Abstract
BACKGROUND
The Management of Myelomeningocele Study (MOMS) was a multicenter, randomized controlled trial that compared prenatal repair with standard postnatal repair for fetal myelomeningocele.
OBJECTIVE
We sought to describe the long-term impact on the families of the women who participated and to evaluate how the timing of repair influenced the impact on families and parental stress.
STUDY DESIGN
Randomized women completed the 24-item Impact on Family Scale (IFS) and the 36-item Parenting Stress Index Short Form (PSI-SF) at 12 and 30 months after delivery. A revised 15-item score of the IFS (RIFS) describing overall impact was also computed. Higher scores reflect more negative impacts or greater stress. In addition, we examined Family Support Scale (FSS) and Family Resource Scale (FRS) scores along with various neonatal outcomes. Repeated measures analysis was conducted for each scale and subscale.
RESULTS
Of 183 women randomized, 171 women completed the IFS and 172 completed the PSI at both 12 and 30 months. The prenatal surgery group had significantly lower RIFS scores as well as familial-social impact subscale scores compared to the postnatal surgery group (p=0.02 and 0.004, respectively). There was no difference in total parental stress between the two groups (p=0.89) or in any of the PSI-SF subscales. In addition, walking independently at 30 months and family resources at 12 months are associated with both family impact and parental stress.
CONCLUSION
The overall negative family impact of caring for a child with spina bifida, up to 30 months of age, was significantly lower in the prenatal surgery group compared to the postnatal surgery group. Ambulation status and family resources were predictive of impact on family and parental stress.
Keywords: Family impact, maternal-fetal surgery, myelomeningocele, spina bifida, parental stress
INTRODUCTION
Spina bifida is referred to as one of the most severe congenital malformations compatible with life.(1) The incidence of spina bifida is estimated at 3.5 per 10,000 live births per year in the U.S. (2) Myelomeningocele, or open spina bifida, is the most common and severe form characterized by protrusion of the spinal cord through the open vertebrae into the amniotic fluid. The severity of disability varies in accordance to the level of the neurologic lesion and the extent of the intracranial abnormalities.(3) The malformation is associated with significant lifelong disability including motor and sensory deficits, neurogenic bowel and bladder, hindbrain herniation (the Chiari II malformation) and associated hydrocephalus, orthopedic abnormalities, and cognitive deficits. In addition, children with spina bifida are more likely to have psychosocial adjustment difficulties and report lower physical health related quality of life than peers. (4) Self-reported satisfaction with family functioning has been shown to be an important factor related to improved quality of life.(5)
Prenatal spina bifida repair was developed with the hope that earlier repair would lessen these disabilities by ameliorating the secondary damage from the chronic mechanical and chemical in utero insults. The early experience of prenatal repair of myelomeningocele, first performed in 1997, showed promising results including the reversal of hindbrain herniation and a decreased need for ventriculoperitoneal shunting. (6–7) However, prenatal repair was associated with significant maternal morbidity and the risk of fetal or neonatal death and prematurity. From 2003 to 2011, we conducted the Management of Myelomeningocele Study (MOMS), a multicenter randomized controlled trial to compare the efficacy and safety of prenatal repair of myelomeningocele compared to the standard postnatal repair. Trial recruitment was halted in December 2010 when interim analysis demonstrated efficacy of prenatal repair. (8) The prenatal group showed significant decrease in the rate of shunt placement (40 vs. 82 %) and improved motor outcomes at 30 months of age, including the ability to walk without orthotics (42 vs 21 %). (8) Not all fetuses benefited, however, and maternal and fetal risks included preterm rupture of membranes and prematurity.
The call to address the impact of illness and disability on family functioning and parental stress has become stronger and broader.(9) Previous studies have described the familial impact of caring for children with Trisomy 13(10), brain tumors(11), and congenital diaphragmatic hernia (12). In general, the presence of spina bifida in families is predictive of increased parental stress and psychological strain.(5,13) The effect on families depends on the parental relationship, family climate, and support from social networks.(13) Yet, many families also show increased resilience and adaptation.(14) To further understand the impacts of prenatal surgery, beyond the health consequences to the pregnant woman/fetal dyad, women were asked to complete questionnaires to identify impact on families and psychological stress.
