Abstract
Background
Intestinal metaplasia (IM) is a gastric cancer precursor lesion (GCPL), with the highest risk to progress to gastric cancer (GC). Clinical guidelines recommend gastroscopy every 3 years for extensive IM. Unfortunately, studies on protein biomarkers indicating a transition from IM to GC are lacking. We have recently found that the IFNα-responsive gene Schlafen 4 (Slfn4) present in immune cells correlates with metaplastic changes in Helicobacter-infected mice. Therefore we tested the hypothesis that a human homolog of Slfn4, which is Schlafen 5 (SLFN5) correlates with progression of GCPL to GC.
Methods
Jurkat T-lymphoid and HL-60 myeloid cell lines were treated with IFNα and SLFN5 mRNA was quantified by qPCR. SLFN5 protein expression in the inflamed gastric mucosa was co-localized to specific immune cell types by immunohistochemistry using CD20, CD2 and MAC2 antibodies. SLFN5 expression was also determined by immunohistochemistry in FFPE samples from individuals with non-atrophic, atrophic gastritis, complete and incomplete IM as well as GC.
Results
We demonstrated that IFNα treatment of Jurkat and HL-60 cells induced SLFN5 mRNA. SLFN5 protein was expressed mainly by T lymphocytes in inflamed gastric mucosa. The highest level of SLFN5 expression was observed in subjects with IM that progressed to GC. ROC curves demonstrated that combining SLFN5 expression with the histological diagnosis of IM significantly increased the probability of identifying patients that might progress to GC.
Conclusion
Elevated SLFN5 protein expression in subjects with IM correlated with progression to gastric cancer.
Keywords: Intestinal metaplasia, biomarker, SLFN5, immunohistochemistry, ROC curve
INTRODUCTION
Gastric cancer (GC) is preceded by a series of events that include chronic atrophic gastritis, intestinal metaplasia (IM), and dysplasia [1]. IM is sub-classified as complete (small intestinal type) or incomplete (colonic type), in which the latter is considered to be the most advance stage of IM [2]. The initiating event leading to gastric carcinogenesis is atrophic gastritis typically due to infection with Helicobacter pylori (H. pylori) and subsequent infiltration of cytokine-secreting cells into the lamina propria [3].
GLI1 is a transcription factor and a downstream target of the Hedgehog (Hh) signaling pathway. We recently showed that Helicobacter-infected Gli1−/− mice do not develop gastric atrophy and spasmolytic polypeptide-expressing metaplasia (SPEM), a pre-neoplastic lesion compared to wild type (WT)-infected mice, demonstrating that Hh signaling is required for progression from inflammation to mucosal transformation [4]. Microarray analysis comparing stomach tissue from Helicobacter-infected wild type versus Gli1−/− mice revealed that a myeloid differentiation factor and Gli1 target gene called Schlafen 4 (Slfn4) was up-regulated in the gastric mucosa of wild type mice that develop the metaplasia [4].
Schlafens (SLFNs) are a family of highly conserved type 1 interferon-regulated proteins that control T-cell development and maturation, and when over-expressed impair T-cell proliferation and activation [5]. There are 10 murine and 5 human isoforms divided into three groups based upon their secondary structure. Although Slfn4 in mice correlates with atrophy and metaplasia, this isoform does not exist in the human genome. The predominant type 1 interferon-regulated SCHLAFEN gene in the human genome is SLFN5 [6].
Considering the increased level of pro-inflammatory cytokines in chronic atrophic gastritis [7] and several studies showing that IM increases the risk of GC relative to other gastric lesions [2,8], we hypothesized that SLFN5 might be an IFNα-regulated gene in human immune cells that correlates with a higher likelihood of gastric tumorigenesis. To test this hypothesis, we used samples from a follow-up study to examine whether SLFN5 protein expression in human gastric samples of IM correlated with histologic progression to GC.
MATERIALS AND METHODS
IFNα Induction of SLFN5 in Human Cell Lines
Jurkat cells are a human T lymphoblastoid cell line derived from an acute T cell leukemia (ATCC®, TIB-152). HL-60 cells are human promyelocytic leukemia cell line (ATCC®, CCL-240, Manassas, VA). HL-60 cells were cultured at 37°C with 5% CO2 in Iscove’s Modified Dulbecco’s Medium (IMDM, Life Technologies, Carlsbad, CA) plus 20% fetal bovine serum (FBS). Jurkat cells were cultured in RPMI 1640 media supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/ml streptomycin in 5% CO2 at 37°C. Both cell lines were treated with human recombinant interferon alpha (IFN2α, 1000 IU/ml, #11105-1, Interferon Source, Piscataway, NY). After removing the media, total cell RNA (500 ng) was prepared in Trizol® reagent (Life Technologies) for quantitative polymerase chain reaction (RT-qPCR) in a Bio-Rad C1000 Thermocycler™ with the CFX96™ real time attachment and CFX Manager software v.1.5 (Hercules, CA). TaqMan® probes and the TaqMan Gene Expression Assay (Life Technologies) were purchased for SLFN5 (Hs00288058_m1) and 18S RNA (Hs 99999901_s1) and normalized to beta actin (#Hs01060665_g1). Normalized values for IFNα-treated samples (N=9) were compared to untreated controls (N=9) using Bootsratio statistical software [9].
To validate the specificity of the induction, Jurkat cells were transfected with hSLFN5 siRNA (SC94178, Santa Cruz) or a control siRNA using Lipofectamine (RNAiMAX reagent kit, Life technology) according to the manufacturer’s instructions. Two days after transfection, cells were treated with IFNα (800U/ml) for 24h and then collected on slides by centrifugation for DAB staining with SLFN5 antibody.
