Abstract
Bioterrorism agents that can be easily transmitted with high mortality rates and cause debilitating diseases pose major threats to national security and public health. The recent Ebola virus outbreak in West Africa and ongoing Zika virus outbreak in Brazil, now spreading throughout Latin America, are case examples of emerging infectious pathogens that have incited widespread fear and economic and social disruption on a global scale. Prophylactic vaccines would provide effective countermeasures against infectious pathogens and biological warfare agents. However, traditional approaches relying on attenuated or inactivated vaccines have been hampered by their unacceptable levels of reactogenicity and safety issues, whereas subunit antigen-based vaccines suffer from suboptimal immunogenicity and efficacy. In contrast, particulate vaccine delivery systems offer key advantages, including efficient and stable delivery of subunit antigens, co-delivery of adjuvant molecules to bolster immune responses, low reactogenicity due to the use of biocompatible biomaterials, and robust efficiency to elicit humoral and cellular immunity in systemic and mucosal tissues. Thus, vaccine nanoparticles and microparticles are promising platforms for clinical development of biodefense vaccines. In this review, we summarize the current status of research efforts to develop particulate vaccine delivery systems against bioterrorism agents and emerging infectious pathogens.
Introduction
The United States Centers for Disease Control and Prevention (CDC) has classified bioterrorism agents into three categories.1 Category A pathogens are associated with the highest risk to national security and public health due to their ease of dissemination and high mortality rates. Category B pathogens are of the second highest priority with moderate ease of dissemination and mortality rates while category C includes infectious pathogens that could be modified for widespread dissemination. Many of these emerging infectious pathogens are zoonotic without any available prophylactic vaccines or effective post-exposure treatments, and their natural outbreak or malicious dissemination can have grave consequences, as recently manifested during the outbreaks of Ebola virus in West Africa and Zika virus in Brazil that have caused widespread fear and economic and social disruption on a global scale. Therefore, there is an urgent need for vaccines that can elicit concerted cellular and humoral immune responses and establish protective immunity against pathogens with spared doses and short-term immunization regimens. In addition, it is critical to maintain robust public preparedness programs with sufficient vaccine stockpiles that can be distributed readily to protect the general public. Such organizational preparedness rooted in robust vaccine programs will also have the added benefits of deterring any terrorist organizations from developing and deploying biological weapons.
For decades, research efforts on countermeasures against emerging infectious pathogens and biological warfare agents have been focused on attenuated or inactivated whole-bacteria or whole-virus vaccines. Despite their strong immunostimulatory efficacy, pre-clinical and clinical studies performed with these traditional vaccines have raised serious concerns, as they have induced unacceptable levels of reactogenicity and caused inadvertent pathogenic infections with ill-prepared live-cell vaccines in the past.2–4 In contrast, molecularly defined subunit antigens derived from whole pathogens offer safer alternatives. However, subunit antigens are usually far less immunogenic than live and attenuated vaccines and are also more susceptible to deactivation and degradation. Recent advances in particulate vaccine delivery systems have addressed these challenges faced by subunit antigen-based vaccines.5–8 In particular, various biomaterial-based particulate carriers have been shown to protect encapsulated antigens from enzymatic degradation, co-deliver antigens together with adjuvants to lymphatic organs, and prolong the stability of vaccine products without the infrastructure for cold-chain.9 Synthetic delivery systems such as poly(lactic-co-glycolic acid) (PLGA) microparticles, liposomes, and lipid-based particles composed of FDA-approved materials have been intensely investigated by us and others for delivery of peptides, proteins, and DNA antigens.10–13 Use of biocompatible polymers and lipids can alleviate safety concerns often associated with viral vectors. Display of antigens on particle surfaces and formation of antigenic depots in situ can promote humoral immunity, characterized by robust, long-term, and balanced Th1/Th2 antibody responses.14, 15 Compared with soluble antigens which predominantly elicit humoral immune responses, particulate vaccines can enhance uptake and cross-presentation of antigens by dendritic cells and activate cytotoxic CD8+ T lymphocyte responses that are critical to eradicate intracellular infections.16–19 In addition, mucosal administration of nanoparticle vaccines can induce both mucosal and systemic immunity,20–23 thereby fortifying mucosal surfaces as the frontline of immunological defense against aerosolized bioterrorism agents or contaminated food and water supplies.
This review is focused on particulate vaccine delivery systems developed against emerging infectious pathogens, including Category A (Table 1) and B agents (Table 2) as defined and classified by the U.S. CDC. Each agent/disease is introduced with basic pathological facts and a brief history of vaccination approaches, followed by an overview on research efforts to develop particulate vaccines.
Table 1.
Particulate Vaccine Delivery Systems Investigated for Category A Bioterrorism Agents.
| Disease | Antigen | Delivery System | Adjuvants | Animal Model | Dosing Scheme | Major Results | Ref. |
|---|---|---|---|---|---|---|---|
| Anthrax | PA | PLA microparticle | None | Mice | i.m. or i.n., two doses | Complete protection mediated by robust levels of anti-PA IgG | 27 |
| A subunit of PA | PLGA nanoparticle | None | Mice | i.p., single dose | Balanced Th1/Th2 humoral immune responses; prolonged survival compared with a soluble vaccine | 28 | |
| PA | Nanoemulsion | None | Mice, guinea pigs | i.n., two doses | More than 3-fold higher anti-PA IgG and IgA titers than vaccines composed of other adjuvants including MPLA, CpG or Alum; protection rates of 70% and 40% following a low and high dose of pathogen challenge, respectively | 31 | |
| PA | Chitosan nanoparticle | Compound 48/80 | Mice | i.n., three doses | Enhanced mucosal and systemic humoral immune responses compared with soluble vaccines | 29 | |
| PA | Liposome | MPLA | Rabbits | i.m., two or three doses | Complete protection mediated by robust levels of neutralizing antibodies | 32 | |
| PA | Dextran microparticle | Resiquimod | Mice | s.c., two doses | Complete protection possibly by cellular immune responses | 30 | |
| PA | Aluminum nanoparticle | None | Mice | s.c., two doses | Durable anti-PA IgG titer for a month after the booster dose; enhanced antigen uptake, and milder inflammation at the injection site compared with the microparticle counterpart | 34 | |
|
| |||||||
| Plague | F1 and LcrV | PLA microparticle | None | Mice | i.t., i.m., or i.n., two doses | Successful elicitation of antigen-specific antibodies | 38 |
| F1 | PLGA/PEG microparticle | None | Mice | s.c., single dose | Complete protection mediated by robust anti-F1 IgG titers | 39 | |
| B- and T-cell epitopes of LcrV | PLGA microparticle | None | Mice | i.n., single dose | Balanced Th1/Th2 responses; protection rates varied between 0–90% depending on epitope sequences | 40 | |
| F1-V | Poly(anhydride) nanoparticle | None | Mice | i.n., single dose | Complete protection mediated by high avidity, antigen-specific IgG | 42 | |
|
| |||||||
| Plague | F1 | Gold nanoparticle | Alhydrogel® | Mice | s.c., single dose | Two- to four-fold higher titers and avidity of anti-F1 IgG than those elicited by a soluble vaccine or the particulate vaccine without adjuvant | 43 |
| LcrV | Lipoprotein nanoparticle | MPLA or CpG | Mice | i.p., single dose | Four-fold higher anti-V IgG titer than that elicited by soluble vaccines | 44 | |
| F1-V | Cationic lipid/hyaluronic acid hybrid nanoparticle | MPLA | Mice | i.n., three doses | Enhanced biocompatibility and 11-fold increase in serum titers of F1-V-specific IgG with balanced Th1/Th2 IgG subtypes | 23 | |
|
| |||||||
| Hemorrhagic fever caused by filoviruses | Irradiated whole Ebola virus | Liposome | MPLA | Mice, NHP | i.v. or i.m., two doses | Elicitation of cytotoxic T-lymphocyte responses; complete protection was achieved in the murine but not the NHP model | 59, 60 |
| Ebola GP and VP40 | VLP | None | Mice | i.m. or i.p., three doses | Activation of dendritic cells in vitro; elicitation of cellular and humoral immune responses in vivo; complete protection | 54 | |
| Ebola GP, NP, and VP40 | VLP | Ribi adjuvant | NHP | i.m., three doses | Elicitation of humoral and cellular immune responses; complete protection | 55 | |
| Ebola GP, Marburg GP, and VP40 | VLP | Poly I:C | Guinea pigs | Unknown route, two doses | The protection rate against a Marburg viral challenge was higher than 70%; protection rates against an Ebola viral challenge varied between 20%–70% for Ebola GP subunits with different immunogenicity | 58 | |
|
| |||||||
| Hemorrhagic fever caused by flaviviruses | WNV envelope protein | Gold nanoparticles of different sizes and shapes | None | Mice | i.p., two doses | Rod-like particles facilitated antigen uptake by antigen-presenting cells, whereas 40-nm nanospheres elicited the highest levels of antigen-specific antibodies and inflammatory cytokines in vivo | 65 |
| WNV envelope protein | PLGA nanoparticle | CpG | Mice | s.c., two doses | Th1-skewed humoral immune responses; a protection rate of 94%. | 66 | |
|
| |||||||
| Botulism | Subunit of the botulinum toxin | Pullulan nanogel | None | Mice | i.n., single dose | Prolonged nasal residence of the antigen within 12 h post immunization; robust titers of antigen-specific mucosal IgA and systemic IgG; complete protection | 72 |
|
| |||||||
| Tularemia | Membrane proteins of LVS | Liposome | IL-12 and Alum | Mice | s.c, i.p., or i.n., three doses | Complete protection against LVS but not a virulent strain | 73 |
| LVS lysates | Catanionic vesicle | None | Mice | s.c, i.p., or i.n., two to four doses | Protection rates were 100% and < 25% against LVS and a virulent strain, respectively | 74 | |
Abbreviations: PA, the protective antigen of Bacillus anthracis; PLA, poly-L-lactide; PLGA, poly(lactic-co-glycolic acid); PEG, polyethylene glycol; LcrV: low-calcium response V antigen of Yersinia pestis; F1-V, recombinant protein of capsular portion F1 and LcrV of Yersinia pestis; MPLA, monophosphoryl lipid A; LVS, live vaccine strain of attenuated Francisellar tularensis; GP, glycoprotein; NP, nucleoprotein; VP40, viral matrix protein 40 of filoviruses; VLP, virus-like particle; poly I:C, polyinosinic-polycytidylic acid; NHP, non-human primate; WNV, West Nile virus; i.m., intramuscular; i.n., intranasal; i.p., intraperitoneal; s.c., subcutaneous, i.t., intratracheal; i.v., intravenous.
Table 2.
