Structured Abstract
Objective
MPNSTs are an aggressive group of soft tissue sarcomas that can arise sporadically, in the context of NF1, or at a site of prior irradiation. Large series profiling the features and outcomes of sporadic, neurofibromatosis type 1 (NF1)-associated, and radiation (RT)-associated malignant peripheral nerve sheath tumor (MPNST) are limited. The goal of this study was to elucidate differences between MPNST etiologies in a large single-institution retrospective study.
Methods
Patients (n = 317) were identified through our institutional tumor registry. Clinicopathologic features were retrospectively collected. Features were compared among MPNST subtypes for patients who had sufficient clinical history (n = 289), and clinicopathologic features were used to identify adverse predictors of recurrence and survival outcomes.
Results
Five-year local recurrence-free survival (LRFS), distant recurrence-free survival (DRFS), and disease-specific survival (DSS) estimates were 56.6%, 49.6%, and 53.6% for the high-grade MPNST cohort, respectively. Five-year DSS was lower in NF1-associated and RT-associated compared to sporadic MPNST (48.7%, 40.9%, and 63.0%, respectively; p = 0.140). RT-associated MPNST had worse LRFS than sporadic and NF1-associated subtypes (p = 0.047). Truncally located tumors, positive surgical margins, local recurrence, and metastasis were predictors of adverse DSS in multivariate analysis.
Conclusion
RT-associated MPNSTs demonstrate poorer local recurrence-free and disease-specific survival than sporadic and NF1-associated tumors. NF1-associated MPNSTs may have worse survival outcomes owing to large tumor size, compromising truncal location, and lower rate of negative resection margins compared to sporadic tumors.
Keywords: Malignant peripheral nerve sheath tumor, MPNST, neurofibromatosis type 1, radiation-induced sarcoma
Introduction
Accounting for 2–5% of soft tissue sarcomas, malignant peripheral nerve sheath tumors (MPNSTs) are a complex group of tumors that arise from the peripheral nerve sheath. 4,9 Approximately 40–50% of MPNSTs arise within the setting of neurofibromatosis type 1 (NF1), a highly penetrant autosomal dominant genetic disorder caused by a loss of function mutation in the NF1 gene. The lifetime incidence of malignant transformation of existing neurofibromas in NF1 patients is approximately 10%.8,24 An additional 40–47% of MPNSTs develop sporadically and 10–13% arise in a prior field of therapeutic radiation.8,15 Management of these tumors is challenging, as the benefit of chemotherapy has not been widely demonstrated and the success of radiotherapy for local control has not consistently been reported.1,7,19,23,25 Negative margin resection – often impeded by large tumor size and extensive nerve involvement – remains the mainstay of curative treatment.3,11 MPNSTs have a high propensity for local relapse without complete surgical resection and a high risk for metastatic spread. Prognosis in patients with MPNST remains poor with reported 5-year disease-specific survival rates ranging from 39–60% in multiple single-institution series over the last 15 years.1,7,19,23,25 However, some small studies have demonstrated superior survival in patients with low-grade variants of MPNST, suggesting a benign natural history in these rare lesions compared to their high-grade counterparts.2,22 Whether NF1 is adversely associated with survival remains controversial.14,19,23 Additionally, studies comparing outcomes of radiation-associated (RT-associated) MPNST to other subtypes are sparse as a consequence of their rarity.15 The objective of this study was to identify adverse predictors of recurrence and survival in patients with sporadic, NF1-associated, and RT-associated MPNST. As a secondary goal, low-grade MPNSTs were interrogated separately from high-grade MPNSTs to determine their natural history and optimal clinical management.
Methods
With approval of The University of Texas MD Anderson Cancer Center Institutional Review Board, a retrospective database containing 317 patients with a pathologically confirmed MPNST diagnosis between 1990 and 2014 was constructed. Patients that had adequate clinical history (n = 289) were included for statistical analysis (Figure 1). Clinicopathologic characteristics were collected in a comprehensive medical record review. A diagnosis of sporadic MPNST was made as previously described and in the absence of NF1 or prior local radiotherapy for a different malignancy.25 A tumor was classified as NF1-associated if documented genetic testing confirmed a germline NF1 mutation or by clinical evaluation based on the 1987 NIH consensus criteria for diagnosis of NF1.18 Patients with a history of therapeutic radiotherapy at least six months prior for an unrelated malignancy within the local field containing the newly arising MPNST were considered to have a RT-associated MPNST.10
Figure 1.
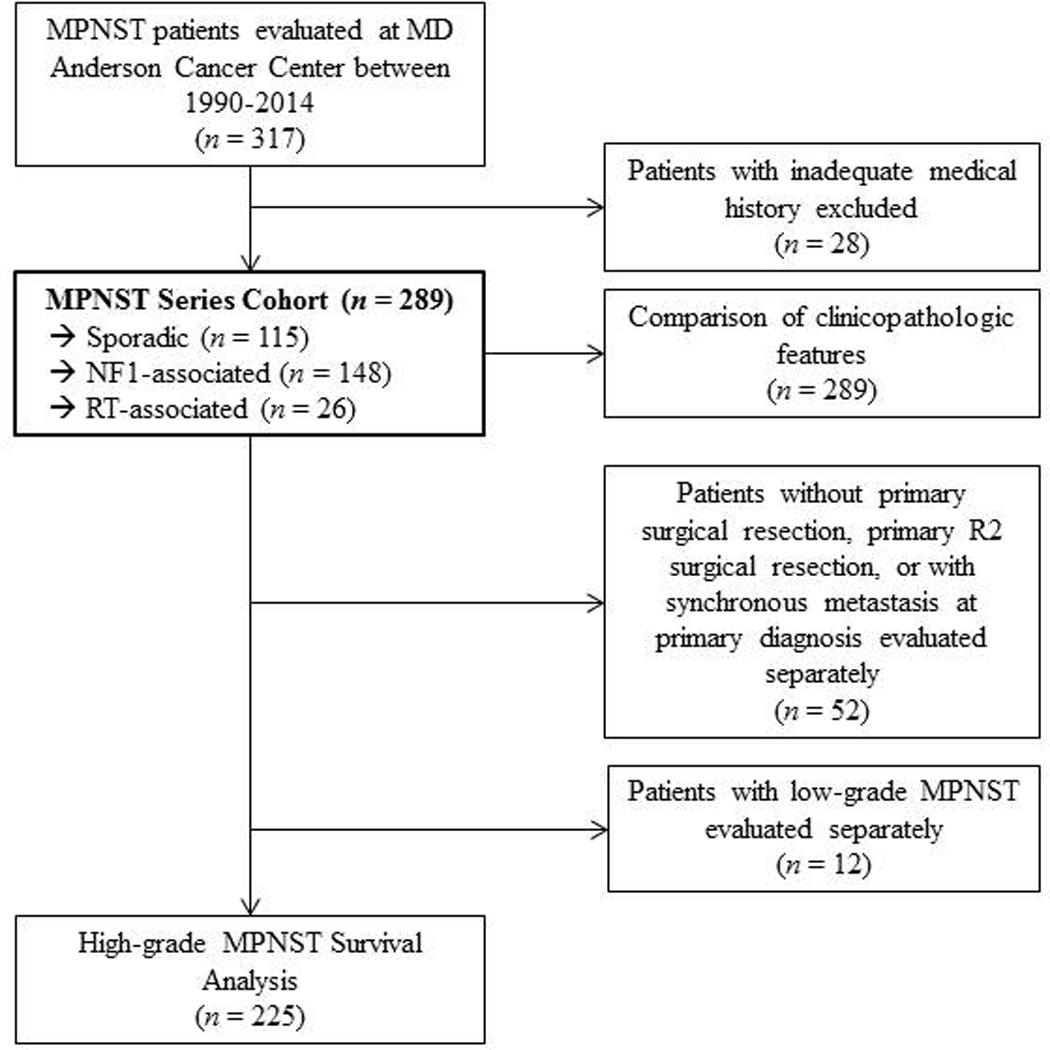
Patient selection and study analysis flowchart
Resection margins were obtained from pathology notes. A resection margin was considered microscopically negative (R0) if the closest margin was > 1 mm from the inked surface, microscopically positive (R1) if the closest margin was ≤ 1 mm from the inked surface, or macroscopically positive (R2) for any subtotal resection where gross disease was present at inked margins. Tumors were graded as high or low on the basis of nuclear atypia, presence of hyperchromasia, mitotic activity, cellularity, and growth pattern.6,21 Select studies have demonstrated more favorable outcomes in low-grade MPNSTs compared to high grade MPNSTs; therefore, we reviewed patients with low-grade MPNSTs independently (n =12).2,22 Patients in the high-grade cohort who did not receive surgical intervention as part of their primary treatment (n = 22) or who received subtotal R2 resection (n = 23) were evaluated independently owing to their anticipated poor prognosis. Only patients who had primary tumors in the absence of synchronous metastasis at diagnosis were included in the outcomes analysis (Figure 1).
