Abstract
Treatment of cancer has come a long way from the initial “radical surgeries” to the multi-modality treatments. For the major part of the last century, cancer was considered as a mono-cellular disorder, and treatment strategies were designed according to that hypothesis. However, the mortality rate from cancer continued to be high and a comprehensive treatment remained elusive. Recent progress in research has demonstrated that tumors are a complex network of neoplastic and non-neoplastic cells. The non-neoplastic cells, which are collectively called stroma, assist in tumor survival and progression. It has been shown that disrupting the tumor-stromal balance leads to significant effects on the tumor survival, and effective treatment can be achieved by targeting one or more of the stromal components. In this review, we summarize the roles of various stromal components in promoting tumor progression, and discuss innovative nanoparticle-mediated drug targeting strategies for stromal depletion and the subsequent effects on the tumors. Perspectives and the future directions are also provided.
Keywords: Nanoparticle, tumor stroma, modification of tumor microenvironment
1. Introduction
Cancer has long been regarded as an independent and autonomous disease of only neoplastic cells, i.e., a disease of a single cell type. The central hypothesis of cancer generation, survival and metastasis were linked to genotypic and phenotypic changes only in the cancerous cells. Significant knowledge has been gathered ranging from genetic changes in the neoplastic cells that promote tumorigenesis and metastasis. Although the “seed and soil” hypothesis was presented more than a century ago by Stephen Paget in 18891 emphasizing the tumor microenvironment for tumor progression, until recently the role of non-neoplastic cells in the tumor microenvironment has largely been neglected. Owing to many advanced studies, it has now been established that tumors are, in fact, a complex network of neoplastic and non-neoplastic cells intertwined with each other in a strong symbiotic relationship which provides the condition permissive for the growth and progression of malignant cells (Figure 1). Cancerous cells modify and recruit non-cancerous cells from local or distant host tissue to the tumor microenvironment, and survival of the neoplastic cells are highly dependent on the nature and balance of these supporting cells. These non-neoplastic cells that constitute the tumor microenvironment facilitate tumor development by providing extracellular matrices, cytokines, growth factors, and vascular networks. In addition to the cellular component, the extracellular matrix (ECM) and secreted extracellular molecules act in autocrine and/or paracrine manners to support and sustain tumor progression. A large part of the tumor is made up from these supporting cells. As accumulating research points to the importance of these cells for tumor survival, a better understanding of the tumor microenvironment and how to manipulate it into one that is less or non-permissive to tumor development attracts significant interest.2, 3 Nanotherapeutics have been demonstrated to be a promising platform to perform this type of therapy.
Figure 1.
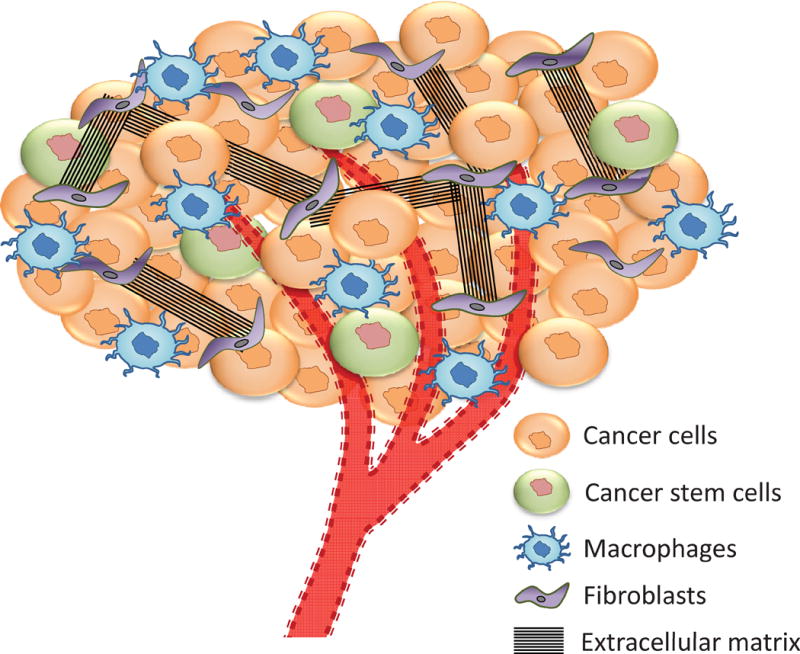
Components of a tumor stroma. A complex network of neoplastic and non-neoplastic cells constitute tumor stroma. A strong symbiotic relationship exists among them which help the tumor growth and survival. Just like an organ, tumor has its own support cells, blood vessels, residing immune cells and even stem cells that helps its survival and propagation.
This review provides an overview of the cellular and extracellular components that constitute the tumor microenvironment, and the nanoparticle strategies used to deliver therapeutics aimed at disrupting the tumor microenvironment.
2. Role of tumor microenvironment in tumor initiation and progression
The tumor microenvironment (the non-cancerous components of which are collectively called stroma) is primarily moulded by the developing tumor, and both the tumor and stroma cells co-evolve to the benefit of the tumor progression. The logistics of the development of the stroma, its initiation and recruitment at the tumor site, has not yet been fully understood. The stroma consists of a collection of cells, including fibroblasts/myofibroblasts, endothelial, vascular, smooth muscle, and immune cells along with the extracellular matrix (ECM) and secreted extracellular molecules (Figure 1), which act in autocrine and/or paracrine manners to increase the tumor cell survival.
None of the components of stroma are malignant, and in normal tissue, they maintain tissue structure and function. However, through intercellular interactions or paracrine secretions from cancer cells, stromal cells acquire abnormal phenotypes that support cancer cell growth and tumor progression. Additionally, the abnormal interaction between different stroma components further drives the cancer stroma phenotype, and may result in permanent alterations in cell function of tumor stroma. Tumor growth is stimulated by growth factors and chemokines produced by the altered fibroblasts and immune cells in the stroma, which in turn recruit more stromal cells, continuing into a vicious cycle. Tumor stroma is also found to confer resistance to diverse chemotherapeutic drugs.4–6 Miao et al. has reported that repeated treatments with cisplatin loaded DSPE-PEG nanoparticles lead to heightened tumor cell resistance and stroma reconstruction due to Wnt16 upregulation.7
We will discuss the role of the different tumor stromal cells, particularly those that have been exploited for cancer therapies, how they contribute to tumor progression and metastasis and how nanotherapeutic strategies are being explored to target these cells.
2.1. Tumor associated fibroblasts
Fibroblasts are essential to maintain the integrity of normal epithelial tissues and are the primary producers of the non-cellular scaffold - the extracellular matrix (ECM). They also produce different collagen subtypes and fibronectin, which maintains the tissue basement membrane. Fibroblasts also remodel the overall architecture of tissues by modifying the ECM through secretion of different matrix metalloproteinases (MMPs) and various proteases.8
During wound repair, fibroblasts become “activated”, with increased proliferation and alterations in both phenotype and secretory capacity. They start expressing alpha-smooth muscle actin (α-SMA) to migrate into areas of damage and assist in tissue restoration. They also secrete increased levels of ECM proteins that eventually build a scaffold. They help to recruit immune cells and vascular progenitor cells at the wound site for a faster recovery. After tissue recovery, the activated fibroblasts generally transpose into the inactive state or undergo apoptosis.
There are lots of similarities between activated fibroblasts and cancer associated fibroblasts (CAFs), reported as early as in the 1980s by Dvorak.9 Both types of fibroblasts proliferate rapidly and produce a complex ECM, with significant fibrin deposition. Similar to the activated fibroblasts, CAFs also overexpress α-SMA and secrete various growth factors. However, unlike activated fibroblasts, CAFs do not revert back to their inactivated state, or undergo apoptosis. Moreover, CAFs produce many other growth factors like transforming growth factor β, hepatocyte growth factor, insulin-like growth factor 1/2, which facilitate proliferation and invasion of cancer cells (Figure 2).10 They also secrete chemokines such as monocyte chemotactic protein 1 and interleukin 1 to stimulate proliferation of tumor cells.11 In addition, CAFs produce MMPs, mostly MMP-9 and MMP-2, and other matrix-modifying enzymes, including urokinase-type plasminogen activator (uPA), that degrade the ECM and support tumor invasion and metastasis.12 Further, CAFs also produce stromal cell-derived factor 1 (SDF-1) which stimulates tumor growth directly, acting through its receptor CXCR4 expressed in many cancer cells.13 SDF-1 signaling can also stimulate angiogenesis by recruiting circulating endothelial progenitor cells (EPCs) into the tumor stroma.14 CAFs also produce a significant amount of Secreted Protein Acidic and Rich in Cysteine (SPARC). SPARC expression in CAFs has been associated with disease progression.15 Functions of SPARC include cell–cell de-adhesion, regulation of angiogenesis, and modulation of ECM production and composition.16, 17 Overexpression of SPARC suppresses immune cell infiltration into tumors.17
Figure 2.
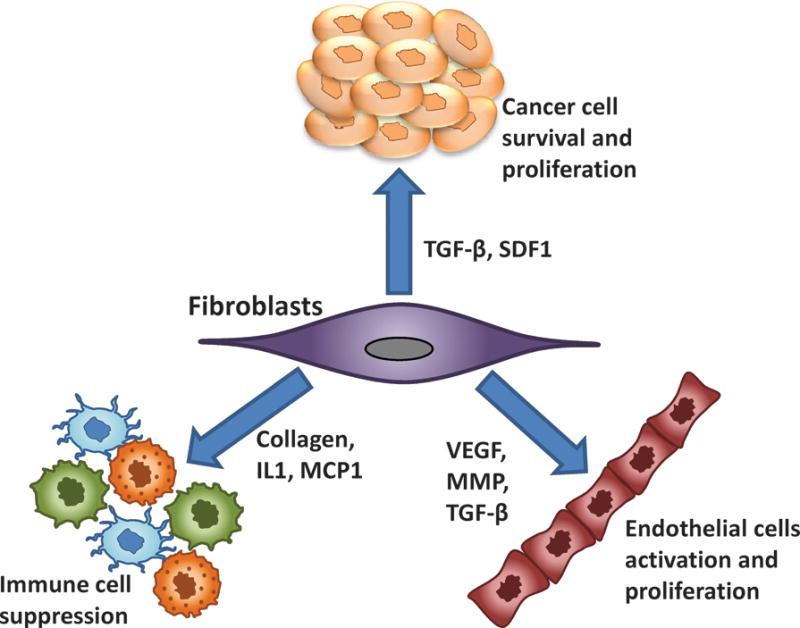
Effect of cancer associated fibroblasts (CAF) on tumor and other stromal cells. CAFs can promote tumorigenesis directly through multiple mechanisms, including increased angiogenesis, proliferation, invasion, and inhibition of tumor cell death. These effects are mediated through the expression and secretion of numerous growth factors, cytokines, proteases, and extracellular matrix proteins.