Patient Samples
SLFN5 protein expression was determined by immunohistochemistry on three sources of human tissue: a commercial tissue microarray of human gastric mucosa, archived clinical samples from the Soria follow-up study [8], and from Marañon Hospital. The four commercial tissue microarrays were from US Biomax, Inc., (http://www.biomax.us/tissue-arrays; #ST1001, #ST805, #ST806, #ST00011b) and contained normal gastric mucosa (N=15), superficial and chronic gastritis (N=76), atrophy (N=57), dysplasia (N=11) and GC (N=106).
Clinical samples were FFPE blocks from precancerous lesions at recruitment from a follow-up study with GC as the end-point, carried out in the Province of Soria, which exhibits one of the highest risks for GC in Spain [8]. Briefly, patients who underwent endoscopy and gastric biopsies from 1988–1994 with the diagnosis of non-atrophic gastritis (NAG, N=26), multifocal atrophic gastritis (MAG, N=49) and complete (CIM, N=44) or incomplete intestinal metaplasia (IIM, N=54) were invited to undergo a follow-up biopsy between 2005 and 2007. This permitted documentation of the histologic changes in the gastric mucosa after an average follow-up interval of 12.5-years after the first endoscopy. Since none of the original IM samples analyzed were from lesions that progressed to GC, we included additional samples of CIM (n=6) and IIM (n=19) from the same hospital that after a similar mean follow-up of 12 years progressed to GC. Normal gastric mucosa (n=41) and GC (n=67) tissue from the same hospital were also analyzed by immunohistochemistry to assess the level of SLFN5 protein expression. A total of 281 clinical samples were analyzed and the demographics are presented in Table 1. H. pylori infection was determined by Giemsa staining, urea breath tests or documentation of a previous infection. An independent series of archived gastric specimens from the Gregorio Marañón Hospital (Madrid) consisting of CIM→GC (n=21) and CIM→No GC (n=29) was included to independently validate the results from the Soria study. The samples originate from 1518 Marañon samples diagnosed with IM from 4310 pathology reports from 1990 to 2008. Of the 1518 subjects with IM, 181 developed GC. However, paraffin blocks were recovered for only 21 of the patients with GC. The 29 samples forming the control group were selected from the 1518 that did not develop GC, were age-matched and were collected at approximately the same time as the cases with GC. The Helicobacter status is not known for the Marañon cases. All patients from the Soria study signed an informed consent giving permission for the procedures performed in the study. No informed consent was required for the use of the Marañón FFPE samples because all of archived tissue used in this study was acquired before the Spanish Biomedical Research law, 14/2007. The protocols were approved by the Ethical Committees of Hospital General Virgen del Mirón in Soria, the University Hospital of Bellvitge-Catalan Institute of Oncology and the Hospital Gregorio Marañón in Madrid.
Table 1.
Patient Diagnosis, H. pylori status and SLFN5 Histologic Scores
| Histologic Diagnosisa | N | Stromal H Score Median (P25,P75) | Categorized Stromal H Score b N(%) | Age Mean (±SD) | Gender (%Male) | ||
|---|---|---|---|---|---|---|---|
| Negative | Weak positive | Moderate positive | |||||
| Normal | 41 | 1.25 (0.17, 4.07) | 37 (90.24) | 4 (9.76) | 0 (0) | 42.5 (18.8) | 51.2 |
| NAG | 26 | 2.53 (0.33, 6.33) | 21 (80.77) | 5 (19.23) | 0 (0) | 44.2 (12.3) | 46.1 |
| MAG | 49 | 0.84 (0, 3.00) | 40 (81.63) | 9 (18.37) | 0 (0) | 48.5 (12.6) | 38.8 |
| CIM | 44 | 5.73 (1.00, 24.64) | 25 (56.82) | 18 (40.91) | 1 (2.27) | 53.1 (10.5) | 45.4 |
| CIM→GC | 6 | 50.68 (6.5, 92.31) | 2 (33.33) | 4 (66.67) | 0 (0) | 62.0 (6.6) | 50 |
| CIM→Not GC | 38 | 2.53 (1.00, 14.4) | 23 (60.53) | 14 (36.84) | 1 (2.63) | 51.7 (10.4) | 44.7 |
| IIM | 54 | 5.11 (0.13, 34.25) | 32 (59.26) | 20 (37.04) | 2 (3.7) | 58.3 (12.8) | 50 |
| IIM→GC *** | 19 | 40.00 (15.67, 70.0) | 3 (15.79) | 14 (73.68) | 2 (10.53) | 66.5 (13.6) | 63.2 |
| IIM→Not GC | 35 | 1.14 (0, 7.58) | 29 (82.86) | 6 (17.14) | 0 (0) | 53.8 (9.9) | 42.9 |
| Total IM | 98 | 5.43 (0.7, 25.29) | 57 (58.04) | 38 (38.97) | 3 (2.98) | 55.9 (12.1) | 48 |
| IM→GC *** | 25 | 40.18 (15.67, 70.0) | 5 (24.56) | 18 (70.17) | 2 (5.26) | 65.4 (12.4) | 60 |
| IM→Not GC | 73 | 2.00 (0.4, 11.60) | 52 (71.69) | 20 (26.99) | 1 (1.31) | 52.7 (10.1) | 43.8 |
| GC | 67 | 1.38 (0, 9.67) | 51 (76.12) | 16 (23.88) | 0 (0) | 72.6 (11.0) | 61.2 |
| Intestinal | 41 | 8.3 (0, 10.38) | 31 (75.6) | 10 (24.39) | 0 (0) | 72.97 (10.15) | 56.1 |
| Diffuse | 25 | 7.46 (0, 9.76) | 19 (76) | 6 (24) | 0 (0) | 70.6 (12.43) | 17 (68) |
| Mixed | 1 | 2.38 | 1 (100) | 0 (0) | 0 (0) | 66 | 1 (100) |
Normal= Normal gastric mucosa not infected by H. pylori; NAG= Non-atrophic gastritis; MAG= multi-focal atrophic gastritis; CIM= Complete Intestinal Metaplasia; CIM→GC= CIM that progressed to gastric cancer; CIM→No GC= CIM that did not progress to gastric cancer; IIM= Incomplete Intestinal Metaplasia; IIM→GC= IIM that progressed to gastric cancer; IIM→No GC= IIM that did not progress to gastric cancer; IM= Intestinal Metaplasia; IM→GC Intestinal Metaplasia (CIM+IIM) that progressed to Gastric Cancer; IM→No GC= Intestinal Metaplasia that did not progress to gastric cancer; GC= gastric cancer.