In this article we describe the impact on families caring for a child with spina bifida and to compare the difference in impact between those who were randomized to prenatal versus postnatal repair. In addition, we explore other factors associated with the impact on families and parental stress. We hypothesized that families will experience less negative impacts and less parental stress at 12 and 30 months after prenatal myelomeningocele repair compared to postnatal repair because of the decreased shunt rate and improved neuromotor function.
MATERIALS AND METHODS
The MOMS trial was conducted at three maternal-fetal surgery centers, The Children’s Hospital of Philadelphia, Vanderbilt University, and the University of California, San Francisco in collaboration with an independent data-coordinating center at George Washington University and with the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The trial was approved by the Institutional Review Board at each center. Details of the trial design and proceedings have been previously described. (8) Briefly, pregnant women between 19 and 25 weeks gestation with a fetus diagnosed with myelomeningocele who met eligibility criteria were randomly assigned to either prenatal surgery or postnatal surgery. All women randomized were planned to deliver at 37 weeks of gestation at the MOMS Center where randomized, if not already delivered. (Those randomized to prenatal surgery remained nearby while those randomized to postnatal went home and returned.) Families returned to the maternal–fetal surgery centers for follow-up of the children at 12 and 30 months of age at which time the objective measures were completed.
The primary outcome of interest was the impact on families caring for a child with spina bifida. The mother or the primary caregiver of the affected child completed the Impact on Family Scale (IFS) at 12 and 30 months after birth. The original IFS was developed by Drs. Stein and Riessman to measure the impacts of chronic childhood illnesses on families. (15) The measure consists of 24-items that assess impact in four domains: financial burden, social interactions within and outside the home, personal strain/distress felt by the parent, and sense of a lack of mastery resulting from stress. Response categories for each item include: strongly agree, agree, disagree, and strongly disagree. Items are scored 1 to 4 to compute four domain scores. Higher scores are reflective of a more negative impact in the particular domain. Since the development of the original scale, additional validation studies support the use of 15 of the original questions to assess the overall social and familial impacts of childhood illness. (16–17) Therefore, we also computed a revised 15-item IFS (RIFS) score describing the overall impact. Scores for the RIFS ranged from 15–60, with higher scores indicating more negative impact.
The mother or primary caregiver also completed the Parenting Stress Index Short Form (PSI-SF). The PSI-SF is a 36-item measure taken directly from the Parenting Stress Index full length test developed by Dr. Abidin in the early 1980’s.(18) The short form has three sub-scales: parental distress, parent-child dysfunctional interaction, and difficult child behavioral characteristics. In addition, a total stress score is computed to indicate the overall level of parenting stress the participant is experiencing. Response categories for each item include: strongly agree, agree, not sure, disagree, and strongly disagree. Items are scored 1 to 5 with composite scores ranging from 36–180. Higher scores reflect greater stress. In addition to the three sub-scales and the total stress score, a defensive responding scale can be calculated. This scale assesses the extent to which the parent is simply trying to answer the items in a socially desirable way. A low score indicates responding in a defensive manner.
In addition to the IFS and PSI-SF, participants also completed a Family Support Scale (FSS) and a Family Resource Scale (FRS). The FSS is an 18-item measure that assesses perceived helpfulness of different social resources. (19) The FSS assesses family support in the following areas: informal kinship, social organizations, formal kinship, nuclear family, specialized professional services, and generic professional services. Response categories to measure the degree of helpfulness include: not at all helpful, sometimes helpful, generally helpful, very helpful, and extremely helpful. The FRS is a 30-item measure that assesses perceived family resources in four domains: basic needs, money, time for self, time for family. (20) Responses are rated on a 5-point Likert scale and includes: not at all adequate, seldom adequate, sometimes adequate, usually adequate, and almost always adequate.
Demographic and socioeconomic covariates used in this analysis includes: maternal age at screening, years of schooling completed, number of live births prior to MOMS pregnancy, and household income at randomization. Fetal/neonatal covariates used includes sex, spina bifida lesion level on ultrasound, whether the child required a shunt by 12 months of age, and walking status at 30 months.