2.2. Vascular Endothelial Cells
Angiogenesis is critical for cancer cell growth and survival as it supplies the nutrients and oxygen needed for tumor growth.18 Initiation of angiogenesis requires increased matrix metalloproteinase (MMP) production leading to degradation of the basement membrane, sprouting of endothelial cells, and regulation of pericyte attachment. Intratumoral hypoxia, induced by rapid tumor growth facilitates angiogenesis by promoting production of many secreted factors like hypoxia-inducible factors (HIFs), angiopoietin 2, angiopoietin 4, placental growth factor, platelet-derived growth factor B, stem cell factor, stromal-derived factor 1, and vascular endothelial growth factor (VEGF).19 CAFs play an important role in synchronizing the angiogenesis events through the expression of numerous ECM molecules and growth factors, including transforming growth factor (TGF)-β, VEGF, and fibroblast growth factor (FGF) 2.
These angiogenic vessels are generally formed by one of several mechanisms including budding of existing vascular networks, recruitment of vascular progenitor cells to form new vascular channels, or vascular mimicry - a process by which tumour cells, without the presence of endothelial cells, line up to form a capillary allowing blood transport.20 These neo-angiogenic vessels are abnormal; they are non-uniformly distributed and irregularly shaped, inappropriately branched and tortuous, often ending blindly. They do not have the classic hierarchical arrangement of arterioles, venules, and capillaries and often form arterio-venous shunts. These vessels are variably fenestrated and leaky which are routes for cancer cells to enter circulation to initiate metastasis (Figure 3).
Figure 3.
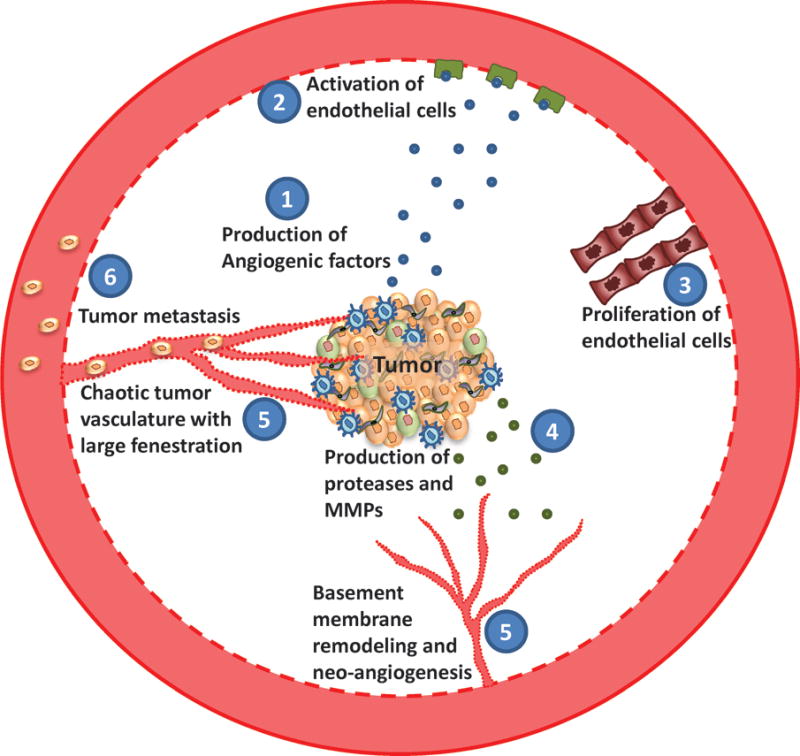
Tumor angiogenesis and metastasis. Tumor cells proliferate rapidly and have a high metabolic demand. To fulfill their energy requirements, tumor cells and other stromal cells produce different angiogenic factors including VEGF, which activates the endothelial cells and stimulate proliferation. Tumor stroma also produce a host of proteases and MMPs which helps basement membrane remodeling. Following the breakdown of basement membrane, proliferating endothelial cells lead to uncontrolled neovascularization. These newly formed vessels are irregular and leaky, through which tumor cells escape and metastasize to distant organs.
2.3. Tumor-associated immune cells
Since its inception, tumor cells recruit and interact with different immune cells in the stroma. The famous tumor immune editing hypothesis emphasizes three stages during this interaction, the three Es: elimination, equilibrium and escape. At the very initial stage of tumorigenesis, the immune defence mechanism of the host can eliminate a significant portion of the pre-cancerous or cancerous cells. Most of the spontaneous pre-cancers are thus eradicated even before their initiation. Sometimes the cancer cells evade immune attack and stay at a dormant stage for a long time, which is called the equilibrium stage. When these dormant cells become significantly mutated, they can escape the immune surveillance and proliferate vigorously to form a tumor. Even at the escape stage, tumor stroma is not devoid of the immune cells. On the contrary, a very significant part of the tumor stroma is composed of different immune cells. Tumor cells and other stromal cells like fibroblasts secrete a high amount of immune inhibitory cytokines to keep the tumor microenvironment in an immunosuppressive state. The tumor cells in fact use the suppressed immune cells for their benefit: cytokines secreted from these immune cells promote angiogenesis, which helps tumor cell survival and metastasis.
Tumor associated macrophages (TAMs) constitute a major portion of the immune cells in the tumor stroma. In fact, TAMs are among the most abundant non-neoplastic cells in the tumor microenvironment.21 Substantial evidence indicates that TAMs, rather than being tumoricidal, adopt a pro-tumoral phenotype both in the primary and metastatic sites.22 TAMs promote many important features of tumor progression including angiogenesis, tumor cell invasion, motility, stimulation of tumor cell extravasation as well as suppression of T cell responses.23 Normally, macrophages are viewed as proinflammatory, anti-cancerous cells which would fight against cancer growth. However, biological functions of macrophages are dictated by their phenotypes. Macrophages exhibit activational and functional plasticity, and can differentiate into two distinct phenotypes. Macrophages activated by bacterial lipopolysaccharide (LPS) and interferon gamma (IFN-γ) are termed M1 and are pro-inflammatory in nature. M1 macrophages are phagocytotic and cytotoxic, inhibiting tumor progression. On the other hand, macrophages activated by IL-4, IL-10, IL-13, M-CSF etc. are called M2 macrophages, which are immunosuppressive in nature. During the immune escape stage, cancer cells secrete a host of cytokines and growth factors including IL-4, IL-13, colony stimulating factor-1 (CSF1) and GM-CSF.21 These cytokines and growth factors maintain an immunosuppressive state in the tumor microenvironment, converting the macrophages to the M2 phenotype. The M2 macrophages promote tissue repair and produce cytokines that suppress the adaptive immune system, thereby supporting tumor progression.24 The M2 macrophages also support angiogenesis in tumors, by secreting VEGF and IL-8, which stimulate the proliferation of tumor-associated endothelial cells (Figure 4). In addition to secreting cytokines and promoting angiogenesis, TAMs also have a role in ECM remodelling by producing MMPs, uPA and uPA receptor, which facilitate ECM remodelling in collaboration with CAFs.25 TAMs also express PD-L1 and produce chemokine CCL22, which further inhibit T cell proliferation and promote trafficking of immunosuppressive T regulatory (Treg) cells to the tumor.26
Figure 4.
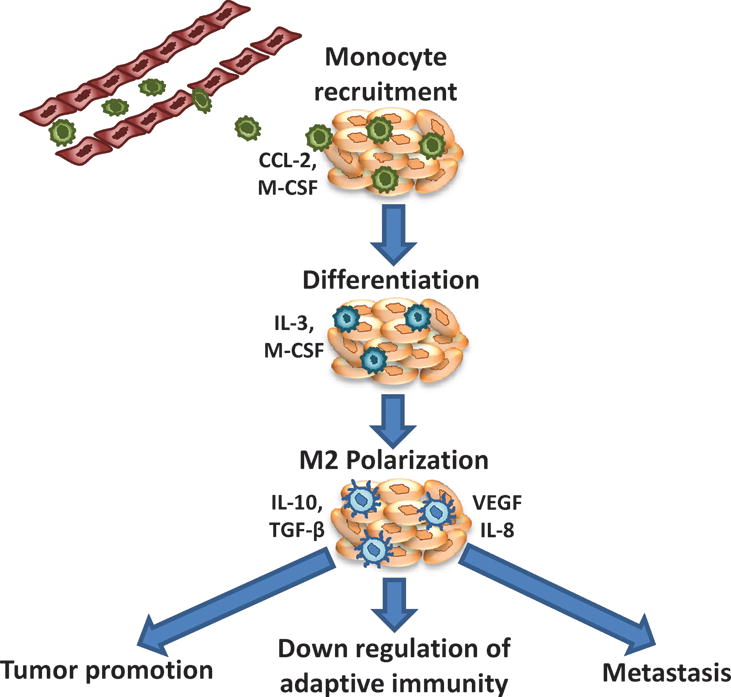
Tumor associated macrophage (TAM) – recruitment, transformation and activity. Due to secretion of different factors (CCL-2, M-CSF) from the tumor stroma, monocytes accumulate at the tumor microenvironment (TME). Due to the primarily immunosupressive nature of TME, these monocytes differentiate into M2 phenotype. M2 macrophages assist tumor survival and metastasis as well as downregulates immune response at the TME by secretion of different cytokines.
TAMs are highly immune suppressive in nature and produce a host of immune inhibitory cytokines including IL-10, TGF-β, CCL2 etc. This immunosuppressive milieu converts cytotoxic T cells to Treg cells. Within the tumor, Treg cells produce TGF-β and IL-10, which contribute to an immunosuppressive environment through the inhibition of cytotoxic T-cells and natural killer cells.