Categorized Stromal score H was compared between IIM→GC vs IIM→No GC (Mann-Whitney p<0.0001 ***) and between IM→GC vs IM→No GC (Mann-Whitney p<0.0001 *). The Kruskal-Wallis test was performed to compare groups of Normal, NAG, MAG, CIM, IIM and GC, yielding significant results (p=0.0033)
SLFN5 detection by immunohistochemistry
The FFPE samples from clinical studies were stained using the rabbit specific streptavidin-biotin immune-enzymatic antigen detection system (Abcam, ab64261). Four micron sections were prepared and incubated overnight at 60 °C prior to deparaffinization in xylene-alcohol series followed by rehydration in 1X PBS. Antigen retrieval was performed by boiling the slides in 10 mM sodium citrate buffer, pH 6 for 45 min. After cooling to room temperature, the slides were washed twice in 1X TBS-T (TBS, 0.1% Triton X-100), immersed for 10 min in H2O2 to deplete endogenous peroxidase and then rinsed three times in TBS-T. Nonspecific binding sites were blocked with Protein Block (ThermoFisher Scientific) for 10 min. The slides were then incubated for 90 min with rabbit anti-human SLFN5 (Abcam, ab121537) antibody at a 1:500 dilution in 1X TBS-T followed by two rinses. After incubating with the biotinylated goat secondary antibody for 10 min, the slides were rinsed, and then incubated for 10 min with streptavidin peroxidase followed by two additional rinses. The reaction product was visualized with diaminobenzidine (DAB) substrate for 10 min and counterstained with Gill’s hematoxylin. The secondary antibody without SLFN5 primary antibody was used as a negative control and the positive control was human nasopharynx as recommended by the manufacturer.
An independent series of samples from the Marañon hospital was performed on a Dako IHC machine using the same protocol described previously with a minor modification. The SLFN5 staining pattern was confirmed using 10 additional human gastric samples with SLFN5 antibody (HPA 017760, Sigma-Aldrich) [6]. Replicate IHC scoring was blindly performed by OC and by a pathologist (MLP).
Immunohistochemical scoring of SLFN5
SLFN5 expression on the tissue arrays and clinical samples at 200X magnification was scored by a researcher (OC) blinded to the diagnoses using an Olympus BX60 microscope. SLFN5 nuclear staining was quantified independently for stroma and gastric glands using the semi-quantitative method of evaluating positively-stained cells called the H score (% of low intensity-stained cells x 1 +% medium intensity-stained cells x 2 + % of high-intensity-stained cells x 3) [10]. Intraepithelial lymphocytes were included in the stromal score. Depending on the size of the tissue section, 5 to 20 fields were examined. The H score for each stained slide was calculated as the mean of all fields analyzed. When more than one slide per subject was stained, the final H score was the mean of the scores from the different slides analyzed. The mean H score for each subject was plotted against the histologic diagnosis, e.g., normal, NAG, MAG, IM subtypes and GC.
Colocalization of SLFN5, CD2, CD20 and Mac2
To determine which cell type expressed SLFN5 in the gastric mucosa, cell surface markers CD20 (B-cells), CD2 (T-cells), or Mac2 (myeloid cells) was colocalized with SLFN5 using a section from chronic atrophic gastritis. Antigen retrieval was made in sodium citrate 10 mM, pH 6 for 15 min, washing with 1X TBS and then blocked with 20% goat serum. The primary CD20 Ab (Abcam, ab9475) was diluted 1:50 then applied to the tissue at 4°C to incubate overnight. After washing with 1X TBS, a 1:200 dilution of biotinylated goat anti-mouse secondary antibody, (BA-9200, Vector Laboratories) was applied for 30 min and then washed with 1X TBS. Tissue slides were incubated with ABC-AP reagent (Vectastain ABC-AP kit, Vector Labs) for 30 min and then washed with 1X TBS. The reaction product was visualized by incubating with alkaline phosphatase for ~20 min (SK-5100, Red AP Substrate Kit, Vector Labs) after diluting in 100mM Tris-HCl, pH 8.5 followed by a water rinse with 1X TBS. After this step, the same protocol for SLFN5 staining was performed after the H2O2 block for SLFN5. 1X TBS was used for all final rinses before air-drying. To co-localize with the T-cell marker, CD2 (Abcam, ab131276) antibody was incubated at a 1:100 dilution in 1X TBS with 1% BSA overnight at 4°C. The sections were incubated with biotinylated goat anti-rabbit secondary antibody for 10 min and then rinsed in 1X TBS. To detect myeloid/macrophage lineages, the Mac2 antibody (Cat. #14-5301, eBioscience) was incubated at a 1:200 dilution in 1X TBS with 1% BSA for 1 h at RT. The sections were incubated with a 1:200 dilution of biotinylated goat anti-rat secondary (BA-9400, Vector Labs) for 30 min. The slides were incubated with the Vectastain ABC-AP reagent for 30 min and incubated in the dark for 30 min using the BCIP/NBT substrate (SK-5400, Vector Labs) diluted in 100 mM Tris-HCl with 0.1% Tween, pH 9. The stained slides were rinsed in H2O and dehydrated but were not counterstained with hematoxylin prior to placing the coverslip.
