al. 2004, Binder 2009), which are thought to contribute to an altered HPA axis regulation. The association of FKBP5 genotype and several stress-related psychiatric symptoms depends on an interaction of genotype with exposure to stressful life events (Zannas and Binder 2014). FKBP5 rs1360780 genotype interacts with early life but not adult trauma in predicting PTSD symptom severity (Binder et al. 2008, Xie et al. 2010, Boscarino et al. 2012), while FKPB5 genotype interactions with both early and lifetime stress have been reported for depression and suicide (Roy et al. 2010, Appel et al. 2011, Zimmermann et al. 2011). In the setting of childhood adversity, rs1360780 is associated with epigenetic effects including altered chromatin looping and DNA methylation patterns in FKBP5 (Klengel et al. 2013), changes that alter the expression of the FKBP5 gene. Interactions between rs1360780 genotype and self-reported childhood emotional neglect predicted greater threat-related amygdala reactivity in adolescents (White et al. 2012) and adults (Holz et al. 2014), suggesting that FKBP5 genotype × environment interactions may lead to developmental changes in neural circuits.
Importantly, the specific effects of FKBP5 genotype and its interaction with stress may vary by developmental age. For example, in a study of 310 infants, the rs1360780 minor T-allele was associated with greater cortisol reactivity to minor stress (Luijk et al. 2010). However, no main effect of genotype was observed on salivary cortisol levels in a sample of 368 healthy adults in response to the Trier Social Stress Test (TSST) (Mahon et al. 2013). In contrast, among young adults homozygous for the rs1360780*C allele, those with greater childhood adversity showed an attenuated cortisol response to the TSST. In this study, cortisol response to the TSST in T-allele carriers did not differ as a function of childhood adversity (Buchmann et al. 2014), which suggests that the C/C genotype confers protective effects on the stress-activated cortisol response in adults with a history of childhood adversity.
Prior studies examining FKBP5 genotype × life stress interactions have reported greater depressive or PTSD symptoms in subjects with a history of life trauma carrying the rs1360780 T-allele, and frequently have referred to the rs1360780 T-allele as the risk allele. However, the design and results of these studies do not allow for a clear distinction as to whether the observed differences in symptomology between genotypes is due to a protective-effect of the rs1360780 C/C genotype or a risk-effect of the T-allele. In the present study, we investigated the association of heavy drinking in a large sample of African-American and Caucasian college students based on self-reported early life trauma, past year trauma or negative life events, and the FKBP5 rs1360780 polymorphism. Examining a sample of college students who regularly use alcohol offers the potential to distinguish between a protective vs. risk effect of the rs1360780 polymorphism in the setting of prior life trauma, since a basal level of alcohol consumption in this sample is expected.
Methods
Participants and procedure
Undergraduate students were recruited over a 4-year period (2008–2011) at a Historically Black College and University (HBCU) and a New England Public University (NEPU) through the psychology research pool and campus-wide broadcast emails and flyers inviting students to participate in a study about daily experiences and health-related behavior. Only students who reported drinking alcohol at least twice in the past month and had not received treatment for alcohol use were eligible. Participants were compensated for their participation after giving written, informed consent to participate in the protocol, which was approved by the institutional review boards at each university.
Participants at both universities first completed a web-based baseline survey that included various demographic questions, an inventory of traumatic life experiences prior to age 6 and negative life events in the past year. Participants also provided saliva samples for genotyping. Approximately two weeks after completing the baseline survey, students accessed a secure website each day from 2:30–7:00 PM for 30 days to complete a brief survey. This time window was selected to coincide with most undergraduate students’ naturally occurring end of the school day, but before typical evening activities began (including drinking). Relevant to our study, participants were asked each day to report on their alcohol consumption for the past evening (i.e., after the previous day’s survey) and for the current day. If a daily survey was not completed, the participant was reminded by email to complete the next day's survey. Further, if participants failed to complete a daily survey, during their next login, the server queried them about their drinking during missed intervals lasting up to 3 days.
The baseline sample at the HBCU consisted of 741 students of which 53% were female and 96% were self-identified African Americans (or of African descent). Three-hundred three individuals were excluded from analysis because they did not complete at least 15 daily diary entries or had missing data in the relevant baseline measures, resulting in a final sample of 438 students (58% female and 97% of African descent) for analysis with a mean age of 20.0 years (SD = 1.6). Approximately half of the students (48%) were freshmen or sophomores.
The baseline NEPU sample consisted of 1815 students, 78.0 of which were self-identified European Americans, 11.0% Asians, 4.5% African Americans, 4.0% Hispanic/Latinos and 2.8% misc./other. Exclusion of Asian and misc./other students resulted in a sample of 1600. An additional 190 participants were excluded because they did not complete at least 15 daily diary entries and 3 additional students had missing data in the relevant baseline measures, resulting in 1407 participants eligible for analysis. The final NEPU sample was 55% female; had a mean age of 19.2 years (SD = 1.4); was comprised mostly of freshmen or sophomores (74%), and was predominantly Caucasian [91% (n = 1278)], with small percentages of African Americans [4.9% (n = 69)] and Hispanics/Latinos [4.3% (n = 60)]. Across both universities, we had a final sample of 1845 (54% female).