2.4. Cancer stem cells
Cancer cells differentiate themselves from normal host cells in their astonishing ability of multiplying at an extraordinarily high rate. Stem cells are the only type of normal cells that can reproduce and self-renew. This similarity in the ability of self-renewal of the normal healthy stem cells and the tumor cells propagated the idea of cancer stem cell (CSC). CSCs are proposed to be a very small subpopulation of the cancer cells that reproduce and sustain the cancer. CSCs can arise from normal tissue resident stem cells through oncogenic mutations or may be normal somatic cells that acquire oncogenic mutations, which prevent them from entering post-mitotic differentiation states.27 Stem cells can also be recruited from the circulation and/or from nearby tissues into the tumor stroma.28 It has been proposed that CSCs are mainly responsible for sustaining the tumor growth. Most of the tumors are highly heterogeneous and this heterogeneity is believed to arise from the differentiation of the stem cells.27 Due to the self-renewal properties, CSCs maintain their stem cell characteristics and are able to form tumors when transplanted in another host.29 They are resistant to conventional chemotherapy and radiation therapy and due to their ease of transplantation, CSCs are very likely to be the origin of cancer metastasis.30
2.5. Extracellular matrix
Although long viewed as a stable structure that plays a mainly supportive role in maintaining tissue morphology, the tumor extracellular matrix (ECM) is an essential part of tumor stroma. The ECM mainly consists of structural proteins (collagen and elastin), specialized proteins (fibrilin, fibronectin, and elastin), and proteoglycans.31 They provide the structural scaffold for the cancer cells to propagate. ECM affects cancer progression by directly promoting cellular transformation and metastasis. An increase in ECM stiffness up-regulates integrin signaling and can thus promote cell survival and proliferation.32, 33 Abnormal ECM also indirectly affects cancer cells by influencing the behavior of other stromal cells, including endothelial cells, immune cells, and fibroblasts, leading to generation of a tumorigenic microenvironment.13, 34–36 Therefore, targeting the tumor ECM may provide yet another effective avenue to combat cancer.
3. Regulating the tumor stroma with nanoparticle therapeutics for effective treatment
As has been discussed above, tumor stroma plays a critical role for the maintenance and progression of tumors. Without an active support from the stromal component, tumor progression can be halted or eliminated. Since tumors have long been regarded as a mono-cellular disorder, almost all of the approved anticancer therapeutics have been designed to target the neoplastic cells exclusively. Advanced studies with some of these drugs have shown that apart from the tumor cell itself, they also have significant activity against various stroma components. Nanoparticle therapeutics have been designed to take advantage of the morphological and physiological abnormalities of tumor microenvironment for drug targeting, namely the fenestrated and leaky vasculature. Due to the leaky vasculature in the tumor vessels, intravenously administered nanoparticles preferentially accumulate in the tumor interstitium whereas their penetration through the tight endothelial junctions of normal blood vessels is limited. In addition to targeting therapeutics to tumors, most nanoparticle drug delivery systems provide enhanced stability and controlled drug release.
3.1. Targeting tumor associated fibroblasts
Fibroblasts are one of the major components of tumor stroma and play a crucial role for tumor survival. Several strategies have been adapted for nanoparticle mediated targeting of CAFs. Anisamide, a ligand for the sigma receptor overexpressed on the surface of CAFs, was coated on the liposomes to enhance receptor mediated endocytosis. Delivery of cisplatin in liposomes decorated with anisamide resulted in a significant increase in CAF uptake compared to the non-decorated liposomes (from ~ 20% to ~ 30%), leading to a decrease in the percentage of CAF cells in the tumor.7 Tumor cells also exhibited higher uptake of the targeted liposomes compared to the non-targeted one. The anisamide decorated liposomes induced enhanced antitumor efficacy compared to the non-targeted liposomes against a stroma rich bladder cancer model, possibly due to increased targeting and activity against both the tumor cells and CAFs.
Iron oxide (Fe3O4) nanoparticles (~ 65 nm) have been shown to prevent the expression of α-SMA and therefore decrease the number of myofibroblastic cells.37 However, due to the pro-invasive effect of iron oxide, it was not effective as therapy.37 In contrast, polymer-coated cerium oxide nanoparticles (size of 3–5 nm) reduced both the expression of α-SMA in the stromal cells and the invasion of tumor cells.38
A cleavable amphiphilic peptide (CAP), which is specifically responsive to fibroblast activation protein-α (FAP-α), a protease expressed on the surface of cancer-associated fibroblasts, can self-assemble into drug-loaded spherical nanoparticles in the presence of hydrophobic chemotherapeutic drugs. The disassembly of these nanoparticles (CAP-NPs) upon FAP-α cleavage resulted in rapid and efficient release of the encapsulated drugs specifically at the tumor site. Treatment of mice bearing PC3 tumor with DOX loaded CAP-NPs resulted in significant tumor regression and tumor core penetration of DOX compared to un-cleavable amphiphilic peptide loaded DOX NPs.39
Targeted delivery of chemotherapeutics to CAFs also led to significant antitumor activity along with reduced metastasis. Ernsting et al. has developed a nanoparticulate system by conjugating docetaxel with PEGylated acetyl-carboxymethyl-cellulose, known as Cellax.40 Cellax has demonstrated major CAF targeted delivery with more than 85% of the NPs targeted to the CAFs in an orthotopic breast tumor in mice.41 A significant decrease in CAF population was noted with Cellax therapy in multiple tumor models, leading to a significant increase in tumor perfusion, a decrease in tumor interstitial fluid pressure and significantly enhanced anti-metastatic effect (Figure 5).41, 42 The possible mechanism of CAF targeting by Cellax may be through an albumin and SPARC dependent internalization mechanism. Hoang et al. have demonstrated that serum albumin efficiently adsorbed onto the Cellax particles and significantly higher internalization was noted in SPARC positive cells compared to SPARC negative cells (Figure 6).43 As SPARC is overexpressed by CAFs, Cellax could be efficiently internalized by SPARC positive CAFs.
Figure 5.
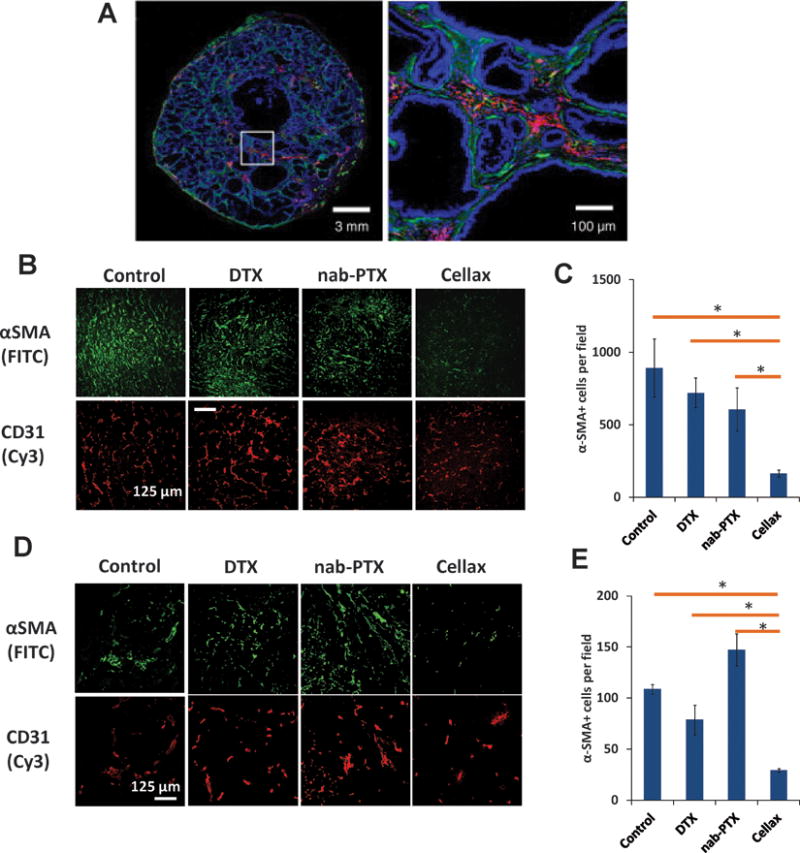
A. Colocalization of Cellax nanoparticles with α-SMA+ CAF cells (green: α-SMA; red: Cellax-DiI; blue: DAPI). B-E: Effects of native docetaxel (DTX), nab-paclitaxel (nab-PTX), and Cellax on the CAF cells in different orthotopic breast cancer models (4T1 [B, C] and MDA-MB-231 [D, E]). Treatment with Cellax lead to marked reduction in the α-SMA+ CAF cells in both of the models. Adapted from Ernsting et al., Journal of Controlled Release. 2015 and Murakami et al., Cancer Res. 2013.
Figure 6.
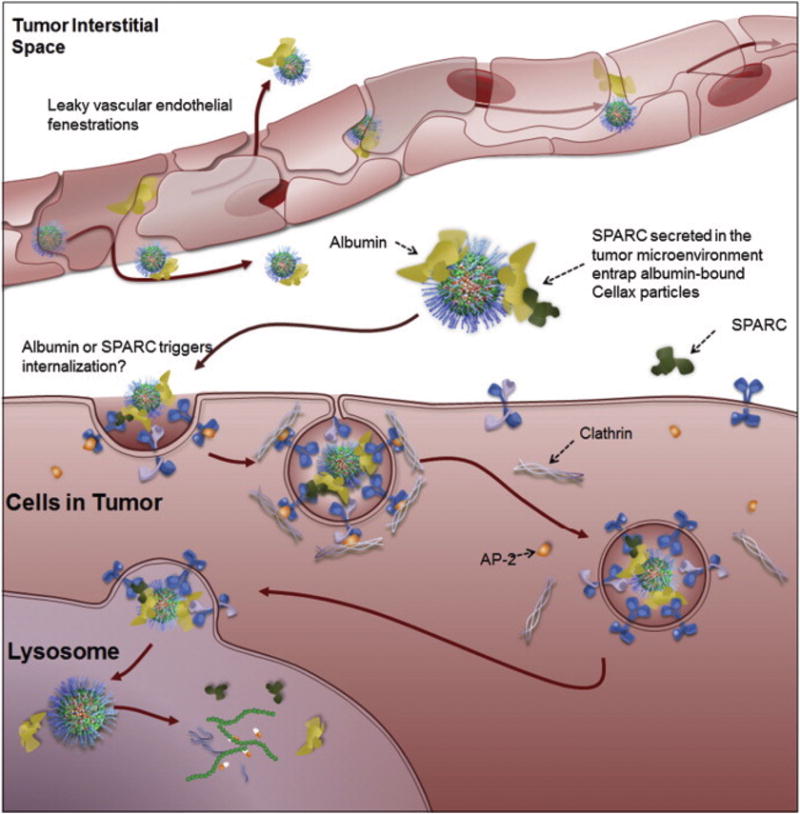
Proposed mechanism for Cellax intracellular internalization. Cellax adsorbs albumin in circulation and accumulated within the tumor interstitium through the leaky vasculature of the tumor. SPARC produced in the tumor microenvironment binds to the surface albumin on the Cellax nanoparticles and thus traps the particles in the tumor. Cellax is internalized via a clathrin-mediated mechanism and finally ends up in the endo-lysosomal compartment, where the polymer is broken down and the drug is released. Adapted from Hoang et al., Biomaterials. 2015.