Baseline and other covariates were compared by univariable analysis; continuous variables were compared with the Wilcoxon test, categorical variables were compared with the chi-square or Fisher’s exact test as appropriate. The outcomes (IFS and PSI-SF) obtained at 12 and 30 months were analyzed together, as repeated measures, using generalized estimating equations to account for the correlation between time points. For the RIFS and total PSI, generalized linear models were used in a multivariable analysis adjusting for treatment group, maternal age at screening, years of schooling completed, number of live births prior to MOMS pregnancy, and household income at randomization, FSS and FRS. For all tests a nominal p value of less than 0.05 was considered to indicate statistical significance. No adjustment was made for multiple comparisons.
RESULTS
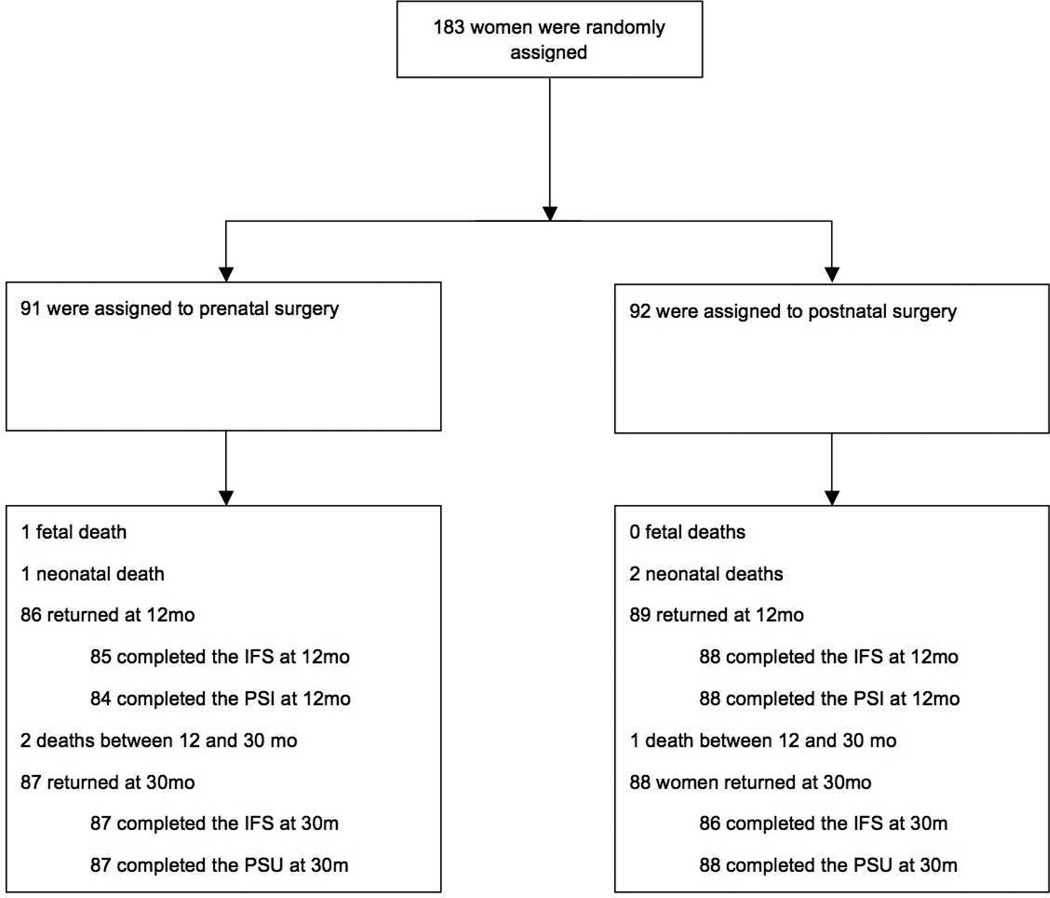
A total of 183 patients were randomized, 91 in the prenatal surgery group and 92 in the postnatal surgery group (Figure 1). The baseline covariates of the cohort are presented in Table 1. There were no differences between the surgery groups except for fetal sex (46% female in the prenatal surgery group vs 62% in the postnatal surgery group; p = 0.03). There were no differences in FSS or FRS at 12 months between the surgery groups (Table 2).
