Abstract
Antibiotic resistance is a worldwide problem that needs to be addressed. Methicillin-resistant Staphylococcus aureus (MRSA) is one of the dangerous “ESKAPE” pathogens that rapidly evolve and evade many current FDA-approved antibiotics. Thus, there is an urgent need for new anti-MRSA compounds. Ebselen (also known as 2-phenyl-1,2-benzisoselenazol-3(2H)-one) has shown promising activity in clinical trials for cerebral ischemia, bipolar disorder, and noise-induced hearing loss. Recently, there has been a renewed interest in exploring the antibacterial properties of ebselen. In this study, we synthesized an ebselen-inspired library of 33 compounds where the selenium atom has been replaced by sulfur (ebsulfur derivatives) and evaluated them against a panel of drug sensitive and drug resistant S. aureus and non-S. aureus strains. Within our library, we identified three outstanding analogues with potent activity against all S. aureus strains tested (MIC values mostly ≤2 μg/mL), and numerous additional ones with overall very good to good antibacterial activity (1–7.8 μg/mL). We also characterized the time-kill analysis, anti-biofilm ability, hemolytic activity, mammalian cytotoxicity, membrane-disruption ability, and reactive oxygen species (ROS) production of some of these analogues.
Keywords: Antibiotic, Benzisothiazolinone, Biofilm, ESKAPE, Reactive oxygen species (ROS), Resistance
Graphical Abstract
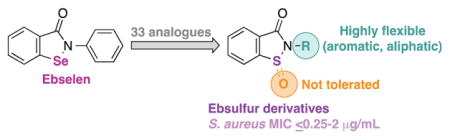
1. Introduction
Only two years after its introduction on the market in 1959, methicillin had experienced resistance by Staphylococcus aureus.1 Ever since, methicillin-resistant S. aureus (MRSA) strains have spread worldwide and have become resistant to many additional FDA approved antibiotics causing great harm to infected patients. In 2011, the Centers for Disease Control (CDC) estimated that the national incidence of invasive MRSA infections was 80,461 cases including 650 cases of death, which was the highest mortality rate among bacterial infections.2 S. aureus is a Gram-positive bacterium residing mostly on the skin and nasal lining of up to one-third of healthy individuals and can be transferred from one host to another by skin contact. Typically, the bacterium causes no symptoms. However, once the skin layer is broken due to scratches or cuts, S. aureus may lead to many problems varying from mild acne to life-threatening conditions such as bacteremia, pneumonia, endocarditis, and osteomyelitis.3 Furthermore, MRSA is one of the “ESKAPE” pathogens, which also include Enterococcus faecium, Klebsiella pneumoniae, Acinetobacter baumanii, Pseudomonas aeruginosa, and Enterobacter species. The “ESKAPE” pathogens are termed that way because of their ability to escape the therapeutic effects of many known antibiotics and are responsible for the majority of hospital infections. In fact, the Infectious Diseases Society of America (IDSA) has expressed concerns about the empty pipeline for novel antibacterials that can target these pathogens.4 Thus, there is a need for new drug candidates to combat MRSA and the rest of the “ESKAPE” pathogens.
In recent years, a number of novel scaffolds with promising activities and mechanism of actions against MRSA have been described. Among them, the compound 5-nitro-2-phenyl-(1H)-indole was discovered as a NorA, efflux pump, inhibitor at IC50 values lower than 5.0 μM.5 We also reported 6″-thioether tobramycin and kanamycin B analogues with long linear alkyl chains disrupting bacterial cell membranes and displaying good activity against S. aureus strains.6–8 Another compound, AFN-125, was found to selectively inhibit S. aureus enoyl-ACP reductase, and has even been tested in clinical trials.9 These recent advancements have certainly contributed towards our efforts of eradicating MRSA. However, the concerns about MRSA and “ESKAPE” pathogens have not been completely alleviated and novel scaffolds with potent antibacterial activities are still urgently needed.
Ebselen (also known as 2-phenyl-1,2-benzisoselenazol-3(2H)-one) was developed by Daiichi Pharmaceuticals in 1997 for cerebral ischemia in Japan, but failed during phase 3 clinical trial due to insufficient efficacy.10,11 Since then, there has been a renewed interest in this compound; in fact, ebselen is currently being evaluated in clinical trials for treatments of bipolar disorder12 and noise-induced hearing loss.13 Clinical applications of ebselen are hypothesized to be related to its ability to covalently bind to cysteine residues on targeted proteins or its antioxidant activity via mimicking glutathione peroxidase.14,15 In addition, ebselen was found to inhibit the growth of various Gram-positive and Gram-negative bacterial strains.16 In 2014, the crystal structure of antigen 85C, a putative drug target in Mycobacterium tuberculosis (Mtb), was solved and revealed that ebselen covalently inhibit the antigen 85 complex and hence, explained its activity against Mtb.17 From a high-throughput screen, ebselen was discovered to target the cysteine protease domain within the major virulence factors A and B of Clostridium difficile.18 Futhermore, ebselen and ebsulfur (2a) were found to inhibit bacterial thioredoxin reductase, suggesting that they may be useful agents against bacteria lacking the glutathione redox system.19 Finally, ebselen especially caught our attention because it demonstrated potent bactericidal activity against many clinical isolates of drug-resistant S. aureus and was effective in a murine model of MRSA skin infection.20 These findings prompted us to investigate the antibacterial activity of our own library of ebselen-inspired compounds.
Although no clear evidence has yet been established during clinical studies, there were initial concerns about selenium toxicity of ebselen as some systemic accumulation was observed.11 In lieu of this potential adverse effect, we decided to study ebsulfur, in which the selenium of ebselen is replaced by a sulfur atom. Additionally, in terms of synthesis, the ebsulfur scaffold or 2-phenyl-1,2-benzisothiazol-3(2H)-one is readily accessible via a convenient 2-step synthesis, which allows for simple scale-up and derivatization. Herein, we synthesized and evaluated ebsulfur (2a) and 32 of its analogues (2b–4n) against a panel of methicillin-sensitive S. aureus (MSSA), MRSA, and other bacterial strains. We also performed the time-kill analysis, established the anti-biofilm ability, hemolytic activity, membrane-disruption ability, and ROS production of some of these analogues.
2. Results and discussion
2.1. Chemistry
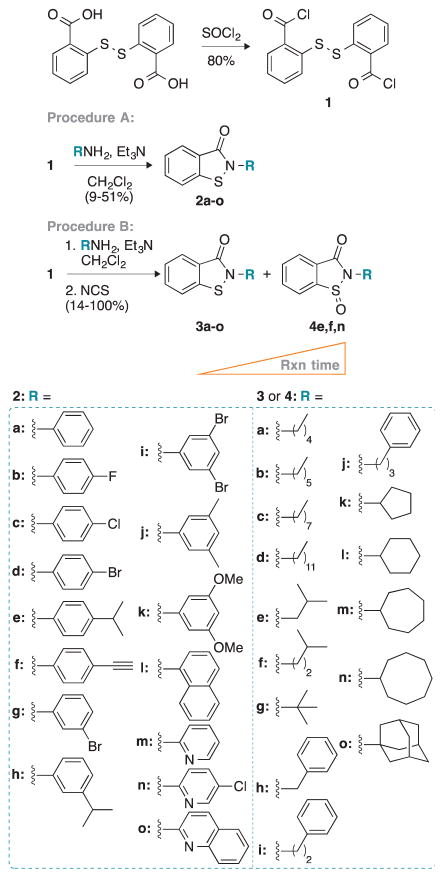
To synthesize the desired ebsulfur (2a) and its derivatives 2b–3o, we first prepared the common intermediate compound 1 in 80% yield by refluxing 2,2′-dithio-dibenzoic acid with thionyl chloride (Scheme 1). Compound 1 was then treated with a variety of aniline analogues and triethylamine in dichloromethane to afford compounds 2a–o in 9–51% yield, as shown in procedure A. We initially tried to use procedure A for the preparation of ebsulfur analogues with aliphatic amines, but were unsuccessful as the undesired 2,2′-dithio-dibenzamide products were typically the major products observed. Thus, the undesired 2,2′-dithio-dibenzamide products were converted to the desired products 3a–o in 14% to quantitative yield by subsequent addition of N-chlorosuccinimide to the reaction mixture, as shown in procedure B. During our synthesis, we also noticed that extra-long reaction time or poor-quality anhydrous dichloromethane led to the formation of the oxidized sulfoxide byproducts. On silica gel, the oxidized byproducts actually displayed retention times that were similar to the desired product and could be mistakenly isolated. Thus, we carefully monitored our reactions by TLC and verified their masses either by LRMS or HRMS. To test the effect of oxidation of the sulfur on the biological activity of our ebsulfur (2a) derivatives, we randomly selected three compounds (3e, f, and n) for which we let the reaction go longer to isolate the corresponding oxidized derivatives 4e, f, and n. All new molecules were characterized by 1H and 13C NMR as well as by mass spectrometry and were confirmed to be ≥95% purity.