Particulate Vaccine Delivery Systems Investigated for Category B Bioterrorism Agents
| Disease | Antigen | Delivery system | Adjuvants | Animal Model | Dosing Scheme | Major Results | Ref. |
|---|---|---|---|---|---|---|---|
| Brucellosis | Subcellular extraction | PCL microparticle | None | Mice | s.c., single dose | Similar protection efficacy as a live attenuated vaccine | 82 |
| Subcellular extraction | Mannosylated poly(anhydride) nanoparticle | None | Mice | Eye drop, single dose | Two-fold higher mucosal IgA titers and increased protective efficacy than those elicited by a live attenuated vaccine | 83 | |
| Subunit bacterial nucleoprotein | PLGA microparticle | None | Mice | i.p., two doses | High level of IFN-γ secreted by splenocytes from immunized mice; suboptimal protection compared with a live attenuated vaccine | 85 | |
| Bacterial T-cell epitopes | PLGA microparticle | None | Mice | s.c., two doses | Suboptimal protection compared with a live-attenuated vaccine | 86 | |
|
| |||||||
| Salmonellosis | Subcellular extraction | PVM/MA nanoparticle | None | Mice | i.p., single dose | Release of IFN-γ from splenocytes; complete protection | 92 |
| Vi polysaccharide | PLA nanoparticle or microparticle | Alhydrogel® | Mice | i.m., two doses (boosted with a low dose of soluble Vi) | Two-fold higher memory antibody responses compared with a soluble vaccine; humoral immune responses were further enhanced by display of antigens on the particle surface | 93 | |
|
| |||||||
| Shigellosis | OMVs | PVM/MA nanoparticle | None | Mice | i.d., i.n., oral, or ocular delivery, single dose | Complete protection through four vaccination routes | 97, 98 |
|
| |||||||
| Enterohemorrhage caused by E. coli O157:H7 | Killed whole bacteria | Liposome | MPLA | Mice | Oral, three doses | Elicitation of systemic and mucosal antigen-specific IgG and IgA | 103 |
| Stx | Liposome | None | Mice, NHP | i.p. single dose for mice, i.m. four doses for NHP | Complete protection against a challenge by Stx due to robust antigen-specific serum IgG levels | 104, 105 | |
|
| |||||||
| Poisoning by staphylococcal toxins | Inactivated SEB | PLGA microparticle | None | Monkey | i.m., i.t., or oral, two doses with different routes | Booster dose through the i.t. route achieved the highest antibody titers and best protection | 110 |
| Inactivated SEB | PLGA nanoparticle | None | Rabbit | s.c., single dose | Humoral immune responses were comparable to those elicited by a soluble vaccine using Alum as adjuvant | 111 | |
| α-haemolysin | Erythrocyte membrane-coated PLGA nanoparticle | None | Mice | s.c., single or three doses | Decreased skin toxicity, 10-fold higher antigen-specific IgG titers after booster doses, and increased protection rates compared with heat-inactivated toxin | 112 | |
|
| |||||||
| Cholera | Inactivated whole bacteria | PLGA microparticle | Amphotericin B | Mice | Oral, single dose | Antigen-specific serum IgG and IgM levels were 10- and 4-fold higher, compared with the soluble vaccine and were further enhanced by the adjuvant | 118 |
| Inactivated whole bacteria | PLGA or PLA/PEG microparticle | None | Mice | Oral, single dose | PLA/PEG particulate vaccine achieved the strongest humoral immune responses and the highest protection rate compared with PLGA counterparts | 119 | |
| Inactivated whole bacteria | Eudragit® plus alginate microparticle | None | Rat | Oral, two doses | Slightly higher vibriocidal titers than those induced by a soluble vaccine | 121 | |
|
| |||||||
| Melioidosis and Glanders | None | Cationic liposomes | CpG | Mice | i.n., single dose | Animals were 100% protected from an aerosol challenge with B. pseudomallei or B. mallei by enhanced IFN-γ secretion and activation of NK cells, but chronic diseases still occurred | 129 |
| Bacterial LPS | Gold nanoparticle | Alhydrogel® | Mice, NHP | i.n. for mice, s.c. for NHP, three doses | Protection was observed in the murine but not the NHP model | 130, 131 | |
|
| |||||||
| Intoxication with ricin toxin | Inactivated toxin | PLGA microparticle | None | Mice | s.c. for a single dose; i.n. for two doses | A single s.c. dose elicited systemic humoral immune responses, lasting for 1 year post immunization; two i.n. doses achieved 100% protection | 137, 138 |
| Inactivated toxin | Liposome | None | Rat | i.t., two doses | Similar humoral immune responses and protection rates compared with soluble vaccines; absence of lung inflammation post an aerosol challenge | 140, 141 | |
|
| |||||||
| Viral encephalitis caused by alphaviruses | Inactivated VEEV | PLGA microparticle | None | Mice | s.c. or i.t. for the prime dose, s.c., i.t., or oral delivery for one booster dose | Higher activity of antigen-specific IgG and IgA, and 100% protection by the i.t. route than other routes for the booster dose | 145 |
Abbreviations: PCL, poly(ε-caprolactone); PVM/MA, poly(methyl vinyl ester/maleic acid); PLA, poly-L-lactide; PLGA, poly(lactic-co-glycolic acid); OMVs, outer membrane vesicles; Stx, Shiga toxin; SEB, staphylococcal enterotoxin B; PEG, polyethylene glycol; LPS, lipopolysaccharides; NHP, non-human primate; VEEV, Venezuelan equine encephalitis virus; s.c., subcutaneous; i.p., intraperitoneal; i.m., intramuscular; i.d., intradermal; i.n., intranasal; i.t., intratracheal.
PARTICULATE VACCINES AGAINST CATEGORY A BIOTERRORISM AGENTS
Category A agents include bacteria causing anthrax, plague, botulism, and tularemia as well as viruses leading to smallpox and viral hemorrhagic fevers. Although there are successful vaccines, perhaps best exemplified by the control and elimination of smallpox epidemic, vaccines for other causative pathogens in Category A are still in investigational status and are of the highest priority for biodefense research. To protect the general public from unpredictable events such as documented anthrax terror attacks and recent outbreaks of emerging infectious diseases, caused by Ebola virus and Zika virus, there is an urgent need to expedite pre-clinical development and clinical translation of promising vaccine candidates. Synthetic particulate systems for delivery of subunit antigens have been widely examined to potentiate both humoral and cellular immune responses. Particulate vaccines under investigation for Category A agents are summarized in Table 1.
Anthrax
Anthrax is an epizootic disease commonly affecting hoofed animals, and humans can also be infected upon contact with infected animals or their products. Anthrax is one of the most dangerous bioterror agents because spores of its pathogen, Bacillus anthracis, can survive for decades or even centuries under extreme temperature or chemical treatment and can be easily aerosolized and disseminated.24 The threat of anthrax was realized by the 2001 U.S. postal attack that left five people killed and thousands exposed to the pathogen.25 To date, two prophylactic vaccines against anthrax have been developed. A live-spore vaccine was an effective formulation but often caused severe toxicity at injection sites; therefore, it has since been replaced by the current anthrax vaccine, AVA, registered as BioThrax®.26 AVA is produced by adsorbing the formalin-treated culture filtrates of a toxigenic but avirulent anthrax strain onto aluminium hydroxide. Although AVA can elicit robust humoral immune responses, it requires at least three initial vaccinations and yearly boosts for generation of long-term memory immune responses.24 To address the limitations of current anthrax vaccines, subunit vaccines delivered by various particulate systems including polymeric nano/microparticles,27–30 nanoemulsions,31 and liposomes32 have been investigated and shown to improve the protective efficacy while reducing the number of administrations necessary to produce robust immune responses. In particular, the anthrax protective antigen (PA), which is a non-toxic, cell-binding component derived from the anthrax toxin, has been demonstrated to induce protection by elicitation of robust antigen-specific antibodies in both animal models and humans.33 A pilot study demonstrated that encapsulation or attachment of PA to poly-L-lactide (PLA) microparticles enhanced immune responses.27 When delivered through either the intramuscular or intranasal route, the vaccine particles protected all immunized mice against anthrax infection. In another approach, an intranasal vaccine was prepared by formulating PA with a water-in-oil nanoemulsion system, which elicited higher levels of mucosal anti-PA IgA and IgG than conventional adjuvants and conferred robust protection against an intranasal challenge with B. anthracis live spores (Figure 1).31 Immunization of PA absorbed on aluminium hydroxide nanoparticles of ~0.1 μm in diameter increased the serum level of anti-PA IgG and reduced skin inflammation at the injection site, compared with vaccination with microparticles of ~10 μm.34 In addition to serving as adjuvants, particulate carriers can mediate co-delivery of antigen and danger signals, therefore amplifying immune responses. Intranasal immunization with chitosan nanoparticles co-loaded with PA and the adjuvant compound 48/80 achieved both mucosal and systemic humoral immune responses with a lower dose of PA, compared with soluble vaccines.29 Liposomes co-encapsulating PA and monophosphoryl lipid A (MPLA), a Toll-like receptor (TLR) 4 agonist, were shown to elicit higher levels of toxin-neutralizing antibodies, compared with PA adsorbed on Alhydrogel® or PA displayed on bacteriophages.32 Interestingly, dextran microparticles co-encapsulating PA and resiquimod, a TLR 7/8 agonist, exhibited robust protective efficacy without raising high neutralizing antibody responses. Upon re-stimulation, splenocytes from immunized mice secreted high levels of IL-2 and IFN-γ,30 suggesting that cellular immune responses may contribute to a successful anthrax vaccine.
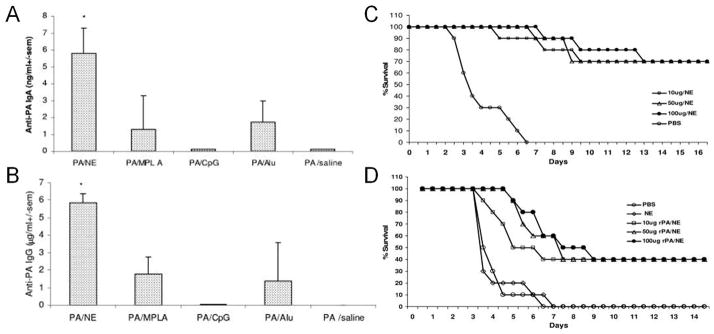
Figure 1.
A nanoemulsion (NE) system formulated with the anthrax protective antigen (PA) enhanced mucosal humoral immune responses and improved protection against bacterial spore challenges. Compared with conventional adjuvants, such as MPLA, CpG, and aluminium hydroxide, the NE vaccine administered via the intranasal route elicited higher titers of anti-PA IgA (A) and IgG (B) in bronchial alveolar lavage fluids from immunized mice. Vaccination with the NE vaccine also protected guinea pigs against an intranasal challenge with a 10-fold (C) or 100-fold (D) LD50 dose of bacterial spores. Reproduced with permission from ref. 31.
Plague
Plague, caused by infection with Yersinia pestis, is an ancient zoonotic disease that is naturally carried in rodent reservoirs. The disease can be transmitted to humans by direct contact with infected rodents or bites from inflected fleas from these rodents that cause bubonic plague, or by pathogenic aerosols that cause pneumonic plague. Historically, plague had been one of the most devastating epidemic diseases, including the infamous Black Death pandemic in the 14th century that killed one third of the European population. In particular, aerosolized Yersinia pestis can result in deadly pneumonic diseases with a mortality rate of 50–90% without treatment.35 Although an intensive antibiotic therapy can reduce the mortality rate associated with pneumonic plague down to ~15%, it must be given within 24–36 h after exposure.35 In addition, there are reported cases of multidrug-resistant strains, raising concerns about antibiotic therapies.36 Therefore, vaccine development against plague is an indispensable strategy for biodefense. Two types of plague vaccines have been used since the late 19th century: a killed whole-cell vaccine, which is only protective against bubonic plague, and a live whole-cell vaccine, which generates protective immunity against both bubonic and pneumonic plagues.2 However, both vaccines have been discontinued due to local and systemic side effects, such as regional lymphadenopathy, anorexia, and mild fever, long-term booster doses required, and safety concerns with the live bacterial vectors. Current research efforts have been mainly focused on the development of safer subunit vaccines utilizing the capsular subunit protein F1 and low-calcium response V antigen (LcrV). F1 and V, screened from a panel of key virulent factors from Yersinia pestis, have been reported to be promising antigens against bubonic and pneumonic plagues in various animal models.37 Polymeric microspheres have been tested as vaccine carriers to potentiate the efficacy of subunit plague antigens.38–40 PLA microspheres co-encapsulating F1 and V were shown to elicit superior humoral immune responses, compared with soluble vaccines irrespective of administration routes.38 A recent study on F1-loaded PLGA/PEG microspheres showed that a single vaccine dose was sufficient to protect mice from challenge.39 Nanoparticle delivery systems composed of poly(anhydride),41, 42 gold,43 lipoproteins,44 or a hybrid of lipids and biopolymers23 have been developed for plague vaccines. A single intranasal dose of recombinant F1-V-loaded poly(anhydride) nanoparticles led to prolonged lung disposition41 and generated high levels of antigen-specific antibody responses,42 with the overall kinetics dictated by the chemical composition, hydrophobicity, and degradation rate of poly(anhydride) particles.45 In another approach, F1 antigen was conjugated on the surfaces of gold nanoparticles via EDC/NHS chemistry, and the conjugates were resuspended in Alhydrogel®.43 This vaccine system elicited higher titers of both anti-F1 IgG1 and IgG2a than those elicited by the vaccine without Alhydrogel® as well as the soluble F1 mixed with the adjuvant. Lipid-based delivery systems have also shown promising results for delivery of subunit plague antigens.23, 44 Nanolipoprotein particles were constructed with lipids, cholate, and apolipoprotein that self-assembled into nanostructures mimicking high density lipoproteins (Figure 2A).44 V antigen was terminally modified with poly-histidine for complexation with nickel-modified lipids while MPLA or cholesterol-modified CpG was co-encapsulated via lipid insertion. The resulting vaccine particles significantly enhanced V-specific IgG titers in mice, compared with the physical mixture of V antigen and soluble or particulate adjuvants (Figure 2B). Recently, we have developed a lipid/biopolymer hybrid nanoparticle system, composed of cationic lipids and an anionic polymer hyaluronic acid, for intranasal delivery of F1-V (Figure 2C).23 Shielding of cationic liposomes with hyaluronic acid significantly reduced cytotoxicity of cationic lipids by at least 20-fold in dendritic cells. When administered via intranasal route in mice, the hybrid nanoparticles co-loaded with F1-V and MPLA generated potent humoral immune responses with 11-, 23-, and 15-fold higher titers of anti-F1-V total IgG, IgG1, and IgG2c, respectively, and a more balanced Th1/Th2 response, compared with the soluble vaccine (Figure 2D). It remains to be seen how these lipid-based nanoparticles perform against pneumonic plague.