Characteristics between MPNST subtypes were compared using χ2 or Fisher’s exact test for categorical variables and the non-parametric Mann-Whitney U test or Kruskal-Wallis test for continuous variables, as appropriate. Log-rank methods were employed to estimate local recurrence-free survival (LRFS), distant recurrence-free survival (DRFS), and disease-specific survival (DSS) outcomes. A disease-specific event was recorded if the patient experienced MPNST-specific death. Multivariate Cox regression models were constructed by including any variables with statistical significance below a p value cutoff of 0.05 at the univariate level. All two-sided p values < 0.05 were considered statistically significant. All computations were performed using SPSS version 22.0 (IBM Corp. Armonk, NY).
Results
Patient characteristics and presentation
A total of 289 patients with MPNST treated at our institution between 1990 and 2014 were included. Tumor and demographic characteristics of these patients are summarized in Table 1. Median follow-up time was 2.13 years (range, 0.05–36.0 years), 2.18 years (range, 0.03–29.8 years), and 1.74 years (range, 0.25–13.7 years) for sporadic, NF1-associated, and RT-associated MPNSTs, respectively. One-hundred fifteen (40%) patients presented with sporadic disease, 148 (51%) in association with NF1, and 26 (9%) subsequent to previous local radiation therapy for a different malignancy. The median latency period for the development of RT-associated MPNST from prior irradiation was 16 years (range, 0.86 – 37 years); the most common indications for prior radiotherapy were breast carcinoma (33%) and Hodgkin’s lymphoma (22%).
Table 1.
Patient and tumor characteristics of 289 patients with MPNST
| N (%) of column total | Overall | Sporadic | NF1-associated | RT-associated | P Valuea (P Value)b |
|---|---|---|---|---|---|
| 289(100) | 115(39.8) | 148(51.2) | 26(8.9) | ||
| Median age, years (range) | 37(1–81) | 43(5–81) | 33(1–75) | 52(20–77) | <0.001 (<0.001) |
| Median tumor size, cm (range) | 8.0(1.0–35.0) | 6.0(1.0–32.0) | 10(2.5–35.0) | 6.0(1.5–15.0) | <0.001 (<0.001) |
| Sex | |||||
| Female | 134(46.4) | 52(45.2) | 68(45.9) | 14(53.8) | 0.720 (0.906) |
| Male | 155(53.6) | 63(54.8) | 80(54.1) | 12(46.2) | |
| Disease at primary diagnosis | |||||
| Localized | 274(94.8) | 105(91.3) | 143(96.6) | 26(100.0) | 0.071 (0.065) |
| Metastatic | 15(5.2) | 10(8.7) | 5(3.4) | 0(0.0) | |
| Location | |||||
| Head/neck | 43(14.9) | 23(20.0) | 13(8.8) | 7(26.9) | 0.008c (0.211) |
| Cervical nerve root | 15(5.2) | 4(3.5) | 5(3.4) | 6(23.0) | |
| Cranial nerve | 13(4.5) | 8(6.9) | 4(2.7) | 1(3.8) | |
| Other nerve | 9(3.1) | 8(6.9) | 1(0.7) | 0(0.0) | |
| Nerve unknown | 6(2.1) | 3(2.6) | 3(2.0) | 0(0.0) | |
| Trunk | 157(54.3) | 54(47.0) | 85(57.4) | 18(69.2) | |
| Brachial plexus trunks/divisions | 21(7.3) | 5(4.3) | 9(6.1) | 9(34.6) | |
| Brachial plexus cords | 9(3.1) | 4(3.5) | 2(1.3) | 3(11.5) | |
| Lumbosacral nerve root | 37(12.8) | 19(16.5) | 18(12.2) | 0(0.0) | |
| Thoracic nerve root | 17(5.9) | 3(2.6) | 12(8.1) | 2(7.6) | |
| Sciatic nerve | 12(4.2) | 1(0.8) | 10(6.8) | 1(3.8) | |
| Phrenic | 4(1.4) | 1(0.8) | 3(2.0) | 0(0.0) | |
| Obturator | 4(1.4) | 0(0.0) | 4(2.7) | 0(0.0) | |
| Femoral | 6(2.7) | 2(1.7) | 3(2.0) | 1(5.9) | |
| Other nerve | 25(8.7) | 7(6.1) | 16(10.8) | 2(11.8) | |
| Nerve unknown | 20(6.9) | 12(10.4) | 8(5.4) | 0(0.0) | |
| Upper extremity | 26(8.9) | 13(11.3) | 12(8.1) | 1(3.8) | |
| Radial nerve | 4(1.4) | 2(1.7) | 2(1.3) | 0(0.0) | |
| Median nerve | 7(2.4) | 3(2.6) | 3(2.0) | 1(3.8) | |
| Ulnar nerve | 7(2.4) | 3(2.6) | 4(2.7) | 0(0.0) | |
| Other nerve | 4(1.4) | 3(2.6) | 1(0.6) | 0(0.0) | |
| Nerve unknown | 4(1.4) | 2(1.7) | 2(1.3) | 0(0.0) | |
| Lower extremity | 63(21.8) | 25(21.7) | 38(25.7) | 0(0.0) | |
| Sciatic nerve | 25(8.7) | 6(5.2) | 19(12.8) | 0(0.0) | |
| Femoral nerve | 7(2.4) | 3(2.6) | 4(1.4) | 0(0.0) | |
| Obturator nerve | 2(0.7) | 0(0.0) | 2(1.3) | 0(0.0) | |
| Tibial nerve | 9(3.1) | 5(4.3) | 4(2.7) | 0(0.0) | |
| Other nerve | 10(3.5) | 3(2.6) | 6(4.1) | 0(0.0) | |
| Nerve unknown | 10(3.5) | 8(6.9) | 2(1.3) | 0(0.0) | |
| Depth d | |||||
| Deep | 272(94.1) | 104(90.4) | 143(96.6) | 25(96.2) | 0.096 (0.037) |
| Superficial | 17(5.9) | 11(9.6) | 5(3.4) | 1(3.8) | |
| Histologic subclassification | |||||
| MPNST, not further classified | 258(89.3) | 94(81.7) | 140(94.6) | 24(92.3) | 0.001 (<0.001) |
| Epithelioid | 20(6.9) | 17(14.8) | 2(1.4) | 1(3.8) | |
| Triton tumor | 11(3.8) | 4(3.5) | 6(4.1) | 1(3.8) | |
| Grade | |||||
| Low-intermediate | 12(4.2) | 6(5.2) | 5(3.4) | 1(3.8) | 0.791 (0.480) |
| High (or not evaluated) | 277(95.8) | 109(94.8) | 143(96.6) | 25(96.2) | |
Percentages may not add to 100 due to rounding. Bold indicates statistical significance of p < 0.05
Three group comparison between sporadic, NF1-associated, and RT-associated characteristics
Two group comparison between sporadic and NF1-associated characteristics
Comparisons between four major anatomical locations (head/neck, trunk, upper extremity, lower extremity)
Tumors with extension through or seated below the superficial fascia were considered deep.