Statistical Analysis
Baseline quantitative variables were expressed as the mean ± standard deviation (SD) or as the median with the 25th and 75th percentiles, depending if the variable followed a normal distribution or not, using the D’Agostino & Pearson omnibus normality test. Qualitative variables were expressed as frequencies and percentages. The Kruskal-Wallis test was performed to assess differences in the SLFN5 stromal score among the Soria samples at recruitment (Normal, NAG, MAG, CIM, IIM, and GC). The Mann-Whitney test was used to compare SLFN5 stromal scores of each IM subtype that progressed to GC versus those that did not progress to GC (CIM→GC vs CIM→Not GC, IIM→GC vs IIM→Not GC). We clustered the H scores into four categories: 0<= H score <10 (Negative), 10<= H score <=100 (Weakly Positive), 100<= H score <=200 (Moderately Positive), 200 < H score <= 200 (Strongly Positive) [11]. To assess the role of SLFN5 stromal expression as a prognostic indicator of IM progression to GC, we constructed two logistic regression models with or without the categorized SLFN5 stromal H score, adjusting by sex, age and type of IM (CIM, IIM), as potential confounding variables that could influence GC progression. H. pylori infection was not included as a confounding factor because the bacterium is not present in the advanced stages of gastric carcinogenesis. In order to increase statistical power, the odds ratios (OR) and confidence intervals (95% CI) for the categorical stromal H scores were calculated using the positive versus the negative H score, with the negative one used as the reference group. We constructed ROC curves for both logistic regression models (with and without SLFN5 protein expression data) and calculated the area under the curve (AUC). To determine whether adding the SLFN5 stromal H score significantly improved the likelihood of IM progression to gastric cancer, the Likelihood-Ratio Test (LRT) was applied. All analyses were performed on the IIM and IM samples and not the CIM samples separately due to the low number of CIM cases progressing to GC (n=6, Table 1) in the Soria follow-up study. SAS v9.0 software was used for the statistical analysis and significance was set at p< 0.05.
RESULTS
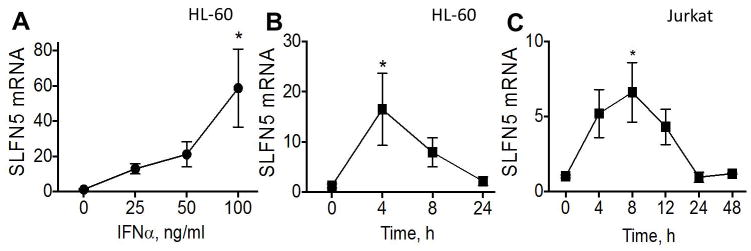
Type I interferons induce expression of both the murine Slfn4 gene in myeloid cells [12] and human SLFN5 gene in malignant melanoma [6]. Therefore, we examined whether IFNα induces SLFN5 expression in human immune cells and could play a similar role as Slfn4 in the inflamed stomachs of Helicobacter-infected mice. A dose-response curve and time course for IFNα in the HL-60 monocyte and Jurkat T-lymphocyte lines revealed a rapid and significant (p<0.05) induction of SLFN5 mRNA within 4–8h (Fig. 1A–C). SLFN5 protein was specifically detected in Jurkat cells by IHC since siRNA knockdown of the protein abolished staining (Fig. 2A–B). The same antibody was used to stain human tissue sections.
Fig. 1.
IFNα induction of SLFN5 in cell lines. Dose response curve for SLFN5 mRNA in HL-60 cells after IFNα treatment (A). Time course of SLFN5 induction in HL-60 cells (B) and Jurkat cells (C).
Fig. 2.
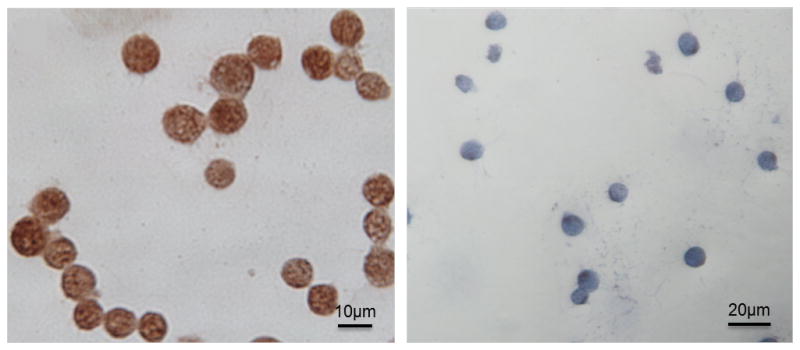
SLFN5 knock-down in Jurkat cells. SLFN5 expression was determined by immunohistochemistry in Jurkat cells before (A) or after SLFN5 siRNA knockdown (B).
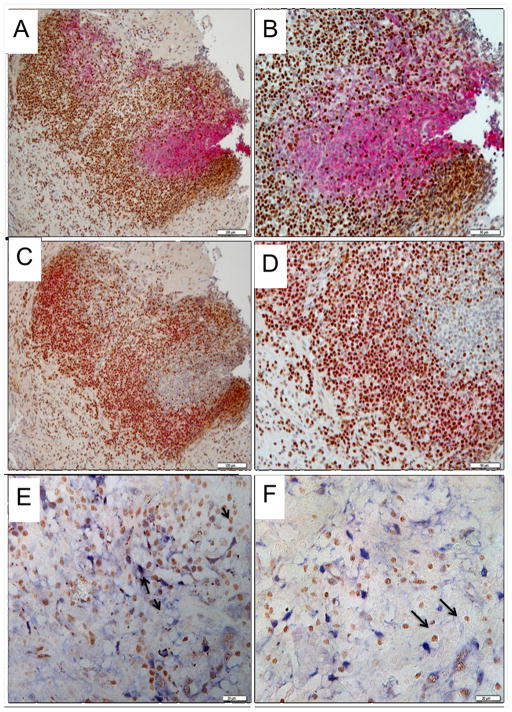
When we co-stained for SLFN5 in clinical samples from a patient with IIM→GC (also see Fig. 4F for area with IIM) immune surface markers, most of the SLFN5 positive cells in the lymphoid follicle were T cells and not B cells since there was co-localization with CD2 (Fig. 3C–D) but not CD20 (Fig. 3A–B). SLFN5-expressing macrophage cells were scattered through the gastric lamina propria and co-localized with the Mac2 macrophage protein marker (Fig. 3E–F).