Scheme 1.
Synthetic scheme for the preparation of compounds 2a–o, 3a–o, and 4e, 4f, and 4n following two different experimental procedures (A and B).
2.2. Evaluation of compounds 2a–4n as antibacterial agents
The antibacterial activity of 2a–4n was evaluated against a panel of S. aureus strains comprised of three MSSA strains (A–C) and 25 MRSA strains (D-AB) (Table 1).21 We also tested the activity of all these analogues against non-S. aureus strains such as S. epidermidis, E. faecalis, E. faecium, VRE, L. monocytogenes, and M. smegmatis (AC–AI) (Table 2). Furthermore, the controls amikacin (AMK), ebselen, and ebsulfur (2a), along with our best compound, 2h, were tested against a panel of additional bacterial strains, which included A. baumannii, E. cloacae, E. coli, K. pneumoniae, P. aeruginosa, S. enterica, and S. epidermidis (Table 3). The strains tested ranged from completely resistant to the control AMK (≥125 μg/mL, AD, AE, and AF) to very susceptible to AMK (≤0.50 μg/mL, B, T, and AI). Minimum inhibitory concentration (MIC) values were determined using the broth double dilution method.
Table 1.
MIC valuesa (in μg/mL) determined for all compounds and for the control antibacterial agent (AMK) against various S. aureus strains.
| Cpd # | A | B | C | D | E | F | G | H | I | J | K | L | M | N |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ebselen | 2 | 3.9 | 2 | 0.5 | ≤0.25 | 2 | 7.8 | 2 | 1 | 0.5 | 2 | 3.9 | 1 | 2 |
| 2a | 7.8 | 3.9 | 3.9 | 0.5 | 2 | 7.8 | 7.8 | 3.9 | 2 | 2 | 7.8 | 3.9 | 2 | 3.9 |
| 2b | 3.9 | 2 | 3.9 | 1 | 3.9 | 2 | 3.9 | 2 | 3.9 | 1 | 3.9 | 3.9 | 2 | 2 |
| 2c | 3.9 | 2 | 2 | 1 | 1 | 1 | 3.9 | 2 | 1 | 1 | 3.9 | 3.9 | 1 | 2 |
| 2d | 2 | 2 | 2 | 0.5 | 3.9 | 2 | 3.9 | 2 | 2 | 1 | 3.9 | 3.9 | 2 | 2 |
| 2e | 2 | 2 | ≤0.25 | ≤0.25 | ≤0.25 | 1 | 2 | ≤0.25 | 0.5 | 0.5 | 1 | 1 | 0.5 | ≤0.25 |
| 2f | 7.8 | 3.9 | 1 | 1 | 3.9 | 2 | 7.8 | 2 | 2 | 2 | 7.8 | 7.8 | 2 | 2 |
| 2g | 7.8 | 7.8 | 3.9 | 2 | 7.8 | 3.9 | 7.8 | 3.9 | 3.9 | 3.9 | 7.8 | 3.9 | 3.9 | 3.9 |
| 2h | 3.9 | 0.5 | ≤0.25 | ≤0.25 | ≤0.25 | ≤0.25 | 1 | ≤0.25 | ≤0.25 | ≤0.25 | 0.5 | ≤0.25 | ≤0.25 | ≤0.25 |
| 2j | 7.8 | 7.8 | 3.9 | 1 | 7.8 | 7.8 | 7.8 | 3.9 | 3.9 | 3.9 | 15.6 | 7.8 | 7.8 | 7.8 |
| 2k | 1 | 0.5 | ≤0.25 | ≤0.25 | ≤0.25 | 1 | 2 | 0.5 | ≤0.25 | 0.5 | 2 | 2 | ≤0.25 | 1 |
| 2l | >125 | 2 | 0.5 | 0.5 | 1 | 0.5 | 2 | 2 | 0.5 | 1 | 2 | 15.6 | 0.5 | 2 |
| 2m | 7.8 | 7.8 | 7.8 | 3.9 | 15.6 | 7.8 | 15.6 | 3.9 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 |
| 3a | 7.8 | 3.9 | 3.9 | 1 | 0.5 | 2 | 3.9 | 2 | 2 | 2 | 3.9 | 7.8 | 1 | 0.5 |
| 3b | 7.8 | 3.9 | 3.9 | 2 | 2 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 7.8 | 2 | 0.5 |
| 3c | 1 | 1 | 1 | 0.5 | 0.5 | 1 | 1 | 1 | 0.5 | 1 | 1 | 2 | 0.5 | ≤0.25 |
| 3d | 62.5 | 3.9 | 3.9 | 2 | 0.25 | 1 | 15.6 | 3.9 | 1 | 2 | 15.6 | 3.9 | 1 | 0.5 |
| 3e | 7.8 | 125 | 15.6 | >125 | 7.8 | 15.6 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 3.9 |
| 3f | 3.9 | >125 | 3.9 | >125 | 3.9 | 7.8 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 7.8 | 3.9 | 2 |
| 3g | 15.6 | 15.6 | 15.6 | 3.9 | 7.8 | 15.6 | 15.6 | 7.8 | 7.8 | 15.6 | 15.6 | 15.6 | 7.8 | 7.8 |
| 3h | 2 | 3.9 | 1 | 1 | 0.5 | 2 | 2 | 3.9 | 1 | 1 | 2 | 3.9 | 1 | 1 |
| 3i | 3.9 | 1 | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 3.9 | 1 | 0.5 |
| 3j | 3.9 | 2 | 2 | 1 | 0.5 | 2 | 3.9 | 2 | 2 | 2 | 3.9 | 3.9 | 2 | 0.5 |
| 3k | 7.8 | 3.9 | 2 | 3.9 | 2 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 3,9 | 3,9 | 3.9 | 2 |
| 3l | 7.8 | 3.9 | 3.8 | 2 | 2 | 3.9 | 7.8 | 2 | 3.9 | 3.9 | 2 | 3.9 | 3.9 | 2 |
| 3m | 7.8 | 3.9 | 3.9 | 1 | 2 | 3.9 | 2 | 2 | 2 | 3.9 | 2 | 3.9 | 1 | 0.5 |
| 3n | 2 | >125 | 2 | >125 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 2 | 3.9 | 2 | 1 |
