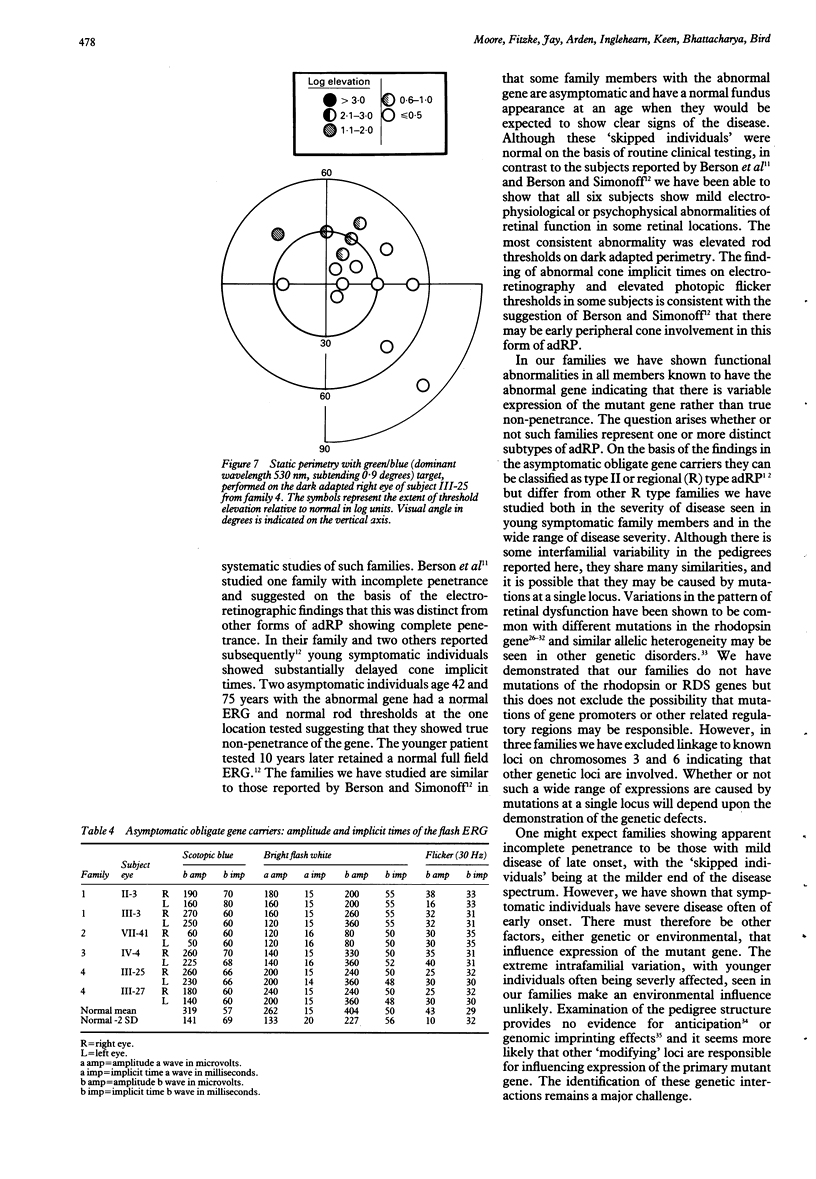
Abstract
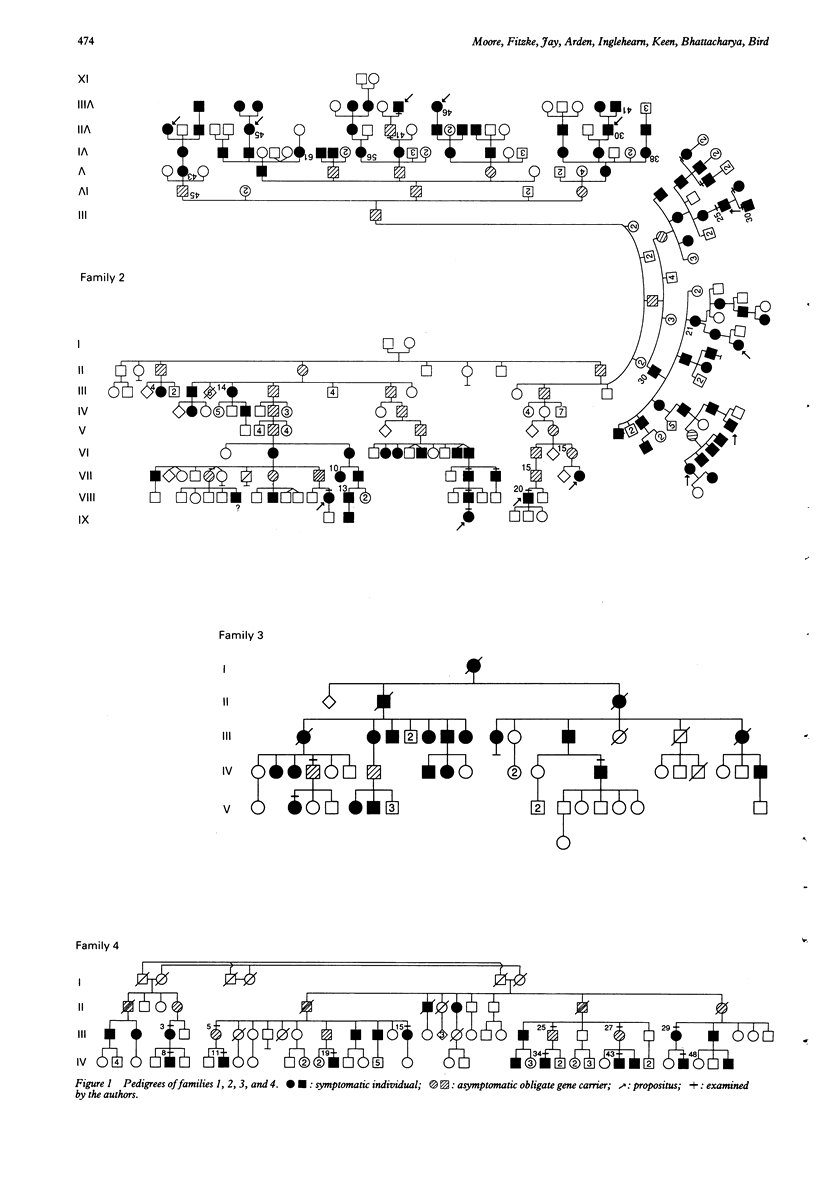
Twenty five symptomatic individuals and six asymptomatic obligate gene carriers from four families with autosomal dominant retinitis pigmentosa (adRP) showing apparent incomplete penetrance have been studied. Symptomatic individuals from three families showed early onset of night blindness, non-recordable rod electroretinograms, and marked elevation of both rod and cone thresholds in all subjects tested. In the fourth family, there was more variation in the age of onset of night blindness and some symptomatic individuals showed well preserved rod and cone function in some retinal areas. All asymptomatic individuals tested had evidence of mild abnormalities of rod and cone function, indicating that these families show marked variation in expressivity rather than true non-penetrance of the adRP gene. No mutations of the rhodopsin or RDS genes were found in these families and the precise genetic mutation(s) remain to be identified.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arden G. B., Carter R. M., Hogg C. R., Powell D. J., Ernst W. J., Clover G. M., Lyness A. L., Quinlan M. P. A modified ERG technique and the results obtained in X-linked retinitis pigmentosa. Br J Ophthalmol. 1983 Jul;67(7):419–430. doi: 10.1136/bjo.67.7.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berson E. L., Gouras P., Gunkel R. D., Myrianthopoulos N. C. Dominant retinitis pigmentosa with reduced penetrance. Arch Ophthalmol. 1969 Feb;81(2):226–234. doi: 10.1001/archopht.1969.00990010228013. [DOI] [PubMed] [Google Scholar]
- Berson E. L., Rosner B., Sandberg M. A., Dryja T. P. Ocular findings in patients with autosomal dominant retinitis pigmentosa and a rhodopsin gene defect (Pro-23-His). Arch Ophthalmol. 1991 Jan;109(1):92–101. doi: 10.1001/archopht.1991.01080010094039. [DOI] [PubMed] [Google Scholar]
- Berson E. L., Simonoff E. A. Dominant retinitis pigmentosa with reduced penetrance. Further studies of the electroretinogram. Arch Ophthalmol. 1979 Jul;97(7):1286–1291. doi: 10.1001/archopht.1979.01020020028006. [DOI] [PubMed] [Google Scholar]