The diagnosis of MPNST was established histologically in all cases (MPNST, 89%; epithelioid MPNST, 7%; triton tumor, 4%). Primary disease was most commonly located in the trunk (54%), followed by the extremities (31%), but location varied between MPNST subtypes (p = 0.008). The most commonly affected nerve for truncally located disease was within the lumbosacral nerve roots (n = 37/157, 24%) followed by the brachial plexus (n = 30/157, 19%) and the thoracic nerve roots (n = 17/157, 11%). The sciatic nerve was affected in 8% (n = 12/157) of truncally located cases and 40% (n = 25/63) of lower extremity cases; 50% (n = 19/38) of NF1-associated MPNSTs located within the lower extremity arose from the sciatic nerve. Symptoms at primary diagnosis were available for 245 (85%) patients; 16% (n = 38/245) of patients were asymptomatic but were able to palpate mass effect and 2% (n = 4/245) were asymptomatic with incidental discovery of their disease. Two-hundred and three patients of 245 (83%) patients were symptomatic and 49% (n = 120/245) reported more than one symptom. The most common symptom was pain (71%, n = 173/245), followed by extremity weakness (13%, n = 32/245), extremity numbness (9%, n = 22/245), paresthesia (6%, n = 14/245), and gait instability or footdrop (5%, n = 13/245). There were no statistically significant differences between symptoms at presentation and MPNST subtype; however, patients with NF1-associated tumors were more likely to be symptomatic at presentation than patients with sporadic or RT-associated tumors (90% versus 75% and 73%, respectively; p = 0.006).
Twelve (4 %) tumors were determined to be low grade. Low grade MPNSTs were more likely to be superficially located compared to high grade MPNSTs (31% versus 5% in low-grade and high-grade MPNST, respectively; p = 0.004). All NF1-associated low grade MPNSTs arose within a neurofibroma.
Treatment Characteristics
Two-hundred and sixty-seven (92%) patients had surgical resection of primary disease. One-hundred and fifty-nine (59%) patients underwent definitive resection of their primary tumor at outside institutions and 108 (41%) were excised at our institution. The margin status was reviewed by a sarcoma pathologist at our institution. The remaining patients did not receive surgical resection due to advanced disease (n = 17) or medical comorbidities (n = 5). Nine (3%) patients with localized primary tumors required amputation and an additional 6 (2%) required amputation after local recurrence (LR). Twenty-three (8%) patients received R2 resections (Table 2). Of these, 17 were resected with curative intent; however, additional resection to obtain negative margins was not pursued owing to rapidly progressive disease (n = 9) or prohibitive location (n = 8). Median time to disease-specific death following incomplete (R2) resection was 0.8 years. Six patients received palliative R2 resection. Indications for palliative resection were pain (n = 3), spinal cord compression (n = 2), and bowel obstruction (n = 1). Five of 6 patients reported temporary alleviation of symptom; however, all patients had progressive disease resulting in death following palliative procedures. Median time to disease-specific death following palliative (R2) resection was 1.19 years.
Table 2.
Treatment characteristics of 274 patients with localized disease at presentation
| N (%) column total | Overall | Sporadic | NF1- associated |
RT- associated |
P Valuea (P Value)b |
|---|---|---|---|---|---|
| 274 (100) | 105 (38.3) | 143 (52.2) | 26 (9.5) | ||
| Surgery | |||||
| Yes | 259 (94.5) | 102 (97.1) | 133 (93.0) | 24 (92.3) | 0.321 (0.248) |
| No | 15 (5.5) | 3 (2.9) | 10 (7.0) | 2 (92.3) | |
| Resection Margins (n = 259) | |||||
| R0 | 145 (60.0) | 66 (64.7) | 70 (52.6) | 9 (37.5) | <0.001c (<0.001) |
| R1 | 54 (20.8) | 13 (12.7) | 36 (27.1) | 5 (20.8) | |
| R2 | 23 (8.9) | 7 (6.9) | 9 (6.8) | 7 (29.2) | |
| Unknown | 37 (14.3) | 16 (15.7) | 18 (13.5) | 3 (12.5) | |
| Therapy-surgically resected (n = 259) | 0.312d (0.590) | ||||
| Surgery alone | 75(27.3) | 31(29.5) | 36(25.2) | 8(30.8) | |
| Chemotherapy alone + surgery | 45(16.4) | 19(18.1) | 19(13.3) | 7(26.9) | |
| Neoadjuvant | 18(6.6) | 5(4.7) | 10(6.9) | 3(11.5) | |
| Adjuvant | 26(9.5) | 14(13.3) | 9(6.3) | 3(11.5) | |
| Both neo-/adjuvant | 1(0.3) | 0(0.0) | 0(0.0) | 1(3.8) | |
| Radiation alone + surgery | 75(27.4) | 29(27.6) | 39(27.3) | 7(26.9) | |
| Neoadjuvant | 11(4.0) | 3(2.8) | 8(5.9) | 0(0.0) | |
| Adjuvant | 63(22.9) | 26(24.8) | 31(21.7) | 6(23.1) | |
| Other (IORT) | 1(0.3) | 0(0.0) | 0(0.0) | 1(3.8) | |
| Chemoradiotherapy + surgery | 64(24.4) | 23(21.9) | 39(27.3) | 2(7.7) | |
| Neoadjuvant | 22(8.0) | 8(7.6) | 14(9.8) | 0(0.0) | |
| Adjuvant | 29(10.6) | 14(13.3) | 14(9.8) | 1(3.8) | |
| Other combination | 13(4.7) | 1(1.0) | 11(7.7) | 1(3.8) | |
| Therapy-no surgical resection (n = 15) | 0.240 (0.296) | ||||
| Palliative chemotherapy | 9 (3.3) | 3 (2.9) | 5 (3.5) | 1 (3.8) | |
| Palliative radiotherapy | 1 (0.3) | 0 (0.0) | 1 (0.6) | 0 (0.0) | |
| Palliative chemoradiotherapy | 5 (1.8) | 0 (0.0) | 4 (2.7) | 1 (3.8) | |
| Local recurrence | 98 (35.7) | 33 (31.4) | 51 (35.7) | 14 (53.8) | 0.102 (0.486) |
| Distant metastasis | 120 (43.8) | 47 (44.8) | 65 (45.5) | 8 (30.8) | 0.369 (0.914) |
Abbreviations: IORT, intraoperative radiotherapy. Percentages may not add to 100 due to rounding. Bold indicates statistical significance of p < 0.05
Three group comparison between sporadic, NF1-associated, and RT-associated characteristics
Two group comparison between sporadic and NF1-associated characteristics
Comparisons exclude unknown resection margins
Overall comparison between surgery alone, chemotherapy + surgery, radiation + surgery, or chemoradiotherapy + surgery.