| 4e | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 4f | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 4n | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| AMK | 2 | ≤0.25 | 62.5 | 31.3 | 31.3 | 3.9 | 62.5 | 2 | 2 | 7.8 | 31.3 | 31.3 | 3.9 | 31.3 |
| Cpd # | O | P | Q | R | S | T | U | V | W | X | Y | Z | AA | AB |
| Ebselen | 1 | 1 | 1 | 7.8 | 1 | 3.9 | ≤0.25 | 1 | 1 | 0.5 | 2 | 2 | 3.9 | 3.9 |
| 2a | 3.9 | 3.9 | 2 | 7.8 | 3.9 | 3.9 | ≤0.25 | 3.9 | 2 | 7.8 | 3.9 | 3.9 | 3.9 | 3.9 |
| 2b | 3.9 | 2 | 2 | 3.9 | 3.9 | 3.9 | ≤0.25 | 3.9 | 2 | 2 | 2 | 3.9 | 2 | 2 |
| 2c | 2 | 2 | 1 | 3.9 | 3.9 | 3.9 | ≤0.25 | 1 | 2 | 1 | 2 | 2 | 3.9 | 2 |
| 2d | 3.9 | 2 | 2 | 3.9 | 2 | 2 | ≤0.25 | 3.9 | 2 | 2 | 7.8 | 3.9 | 3.9 | 3.9 |
| 2e | 0.5 | 1 | 1 | 2 | 0.5 | 1 | ≤0.25 | ≤0.25 | 0.5 | ≤0.25 | 0.5 | 1 | 2 | 1 |
| 2f | 7.8 | 3.9 | 3.9 | 7.8 | 3.9 | 3.9 | 0.5 | 3.9 | 2 | 2 | 2 | 3.9 | 3.9 | 7.8 |
| 2g | 3.9 | 7.8 | 7.8 | 7.8 | 3.9 | 3.9 | 0.5 | 3.9 | >125 | 3.9 | 3.9 | 7.8 | 3.9 | 7.8 |
| 2h | 0.5 | 0.5 | 0.5 | 1 | ≤0.25 | 0.5 | ≤0.25 | ≤0.25 | 1 | ≤0.25 | 0.5 | 0.5 | ≤0.25 | ≤0.25 |
| 2j | 7.8 | 7.8 | 3.9 | 7.8 | 7.8 | 7.8 | ≤0.25 | 3.9 | 3.9 | 7.8 | 3.9 | 7.8 | 7.8 | 7.8 |
| 2k | 1 | 0.5 | ≤0.25 | 2 | 1 | 2 | ≤0.25 | 1 | 0.5 | ≤0.25 | 0.5 | 0.5 | ≤0.25 | 2 |
| 2l | 0.5 | 0.5 | 2 | 2 | 1 | 2 | ≤0.25 | 1 | 1 | 0.5 | 0.5 | 2 | 2 | 2 |
| 2m | 7.8 | 7.8 | 7.8 | 15.6 | 7.8 | 15.6 | 2 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 15.6 |
| 3a | 2 | 2 | 2 | 2 | 3.9 | 2 | ≤0.25 | 2 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 |
| 3b | 3.9 | 2 | 2 | 3.9 | 3.9 | 3.9 | ≤0.25 | 3.9 | 3.9 | 3.9 | 7.8 | 7.8 | 3.9 | 7.8 |
| 3c | 1 | 1 | 1 | 1 | 1 | 1 | ≤0.25 | 1 | 1 | 1 | 2 | 3.9 | 2 | 3.9 |
| 3d | 1 | 1 | 2 | 3.9 | 3.9 | 2 | 1 | 1 | 3.9 | 1 | 3.9 | 7.8 | 7.8 | 7.8 |
| 3e | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 2 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 | 7.8 |
| 3f | 3.9 | 3.9 | 7.8 | 3.9 | 7.8 | 3.9 | 1 | 3.9 | 7.8 | 3.9 | 7.8 | 7.8 | 7.8 | 7.8 |
| 3g | 15.6 | 15.6 | 15.6 | 15.6 | 7.8 | 15.6 | 2 | 15.6 | 15.6 | 15.6 | 15.6 | 15.6 | 15.6 | 15.6 |
| 3h | 2 | 1 | 1 | 1 | 2 | 1 | ≤0.25 | 1 | 2 | 1 | 2 | 2 | 2 | 2 |
| 3i | 1 | 1 | 1 | 2 | 2 | 2 | 0.5 | 1 | 3.9 | 2 | 3.9 | 2 | 2 | 3.9 |
| 3j | 2 | 2 | 2 | 2 | 2 | 2 | ≤0.25 | 2 | 3.9 | 2 | 2 | 3.9 | 2 | 3.9 |
| 3k | 3,9 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 0.5 | 3.9 | 7.8 | 3.9 | 3.9 | 3.9 | 3.9 | 7.8 |
| 3l | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 0.5 | 7.8 | 7.8 | 3.9 | 7.8 | 7.8 | 7.8 | 7.8 |
| 3m | 2 | 2 | 3.9 | 2 | 3.9 | 2 | 0.5 | 1 | 3.9 | 3.9 | 3.9 | 7.8 | 15.6 | 7.8 |
| 3n | 2 | 2 | 3.9 | 0.5 | 2 | 2 | 1 | 2 | 7.8 | 3.9 | 7.8 | 7.8 | 7.8 | 7.8 |
| 4e | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 4f | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 4n | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | >125 | 125 | >125 | >125 | >125 | >125 |
| AMK | 15.6 | 15.6 | 31.3 | 31.3 | 15.6 | 0.5 | 7.8 | 31.3 | 7.8 | 31.3 | 62.5 | 62.5 | 62.5 | 62.5 |
Bacterial strains: A = S. aureus ATCC 6538, B = S. aureus ATCC 25923, C = S. aureus ATCC 29213, D = S. aureus ATCC 33591, E = MRSA1, F = MRSA2, G = MRSA BRS3, H = MRSA C1, I = MRSA C2, J = MRSA C7, K = MRSA C14, L = MRSA C16, M = MRSA C19, N = MRSA G1, O = MRSA G6, P = MRSA G12, Q = MRSA G14, R = MRSA MRSA NRS4, S = MRSA NRS51, T = MRSA NRS77, U = MRSA S14, V = MRSA S17, W = MRSA S22, X = MRSA S24, Y = MRSA USA100, Z = MRSA USA200, AA = MRSA USA300, AB = MRSA USA600.
Table 2.
MIC valuesa (in μg/mL) determined for all compounds and for the control antibacterial agent (AMK) against various non-S. aureus bacterial strains.