- Dryja T. P., Hahn L. B., Cowley G. S., McGee T. L., Berson E. L. Mutation spectrum of the rhodopsin gene among patients with autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9370–9374. doi: 10.1073/pnas.88.20.9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., McGee T. L., Reichel E., Hahn L. B., Cowley G. S., Yandell D. W., Sandberg M. A., Berson E. L. A point mutation of the rhodopsin gene in one form of retinitis pigmentosa. Nature. 1990 Jan 25;343(6256):364–366. doi: 10.1038/343364a0. [DOI] [PubMed] [Google Scholar]
- Ernst W., Faulkner D. J., Hogg C. R., Powell D. J., Arden G. B., Vaegan An automated statis perimeter/adaptometer using light emitting diodes. Br J Ophthalmol. 1983 Jul;67(7):431–442. doi: 10.1136/bjo.67.7.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar G. J., Kenna P., Jordan S. A., Kumar-Singh R., Humphries M. M., Sharp E. M., Sheils D. M., Humphries P. A three-base-pair deletion in the peripherin-RDS gene in one form of retinitis pigmentosa. Nature. 1991 Dec 12;354(6353):478–480. doi: 10.1038/354478a0. [DOI] [PubMed] [Google Scholar]
- Fishman G. A., Alexander K. R., Anderson R. J. Autosomal dominant retinitis pigmentosa. A method of classification. Arch Ophthalmol. 1985 Mar;103(3):366–374. doi: 10.1001/archopht.1985.01050030062023. [DOI] [PubMed] [Google Scholar]
- Fishman G. A., Stone E. M., Gilbert L. D., Kenna P., Sheffield V. C. Ocular findings associated with a rhodopsin gene codon 58 transversion mutation in autosomal dominant retinitis pigmentosa. Arch Ophthalmol. 1991 Oct;109(10):1387–1393. doi: 10.1001/archopht.1991.01080100067044. [DOI] [PubMed] [Google Scholar]
- Harper P. S., Harley H. G., Reardon W., Shaw D. J. Anticipation in myotonic dystrophy: new light on an old problem. Am J Hum Genet. 1992 Jul;51(1):10–16. [PMC free article] [PubMed] [Google Scholar]
- Heckenlively J. R., Rodriguez J. A., Daiger S. P. Autosomal dominant sectoral retinitis pigmentosa. Two families with transversion mutation in codon 23 of rhodopsin. Arch Ophthalmol. 1991 Jan;109(1):84–91. doi: 10.1001/archopht.1991.01080010086038. [DOI] [PubMed] [Google Scholar]
- Inglehearn C. F., Keen T. J., Bashir R., Jay M., Fitzke F., Bird A. C., Crombie A., Bhattacharya S. A completed screen for mutations of the rhodopsin gene in a panel of patients with autosomal dominant retinitis pigmentosa. Hum Mol Genet. 1992 Apr;1(1):41–45. doi: 10.1093/hmg/1.1.41. [DOI] [PubMed] [Google Scholar]