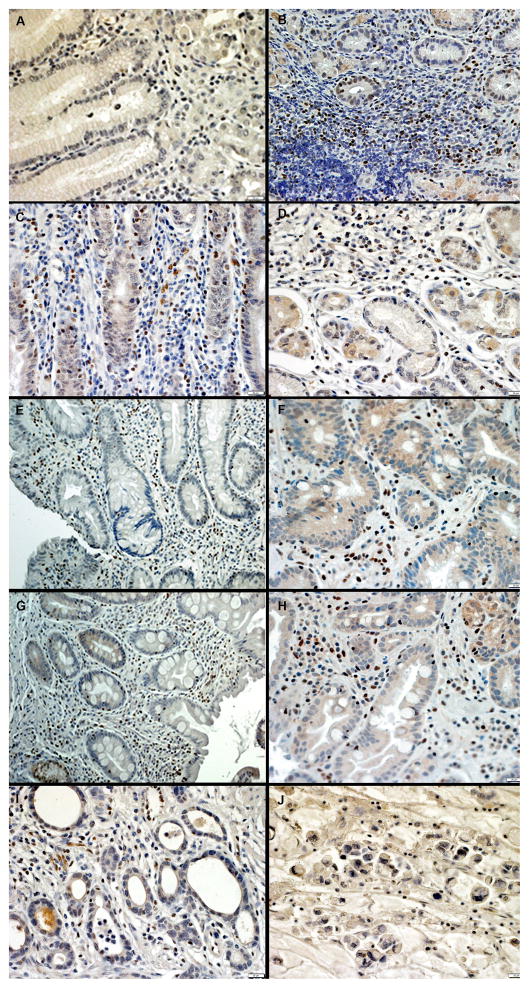
Fig. 4.
Immunohistochemistry of SLFN5 in the human stomach. SLFN5 protein detection was performed on archived gastric tissues from A) Normal; B) Atrophic gastritis, in stroma versus glands; C) Non-atrophic gastritis (NAG); D) Multi-focal atrophic gastritis (MAG); E) Incomplete Intestinal Metaplasia that did not progress to GC (IIM→Not GC); F) Incomplete Intestinal Metaplasia that progressed to gastric cancer (IIM→GC); G) Complete Intestinal Metaplasia that did not progress to GC (CIM→Not GC); H) Complete Intestinal Metaplasia that progressed to gastric cancer (CIM→GC); I) Intestinal-type gastric cancer; J) Diffuse-type gastric cancer
Fig. 3.
Co-localization of SLFN5 with hematopoietic markers in human gastric mucosa of subjects with IM. SLFN5 protein expression was detected using horseradish peroxidase with the diaminobenzidine substrate and co-labeled with antibodies to detect B cells (CD20), T cells (CD2) or macrophages (Mac2) using the alkaline phosphatase secondary and red or blue substrates. The colocalization was performed on a lymphoid aggregate in the gastric tissue sample shown in Fig. 4F (IIM→GC). A) CD20 (B cell stain (pink) + SLFN5 (brown), 200X; B) CD20 + SLFN5, 400X; C) CD2 (T cell stain (pink) + SLFN5 (brown), 200X; D) CD2 + SLFN5, 400X; E and F) Mac2 Myeloid cell stain (Blue) + SLFN5 (brown), 400X.
Since IFNα was able to induce SLFN5 expression in human immune cells, we examined SLFN5 protein expression in human gastric tissue by IHC. First, we examined SLFN5 expression in commercial tissue microarrays of human gastric tissue, which revealed higher SLFN5 protein in the samples with atrophy and frank cancer (data not shown). Although samples with chronic atrophic gastritis (CAG) exhibited an increase in SLFN5 expression, the increase was not statistically significant compared to healthy subjects. Since the commercial array did not provide sufficient clinical details and follow-up information, we analyzed archived tissue from a follow-up study of gastric cancer precursor lesions from Soria, Spain for SLFN5 expression by IHC [8].
In these samples, normal gastric mucosa was either completely negative or weakly positive for SLFN5 protein expression (Fig. 4A). SLFN5 staining was generally higher in the gastric stroma compared to gastric glands (Fig. 4B), as expected based upon known Schlafen gene expression in murine and human hematopoietic cells [4,13]. SLFN5 expression was observed in subjects with atrophic gastritis, intestinal metaplasia, and both intestinal and diffuse subtypes of GC (Fig. 4C–J). We observed more intense staining in areas of organized inflammatory collections, which typically are composed primarily of lymphocytes and myeloid cells.
The widely accepted H score, which is commonly used for semi-quantitation of estrogen receptors in breast cancer by IHC [10], was used here to quantify the changes in gastric SLFN5 expression in the archived specimens from the Soria follow-up study. SLFN5 expression increased with lesion severity until GC developed, when expression was reduced (Table 1, Fig. 5). Significant differences between the stromal H scores for the major histological groups (Normal, NAG, MAG, CIM, IIM and GC, p=0.0033) were observed. In addition, stromal expression of SLFN5 was greatest in intestinal metaplasia that progressed to GC (IM→GC) when compared to IM that did not progress (IM→Not GC; p<0.0001, Table 1, Fig. 5). Similar results were obtained when incomplete intestinal metaplasia that progressed to GC (IIM→GC) was compared to those that did not progress (IIM→Not GC, p<0.0001, Table 1). This analysis was not performed in CIM due to the low sample size of CIM→GC (n=6).