3.2. Targeting vascular endothelial cells
Tumor vasculature is one of the most important components of the tumor stroma, supplying all the necessary nutrients and oxygen for the survival of the tumor. Tumor growth correlates strongly with the extent of angiogenesis.44, 45 Studies in breast cancer patients have showed that angiogenesis positively correlates with the degree of metastasis, tumor recurrence and mortality rates, thus demonstrating the value of angiogenesis as a prognostic cancer marker.46, 47 The vascular endothelial cells are one of the easiest to target among all the different components of the tumor stroma due to their high accessibility. Therapeutic targeting of tumor vasculatures has several advantages over conventional anticancer therapy. Tumor-associated vascular endothelial cells proliferate rapidly and express a variety of angiogenic markers, allowing specific vascular-targeted therapies to cause minimal side effects to normal vasculatures. Compared with tumor cells, endothelial cells are genetically stable, accessible to circulating drugs and share similar phenotypes in most tumors. Therefore, antivascular therapies generally develop low drug resistance and can be applied to a wide range of solid tumors. Currently a vast number of antivascular agents are in development or have been developed for clinical applications, such as bevacizumab, sunitinib, sorafenib and combretastatin A4.48
Endothelial cells in tumor blood vessels express unique integrins, proteoglycans and proteases that can be targeted to deliver cytotoxic drugs.49 Other targeting ligands used for drug delivery to tumor vasculature include antibodies to vascular endothelial growth factor receptors which are overexpressed on the vascular endothelium.50 Although it has been demonstrated that NPs with a surface rich in negatively charged polyelectrolytes are preferentially taken up by endothelial cells without cell-specific targeting ligands,51 passive targeting of the nanotherapeutics to the tumor vasculature was not successful due to the constant flow of blood in the lumen.52
As discussed, endothelial cells express a series of angiogenic markers that provide opportunities for the design of actively targeted nanoparticles. The most prominent marker for tumor endothelial cells are the integrins, especially the αvβ3 subtype, which is upregulated in a number of tumors,53–55 and has been widely exploited for targeted drug delivery.56 The first integrin-targeted nanoparticle was developed using a cationic polymerized lipid that was covalently coupled to a small organic αvβ3 ligand to deliver mutant Raf gene to angiogenic blood vessels. This system induced apoptosis in the tumor endothelial cells and regression of primary and metastatic tumors.57 Xie et al. synthesized a neopentyl derivative of the same ligand which exhibited a superior binding affinity for αvβ3 integrin. Lipid nanoparticles conjugated with this derivative showed increased and selective accumulation in angiogenic tumors.58 A paramagnetic polymerized liposomal nanoparticle was developed by attaching the αvβ3 antibody LM609 to the surface of the nanoparticles,59 and these polymerized nanoparticles showed selective tumor accumulation in the VX2 rabbit tumor model.60 Cyclic or linear derivatives of the RGD peptide (Arg-Gly-Asp) are natural ligands for integrin and have been widely used for drug targeting.61 Murphy et al. prepared cyclic-RGD decorated lipid NPs for the delivery of doxorubicin, which exhibited a 15-fold increase in anti-metastatic activity, again revealing the vital role of angiogenesis in tumor progression and invasion.62 Many such nanoparticle systems have since been developed,63–65 and reviewed elsewhere.61 Tumstatin is another important ligand for αvβ3 integrin66, and a tumstatin-conjugated iron oxide nanoparticle showed enhanced penetration and selective targeting to angiogenic endothelial cells, resulting in significant inhibition of tumor neovascularisation.67
Nucleolin, another angiogenic marker, is highly expressed on tumor blood vessels relative to normal vasculatures.68 A 31-amino-acid (F3 peptide) as well as a DNA aptamer (AS1411) that recognize cell surface nucleolin have been utilized for the development of nucleolin-targeting nanoparticles.69–72 These nanoparticles effectively eliminated angiogenesis and blocked tumor growth.
Vascular endothelial growth factor receptor (VEGFR) is another tumor endothelial marker which has been explored for targeted delivery. Different peptides have been identified to target VEGFR and applied in nanoparticle conjugation. One such peptide (HTMYYHHYQHHL) that binds VEGFR-2 with a high affinity and specificity was conjugated to paclitaxel-loaded nanoparticles, contributing to rapid, long-term and targeted inhibition of tumor vasculature.73 Polyvinylidene fluoride (PVDF) nanoparticles modified with a cyclopeptide (CBO-P11) have shown a high binding affinity with VEGFR-2 and low toxicity, and may represent a useful vector for targeting tumor angiogenesis.74
Apart from using VEGFR agonist, native VEGF has also been tested for its ability to target endothelial cells. Backer et al. have reported the construction of VEGF conjugated, boronated polyamidoamine dendrimer that can be used to target VEGFR on tumor neovasculature.75
Chen et al. have reported that dextran-coated iron-oxide nanoparticles conjugated to radiolabeled anti-VEGF monoclonal antibody significantly increased the imaging resolution as well as inhibited liver cancer in mice.76
Other than these, many other endothelial receptors and markers have been utilized for targeted delivery including CD13,77, 78 CD105,79–81 VE-cadherin82 etc.
3.3. Targeting tumor-associated immune cells
Tumor-associated immune cells are by far the most researched target among the components of tumor stroma. Immune cells are designed to eliminate neoplastic cells from the host, but due to immune editing by the cancer cells, they in turn help cancer cells survive and propagate. They can be reactivated and re-educated to harness their tumoricidal activity. Systemic immune activation is not favoured as the activated immune cells are extremely potent and can lead to very serious side effects. To stimulate the immune cells specifically in the tumor microenvironment, nanoparticle mediated delivery is an attractive strategy. A massive body of research has been done on this topic, a comprehensive discussion of which is beyond the scope of this review but can be found elsewhere.83–85 Here, we focus on major immune cells found in the tumor stroma and the main mechanism to modulate them using nanomedicines for effective tumor therapy.
Because macrophages are the most prominent immune component in the tumor stroma and are a major contributor for tumor immunosuppression due to their M1 → M2 switch, they have been the target of many investigations. Two major approaches have been used to target the TAM population: (1) eradicating TAMs in the tumor stroma and (2) re-stimulating TAMs to M1 macrophages.
Bisphosphonate (BP) molecules such as clodronate, zolindronic acid (ZOL) and pamidronate are effective against TAMs;86 however due to their short half-life and rapid uptake by bone, their use for cancer therapy is limited. To increase their half-life and target them to TAMs, nanoparticulate delivery has been employed. It has been demonstrated that melanoma bearing mice treated with clodronate liposomes were depleted of TAMs, and the tumor growth was inhibited.87 PEGylated self-assembled nanoparticles of ZOL were developed and shown to reduce TAMs and completely regress PC3 prostate tumor, resulting in prolonged overall survival.88, 89 Since M2 macrophages express a high level of mannose receptors, TAMs can also be targeted via mannose decorated nanoparticles. Zhu et al., developed a sheddable PEG decorated, mannose-modified nanoparticle platform to improve TAM targeted delivery. Sheddable PEG was conjugated to mannose decorated PLGA nanoparticles via an acid sensitive linker. In a murine melanoma model (B16F10), improved accumulation of nanoparticles in TAMs was found with the sheddable PEG decorated formulation as compared to nanoparticles conjugated to non-sheddable PEG. PEGylation prevents uptake of the nanoparticles during systemic circulation by the circulating macrophages and other immune cells. However, as the tumor microenvironment is relatively acidic, after tumor accumulation of the nanoparticles by the EPR effect, the acid labile PEG linker was cleaved, releasing PEG and allowing the mannose labeled nanoparticles to be internalized by TAMs.90
Re-stimulation of TAMs is generally achieved by using different adjuvants. Toll like receptor (TLR) agonists are the front runners as adjuvants for macrophage stimulation.91 Although TLRs are mainly involved in innate immunity by sensing pathogenic danger signals, they are crucial for induction of adaptive immune responses as they can promote cross-presentation in antigen presenting cells (APCs) to activate CD8+ T cells or prime APCs to release cytokines that can activate CD4+ T cells.92 Different TLR agonists, including TLR3, TLR4, TLR7 or TLR9, have been tested for their efficacy as cancer vaccines.93, 94 CpG oligonucleotide is a potent TLR9 agonist, and its negatively charged structure has been used to complex with cationic lipids or polymer for tumor delivery.95, 96 It has been shown that co-delivery of an immune adjuvant in combination with an antigen can significantly enhance the efficiency of cross-presentation and induction of CD8+ T cells.96–98 Co-entrapment of a TLR3 agonist poly I:C and an antigen peptide onto gold nanoparticles led to activation of antigen-specific CD8+ T cells in vivo.97 When co-delivered, a strong synergy among different TLR agonists has also been noted.99 For example, CpG and poly I:C have been co-loaded into polyester nanoparticles,100 while the TLR4 agonist glucopyranosyl lipid A and TLR7 agonist imiquimod have been co-encapsulated into liposomes.101 In both cases, the TH1 response was significantly improved by the dual TLR agonist-loaded particles, compared with that elicited by a single adjuvant. siRNA mediated inhibition of immunosuppressive pathways in tumor residing immune cells have also been observed to modulate immune response towards a more immune active state. Co-delivery of CpG and siRNA targeting IL-10, the inducer of TH2 and Treg cells, skewed immune responses to the TH1 type.102 The combination of a peptide epitope of tyrosine-related protein 2 (Trp2) and a CpG-based nanovaccine with siRNA against TGF-β, a cytokine responsible for induction and maintenance of immunosuppressive tumor microenvironment, has significantly improved the therapeutic efficacy of nanoparticle-based cancer vaccine in a late-stage murine melanoma model.103
Some polymeric nanocarriers have been shown to potentiate immune activation themselves without any classical immune adjuvant.104 However, these cationic nanoparticles often exhibit a high level of uptake by macrophages in the liver and spleen when systemically delivered, their selective immunostimulating effect in tumors could be compromised.