Figure 2.
Lipid-based nanoparticles for delivery of subunit plague antigens. (A) A nanolipoprotein particle loaded with adjuvants and LcrV modified with poly-histidine. (B) Co-delivery of LcrV and adjuvants via the intraperitoneal route elicited higher anti-LcrV IgG titers than LcrV admixed with or without soluble or particulate adjuvants. (C) A cationic lipid/hyaluronic acid (HA) hybrid nanoparticle formed by cross-linking of thiolated HA and thiolated polyethylene glycol (PEG). (D) Intranasal vaccination with the hybrid particles co-loaded with F1-V and MPLA elicited significantly higher serum titers of anti-F1-V IgG, compared with the soluble mixture of F1-V and MPLA. (A) and (B) reproduced with permission from ref. 44; (C) and (D) reproduced with permission from ref. 23.
Hemorrhagic fever caused by filoviruses and flaviviruses
Filoviruses
Filoviruses, including Marburg virus and Ebola virus, are the main causative pathogens for hemorrhagic fever in humans, which is a deadly disease transmitted by direct contact with infected subjects. Since their discovery in 1970s, several outbreaks resulted in fatality rates ranging from 25% to 90%. At the time of this writing, no vaccine or specific antiviral drug is available for the disease. The recent Ebola outbreaks in Africa have intensified research efforts to develop Ebola vaccines, resulting in two vaccine candidates, rVSV-EBOV and ChAd3-ZEBOV, both of which have entered Phase III trials as of late 2015.46 Historically, development of vaccines for Ebola began with inactivated whole viruses, and a whole virion inactivated by formalin was shown to provide better protection than the gamma-irradiation approach.47 More recently, a replication-defective whole-virus vaccine showed complete protection in a pilot trial on non-human primates.48 Given the variable potency of inactivated viruses and emergence of mutant strains, the primary vaccine approach has shifted from the direct use of whole virions to over-expression of genes encoding the Ebola glycoprotein (GP) and nucleoprotein (NP) in the host to elicit potent humoral and cellular immune responses. Specifically, immunity can be elicited by replication-deficient recombinant adenoviruses or plasmid vectors that transduce Ebola antigens or by attenuated recombinant viruses bearing Ebola GPs on their surfaces.49 However, booster immunizations are often required, and safety concerns remain for viral vectors.49 For instance, although recombinant vesicular stomatitis virus (rVSV) expressing Ebola GPs achieved complete protection under a “ring vaccination” scheme in a recent clinical trial,50 previous pre-clinical studies in non-human primates have reported cases of vector-induced viremia;51, 52 thus, safety and compliance concerns need to be meticulously addressed in the ongoing clinical trials. As an alternative to the viral vector-based approaches described above, virus-like particles (VLPs) are the most promising vector-free vaccine platform in the pre-clinical pipeline for Ebola vaccines. Filovirus GPs along with a viral matrix protein VP40 have been produced from mammalian cell lines and self-assembled into VLPs (Figure 3A).53 Immunization with Ebola VLPs completely protected mice and non-human primates from a viral challenge (Figure 3B).54, 55 Follow-up studies revealed that the protection was dependent on type I interferons (IFNs),56 and VLPs combined with polyinosinic-polycytidylic acid (polyI:C),57 a TLR3 agonist capable of driving the production of type I IFNs, significantly augmented cellular and humoral immune responses (Figure 3C and D). In addition, a trimeric hybrid VLP was constructed to express GPs of the Marburg virus, Ebola Zaire and Sudan viruses.58 Immunization with these VLPs induced protection rates higher than 70% against a Marburg challenge but varying rates from 20% to 70% against an Ebola challenge depending on subunits of the Ebola GP used for the VLPs. In a separate line of studies, a liposomal formulation encapsulating irradiated Ebola virions and lipid A as adjuvant has been shown to elicit cytotoxic T-lymphocyte responses and achieve a protection rate of ~100% in a murine model; however, this liposomal vaccine failed to protect non-human primates from lethal challenge.59, 60 These results imply the daunting task of moving nanoparticle vaccines from small to large animal models, as various preclinical animal models exhibit different patterns of pathogenesis and susceptibility to a particular viral infection as well as varying degrees of immune responses elicited by vaccines. In addition, it remains to be seen how synthetic nanoparticles compare with widely explored VLPs in terms of safety profiles, reactogenicity, and immunogenicity against viral challenge in large animal models.
Figure 3.
Virus-like particles (VLPs) as an Ebola vaccine candidate. (A) Transmission electron microscope images of Ebola viruses (left) and VLPs (right). (B) Mice were immunized three times with Ebola VLPs (eVLPs), inactivated Ebola viruses (iEBOV) or Marburg viruses (iMARV), or PBS, followed by a challenge with the mouse-adapted Ebola virus. (C) and (D) Humoral and cellular immune responses elicited by Ebola VLPs were augmented by polyI:C. (C) A low dose of VLPs along with 100 ng-100 μg polyI:C elicited high serum titers of antigen-specific IgG. (D) Splenocytes from immunized mice were cultured with Ebola GP, followed by stimulation with an Ebola GP peptide in vitro. Robust effector T cells were induced by immunization with 10 μg VLPs and polyI:C. (A) and (B) reproduced with permission from ref. 54; (C) and (D) reproduced with permission from ref. 57.
Flaviviruses
In contrast to filoviruses that primarily infect primates, flaviviruses are naturally hosted in arthropods and only occasionally transmitted to humans by bites from infected mosquitoes or ticks. The pathogen family includes more than 70 different viruses, of which six agents are mainly responsible for disease burdens in humans: yellow fever virus, dengue virus, Japanese encephalitis virus, West Nile virus (WNVs), tick-borne encephalitis virus, and Zika virus.61 Diseases caused by some of these agents have been controlled by successful live-attenuated or killed whole-virus vaccines.62 In particular, the first tetravalent dengue vaccine that provides protection against all four dengue virus serotypes has been approved in Mexico, Philippines, and Brazil in December 2015.63, 64 However, there are currently no effective therapeutic interventions or prophylactic vaccines against WNV. To address the shortage of available vaccines, particulate platforms have been developed for vaccine delivery of WNV antigens. One study has compared immune stimulatory effects elicited by gold nanoparticles of different sizes and shapes and coated with WNV envelope protein antigen on their surfaces.65 Although rod shape particles were most favorable for antigen uptake by macrophages and dendritic cells, 40-nm spherical gold nanoparticles elicited the highest titer of antigen-specific antibodies, which were 2-fold greater than those induced by gold nanorods. In another approach, PLGA nanoparticles were used to encapsulate WNV envelope protein in the particle core and display CpG on particle surfaces.66 Compared with Alhydrogel® which predominantly drove Th2 responses, the nanoparticle vaccine skewed humoral immune responses to the Th1 type, eliciting antigen-specific cellular immunity, and protecting 94% of animals against a viral challenge in mice. These studies highlight promising engineering approaches of delivery carriers to effective particulate vaccines against WNV.
On the other hand, Zika virus first discovered in 1950s has received little attention67 and remained as one of the neglected tropical diseases without any research effort devoted to the vaccine development until the recent outbreak in Brazil. As of this writing, the World Health Organization has declared an international public health emergency on the ongoing Zika virus outbreak that is now spreading throughout Latin America. Zika infection can easily spread through mosquito bites, and there is also a report of suspected transmission by sexual intercourse.68 While Zika infection does not trigger perceivable symptoms in infected hosts, it may endanger fetus development in pregnant women, causing infant microcephaly birth defects.69 This has prompted world leaders to devote more resources to research on vaccine development and diagnostics as well as disease control and public preparedness to combat against the global health threat.
Botulism
The botulinum toxin, produced by Clostridium botulinum, triggers neuroparalytic diseases and is regarded as one of the most lethal poisons.70 The disease can occur due to accidental food poisoning or inhalation of maliciously dispersed bacterial spores.71 Interestingly, the toxin can also be used to treat neurological disorders such as dystonia when locally injected at an appropriate dose.70 Formalin-inactivated toxoid vaccines have been developed previously, including a pentavalent vaccine registered as an Investigational New Drug in the US.3, 71 However, this vaccine was discontinued in 2011 due to declining potency and increasing reactogenicity following annual boosts, and currently there is no botulism vaccine available.3 For vaccination with a non-toxic subunit of the botulinum toxin, an adjuvant-free nanogel system has been prepared by self-assembly of polysaccharide pullulan modified with cholesteryl and amino groups (Figure 4A).72 Compared with a soluble vaccine, the nanogel significantly increased the residence time of antigen in nasal epithelium upon intranasal vaccination (Figure 4B) and elicited robust antigen-specific mucosal IgA and systemic IgG responses (Figure 4C), achieving complete protection of animals against an intraperitoneal or intranasal challenge with Clostridium botulinum neurotoxin (Figure 4D). Apart from these studies, there have been only limited research efforts for vaccine development against botulinum toxin. Given the promising results presented here, nanoparticle-based vaccine approaches warrant further investigations.
Figure 4.
A cationic nanogel developed for intranasal delivery of a subunit botulism neurotoxin. (A) The nanogel was self-assembled by the polysaccharide pullulan modified with cholesteryl and amino groups. Intranasal immunization with antigen-loaded nanogels significantly enhanced nasal residence of the antigen (B), antigen-specific antibody titers (C), and protection against challenge with the neurotoxin (D), compared with the soluble antigen. Reproduced with permission from ref. 72.
Tularemia
Tularemia is caused by Francisellar tularensis which infects both animals and humans. There are various pathogenic strains that can cause ulceroglandular, gastroinstestinal, oropharyngeal, or pneumonic tularemia.4 Similar to plague, the pneumonic form of tularemia poses the highest public health risk and is the major concern for bioterrorism due to its low lethal dose, high virulence, and ease of aerosol dissemination. Although an attenuated live vaccine strain (LVS) was developed for vaccination, it has been terminated due to potential safety issues associated with pathogenic mutations of the vaccine strain and unsatisfactory efficacy.4 Particulate delivery approaches for tularemia vaccines have been mostly focused on lipid-based nanoparticles and have offered some initial success. In one example, vaccination with synthetic liposomes incorporating the membrane proteins of LVS and alum plus IL-12 protected mice against a LVS challenge but not against the virulent strain.73 In another study, bacterial lysates were loaded into catanionic vesicles formed by surfactants with opposite charges.74 Immunized mice were completely and partially protected from challenge with LVS and a virulent strain, respectively. Transferring the immune sera into naïve mice also protected recipients against a LVS challenge, indicating the importance of humoral immunity. In addition, archaeal lipids have been tested as an adjuvant for LVS lysates.75 Intranasal immunization with this vaccine elicited high antigen-specific antibody titers in serum and bronchial lavage fluids as well as cellular immune responses, characterized by the proliferation of antigen-specific splenocytes and IL-17 secretion. In fact, cellular immunity directed against intracellular Francisellar tularensis may play a vital role in vaccination against tularemia as the bacteria can evade phagocytic degradation and reside within macrophages.76, 77 As demonstrated by these studies, it is relatively easy to show the protective efficacy of vaccines against LVS. Future studies should be directed to enhance immunogenicity of particulate vaccines and assess their potency in more stringent animal models utilizing virulent strains of Francisellar tularensis.