Two-hundred and seventy-four (95%) patients presented with localized disease; of which, 75 (27%) underwent surgical resection alone (Table 2). In addition to surgical resection, 45 (16%) patients received chemotherapy, 75 (27%) patients received radiation, 64 (24%) patients received combination chemo-radiotherapy, and 15 (5%) underwent palliative chemo- or radiotherapy without surgical resection. Chemotherapy regimens varied, but most often included doxorubicin +/− ifosfamide as a first line therapy; others included gemcitabine +/− docetaxel, cisplatin, dacarbazine, cyclophosphamide, epirubicin, vincristine, etoposide, and pazopanib.
Of 15 (5%) patients with metastasis at initial presentation, 7 (2%) received systemic therapy, 4 (1%) received chemotherapy in addition to surgical resection, and 4 (1%) received combination chemo-radiotherapy in addition to resection. Review of the patients with metastatic disease who underwent surgery revealed that two patients required surgical intervention to provide palliation. One patient presented with intense neck pain and the second patient had urinary and bowel obstruction. Six patients underwent resection at outside hospitals; of which, 5 had staging imaging at the time of surgery and were known to have metastatic disease. The median survival of patients with metastatic disease who underwent surgery was 0.78 years versus 1.73 years for those who were treated with systemic therapy.
Low-grade MPNST outcomes
Only 3 of 12 (25%) patients with low-grade MPNST developed LR after surgical resection (Table 3). R0 resection was achieved in 1 of 3 recurring cases and 7 of 9 non-recurring cases. No patients with low-grade lesions developed metastasis. Five-year DSS outcomes were superior to all high-grade cohort outcomes with 100% of patients surviving at the end of the study period (Figure 2).
Table 3.
Characteristics and survival in 12 low-grade MPNSTs
| Subtype | Age | Location | Neurofibroma component |
Depth | Therapy | Surgical Margins |
LR | DR | DOD |
|---|---|---|---|---|---|---|---|---|---|
| NF1 | 26 | Thoracic spine | Spinal | D | None | R0 | − | − | − |
| NF1 | 39 | Intercostal nerve | Plexiform | D | None | R0 | − | − | − |
| NF1 | 47 | Sciatic nerve, thigh | Diffuse | D | None | R0 | − | − | − |
| NF1 | 43 | Brachial plexus | Plexiform | D | None | R1 | − | − | − |
| NF1 | 69 | Back | Plexiform | S | RT | R1 | − | − | − |
| RAS | 60 | Brachial plexus | None | D | None | R2 | + | − | − |
| Sporadic | 68 | Chest | None | S | RT | R0 | − | − | − |
| Sporadic | 13 | Forehead | None | S | None | R1 | + | − | − |
| Sporadic | 60 | Lumbosacral | None | D | CT | R0 | + | − | − |
| Sporadic | 40 | Peritoneum | None | D | None | R0 | − | − | − |
| Sporadic | 64 | Shoulder | None | S | None | R0 | − | − | − |
| Sporadic | 79 | Retroperitoneum | None | D | CT | R0 | − | − | − |
Abbreviations: CT, chemotherapy; D, deep; DOD, MPNST-specific death; DR, distant recurrence; LR, local recurrence; RAS, radiation-associated; RT, radiation therapy; S, superficial
Figure 2.
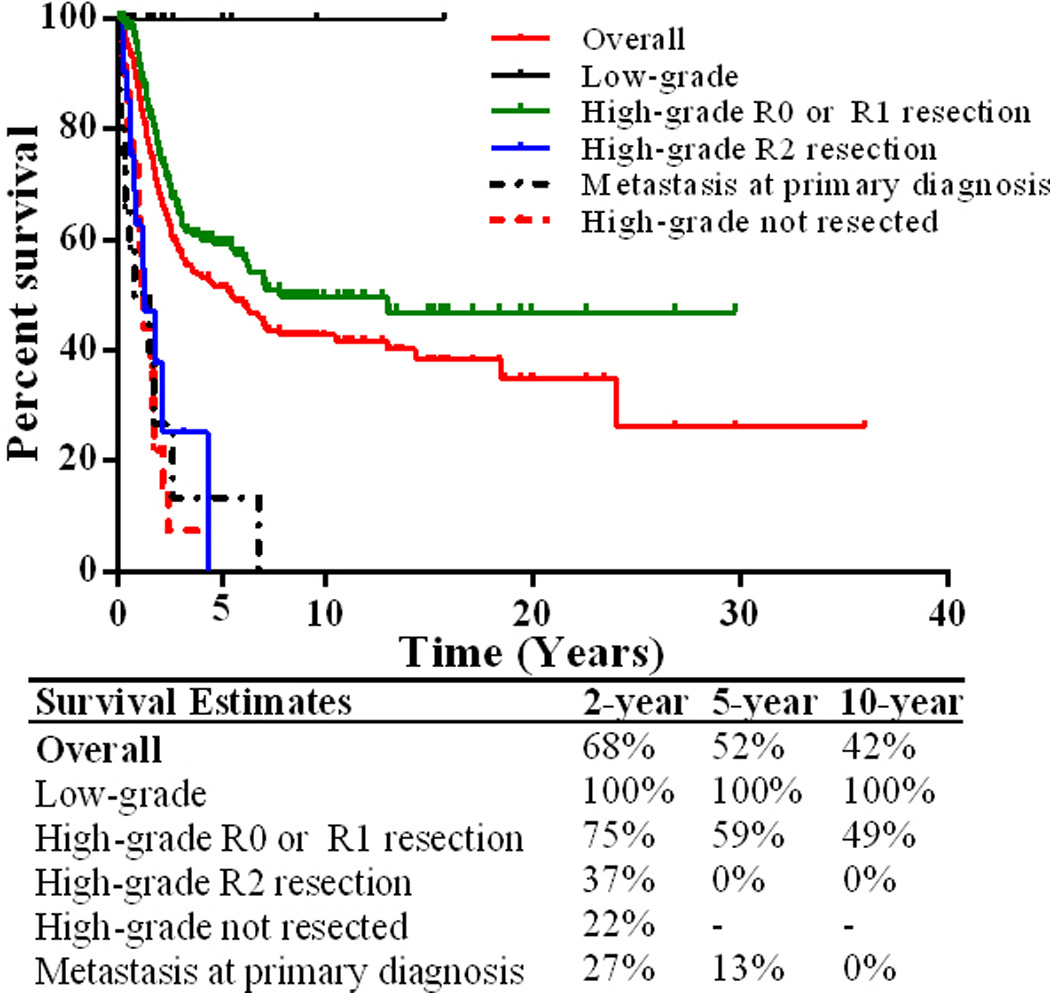
Kaplan-Meier curves of disease-specific survival for MPNST populations
High-grade MPNST local and distant recurrence-free survival
Local and distant recurrence outcomes were assessed in 225 (sporadic, n = 89; NF1-associated, n = 119; RT-associated, n = 17) patients who presented with high-grade localized disease and received R0 or R1 surgical resection. After a median follow-up of 2.7 years, range 0.03–36.0 years, 84 (37%) patients developed LR (34%, 38%, and 53% for sporadic, NF1-associated, and RT-associated subtypes, respectively) after resection. Median time to LR was 0.95 years. Nine patients had amputation; 3 patients recurred at the stump. Of the 84 (37%) patients that experienced LR, 18 (21%) patients received surgical resection alone for recurrent disease, 45 (42%) received chemotherapy or radiotherapy in addition to surgical resection, and 21 (9.3%) did not receive salvage intervention. Comparison of patients that underwent resection at our institution versus outside institutions did not reveal any statistically significant difference in LRFS (3-yr LRFS following R0 resection 84% v. 77%, p = 0.286; 3-yr LRFS following R1 resection 48% v. 38%, p = 0.425).