Fig. 5.
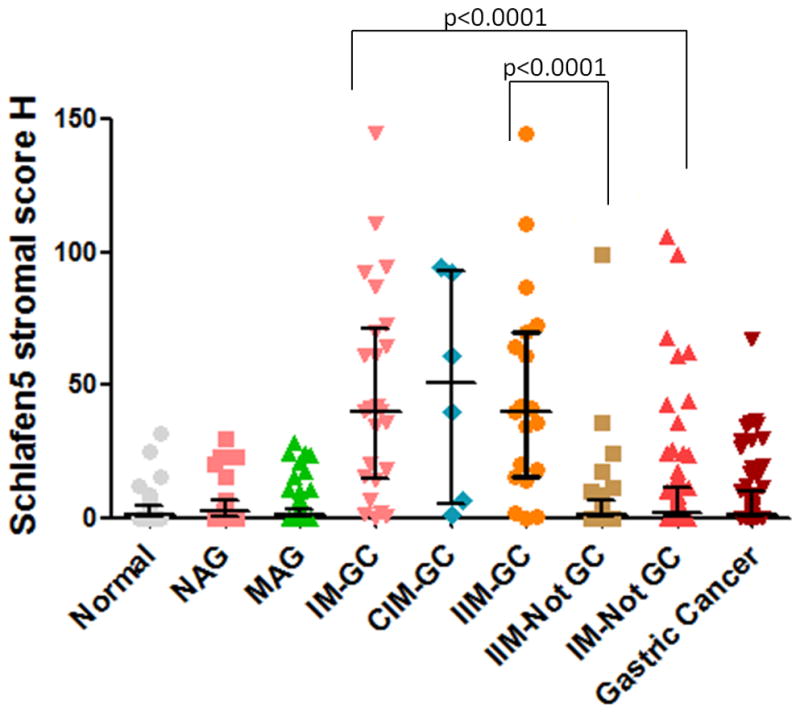
SLFN5 Stromal IHC score.
The distribution of Schlafen5 stromal scores for the indicated categories was plotted as a scatter graph. The mean ± median is shown. The sample size of each group is Normal=41, NAG=26, MAG=49, IM→GC=25, CIM→GC=9, IIM→GC=16, IM→Not GC=73, Gastric Cancer=67.
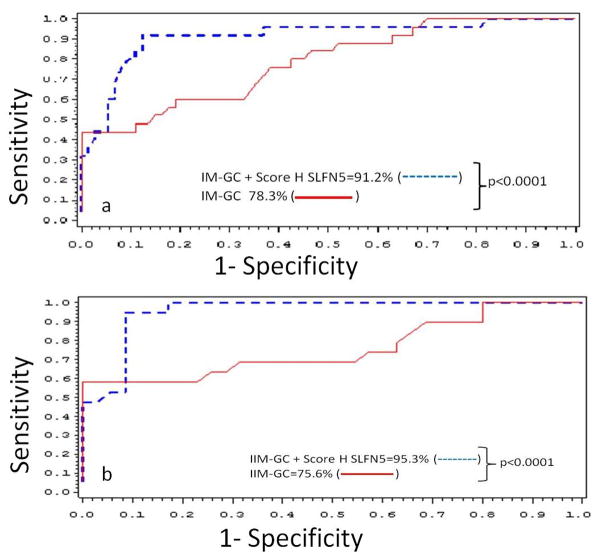
Logistic regression analysis showed that the SLFN5 stromal score was a significant risk factor for IM (CIM+IIM) progression to GC (OR 18.1, CI 95% 4.14–79.1, p=0.0001), as well as for IIM progression (OR 71.3, CI 95% 7.14–712.9, p=0.0003). The ROC curve obtained when SLFN5 expression was included in the logistic regression model for the IM (CIM+IIM) samples indicated an AUC of 0.912, which was 12.9% greater than the AUC obtained when the SLFN5 expression was not included in the model (AUC=0.783). The difference between these two curves was significant (p<0.0001) after applying the LRT test (Fig. 6A). When only IIM samples were considered, the AUC was 0.953 when the SLFN5 H score was included in the model, compared to an AUC of 0.756 when the H score was not included. This 19.7% increase in the AUC was again significant (p<0.0001) after applying the LRT test (Fig. 6B). Therefore in the context of IM of both subtypes, the SLFN5 H score increases the discrimination effect to 91%, and when the IIM subtype is specifically considered, discrimination was higher at 95%.
Fig. 6.
ROC curves for logistic regression models of IM (A) and IIM (B) with or without the SLFN5 stromal score.
Logistic regression models were constructed with histological diagnosis, sex, age and with or without categorized SLFN5 stromal score H, as variables. p-value of the Log-likelihood ratio test between the score with versus without the stromal score is indicated for each model. (
 ). Model with SLFN5 stromal score. (
). Model with SLFN5 stromal score. (
 ). Model without the SLFN5 stromal score. AUC values are also shown as percentages.
). Model without the SLFN5 stromal score. AUC values are also shown as percentages.
A similar analysis for CIM independent of IIM could not be made with the Soria specimens due to the low sample size. Therefore samples from a third hospital (Marañón, Madrid) were collected and stained. In the Marañón hospital samples, analysis of SLFN5 protein expression by two independent researchers (OC and MLP) did not reach statistical significance, although both evaluators showed a trend towards a higher risk of progression in SLFN5 positive samples of CIM when compared to the cases that did not progress to cancer [OR=1.93 (IC 95%: 0.57–6.58) and OR=2.95 (IC 95%: 0.80–10.90)].