| Cpd # | AC | AD | AE | AF | AG | AH | AI |
|---|---|---|---|---|---|---|---|
| Ebselen | 0.25 | 0.5 | ≤0.25 | 0.5 | 1 | 1 | 7.8 |
| 2a | 1 | 31.3 | 15.6 | 7.8 | 1 | 2 | 3.9 |
| 2b | 2 | 62.5 | >125 | 31.3 | 2 | 2 | 1 |
| 2c | 1 | 62.5 | >125 | 31.3 | 0.5 | 1 | 3.9 |
| 2d | 2 | 7.8 | 15.6 | 7.8 | 2 | 2 | 0.5 |
| 2e | 0.25 | 15.6 | 62.5 | 15.6 | ≤0.25 | 0.5 | 0.5 |
| 2f | 2 | 7.8 | <125 | 7.8 | 2 | 3.9 | 3.9 |
| 2g | 3.9 | 15.6 | >125 | 15.6 | 7.8 | 3.9 | 3.9 |
| 2h | 0.25 | >125 | >125 | 3.9 | ≤0.25 | 1 | 1 |
| 2j | 3.9 | 15.6 | 15.6 | 15.6 | 3.9 | 7.8 | 3.9 |
| 2k | 0.25 | 2 | 7.8 | 3.9 | 0.5 | 2 | 2 |
| 2l | 0.25 | >125 | >125 | >125 | 0.5 | 2 | 1 |
| 2m | 3.9 | >125 | >125 | >125 | 7.8 | 7.8 | 7.8 |
| 3a | 3.9 | 7.8 | >125 | 15.6 | 1 | 3.9 | 2 |
| 3b | 2 | >125 | >125 | 7.8 | 2 | 3.9 | 2 |
| 3c | 1 | >125 | >125 | 2 | 0.5 | 1 | 2 |
| 3d | 0.25 | >125 | >125 | 3.9 | 1 | 15.6 | 7.8 |
| 3e | 7.8 | 7.8 | 15.6 | 7.8 | 3.9 | >125 | 3.9 |
| 3f | 3.9 | 7.8 | 15.6 | 7.8 | 2 | >125 | 3.9 |
| 3g | 15.6 | 15.6 | 125 | 7.8 | 3.9 | 3.9 | 3.9 |
| 3h | 1 | 31.3 | >125 | 2 | 1 | 1 | 2 |
| 3i | 2 | >125 | >125 | >125 | 1 | 3.9 | 2 |
| 3j | 2 | 15.6 | >125 | 15.6 | 0.5 | 2 | 3.9 |
| 3k | 2 | >125 | >125 | >125 | 2 | 3.9 | 2 |
| 3l | 2 | >125 | >125 | >125 | 2 | 3.9 | 2 |
| 3m | 2 | >125 | >125 | 7.8 | 2 | 3.9 | 7.8 |
| 3n | 3.9 | 15.6 | 15.6 | 15.6 | 1 | >125 | 3.9 |
| 4e | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 4f | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| 4n | >125 | >125 | >125 | >125 | >125 | >125 | >125 |
| AMK | 15.6 | 125 | >125 | 125 | 62.5 | 3.9 | ≤0.25 |
Bacterial strains: AC = S. epidermidis ATCC 35984, AD = E. faecalis ATCC 29212, AE = E. faecalis ATCC 49533, AF = E. faecium BM4105-RF, AG = VRE, AH = L. monocytogenes ATCC 19115, AI = M. smegmatis MC2-155.
Table 3.
MIC valuesa (in μg/mL) determined for all compounds 2a, 2h, and for the control antibacterial agent (AMK) against additional non-S. aureus bacterial strains.
| Strain | Ebselen | 2a | 2h | AMK |
|---|---|---|---|---|
| A. baumannii ATCC 19606 | 15.6 | 15.6 | 31.3 | 7.8 |
| E. cloacae ATCC 13047 | >125 | 125 | >125 | 1 |
| E. coli MC1061 | 7.8 | 31.3 | 15.6 | 2 |
| K. pneumoniae ATCC 27736 | >125 | 62.5 | >125 | 1 |
| P. aeruginosa ATCC 27853 | >125 | 62.5 | >125 | 31.3 |
| S. enterica ATCC 14028 | >125 | 125 | >125 | 7.8 |
| S. epidermidis ATCC 12228 | >125 | 125 | >125 | 31.3 |
2.2.1. Evaluation of compounds 2a–4n against various S. aureus strains
We commenced our study by evaluating ebselen against a panel of 28 S. aureus strains (Table 1). Ebselen displayed excellent (≤0.50 μg/mL), very good (1–2 μg/mL), and good (3.9–7.8 μg/mL) activity against 5 (D, E, J, U, and X), 16 (A, C, F, H, I, K, M-Q, S, V, W, Y, and Z), and 7 (B, G, L, R, T, AA, and AB) of these S. aureus strains, respectively. We then investigated the replacement of the selenium atom by sulfur by synthesizing and testing ebsulfur (2a). Compound 2a was found to have similar MIC values to those of ebselen against all S. aureus strains tested. As replacement of the selenium atom by sulfur could result in decreased toxicity, we decided to use compound 2a as our model compound for further derivatization and evaluation.
Previous literature on the antibacterial properties of ebselen suggested that the 1,2-benzisothiazol-3(2H)-one core could be required for antibacterial activity.16,19 We also thought of possibly removing the annulated benzene ring of the core to generate 1,2-isothiazolin-3-one analogues. However, we shied away from these analogues once we realized that there were reports suggesting that these compounds could be allergenic and neurotoxic against humans.22,23 Thus, we decided to keep the 1,2-benzisothiazol-3(2H)-one core intact and hypothesized that the phenyl group adjacent to the core scaffold would be a good site for our investigation. In search of chemical modifications to increase the biological activity of 2a, we first replaced the phenyl ring by the following moieties: substituted phenyl and other aromatic rings (2b–o), alkyl chains (3a–g), alkyl chains with a terminal phenyl group (3h–j), and aliphatic rings (3k–o). In general, all of our analogues displayed moderate to excellent activity against S. aureus (15.6 to ≤0.25 μg/mL), except for compounds 4e, 4f, and 4n, which were found to be completely inactive (>125 μg/mL) against all S. aureus strains tested. In addition, compounds 2i, 2n, 2o, and 3o were not evaluated due to solubility issues in liquid Mueller-Hinton medium.
Most of the compounds with mono- (2b, 2c, 2d, 2f, and 2g) and disubstitutions (2i and 2j) at the 3- and 4-positions of the phenyl ring were found to display good to excellent activity (3.9 to ≤0.25 μg/mL), but the activity was very similar to that of 2a. The three overall best compounds of this series of analogues (2e, 2h, and 2k) were found to have very good to excellent activity (≤2.0 μg/mL) across all S. aureus strains tested (Table 1). Among these three, the relatively bulkier 4-isopropylphenyl (2e) and 3-isopropylphenyl (2h) analogues were our two best compounds and displayed up to 16-fold improvement in MIC values when compared to the parent compound 2a. We also noticed that the substitution pattern (p- vs m-isopropylphenyl (2e vs 2h) or p- vs m-bromophenyl (2d vs 2g)) did not have a substantial effect on the MIC values of these compounds (mostly within 2 fold dilutions). Intrigued by this result, we synthesized and tested more analogues with bulky substituents on the phenyl ring. Surprisingly, the 2,3-dimethoxyphenyl analogue (2k) also yielded very good to excellent MIC values (2 to ≤0.25 μg/mL). We decided to add even more bulkiness to the scaffold by synthesizing the naphthyl analogue (2l), which displayed very good to excellent MIC values (2 to ≤0.25 μg/mL). This result further suggested that adding different bulky groups to the phenyl ring is a favorable strategy to increase activity.
To further understand the structure-activity-relationship (SAR) of our ebsulfur scaffold, we decided to introduce a heteroatom into the benzene ring. Previously, the 3-chloropyridyl replacement of the phenyl ring in the ebselen scaffold was reported to greatly reduce toxicity in mammalian HEK293T cell line (IC50 >160 μM).24 We applied this knowledge to our ebsulfur scaffold and synthesized the pyridyl (2m), 3-chloropyridyl (2n), as well as the bulky quinolinyl (2o) analogues. However, only 2m was soluble enough for biological testing, but yielded inferior MIC values (15.6 to 2 μg/mL) relative to the parent compound 2a, suggesting that introduction of a nitrogen atom into the phenyl ring may not be the way to pursue.
