Abstract
Prolonged and/or frequent exposure to psychological stress responses may lead to deterioration of organs and tissues, predisposing to disease. In agreement with this, chronic psychosocial stress is linked to greater cardiovascular risk, including increased incidence of atherosclerosis, myocardial ischemia, coronary heart disease, and death. Thus the association between stress and cardiovascular dysfunction represents an important node for therapeutic intervention in cardiovascular disease. Here we report that 2 weeks of chronic variable stress (CVS) increased indices of vascular stiffness, including increased collagen deposition in the aortic adventitia and increased resting pulse pressure, in male rats. Thus CVS may represent a useful rodent model for stress-associated CVD, especially for aging populations for which widening pulse pressure is a well-known risk factor. Additionally, we report that the thiazolidinedione Rosiglitazone (RSG) blunts chronic stress-associated increases in circulating corticosterone. Despite this, RSG was not protective against adverse cardiovascular outcomes associated with chronic stress. Rather RSG itself is associated with increased pulse pressure, and this is exacerbated by chronic stress—highlighting that chronic stress may represent an additional contributor to RSG-associated cardiovascular risk.
1. INTRODUCTION
A large literature links chronic stress to cardiovascular morbidity and mortality in human populations [reviewed by 1–3]. This relationship between stress and cardiovascular dysfunction represents an important target for the development of therapeutic interventions, since cardiovascular disease (CVD) remains the leading cause of death world wide [4].
In response to an acute psychological stressor, both sympathetic nervous system and hypothalamic-pituitary-adrenocortical (HPA) axis activity is increased, facilitating the mobilization and distribution of fuels. When an individual experiences chronic stress, frequent and/or persistent exposure to these physiological responses over a period of time is thought to create wear and tear on organs and tissues, predisposing to disease [5]. Consequently, we reasoned that pharmacological interventions that blunt sympathetic and hormonal responses to acute stress might provide effective therapy against chronic stress-associated cardiovascular dysfunction.
The peroxisome-proliferator activated receptor-γ (PPARγ) is a nuclear receptor that is activated by fatty acids and fat metabolites, to regulate the transcription of genes involved in lipid and glucose metabolism. Pharmacological agonists of PPARγ include the thiazolidinedione (TZD) class of insulin-sensitizing drugs. Our recent work demonstrates that, in addition to their well-known effects on glucose homeostasis, the TZD Rosiglitazone also effectively blunts both the tachycardic and glucocorticoid response to acute psychological stress in rats [6]. In the present study we tested the hypothesis that chronic psychological stress induces cardiovascular dysfunction, which may be abrogated by concurrent treatment with RSG.
2. MATERIALS AND METHODS
2.1 Animals
The Institutional Animal Care and Use Committees at the University of Cincinnati and the University of California, Davis approved all animal protocols. Male Long-Evans rats (~275g) were obtained from Harlan Labs (Indianapolis, IN), and allowed to acclimate to the vivarium for at least one week prior to surgery or other experimental procedures. Rats were singly-housed with ad libitum access to water and standard rat chow, and maintained on a 12:12 light:dark cycle with lights on at 06:00 h and off at 18:00 h.
2.2 Drugs
RSG (Cayman Chemicals, Ann Arbor, MI) was suspended in 0.2% methylcellulose (Sigma Chemical Co., St. Louis, MO) in water at a concentration of 1 mg/mL, and administered at 10 mg/kg body weight per day by oral gavage. This dose was chosen because it is therapeutic for glycemic control in rats [7] and because our recent work demonstrates that this dose is effective to blunt both corticosterone and heart rate responses to acute restraint stress in rats [6].
2.3 Surgical procedures
Rats were implanted with radiotelemetry transmitters (Data Sciences International, St. Paul, MN) as previously described [6,8]. Briefly, animals were anesthetized using inhaled isoflurane anesthesia. The descending aorta was exposed via an abdominal incision, allowing implantation of a catheter extending from the transmitter. The catheter was secured with tissue adhesive (Vetbond; 3M Animal Care Products, St. Paul, MN) and a cellulose patch. The capsule was sutured to the abdominal musculature, the abdominal musculature was sutured, and wound clips were applied to the skin. Rats recovered for at least 1 wk and wound clips were removed prior to beginning the experiments.