Figure 1.
TABLE 1.
Baseline Covariates by Treatment Group
| Covariate | All N=183 |
Prenatal Repair N=91 |
Postnatal Repair N=92 |
|---|---|---|---|
| Fetal female sex | 99 (54) | 42 (46) | 57 (62) |
| Lesion level on ultrasonography | |||
| Thoracic | 7 (4) | 4 (4) | 3 (3) |
| L1–L2 | 38 (21) | 25 (28) | 13 (14) |
| L3–L4 | 91 (50) | 37 (41) | 54 (59) |
| L5-S1 | 47 (26) | 25 (28) | 22 (24) |
| Maternal age at randomization - y | 28.9 (5.0) | 29.2 (5.2) | 28.7 (4.8) |
| Years of schooling | 14.9 (1.7) | 14.9 (1.7) | 14.9 (1.7) |
|
Number of other children at randomization |
|||
| None | 75 (41) | 38 (42) | 37 (40) |
| 1 | 53 (29) | 23 (25) | 30 (33) |
| More than 1 | 55 (30) | 30 (33) | 25 (27) |
|
Annual household income at randomization |
N=173 | N=84 | N=89 |
| <$30,000 | 17 (10) | 12 (14) | 5 (6) |
| $30,000–<$50,000 | 40 (23) | 19 (23) | 21 (24) |
| $50,000–<$75,000 | 53 (31) | 24 (29) | 29 (33) |
| $75,000–<$100,000 | 24 (14) | 7 (8) | 17 (19) |
| $100,000–<$150,000 | 19 (11) | 10 (12) | 9 (10) |
| >$150,000 | 20 (12) | 12 (14) | 8 (9) |
Data presented as n (%) or mean (standard deviation).
TABLE 2.
Covariates by Treatment Group
| Covariate | All | Prenatal Repair | Postnatal Repair | |||
|---|---|---|---|---|---|---|
| N | N | N | ||||
| FSS at 12 months | 172 | 48.3 (13.1) | 88 | 47.0 (13.4) | 84 | 49.6 (12.8) |
| FRS at 12 months | 174 | 130.7 (12.5) | 89 | 129.4 (13.9) | 85 | 131.9 (10.9) |
Data presented as mean (standard deviation).
Of the 91 families in the prenatal surgery group, 85 (93%) completed the IFS at their 12-month follow-up visit and 87 (95%) completed the IFS at their 30-month visit. Of the 92 families in the postnatal surgery group, 88 (96%) completed the IFS at their 12-month follow-up visit and 86 (93%) completed the IFS at their 30-month visit. There were statistically significant differences between the two groups regarding the familial/social impact sub-scale score over time as well as the overall RIFS scores over time (Table 3). This sub-scale measures the extent of disruption to normal social interactions within and outside the family system due to the child’s disability. The average familial/social impact sub-scores at 12 and 30 months were 13.8 (SD = 3.6) and 14.0 (SD = 3.8) for the prenatal surgery group and 15.2 (SD = 4.1) and 15.3 (SD = 3.7) for the postnatal surgery group, respectively (p=0.004). There was no difference in the other three sub-scale scores (financial impact, personal strain, lack of mastery) between the two surgery groups. The average overall RIFS scores at 12 and 30 months were 24.5 (SD = 6.7) and 24.6 (SD = 6.5) for the prenatal surgery group and 26.5(SD = 6.8) and 26.8 (SD = 6.6) for the postnatal surgery group, respectively (p=0.02).
TABLE 3.