Attrition analysis comparing included vs. excluded participants in the HBCU sample indicated that excluded individuals were more likely to be male, χ2(1) = 13.41, p < 0.001, and fewer excluded subjects reported early life trauma (27%) compared to individuals in the final sample (36%), χ2(1) = 6.01, p = 0.014 (phi = .09). The HBCU final sample did not differ from excluded individuals on age, t(735) = 1.02, p = 0.31, FKBP5 genotype, χ2(1) = 0.207, p = 0.65, or number of past year stressful life events, t(717)=1.30, p = 0.19. Results for the NEPU sample indicated that excluded individuals were also more likely to be male, χ2(1) = 23.7, p < 0.001, and or racial/ethnic minorities, χ2(2) = 7.0, p = 0.03. The final NEPU sample did not differ from excluded individuals on age, t(1595) = .008, p = 0.99, FKBP5 genotype, χ2(1) = 0.147, p = 0.702, or early life trauma χ2(1) = 0.013, p = .909. However, excluded individuals reported more past year stressful life events (M = 4.55, SD = 3.14) compared to individuals in the final sample (M = 3.94, SD = 2.74), t(717)=1593, p = 0.005 (Cohen’s d = .22).
Measures
Alcohol use was measured each day by asking participants how many standard alcoholic drinks they consumed the previous night (from 0 to 15, in one-drink increments, with an option for >15) as well as the number of drinks, if any, they had consumed on the day of the diary report up to the time of logging their response. Students were reminded each day that a standard drink was defined as one 12-oz can or bottle of beer or wine cooler, one 5-oz glass of wine, or a 1.5-oz measure of liquor straight or in a mixed drink. If participants missed a daily survey, they were queried about drinking levels on the missed day(s) on the next occasion that they logged into the system (before they completed that day’s survey); 16.4% and 10.7% of the daily records were backfilled in the HBCU and NEPU samples, respectively. We created full-day records by combining daytime and previous night reports from consecutive days. Lagging values from subsequent day reports allowed us to use only 29 of the reporting days. The number of drinks was summed across daytime and evening drinking and was converted into a binary heavy drinking indicator [4+ drinks for women and 5+ drinks for men (NIAAA 2004)].
Early life trauma (ELT) and past year trauma (PYrTrau) was measured using the Traumatic Events Screening Inventory (TESI)-Adult Screening version 3.2 (Ford et al. 2013). The TESI has 18 items that assess various types of trauma that would qualify for the DSM-IV-TR PTSD diagnosis criterion A1, with a total of nine trauma categories (i.e., accident/illness/disaster, traumatic loss/separation, traumatic physical victimization, traumatic sexual victimization, traumatic emotional victimization, traumatic domestic violence victimization, witnessed trauma, traumatic war victimization, and other traumatic event). The TESI questionnaire has a high inter-rater reliability score, with kappas between 0.73 –1.00, a retest reliability over a 2–4 month period between 0.50 – 0.70 (kappa), and validity coefficients for predicting PTSD symptom severity of 0.26 – 0.32 (Ford et al. 1997, Ford et al. 2000, Ford et al. 2013). Participants indicated (yes/no) as to whether the events occurred before age 6, between ages 6 and 17, above age 18 or within the past year. We created a binary variable reflecting the occurrence of at least one early life traumatic event prior to age 6 or during the past year, as only a small proportion of subjects reported more than one traumatic event in either period with 94% of subjects reporting either 0 or 1 traumatic event prior to age 6.
Past year stressful life events were measured using the Life Events Scale for Students (LESS; (Linden 1984), an empirically derived inventory of common life events adapted from the Social Readjustment Rating Scale (Holmes et al. 1967) for use with college students. In this checklist, students indicated which stressful life events from a list of 36 they experienced during the past year (e.g., broke up with boy/girlfriend, failed a course, family health problems, financial problems). The LESS questionnaire has a high test-retest reliability, with Clements et al. (1996) reporting high consistency of individual events one and six months after initial testing. For the current study, we used the 25 unambiguously negative items selected by Covault et al. (2007) in a prior sample examining college student drinking. Students were asked which of the events occurred in the past year; we created a composite by summing the number of endorsed events with the average student reporting between 4 and 5 past year negative events.
Genotyping
DNA was extracted from saliva samples using the Oragene DNA salivary extraction kit (DNA Genotek, Kanata, Ontario, Canada) per the manufacturer’s instructions. The FKBP5 SNP rs1360780 was genotyped using an Applied Biosystems TaqMan Assay On-Demand probe and primer set (C_8852038_10) with Universal Master Mix II (Life Technologies, Carlsbad, CA) per the manufacturer’s instructions. We used 10µL PCR amplification reactions containing 1µL DNA that were run in 96-well plates with the following PCR conditions: 95° for 10 minutes, followed by 40 cycles of 95° for 15 seconds and 60° for 60 seconds. Post-PCR fluorescent plate reads were carried out on an Applied Biosystems 7500 instrument and analyzed using Applied Biosystems TaqMan Genotyper software. 10% of the samples were repeated with complete concordance. Genotypes for each racial/ethnic group were in Hardy Weinberg equilibrium (p = 0.93; 0.26 and 0.39 respectively for European American, African American and Hispanic groups). The minor allele frequency (MAF) was higher in the African American subject group (0.38; χ2 = 19.6, df=2, p < 0.001) compared with the European American (0.31) or Hispanic (0.29) groups consistent with MAF reported in the 1000 genomes data set for this SNP (European ancestry 0.29 and African ancestry 0.39). The rs1360780genotype counts were as follows: European American C/C = 611, C/T = 547, T/T = 121; African American C/C = 184, C/T = 249, T/T = 68; Hispanic/Latino; C/C = 34, C/T = 24, T/T = 7. The overall sample genotype frequencies were CC =0.449, CT = 0.444, and TT = 0.106 with a minor T-allele frequency of 0.33.