2.4 Cardiovascular parameters
Cardiovascular parameters [heart rate, mean arterial pressure (MAP), and systolic, diastolic and pulse pressures] and general locomotor activity were continuously recorded. AM and PM baseline telemetric measurements were collected for 5 days prior to any intervention, and for each rat changes in these variables were calculated relative to the average of the 5 day baseline. Morning (AM) measurements were collected between 06:30 and 08:30 h. Night (PM) measurements were collected from 18:00 to 06:00 h the next day. Animals were undisturbed by animal care or research staff during these time periods.
2.5 Chronic Variable Stress (CVS)
CVS consisted of twice-daily exposure to one of several stressors, presented in a randomized order between 10:00 and 15:00 h, for 15 days. Stressors included hypoxia (8% O2, 92% N2 for 30 min), cold room (4°C for 1 h), shaker platform (100 rpm for 1 h), restraint (in a well-ventilated Plexiglas tube for 30 min), overnight housing in small cages normally used to house mice, and overnight housing in cages with damp bedding. Unstressed control rats were disturbed only for daily administration of RSG or VEH and for regular husbandry unless otherwise noted.
In cohort 1, rats were first outfitted with telemetry devices and allowed to fully recover from surgery. We collected 5 days of baseline cardiovascular data, and then divided the individuals evenly into 4 treatment groups, matched for baseline MAP: 1) unstressed control rats receiving vehicle (CON-VEH), 2) unstressed control rats receiving RSG (CON-RSG), 3) rats subjected to CVS receiving vehicle (CVS-VEH), and 4) rats subjected to CVS receiving RSG (CVS-RSG). Rats received 4 days of RSG or VEH by gavage prior to the first day of CVS.
In cohort 2, rats were divided evenly into the same 4 treatment groups and subjected to CVS and daily oral gavage, but without the use of telemetry devices: 1) unstressed control rats receiving vehicle (CON-VEH), 2) unstressed control rats receiving RSG (CON-RSG), 3) rats subjected to CVS receiving vehicle (CVS-VEH), and 4) rats subjected to CVS receiving RSG (CVS-RSG).
2.6 Cardiovascular response to acute restraint
The initial stressor on the first day of CVS was a 20-minute restraint stress, allowing us to record the acute cardiovascular response to this challenge in naïve (not previously-stressed) rats. The restraint was performed between 10:00 and 12:00 h. We quickly placed the rats into snug, well-ventilated transparent Plexiglas tubes, noting the exact time at which animals were placed into their restrainers, and cardiovascular responses were recorded continuously starting at this time (T = 0). Rats were quickly removed from the restrainers at 20 minutes following the onset of restraint.
2.7 Serological Assays
Baseline blood samples were collected from the tip of the tail vein within 3 minutes of first disturbing the animal’s cage. We began the bleed at 08:00 h, near the nadir of the circadian rhythm for corticosterone. Blood was centrifuged (3000 rpm, 20 min, 4 °C) and plasma was stored at −80 °C prior to analysis. Immunoreactive corticosterone concentrations were measured by RIA (MP Biomedicals, Santa Ana, CA), according to the manufacturer’s instructions. Inflammatory cytokine levels were measured on a SECTOR Imager 2400 using a custom V-PLEX assay from MESO Scale Discovery (Rockville, MD).
2.8 Vascular Histology
A 2–3 mm portion of the descending aorta, collected 12–15 mm distal to the heart, was embedded in paraffin, sectioned at a thickness of 5 µm, and stained with Verhoeff-Van Gieson (VVG) stain (Cincinnati Children’s Hospital Pathology Research Core) or Masson’s trichrome (Cat# HT-15, Sigma Aldrich, St. Louis, MO) [9]. Luminal circumference, and thickness of the tunica media were quantified from VVG sections using the freehand line and straight-line selection tools, respectively, in ImageJ. Adventitial collagen was quantified with Image J following Masson’s trichrome staining, using the K-means clustering plugin and color thresholding to selectively measure collagen area within the tunica adventitia, as in [10,11]. Display images were adjusted for brightness and contrast.