Comparison of Family Impact by Treatment Group
| Impact on Family Scale | |||||
|---|---|---|---|---|---|
| 12 months* | 30 months * | p-value** | |||
| Prenatal Surgery (N=85) |
Postnatal Surgery (N=88) |
Prenatal Surgery (N=87) |
Postnatal Surgery (N=86) |
||
| Financial impact | 9.3 (2.1) | 9.1 (1.8) | 9.5 (1.8) | 9.4 (1.6) | 0.48 |
| Score range 4–16 | |||||
| Familial/social impact | 13.8 (3.6) | 15.2 (4.1) | 14.0 (3.8) | 15.3 (3.7) | 0.004 |
| Score range 9–36 | |||||
| Personal strain | 10.7 (3.5) | 11.3 (3.3) | 10.6 (3.4) | 11.5 (3.5) | 0.13 |
| Score range 6–24 | |||||
| Mastery (lack of) | 7.1 (1.6) | 7.3 (1.9) | 5.7 (1.5) | 6.1 (1.5) | 0.16 |
| Score range 5– 20 | |||||
| Revised IFS | 24.5 (6.7) | 26.5 (6.8) | 24.6 (6.5) | 26.8 (6.6) | 0.02 |
| Score range 15–60 | |||||
Data presented as means (standard deviation)
Based on infants with assessments at both 12 and 30 months adjusted for covariates
In addition to the IFS, of the 91 families in the prenatal surgery group, 84 also completed the PSI-SF at their 12-month follow-up visit and 87 completed the PSI-SF at their 30-month visit. Of the 92 families in the postnatal surgery group, 88 completed the PSI-SF at their 12-month follow-up visit and at their 30-month visit. The average PSI-SF scores at 12 and 30 months were 56.3 (SD = 13.7) and 61.3 (SD = 21.3) for the prenatal surgery group and 59.4 (SD = 14.2) and 60.3 (SD = 15.4) for the postnatal surgery group, respectively (p=0.89). There were no statistically significant differences between the two groups for the total PSI-SF nor in any of the three stress domains: parental distress, dysfunctional interaction, and difficult child. In addition, there was not a significant difference in defensive responding scores between the two groups.
In a multivariable linear regression model, when adjusting for demographics and other potential confounders, only the ability to walk independently at 30 months and greater family resource score at 12 months were associated with total PSI at 30 months (p=0.0375 and P<0.0001, respectively). In addition, in the multivariable analysis of the RIFS at 30 months, the effect of the treatment group was mediated by the ability to walk at 30 months and greater family resources at 12 months as well (p=0.006 and p<0.0001, respectively).
COMMENT
The overall negative family impact of caring for a child with spina bifida, up to 30 months of age, was significantly lower in the prenatal surgery group compared to the postnatal surgery group. Prenatal repair resulted in a lower familial/social impact. The other family impact subscales, as well as the overall parental stress, were not different between the two groups. Therefore, despite the significant investment of prenatal repair, these families report better family impact or equal family impact/parental stress compared to those who underwent postnatal repair. Finally, walking independently at 30 months and family resources at 12 months are associated with both family impact and parental stress.
The findings in this analysis may play an important role in counseling patients about how the timing of prenatal versus postnatal surgical repair may impact family functioning. This is an important finding given the significant personal and family costs involved with undergoing maternal-fetal surgery. Despite the significant burdens involved with prenatal surgery, these caregivers report less familial/social impact and overall family impact than the women randomized to the postnatal surgery group at 12 and 30 months following delivery. And there were no differences between the groups for the other family impact sub-scales and for the parental stress scales. In addition to prenatal counseling, these findings highlight the need to attend to the experience of families and to find areas for intervention to help families adjust to caring for a child with a disability. Parents may benefit from social worker support, referral to a mental health specialist when indicated, and resource assistance.
Children with spina bifida may require mobility assistance with braces, crutches, walkers or a wheelchair. As previously published, prenatal surgery is associated with improved ambulation at 30 months after delivery. (8) Patients with decreased mobility have reported experiencing a lower quality of life than mobile patients with spina bifida. (21) Our data demonstrates that difficulties with ambulation also affects the larger family unit. In a multivariable linear regression model, adjusting for treatment group, the ability to walk independently at 30 months and a greater family resources score at 12 months were associated with both family impact and parental stress. We believe that it was the timing of the repair that impacts family functioning through the effect of improved mobility. Regular physical therapy and access to Early Intervention Programs is important to help mitigate some of the difficulties families face if their child is unable to ambulate independently.