PARTICULATE VACCINES AGAINST CATEGORY B BIOTERRORISM AGENTS
Category B agents are of the second highest priority due to their moderate ease of dissemination, morbidity, and mortality. Several pathogens of this category, including salmonellosis, shigellosis, and cholera, are mainly transmitted by the fecal-oral route. Although their natural outbreaks can be prevented by improving hygiene resources in epidemic areas, prophylactic vaccines are needed for protection against malicious dissemination by aerosolized pathogens or contaminated food and water supplies. Particulate vaccines investigated for Category B agents are summarized in Table 2.
Brucellosis
Brucellosis is a zoonosis that mainly infects livestock, such as cattle, swine, and goats. Brucellosis is caused by gram-negative Brucella species, among which B. melitensis causes the most severe disease with frequent debilitating relapses.78 Brucella is transmitted to humans by fluid discharges from infected animals, especially dairy products, or by aerosol dissemination.79 Although Brucellosis is currently controlled by vaccination of susceptible livestock, these veterinary vaccines are pathogenic to humans.80 Several attenuated Brucella strains and extracted bacterial fractions have been used as vaccines in humans in the last century. However, none of these are in use nowadays due to suboptimal efficacy.81 To remedy these issues, many particulate delivery systems have been examined. In one study, a hydrophobic portion of the bacterial extract was encapsulated in microparticles composed of poly (ε-caprolactone), β-cyclodextrin, and Pluronic F68.82 This vaccine protected mice against B. melitensis challenge with a similar efficacy as the live attenuated vaccine. In another study, subcellular bacterial extracts were loaded in poly(anhydride) nanoparticles modified with mannose and delivered via the ocular route to target the mucosal immune system.83 A single vaccination dose achieved higher IgA titers and improved protection, compared with the live attenuated vaccine. Since Brucella species predominantly reside in macrophages and monocytes, cellular immunity, supported by the killing of infected cells by cytotoxic CD8+ T lymphocytes and secretion of Th1 cytokines by CD4+ T cells, was shown to be vital for elimination of the bacteria.78, 84 In order to amplify cellular immunity against Brucella, a subunit nucleoprotein or T-cell epitopes derived from the bacteria have been encapsulated in PLGA microparticles. Although these particles offered protection against virulent Brucella infection, their efficacy was suboptimal compared with the live attenuated vaccine.85, 86 Hence, future studies should be devoted to developing particulate systems capable of eliciting potent, concerted humoral and cellular immune responses against Brucellosis.
Food safety threats
Salmonellosis
The gram-negative bacterium Salmonella, especially S. enterica, is an enteric pathogen and a common cause of food-borne diarrheal illness. Four serovars of S. enterica, namely Typhi, Paratyphi A, Typhimurium, and Enteritidis, are responsible for severe infections in humans, with the former two types causing enteric fever and the latter two types causing the invasive nontyphoidal Salmonella (iNTS) disease.87 Every year there are 21.7 million cases of infection and 200,000 fatalities due to typhoid fever from Typhi infection.88 Current vaccines against Salmonella are limited to the Typhi serotype, and they include an inactivated whole-cell vaccine, a live-attenuated vaccine made of a mutant strain Ty21a, and a subunit vaccine made of Vi polysaccharide.89 Among these, the whole-cell vaccine has achieved the highest three-year cumulative efficacy of 73%; however, high reactogenicity limits its general use.87 Ty21a and Vi polysaccharide vaccines have protection rates of ~50% with reduced adverse reactions, but neither is suitable for infants, who suffer most from the disease.87, 90 In addition, the emerging antibiotic-resistant strains provide further motivation for the development of new vaccine products.91 Particulate delivery systems have been examined to improve the efficacy of subunit Salmonella vaccines. Subcellular extracts from the Enteritidis serovar were encapsulated into nanoparticles composed of copolymer poly(methyl vinyl ester/maleic acid) (PVM/MA).92 The particulate formulation elicited robust IFN-γ release from splenocytes in immunized mice and completely protected animals against a lethal challenge. In another study, Vi polysaccharide delivered by PLA nanoparticles or microparticles induced higher levels of antigen-specific IgG and memory antibody responses than a soluble vaccine.93 Furthermore, high density of antigens displayed on the surfaces of PLA particles correlated with robust humoral immune responses. These results suggest that engineering of the interface between antigen-displaying vaccine particles and B-cells is crucial to prime strong humoral immunity. In addition, particulate delivery of compiled subunit antigens for individual serotypes or a common subunit, e.g. a conserved region across all four pathogenic serotypes, may lead to a successful Salmonella vaccine.
Shigellosis
The enteric pathogen Shigella, which is usually transmitted by the fecal-oral route, is another major cause of diarrhea. Four Shigella species have been identified to date: S. dysenteriae, S. flexneri, S. sonnei, and S. boydii, among which the first three are more common for human enteric diseases.94 Specifically, the invasive S. dysenteriae Type 1 causes dysentery and life-threatening kidney damage by releasing Shiga toxin.95 Currently, there is no vaccine available for Shigellosis, but several vaccine candidates are undergoing clinical trials.95 Due to their favorable safety profiles, subunit vaccines are ideal alternatives to live-attenuated vaccines, as vaccine safety is of the utmost importance for Shigella vaccines as children under 5 years old are most vulnerable to Shigellosis.96 In one approach, outer membrane vesicles (OMVs) from S. flexneri have been encapsulated into nanoparticles made of copolymer PVM/MA as a subunit vaccine.97, 98 After intradermal, oral, or intranasal immunization, the vaccine nanoparticles completely protected mice against a pathogen challenge. Notably, future studies should be directed to optimize particulate delivery systems for mucosal vaccination against Shigella as such strategy may offer a key advantage to effectively stop its transmission at mucosal surfaces.
Escherichia coli O157:H7
Although the majority of E. coli strains are benign inhabitants in the human gastrointestinal tract, enterohemorrhagic E. coli strains, which produce the Shiga toxin (Stx), can cause diarrheal illness, even hemorrhagic colitis, and hemolytic uremic syndrome.99 E. coli O157:H7 is the predominant serotype responsible for frequent outbreaks.100 Cattle are the natural host of E. coli O157:H7, and humans can be infected by consumption of contaminated meat.101 Although vaccines for cattle are used to control the disease transmission,102 it is difficult to eliminate the human disease burden due to other animal and environmental reservoirs of the pathogen.101 To develop vaccines for human use, liposomes have been investigated as a vaccine carrier for either the whole bacteria or subunit bacterial proteins. Killed bacteria along with adjuvant MPLA have been co-incorporated into liposomes to produce an oral vaccine, which elicited both systemic and mucosal IgA and IgG antibodies specific to the pathogen.103 Alternatively, Stx was conjugated to amine-modified liposomal surfaces via glutaraldehyde-mediated reaction, which also inactivated the toxin during the coupling process.104 This liposomal vaccine induced robust antigen-specific serum IgG titers and conferred protection against an intravenous toxin challenge in both murine and non-human primate models.104, 105 Recently, a novel anti-bacterial vaccine approach has been developed by coating OMVs on nanoparticle templates.106 OMVs derived from a model pathogen Escherichia coli were stably coated on the surfaces of gold nanoparticles which enhanced activation of dendritic cells, elicited higher serum titers of antigen-specific IgG, and robust cytokine secretion from splenocytes after a single subcutaneous injection in mice, compared with vaccination with native OMVs.
Staphylococcal toxins
Staphylococcal toxins, especially staphylococcal enterotoxin B (SEB) secreted by the gram-positive bacterium Staphylococcus aureus, are a common cause of food poisoning,107 while inhaled SEB provokes more serious syndromes. SEB is a “superantigen” that can lead to hyper T-cell activation and “cytokine storm”, characterized by massive secretion of TNF-α, IFN-γ, IL-1, IL-2, and IL-6.108 Initial attempts to develop SEB vaccines were focused on the formalin-inactivated toxin and recombinant mutant strains, but there are still no agents available to protect against SEB or treat SEB intoxication.109 To address these challenges, PLGA particles have been used to deliver inactivated SEB.110, 111 When multiple routes of vaccine delivery were tested with PLGA microparticles, an intratracheal booster dose offered the highest rate of protection in monkeys against a challenge with aerosolized SEB, compared with the intramuscular or oral route of vaccination.110 Inactivated SEB delivered by PLGA nanoparticles also elicited humoral immune responses comparable to those elicited by a vaccine formulation with alum.111 In an alternative approach, staphylococcal α-haemolysin was trapped by erythrocyte membranes coated on the surfaces of PLGA nanoparticles (Figure 5A).112 Compared with heat-inactivated toxin, this nanotoxoid vaccine alleviated toxicity at injection sites while enhancing antigen-specific serum IgG titers and protecting mice against a toxin challenge (Figure 5B).
Figure 5.
Erythrocyte membrane-coated PLGA nanoparticles for vaccine delivery of staphylococcal α-haemolysin (Hla). (A) Schematic illustration and an image by transmission electron microscope of the nanotoxoid. Scale bar, 80 nm. (B) The nanotoxoid vaccine enhanced humoral immune responses and protected mice against toxin challenge. Empty triangles, vaccine particles without the antigen; solid triangles, unvaccinated control; blue squares, single dose of the heat-inactivated Hla; blue spheres, single dose of the nanotoxoid; red squares, three doses of the heat-inactivated Hla; red spheres, three doses of the nanotoxoid. Reproduced with permission from ref. 112.
Cholera
The gram-negative bacterium Vibrio cholerae is transmitted to humans most often by contaminated drinking water and causes the acute diarrheal disease cholera.113 V. cholerae has been classified into more than 200 serogroups according to different O antigens of the bacterial lipopolysaccharides (LPS), and only O1 and O139 strains are known to cause epidemic cholera.114 Currently there are three oral vaccine products available: Dukoral®, mORC-Vax™, and Shanchol™.115 However, these vaccines require a booster dose, provide only limited and short-term protection, and are not suitable for children under two years old.114, 115 Therefore, their use is mainly restricted to travelers rather than the general population in endemic areas.116 Polymeric particles have been intensely investigated as a delivery system for cholera vaccines.117 A mutant V. cholerae strain was efficiently loaded into PLGA microparticles with encapsulation efficiency of ~98%.118 The particulate vaccine administered orally elicited higher humoral immune responses than a soluble vaccine, and the addition of an anti-fungal drug, amphotericin B, as an adjuvant further enhanced the immune responses. Another study compared the efficacy of whole-bacterium vaccine formulated into polymeric microparticles composed of PLGA (50:50), PLGA (75:25), or PLA/PEG copolymer.119 Those particulate vaccines showed similar size distribution, encapsulation efficiency, and in vitro release profiles of the encapsulated antigen. Upon oral immunization in mice, the PLA/PEG formulation elicited the highest antigen-specific IgG, IgA, and IgM titers and a superior survival rate post-challenge. In an alternative approach, an oral cholera vaccine was produced by encapsulating inactivated V. cholerae into microparticles composed of the enteric excipient Eudragit® and mucoadhesive agents alginate or Carbopol®.120, 121 Following oral vaccination, the formulation incorporating alginate elicited higher vibriocidal titers than a soluble vaccine, demonstrating the promise of mucoadhesive particles for oral vaccination against cholera.