In recent times, simultaneous administration of a chemotherapeutic drug and an immune activator for the treatment of cancer has attracted significant interest. This combined chemo-immunotherapeutic approach is argued to be superior to immunotherapy alone due to several advantages. First, the chemotherapeutic drug will reduce the tumor burden which will help to mount a better immune response as the tumor cells are the major contributor for the immune suppression. Second, chemotherapy-induced cell death can enhance cross-priming of immune cells by providing them potent cancer specific antigens, thereby increasing the antitumor T cell response.105 Third, chemotherapeutic drugs increase the expression of MHC molecules on the tumor cells, making them an easier target for cytotoxic T lymphocytes.106 Based on these hypotheses, Roy et al. developed a nanoparticle formulation to deliver paclitaxel and a TLR-4 agonist (P/SP-LPS, Figure 7).107–109 This nano formulation exhibited significantly better therapeutic efficacy compared to either of the components alone. It also converted the immunosupressive tumor microenvironment into an immune stimulatory state compared to only minor stimulation with the TLR-4 agonist alone, highlighting the potential advantage of combined chemo-immunotherapy.107, 108 However, death of the activated immune cells by the cytotoxic activity of paclitaxel is a possible drawback of this strategy. To gain full advantage of this approach, an innovative targeting strategy is warranted which will deliver a cytotoxic agent specifically to the tumor cells, followed by the release of an immune modulator in the tumor microenvironment.
Figure 7.
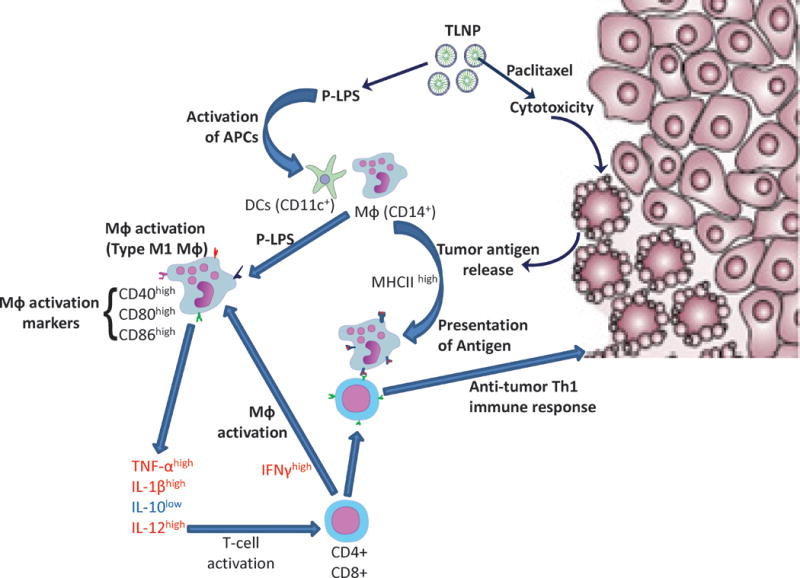
Combined chemo-immuno therapy using a nanoparticle coencapsulating a cytotoxic drug (paclitaxel) and a TLR4 agonist (P-LPS). The cytotoxic drug kill and debulk the tumor as well as produce tumor antigens due to apoptotic tumor cell death. The TLR4 agonist activate the macrophages to the M1 subtype which then present the tumor antigens as well as secrete cytokines to activate cytotoxic T cells, producing a potent anti-tumor Th1 immune response. Adapted from Roy et al., Int J Pharm. 2013.
3.4. Targeting cancer stem cells
Cancer stem cells are among the most researched area of cancer today, and due to many advanced studies, different cancer stem cell markers have been discovered. Formulation scientists have exploited these markers to develop nanoparticle delivery systems targeting these specific cells. Aldehyde dehydrogenases (ALDHs) are overexpressed in different types of cancers and have been identified as stem cell markers.110, 111 NPs loaded with low-dose decitabine, a DNA hypermethylation inhibitor, significantly reduced clonogenic growth and ALDH-positive stem-like population in malignant breast cancer.112 Chenna et al encapsulated a hedgehog inhibitor (HPI) into nanoparticles composed of PEGylated poly(lactic-co-glycolic acid).113 This HPI-incorporated polymeric NP system induced increased apoptotic effects in secondary mutational pancreatic cells by suppressing ALDH-positive CSCs in the orthotopic Pa03C xenograft model.113 Shen et al demonstrated that co-delivery of cisplatin and a siRNA targeting Notch1 using a micellar nanoparticle system led to enhanced anti-proliferation and apoptosis in the cancer stem cell population in an in vitro system. This nanoparticle system suppressed tumor growth while reducing the cancer stem cell population in a mouse tumor model.114 Treatment with antiangiogenic drug bevacizumab along with a camptothecin nanoparticle resulted in enhanced tumor regression, delayed tumor recurrence as well as increased depletion of the stem cell population, suggesting the importance of angiogenesis for the maintenance of the stem cell niche.115 Combination therapy with all-trans-retinoic acid and doxorubicin using a nanoparticle made with PEG-PLA co-polymer reduced the percentage of cancer stem cells in a synergistic manner.116 Hyaluronic acid is a natural ligand for CD44, which has been found to be over-expressed in cancer stem cells in many solid tumors.117–120. Wang et al. reported that treatment with a hyaluronan modified silica fused liposome loaded with 8-hydroxyquinoline produced enhanced cytotoxicity against tumor mammospheres which are characterized to be enriched with cancer stem cells. In vivo, co-treatment with the hyaluronan modified 8-hydroxyquinoline loaded liposome and a liposomal docetaxel (unmodified) produced better antitumor efficacy compared to both liposomal docetaxel and liposomal 8-hydroxyquinoline alone.121 Wang et al. reported that anti-CD44 antibody decorated liposomal NP loaded with a suicide gene or doxorubicin selectively targeted the CD44-positive hepatocellular carcinoma cells and effectively induce apoptotic cell death.122 Down-regulation of CD44 expression using a CD44 siRNA has also been shown to be beneficial for tumor reduction. Shah et al. synthesized a polypropylenimine (PPI) dendrimer containing paclitaxel and anti-CD44 siRNA decorated with a synthetic analog of luteinizing hormone-releasing hormone (LHRH) peptide as a tumor-targeting moiety. Treatment of metastatic tumors, which overexpress CD44 compared to the primary tumor, with this dendrimer led to the suppression of CD44 mRNA and protein, efficient induction of cell death, and effective tumor shrinkage.123 Salinomycin, an antibiotic produced by Streptomyces, has been shown to be highly effective against cancer stem cells.124 A bioconjugate of salinomycin with a polypeptide exhibited increased stem cell elimination in an orthotopic breast cancer model.125
4. Perspectives and Future directions
Cancer was regarded as a mono-cellular disorder, and accordingly, the majority of antitumor therapies were focused on killing or preventing the growth of these cells only. This approach was found to be beneficial in certain types of cancers, but in the majority of the cases, recurrence rate remained high. Previously, surgery was believed to be the only curative therapy for cancer, and surgeons perform “radical surgery”, removing blood supply, lymph nodes and a large part of the adjacent tissues in order to stem out cancerous cells. Unfortunately, this approach was found to be futile and most of the patients reported recurrence. It was proposed that the tumor cells can be “poisoned to death”, initiating the modern era of cancer chemotherapeutics. These cytotoxic drugs exhibit remarkable efficacy in shrinking the primary tumor, and in some exceptional cases, total cure. However, a fundamental treatment for all types of cancer is still elusive. Advanced research has revealed that cancers of different tissues or organs are completely separate disorders and warrant different treatment modalities. More recently it has also become apparent that cancer is a multi-cellular disease which involves a complex interplay between the tumor cells and the stromal cells. These findings refocus the cancer treatment strategy on the nature of the tumor microenvironment. Modulation of the tumor microenvironment has been demonstrated to be beneficial in a number of studies. The importance of multi-dimensional therapy has also attracted significant attention. As cancer is a multi-factorial disorder, a treatment strategy which targets more than one causative factor has obvious advantages over a single targeted approach. In this regard, it would be highly beneficial to stratify the patients after a tumor microenvironment analysis to quantify different stromal components in an individual tumor and recommend the most appropriate treatment modality accordingly. This “personalized therapy” approach can be beneficial over the conventional therapy as it is now well-known that each type of cancer comes with their own individual flavours and the “one size fits all” strategy has not been effective. Analyzing the tumor for different biomarkers can also be an effective method for selecting an appropriate therapy. In our work with Cellax, SPARC expression level in tumors correlated positively with the efficacy of Cellax. Up to now, very few therapeutics are available for targeting non-neoplastic cells in the tumor microenvironment. As tumor survival is highly dependent on the fine balance between the tumor cells and the stromal cells, drugs or delivery systems that can target multiple components of stroma may be desirable. There are many specific markers identified on different tumor cells, and various targeting ligands have been developed accordingly. Nevertheless, specific ligands for tumor stroma targeting have been most successful with the RGD ligands for angiogenic vascular endothelial cells. More efforts should be emphasized on identifying specific makers in other types of stroma for developing specific drugs or delivery methods.
CSCs are the main propagator for the tumor growth, and most commonly used chemotherapeutics have little effect on the CSCs. Including a CSC-depleting agent in a tumor-targeted delivery system that can localize the drug to the tumor microenvironment is an attractive strategy. Zhou et al. have prepared an HPMA copolymer-cyclopamine conjugate nanoparticle to block the hedgehog (Hh) signaling pathway; an important pathway involved in stem cell self-renewal.126 In vitro evaluation of these nanoparticles against a model CSC cell line demonstrated a selective inhibitory effect on the CSCs relative to the bulk cancer cells whereas docetaxel showed cytotoxicity to the bulk cancer cells only. Specific pathways and tumor microenvironmental niches in driving self-renewal of CSCs should be identified for developing effective drugs against CSCs. More understanding on the CSC niche environment and the anatomical location of CSCs within tumors will help design a drug targeting strategy for CSC depletion.
It is still not clear whether nanoparticles are capable of localizing in small metastases of tumors via the EPR effect, and this question requires sophisticated clinical studies to conclude. Developing alternative drug delivery approaches for targeting small metastases should be of priority to this field.