- Jacobson S. G., Kemp C. M., Sung C. H., Nathans J. Retinal function and rhodopsin levels in autosomal dominant retinitis pigmentosa with rhodopsin mutations. Am J Ophthalmol. 1991 Sep 15;112(3):256–271. doi: 10.1016/s0002-9394(14)76726-1. [DOI] [PubMed] [Google Scholar]
- Jacobson S. G., Voigt W. J., Parel J. M., Apáthy P. P., Nghiem-Phu L., Myers S. W., Patella V. M. Automated light- and dark-adapted perimetry for evaluating retinitis pigmentosa. Ophthalmology. 1986 Dec;93(12):1604–1611. doi: 10.1016/s0161-6420(86)33522-x. [DOI] [PubMed] [Google Scholar]
- Jay M., Bird A. C., Moore A. N., Jay B. Nine generations of a family with autosomal dominant retinitis pigmentosa and evidence of variable expressivity from census records. J Med Genet. 1992 Dec;29(12):906–910. doi: 10.1136/jmg.29.12.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara K., Hahn L. B., Mukai S., Travis G. H., Berson E. L., Dryja T. P. Mutations in the human retinal degeneration slow gene in autosomal dominant retinitis pigmentosa. Nature. 1991 Dec 12;354(6353):480–483. doi: 10.1038/354480a0. [DOI] [PubMed] [Google Scholar]
- Keen J., Lester D., Inglehearn C., Curtis A., Bhattacharya S. Rapid detection of single base mismatches as heteroduplexes on Hydrolink gels. Trends Genet. 1991 Jan;7(1):5–5. doi: 10.1016/0168-9525(91)90004-a. [DOI] [PubMed] [Google Scholar]
- Kemp C. M., Jacobson S. G., Faulkner D. J. Two types of visual dysfunction in autosomal dominant retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1988 Aug;29(8):1235–1241. [PubMed] [Google Scholar]
- Kemp C. M., Jacobson S. G., Roman A. J., Sung C. H., Nathans J. Abnormal rod dark adaptation in autosomal dominant retinitis pigmentosa with proline-23-histidine rhodopsin mutation. Am J Ophthalmol. 1992 Feb 15;113(2):165–174. doi: 10.1016/s0002-9394(14)71529-6. [DOI] [PubMed] [Google Scholar]
- Kumar-Singh R., Jordan S. A., Farrar G. J., Humphries P. Poly (T/A) polymorphism at the human retinal degeneration slow (RDS) locus. Nucleic Acids Res. 1991 Oct 25;19(20):5800–5800. doi: 10.1093/nar/19.20.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyness A. L., Ernst W., Quinlan M. P., Clover G. M., Arden G. B., Carter R. M., Bird A. C., Parker J. A. A clinical, psychophysical, and electroretinographic survey of patients with autosomal dominant retinitis pigmentosa. Br J Ophthalmol. 1985 May;69(5):326–339. doi: 10.1136/bjo.69.5.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massof R. W., Finkelstein D. Two forms of autosomal dominant primary retinitis pigmentosa. Doc Ophthalmol. 1981 Nov;51(4):289–346. doi: 10.1007/BF00143336. [DOI] [PubMed] [Google Scholar]