This analysis has some important limitations. This study only assessed family impact and parental stress up to 30 months after delivery. It will be important to study the long term family impact. The MOMS cohort will continue to be studied under the NIH funded MOMS2 study as the children enter school. Second, while the scales used in this study are well validated and widely used to assess family impact and parental stress, the instruments are not able to address all potential impacts on families. Third, we did not assess which families utilized various supportive services and thus cannot determine the potential benefit of the specific available supportive services. Finally, in the future, it will be valuable to assess multiple family members who help care for the child. Not withstanding these limitations, this study represents the first report of family impact and parental stress of prenatal myelomeningocele repair. A major strength of the study is the randomized design. The MOMS trial gives us a unique and unprecedented opportunity to compare family impact between families who were randomized to prenatal versus postnatal repair. Furthermore, our 30-month follow-up rate was excellent which greatly reduced any potential selection bias due to loss to follow-up.
CONCLUSIONS
Prenatal surgery for myelomeningocele has been demonstrated to have benefits over postnatal surgery. Yet, it is important to emphasize that not all families benefited and the potential benefit must be weighed against both the maternal and fetal risks. Furthermore, maternal-fetal surgery requires a significant emotional, physical, and financial commitment from the pregnant woman and her family. A woman’s ability to undergo maternal-fetal surgery is dependent on the social resources that promote familial resilience. Women who are candidates for maternal-fetal surgery should be counseled that prenatal repair is associated with less negative family impact than postnatal repair and that the timing of repair is not associated with increased parental stress at 12 or 30 months. These data support the importance of comprehensive assessment to identify and recognize stressors within the family unit and to provide social support for families who care for children with spina bifida. (22)
TABLE 4.
Parental Stress at 12 and 30 months after birth.
| Parental Stress Index – Short Form | |||||
|---|---|---|---|---|---|
| 12 months* | 30 months* | p-value** | |||
| Prenatal Surgery (N=84) |
Postnatal Surgery (N=88) |
Prenatal Surgery (N=87) |
Postnatal Surgery (N=88) |
||
| Total stress | 56.3 (13.7) | 59.4 (14.2) | 61.3 (21.3) | 60.3 (15.4) | 0.89 |
| Score range 36–180 | |||||
| Parental distress | 21.3 (6.3) | 22.7 (7.1) | 22.3 (9.5) | 22.3 (8.4) | 0.72 |
| Score range 12–60 | |||||
| Dysfunctional interaction |
15.9 (4.6) | 16.5 (4.3) | 16.5 (6.5) | 16.2 (4.0) | 0.91 |
| Score range 12–60 | |||||
| Difficult child | 19.1 (5.2) | 20.2 (5.5) | 22.6 (8.1) | 21.8 (6.2) | 0.85 |
| Score range 12–60 | |||||
| Defensive responding | 12.3 (4.0) | 13.5 (4.5) | 13.3 (5.8) | 13.3 (5.3) | 0.58 |
| Score range 12–60 | |||||
Data presented as means (standard deviation)
Based on infants with assessments at both 12 and 30 months
Acknowledgments
FUNDING: Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U10-HD041669, U10-HD041667, U10-041666, and U01-HD041665) and the National Institute of Diabetes and Digestive and Kidney Diseases. Funded by the National Institutes of Health (identifier NCT00060606).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors indicate they have no potential conflicts of interest to disclose.