Data analysis
All statistical analyses were conducted using SPSS software v21 (IBM, Armonk, NY). We used generalized estimating equations (GEE) to model the additive and interactive effects of early-life trauma, past-year negative life events, and the main and interactive effects of FKBP5 rs1360780 genotype and either early-life trauma or past-year negative life events to predict heavy drinking days. Given the binary nature of the primary outcome variable (heavy drinking day: present/absent), we specified a logit link and binomial error distribution and an unstructured working matrix. Predictors were entered in 2 blocks: main effects and 2-way interactions. Values shown in the tables reflect effects for the block of entry (i.e., without the subsequent blocks included). FKBP5 rs1360780 genotype was collapsed into binary predictors (minor T-allele carrier vs. C/C individuals) due to the relatively small cell sizes for TT trauma exposed students (n=52). Data from both universities were combined into one sample. We controlled for school (0 = HBCU, 1 = NEPU), weekly variation in drinking by including a weekday-weekend contrast (weekdays = 0, weekend = 1), ethnicity (with 2 dummy codes comparing African descent and Latino/Hispanic with Caucasian [the reference group coded 0]), sex, age, and year in school (with 3 dummy codes comparing each year with freshmen [the reference group coded 0]).
Results
Descriptive statistics
We had daily drinking reports on 12,489 days (28.5 days per person) in the HBCU sample and 39,906 days (28.4 days per person) in the NEPU sample. Overall, we had 52,395 person-days for analysis nested within 1845 participants. Mean percentage of days with any drinking reported (i.e., averaged across all participants) in the HBCU and NEPU samples, respectively were 25.8% (SD = 18.2%) and 20.6% (SD = 14.9%) and for heavy drinking days were 11.6% (SD = 13.2%) and 13.1% (SD = 12.3%). Early-life traumatic events were reported by 35.5% and 21.4% of participants in the HBCU and NEPU samples, respectively. Relatively few subjects (6%) reported more than 1 early-life traumatic event. The mean number of stressful life events in the past year was 5.66 (SD = 3.65) in the HBCU sample and 3.94 (SD = 2.74) in the NEPU sample. FKBP5 rs1360780 genotype was unrelated to ELT (χ2(1) = 2.72, p = 0.099) and past year trauma (χ2(1) = 1.36, p = 0.51), but was weakly related to recent negative life events (t(1843) = 2.07, p = 0.038), with C/C participants reporting fewer events (M= 4.19, SD = 2.95) compared to T-allele carriers (M = 4.49, SD = 3.16).
Models predicting heavy drinking days
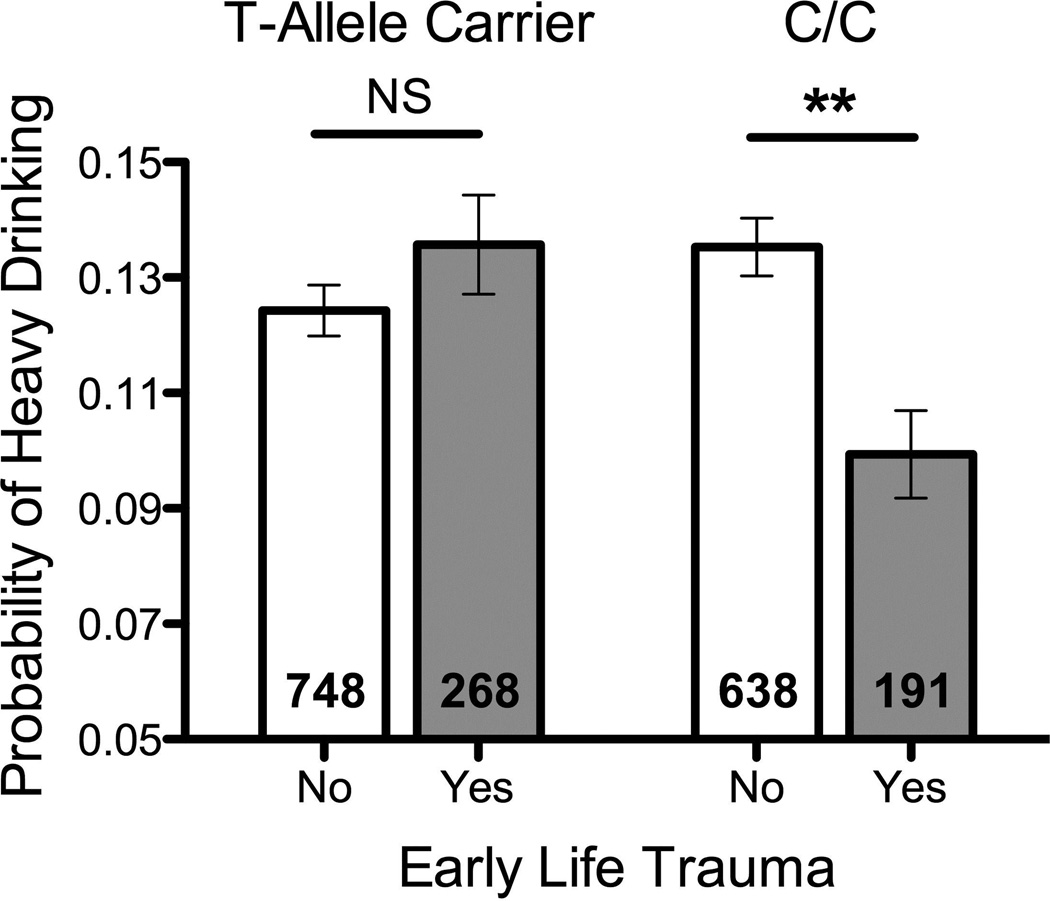
Table 1 shows the results for the GEE model predicting heavy drinking days. In block 1 neither ELT nor FKBP5 genotype were associated with heavy drinking, but recent life stress was a significant predictor, with higher levels of past-year negative events associated with a greater probability of heavy drinking. In block 2, only the interaction between FKBP5 genotype and ELT was significant, the form of which is shown in Figure 1. Follow-up probing of the interaction indicated a non-significant effect of early life trauma on heavy drinking in FKBP5 rs1360780 T-allele carriers (B = 0.10, SE = 0.09, p = 0.29, 95%CI: −.08 to .278, OR = 1.25). In contrast, ELT had a significant effect on heavy drinking in FKBP5 rs1360780 C/C participants, such that C/C participants reporting a positive history of early life trauma had a lower frequency of heavy drinking (B = −0.44, SE = 0.11, p = 0.003, 95%CI: −.65 to −.23, OR = .39). Further probing of the interaction revealed that among participants with no ELT, rs1360780 T-allele carriers were not different from rs1360780 C/C participants in terms of heavy drinking (B = −0.13, SE = 0.07, p = 0.057, 95%CI: −.26 to .004, OR = .88). In contrast, among participants reporting ELT, rs1360780 T-allele carriers reported higher levels of heavy drinking compared to rs1360780 C/C participants (B = 0.41, SE = 0.12, p = 0.001, 95%CI: .17 to .66, OR = 1.51).