Increased understanding of the tumor microenvironment has identified multiple therapeutic targets for inhibiting tumor progression and metastasis. We anticipate that new drugs inhibiting these key pathways will be developed to modulate tumor microenvironment, augmenting the existing anticancer therapies. One successful example is anti-PD-L1 monoclonal antibody MDX-1105 (PD-L1 or CD274 is a trans-membrane protein overexpressed by many tumor cells and plays a major role in immune suppression. PD-L1 binds with activated T cells and induces apoptosis, thereby inhibiting T-cell-mediated immune response. MDX-1105 binds to PD-L1 and inhibits its immune suppressing activity). Apart from inhibition of immune suppression, drugs that can specifically modulate the activation of fibroblasts and the polarization of macrophages and immune cells are to be developed and are expected to provide alternative therapeutics against tumors. Selectivity of these drugs is paramount, as these stromal cells share many common characteristics with the normal host cells, and any non-specific action may induce significant side effects. Targeting CSCs will be a major challenge for tumor microenvironment modulation, as these cells have yet to be fully characterized. Increased characterization of these stromal cells will also provide critical information for designing innovative strategies for drug delivery to different stromal components. It is also expected that robust screening of new drugs and drug delivery systems will be required for drug development, and a validated in vitro system capable of performing high throughput screening will greatly enhance the progress. For example, tumor spheroids share many important characteristics with in-vivo tumors and various stromal components can be included and co-cultured with the tumor cells. With the advance of tumor biology and breakthroughs in drug development and drug delivery technologies, the days of multi-modal therapies targeting the tumor microenvironment for cancer eradication is on the horizon.
Acknowledgments
The Li lab was funded by grants from the Ontario Institute for Cancer Research Intellectual Property Development and Commercialization Fund, the Canadian Institutes of Health Research (PPP-122898), and the National Institutes of Health (CA17633901). S.D. Li is a recipient of a Coalition to Cure Prostate Cancer Young Investigator Award from the Prostate Cancer Foundation, and a New Investigator Award from Canadian Institutes of Health Research (MSH-130195). Jonathan P. May is acknowledged for editing this manuscript.
Footnotes
The authors would like to dedicate this manuscript to Dr. Mark Ernsting, who was the major contributor to the pilot work demonstrating tumor microenvironment modulation by Cellax nanoparticles. Dr. Ernsting passed away in December 2015.
References
- 1.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 2.Jain RK. Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol. 2013;31:2205–2218. doi: 10.1200/JCO.2012.46.3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMillin DW, Delmore J, Weisberg E, Negri JM, Geer DC, Klippel S, Mitsiades N, Schlossman RL, Munshi NC, Kung AL, et al. Tumor cell-specific bioluminescence platform to identify stroma-induced changes to anticancer drug activity. Nat Med. 2010;16:483–489. doi: 10.1038/nm.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teicher BA, Herman TS, Holden SA, Wang YY, Pfeffer MR, Crawford JW, Frei E., 3rd Tumor resistance to alkylating agents conferred by mechanisms operative only in vivo. Science. 1990;247:1457–1461. doi: 10.1126/science.247.4949.1457. [DOI] [PubMed] [Google Scholar]
- 6.Fidler IJ, Wilmanns C, Staroselsky A, Radinsky R, Dong Z, Fan D. Modulation of tumor cell response to chemotherapy by the organ environment. Cancer Metastasis Rev. 1994;13:209–222. doi: 10.1007/BF00689637. [DOI] [PubMed] [Google Scholar]
- 7.Miao L, Wang Y, Lin CM, Xiong Y, Chen N, Zhang L, Kim WY, Huang L. Nanoparticle modulation of the tumor microenvironment enhances therapeutic efficacy of cisplatin. J Control Release. 2015;217:27–41. doi: 10.1016/j.jconrel.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 10.Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012;31:195–208. doi: 10.1007/s10555-011-9340-x. [DOI] [PubMed] [Google Scholar]
- 11.Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol. 2010;21:33–39. doi: 10.1016/j.semcdb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fullar A, Dudas J, Olah L, Hollosi P, Papp Z, Sobel G, Karaszi K, Paku S, Baghy K, Kovalszky I. Remodeling of extracellular matrix by normal and tumor-associated fibroblasts promotes cervical cancer progression. BMC Cancer. 2015;15:256. doi: 10.1186/s12885-015-1272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 14.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, et al. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107:20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Infante JR, Matsubayashi H, Sato N, Tonascia J, Klein AP, Riall TA, Yeo C, Iacobuzio-Donahue C, Goggins M. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 16.Motamed K, Sage EH. SPARC inhibits endothelial cell adhesion but not proliferation through a tyrosine phosphorylation-dependent pathway. J Cell Biochem. 1998;70:543–552. doi: 10.1002/(sici)1097-4644(19980915)70:4<543::aid-jcb10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Framson PE, Sage EH. SPARC and tumor growth: where the seed meets the soil? J Cell Biochem. 2004;92:679–690. doi: 10.1002/jcb.20091. [DOI] [PubMed] [Google Scholar]
- 18.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 19.Semenza GL. Cancer-stromal cell interactions mediated by hypoxia-inducible factors promote angiogenesis, lymphangiogenesis, and metastasis. Oncogene. 2013;32:4057–4063. doi: 10.1038/onc.2012.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 21.Burri BJ, Edgington TS, Fair DS. Molecular interactions of the intrinsic activation complex of coagulation: binding of native and activated human factors IX and X to defined phospholipid vesicles. Biochim Biophys Acta. 1987;923:176–186. doi: 10.1016/0304-4165(87)90002-x. [DOI] [PubMed] [Google Scholar]
- 22.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013;35:585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 23.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor associated macrophages and neutrophils in tumor progression. J Cell Physiol. 2013;228:1404–1412. doi: 10.1002/jcp.24260. [DOI] [PubMed] [Google Scholar]
- 25.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–126. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–296. doi: 10.1016/j.ccr.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem. 2007;101:805–815. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- 29.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int J Biochem Cell Biol. 2012;44:2144–2151. doi: 10.1016/j.biocel.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ronnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: importance of the stromal reaction. Physiol Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 32.Wozniak MA, Desai R, Solski PA, Der CJ, Keely PJ. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J Cell Biol. 2003;163:583–595. doi: 10.1083/jcb.200305010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–272. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alili L, Chapiro S, Marten GU, Schmidt AM, Zanger K, Brenneisen P. Effect of Fe3O4 Nanoparticles on Skin Tumor Cells and Dermal Fibroblasts. Biomed Res Int. 2015;2015:530957. doi: 10.1155/2015/530957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alili L, Sack M, Karakoti AS, Teuber S, Puschmann K, Hirst SM, Reilly CM, Zanger K, Stahl W, Das S, et al. Combined cytotoxic and anti-invasive properties of redox-active nanoparticles in tumor-stroma interactions. Biomaterials. 2011;32:2918–2929. doi: 10.1016/j.biomaterials.2010.12.056. [DOI] [PubMed] [Google Scholar]
- 39.Ji T, Zhao Y, Ding Y, Wang J, Zhao R, Lang J, Qin H, Liu X, Shi J, Tao N, et al. Transformable Peptide Nanocarriers for Expeditious Drug Release and Effective Cancer Therapy via Cancer-Associated Fibroblast Activation. Angew Chem Int Ed Engl. 2015 doi: 10.1002/anie.201506262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ernsting MJ, Tang WL, MacCallum N, Li SD. Synthetic modification of carboxymethylcellulose and use thereof to prepare a nanoparticle forming conjugate of docetaxel for enhanced cytotoxicity against cancer cells. Bioconjug Chem. 2011;22:2474–2486. doi: 10.1021/bc200284b. [DOI] [PubMed] [Google Scholar]
- 41.Murakami M, Ernsting MJ, Undzys E, Holwell N, Foltz WD, Li SD. Docetaxel conjugate nanoparticles that target alpha-smooth muscle actin-expressing stromal cells suppress breast cancer metastasis. Cancer Res. 2013;73:4862–4871. doi: 10.1158/0008-5472.CAN-13-0062. [DOI] [PubMed] [Google Scholar]
- 42.Ernsting MJ, Hoang B, Lohse I, Undzys E, Cao P, Do T, Gill B, Pintilie M, Hedley D, Li SD. Targeting of metastasis-promoting tumor-associated fibroblasts and modulation of pancreatic tumor-associated stroma with a carboxymethylcellulose-docetaxel nanoparticle. J Control Release. 2015;206:122–130. doi: 10.1016/j.jconrel.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoang B, Ernsting MJ, Roy A, Murakami M, Undzys E, Li SD. Docetaxel-carboxymethylcellulose nanoparticles target cells via a SPARC and albumin dependent mechanism. Biomaterials. 2015;59:66–76. doi: 10.1016/j.biomaterials.2015.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle. 2006;5:1779–1787. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- 45.Li CY, Shan S, Huang Q, Braun RD, Lanzen J, Hu K, Lin P, Dewhirst MW. Initial stages of tumor cell-induced angiogenesis: evaluation via skin window chambers in rodent models. J Natl Cancer Inst. 2000;92:143–147. doi: 10.1093/jnci/92.2.143. [DOI] [PubMed] [Google Scholar]
- 46.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 47.Weidner N, Folkman J, Pozza F, Bevilacqua P, Allred EN, Moore DH, Meli S, Gasparini G. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 48.Jackson AL, Eisenhauer EL, Herzog TJ. Emerging therapies: angiogenesis inhibitors for ovarian cancer. Expert Opin Emerg Drugs. 2015;20:331–346. doi: 10.1517/14728214.2015.1036739. [DOI] [PubMed] [Google Scholar]