We next investigated the effect of replacing the phenyl ring by linear alkyl chains, which were previously shown to improve the antibacterial activity of another class of antibiotics, the aminoglycosides.6–8,25,26 Compounds 3a–d were synthesized to contain n-pentyl, n-hexyl, n-octyl, and n-dodecyl side chains instead of the typical phenyl ring found in 2a. Among these compounds, the n-octyl analogue (3c) displayed the best MIC values (3.9 to ≤0.25 μg/mL). Compounds 3a, 3b, and 3d displayed MIC values (62.5 to 0.25 μg/mL) that were similar to that of the parent compound 2a. Inspired by our best compounds in series 2 containing an isopropyl moiety, compounds 2e and 2h, we decided to explore the effect of branched alkyl chains by synthesizing and testing compounds 3e–g. These compounds displayed mostly good to moderate activity (3.9 to 15.6 μg/mL). However, they were definitely inferior when comparing to compounds 2e and 2h. Additionally, we were also able to isolate the sulfoxide analogues of these compounds (4e and 4f) and evaluated them. We were surprised to find that these oxidized compounds completely lost their antibacterial activity against S. aureus. This finding was consistent with previous reports that the S-N bond is essential for biological activity by covalently binding to cysteine residues of targeted enzymes.17,19
In an attempt to further understand the SAR of the phenyl ring, we explored whether having this ring directly attached to (compound 2a) or at a distance from the 1,2-benzisothiazol-3(2H)-one core made any difference. We synthesized compounds 3h–j with 1–3 carbon linkers separating the phenyl ring and the core. These compounds displayed good to excellent activity (3.9 to ≤0.25 μg/mL). Lastly, to confirm that the aromaticity of the substituent is not required for antibacterial activity, we synthesized a series of compounds containing different-sized aliphatic rings (3k–o). We found that compound 3l with a cyclohexyl ring displayed very similar MIC values (7.8 to 0.5 μg/mL) to its aromatic counterpart 2a. We noted that all of these compounds retained good to excellent antibacterial activity against S. aureus, with the exception of the adamantyl derivative 3o, which could not be tested due to solubility issues.
2.2.2. Evaluation of compounds 2a–4n against various non-S. aureus strains
To further examine the antibacterial spectrum of compounds 2a–4n, we also tested them against a panel of non-S. aureus strains. Overall, we found that many of our ebsulfur (2a) analogues were more specific towards S. aureus strains. We did observe that a few analogues actually displayed moderate to excellent activity (15.6 to ≤0.25 μg/mL) against certain non-S. aureus strains (Table 2). Interestingly, we found that ebselen still displayed good to excellent activity against many non-S. aureus strains (7.8 to ≤0.25 μg/mL), in contrary to what was previously observed when ebselen was tested against a different panel of bacterial strains.16 We were especially enlightened to find that our best compounds, 2e and 2h, still retained their excellent activity (1 to ≤0.25 μg/mL) against selected strains such as S. epidermidis (AC), VRE (AG), L. monocytogenes (AH), and M. smegmatis (AI).
To gain a better understanding of the antibacterial spectrum of our ebsulfur (2a) analogues, we decided to test them against a biofilm-forming S. epidermidis (AC), another Gram-positive bacterium of the genus Staphylococcus. As expected, other than the previously inactive oxidized compounds 4e, 4f, and 4n, we found that our entire library of analogues still retained moderate to excellent activity that was similarly observed during our evaluation of these compounds against the panel of S. aureus strains presented in Table 1.
Next, we explored the activity of our analogues against examples of the genus Enterococcus. Against the E. faecalis strains (AD and AE) our ebsulfur analogues mostly showed moderate to poor activity (≥15.6 μg/mL), except in the case of compound 2k, which still displayed good to very good activity (7.8 to 2 μg/mL) (Table 2). When comparing the activity of our compounds against E. faecalis (strains AD and AE) and E. faecium (strain AF), we observed a slightly improved activity (lower MIC values) against E. faecium, with compounds 2a, 2d, 2f, 2h, 2k, 3b–f, 3g, 3h, and 3m–n displaying good to very good activity (7.8 to 2 μg/mL). Surprisingly, against the vancomycin-resistant Enterococcus (VRE) strain (AG), all of our analogues (except for 4e, 4f, and 4n) displayed good to excellent activity (7.8 to ≤0.25 μg/mL).
To continue our study, we explored the activity of our library against L. monocytogenes (AH) and M. smegmatis (AI). We found that most of our analogues (2a–h, 2k–m, 3a–f (except 3e,f against strain AH), and 3h–m) displayed good to excellent activity (7.8 to 0.5 μg/mL) against these strains.
Intrigued by our results against the non-S. aureus panel, we further investigated the activity of our best compound, 2h, along with ebselen and ebsulfur (2a) against some additional non-S. aureus strains (A. baumannii, E. cloacae, E. coli, K. pneumoniae, P. aeruginosa, S. enterica, and an additional non-biofilm-forming S. epidermidis). However, we found that these compounds were mostly inactive against these strains (Table 3).
2.3. Evaluation of time-kill curve of compounds 2a, 3b, and 3c
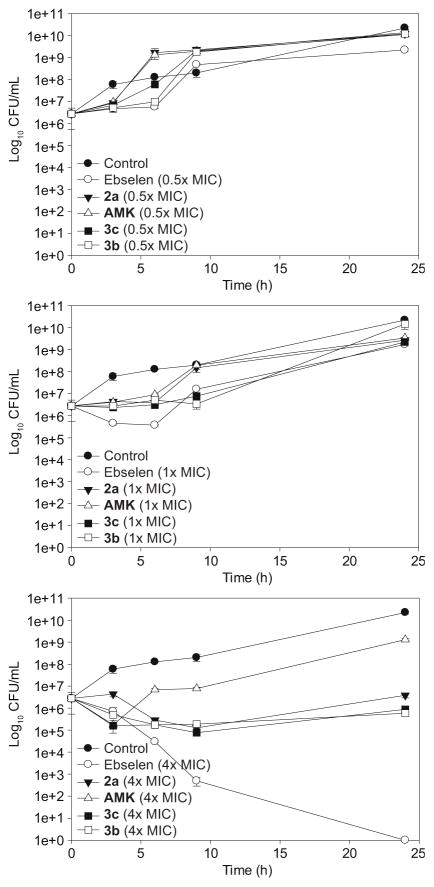
To gain more insights into the antibacterial activity kinetics of our ebsulfur analogues, we performed time-kill assays27 of compounds 3b and 3c on MRSA S22 (strain W) over a period of 24 h and compared that to ebselen, ebsulfur (2a), and AMK (Fig. 1). At sub-MIC concentration (0.5× MIC), as expected, we observed that strain W grew well in the presence of all these compounds. Unlike the control (untreated bacteria), we found that all of the compounds were bacteriostatic up until 3 to 6 h. At MIC concentration, we observed similar bacteriostatic profiles as described previously in our experiment at sub-MIC. We decided to conduct another study at 4× MIC, where we were surprised to find that ebselen was bactericidal and led to complete cell death at 24 h. Compounds 2a, 3b, and 3c reduced bacterial load by 1.5 orders of magnitude, but they still remained bacteriostatic. These results are interesting and may suggest that the selenium atom has a role in promoting the bactericidal effect. However, it should be noted that the time-kill curve assays were conducted under different conditions than those used in the MIC broth double-dilution assays (inoculum size, air flow, and volumes of medium). These differences could lead to a change in the effective or therapeutic doses and thus, potentially explain why the 4× MIC dosages were required for strong efficacy. It is well established that increasing the inoculum size will require a different MIC to achieve therapeutic efficacy. To investigate whether the inoculum size affected our MIC values, we re-performed the MIC broth double-dilution assay on eight compounds (AMK, ebselen, 2a, 2g, 2h, and 3a–c) with the inoculum size used in time-kill assays (1×106 CFU/mL) and found that the MIC values remained identical. Therefore, the selenium atom might indeed play a role in the bactericidal effect.
Fig. 1.
Representative time-kill studies of ebselen, AMK, 2a, 3c, and 3b against MRSA S22 (strain W). Bacterial cells were either treated with ebselen (white circle), AMK (white triangle), 2a (black inverted triangle), 3c (black square), and 3b (white square) at 0.5× MIC (top panel), 1× MIC (middle panel) and 4× MIC (bottom panel), respectively or no drug (black circle).