One-hundred and five (47%) patients developed distant recurrence (47%, 47%, and 41% for sporadic, NF1-associated, and RT-associated subtypes, respectively). Median time to distant recurrence was 1.4 years. Thirty-one percent (n = 33/105) of patients had metastases to multiple organs. The most common locations included the lungs (n = 85), paraspinal region (n = 12), bone (n = 10), liver (n = 5), lymph nodes (n = 8), brain (n = 7), pelvis (n = 5) and leptomeningeal metastasis (n = 4). Less common sites involved the mediastinum, retroperitoneum, musculature of the extremities, bladder, vagina, and spleen.
Univariate analyses for LRFS and DRFS revealed no significant differences in outcomes between neoadjuvant and adjuvant radiation or chemotherapy. Therefore, neoadjuvant and adjuvant therapy variables were combined to strengthen statistical power (Table 4). Five-year LRFS was 59%. Univariate analysis revealed sporadic tumors had better LRFS compared to RT-associated tumors (p = 0.010, HR 0.43; Table 4, Figure 3). Patients that received radiation therapy in addition to surgical resection had a better LRFS compared to patients that did not receive radiation (p = 0.040, HR 0.64; Table 4). In multivariate analysis, tumors ≥ 10 cm and positive margins remained significant prognosticators of adverse LRFS (p = 0.056, HR 2.09; p < 0.001 HR 3.13; Table 4).
Table 4.
Univariate and multivariate analysis of local recurrence-free and distant recurrence-free survival
| Local recurrence-free survival | Distant recurrence-free survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Age, ≥ 45 v. < 45 | 1.03 (0.65–1.63) | 0.888 | 1.36 (0.91–2.01) | 0.132 | ||||
| Sex, female v. male | 0.59 (0.38–0.91) | 0.018 | 0.65 (0.30–1.39) | 0.269 | 0.63 (0.42–0.93) | 0.022 | 0.83 (0.50–1.35) | 0.450 |
| Subtype | ||||||||
| Sporadic v. NF1-associated | 0.85 (0.54–1.33) | 0.463 | 0.85 (0.58–1.25) | 0.413 | ||||
| Sporadic v. RT-associated | 0.42 (0.23–0.82) | 0.010 | 0.36 (0.12–1.79) | 0.082 | 1.17 (0.54–2.51) | 0.686 | ||
| Histology, other a v. MPNST | 0.86 (0.43–1.71) | 0.658 | 0.46 (0.21–1.00) | 0.050 | 0.62 (0.26–1.44) | 0.266 | ||
| Size, ≥ 10 cm v. < 10 cm | 2.26 (1.23–4.16) | 0.008 | 2.09 (0.98–4.49) | 0.056 | 2.06 (1.26–3.36) | 0.004 | 1.76 (1.04–2.97) | 0.034 |
| Location, trunk v. other b | 2.01 (1.29–3.14) | 0.002 | 1.43 (0.67–3.04) | 0.353 | 1.06 (0.72–1.57) | 0.749 | ||
| Depth, deep v. superficial | 3.25 (0.79–13.2) | 0.100 | 9.39 (1.31–67.5) | 0.026 | 1.79 (0.93–15.3) | 0.566 | ||
| Chemotherapy, yes v. no | 0.90 (0.57–1.38) | 0.599 | 1.51 (1.02–2.22) | 0.037 | 1.15 (0.68–1.94) | 0.596 | ||
| Radiation therapy, yes v. no | 0.64 (0.41–0.98) | 0.040 | 0.84 (0.41–1.68) | 0.619 | 0.84 (0.56–1.24) | 0.365 | ||
| Margins, positive v. negative | 3.62 (2.18–6.04) | <0.001 | 3.13 (1.56–6.25) | <0.001 | 0.97 (0.58–1.62) | 0.631 | ||
Abbreviations: P, P Value. Bold indicates statistical significance p < 0.05.
Epithelioid type or triton tumor
extremity or head/neck
Figure 3.
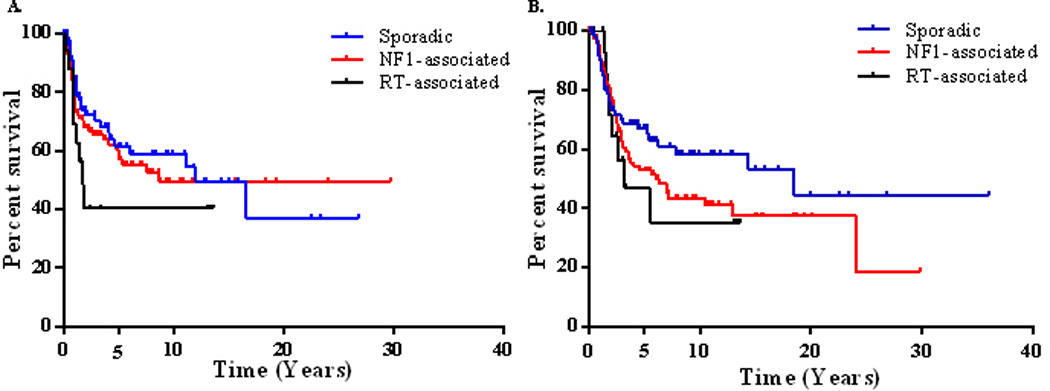
Kaplan-Meier curves for (left) local recurrence-free survival stratified by MPNST subtype (overall comparison, p = 0.278), and (right) disease-specific survival stratified by MPNST subtype (overall comparison, p = 0.129)
At our institution, radiation therapy is typically not offered to patients that present with RT-associated MPNST as they cannot be meaningfully irradiated without unacceptable toxicity in the vast majority of cases. Therefore, an alternative local relapse outcome analysis including patients that were treated at our institution for their primary sporadic or NF1-associated disease was constructed to evaluate the utility of radiation therapy in this group (Table 5). In univariate analysis we observed a LR risk reduction in patients who received radiation and surgical resection (p = 0.058, HR 0.49). The 5- and 10-year LRFS for patients that received radiation was 82% and 71%, respectively versus 67% and 59%, respectively for those that were not radiated. Adjusted multivariate analyses for this cohort revealed positive margins and males to be associated with worse LRFS.
Table 5.