Table 1.
Model predicting heavy drinking days
| 95% CI | |||||||
|---|---|---|---|---|---|---|---|
| Block | B | SE | p | Lower | Upper | Odds Ratio |
|
| 1 | African American (vs. White) | −0.997 | 0.158 | <.001 | −1.306 | −0.688 | 0.369 |
| Latino/Latina (vs. White) | −0.538 | 0.204 | .008 | −0.939 | −0.138 | 0.584 | |
| School (0 = HBCU, 1 = NEPU) | −0.495 | 0.164 | .002 | −0.816 | −0.174 | 0.610 | |
| Weekend (0=weekday; 1 = weekend) | 2.124 | 0.054 | <.001 | 2.018 | 2.231 | 8.368 | |
| Age | −0.137 | 0.053 | .010 | −0.242 | −0.033 | 0.872 | |
| Sophomore | 0.314 | 0.085 | <.001 | 0.147 | 0.481 | 1.369 | |
| Junior | 0.600 | 0.135 | <.001 | 0.335 | 0.866 | 1.823 | |
| Senior | 0.883 | 0.193 | <.001 | 0.504 | 1.262 | 2.418 | |
| Beyond senior | 1.139 | 0.273 | <.001 | 0.605 | 1.674 | 3.124 | |
| Sex | −0.426 | 0.059 | <.001 | −0.542 | −0.309 | 0.653 | |
| Negative life events (NLE) | 0.043 | 0.010 | <.001 | 0.024 | 0.062 | 1.044 | |
| Early trauma | −0.127 | 0.071 | .074 | −0.267 | 0.012 | 0.881 | |
| FKBP5 T-carrier | −0.006 | 0.058 | .918 | −0.120 | 0.108 | 0.994 | |
| 2 | Early trauma × FKBP5 T-carrier | 0.524 | 0.143 | <.001 | 0.244 | 0.804 | 1.689 |
| NLE × FKBP5 T-carrier | 0.014 | 0.019 | .458 | −0.023 | 0.051 | 1.014 | |
| NLE × Early trauma | −0.028 | 0.020 | .173 | −0.068 | 0.012 | 0.973 | |
Note. B = change in log of odds for a unit change in predictor. Early trauma 0 = no, 1 = yes; FKBP5: 0 = CC genotype, 1 = T-allele carrier; Sex: 0 = males, 1 = females; Weekend -1 = weekday, 1 = weekend.
Figure 1. Interactive effect of FKBP5 rs1360780 and early life trauma in predicting heavy drinking.
Daily self-reports of alcohol consumption in a diverse sample of 1845 college students revealed no significant effect of early life trauma on the probability of heavy drinking in carriers of the FKBP5 rs1360780*T-allele (B = −0.23, SE = 0.14, p = 0.51, OR = 1.25). In contrast, a significant effect of early life trauma on the probability of heavy drinking was observed in FKBP5 rs1360780*C/C genotype individuals (B = −0.29, SE = 0.15, p = 0.003, OR = 0.747). The depicted probability of heavy drinking were calculated with the levels for covariates held at their mean. Number of participants within each group is depicted in each bar. (** p < 0.01, NS = not significant).
As recommended by Keller (2014), in order to rule out the possible confounding interactive effects of the covariates with regard to the FKBP5 genotype × ELT interaction, we estimated a model that included all of the product terms between the covariates (ethnicity, college, age, sex and year in school) and both FKBP5 genotype and ELT. None of the covariate interactions were significant (all p’s > 0.12), and importantly the coefficient for the FKBP5 genotype × ELT interaction (B = .55, SE = 0.15, p <.001) was not altered with all of the covariate interactions included in the model.
We also examined several supplemental models to better understand the findings. First, we re-estimated the model using the 3-level FKBP5 rs1360780 genotype instead of the binary coding described above. Specifically, we used two Helmert contrasts to code for genotype: the first contrast was C/C coded 2 and t-carriers coded −1 and the second contrast was T/T coded 1, C/T coded −1 and C/C coded 0. The second contrast allowed us to examine whether the effect of ELT differed across C/T and T/T individuals. Results indicated that only the first contrast interacted with ELT (B = −0.16, SE = 0.05, p = 0.002, 95%CI: −.26 to −.06), the form of which was identical to that shown in Figure 1. The contrast comparing C/T and T/T individuals did not interact with ELT (B = −0.06, SE = 0.11, p = 0.57, 95%CI: −.26 to .06).