DISCUSSION
Based upon our prior characterization of murine Slf4 over-expression in Helicobacter-induced metaplasia [4], we examined whether a human SCHLAFEN gene homolog correlated with pre-neoplastic changes in the stomach. We report here that SLFN5 protein expression can indeed improve the identification of patients at greater risk for GC once they have developed IM. GC is a slow, progressive process that emerges from metaplastic changes occurring as a result of subsequent sustained inflammation initiated by H. pylori infection. With the exception of histology demonstrating IM on endoscopic biopsies, there are no clinical biomarkers for this deadly disease. Therefore, we evaluated samples from a long-term follow-up study of GCPL with histological diagnoses made at the time of recruitment and at the end of a 12.5-year follow-up period. Guidelines for the management of GCPL include endoscopic surveillance every 3 years for extensive IM [14], however there are no biomarkers for to predict which patients with IM are most likely to develop GC over time. Taking into account that the incidence rate for GC is 3.77 cases for every 1000 IM patients after 5 years observed in the Soria study [8], highly expressed proteins might identify which IM patients are predisposed to eventually develop cancer.
We first demonstrated that IFNα, which is increased in patients infected by H. pylori [7], induces the in vitro expression of SLFN5 in human T and monocyte cell lines. Next we confirmed that SLFN5 is expressed in human gastric T and macrophage cells of the inflamed stomach in vivo. Next, we examined SLFN5 expression in the gastric mucosa of human subjects and found that SLFN5 expression was higher in IM and IIM that progressed to GC (IM→GC and IIM→GC) compared to the same lesions that do not progress to GC (IM→Not GC, IIM→Not GC). Therefore, adding scoring for SLFN5 protein expression in immune cells to the histological subtype of IM or even total IM could increase the probability of identifying patients that would eventually develop GC, based on AUCs. This conclusion was reached by comparing ROC curves with and without the SLFN5 stromal H score. When we analyzed SLFN5 staining in independent samples from another population (Marañón Hospital), the differences were suggestive but did not reach statistical significance, which we speculate is due to the relative small sample size. However, only CIM samples were identified in those cases, which might reflect the fact that the prevalence of GC in Madrid is lower than in Soria [15]. Using the Madrid samples, we that the IHC scoring employed showed a similar trend of higher expression in those that progress to GC compared to those that do not progress.
Few studies have examined whether protein biomarkers are present in patients with GCPL that eventually progress to GC. In a study of 93 patients with gastric atrophy and metaplasia, it was found that the expression of inducible nitric oxide synthase and nitrotyrosine were significantly higher in 34 patients that developed gastric cancer 2 years later [16]. Another study of 396 patients with GCPL, showed elevated MG7-Ag and COX-2 expression by IHC in IM that progressed to dysplasia compared to non-IM controls. Samples positively staining for both markers had a significantly higher risk of progression to GC (OR 22.7, p<0.005) than those without positive staining [17].
Our earlier observations in a mouse model of Helicobacter infection showed that Slfn4 was expressed in a myeloid population that also expressed IL-1β and TNFα proinflammatory cytokines [4]. Surface marker analysis of these cytokine-producing cells indicated that they were CD11b+ and Gr-1+ suggestive of myeloid derived suppressor cells, a population of immature myeloid cells that inhibit proliferation of cytotoxic T cells. Suppression of cytotoxic T cells by SLFN5 producing cells could create a permissive environment for tumor cell growth once IM develops [18]. In the mouse model, Slfn4 expression was Gli1-dependent, indicating that the Hedgehog signaling pathway regulates the quality of the gastric immune response [4].
Previous research of Schlafen genes (including SLFN5) indicates that they are up-regulated in mature CD4+ and CD8+ thymocytes and in peripheral resting T cells; and down-regulated after T-cell stimulation [13,19]. In the SLFN8 (group III) transgenic mouse, elevated T-cell expression partially blocked thymocyte transition to mature T cells; and transgenic T cells exhibited a significant reduction in the proliferative response [19]. Furthermore, transgenic expression of SLFN1 in thymocytes and fibroblasts causes cell cycle arrest [20]. SLFN5 expression is down-regulated in peripheral T cells-stimulated by TCR suggesting that it negatively regulates T cell growth [20]. In addition, a recent article shows evidence that IFNα signaling induces Schlafen genes precluding T-cell activation; and that the high basal expression of SLFN5 in resting T-cells is down-regulated suggesting that this gene plays an active role in maintaining T-cell quiescence [13]. Based on our results and the previous literature, we speculate that IFNα induction of SLFN5 in T-cells and possibly macrophages might maintain T-cells in a non-activated or quiescent state dampening their response to environmental cues. Suppression of CD8+-cytotoxic T cell activation can create a permissive environment for neoplastic transformation [18]. A recent study has suggested that SLFN 5 like SLFN12L is associated with T cell suppression, a function consistent with MDSC function that creates an immune environment permissive for cancer development [13]. Additional studies suggest that the T cells that do not attack the tumor might express programmed death -ligand 1 (PD-L1) or its receptor and would be amenable to anti-PD therapies [22]. We can only speculate at this point what additional markers these SLFN5 cells express and suggest that future studies with freshly isolated tissue will further characterize the function of these cells. Immunohistochemical analysis of SLFN5 protein on endoscopic biopsies might provide clues as to the likelihood of progression to GC. However, implementation of such analyses into clinical practice will require a prospective study of SLFN5 expression in patients at risk for developing GC.
Acknowledgments
The authors acknowledge technical contribution from Mrs. Nadia Garcia from the Molecular Epidemiology Group, Translational Research Laboratory, Catalan Institute of Oncology. Sources of funding: National Institutes of Health P01 DK062041 (JLM); Health Research Funds; Grant number: FIS Exp030077; Grant sponsor: RETICC, Spanish Ministry of Health; Grant number: DR06/0020-0015, RD12/0036/0018 and PI10/01089.
Instituto de Salud Carlos III, Ministerio de Economía y Competitividad; Cofunded by FEDER funds/European Regional Development Fund (ERDF).