Univariate and multivariate predictors of adverse local recurrence-free survival in patients with sporadic or NF1-asscociated MPNST that presented to our institution with primary disease (n = 128)a
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, ≥ 45 v. <45 | 0.85 (0.38–1.91) | 0.691 | ||
| Sex, female v. male | 0.37 (0.17–0.82) | 0.014 | 0.34 (0.12–0.94) | 0.039 |
| Subtype, sporadic v. NF1-associated | 0.40 (0.17–0.84) | 0.036 | 0.38 (0.13–1.15) | 0.385 |
| Histology, other b v. MPNST | 0.67 (0.34–2.32) | 0.805 | ||
| Size, ≥ 10 cm v. <10 cm | 2.35 (0.92–5.99) | 0.074 | ||
| Location, trunk v. other c | 2.06 (0.99–4.27) | 0.051 | 1.17 (0.48–2.84) | 0.721 |
| Chemotherapy, yes (n = 59) v. no (n = 69) | 1.06 (0.52–2.19) | 0.862 | ||
| Radiation, yes (n = 69) v. no (n = 59) | 0.49 (0.24–1.02) | 0.058 | 0.49 (0.20–1.23) | 0.130 |
| Margins, positive v. negative | 5.08 (2.27–11.4) | <0.001 | 3.90 (1.69–8.97) | 0.001 |
Bold indicates statistical significance p < 0.05
Patients with localized primary sporadic or NF1-associated MPNST who underwent R0 or R1 resection
Epithelioid type or triton tumor
Extremity or head/neck
Five-year DRFS was 50%. Adverse predictors of distant recurrence-free survival in univariate analysis included males, non-epithelioid or triton MPNSTs, tumors ≥ 10 cm, deep location, and treatment with chemotherapeutics (Table 4). Only tumor size (≥ 10 cm) remained a significant predictor of poor DRFS in multivariate analysis (p = 0.034, HR 1.76; Table 4).
High-grade MPNST Disease-specific survival
Of the entire cohort (n = 289), 131 of patients died of disease, 17 died of unrelated causes, 86 had no evidence of active malignancy, and 55 were alive with disease at the end of the follow-up period. Median disease-specific survival time was 5.5 years and 5-year DSS was 51% (Figure 2). Patients with high-grade MPNST who had incomplete (R2) resection (n = 23), did not receive surgical intervention (n = 22), or had metastasis at presentation (n = 15) had similar outcomes with significantly worse prognosis than patients who presented with localized disease and received R0 or R1 surgical resection; therefore, these patients were excluded from subsequent univariate and multivariate survival analyses. Survival was worse for patients that had amputation compared to those that had R0 limb sparing surgery (p = 0.003, HR 4.5; 5-year DSS, 25% and 78%, respectively).
Individual interrogation between MPNST subtypes revealed superior DSS in sporadic compared to NF1-associated and RT-associated patients (p = 0.016, HR 0.59; p = 0.058, HR 0.52; Table 6, Figure 3). Truncal location, positive resection margins, local recurrence, and metastasis were adverse predictors of DSS in multivariate analysis (p = 0.039, HR 1.92; p = 0.014, HR 2.41; p = 0.059, HR 1.95; p < 0.001, HR 10.7).
Table 6.
Univariate and multivariate predictors of adverse disease-specific survival
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age, ≥ 45 v. < 45 | 1.18 (0.77–1.81) | 0.438 | ||
| Sex, female v. male | 0.66 (0.43–0.99) | 0.047 | 1.53 (0.81–2.91) | 0.194 |
| Subtype | ||||
| Sporadic v. NF1-associated | 0.59 (0.39–0.91) | 0.016 | 1.00 (0.48–2.06) | 0.997 |
| Sporadic v. RT-associated | 0.52 (0.26–1.08) | 0.058 | 1.35 (0.48–3.75) | 0.568 |
| Histology, epithelioid/Triton v. MPNST | 0.59 (0.27–1.29) | 0.192 | ||
| Size, ≥ 10 cm v. < 10 cm | 3.14 (1.88–5.23) | <0.001 | 1.78 (0.91–3.84) | 0.092 |
| Location, trunk v. extremity or head/neck | 1.56 (1.07–2.27) | 0.021 | 1.92 (1.03–3.58) | 0.039 |
| Depth, deep v. superficial | 2.37 (0.75–7.52) | 0.141 | ||
| Chemotherapy, yes v. no | 1.68 (1.12–2.53) | 0.012 | 1.46 (0.76–2.08) | 0.261 |
| Radiation therapy, yes v. no | 0.64 (0.43–0.97) | 0.035 | 0.81 (0.46–1.47) | 0.501 |
| Margins, positive v. negative | 2.14 (1.32–3.45) | 0.002 | 2.41 (1.19–4.84) | 0.014 |
| Local recurrence, yes v. no | 2.46 (1.63–3.72) | <0.001 | 1.95 (0.96–3.89) | 0.059 |
| Distant recurrence, yes v. no | 4.61 (2.83–7.52) | <0.001 | 10.7 (4.89–23.5) | <0.001 |
Bold indicates statistical significance p < 0.05.
Discussion
In the present study we aimed to identify and contrast features predictive of recurrence and survival in patients with sporadic, NF1-associated, and RT-associated MPNST. First, we found that low-grade MPNSTs have a benign natural history with a low risk of recurrence with negative margin resection and better survival outcomes compared to their high-grade counterparts. In our investigation of high-grade MPNST, the importance of negative margin resection was confirmed to be a key determinant of local control and disease-specific outcomes. We found that local recurrence patterns are more aggressive in RT-associated MPNST compared to sporadic or NF1-associated MPNST, which may in part be attributed to the propensity for these tumors to be located deeply in the trunk where negative margins were less likely to be achieved. Lower survival estimates in NF1- and RT-associated MPNST may in part be explained by their differences in location, size, and positive margin rate compared to sporadic MPNST.
A review of 6 recent similar single-institution retrospective series that had at minimum 100 patients in their cohort is summarized in Table 7. Including our series, the most common adverse prognostic factor associated with survival was large tumor size (6/7 studies), followed by positive resection margins (3/7 studies), and truncal location (2/7). Only Porter et al19 identified NF1 as an adverse prognostic factor associated with survival, while Stuckey et al23 and our study identified a trend. In our cohort, a shorter disease-specific survival trend for RT-associated MPNST was noted when compared to sporadic and NF1-associated MPNST, which supports findings by LaFemina et al15 in which RT-associated MPNST patients were found to have a poorer prognosis. Six of the 7 series investigated the impact of chemotherapy and radiotherapy on survival outcomes and only Anghileri et al1 reported radiation to be associated with a significant survival benefit.
Table 7.
MPNST: a review of select recent single-institution retrospective series
| Authors & Year | No. Patients |
5 year survival |
Adverse prognostic features | Therapy Benefit |
|||||
|---|---|---|---|---|---|---|---|---|---|
| NF1 | RAS | Location | Size | Positive Margins |
RT | CT | |||
| Present series | 289 | 52%a | T | NS | T (trunk) | S (≥ 10 cm) | S | NS | NS |
| LaFemina et al., 2013 | 105 | - | NS | S | NS | S (larger) | S | - | - |
| Fan et al., 2013 | 146 | 57% b | NS | - | NS | NS | NS | NS | NS |
| Stucky et al., 2011 | 175 | 60% a | T | - | S (trunk) | S (≥ 5cm) | NS | NS | NS |
| Zou et al., 2009 | 140 | 39% a | NS | NS | NS | S (≥ 10 cm) | NS | NS | NS |
| Porter et al., 2008 | 123 | 51% b | S | - | NS | S (> 200 ml) | NS | NS | NS |
| Anghileri et al., 2006 | 205 | 40% a | NS | - | S (trunk, HN) | S (larger) | S | S | NS |
Abbreviations: CT, chemotherapy; HN, head/neck; NF1, neurofibromatosis type1-associated MPNST; NS, not statistically significant; RAS, radiation-associated MPNST; RT, radiation therapy ; S, statistically significant; T, trend
Disease-specific survival
Overall survival
Tri-site series for a single-institution
MPNSTs pose a challenging management dilemma. Patients often present with large tumors that infiltrate multiple segments of nerve which makes obtaining negative margins and maintaining acceptable neural functionality with surgical intervention problematic. Similar to others, we found that patients that underwent resection with negative margins and tumors < 10 cm had better LRFS outcomes.6,8,10 Additionally, our study demonstrates that radiation therapy in combination with an R0/R1 resection is associated with better local control (5-year LRFS, 82% versus 67% for those that were not radiated). Despite this finding, the use of radiation failed to definitively show benefits associated to disease-specific outcomes (similar to previous reports). 1,7,19,23,25
Current chemotherapeutics have not been consistently shown to provide a significant survival benefit.1,7,19,23,25 We found that patients treated with chemotherapy had worse outcomes than those who did not receive chemotherapy. However, in our cohort chemotherapy was administered more often to patients with truncally located or large tumors and to patients with advanced disease. The therapeutic benefits of chemotherapy therefore remain unclear. Three patients had complete response to therapy, which illustrates that chemotherapy can be of significant benefit in select patients. Additional research is needed to identify predictive biomarkers for therapeutic response to improve outcomes for patients with MPNST.