2.9 Statistics
Data were analyzed by ANOVA, with repeated measures as appropriate, and with Tukey’s posthoc analyses using Prism v. 6.0 (Graphpad Software, La Jolla, CA) or SigmaStat (SYSTAT software, San Jose, CA). α = 0.05. Data are shown as mean +/− standard error.
3. RESULTS
3.1 Cardiovascular response to acute restraint stress
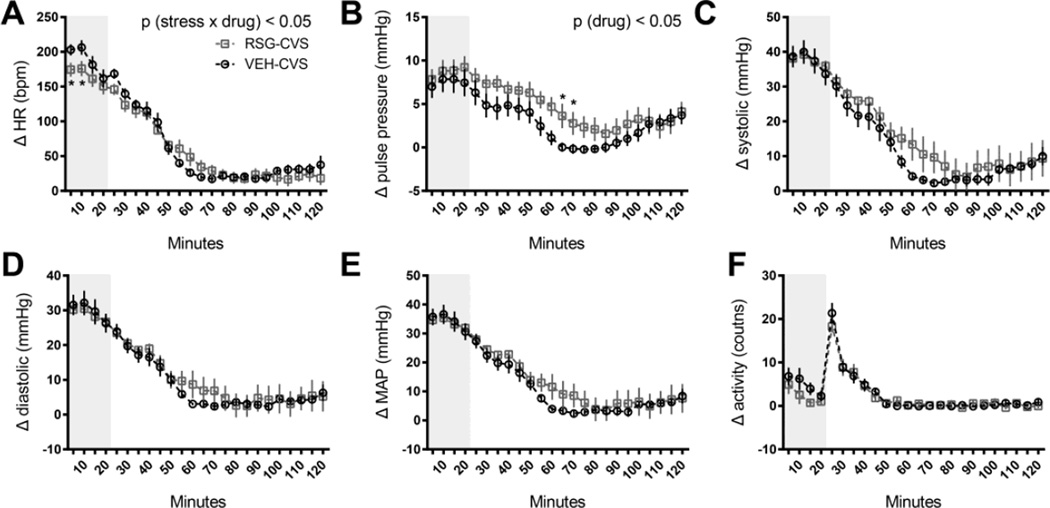
We performed an acute restraint challenge for the VEH-CVS and RSG-CVS groups as the first stressor in the CVS paradigm. The cardiovascular response to this stressor was measured as the change from that morning’s baseline, defined here as the 60 min before the investigators entered the room to begin the experiment. There were no differences during this 1-h interval between the groups, for any of the variables (data not shown). In response to restraint, we observed a rapid increase in both heart rate and blood pressure. Consistent with our previous report [6], the acute heart rate response was blunted in RSG-treated rats. RM ANOVA indicated a significant interaction between time and the RSG treatment [p (stress × drug) < 0.05], and Tukey’s post hoc tests indicated that the increase in heart rate over the first 10 min was significantly lower in RSG-treated rats [p < 0.05, (Fig 1A)].
Figure 1. Rosiglitazone (RSG) and the cardiovascular response to acute stress.
Rats treated with oral RSG (10 mg/kg body weight, once daily) exhibited a significantly blunted heart rate (A), and a significantly elevated pulse pressure (B) response to a 20-minute restraint challenge (represented by gray shading), compared to rats treated with vehicle alone (VEH). There were no significant effects of RSG on the systolic (C), diastolic (D), mean arterial (MAP) pressure (E) or locomotor (F) response to this acute stressor. Data are represented as means of 5 minute time bins ±SEM for 120 minutes during and immediately following restraint. * p<0.05 by Tukey’s posthoc test; n =8 rats per group.
In addition, more detailed analysis in this study indicate that RSG-treated rats exhibited an impaired recovery from the stress-induced increase in pulse pressure, relative to VEH-treated controls [RM ANOVA, p (drug) < 0.05 (Fig 1B)]; this novel effect was due primarily to a non-significant increase in systolic pressure (Fig 1C). Tukey’s posthoc tests indicated a significantly greater pulse pressure in RSG-CVS compared to VEH-CVS rats at 60–70 minutes after the onset of restraint [p< 0.05].