- 49.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56:1649–1659. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 51.Voigt J, Christensen J, Shastri VP. Differential uptake of nanoparticles by endothelial cells through polyelectrolytes with affinity for caveolae. Proc Natl Acad Sci U S A. 2014;111:2942–2947. doi: 10.1073/pnas.1322356111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banerjee D, Harfouche R, Sengupta S. Nanotechnology-mediated targeting of tumor angiogenesis. Vasc Cell. 2011;3:3. doi: 10.1186/2045-824X-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Anderson SA, Rader RK, Westlin WF, Null C, Jackson D, Lanza GM, Wickline SA, Kotyk JJ. Magnetic resonance contrast enhancement of neovasculature with alpha(v)beta(3)-targeted nanoparticles. Magn Reson Med. 2000;44:433–439. doi: 10.1002/1522-2594(200009)44:3<433::aid-mrm14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 54.Park JH, Kwon S, Nam JO, Park RW, Chung H, Seo SB, Kim IS, Kwon IC, Jeong SY. Self-assembled nanoparticles based on glycol chitosan bearing 5beta-cholanic acid for RGD peptide delivery. J Control Release. 2004;95:579–588. doi: 10.1016/j.jconrel.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 55.Waters EA, Chen J, Yang X, Zhang H, Neumann R, Santeford A, Arbeit J, Lanza GM, Wickline SA. Detection of targeted perfluorocarbon nanoparticle binding using 19F diffusion weighted MR spectroscopy. Magn Reson Med. 2008;60:1232–1236. doi: 10.1002/mrm.21794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Foubert P, Varner JA. Integrins in tumor angiogenesis and lymphangiogenesis. Methods Mol Biol. 2012;757:471–486. doi: 10.1007/978-1-61779-166-6_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hood JD, Bednarski M, Frausto R, Guccione S, Reisfeld RA, Xiang R, Cheresh DA. Tumor regression by targeted gene delivery to the neovasculature. Science. 2002;296:2404–2407. doi: 10.1126/science.1070200. [DOI] [PubMed] [Google Scholar]
- 58.Mastroberardino G, Costa C, Gavelli MS, Vitaliano E, Rossi F, Catalano A, Barletta R, Guarini G. Plasma cortisol and testosterone in hypercholesterolaemia treated with clofibrate and lovastatin. J Int Med Res. 1989;17:388–394. doi: 10.1177/030006058901700413. [DOI] [PubMed] [Google Scholar]
- 59.Storrs RW, Tropper FD, Li HY, Song CK, Kuniyoshi JK, Sipkins DA, Li KCP, Bednarski MD. Paramagnetic Polymerized Liposomes: Synthesis, Characterization, and Applications for Magnetic Resonance Imaging. Journal of the American Chemical Society. 1995;117:7301–7306. [Google Scholar]
- 60.Sipkins DA, Cheresh DA, Kazemi MR, Nevin LM, Bednarski MD, Li KC. Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat Med. 1998;4:623–626. doi: 10.1038/nm0598-623. [DOI] [PubMed] [Google Scholar]
- 61.Danhier F, Le Breton A, Preat V. RGD-based strategies to target alpha(v) beta(3) integrin in cancer therapy and diagnosis. Mol Pharm. 2012;9:2961–2973. doi: 10.1021/mp3002733. [DOI] [PubMed] [Google Scholar]
- 62.Murphy EA, Majeti BK, Barnes LA, Makale M, Weis SM, Lutu-Fuga K, Wrasidlo W, Cheresh DA. Nanoparticle-mediated drug delivery to tumor vasculature suppresses metastasis. Proc Natl Acad Sci U S A. 2008;105:9343–9348. doi: 10.1073/pnas.0803728105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhan C, Gu B, Xie C, Li J, Liu Y, Lu W. Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J Control Release. 2010;143:136–142. doi: 10.1016/j.jconrel.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 64.Borgman MP, Aras O, Geyser-Stoops S, Sausville EA, Ghandehari H. Biodistribution of HPMA copolymer-aminohexylgeldanamycin-RGDfK conjugates for prostate cancer drug delivery. Mol Pharm. 2009;6:1836–1847. doi: 10.1021/mp900134c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Danhier F, Vroman B, Lecouturier N, Crokart N, Pourcelle V, Freichels H, Jerome C, Marchand-Brynaert J, Feron O, Preat V. Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with paclitaxel. J Control Release. 2009;140:166–173. doi: 10.1016/j.jconrel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 66.Maeshima Y, Colorado PC, Kalluri R. Two RGD-independent alpha vbeta 3 integrin binding sites on tumstatin regulate distinct anti-tumor properties. J Biol Chem. 2000;275:23745–23750. doi: 10.1074/jbc.C000186200. [DOI] [PubMed] [Google Scholar]
- 67.Ho DN, Kohler N, Sigdel A, Kalluri R, Morgan JR, Xu C, Sun S. Penetration of endothelial cell coated multicellular tumor spheroids by iron oxide nanoparticles. Theranostics. 2012;2:66–75. doi: 10.7150/thno.3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Christian S, Pilch J, Akerman ME, Porkka K, Laakkonen P, Ruoslahti E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J Cell Biol. 2003;163:871–878. doi: 10.1083/jcb.200304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu Q, Gu G, Liu Z, Jiang M, Kang T, Miao D, Tu Y, Pang Z, Song Q, Yao L, et al. F3 peptide-functionalized PEG-PLA nanoparticles co-administrated with tLyp-1 peptide for anti-glioma drug delivery. Biomaterials. 2013;34:1135–1145. doi: 10.1016/j.biomaterials.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 70.Prickett WM, Van Rite BD, Resasco DE, Harrison RG. Vascular targeted single-walled carbon nanotubes for near-infrared light therapy of cancer. Nanotechnology. 2011;22:455101. doi: 10.1088/0957-4484/22/45/455101. [DOI] [PubMed] [Google Scholar]
- 71.Guo J, Gao X, Su L, Xia H, Gu G, Pang Z, Jiang X, Yao L, Chen J, Chen H. Aptamer-functionalized PEG-PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32:8010–8020. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 72.Winer I, Wang S, Lee YE, Fan W, Gong Y, Burgos-Ojeda D, Spahlinger G, Kopelman R, Buckanovich RJ. F3-targeted cisplatin-hydrogel nanoparticles as an effective therapeutic that targets both murine and human ovarian tumor endothelial cells in vivo. Cancer Res. 2010;70:8674–8683. doi: 10.1158/0008-5472.CAN-10-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu DH, Lu Q, Xie J, Fang C, Chen HZ. Peptide-conjugated biodegradable nanoparticles as a carrier to target paclitaxel to tumor neovasculature. Biomaterials. 2010;31:2278–2292. doi: 10.1016/j.biomaterials.2009.11.047. [DOI] [PubMed] [Google Scholar]
- 74.Deshayes S, Maurizot V, Clochard MC, Baudin C, Berthelot T, Esnouf S, Lairez D, Moenner M, Deleris G. “Click” conjugation of peptide on the surface of polymeric nanoparticles for targeting tumor angiogenesis. Pharm Res. 2011;28:1631–1642. doi: 10.1007/s11095-011-0398-5. [DOI] [PubMed] [Google Scholar]
- 75.Backer MV, Gaynutdinov TI, Patel V, Bandyopadhyaya AK, Thirumamagal BT, Tjarks W, Barth RF, Claffey K, Backer JM. Vascular endothelial growth factor selectively targets boronated dendrimers to tumor vasculature. Mol Cancer Ther. 2005;4:1423–1429. doi: 10.1158/1535-7163.MCT-05-0161. [DOI] [PubMed] [Google Scholar]
- 76.Chen J, Wu H, Han D, Xie C. Using anti-VEGF McAb and magnetic nanoparticles as double-targeting vector for the radioimmunotherapy of liver cancer. Cancer Lett. 2006;231:169–175. doi: 10.1016/j.canlet.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 77.Murase Y, Asai T, Katanasaka Y, Sugiyama T, Shimizu K, Maeda N, Oku N. A novel DDS strategy, “dual-targeting”, and its application for antineovascular therapy. Cancer Lett. 2010;287:165–171. doi: 10.1016/j.canlet.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 78.Pastorino F, Brignole C, Marimpietri D, Cilli M, Gambini C, Ribatti D, Longhi R, Allen TM, Corti A, Ponzoni M. Vascular damage and anti-angiogenic effects of tumor vessel-targeted liposomal chemotherapy. Cancer Res. 2003;63:7400–7409. [PubMed] [Google Scholar]
- 79.Hong H, Zhang Y, Engle JW, Nayak TR, Theuer CP, Nickles RJ, Barnhart TE, Cai W. In vivo targeting and positron emission tomography imaging of tumor vasculature with (66)Ga-labeled nano-graphene. Biomaterials. 2012;33:4147–4156. doi: 10.1016/j.biomaterials.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hong H, Yang K, Zhang Y, Engle JW, Feng L, Yang Y, Nayak TR, Goel S, Bean J, Theuer CP, et al. In vivo targeting and imaging of tumor vasculature with radiolabeled, antibody-conjugated nanographene. ACS Nano. 2012;6:2361–2370. doi: 10.1021/nn204625e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang D, Feng XY, Henning TD, Wen L, Lu WY, Pan H, Wu X, Zou LG. MR imaging of tumor angiogenesis using sterically stabilized Gd-DTPA liposomes targeted to CD105. Eur J Radiol. 2009;70:180–189. doi: 10.1016/j.ejrad.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 82.Ruggiero A, Villa CH, Holland JP, Sprinkle SR, May C, Lewis JS, Scheinberg DA, McDevitt MR. Imaging and treating tumor vasculature with targeted radiolabeled carbon nanotubes. Int J Nanomedicine. 2010;5:783–802. doi: 10.2147/IJN.S13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kapadia CH, Perry JL, Tian S, Luft JC, DeSimone JM. Nanoparticulate immunotherapy for cancer. J Control Release. 2015;219:167–180. doi: 10.1016/j.jconrel.2015.09.062. [DOI] [PubMed] [Google Scholar]
- 84.Fan Y, Moon JJ. Nanoparticle Drug Delivery Systems Designed to Improve Cancer Vaccines and Immunotherapy. Vaccines (Basel) 2015;3:662–685. doi: 10.3390/vaccines3030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Craparo EF, Bondi ML. Application of polymeric nanoparticles in immunotherapy. Curr Opin Allergy Clin Immunol. 2012;12:658–664. doi: 10.1097/ACI.0b013e3283588c57. [DOI] [PubMed] [Google Scholar]
- 86.Baay M, Brouwer A, Pauwels P, Peeters M, Lardon F. Tumor cells and tumor-associated macrophages: secreted proteins as potential targets for therapy. Clin Dev Immunol. 2011;2011:565187. doi: 10.1155/2011/565187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, Vecchi A, Mantovani A, Mordoh J, Wainstok R. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Invest Dermatol. 2007;127:2031–2041. doi: 10.1038/sj.jid.5700827. [DOI] [PubMed] [Google Scholar]