Next, we re-estimated the model predicting the number of drinks as opposed to the binary heavy drinking outcome (using a log link and negative binomial error distribution and an unstructured working matrix). The results were essentially the same, with a significant interaction between FKBP5 rs1360780 genotype and ELT (B = .448, SE = .119, p <.001, 95%CI: .214 to .682). Specifically, we found a non-significant effect of ELT on drinking in FKBP5 rs1360780 T-allele carriers (B = 0.15, SE = 0.08, p = 0.054, 95%CI: −.002 to .304), but a significant effect of ELT on heavy drinking in FKBP5 rs1360780 C/C participants (B = −0.30, SE = 0.09, p = 0.001, 95%CI: −.49 to −.12) with C/C participants reporting a positive history of ELT drinking less.
Finally, to further probe the importance of timing of traumatic experiences for the interaction with FKBP5 genotype in predicting heavy drinking, we replaced past year negative life events (LESS) with presence or absence of trauma during the past year (TESI). Past year traumatic events were reported by 39% and 29% of participants in the HBCU and NEPU sample, respectively. In contrast to early life trauma, presence of a past year traumatic event did not interact with FKBP5 rs1360780 genotype in predicting probability of heavy drinking (B = 0.05, SE = 0.14, p = 0.76, 95%CI: −.24 to .33, OR = 1.05).
Discussion
In the current study we examined heavy drinking, a risk factor for alcohol-related health problems and the development of alcohol use disorder (Kranzler et al. 1990, Dawson et al. 1993), in 1845 participants from two universities as a function of FKBP5 rs1360780 genotype. We found no main effect of FKBP5 rs1360780 genotype on the probability of heavy drinking but found a significant interaction effect of FKBP5 genotype and early-life trauma on the probability of heavy drinking in students of African or European ancestry. In contrast, we did not see an interaction of genotype with the number of past-year negative life events or the presence of a past year traumatic experience in predicting heavy drinking. These findings are in agreement with prior research reporting no main effect of FKBP5 genotype, but a genotype × early-life trauma interaction effect on the subsequent occurrence of psychiatric symptoms (Zannas and Binder, 2014). Our results complement previous findings in relation to gene by environment effects of FKBP5 and early-life trauma and expand them to include a link between these factors and heavy drinking in young adults.
Previous work has implicated the FKBP5 rs1360780 minor T-allele as a risk allele for psychiatric symptoms in individuals exposed to early life trauma. We observed that the frequency of heavy drinking days did not differ by rs1360780 genotype in the absence of early life trauma. Furthermore, we did not observe a significant difference in the frequency of heavy drinking days comparing T-allele carriers exposed to early life trauma and those without a history of early adversity. Rather, we observed a lower frequency of heavy drinking days among rs1360780 C-allele homozygotes that had been exposed to childhood trauma compared to those without a positive history. This result suggests that individuals with the FKBP5 rs1360780 C/C genotype who experience early adversity may be protected from heavy drinking behavior as young adults.
Resilience, defined as an individual’s ability to adapt successfully to adversity, is an active process involving changes at the molecular level that result in normalization of biological and behavioral functions in the setting of stress (Russo et al. 2012). Resilience develops in approximately 10–25% of maltreated children (Walsh et al. 2010). The biological mechanisms leading to resilience in some children but not others exposed to early-life trauma are not understood but likely involve interactions between the type and timing of trauma/adversity and genetic variation, epigenetic responses, social supports and psychological factors. Related to our finding, Buchmann et al. (2014) examined the interaction between FKBP5 rs1360780 genotype and early life trauma on cortisol increases following the Trier Social Stress Test (TSST) in healthy young adults. They found that a history of early life trauma interacted with the FKBP5 rs1360780*C/C genotype to moderate TSST-induced increases in cortisol levels (Buchmann et al. 2014). This group also reported that higher levels of self-reported childhood emotional neglect were associated with reduced threat-related amygdala reactivity in rs1360780*C/C homozygotes (Holz et al. 2014). Interestingly, polymorphisms in the HPA axis-related gene encoding the corticotropin releasing hormone receptor 1 (CRHR1) have been shown to be protective with respect to effects of early-life trauma on the development of depression in adulthood (Bradley et al. 2008, Polanczyk et al. 2009, Grabe et al. 2010, Laucht et al. 2013). Similarly, functional genetic variation in the NPY gene, which encodes the anxiolytic neuropeptide Y, has been implicated in resiliency to stress (Zhou et al. 2008), and higher plasma levels of neuropeptide Y promote resilience to PTSD in combat-exposed veterans (Yehuda et al. 2006). Neuropeptide Y has also been implicated as protective from greater alcohol consumption in stress-exposed primates (Lindell et al. 2010). Although the drinking motives potentially linking the interactive effects of early-life stress and FKBP5 genotype on the frequency of heavy drinking in college students are not clear, the emerging literature on the effects of FKBP5 genotype suggest that they may relate to developmental effects of early-life stress × genotype interactions on hormonal stress response regulation (Buchmann et al. 2014) and/or developmental effects on neural systems related to threat/stress reactivity (White et al. 2012, Holz et al. 2014).