REFERENCES
- 1.Detrait ER, George TM, Etchevers HC, Gilbert JR, Vekemans M, Speer MC. Human neural tube defects: Developmental biology, epidemiology, and genetics. Neurotoxicol Teratol. 2005;27:515–524. doi: 10.1016/j.ntt.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parker SE, Mai CT, Canfield MA, et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res A Clin Mol Teratol. 2010;88:1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell LE, Adzick NS, Melchionne J, et al. Spina bifida. Lancet. 2004;364:1885–1895. doi: 10.1016/S0140-6736(04)17445-X. [DOI] [PubMed] [Google Scholar]
- 4.Holmbeck GN. J Consult Clin Psychol. 2010;78:511–525. doi: 10.1037/a0019599. [DOI] [PubMed] [Google Scholar]
- 5.Vermaes IP, Gerris JR, Janssens JM. Parents' social adjustment in families of children with spina bifida: a theory-driven review. J Pediatr Psychol. 2007;32:1214–1226. doi: 10.1093/jpepsy/jsm054. [DOI] [PubMed] [Google Scholar]
- 6.Tulipan N, Bruner JP, Hernanz-Schulman M, et al. Effect of intrauterine myelomeningocele repair on central nervous system structure and function. Pediatr Neurosurg. 1999;31:183–188. doi: 10.1159/000028859. [DOI] [PubMed] [Google Scholar]
- 7.Sutton LN, Adzick NS, Bilaniuk LT, Johnson MP, Crombleholme TM, Flake AW. Improvement in hindbrain herniation demonstrated by serial fetal magnetic resonance imaging following fetal surgery for myelomeningocele. JAMA. 1999;282:1826–1831. doi: 10.1001/jama.282.19.1826. [DOI] [PubMed] [Google Scholar]
- 8.Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med. 2011;364:993–1004. doi: 10.1056/NEJMoa1014379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindemann H. Why families matter. Pediatrics. 2014;134:S97–S103. doi: 10.1542/peds.2014-1394E. [DOI] [PubMed] [Google Scholar]
- 10.Janvier A, Farlow B, Wilfond BS. The experience of families with children with trisomy 13 and 18 in social networks. Pediatrics. 2012;130:293–298. doi: 10.1542/peds.2012-0151. [DOI] [PubMed] [Google Scholar]
- 11.Houtrow AJ, Yock TI, Delahaye J, Kuhlthau K. The family impacts of proton radiation therapy for children with brain tumors. J Pediatr Oncol Nurs. 2012;29:171–179. doi: 10.1177/1043454212446345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C, Jeruss S, Terrin N, Tighiouart H, Wilson JM, Parsons SK. Impact on family of survivors of congenital diaphragmatic hernia repair: a pilot study. J Pediatr Surg. 2007;42:1845–1852. doi: 10.1016/j.jpedsurg.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Vermaes IP, Janssens JM, Bosman AM, Gerris JR. Parents' psychological adjustment in families of children with Spina Bifida: a meta-analysis. BMC Pediatrics. 2005;5:1–13. doi: 10.1186/1471-2431-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmbeck GN, Devine KA. Psychosocial and family functioning in spina bifida. Dev Disabil Res Rev. 2010;16:40–46. doi: 10.1002/ddrr.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein RE, Riessman CK. The development of an impact-on-family scale: preliminary findings. Med Care. 1980;18:465–472. doi: 10.1097/00005650-198004000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Stein RE, Jessop DJ. The impact on family scale revisited: further psychometric data. J Dev Behav Pediatr. 2003;24:9–16. [PubMed] [Google Scholar]
- 17.Williams AR, Piamjariyakul U, Williams PD, Bruggeman SK, Cabanela RL. Validity of the revised Impact on Family (IOF) scale. J Pediatr. 2006;149:257–261. doi: 10.1016/j.jpeds.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Abidin RR. Parenting Stress Index. 3rd. Odessa, FL: Psychological Assessment Resources; 1995. [Google Scholar]
- 19.Dunst CJ, Jenkins V, Trivette CM. Family Support Scale Reliability and Validity. Asheville, NC: Winterberry Press; 2007. [Google Scholar]
- 20.Dunst CJ, Leet HE. Measuring the adequacy of resources in households with young children. Child Care Health Dev. 1987;3:111–125. doi: 10.1111/j.1365-2214.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]
- 21.Danielsson AJ, Bartonek A, Levey E, McHale K, Sponseller P, Saraste H. Associations between orthopaedic findings, ambulation and health-related quality of life in children with myelomeningocele. J Child Orthop. 2008;2:45–54. doi: 10.1007/s11832-007-0069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rofail D, Maguire L, Kissner M, Colligs A, Abetz-Webb L. A review of the social, psychological, and economic burdens experienced by people with spina bifida and their caregivers. Neurol Ther. 2013;2:1–12. doi: 10.1007/s40120-013-0007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]