- Moore A. T., Fitzke F. W., Kemp C. M., Arden G. B., Keen T. J., Inglehearn C. F., Bhattacharya S. S., Bird A. C. Abnormal dark adaptation kinetics in autosomal dominant sector retinitis pigmentosa due to rod opsin mutation. Br J Ophthalmol. 1992 Aug;76(8):465–469. doi: 10.1136/bjo.76.8.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T., Haig D. Genomic imprinting in mammalian development: a parental tug-of-war. Trends Genet. 1991 Feb;7(2):45–49. doi: 10.1016/0168-9525(91)90230-N. [DOI] [PubMed] [Google Scholar]
- Sung C. H., Davenport C. M., Hennessey J. C., Maumenee I. H., Jacobson S. G., Heckenlively J. R., Nowakowski R., Fishman G., Gouras P., Nathans J. Rhodopsin mutations in autosomal dominant retinitis pigmentosa. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6481–6485. doi: 10.1073/pnas.88.15.6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suthers G. K., Davies K. E. Phenotypic heterogeneity and the single gene. Am J Hum Genet. 1992 May;50(5):887–891. [PMC free article] [PubMed] [Google Scholar]
- Travis G. H., Christerson L., Danielson P. E., Klisak I., Sparkes R. S., Hahn L. B., Dryja T. P., Sutcliffe J. G. The human retinal degeneration slow (RDS) gene: chromosome assignment and structure of the mRNA. Genomics. 1991 Jul;10(3):733–739. doi: 10.1016/0888-7543(91)90457-p. [DOI] [PubMed] [Google Scholar]
- Tyler C. W., Ernst W., Lyness A. L. Photopic flicker sensitivity losses in simplex and multiplex retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1984 Sep;25(9):1035–1042. [PubMed] [Google Scholar]
- Weber J. L., May P. E. Abundant class of human DNA polymorphisms which can be typed using the polymerase chain reaction. Am J Hum Genet. 1989 Mar;44(3):388–396. [PMC free article] [PubMed] [Google Scholar]
- Wells J., Wroblewski J., Keen J., Inglehearn C., Jubb C., Eckstein A., Jay M., Arden G., Bhattacharya S., Fitzke F. Mutations in the human retinal degeneration slow (RDS) gene can cause either retinitis pigmentosa or macular dystrophy. Nat Genet. 1993 Mar;3(3):213–218. doi: 10.1038/ng0393-213. [DOI] [PubMed] [Google Scholar]