Sequencing of MPNST has identified several recurring genetic aberrations (such as NF1, TP53, CDKN1B, PDGFRA, and HGF) and irregular receptor tyrosine kinase activity that could be exploited for treatment or highlight other targetable molecular dysregulations.12,17,20 However, several clinical trials using the targeted inhibitors erlotinib, sorafenib, and imatinib, among others, have been conducted in sarcoma patients, including those with MPNST, with minimal observed responses.3,8 Combination therapy to achieve a more complete signaling blockade, such as co-inhibition of signaling pathways and upstream activators; the conversion of promising preclinical targets into clinical trials, such as histone deacetylase inhibitors and PI3K/mTOR inhibitors; or further identification of targetable nodes may improve outcomes for MPNST patients.13,16,26
Our study is limited by its retrospective nature, the inherent heterogeneity of primary management, and small number of radiation-associated MPNSTs meeting the eligibility criteria. Furthermore, as a high-volume tertiary center we were able to obtain, to our knowledge, the largest single-institution MPNST cohort to date but follow-up data is variable between survivors. However, our cohort was subjected to thorough subset analysis and offers insights on management of a rare disease that is understudied as a result of the difficulties obtaining reasonably sized cohorts. Few studies reporting the natural history of MPNST have described the features and outcomes of these aggressive tumors with specific attention to MPNST etiology as exhaustively as the present series. In addition to reporting the treatment and outcomes for our cohort, we profile the nerves of involvement and accompanying symptoms with respect to MPNST subtype, profile the sites of metastasis, and describe the behavior of low-grade MPNSTs separately from high-grade MPNSTs. No predictive measures for malignant transformation in pre-existing neurofibroma of NF1 patients exist; however, our data reveal that 57% of NF1-associated MPNSTs develop in the trunk with 20% arising from the lumbosacral or thoracic nerve roots. In addition to routine follow-up evaluating changes in pain, onset of extremity numbness, weakness, or paresthesia, annual 18F-FDG PET/CT may be considered in patients with spinal or pelvic neurofibromas.
Conclusion
The goal of curative management for all MPNSTs should be negative margin resection whenever possible. Additionally, supplemental radiation therapy for local control should be considered. Large index tumor size was implicated as a major adverse prognostic factor which recapitulates the importance of early diagnosis and intervention in these aggressive tumors, particularly in the NF1 population where 18F-FDG PET/CT has been shown to appropriately discriminate between benign peripheral nerve sheath tumors and MPNSTs.5 Finally, differences in clinicopathologic features and patterns of local recurrence and survival do exist between sporadic, NF1-asssociated, and RT-associated MPNST; therefore, the etiology of these tumors should be considered in the management of these patients and in future studies evaluating their biology.
Table 1.
Clinical parameters of MPNST cohorts.
| MPNST Cohort 1 MDACC (n=80) |
MPNST Cohort 2 Stanford (n=66) |
MPNST Cohort 3 LUMC (n=16) |
|
|---|---|---|---|
| Age at diagnosis | |||
| -median (range) | 40 (3–73) | n.a. | 28 (15–68) |
| Gender | |||
| -Female | 27 (34%) | n.a. | 9 (56%) |
| -Male | 37 (46%) | n.a. | 7 (44%) |
| -Not available | 16 (20%) | ||
| MPNSTs | |||
| -NF1 associated | 34 (43%) | 43 (65%) | 4 (25%) |
| -Sporadic | 22 (27%) | 21 (32%) | 12 (75%) |
| -Radiation associated | 5 (6%) | 0 (0%) | 0 (0%) |
| -Data n.a. | 19 (24%) | 2 (3%) | 0 (0%) |
| Treatment | |||
| -Resection | 75 (93%) | n.a. | 16 (100%) |
| -Radiotherapy | 6 (8%) | n.a. | 10 (63%) |
| -Chemotherapy | 16 (20%) | n.a. | 4 (25%) |
| -Not available | 48 (60%) | 1 (6%) | |
| Mean survival Time (years) | 4,9 | n.a. | 5,8 |
| Events | 35 (56%) | n.a. | 9 (56%) |
MPNST=malignant peripheral nerve sheath tumor; MDACC= The University of Texas MD Anderson Cancer Center; SUMC= Stanford University Medical Center; LUMC= Leiden University Medical Center. Events = death due to disease. n.a.=not available.
Table 2.
Distribution of loss or intact H3K27me3 according to tumor subtype.
| Loss of H3K27me3 | Intact H3K27me3 | |
|---|---|---|
| MPNSTs | 55 (34%) | 107 (66%) |
| -Triton | 0 (0%) | 5 (100%) |
| -NF1 associated | 33(41%) | 47(59%) |
| -Sporadic | 17(32%) | 37(68%) |
| Neurofibroma | 0 (0%) | 97 (100%) |
| -Atypical | 0 (0%) | 8 (100%) |
| -Plexiform | 0 (0%) | 24 (100%) |
| Schwannoma | 1 (2%) | 43 (98%) |
| Perineurioma | 0 (0%) | 4 (100%) |
| Sarcoma NOS | 0 (0%) | 26 (100%) |
| Undifferentiated pleomorphic or spindle cell sarcoma | 5 (3%) | 172 (97%) |
| Angiosarcoma | 2 (10%) | 19 (90%) |
| Myxofibrosarcoma | 0 (0%) | 17 (100%) |
| Synovial sarcoma | 9 (60%) | 6 (40%) |
| Melanoma | 1 (11%) | 8 (89%) |
| DFSP | 3 (38%) | 5 (62%) |
| Clear cell sarcoma | 2 (40%) | 3 (60%) |
| Leiomyosarcoma | 0 (0%) | 5 (100%) |
| Dedif. Liposarcoma | 0 (0%) | 1 (100%) |
| Pleomorphic liposarcoma | 0 (0%) | 5 (100%) |
| Rhabdomyosarcoma | 0 (0%) | 2 (100%) |
| Osteosarcoma | 1 (50%) | 1 (50%) |
MPNST=malignant peripheral nerve sheath tumor; NOS= not otherwise specified; DFSP=dermatofibrosarcoma protuberans.
Acknowledgments
We thank Jill Designe for review and editing of the manuscript. We also thank Geoffrey Giacco for identification of the study cohort.
Sources of Support: Funding for this research was provided in part by NIH/NCI K08CA160443 (KET), The Sally M. Kingsbury Sarcoma Research Foundation (KET), and Amschwand Sarcoma Cancer Foundation (supporting CMK).