2.4. Activity against biofilms of S. aureus and S. epidermidis
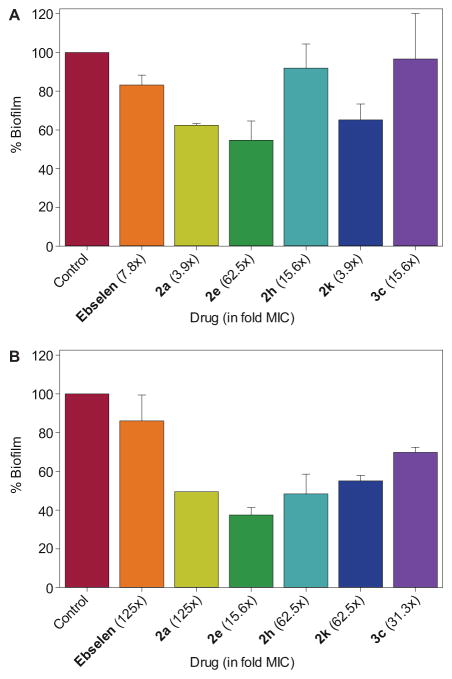
Having established that our compounds displayed activity against biofilm-forming S. aureus (strain A) and S. epidermidis (strain AC), we wanted to explore whether these compounds could also reduce the biofilm mass of these strains. Bacterial biofilm is a matrix of bacterial cells that are more tolerant to most antibacterial compounds and are highly associated with chronic persistent infections.28 Recently, ebselen was reported to display potent biofilm reduction properties against established biofilms of S. aureus and S. epidermidis. When compared to the biofilm reduction activity of conventional antibiotics (linezolid, mupirocin, vancomycin, and rifampicin), the activity of ebselen was found to be significantly superior.20 We were interested in finding out whether our ebsulfur analogues also possess the same anti-biofilm property, which would make them highly desirable in combating pathogenic bacteria associated with chronic infectious diseases. To evaluate the biofilm reduction activity,20 ebselen, ebsulfur (2a), as well as some of our best compounds (2e, 2h, 2k, and 3c) were tested against the established biofilm of two biofilm-forming strains of S. aureus ATCC 6538 (strain A) and S. epidermidis ATCC 35984 (strain AC) at 125×, 62.5×, 31.3×, 15.6×, 7.8×, 3.9×, and 1× MIC (Fig. 2). Due to the observed ceiling effect (where the biofilm reduction plateaus), for each compound, we are reporting the lowest MIC folds at which the plateau begins.
Fig. 2.
Bar graphs showing the ability of selected compounds to reduce the amount of biofilm observed for A. S. aureus ATCC 6538 (strain A) and B. S. epidermidis ATCC 35984 (strain AC). The fold MIC for the optimal reduction for each compound is shown into parenthesis beside the compound name/number.
Against the established biofilm of strain A, we found that ebselen (at 15.6 μg/mL or 7.8× MIC) reduced approximately 20% of the biofilm when comparing to the biofilm of the control (untreated biofilm) (Fig. 2A). This is different from previous finding about the biofilm reduction of this MRSA strain by ebselen, which was reported to be approximately 60% at 2 μg/mL.20 Ebsulfur (2a) (at 31.3 μg/mL or 3.9× MIC) displayed superior biofilm reduction, approximately 40%. Compound 2e also resulted in ~50% biofilm reduction at a much higher concentration of 125 μg/mL or 62.5× MIC. Meanwhile, compound 2h (at 62.5 μg/mL or 15.6× MIC) only reduced the biofilm by ~10%. Compound 3c was also found to result in low to no biofilm reduction. However, we found that compound 2k displayed 40% biofilm reduction at 3.9 μg/mL or 3.9× MIC, which is the lowest concentration tested in our assay.
Against the biofilm of strain AC, ebselen (at 31.3 μg/mL or 125× MIC) was found to result in ~20% reduction of the biofilm (Fig. 2B). Ebsulfur (2a) was found to be slightly more effective, reducing the biofilm by ~50%, but at a much higher concentration (125 μg/mL or 125× MIC). Compounds 2e and 2h were found to have similar biofilm reduction activity (~60–50%) at 3.9 and 15.6 μg/mL (15.6× and 62.5× MIC), respectively. Compound 2k displayed about 50% biofilm reduction at 15.6 μg/mL or 62.5× MIC. Finally, we found that compound 3c displayed about 40% biofilm reduction at 31.3 μg/mL or 31.3× MIC.
Overall, when compared to ebselen, we found that ebsulfur (2a) and compound 2e displayed more potent biofilm reduction, but at higher concentrations. Compounds 2h and 3c were found to be active against the biofilm of strain AC, but not against the biofilm of strain A. Finally, compound 2k displayed good biofilm disruption (40–50%) against both biofilm-forming strains at relatively low concentrations (3.9 and 15.6 μg/mL).
2.5. Evaluation of the hemolytic potential of compounds 2a, 2h, and 3c
As red blood cells are some of the more fragile mammalian cells, we were interested in assessing our analogues for their general mammalian cell cytotoxicity and establishing whether these compounds would be suitable for any applications in humans. Hemolytic assays29,30 were performed by testing ebselen, compounds 2a, 2h, and 3c, as well as AMK (as a negative control) at various concentrations against murine red blood cells (mRBCs) (Fig. 3). We observed that our compounds tested did not show any significant hemolytic activity (≤20% hemolysis) up to 7.8 μg/mL. This result is relatively encouraging knowing that against S. aureus strains, compound 2h was found mostly to display MIC values ranging from ≤0.25 to 1 μg/mL, while compound 3c mostly displayed MIC values ranging from ≤0.25 to 2 μg/mL. Another interesting observation was that 2a and its analogues 2h and 3c were less hemolytic when compared to ebselen (100% hemolytic at 15.6 μg/mL). Amongst the ebsulfur analogues tested, compound 2h was found to be the least hemolytic.
Fig. 3.
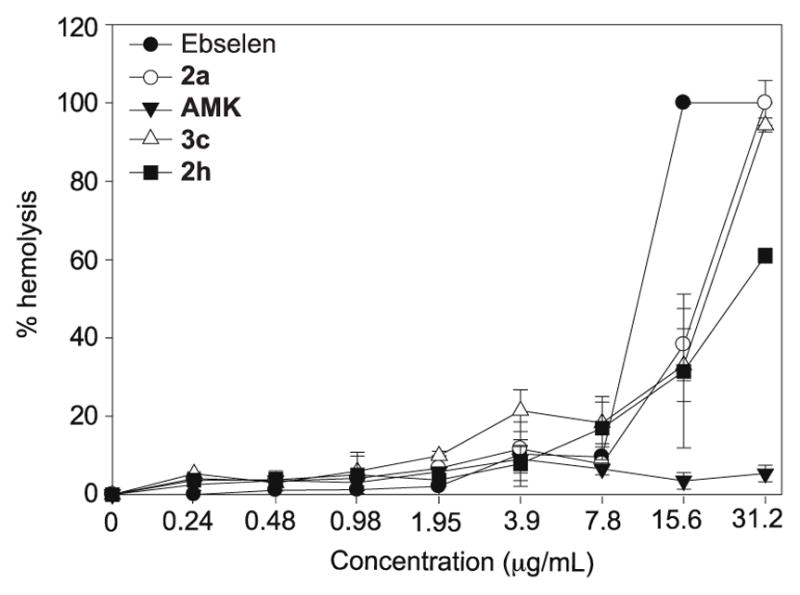
Hemolytic activity of ebselen (black circle), 2a (white circle), AMK (black inverted triangle), 3c (white triangle), and 2h (black square) on mouse red blood cells. The positive control (untreated) was found to display no hemolysis while the negative control (1% Triton X) was found to display 100% hemolysis (not shown on the graph).