Melioidosis and glanders
Burkholderia pseudomallei and Burkholderia mallei are the causative agents of melioidosis and glanders, respectively. Both pathogens are gram-negative bacteria that reside in host immune cells and establish infection after oral ingestion, aerosol inhalation, or skin contact with cutaneous wounds.122 Melioidosis is a severe human endemic disease in Southeast Asia and Northern Australia, with mortality rates of 50% and 20%, respectively.123 In contrast, glanders mainly infects solipeds, such as horses, mules, and donkeys, but rarely infects humans.124 Although it is reported that glanders has been eradicated in the developed world, it still poses a threat to public health, and B. mallei has a documented history as a bio-warfare agent.125 In addition, both pathogens are resistant to most antibiotics, and there is no vaccine product available.125, 126 Early vaccine approaches include live-attenuated or inactivated bacteria and subunit vaccines for both diseases; however, none have achieved complete protection.122 Since an intracellular cycle is indispensable for the infectivity of these pathogens, induction of cellular immune responses by specific adjuvants are of particular interest. For instance, pre-treatment with CpG, a TLR9 agonist, has been shown to promote protection against B. pseudomallei.127 Furthermore, CpG complexed with cationic DOTAP liposomes showed better protection than CpG complexed with zwitterionic DOPC liposomes or soluble CpG.128 The CpG/DOTAP vaccine delivered intranasally in mice conferred partial protection against a lethal challenge of B. mallei or B. pseudomallei, and the protection relied on an elevated level of IFN-γ and activation of NK cells, suggesting the important roles of adaptive and innate immune responses in disease control.129 However, mice protected from the acute challenge still developed chronic infections in spleen and liver. Recently, a subunit particulate vaccine has been developed for B. mallei by conjugating LPS from a non-virulent strain to the surfaces of gold nanoparticles.130, 131 Vaccinated mice were later challenged with aerosolized B. mallei. Compared with the soluble LPS, the nanoparticle vaccine elicited higher anti-LPS IgG titers, reduced bacterial burdens in the spleen, and enhanced protection.130 However, when tested in non-human primates, the vaccine failed to induce favorable protection compared with non-immunized controls, despite elicitation of anti-LPS IgG.131
Ricin toxin
Ricin toxin is derived from the common plant Ricinus communis, or castor beans, and can be a byproduct during the production of castor oil. Despite multiple routes that can induce poisoning, ricin toxin is rarely transmitted to humans, and interpersonal transmission is negligible.132 Nevertheless, ease of production, storage, and dissemination, as well as the lack of effective protective and therapeutic options still make ricin toxin a potential bioterror threat.133 Indeed, it has been utilized in espionage incidents and malicious mailing attacks.134, 135 Ricin toxin is a heterodimeric glycoprotein consisting of an A chain (RTA) which enzymatically inactivates ribosomes, and a B chain (RTB) which facilitates entry of the toxin into target cells.136 Since RTB is a poor inducer of humoral immune responses, inactivated forms of the toxin and RTA become the main antigen sources tested for vaccine development.109, 133 In one approach, a single subcutaneous immunization of PLGA microparticles encapsulating the inactivated toxin induced similar serum levels of anti-ricin IgG and protection rates as three doses of a soluble vaccine.137 Notably, humoral immune responses elicited by the particulate vaccine lasted as long as one year after immunization. In a follow-up study with single or dual doses delivered through the intranasal route, the microparticle vaccine elicited higher serum anti-ricin IgG and IgA titers and achieved a higher protection rate against an aerosol challenge, compared with the soluble vaccine.138 The enhanced protection was observed when the challenge was performed at six weeks or one year after immunization. As an alternative strategy, liposomes encapsulating the inactivated toxin have been examined for intratracheal delivery.139, 140 The liposomal vaccine elicited similar humoral immune responses and slightly better short-term protection against an aerosol challenge, compared with soluble vaccines with or without the Alhydrogel® adjuvant.140 Further pathological analyses post-challenge revealed that the liposomal vaccine reduced infiltration of neutrophils into lungs and decreased pulmonary edema.141
Viral encephalitis caused by alphaviruses
Encephalitic alphaviruses, including eastern equine encephalitis virus (EEEV), western equine encephalitis virus (WEEV), and Venezuelan equine encephalitis virus (VEEV), infect both horses and humans in an epidemic area restricted to the Americas.142 The mortality rates are 3–7% for WEEV and 50–75% for EEEV, and there are no available therapeutics or vaccines.143, 144 Both attenuated and formalin-inactivated vaccines have been tested for three encephalitic alphaviruses, but none of the candidates were viable due to high reactogenicity of the attenuated vaccines and poor efficacy of the inactivated vaccines.144 To improve the efficacy, inactivated VEEV was encapsulated into PLGA microparticles and delivered via multiple routes.145 Mice were immunized through the subcutaneous or intratracheal route, followed by a booster dose through the oral, subcutaneous, or intratracheal route. The intratracheal route elicited mucosal anti-VEEV IgG and IgA with higher activity and also enhanced protection, compared with the other two routes tested for the booster dose.
Conclusion
Most particulate delivery systems examined in the past for biodefense vaccines are composed of biodegradable materials, such as PLGA and phospholipids, which are already approved for pharmaceutical products and would therefore facilitate clinical translation of promising vaccine candidates. Particulate vaccine vehicles can stably encapsulate or surface-display antigens while also serving additional roles as adjuvants or carriers for adjuvant molecules. As shown in studies discussed above, successful seroconversion usually correlates with high protection rates against lethal challenges, while cellular immunity is vital for elimination of intracellular reservoirs of pathogens.
It is the authors’ opinion that more resources and research efforts should be devoted to the development of mucosal vaccine delivery systems for biodefense vaccines. Mucosal vaccination would preferentially elicit T- and B-cell immune responses in local and distal mucosal surfaces, including the respiratory tract, thereby establishing immunity in the frontline of protection against aerosol transmission of bioterrorism agents.20–23 Compared with soluble antigens that are subjected to fast clearance or degradation, particulate vaccines could improve colloidal stability, increase residence time in mucosal tissues, and promote induction of antigen-specific IgA titers and peripheral tissue-resident effector CD4+ and CD8+ T-cells. Among various mucosal delivery strategies, oral vaccines may be most amenable to mass vaccination campaigns in the event of biological terror attacks and also most efficacious for immunization against enteric pathogens.146 Indeed, oral vaccine delivery has been exploited for currently licensed and candidate cholera vaccines. However, one of the major challenges in oral vaccination lies in rapid denaturation and degradation of antigens in response to acidic pH and abundant proteases in the gastrointestinal tract. Thus, it would be valuable to investigate particulate carriers composed of biopolymers that can endure the harsh gastric environment or liposomes stabilized by bile salts or archaeobacterial lipids that can maintain antigenicity and immunogenicity of antigens.147 In addition, vaccine carriers targeted to M cells and/or intestinal dendritic cells should be examined to further improve the efficiency of oral vaccination. Another mucosal route of vaccine delivery that warrants further investigation is transcutaneous vaccination. Notably, microneedle patches have recently garnered much attention as a promising vaccine delivery tool that could offer good patient compliance and facile self-administration approaches, while achieving dose sparing by targeting of antigens and adjuvants to skin-resident antigen-presenting cells.148–150 As in the case with oral dosage forms, pre-formulated microneedle patches could be easily stored for long-term preparedness and rapid distribution among susceptible populations in response to bioterror attacks. New microneedle technologies permit long-term storage of vaccines and allow precise tuning of drug release profiles by employing dissolvable polymeric patches,151 layer-by-layer coatings on needles,152 or therapeutic depots responsive to external tensile strength.153 Future research efforts should be directed to exploit these technologies to achieve efficient and stable loading of antigens, to attract skin-resident DCs to vaccination sites with the antigen depot deployed during administration, and to augment mucosal immune responses. These advances will allow an unprecedented control over dosing and immunization schemes for transcutaneous vaccination.
There are numerous challenges that need to be overcome for clinical translation of particulate vaccines against biological warfare agents. It is crucial to streamline the manufacturing processes for industrial scale-up of particulate vaccines with minimal batch-to-batch variability. In addition, since large vaccine stockpiles and rapid and facile distribution of vaccines are vital components of countermeasures against outbreaks and bioterror attacks, it is critical to develop vaccine products that are stable for a long term without the cold-chain. Furthermore, as illustrated in this article, the majority of particulate vaccine candidates is still in the early stages of pre-clinical development and tested only in murine models. However, many vaccine candidates that may be adequate in small animals fail to exhibit strong immune responses in large animals or humans. Murine species cannot be naturally infected by many emergent pathogens introduced above. The pathogenesis, dosing for immunization and challenge, and types and durability of immune responses demonstrated in murine models may not correlate with those in natural hosts, including humans. Furthermore, following the disease challenge, small animals may clear pathogens by one-wave immune responses or easily succumb to death, making them inappropriate models to evaluate vaccine efficacy against latent intracellular infections or recurring and debilitating diseases.154, 155 Therefore, more stringent screening process should be implemented in the early cycles of product development; this may involve direct comparison of particulate vaccine candidates against other strong benchmarks in vaccine research, including gold standard adjuvants, VLPs, or live vectors. In parallel, evaluation of vaccine candidates in more rigorous animal models, including non-human primates, should be considered early in the vaccine design and development.
In conclusion, particulate delivery systems have shown great promise for addressing current limitations in vaccine technologies against bioterrorism and emerging infectious agents, and they should be strongly considered for public health preparedness and countermeasures against potential outbreaks and bioterror threats.
Acknowledgments
This work is supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000433, the John S. and Suzanne C. Munn Cancer Fund of the University of Michigan Comprehensive Cancer Center, and National Science Foundation CAREER award. J.J.M. is supported by the Melanoma Research Alliance Young Investigator Award. Y.F. is supported by the Broomfield International Student Fellowship. We thank Joseph Bazzill and Cameron Louttit for critical review of the manuscript.
Footnotes
Conflict of interests: The authors declare no conflict of interests
Contributor Information
Yuchen Fan, Department of Pharmaceutical Sciences, Biointerfaces Institute, University of Michigan, Ann Arbor, MI 48109, USA.
James J. Moon, Department of Pharmaceutical Sciences, Department of Biomedical Engineering, Biointerfaces Institute, University of Michigan, Ann Arbor, MI 48109, USA.