Strengths of the current study include: i) a large sample that was diverse in gender, race/ethnicity, and socioeconomic background, ii) self-reporting of alcohol consumption near its real-time occurrence, thus limiting recall error and bias, and iii) the examination of both the number of drinks per occasion as well as the frequency of heavy drinking in college students, which is a public health concern. We chose to examine the frequency of heavy drinking days as a primary outcome, as opposed to the number of standard drinks per day, as heavy drinking in college students has been associated with increased negative consequences, including academic, legal, and interpersonal problems (Engs et al. 1988, Wechsler et al. 1994). Furthermore, there is the potential for the pattern of heavy drinking to persist into adulthood (Schulenberg et al. 1996, Gotham et al. 1997, Schulenberg et al. 2002), which may increase risk for developing AUD. Weaknesses of the study include: i) the retrospective self-reporting of both early-life trauma and past-year life stress via questionnaire, ii) the variable attrition between the two study sites, iii) differences between included and excluded individuals regarding proportion reporting early life trauma (HBCU) or number of past year stressful life events (NEPU), and iv) the lack of distinction between the proportion of heavy drinking related to stress vs. social enhancement or emotion-related drinking. Because the frequency of stress-related drinking was not well defined, it is unclear whether FKBP5 genotype interacts with early-life trauma to produce differences in coping-related drinking or social-related activities in the college student sample examined. Recent reviews of the gene × environment interaction research literature have raised concerns that many studies have been underpowered, that there may be a publication bias where only significant interactions are reported, and that there has been a failure to replicate significant findings in many cases (Duncan et al. 2011, Dick et al. 2015). While these limitations must be considered when interpreting the results from our study, it is important to note that while other studies have attempted to identify novel gene × environment interactions, we chose to examine the widely-studied FKBP5 gene, which has reproducibly been reported to interact with stress to predict psychopathology (Zannas et al. 2014). Additionally, our study has more than five times the median sample size of the analysis conducted by Duncan et al. (2011) of 103 gene × environment studies. Notwithstanding, it is important to acknowledge the limitations of gene × environment interaction studies outlined above, and suggest that the research community attempt to replicate our findings to confirm the interactive effects of the FKBP5 rs1360780 polymorphism and early life trauma on heavy alcohol consumption.
In conclusion, we found that the FKBP5 rs1360780*C/C genotype interacts with self-reported history of early-life trauma to predict fewer days of heavy alcohol consumption in young adult college students. To our knowledge, this is the first study to implicate interactions between early life trauma and variation in FKBP5 with alcohol consumption. Importantly, these results are consistent with the assignment of risk and protective alleles at this locus with prior reports identifying interactions of rs1360780 with childhood trauma on risk of PTSD and depression.
Acknowledgments
We would like to thank Dr. Julian Ford from the Department of Psychiatry at the University of Connecticut Medical School in Farmington, CT, for providing the TESI questionnaire. We greatly appreciate the work of Kaitlin Clinton for her assistance in DNA extraction and genotyping and Jennifer Kim, Aquil Meeks, Nnenna Kalu, Gloria Cain, and Vanessa Marshall for assisting in subject recruitment and phenotype data collection. Supported by NIH grants P60 AA03510 (Alcohol Research Center), R21 AA017584 (JC and HT), K24 AA13736 (HRK), M01 RR06192 (UConn GCRC), M01 RR10284 (HU-GCRC). Dr. Kranzler has served as a consultant or advisory board member for the following companies: Alkermes, Lilly, Lundbeck, and Otsuka. He is a member of the Alcohol Clinical Trials Group of the American Society of Clinical Psychopharmacology, which is supported by AbbVie, Alkermes, Ethypharm, Lilly, Lundbeck, and Pfizer.
Footnotes
The authors declare no conflict of interests related to this work.
References
- Appel K, Schwahn C, Mahler J, Schulz A, Spitzer C, Fenske K, Stender J, Barnow S, John U, Teumer A, Biffar R, Nauck M, Volzke H, Freyberger HJ, Grabe HJ. Moderation of adult depression by a polymorphism in the FKBP5 gene and childhood physical abuse in the general population. Neuropsychopharmacology. 2011;36:1982–1991. doi: 10.1038/npp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua L, Carli V, Sarchiapone M, George DK, Goldman D, Roy A, Enoch MA. Interaction between FKBP5 and childhood trauma and risk of aggressive behavior. Arch Gen Psychiatry. 2012;69:62–70. doi: 10.1001/archgenpsychiatry.2011.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B, Papiol S, Seaman S, Lucae S, Kohli MA, Nickel T, Kunzel HE, Fuchs B, Majer M, Pfennig A, Kern N, Brunner J, Modell S, Baghai T, Deiml T, Zill P, Bondy B, Rupprecht R, Messer T, Kohnlein O, Dabitz H, Bruckl T, Muller N, Pfister H, Lieb R, Mueller JC, Lohmussaar E, Strom TM, Bettecken T, Meitinger T, Uhr M, Rein T, Holsboer F, Muller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Boscarino JA, Erlich PM, Hoffman SN, Zhang X. Higher FKBP5, COMT, CHRNA5, and CRHR1 allele burdens are associated with PTSD and interact with trauma exposure: implications for neuropsychiatric research and treatment. Neuropsychiatr Dis Treat. 2012;8:131–139. doi: 10.2147/NDT.S29508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ, Stowe ZN, Heim CM, Nemeroff CB, Schwartz A, Cubells JF, Ressler KJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65:190–200. doi: 10.1001/archgenpsychiatry.2007.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann AF, Holz N, Boecker R, Blomeyer D, Rietschel M, Witt SH, Schmidt MH, Esser G, Banaschewski T, Brandeis D, Zimmermann US, Laucht M. Moderating role of FKBP5 genotype in the impact of childhood adversity on cortisol stress response during adulthood. Eur Neuropsychopharmacol. 2014;24:837–845. doi: 10.1016/j.euroneuro.2013.12.001. [DOI] [PubMed] [Google Scholar]