2.6 Evaluation of mammalian toxicity potential of ebselen and compounds 2e, 2h, 2k, 2l, 3c, 3i, and 3k
Although the hemolysis data was encouraging, we realized that a more extensive in vitro cytotoxicity study would still be needed to further understand the mammalian toxicity potential of our compounds.30 Given that many current antibiotics are nephrotoxic, we selected the human embryonic kidney cell line HEK-293 as the model mammalian cell line for our cytotoxicity evaluation (Fig. 4). Ebselen was used as the control because it was shown to be safe for human use.13 Compounds 2e, 2h, and 2l all did not display any significant cytotoxicity (>20% cell death) up until 10 μg/mL, which was very similar to what was found with ebselen. On the other hand, compounds 2k, 3c, 3i, and 3k were slightly more toxic against HEK-293 with >20% cell death observed at 1.25 μg/mL. Collectively, it was intriguing and encouraging that our best analogues 2e and 2h displayed stronger selectivity for bacterial cells. Depending on the MRSA strains, there could be up to 40-fold difference when compared to their MIC values.
Fig. 4.
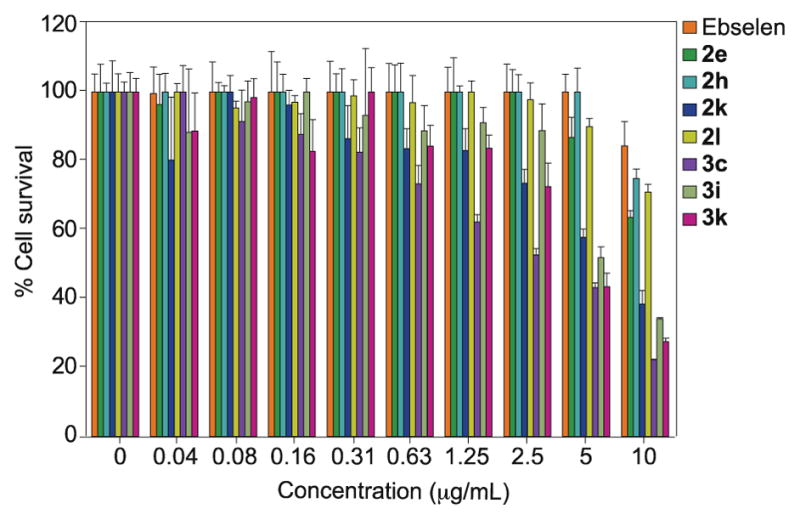
Mammalian cytotoxicity of ebselen (orange bars), 2e (green bars), 2h (teal bars), 2k (blue bars), 2l (yellow bars), 3c (purple bars), 3i (light green bars), and 3k (pink bars). Untreated cells were used as positive control and cells treated with 1% Triton X were used as negative control (not shown on graph).
2.7. Evaluation of ebselen and compounds 2a and 3b,c as bacterial membrane disruptors
Based on previous literature on membrane-disrupting cationic amphiphilic aminoglycosides,6–8 we suspected that our linear alkyl ebsulfur analogues (3b,c) could potentially exert their antibacterial activity by their ability to disrupt bacterial cell membranes. Thus, we tested compounds 3b,c along with 2a and ebselen against S. epidermidis ATCC 35984 (strain AC) at their 1× and 4× MIC values and evaluated them for membrane disruption. We also used a tobramycin derivative with a linear alkyl chain of 14 carbons in length attached in a thioether linkage to the 6″-position (C14-TOB) and AMK as positive and negative controls, respectively. We used propidium iodide (PI) staining to visualize any damage to the bacterial cell membrane. If the bacterial cell membrane was compromised, the PI would penetrate and stain the cells red. Based on our results, we saw no sign of membrane disruption from any of our compounds (Fig. 5). Thus, membrane disruption is likely not a viable antibacterial mechanism of action for these analogues. We then decided to switch our attention to study another possible mechanism of action for these compounds, the production of ROS.
Fig. 5.
Effect of ebsulfur (2a) and its analogues 3b and 3c on cell membrane integrity of S. epidermidis ATCC 35984 (strain AC). Bacterial cells were treated with no drug or AMK (negative controls), C14-TOB (positive control), or ebselen, 2a, 3b, and 3c, at their 1× and 4× respective MIC values. Propidium iodine (PI) dye was used to monitor the uptake by bacterial cells.
2.8. Detection of reactive oxygen species (ROS) production
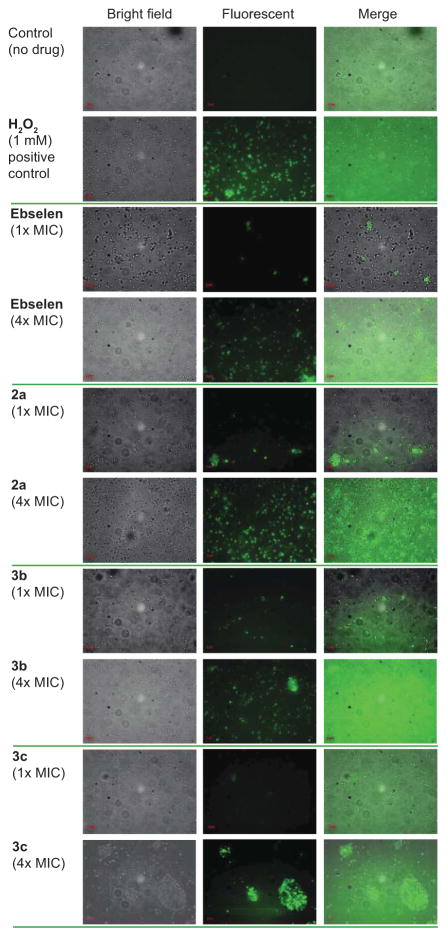
In bacteria lacking glutathione and glutaredoxin such as S. aureus and others, the thioredoxin system is crucial for ROS regulation and thus, bacterial survival and proliferation.31–33 Ebselen was identified previously to inhibit bacterial thioredoxin reductase.19 To test the hypothesis that our analogues also inhibit thioredoxin reductase, we treated S. epidermidis ATCC 35984 (strain AC) with ebselen, compounds 2a and 3b,c at various concentrations (1× and 4× their respective MIC values). 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA)34 was then used to detect and visualize ROS production (Fig. 6). We found that ebselen and all of our analogues led to production of ROS and that inhibition of thioredoxin reductase is possibly the target responsible for antibacterial activity, which will be the subject of future studies.
Fig. 6.
Effect of ebsulfur (2a) and its analogues 3b and 3c on intracellular ROS production by S. epidermidis ATCC 35984 (strain AC). Bacterial cells were treated with no drug (negative control), 1 mM of H2O2 (positive control), or ebselen, 2a, 3b, and 3c, at their 1× and 4× respective MIC values for 1 h at 37 °C. After staining with DCFH-DA (40 μg/mL), the samples were analyzed using a Zeiss Axovert 200M fluorescence microscope.
3. Conclusions
We synthesized an ebselen-inspired (ebsulfur) library comprised of 33 molecules based on previous reports of the antibacterial activity of ebselen against drug-resistant S. aureus clinical isolates. Our SAR analysis suggested that replacing the selenium atom with the sulfur atom in the 1,2-benzisoselenazol-3(2H)-one core did not significantly alter antibacterial activity. However, oxidizing the sulfur atom of the 1,2-benzisothiazol-3(2H)-one core, as in compounds 4e, 4f, and 4n, completely obliterated antibacterial activity. This finding demonstrated that the stereoelectronic nature of the S-N bond is indeed important for biological activity, which is consistent with previous reports.17,19 During our search for analogues with improved antibacterial activity, we identified three compounds (2e, 2h, and 2k) with remarkably potent activity (MIC values mostly ≤2 μg/mL) against S. aureus clinical isolates. We then evaluated our compounds for their antibacterial spectrum by testing them against non-S. aureus strains. Our analogues were generally more selective towards Staphylococcus strains, but some of them also displayed good activities against VRE, L. monocytogenes, and M. smegmatis. Compounds with biofilm reduction are currently in high demand. Our evaluation showed that albeit at high concentrations, our analogues were able to reduce Staphylococcal established biofilms. We showed that the antibacterial activity of our compounds was highly correlated with ROS production. Lastly, we assessed our compounds for general mammalian toxicity by testing them against murine RBCs and HEK293. The mammalian toxicities of our most potent compounds were found to be acceptable. With further optimizations, these ebsulfur analogues could potentially have clinical utilities in our fight against bacterial resistance.