- 88.Marra M, Salzano G, Leonetti C, Porru M, Franco R, Zappavigna S, Liguori G, Botti G, Chieffi P, Lamberti M, et al. New self-assembly nanoparticles and stealth liposomes for the delivery of zoledronic acid: a comparative study. Biotechnol Adv. 2012;30:302–309. doi: 10.1016/j.biotechadv.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 89.Salzano G, Marra M, Porru M, Zappavigna S, Abbruzzese A, La Rotonda MI, Leonetti C, Caraglia M, De Rosa G. Self-assembly nanoparticles for the delivery of bisphosphonates into tumors. Int J Pharm. 2011;403:292–297. doi: 10.1016/j.ijpharm.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 90.Zhu S, Niu M, O’Mary H, Cui Z. Targeting of tumor-associated macrophages made possible by PEG-sheddable, mannose-modified nanoparticles. Mol Pharm. 2013;10:3525–3530. doi: 10.1021/mp400216r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Steinhagen F, Kinjo T, Bode C, Klinman DM. TLR-based immune adjuvants. Vaccine. 2011;29:3341–3355. doi: 10.1016/j.vaccine.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaczanowska S, Joseph AM, Davila E. TLR agonists: our best frenemy in cancer immunotherapy. J Leukoc Biol. 2013;93:847–863. doi: 10.1189/jlb.1012501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mandraju R, Murray S, Forman J, Pasare C. Differential ability of surface and endosomal TLRs to induce CD8 T cell responses in vivo. J Immunol. 2014;192:4303–4315. doi: 10.4049/jimmunol.1302244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oh JZ, Kurche JS, Burchill MA, Kedl RM. TLR7 enables cross-presentation by multiple dendritic cell subsets through a type I IFN-dependent pathway. Blood. 2011;118:3028–3038. doi: 10.1182/blood-2011-04-348839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Titta A, Ballester M, Julier Z, Nembrini C, Jeanbart L, van der Vlies AJ, Swartz MA, Hubbell JA. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc Natl Acad Sci U S A. 2013;110:19902–19907. doi: 10.1073/pnas.1313152110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilson JT, Keller S, Manganiello MJ, Cheng C, Lee CC, Opara C, Convertine A, Stayton PS. pH-Responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano. 2013;7:3912–3925. doi: 10.1021/nn305466z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang P, Chiu YC, Tostanoski LH, Jewell CM. Polyelectrolyte Multilayers Assembled Entirely from Immune Signals on Gold Nanoparticle Templates Promote Antigen-Specific T Cell Response. ACS Nano. 2015;9:6465–6477. doi: 10.1021/acsnano.5b02153. [DOI] [PubMed] [Google Scholar]
- 98.Molino NM, Anderson AK, Nelson EL, Wang SW. Biomimetic protein nanoparticles facilitate enhanced dendritic cell activation and cross-presentation. ACS Nano. 2013;7:9743–9752. doi: 10.1021/nn403085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Silva JM, Zupancic E, Vandermeulen G, Oliveira VG, Salgado A, Videira M, Gaspar M, Graca L, Preat V, Florindo HF. In vivo delivery of peptides and Toll-like receptor ligands by mannose-functionalized polymeric nanoparticles induces prophylactic and therapeutic anti-tumor immune responses in a melanoma model. J Control Release. 2015;198:91–103. doi: 10.1016/j.jconrel.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 101.Fox CB, Sivananthan SJ, Duthie MS, Vergara J, Guderian JA, Moon E, Coblentz D, Reed SG, Carter D. A nanoliposome delivery system to synergistically trigger TLR4 AND TLR7. J Nanobiotechnology. 2014;12:17. doi: 10.1186/1477-3155-12-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pradhan P, Qin H, Leleux JA, Gwak D, Sakamaki I, Kwak LW, Roy K. The effect of combined IL10 siRNA and CpG ODN as pathogen-mimicking microparticles on Th1/Th2 cytokine balance in dendritic cells and protective immunity against B cell lymphoma. Biomaterials. 2014;35:5491–5504. doi: 10.1016/j.biomaterials.2014.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu Z, Wang Y, Zhang L, Huang L. Nanoparticle-delivered transforming growth factor-beta siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano. 2014;8:3636–3645. doi: 10.1021/nn500216y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tamayo I, Irache JM, Mansilla C, Ochoa-Reparaz J, Lasarte JJ, Gamazo C. Poly(anhydride) nanoparticles act as active Th1 adjuvants through Toll-like receptor exploitation. Clin Vaccine Immunol. 2010;17:1356–1362. doi: 10.1128/CVI.00164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Anyaegbu CC, Lake RA, Heel K, Robinson BW, Fisher SA. Chemotherapy enhances cross-presentation of nuclear tumor antigens. PLoS One. 2014;9:e107894. doi: 10.1371/journal.pone.0107894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wan S, Pestka S, Jubin RG, Lyu YL, Tsai YC, Liu LF. Chemotherapeutics and radiation stimulate MHC class I expression through elevated interferon-beta signaling in breast cancer cells. PLoS One. 2012;7:e32542. doi: 10.1371/journal.pone.0032542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Roy A, Singh MS, Upadhyay P, Bhaskar S. Nanoparticle mediated co-delivery of paclitaxel and a TLR-4 agonist results in tumor regression and enhanced immune response in the tumor microenvironment of a mouse model. Int J Pharm. 2013;445:171–180. doi: 10.1016/j.ijpharm.2013.01.045. [DOI] [PubMed] [Google Scholar]
- 108.Roy A, Chandra S, Mamilapally S, Upadhyay P, Bhaskar S. Anticancer and immunostimulatory activity by conjugate of paclitaxel and non-toxic derivative of LPS for combined chemo-immunotherapy. Pharm Res. 2012;29:2294–2309. doi: 10.1007/s11095-012-0756-y. [DOI] [PubMed] [Google Scholar]
- 109.Roy A, Singh MS, Upadhyay P, Bhaskar S. Combined chemo-immunotherapy as a prospective strategy to combat cancer: a nanoparticle based approach. Mol Pharm. 2010;7:1778–1788. doi: 10.1021/mp100153r. [DOI] [PubMed] [Google Scholar]
- 110.Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ, Guan XY. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6:1146–1153. doi: 10.1158/1541-7786.MCR-08-0035. [DOI] [PubMed] [Google Scholar]
- 111.Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li SY, Sun R, Wang HX, Shen S, Liu Y, Du XJ, Zhu YH, Jun W. Combination therapy with epigenetic-targeted and chemotherapeutic drugs delivered by nanoparticles to enhance the chemotherapy response and overcome resistance by breast cancer stem cells. J Control Release. 2015;205:7–14. doi: 10.1016/j.jconrel.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 113.Chenna V, Hu C, Pramanik D, Aftab BT, Karikari C, Campbell NR, Hong SM, Zhao M, Rudek MA, Khan SR, et al. A polymeric nanoparticle encapsulated small-molecule inhibitor of Hedgehog signaling (NanoHHI) bypasses secondary mutational resistance to Smoothened antagonists. Mol Cancer Ther. 2012;11:165–173. doi: 10.1158/1535-7163.MCT-11-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shen S, Sun CY, Du XJ, Li HJ, Liu Y, Xia JX, Zhu YH, Wang J. Co-delivery of platinum drug and siNotch1 with micelleplex for enhanced hepatocellular carcinoma therapy. Biomaterials. 2015;70:71–83. doi: 10.1016/j.biomaterials.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 115.Conley SJ, Baker TL, Burnett JP, Theisen RL, Lazarus D, Peters CG, Clouthier SG, Eliasof S, Wicha MS. CRLX101, an investigational camptothecin-containing nanoparticle-drug conjugate, targets cancer stem cells and impedes resistance to antiangiogenic therapy in mouse models of breast cancer. Breast Cancer Res Treat. 2015;150:559–567. doi: 10.1007/s10549-015-3349-8. [DOI] [PubMed] [Google Scholar]
- 116.Sun R, Liu Y, Li SY, Shen S, Du XJ, Xu CF, Cao ZT, Bao Y, Zhu YH, Li YP, et al. Co-delivery of all-trans-retinoic acid and doxorubicin for cancer therapy with synergistic inhibition of cancer stem cells. Biomaterials. 2015;37:405–414. doi: 10.1016/j.biomaterials.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 117.Feng D, Peng C, Li C, Zhou Y, Li M, Ling B, Wei H, Tian Z. Identification and characterization of cancer stem-like cells from primary carcinoma of the cervix uteri. Oncol Rep. 2009;22:1129–1134. doi: 10.3892/or_00000545. [DOI] [PubMed] [Google Scholar]
- 118.Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Haraguchi N, Ohkuma M, Sakashita H, Matsuzaki S, Tanaka F, Mimori K, Kamohara Y, Inoue H, Mori M. CD133+CD44+ population efficiently enriches colon cancer initiating cells. Ann Surg Oncol. 2008;15:2927–2933. doi: 10.1245/s10434-008-0074-0. [DOI] [PubMed] [Google Scholar]
- 120.Yang YM, Chang JW. Bladder cancer initiating cells (BCICs) are among EMA-CD44v6+ subset: novel methods for isolating undetermined cancer stem (initiating) cells. Cancer Invest. 2008;26:725–733. doi: 10.1080/07357900801941845. [DOI] [PubMed] [Google Scholar]
- 121.Wang D, Huang J, Wang X, Yu Y, Zhang H, Chen Y, Liu J, Sun Z, Zou H, Sun D, et al. The eradication of breast cancer cells and stem cells by 8-hydroxyquinoline-loaded hyaluronan modified mesoporous silica nanoparticle-supported lipid bilayers containing docetaxel. Biomaterials. 2013;34:7662–7673. doi: 10.1016/j.biomaterials.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 122.Wang L, Su W, Liu Z, Zhou M, Chen S, Chen Y, Lu D, Liu Y, Fan Y, Zheng Y, et al. CD44 antibody-targeted liposomal nanoparticles for molecular imaging and therapy of hepatocellular carcinoma. Biomaterials. 2012;33:5107–5114. doi: 10.1016/j.biomaterials.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 123.Shah V, Taratula O, Garbuzenko OB, Taratula OR, Rodriguez-Rodriguez L, Minko T. Targeted nanomedicine for suppression of CD44 and simultaneous cell death induction in ovarian cancer: an optimal delivery of siRNA and anticancer drug. Clin Cancer Res. 2013;19:6193–6204. doi: 10.1158/1078-0432.CCR-13-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Naujokat C, Steinhart R. Salinomycin as a drug for targeting human cancer stem cells. J Biomed Biotechnol. 2012;2012:950658. doi: 10.1155/2012/950658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhao P, Dong S, Bhattacharyya J, Chen M. iTEP nanoparticle-delivered salinomycin displays an enhanced toxicity to cancer stem cells in orthotopic breast tumors. Mol Pharm. 2014;11:2703–2712. doi: 10.1021/mp5002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou Y, Yang J, Kopecek J. Selective inhibitory effect of HPMA copolymer-cyclopamine conjugate on prostate cancer stem cells. Biomaterials. 2012;33:1863–1872. doi: 10.1016/j.biomaterials.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