- Chatterjee S, Hadi A, Price B. The use of regression analysis by example. New York: John Wiley and Sons; 2000. [Google Scholar]
- Clements K, Turpin G. The life events scale for students: Validation for use with British samples. Personality and Individual Differences. 1996;20:747–751. [Google Scholar]
- Covault J, Tennen H, Armeli S, Conner TS, Herman AI, Cillessen AH, Kranzler HR. Interactive effects of the serotonin transporter 5-HTTLPR polymorphism and stressful life events on college student drinking and drug use. Biol Psychiatry. 2007;61:609–616. doi: 10.1016/j.biopsych.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Archer LD. Relative frequency of heavy drinking and the risk of alcohol dependence. Addiction. 1993;88:1509–1518. doi: 10.1111/j.1360-0443.1993.tb03136.x. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Dick DM, Agrawal A, Keller MC, Adkins A, Aliev F, Monroe S, Hewitt JK, Kendler KS, Sher KJ. Candidate gene-environment interaction research: reflections and recommendations. Perspect Psychol Sci. 2015;10:37–59. doi: 10.1177/1745691614556682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engs RC, Hanson DJ. University students' drinking patterns and problems: examining the effects of raising the purchase age. Public Health Rep. 1988;103:667–673. [PMC free article] [PubMed] [Google Scholar]
- Enoch MA. The role of early life stress as a predictor for alcohol and drug dependence. Psychopharmacology (Berl) 2011;214:17–31. doi: 10.1007/s00213-010-1916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J, Rogers K. Empirically-based assessment of trauma and PTSD with children and adolescents; Proceedings from the International Society for Traumatic Stress Studies Annual Meeting; 1997. [Google Scholar]
- Ford JD, Grasso DJ, Hawke J, Chapman JF. Poly-victimization among juvenile justice-involved youths. Child Abuse Negl. 2013;37:788–800. doi: 10.1016/j.chiabu.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Ford JD, Racusin R, Ellis CG, Daviss WB, Reiser J, Fleischer A, Thomas J. Child maltreatment, other trauma exposure, and posttraumatic symptomatology among children with oppositional defiant and attention deficit hyperactivity disorders. Child Maltreat. 2000;5:205–217. doi: 10.1177/1077559500005003001. [DOI] [PubMed] [Google Scholar]
- Gotham HJ, Sher KJ, Wood PK. Predicting stability and change in frequency of intoxication from the college years to beyond: individual-difference and role transition variables. J Abnorm Psychol. 1997;106:619–629. doi: 10.1037//0021-843x.106.4.619. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Schwahn C, Appel K, Mahler J, Schulz A, Spitzer C, Fenske K, Barnow S, Lucht M, Freyberger HJ, John U, Teumer A, Wallaschofski H, Nauck M, Volzke H. Childhood maltreatment, the corticotropin-releasing hormone receptor gene and adult depression in the general population. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:1483–1493. doi: 10.1002/ajmg.b.31131. [DOI] [PubMed] [Google Scholar]
- Grad I, Picard D. The glucocorticoid responses are shaped by molecular chaperones. Mol Cell Endocrinol. 2007;275:2–12. doi: 10.1016/j.mce.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund PA, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes TH, Rahe RH. The Social Readjustment Rating Scale. J Psychosom Res. 1967;11:213–218. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- Holz NE, Buchmann AF, Boecker R, Blomeyer D, Baumeister S, Wolf I, Rietschel M, Witt SH, Plichta MM, Meyer-Lindenberg A, Banaschewski T, Brandeis D, Laucht M. Role of FKBP5 in emotion processing: results on amygdala activity, connectivity and volume. Brain Struct Funct. 2014 doi: 10.1007/s00429-014-0729-5. [DOI] [PubMed] [Google Scholar]
- Huang MC, Schwandt ML, Chester JA, Kirchhoff AM, Kao CF, Liang T, Tapocik JD, Ramchandani VA, George DT, Hodgkinson CA, Goldman D, Heilig M. FKBP5 moderates alcohol withdrawal severity: human genetic association and functional validation in knockout mice. Neuropsychopharmacology. 2014;39:2029–2038. doi: 10.1038/npp.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KP, Herman AI, Morean ME, Kranzler HR, Gelernter J, Sofuoglu M. FKBP5 variation is associated with the acute and chronic effects of nicotine. Pharmacogenomics J. 2015;15:340–346. doi: 10.1038/tpj.2014.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MC. Gene × environment interaction studies have not properly controlled for potential confounders: the problem and the (simple) solution. Biol Psychiatry. 2014;75:18–24. doi: 10.1016/j.biopsych.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM, Pace TW, Mercer KB, Mayberg HS, Bradley B, Nemeroff CB, Holsboer F, Heim CM, Ressler KJ, Rein T, Binder EB. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Babor TF, Lauerman RJ. Problems associated with average alcohol consumption and frequency of intoxication in a medical population. Alcohol Clin Exp Res. 1990;14:119–126. doi: 10.1111/j.1530-0277.1990.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Laucht M, Treutlein J, Blomeyer D, Buchmann AF, Schmidt MH, Esser G, Jennen-Steinmetz C, Rietschel M, Banaschewski T. Interactive effects of corticotropin-releasing hormone receptor 1 gene and childhood adversity on depressive symptoms in young adults: findings from a longitudinal study. Eur Neuropsychopharmacol. 2013;23:358–367. doi: 10.1016/j.euroneuro.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Lavebratt C, Aberg E, Sjoholm LK, Forsell Y. Variations in FKBP5 and BDNF genes are suggestively associated with depression in a Swedish population-based cohort. J Affect Disord. 2010;125:249–255. doi: 10.1016/j.jad.2010.02.113. [DOI] [PubMed] [Google Scholar]