4. Experimental section/Supplementary material
Details of all experimental procedures for (i) synthesis of and characterization of compounds 1–4n, (ii) determination of MIC values, as well as (iii) time-kill curves, (iv) biofilm disruption, (v) hemolysis assays, (vi) mammalian cytotoxicity, (vii) cell membrane permeabilization, and (viii) detection of ROS production are included in the Supplemental Information. The Supplemental Information also includes all 1H and 13C NMR spectra (Figs. S1–S57) for the molecules generated.
Supplementary Material
Acknowledgments
This work was supported by a grant from the National Institute of Health NIH AI90048 (to S.G.-T.) and by startup funds from the University of Kentucky (to S.G.-T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barber M. J Clin Pathol. 1961;14:385. doi: 10.1136/jcp.14.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, Lessa FC, Lynfield R, Nadle J, Petit S, Ray SM, Schaffner W, Townes J, Fridkin S Emerging Infections Program-Active Bacterial Core Surveillance, MSI. JAMA Int Med. 2013;173:1970. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harris A. UpToDate post TW, editor. UpToDate. Waltham, MA: 2016. Methicillin-resistant Staphylococcus aureus (MRSA) (Beyond the Basics) [Google Scholar]
- 4.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. Clin Infect Dis. 2009;48:1. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 5.Lepri S, Buonerba F, Goracci L, Velilla I, Ruzziconi R, Schindler BD, Seo SM, Kaatz GW, Cruciani G. J Med Chem. 2016 doi: 10.1021/acs.jmedchem.5b01219. [DOI] [PubMed] [Google Scholar]
- 6.Herzog IM, Green KD, Berkov-Zrihen Y, Feldman M, Vidavski RR, Eldar-Boock A, Satchi-Fainaro R, Eldar A, Garneau-Tsodikova S, Fridman M. Angew Chem. 2012;51:5652. doi: 10.1002/anie.201200761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fosso MY, Shrestha SK, Green KD, Garneau-Tsodikova S. J Med Chem. 2015;58:9124. doi: 10.1021/acs.jmedchem.5b01375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shrestha SK, Fosso MY, Green KD, Garneau-Tsodikova S. Antimicrob Agents Chemother. 2015;59:4861. doi: 10.1128/AAC.00229-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hafkin B, Kaplan N, Murphy B. Antimicrob Agents Chemother. 2015 doi: 10.1128/AAC.01741/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, Yasuhara H. Stroke. 1998;29:12. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- 11.Parnham MJ, Sies H. Biochem Pharmacol. 2013;86:1248. doi: 10.1016/j.bcp.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Singh N, Halliday AC, Thomas JM, Kuznetsova OV, Baldwin R, Woon EC, Aley PK, Antoniadou I, Sharp T, Vasudevan SR, Churchill GC. Nat Commun. 2013;4:1332. doi: 10.1038/ncomms2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch E, Kil J. Semin Hear. 2009;30:047. [Google Scholar]
- 14.Abdul-Hay SO, Bannister TD, Wang H, Cameron MD, Caulfield TR, Masson A, Bertrand J, Howard EA, McGuire MP, Crisafulli U, Rosenberry TR, Topper CL, Thompson CR, Schurer SC, Madoux F, Hodder P, Leissring MA. ACS Chem Biol. 2015;10:2716. doi: 10.1021/acschembio.5b00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhowmick D, Srivastava S, D’Silva P, Mugesh G. Angew Chem. 2015;54:8449. doi: 10.1002/anie.201502430. [DOI] [PubMed] [Google Scholar]
- 16.Nozawa R, Yokota T, Fujimoto T. Antimicrob Agents Chemother. 1989;33:1388. doi: 10.1128/aac.33.8.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favrot L, Lajiness DH, Ronning DR. J Biol Chem. 2014;289:25031. doi: 10.1074/jbc.M114.582445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bender KO, Garland M, Ferreyra JA, Hryckowian AJ, Child MA, Puri AW, Solow-Cordero DE, Higginbottom SK, Segal E, Banaei N, Shen A, Sonnenburg JL, Bogyo M. Sci Transl Med. 2015;7:306ra148. doi: 10.1126/scitranslmed.aac9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J, Vlamis-Gardikas A, Kandasamy K, Zhao R, Gustafsson TN, Engstrand L, Hoffner S, Engman L, Holmgren A. FASEB J. 2013;27:1394. doi: 10.1096/fj.12-223305. [DOI] [PubMed] [Google Scholar]
- 20.Thangamani S, Younis W, Seleem MN. Sci Rep. 2015;5:11596. doi: 10.1038/srep11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gajadeera C, Willby MJ, Green KD, Shaul P, Fridman M, Garneau-Tsodikova S, Posey JE, Tsodikov OV. J Antibiotics. 2015;68:153. doi: 10.1038/ja.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnuch A, Geier J, Uter W, Frosch PJ. Brit J Dermatol. 1998;138:467. doi: 10.1046/j.1365-2133.1998.02126.x. [DOI] [PubMed] [Google Scholar]
- 23.Du S, McLaughlin B, Pal S, Aizenman E. J Neurosci. 2002;22:7408. doi: 10.1523/JNEUROSCI.22-17-07408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu J, Vodnala SK, Gustavsson AL, Gustafsson TN, Sjoberg B, Johansson HA, Kumar S, Tjernberg A, Engman L, Rottenberg ME, Holmgren A. J Biol Chem. 2013;288:27456. doi: 10.1074/jbc.M113.495101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benhamou RI, Shaul P, Herzog IM, Fridman M. Angew Chem. 2015;54:13617. doi: 10.1002/anie.201506814. [DOI] [PubMed] [Google Scholar]
- 26.Bera S, Zhanel GG, Schweizer F. J Med Chem. 2010;53:3626. doi: 10.1021/jm1000437. [DOI] [PubMed] [Google Scholar]
- 27.Motyl M, Dorso K, Barrett J, Giacobbe R. Curr Protoc Pharmacol. 2006;Chapter 13(Unit13A):3. doi: 10.1002/0471141755.ph13a03s31. [DOI] [PubMed] [Google Scholar]
- 28.Bjarnsholt T. Acta Pathol Microbiol Immunol Scand A. 2013;121:1. [Google Scholar]
- 29.Dartois V, Sanchez-Quesada J, Cabezas E, Chi E, Dubbelde C, Dunn C, Granja J, Gritzen C, Weinberger D, Ghadiri MR, Parr TR., Jr Antimicrob Agents Chemother. 2005;49:3302. doi: 10.1128/AAC.49.8.3302-3310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shrestha SK, Fosso MY, Garneau-Tsodikova S. Sci Rep. 2015;5:17070. doi: 10.1038/srep17070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uziel O, Borovok I, Schreiber R, Cohen G, Aharonowitz Y. J Bacteriol. 2004;186:326. doi: 10.1128/JB.186.2.326-334.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gustafsson TN, Sahlin M, Lu J, Sjoberg BM, Holmgren A. The J Biol Chem. 2012;287:39686. doi: 10.1074/jbc.M112.413427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scharf C, Riethdorf S, Ernst H, Engelmann S, Volker U, Hecker M. J Bacteriol. 1998;180:1869. doi: 10.1128/jb.180.7.1869-1877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Chang W, Zhang M, Li X, Jiao Y, Lou H. PloS one. 2015;10:e0128693. doi: 10.1371/journal.pone.0128693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.