References
- 1.Rotz LD, Khan AS, Lillibridge SR, Ostroff SM, Hughes JM. Public health assessment of potential biological terrorism agents. Emerg Infect Dis. 2002;8:225–230. doi: 10.3201/eid0802.010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feodorova VA, Motin VL. Plague vaccines: current developments and future perspectives. Emerging Microbes & Infections. 2012;1:e36. doi: 10.1038/emi.2012.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webb RP, Smith LA. What next for botulism vaccine development? Expert Rev Vaccines. 2013;12:481–492. doi: 10.1586/erv.13.37. [DOI] [PubMed] [Google Scholar]
- 4.Isherwood KE, Titball RW, Davies DH, Felgner PL, Morrow WJ. Vaccination strategies for Francisella tularensis. Adv Drug Deliv Rev. 2005;57:1403–1414. doi: 10.1016/j.addr.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Moon JJ, Huang B, Irvine DJ. Engineering nano- and microparticles to tune immunity. Adv Mater. 2012;24:3724–3746. doi: 10.1002/adma.201200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yue H, Ma G. Polymeric micro/nanoparticles: Particle design and potential vaccine delivery applications. Vaccine. 2015;33:5927–5936. doi: 10.1016/j.vaccine.2015.07.100. [DOI] [PubMed] [Google Scholar]
- 7.Irvine DJ, Hanson MC, Rakhra K, Tokatlian T. Synthetic Nanoparticles for Vaccines and Immunotherapy. Chem Rev. 2015;115:11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan Y, Moon JJ. Nanoparticle Drug Delivery Systems Designed to Improve Cancer Vaccines and Immunotherapy. Vaccines (Basel) 2015;3:662–685. doi: 10.3390/vaccines3030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahdev P, Ochyl LJ, Moon JJ. Biomaterials for nanoparticle vaccine delivery systems. Pharm Res. 2014;31:2563–2582. doi: 10.1007/s11095-014-1419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen D, Korsholm KS, Andersen P, Agger EM. Cationic liposomes as vaccine adjuvants. Expert Rev Vaccines. 2011;10:513–521. doi: 10.1586/erv.11.17. [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005;57:391–410. doi: 10.1016/j.addr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Bramwell VW, Eyles JE, Oya Alpar H. Particulate delivery systems for biodefense subunit vaccines. Adv Drug Deliv Rev. 2005;57:1247–1265. doi: 10.1016/j.addr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 13.Kuai R, Ochyl LJ, Schwendeman A, Moon JJ. Lipid-based nanoparticles for vaccine applications. In: Jo H, Jun HW, Shin J, Lee SH, editors. Biomedical Engineering: Frontier Research and Converging Technologies. Springer; 2015. pp. 177–197. [Google Scholar]
- 14.Moon JJ, Suh H, Li AV, Ockenhouse CF, Yadava A, Irvine DJ. Enhancing humoral responses to a malaria antigen with nanoparticle vaccines that expand Tfh cells and promote germinal center induction. Proc Natl Acad Sci U S A. 2012;109:1080–1085. doi: 10.1073/pnas.1112648109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moon JJ, Suh H, Polhemus ME, Ockenhouse CF, Yadava A, Irvine DJ. Antigen-displaying lipid-enveloped PLGA nanoparticles as delivery agents for a Plasmodium vivax malaria vaccine. PLoS One. 2012;7:e31472. doi: 10.1371/journal.pone.0031472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith DM, Simon JK, Baker JR., Jr Applications of nanotechnology for immunology. Nat Rev Immunol. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson JT, Keller S, Manganiello MJ, Cheng C, Lee CC, Opara C, Convertine A, Stayton PS. pH-Responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano. 2013;7:3912–3925. doi: 10.1021/nn305466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, Sohail M, Luo S, Um SH, Khant H, et al. Interbilayer-crosslinked multilamellar vesicles as synthetic vaccines for potent humoral and cellular immune responses. Nat Mater. 2011;10:243–251. doi: 10.1038/nmat2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ochyl LJ, Moon JJ. Whole-animal imaging and flow cytometric techniques for analysis of antigen-specific CD8+ T cell responses after nanoparticle vaccination. J Vis Exp. 2015:e52771. doi: 10.3791/52771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 21.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 22.Li AV, Moon JJ, Abraham W, Suh H, Elkhader J, Seidman MA, Yen M, Im EJ, Foley MH, Barouch DH, et al. Generation of effector memory T cell-based mucosal and systemic immunity with pulmonary nanoparticle vaccination. Sci Transl Med. 2013;5:204ra130. doi: 10.1126/scitranslmed.3006516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan Y, Sahdev P, Ochyl LJ, JJA, Moon JJ. Cationic liposome-hyaluronic acid hybrid nanoparticles for intranasal vaccination with subunit antigens. J Control Release. 2015;208:121–129. doi: 10.1016/j.jconrel.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur M, Singh S, Bhatnagar R. Anthrax vaccines: present status and future prospects. Expert Rev Vaccines. 2013;12:955–970. doi: 10.1586/14760584.2013.814860. [DOI] [PubMed] [Google Scholar]
- 25.Guarner J, Jernigan JA, Shieh WJ, Tatti K, Flannagan LM, Stephens DS, Popovic T, Ashford DA, Perkins BA, Zaki SR, et al. Pathology and pathogenesis of bioterrorism-related inhalational anthrax. Am J Pathol. 2003;163:701–709. doi: 10.1016/S0002-9440(10)63697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopkins RJ, Howard C, Hunter-Stitt E, Kaptur PE, Pleune B, Muse D, Sheldon E, Davis M, Strout C, Vert-Wong K. Phase 3 trial evaluating the immunogenicity and safety of a three-dose BioThrax(R) regimen for post-exposure prophylaxis in healthy adults. Vaccine. 2014;32:2217–2224. doi: 10.1016/j.vaccine.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 27.Flick-Smith HC, Eyles JE, Hebdon R, Waters EL, Beedham RJ, Stagg TJ, Miller J, Alpar HO, Baillie LW, Williamson ED. Mucosal or parenteral administration of microsphere-associated Bacillus anthracis protective antigen protects against anthrax infection in mice. Infect Immun. 2002;70:2022–2028. doi: 10.1128/IAI.70.4.2022-2028.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manish M, Rahi A, Kaur M, Bhatnagar R, Singh S. A single-dose PLGA encapsulated protective antigen domain 4 nanoformulation protects mice against Bacillus anthracis spore challenge. PLoS One. 2013;8:e61885. doi: 10.1371/journal.pone.0061885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bento D, Staats HF, Borges O. Effect of particulate adjuvant on the anthrax protective antigen dose required for effective nasal vaccination. Vaccine. 2015;33:3609–3613. doi: 10.1016/j.vaccine.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 30.Schully KL, Sharma S, Peine KJ, Pesce J, Elberson MA, Fonseca ME, Prouty AM, Bell MG, Borteh H, Gallovic M, et al. Rapid vaccination using an acetalated dextran microparticulate subunit vaccine confers protection against triplicate challenge by bacillus anthracis. Pharm Res. 2013;30:1349–1361. doi: 10.1007/s11095-013-0975-x. [DOI] [PubMed] [Google Scholar]
- 31.Bielinska AU, Janczak KW, Landers JJ, Makidon P, Sower LE, Peterson JW, Baker JR., Jr Mucosal immunization with a novel nanoemulsion-based recombinant anthrax protective antigen vaccine protects against Bacillus anthracis spore challenge. Infect Immun. 2007;75:4020–4029. doi: 10.1128/IAI.00070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peachman KK, Li Q, Matyas GR, Shivachandra SB, Lovchik J, Lyons RC, Alving CR, Rao VB, Rao M. Anthrax vaccine antigen-adjuvant formulations completely protect New Zealand white rabbits against challenge with Bacillus anthracis Ames strain spores. Clin Vaccine Immunol. 2012;19:11–16. doi: 10.1128/CVI.05376-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keitel WA. Recombinant protective antigen 102 (rPA102): profile of a second-generation anthrax vaccine. Expert Rev Vaccines. 2006;5:417–430. doi: 10.1586/14760584.5.4.417. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Aldayel AM, Cui Z. Aluminum hydroxide nanoparticles show a stronger vaccine adjuvant activity than traditional aluminum hydroxide microparticles. J Control Release. 2014;173:148–157. doi: 10.1016/j.jconrel.2013.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenzweig JA, Jejelowo O, Sha J, Erova TE, Brackman SM, Kirtley ML, van Lier CJ, Chopra AK. Progress on plague vaccine development. Appl Microbiol Biotechnol. 2011;91:265–286. doi: 10.1007/s00253-011-3380-6. [DOI] [PubMed] [Google Scholar]
- 36.Inglesby TV, Dennis DT, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Koerner JF, et al. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 2000;283:2281–2290. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- 37.Williamson ED, Oyston PCF. Protecting against plague: towards a next-generation vaccine. Clinical and Experimental Immunology. 2013;172:1–8. doi: 10.1111/cei.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eyles JE, Spiers ID, Williamson ED, Alpar HO. Analysis of local and systemic immunological responses after intra-tracheal, intra-nasal and intra-muscular administration of microsphere co-encapsulated Yersinia pestis sub-unit vaccines. Vaccine. 1998;16:2000–2009. doi: 10.1016/s0264-410x(98)00089-9. [DOI] [PubMed] [Google Scholar]
- 39.Huang SS, Li IH, Hong PD, Yeh MK. Development of Yersinia pestis F1 antigen-loaded microspheres vaccine against plague. Int J Nanomedicine. 2014;9:813–822. doi: 10.2147/IJN.S56260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uppada JB, Khan AA, Bhat AA, Deshmukh R, Rao DN. Humoral immune responses and protective efficacy of sequential B- and T-cell epitopes of V antigen of Yersinia pestis by intranasal immunization in microparticles. Med Microbiol Immunol. 2009;198:247–256. doi: 10.1007/s00430-009-0124-7. [DOI] [PubMed] [Google Scholar]
- 41.Ross KA, Haughney SL, Petersen LK, Boggiatto P, Wannemuehler MJ, Narasimhan B. Lung deposition and cellular uptake behavior of pathogen-mimicking nanovaccines in the first 48 hours. Adv Healthc Mater. 2014;3:1071–1077. doi: 10.1002/adhm.201300525. [DOI] [PubMed] [Google Scholar]
- 42.Ulery BD, Kumar D, Ramer-Tait AE, Metzger DW, Wannemuehler MJ, Narasimhan B. Design of a protective single-dose intranasal nanoparticle-based vaccine platform for respiratory infectious diseases. PLoS One. 2011;6:e17642. doi: 10.1371/journal.pone.0017642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregory AE, Williamson ED, Prior JL, Butcher WA, Thompson IJ, Shaw AM, Titball RW. Conjugation of Y. pestis F1-antigen to gold nanoparticles improves immunogenicity. Vaccine. 2012;30:6777–6782. doi: 10.1016/j.vaccine.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fischer NO, Rasley A, Corzett M, Hwang MH, Hoeprich PD, Blanchette CD. Colocalized delivery of adjuvant and antigen using nanolipoprotein particles enhances the immune response to recombinant antigens. J Am Chem Soc. 2013;135:2044–2047. doi: 10.1021/ja3063293. [DOI] [PubMed] [Google Scholar]
- 45.Haughney SL, Ross KA, Boggiatto PM, Wannemuehler MJ, Narasimhan B. Effect of nanovaccine chemistry on humoral immune response kinetics and maturation. Nanoscale. 2014;6:13770–13778. doi: 10.1039/c4nr03724c. [DOI] [PubMed] [Google Scholar]
- 46.de La Vega MA, Stein D, Kobinger GP. Ebolavirus Evolution: Past and Present. PLoS Pathog. 2015;11:e1005221. doi: 10.1371/journal.ppat.1005221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geisbert TW, Bausch DG, Feldmann H. Prospects for immunisation against Marburg and Ebola viruses. Rev Med Virol. 2010;20:344–357. doi: 10.1002/rmv.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marzi A, Halfmann P, Hill-Batorski L, Feldmann F, Shupert WL, Neumann G, Feldmann H, Kawaoka Y. Vaccines. An Ebola whole-virus vaccine is protective in nonhuman primates. Science. 2015;348:439–442. doi: 10.1126/science.aaa4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Choi JH, Croyle MA. Emerging targets and novel approaches to Ebola virus prophylaxis and treatment. BioDrugs. 2013;27:565–583. doi: 10.1007/s40259-013-0046-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, Camacho A, Carroll MW, Doumbia M, Draguez B, Duraffour S, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet. 2015;386:857–866. doi: 10.1016/S0140-6736(15)61117-5. [DOI] [PubMed] [Google Scholar]
- 51.Jones SM, Feldmann H, Stroher U, Geisbert JB, Fernando L, Grolla A, Klenk HD, Sullivan NJ, Volchkov VE, Fritz EA, et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat Med. 2005;11:786–790. doi: 10.1038/nm1258. [DOI] [PubMed] [Google Scholar]