- Levran O, Randesi M, Li Y, Rotrosen J, Ott J, Adelson M, Kreek MJ. Drug addiction and stress-response genetic variability: association study in African Americans. Ann Hum Genet. 2014;78:290–298. doi: 10.1111/ahg.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindell SG, Schwandt ML, Sun H, Sparenborg JD, Bjork K, Kasckow JW, Sommer WH, Goldman D, Higley JD, Suomi SJ, Heilig M, Barr CS. Functional NPY variation as a factor in stress resilience and alcohol consumption in rhesus macaques. Arch Gen Psychiatry. 2010;67:423–431. doi: 10.1001/archgenpsychiatry.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden W. Development and initial validation of a life event scale for students. Canadian Counsellor. 1984;18:106–110. [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma Family Health Patterns Project. Biol Psychiatry. 2012;71:344–349. doi: 10.1016/j.biopsych.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijk MP, Velders FP, Tharner A, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Jaddoe VW, Hofman A, Verhulst FC, Tiemeier H. FKBP5 and resistant attachment predict cortisol reactivity in infants: gene-environment interaction. Psychoneuroendocrinology. 2010;35:1454–1461. doi: 10.1016/j.psyneuen.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Mahon PB, Zandi PP, Potash JB, Nestadt G, Wand GS. Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology (Berl) 2013;227:231–241. doi: 10.1007/s00213-012-2956-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA. NIAAA Newsletter. 2004;3:3. [Google Scholar]
- Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001349. doi: 10.1371/journal.pmed.1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, Uher R, Poulton R, Moffitt TE. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry. 2009;66:978–985. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA. Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology. 2010;35:1674–1683. doi: 10.1038/npp.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Murrough JW, Han MH, Charney DS, Nestler EJ. Neurobiology of resilience. Nat Neurosci. 2012;15:1475–1484. doi: 10.1038/nn.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzberg AF, Keller J, Tennakoon L, Lembke A, Williams G, Kraemer FB, Sarginson JE, Lazzeroni LC, Murphy GM. HPA axis genetic variation, cortisol and psychosis in major depression. Molecular Psychiatry. 2014;19:220–227. doi: 10.1038/mp.2013.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenberg J, O'Malley PM, Bachman JG, Wadsworth KN, Johnston LD. Getting drunk and growing up: trajectories of frequent binge drinking during the transition to young adulthood. J Stud Alcohol. 1996;57:289–304. doi: 10.15288/jsa.1996.57.289. [DOI] [PubMed] [Google Scholar]
- Schulenberg JE, Maggs JL. A developmental perspective on alcohol use and heavy drinking during adolescence and the transition to young adulthood. J Stud Alcohol. 2002;(Suppl):54–70. doi: 10.15288/jsas.2002.s14.54. [DOI] [PubMed] [Google Scholar]
- Sinclair D, Fillman SG, Webster MJ, Weickert CS. Dysregulation of glucocorticoid receptor co-factors FKBP5, BAG1 and PTGES3 in prefrontal cortex in psychotic illness. Sci Rep. 2013;3:3539. doi: 10.1038/srep03539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stechschulte LA, Sanchez ER. FKBP51-a selective modulator of glucocorticoid and androgen sensitivity. Curr Opin Pharmacol. 2011;11:332–337. doi: 10.1016/j.coph.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh WA, Dawson J, Mattingly MJ. How are we measuring resilience following childhood maltreatment? Is the research adequate and consistent? What is the impact on research, practice, and policy? Trauma Violence Abuse. 2010;11:27–41. doi: 10.1177/1524838009358892. [DOI] [PubMed] [Google Scholar]
- Wechsler H, Davenport A, Dowdall G, Moeykens B, Castillo S. Health and behavioral consequences of binge drinking in college. A national survey of students at 140 campuses. JAMA. 1994;272:1672–1677. [PubMed] [Google Scholar]
- White MG, Bogdan R, Fisher PM, Munoz KE, Williamson DE, Hariri AR. FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity. Genes Brain Behav. 2012;11:869–878. doi: 10.1111/j.1601-183X.2012.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA, Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Brand S, Yang RK. Plasma neuropeptide Y concentrations in combat exposed veterans: relationship to trauma exposure, recovery from PTSD, and coping. Biol Psychiatry. 2006;59:660–663. doi: 10.1016/j.biopsych.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Zannas AS, Binder EB. Gene-environment interactions at the FKBP5 locus: sensitive periods, mechanisms and pleiotropism. Genes Brain and Behavior. 2014;13:25–37. doi: 10.1111/gbb.12104. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Zhu G, Hariri AR, Enoch MA, Scott D, Sinha R, Virkkunen M, Mash DC, Lipsky RH, Hu XZ, Hodgkinson CA, Xu K, Buzas B, Yuan Q, Shen PH, Ferrell RE, Manuck SB, Brown SM, Hauger RL, Stohler CS, Zubieta JK, Goldman D. Genetic variation in human NPY expression affects stress response and emotion. Nature. 2008;452:997–1001. doi: 10.1038/nature06858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Bruckl T, Nocon A, Pfister H, Binder EB, Uhr M, Lieb R, Moffitt TE, Caspi A, Holsboer F, Ising M. Interaction of FKBP5 gene variants and adverse life events in predicting depression onset: results from a 10-year prospective community study. Am J Psychiatry. 2011;168:1107–1116. doi: 10.1176/appi.ajp.2011.10111577. [DOI] [PMC free article] [PubMed] [Google Scholar]