Footnotes
Disclosures: We have no disclosures to report. None of the funding organizations had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- 1.Anghileri M, Miceli R, Fiore M, Mariani L, Ferrari A, Mussi C, et al. Malignant peripheral nerve sheath tumors: prognostic factors and survival in a series of patients treated at a single institution. Cancer. 2006;107:1065–1074. doi: 10.1002/cncr.22098. [DOI] [PubMed] [Google Scholar]
- 2.Bernthal NM, Putnam A, Jones KB, Viskochil D, Randall RL. The effect of surgical margins on outcomes for low grade MPNSTs and atypical neurofibroma. J Surg Oncol. 2014;110:813–816. doi: 10.1002/jso.23736. [DOI] [PubMed] [Google Scholar]
- 3.Bradford D, Kim A. Current treatment options for malignant peripheral nerve sheath tumors. Curr Treat Options Oncol. 2015;16:12. doi: 10.1007/s11864-015-0328-6. [DOI] [PubMed] [Google Scholar]
- 4.Brennan MF, Antonescu CR, Moraco N, Singer S. Lessons learned from the study of 10,000 patients with soft tissue sarcoma. Ann Surg. 2014;260:416–422. doi: 10.1097/SLA.0000000000000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chirindel A, Chaudhry M, Blakeley JO, Wahl R. 18F-FDG PET/CT qualitative and quantitative evaluation in neurofibromatosis type 1 patients for detection of malignant transformation: comparison of early to delayed imaging with and without liver activity normalization. J Nucl Med. 2014;56:379–385. doi: 10.2967/jnumed.114.142372. [DOI] [PubMed] [Google Scholar]
- 6.Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57:2006–2021. doi: 10.1002/1097-0142(19860515)57:10<2006::aid-cncr2820571022>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Fan Q, Yang J, Wang G. Clinical and molecular prognostic predictors of malignant peripheral nerve sheath tumor. Clin Trans Oncol. 2014;16:191–199. doi: 10.1007/s12094-013-1061-x. [DOI] [PubMed] [Google Scholar]
- 8.Farid M, Demicco EG, Garcia R, Ahn L, Merola PR, Cioffi A, et al. Malignant peripheral nerve sheath tumors. Oncologist. 2014;19:193–201. doi: 10.1634/theoncologist.2013-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher CDM, Bridge JA, Hogendoorn P, Mertens F, editors. WHO Classification of Tumours of Soft Tissue and Bone. Lyon, France: IARC; 2013. [Google Scholar]
- 10.Gladdy RA, Qin LX, Moraco N, Edgar MA, Antonescu CR, Alektiar KM, et al. Do radiation-associated soft tissue sarcomas have the same prognosis as sporadic soft tissue sarcomas? J Clin Oncol. 2010;28:2064–2069. doi: 10.1200/JCO.2009.25.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grobmyer SR, Reith JD, Shahlaee A, Bush CH, Hochwald SN. Malignant peripheral nerve sheath tumor: molecular pathogenesis and current management considerations. J Surg Oncol. 2008;97:340–349. doi: 10.1002/jso.20971. [DOI] [PubMed] [Google Scholar]
- 12.Jhanwar SC, Chen Q, Li FP, Brennan MF, Woodruff JM. Cytogenetic analysis of soft tissue sarcomas: recurrent chromosome abnormalities in malignant peripheral nerve sheath tumors (MPNST) Cancer Genet Cytogenet. 1994;78:138–144. doi: 10.1016/0165-4608(94)90081-7. [DOI] [PubMed] [Google Scholar]
- 13.Johansson G, Mahller YY, Collins MH, et al. Effective in vivo targeting of the mammalian target of rapamycin pathway in malignant peripheral nerve sheath tumors. Mol Cancer Ther. 2008;7:1237–1245. doi: 10.1158/1535-7163.MCT-07-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolberg M, Holand M, Agesen TH, Brekke HR, Liestol K, Hall KS, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:135–147. doi: 10.1093/neuonc/nos287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaFemina J, Qin LX, Moraco NH, Antonescu CR, Fields RC, Crago AM, et al. Oncologic outcomes of sporadic, neurofibromatosis-associated, and radiation-induced malignant peripheral nerve sheath tumors. Ann Surg Oncol. 2013;20:66–72. doi: 10.1245/s10434-012-2573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez G, Torres KE, Liu J, Hernandez B, Young E, Belousov R, et al. Autophagic survival in resistance to histone deacetylase inhibitors: novel strategies to treat malignant peripheral nerve sheath tumors. Cancer Res. 2010;71:185–196. doi: 10.1158/0008-5472.CAN-10-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mertens F, Dal Cin P, De Wever I, Fletcher CDM, Mandahl N, Mitelman F, et al. Cytogenetic characterization of peripheral nerve sheath tumours: a report of the CHAMP study group. J Pathol. 2000;190:31–38. doi: 10.1002/(SICI)1096-9896(200001)190:1<31::AID-PATH505>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Neurofibromatosis. NIH Consens Statement. 1987 Jul 13–15;6(12):1–19. [Google Scholar]
- 19.Porter DE, Prasad V, Foster L, Dall GF, Birch R, Grimer RJ. Survival in Malignant Peripheral Nerve Sheath Tumours: A Comparison between Sporadic and Neurofibromatosis Type 1-Associated Tumours. Sarcoma. 2009;2009 doi: 10.1155/2009/756395. Article ID 756395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahrmann EP, Watson AL, Keng VW, Choi K, Moriarity BS, Beckmann DA. Forward genetic screen for malignant peripheral nerve sheath tumor formation identifies new genes and pathways driving tumorigenesis. Nat Genet. 2013;45:756–766. doi: 10.1038/ng.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez FJ, Folpe AL, Giannini C, Perry A. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta neuropathol. 2012;123:295–319. doi: 10.1007/s00401-012-0954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaefer IM, Fletcher CD. Malignant peripheral nerve sheath tumor (MPNST) arising in diffuse-type neurofibroma: clinicopathologic characterization in a series of 9 cases. Am J Surg Pathol. 2015;39:1234–1241. doi: 10.1097/PAS.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 23.Stucky CC, Johnson KN, Gray RJ, Pockaj BA, Ocal IT, Rose PS, et al. Malignant peripheral nerve sheath tumors (MPNST): the Mayo Clinic experience. Ann Surg Oncol. 2012;19:878–885. doi: 10.1245/s10434-011-1978-7. [DOI] [PubMed] [Google Scholar]
- 24.Weiss SW, Goldblum JR. Enzinger and Weiss's Soft Tissue Tumors. 5. St. Louis: Mosby Elsevier; 2008. Malignant tumors of the peripheral nerves; pp. 903–925. [Google Scholar]
- 25.Zou C, Smith KD, Liu J, Lahat G, Myers S, Wang WL, et al. Clinical, pathological, and molecular variables predictive of malignant peripheral nerve sheath tumor outcome. Ann Surg. 2009;249:1014–1022. doi: 10.1097/SLA.0b013e3181a77e9a. [DOI] [PubMed] [Google Scholar]
- 26.Zou CY, Smith KD, Zhu QS, Liu J, McCutcheon IE, Slopis JM, et al. Dual targeting of AKT and mammalian target of rapamycin: a potential therapeutic approach for malignant peripheral nerve sheath tumor. Mol Cancer Ther. 2012;8:1157–1168. doi: 10.1158/1535-7163.MCT-08-1008. [DOI] [PubMed] [Google Scholar]