- 52.Mire CE, Matassov D, Geisbert JB, Latham TE, Agans KN, Xu R, Ota-Setlik A, Egan MA, Fenton KA, Clarke DK, et al. Single-dose attenuated Vesiculovax vaccines protect primates against Ebola Makona virus. Nature. 2015;520:688–691. doi: 10.1038/nature14428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warfield KL, Aman MJ. Advances in virus-like particle vaccines for filoviruses. J Infect Dis. 2011;204(Suppl 3):S1053–1059. doi: 10.1093/infdis/jir346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warfield KL, Bosio CM, Welcher BC, Deal EM, Mohamadzadeh M, Schmaljohn A, Aman MJ, Bavari S. Ebola virus-like particles protect from lethal Ebola virus infection. Proc Natl Acad Sci U S A. 2003;100:15889–15894. doi: 10.1073/pnas.2237038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warfield KL, Swenson DL, Olinger GG, Kalina WV, Aman MJ, Bavari S. Ebola virus-like particle-based vaccine protects nonhuman primates against lethal Ebola virus challenge. J Infect Dis. 2007;196(Suppl 2):S430–437. doi: 10.1086/520583. [DOI] [PubMed] [Google Scholar]
- 56.Ayithan N, Bradfute SB, Anthony SM, Stuthman KS, Bavari S, Bray M, Ozato K. Virus-like particles activate type I interferon pathways to facilitate post-exposure protection against Ebola virus infection. PLoS One. 2015;10:e0118345. doi: 10.1371/journal.pone.0118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martins KA, Steffens JT, van Tongeren SA, Wells JB, Bergeron AA, Dickson SP, Dye JM, Salazar AM, Bavari S. Toll-like receptor agonist augments virus-like particle-mediated protection from Ebola virus with transient immune activation. PLoS One. 2014;9:e89735. doi: 10.1371/journal.pone.0089735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martins K, Carra JH, Cooper CL, Kwilas SA, Robinson CG, Shurtleff AC, Schokman RD, Kuehl KA, Wells JB, Steffens JT, et al. Cross-protection conferred by filovirus virus-like particles containing trimeric hybrid glycoprotein. Viral Immunol. 2015;28:62–70. doi: 10.1089/vim.2014.0071. [DOI] [PubMed] [Google Scholar]
- 59.Rao M, Matyas GR, Grieder F, Anderson K, Jahrling PB, Alving CR. Cytotoxic T lymphocytes to Ebola Zaire virus are induced in mice by immunization with liposomes containing lipid A. Vaccine. 1999;17:2991–2998. doi: 10.1016/s0264-410x(99)00170-x. [DOI] [PubMed] [Google Scholar]
- 60.Rao M, Bray M, Alving CR, Jahrling P, Matyas GR. Induction of immune responses in mice and monkeys to Ebola virus after immunization with liposome-encapsulated irradiated Ebola virus: protection in mice requires CD4(+) T cells. J Virol. 2002;76:9176–9185. doi: 10.1128/JVI.76.18.9176-9185.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heinz FX, Stiasny K. Flaviviruses and flavivirus vaccines. Vaccine. 2012;30:4301–4306. doi: 10.1016/j.vaccine.2011.09.114. [DOI] [PubMed] [Google Scholar]
- 62.Ishikawa T, Yamanaka A, Konishi E. A review of successful flavivirus vaccines and the problems with those flaviviruses for which vaccines are not yet available. Vaccine. 2014;32:1326–1337. doi: 10.1016/j.vaccine.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 63.Villar L, Dayan GH, Arredondo-Garcia JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramirez JO, Carrasquilla G, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med. 2015;372:113–123. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 64.Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N Engl J Med. 2015;373:1195–1206. doi: 10.1056/NEJMoa1506223. [DOI] [PubMed] [Google Scholar]
- 65.Niikura K, Matsunaga T, Suzuki T, Kobayashi S, Yamaguchi H, Orba Y, Kawaguchi A, Hasegawa H, Kajino K, Ninomiya T, et al. Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7:3926–3938. doi: 10.1021/nn3057005. [DOI] [PubMed] [Google Scholar]
- 66.Demento SL, Bonafe N, Cui W, Kaech SM, Caplan MJ, Fikrig E, Ledizet M, Fahmy TM. TLR9-targeted biodegradable nanoparticles as immunization vectors protect against West Nile encephalitis. J Immunol. 2010;185:2989–2997. doi: 10.4049/jimmunol.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 68.Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015;21:359–361. doi: 10.3201/eid2102.141363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg? Ultrasound Obstet Gynecol. 2016;47:6–7. doi: 10.1002/uog.15831. [DOI] [PubMed] [Google Scholar]
- 70.Simpson LL. Identification of the major steps in botulinum toxin action. Annu Rev Pharmacol Toxicol. 2004;44:167–193. doi: 10.1146/annurev.pharmtox.44.101802.121554. [DOI] [PubMed] [Google Scholar]
- 71.Karalewitz AP, Barbieri JT. Vaccines against botulism. Curr Opin Microbiol. 2012;15:317–324. doi: 10.1016/j.mib.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 72.Nochi T, Yuki Y, Takahashi H, Sawada S, Mejima M, Kohda T, Harada N, Kong IG, Sato A, Kataoka N, et al. Nanogel antigenic protein-delivery system for adjuvant-free intranasal vaccines. Nat Mater. 2010;9:572–578. doi: 10.1038/nmat2784. [DOI] [PubMed] [Google Scholar]
- 73.Hickey AJ, Hazlett KR, Kirimanjeswara GS, Metzger DW. Identification of Francisella tularensis outer membrane protein A (FopA) as a protective antigen for tularemia. Vaccine. 2011;29:6941–6947. doi: 10.1016/j.vaccine.2011.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Richard K, Mann BJ, Stocker L, Barry EM, Qin A, Cole LE, Hurley MT, Ernst RK, Michalek SM, Stein DC, et al. Novel catanionic surfactant vesicle vaccines protect against Francisella tularensis LVS and confer significant partial protection against F. tularensis Schu S4 strain. Clin Vaccine Immunol. 2014;21:212–226. doi: 10.1128/CVI.00738-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel GB, Zhou H, Ponce A, Harris G, Chen W. Intranasal immunization with an archaeal lipid mucosal vaccine adjuvant and delivery formulation protects against a respiratory pathogen challenge. PLoS One. 2010;5:e15574. doi: 10.1371/journal.pone.0015574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clemens DL, Lee BY, Horwitz MA. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect Immun. 2004;72:3204–3217. doi: 10.1128/IAI.72.6.3204-3217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lindgren H, Golovliov I, Baranov V, Ernst RK, Telepnev M, Sjostedt A. Factors affecting the escape of Francisella tularensis from the phagolysosome. J Med Microbiol. 2004;53:953–958. doi: 10.1099/jmm.0.45685-0. [DOI] [PubMed] [Google Scholar]
- 78.Cannella AP, Tsolis RM, Liang L, Felgner PL, Saito M, Sette A, Gotuzzo E, Vinetz JM. Antigen-specific acquired immunity in human brucellosis: implications for diagnosis, prognosis, and vaccine development. Front Cell Infect Microbiol. 2012;2:1. doi: 10.3389/fcimb.2012.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Perkins SD, Smither SJ, Atkins HS. Towards a Brucella vaccine for humans. FEMS Microbiol Rev. 2010;34:379–394. doi: 10.1111/j.1574-6976.2010.00211.x. [DOI] [PubMed] [Google Scholar]
- 80.Ficht TA, Kahl-McDonagh MM, Arenas-Gamboa AM, Rice-Ficht AC. Brucellosis: the case for live, attenuated vaccines. Vaccine. 2009;27(Suppl 4):D40–43. doi: 10.1016/j.vaccine.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Avila-Calderon ED, Lopez-Merino A, Sriranganathan N, Boyle SM, Contreras-Rodriguez A. A history of the development of Brucella vaccines. Biomed Res Int. 2013;2013:743509. doi: 10.1155/2013/743509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Estevan M, Gamazo C, Grillo MJ, Del Barrio GG, Blasco JM, Irache JM. Experiments on a sub-unit vaccine encapsulated in microparticles and its efficacy against Brucella melitensis in mice. Vaccine. 2006;24:4179–4187. doi: 10.1016/j.vaccine.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 83.Da Costa Martins R, Gamazo C, Sanchez-Martinez M, Barberan M, Penuelas I, Irache JM. Conjunctival vaccination against Brucella ovis in mice with mannosylated nanoparticles. J Control Release. 2012;162:553–560. doi: 10.1016/j.jconrel.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 84.Goel D, Rajendran V, Ghosh PC, Bhatnagar R. Cell mediated immune response after challenge in Omp25 liposome immunized mice contributes to protection against virulent Brucella abortus 544. Vaccine. 2013;31:1231–1237. doi: 10.1016/j.vaccine.2012.12.043. [DOI] [PubMed] [Google Scholar]
- 85.Singh D, Goel D, Bhatnagar R. Recombinant L7/L12 protein entrapping PLGA (poly lactide-co-glycolide) micro particles protect BALB/c mice against the virulent B. abortus 544 infection. Vaccine. 2015;33:2786–2792. doi: 10.1016/j.vaccine.2015.04.030. [DOI] [PubMed] [Google Scholar]
- 86.Afley P, Dohre SK, Prasad GB, Kumar S. Prediction of T cell epitopes of Brucella abortus and evaluation of their protective role in mice. Appl Microbiol Biotechnol. 2015;99:7625–7637. doi: 10.1007/s00253-015-6787-7. [DOI] [PubMed] [Google Scholar]
- 87.MacLennan CA, Martin LB, Micoli F. Vaccines against invasive Salmonella disease: current status and future directions. Hum Vaccin Immunother. 2014;10:1478–1493. doi: 10.4161/hv.29054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 89.Simon R, Levine MM. Glycoconjugate vaccine strategies for protection against invasive Salmonella infections. Hum Vaccin Immunother. 2012;8:494–498. doi: 10.4161/hv.19158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paterson GK, Maskell DJ. Recent advances in the field of Salmonella Typhi vaccines. Hum Vaccin. 2010;6:379–384. doi: 10.4161/hv.6.5.10755. [DOI] [PubMed] [Google Scholar]
- 91.Parry CM, Threlfall EJ. Antimicrobial resistance in typhoidal and nontyphoidal salmonellae. Curr Opin Infect Dis. 2008;21:531–538. doi: 10.1097/QCO.0b013e32830f453a. [DOI] [PubMed] [Google Scholar]
- 92.Ochoa J, Irache JM, Tamayo I, Walz A, DelVecchio VG, Gamazo C. Protective immunity of biodegradable nanoparticle-based vaccine against an experimental challenge with Salmonella Enteritidis in mice. Vaccine. 2007;25:4410–4419. doi: 10.1016/j.vaccine.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 93.Anish C, Goswami DG, Kanchan V, Mathew S, Panda AK. The immunogenic characteristics associated with multivalent display of Vi polysaccharide antigen using biodegradable polymer particles. Biomaterials. 2012;33:6843–6857. doi: 10.1016/j.biomaterials.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 94.Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol. 2007;5:540–553. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Walker RI. An assessment of enterotoxigenic Escherichia coli and Shigella vaccine candidates for infants and children. Vaccine. 2015;33:954–965. doi: 10.1016/j.vaccine.2014.11.049. [DOI] [PubMed] [Google Scholar]
- 96.Camacho AI, Irache JM, Gamazo C. Recent progress towards development of a Shigella vaccine. Expert Rev Vaccines. 2013;12:43–55. doi: 10.1586/erv.12.135. [DOI] [PubMed] [Google Scholar]
- 97.Camacho AI, de Souza J, Sanchez-Gomez S, Pardo-Ros M, Irache JM, Gamazo C. Mucosal immunization with Shigella flexneri outer membrane vesicles induced protection in mice. Vaccine. 2011;29:8222–8229. doi: 10.1016/j.vaccine.2011.08.121. [DOI] [PubMed] [Google Scholar]
- 98.Camacho AI, Irache JM, de Souza J, Sanchez-Gomez S, Gamazo C. Nanoparticle-based vaccine for mucosal protection against Shigella flexneri in mice. Vaccine. 2013;31:3288–3294. doi: 10.1016/j.vaccine.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 99.Garcia-Angulo VA, Kalita A, Torres AG. Advances in the development of enterohemorrhagic Escherichia coli vaccines using murine models of infection. Vaccine. 2013;31:3229–3235. doi: 10.1016/j.vaccine.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bavaro MF. E. coli O157:H7 and other toxigenic strains: the curse of global food distribution. Curr Gastroenterol Rep. 2012;14:317–323. doi: 10.1007/s11894-012-0264-6. [DOI] [PubMed] [Google Scholar]
- 101.Bach SJ, McAllister TA, Veira DM, Gannon VPJ, Holley RA. Transmission and control of Escherichia coli O157: H7 - A review. Canadian Journal of Animal Science. 2002;82:475–490. [Google Scholar]
- 102.Vande Walle K, Vanrompay D, Cox E. Bovine innate and adaptive immune responses against Escherichia coli O157:H7 and vaccination strategies to reduce faecal shedding in ruminants. Vet Immunol Immunopathol. 2013;152:109–120. doi: 10.1016/j.vetimm.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 103.Tana, Watarai S, Isogai E, Oguma K. Induction of intestinal IgA and IgG antibodies preventing adhesion of verotoxin-producing Escherichia coli to Caco-2 cells by oral immunization with liposomes. Lett Appl Microbiol. 2003;36:135–139. doi: 10.1046/j.1472-765x.2003.01278.x. [DOI] [PubMed] [Google Scholar]