Osmel Companioni was supported by a PhD fellowship from the Research Institute of Bellvitge, IDIBELL.
Abbreviations
- AUC
Area under the curve
- CIM
Complete intestinal metaplasia
- CIM→GC
Complete intestinal metaplasia that progress to Gastric Cancer
- CIM→Not GC
Complete intestinal metaplasia that does not progress to Gastric Cancer
- GCPL
Gastric cancer precursor lesion
- IIM
Incomplete intestinal metaplasia
- IIM→GC
Incomplete intestinal metaplasia that progress to Gastric Cancer
- IIM→Not GC
Incomplete intestinal metaplasia that does not progress to Gastric Cancer
- GC
Gastric cancer
- IM
Intestinal metaplasia
- IHC
Immunohistochemistry
- LRT
Likelihood-Ratio Test
- NAG
Non-atrophic gastritis
- MAG
Multifocal atrophic gastritis
- SLFN
human
- Slfn
mouse, Schlafen
- OR
Odds ratio
- HR
Hazard ratio
- ROC
References
- 1.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–40. [PubMed] [Google Scholar]
- 2.González CA, Sanz-Anquela JM, Gisbert JP, Correa P. Utility of subtyping intestinal metaplasia as marker of gastric cancer risk. A review of the evidence. Int J Cancer. 2013;133:1023–32. doi: 10.1002/ijc.28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merchant JL. Inflammation, atrophy, gastric cancer: Connecting the Molecular Dots. Gastroenterology. 2005;129:1079–82. doi: 10.1053/j.gastro.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 4.El-Zaatari M, Kao JY, Tessier A, et al. Gli1 Deletion Prevents Helicobacter-Induced Gastric Metaplasia and Expansion of Myeloid Cell Subsets. PloS One. 2013;8:e58935. doi: 10.1371/journal.pone.0058935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mavrommatis E, Fish EN, Platanias LC. The Schlafen Family of Proteins and Their Regulation by Interferons. J Interferon Cytokine Res. 2013;33:206–10. doi: 10.1089/jir.2012.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katsoulidis E, Mavrommatis E, Woodard J, et al. Role of Interferon alfa inducible Schlafen-5 in Regulation of Anchorage-independent Growth and Invasion of Malignant Melanoma Cells. J Biol Chem. 2010;285:40333–41. doi: 10.1074/jbc.M110.151076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindholm C, Quiding-Järbrink M, Lönroth H, et al. Local cytokine response in Helicobacter pylori-infected subjects. Infect Immun. 1998;66:5964–5971. doi: 10.1128/iai.66.12.5964-5971.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gonzalez CA, Pardo ML, Ruiz Liso JM, et al. Gastric cancer occurrence in preneoplastic lesions: a long-term follow-up in a high-risk area in Spain. Int J Cancer. 2010;127:2654–60. doi: 10.1002/ijc.25273. [DOI] [PubMed] [Google Scholar]
- 9.Clèries R, et al. BootstRatio: A web-based statistical analysis of fold-change in qPCR and RT-qPCR data using resampling methods. Comput Biol Med. 2012;42:438–445. doi: 10.1016/j.compbiomed.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 10.McCarty KS, Miller LS, Cox EB, Konrath J. Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109:716–721. [PubMed] [Google Scholar]
- 11.Shousha S. Oestrogen receptor status of breast carcinoma: Allred/H score conversion table. Histopathology. 2008;53:346–7. doi: 10.1111/j.1365-2559.2008.03075.x. [DOI] [PubMed] [Google Scholar]
- 12.van Zuylen WJ, Garceau V, Idris A, Schroder K, Irvine KM, Lattin JE, et al. Macrophage Activation and Differentiation Signals Regulate Schlafen-4 Gene Expression: Evidence for Schlafen-4 as a Modulator of Myelopoiesis. PLoS ONE. 2011;6:e15723. doi: 10.1371/journal.pone.0015723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puck A, Aigner R, Modak M, Cejka P, Blaas D, Stöckl J. Expression and regulation of Schlafen (SLFN) family members in primary human monocytes, monocyte-derived dendritic cells and T cells. Results Immunol. 2015;5:23–32. doi: 10.1016/j.rinim.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPED) Endoscopy. 2012;44:74–94. doi: 10.1055/s-0031-1291491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aragonés N, Pérez-Gómez B, Pollán M, Ramis R, Vidal E, Lope V, García-Pérez J, Boldo E, López-Abente G. The striking geographical pattern of gastric cancer mortality in Spain: environmental hypotheses revisited. BMC Cancer. 2009;8:316. doi: 10.1186/1471-2407-9-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goto T, Haruma K, Kitadai Y, et al. Enhanced Expression of Inducible Nitric Oxide Synthase and Nitrotyrosine in Gastric Mucosa of Gastric Cancer Patients. Clin Cancer Res. 1999;5:1411–5. [PubMed] [Google Scholar]
- 17.Hong Liu, Li Shujun, Liu L, et al. The value of MG7-Ag and COX-2 for predicting malignancy in gastric precancerous lesions. Cell Biol Int. 2010;34:873–6. doi: 10.1042/CBI20100149. [DOI] [PubMed] [Google Scholar]
- 18.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125:3356–64. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geserick P, Kaiser F, Klemm U, Kaufmann SH, et al. Modulation of T cell development and activation by novel members of the Schlafen (slfn) gene family harbouring an RNA helicase-like motif. Int Immunol. 2004;16:1535–48. doi: 10.1093/intimm/dxh155. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz DA, Katayama CD, Hedrick SM. Schlafen, a New Family of Growth Regulatory Genes that Affect Thymocyte Development. Immunity. 1998;9:657–68. doi: 10.1016/s1074-7613(00)80663-9. [DOI] [PubMed] [Google Scholar]
- 22.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14:847–56. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]