- 104.Fukuda T, Kimiya T, Takahashi M, Arakawa Y, Ami Y, Suzaki Y, Naito S, Horino A, Nagata N, Satoh S, et al. Induction of protection against oral infection with cytotoxin-producing Escherichia coli O157:H7 in mice by shiga-like toxin-liposome conjugate. Int Arch Allergy Immunol. 1998;116:313–317. doi: 10.1159/000023961. [DOI] [PubMed] [Google Scholar]
- 105.Suzaki Y, Ami Y, Nagata N, Naito S, Kato H, Taneichi M, Takahashi M, Komiya T, Satoh S, Gondaira F, et al. Protection of monkeys against Shiga toxin induced by Shiga toxin-liposome conjugates. Int Arch Allergy Immunol. 2002;127:294–298. doi: 10.1159/000057746. [DOI] [PubMed] [Google Scholar]
- 106.Gao W, Fang RH, Thamphiwatana S, Luk BT, Li J, Angsantikul P, Zhang Q, Hu CM, Zhang L. Modulating antibacterial immunity via bacterial membrane-coated nanoparticles. Nano Lett. 2015;15:1403–1409. doi: 10.1021/nl504798g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Henghold WB., 2nd Other biologic toxin bioweapons: ricin, staphylococcal enterotoxin B, and trichothecene mycotoxins. Dermatol Clin. 2004;22:257–262. doi: 10.1016/j.det.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 108.Krakauer T, Stiles BG. The staphylococcal enterotoxin (SE) family: SEB and siblings. Virulence. 2013;4:759–773. doi: 10.4161/viru.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mantis NJ. Vaccines against the category B toxins: Staphylococcal enterotoxin B, epsilon toxin and ricin. Adv Drug Deliv Rev. 2005;57:1424–1439. doi: 10.1016/j.addr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 110.Tseng J, Komisar JL, Trout RN, Hunt RE, Chen JY, Johnson AJ, Pitt L, Ruble DL. Humoral immunity to aerosolized staphylococcal enterotoxin B (SEB), a superantigen, in monkeys vaccinated with SEB toxoid-containing microspheres. Infect Immun. 1995;63:2880–2885. doi: 10.1128/iai.63.8.2880-2885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Desai MP, Hilfinger JM, Amidon GL, Levy RJ, Labhasetwar V. Immune response with biodegradable nanospheres and alum: studies in rabbits using staphylococcal enterotoxin B-toxoid. J Microencapsul. 2000;17:215–225. doi: 10.1080/026520400288454. [DOI] [PubMed] [Google Scholar]
- 112.Hu CM, Fang RH, Luk BT, Zhang L. Nanoparticle-detained toxins for safe and effective vaccination. Nat Nanotechnol. 2013;8:933–938. doi: 10.1038/nnano.2013.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bishop AL, Camilli A. Vibrio cholerae: lessons for mucosal vaccine design. Expert Rev Vaccines. 2011;10:79–94. doi: 10.1586/erv.10.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clemens J, Shin S, Sur D, Nair GB, Holmgren J. New-generation vaccines against cholera. Nat Rev Gastroenterol Hepatol. 2011;8:701–710. doi: 10.1038/nrgastro.2011.174. [DOI] [PubMed] [Google Scholar]
- 115.Martin S, Lopez AL, Bellos A, Deen J, Ali M, Alberti K, Anh DD, Costa A, Grais RF, Legros D, et al. Post-licensure deployment of oral cholera vaccines: a systematic review. Bull World Health Organ. 2014;92:881–893. doi: 10.2471/BLT.14.139949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lopez AL, Gonzales ML, Aldaba JG, Nair GB. Killed oral cholera vaccines: history, development and implementation challenges. Ther Adv Vaccines. 2014;2:123–136. doi: 10.1177/2051013614537819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pastor M, Pedraz JL, Esquisabel A. The state-of-the-art of approved and under-development cholera vaccines. Vaccine. 2013;31:4069–4078. doi: 10.1016/j.vaccine.2013.06.096. [DOI] [PubMed] [Google Scholar]
- 118.Yeh MK, Liu YT, Chen JL, Chiang CH. Oral immunogenicity of the inactivated Vibrio cholerae whole-cell vaccine encapsulated in biodegradable microparticles. J Control Release. 2002;82:237–247. doi: 10.1016/s0168-3659(02)00140-2. [DOI] [PubMed] [Google Scholar]
- 119.Yeh M, Chiang C. Inactive Vibrio cholerae whole-cell vaccine-loaded biodegradable microparticles: in vitro release and oral vaccination. J Microencapsul. 2004;21:91–106. doi: 10.1080/02652040310001619794. [DOI] [PubMed] [Google Scholar]
- 120.Ano G, Esquisabel A, Pastor M, Talavera A, Cedre B, Fernandez S, Sifontes S, Aranguren Y, Falero G, Garcia L, et al. A new oral vaccine candidate based on the microencapsulation by spray-drying of inactivated Vibrio cholerae. Vaccine. 2011;29:5758–5764. doi: 10.1016/j.vaccine.2011.05.098. [DOI] [PubMed] [Google Scholar]
- 121.Pastor M, Esquisabel A, Talavera A, Ano G, Fernandez S, Cedre B, Infante JF, Callico A, Pedraz JL. An approach to a cold chain free oral cholera vaccine: in vitro and in vivo characterization of Vibrio cholerae gastro-resistant microparticles. Int J Pharm. 2013;448:247–258. doi: 10.1016/j.ijpharm.2013.02.057. [DOI] [PubMed] [Google Scholar]
- 122.Silva EB, Dow SW. Development of Burkholderia mallei and pseudomallei vaccines. Front Cell Infect Microbiol. 2013;3:10. doi: 10.3389/fcimb.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wiersinga WJ, van der Poll T, White NJ, Day NP, Peacock SJ. Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol. 2006;4:272–282. doi: 10.1038/nrmicro1385. [DOI] [PubMed] [Google Scholar]
- 124.Bondi SK, Goldberg JB. Strategies toward vaccines against Burkholderia mallei and Burkholderia pseudomallei. Expert Rev Vaccines. 2008;7:1357–1365. doi: 10.1586/14760584.7.9.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Van Zandt KE, Greer MT, Gelhaus HC. Glanders: an overview of infection in humans. Orphanet J Rare Dis. 2013;8:131. doi: 10.1186/1750-1172-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Estes DM, Dow SW, Schweizer HP, Torres AG. Present and future therapeutic strategies for melioidosis and glanders. Expert Rev Anti Infect Ther. 2010;8:325–338. doi: 10.1586/eri.10.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wongratanacheewin S, Kespichayawattana W, Intachote P, Pichyangkul S, Sermswan RW, Krieg AM, Sirisinha S. Immunostimulatory CpG oligodeoxynucleotide confers protection in a murine model of infection with Burkholderia pseudomallei. Infect Immun. 2004;72:4494–4502. doi: 10.1128/IAI.72.8.4494-4502.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Puangpetch A, Anderson R, Huang YY, Sermswan RW, Chaicumpa W, Sirisinha S, Wongratanacheewin S. Cationic liposomes extend the immunostimulatory effect of CpG oligodeoxynucleotide against Burkholderia pseudomallei infection in BALB/c mice. Clin Vaccine Immunol. 2012;19:675–683. doi: 10.1128/CVI.05545-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goodyear A, Kellihan L, Bielefeldt-Ohmann H, Troyer R, Propst K, Dow S. Protection from pneumonic infection with burkholderia species by inhalational immunotherapy. Infect Immun. 2009;77:1579–1588. doi: 10.1128/IAI.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gregory AE, Judy BM, Qazi O, Blumentritt CA, Brown KA, Shaw AM, Torres AG, Titball RW. A gold nanoparticle-linked glycoconjugate vaccine against Burkholderia mallei. Nanomedicine. 2015;11:447–456. doi: 10.1016/j.nano.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Torres AG, Gregory AE, Hatcher CL, Vinet-Oliphant H, Morici LA, Titball RW, Roy CJ. Protection of non-human primates against glanders with a gold nanoparticle glycoconjugate vaccine. Vaccine. 2015;33:686–692. doi: 10.1016/j.vaccine.2014.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Audi J, Belson M, Patel M, Schier J, Osterloh J. Ricin poisoning: a comprehensive review. JAMA. 2005;294:2342–2351. doi: 10.1001/jama.294.18.2342. [DOI] [PubMed] [Google Scholar]
- 133.Smallshaw JE, Vitetta ES. Ricin vaccine development. Curr Top Microbiol Immunol. 2012;357:259–272. doi: 10.1007/82_2011_156. [DOI] [PubMed] [Google Scholar]
- 134.Olsnes S. The history of ricin, abrin and related toxins. Toxicon. 2004;44:361–370. doi: 10.1016/j.toxicon.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 135.Schier JG, Patel MM, Belson MG, Patel A, Schwartz M, Fitzpatrick N, Drociuk D, Deitchman S, Meyer R, Litovitz T, et al. Public health investigation after the discovery of ricin in a South Carolina postal facility. Am J Public Health. 2007;97(Suppl 1):S152–157. doi: 10.2105/AJPH.2006.099903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pincus SH, Smallshaw JE, Song K, Berry J, Vitetta ES. Passive and active vaccination strategies to prevent ricin poisoning. Toxins (Basel) 2011;3:1163–1184. doi: 10.3390/toxins3091163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yan C, Rill WL, Malli R, Hewetson J, Tammariello R, Kende M. Dependence of ricin toxoid vaccine efficacy on the structure of poly(lactide-co-glycolide) microparticle carriers. Vaccine. 1995;13:645–651. doi: 10.1016/0264-410x(94)00026-j. [DOI] [PubMed] [Google Scholar]
- 138.Yan C, Rill WL, Malli R, Hewetson J, Naseem H, Tammariello R, Kende M. Intranasal stimulation of long-lasting immunity against aerosol ricin challenge with ricin toxoid vaccine encapsulated in polymeric microspheres. Vaccine. 1996;14:1031–1038. doi: 10.1016/0264-410x(96)00063-1. [DOI] [PubMed] [Google Scholar]
- 139.Griffiths GD, Bailey SC, Hambrook JL, Keyte MP. Local and systemic responses against ricin toxin promoted by toxoid or peptide vaccines alone or in liposomal formulations. Vaccine. 1998;16:530–535. doi: 10.1016/s0264-410x(97)80007-2. [DOI] [PubMed] [Google Scholar]
- 140.Griffiths GD, Bailey SC, Hambrook JL, Keyte M, Jayasekera P, Miles J, Williamson E. Liposomally-encapsulated ricin toxoid vaccine delivered intratracheally elicits a good immune response and protects against a lethal pulmonary dose of ricin toxin. Vaccine. 1997;15:1933–1939. doi: 10.1016/s0264-410x(97)00123-0. [DOI] [PubMed] [Google Scholar]
- 141.Griffiths GD, Phillips GJ, Bailey SC. Comparison of the quality of protection elicited by toxoid and peptide liposomal vaccine formulations against ricin as assessed by markers of inflammation. Vaccine. 1999;17:2562–2568. doi: 10.1016/s0264-410x(99)00054-7. [DOI] [PubMed] [Google Scholar]
- 142.Stahl JP, Mailles A, Dacheux L, Morand P. Epidemiology of viral encephalitis in 2011. Med Mal Infect. 2011;41:453–464. doi: 10.1016/j.medmal.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 143.Zacks MA, Paessler S. Encephalitic alphaviruses. Vet Microbiol. 2010;140:281–286. doi: 10.1016/j.vetmic.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nagata LP, Wong JP, Hu WG, Wu JQ. Vaccines and therapeutics for the encephalitic alphaviruses. Future Virology. 2013;8:661–674. [Google Scholar]
- 145.Greenway TE, Eldridge JH, Ludwig G, Staas JK, Smith JF, Gilley RM, Michalek SM. Induction of protective immune responses against Venezuelan equine encephalitis (VEE) virus aerosol challenge with microencapsulated VEE virus vaccine. Vaccine. 1998;16:1314–1323. doi: 10.1016/s0264-410x(98)00008-5. [DOI] [PubMed] [Google Scholar]
- 146.Davitt CJ, Lavelle EC. Delivery strategies to enhance oral vaccination against enteric infections. Adv Drug Deliv Rev. 2015;91:52–69. doi: 10.1016/j.addr.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 147.Marasini N, Skwarczynski M, Toth I. Oral delivery of nanoparticle-based vaccines. Expert Rev Vaccines. 2014;13:1361–1376. doi: 10.1586/14760584.2014.936852. [DOI] [PubMed] [Google Scholar]
- 148.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547–1568. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012;161:645–655. doi: 10.1016/j.jconrel.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 150.Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–459. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 151.Sullivan SP, Koutsonanos DG, Del Pilar Martin M, Lee JW, Zarnitsyn V, Choi SO, Murthy N, Compans RW, Skountzou I, Prausnitz MR. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16:915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.DeMuth PC, Moon JJ, Suh H, Hammond PT, Irvine DJ. Releasable layer-by-layer assembly of stabilized lipid nanocapsules on microneedles for enhanced transcutaneous vaccine delivery. ACS Nano. 2012;6:8041–8051. doi: 10.1021/nn302639r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Di J, Yao S, Ye Y, Cui Z, Yu J, Ghosh TK, Zhu Y, Gu Z. Stretch-Triggered Drug Delivery from Wearable Elastomer Films Containing Therapeutic Depots. ACS Nano. 2015;9:9407–9415. doi: 10.1021/acsnano.5b03975. [DOI] [PubMed] [Google Scholar]
- 154.Griffin JF. A strategic approach to vaccine development: animal models, monitoring vaccine efficacy, formulation and delivery. Adv Drug Deliv Rev. 2002;54:851–861. doi: 10.1016/s0169-409x(02)00072-8. [DOI] [PubMed] [Google Scholar]
- 155.Dasgupta G, BenMohamed L. Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine. 2011;29:5824–5836. doi: 10.1016/j.vaccine.2011.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]