There were no significant differences between the two groups in the systolic, diastolic, MAP, or locomotor responses to restraint (Fig 1C–F).
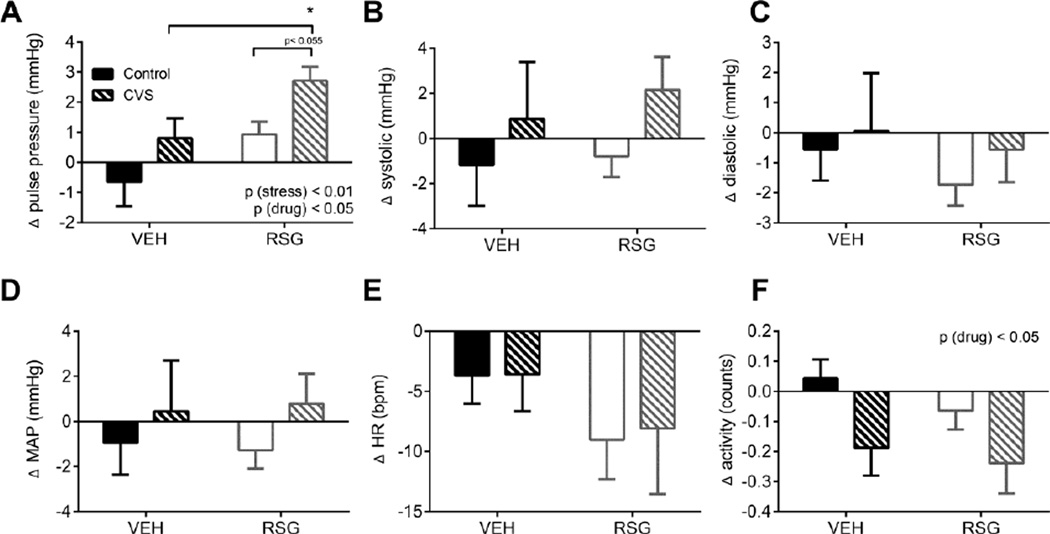
3.2 Changes in cardiovascular parameters during chronic stress (AM)
To examine the effects of CVS and RSG on the cardiovascular system, we first measured the change in blood pressures and heart rate of undisturbed, resting subjects in the early part of the light cycle. CVS induced an increase in pulse pressure [2-way ANOVA, p (stress) < 0.01] and this was further enhanced by RSG treatment [p (drug) < 0.05 (Fig 2A)], with no significant interaction [p (stress × drug), ns]. Tukey’s posthoc test indicated that the RSG-CVS rats exhibited significantly greater pulse pressure compared to the VEH-CVS rats [p< 0.05].
Figure 2. Cardiovascular response to chronic variable stress (CVS) and Rosiglitazone (RSG) during the inactive period.
Both CVS and RSG (10 mg/kg body weight, once daily) elicited a significant increase in resting pulse pressure (A). Locomotor activity was significantly decreased by RSG (F). There was no significant effect of either CVS or RSG on systolic (B), diastolic (C), mean arterial pressure (MAP) (D), or heart rate (E). Data were collected between 6:30–8:30 h (in rats maintained on a 06:00–18:00 h light-dark cycle) and averaged over 14 days of CVS and RSG treatment. Bars represent mean ± SEM. * p<0.05 by Tukey’s posthoc test; n =7–8 rats per group.
RSG also elicited a slight, but significant, decrease in general locomotor activity during the AM [2-way ANOVA, p (drug) < 0.05) (Fig 2F)], but this is unlikely to contribute to the observed differences in pulse pressure since decreased locomotor activity would be expected to have the opposite (if any) effect on pulse pressure.
We observed no significant effects of CVS or RSG on heart rate, or on systolic, diastolic, or MAP (Fig 2B–E).
Day-to-day analysis of cardiovascular parameters revealed a substantial increase in AM heart rate, MAP, and locomotor activity in all CVS animals during exposure to damp bedding, relative to other mornings. Therefore these data were excluded from the AM analyses. Lastly, one rat in the CVS-RSG group was injured during the CVS; its data were excluded from the CVS analyses.
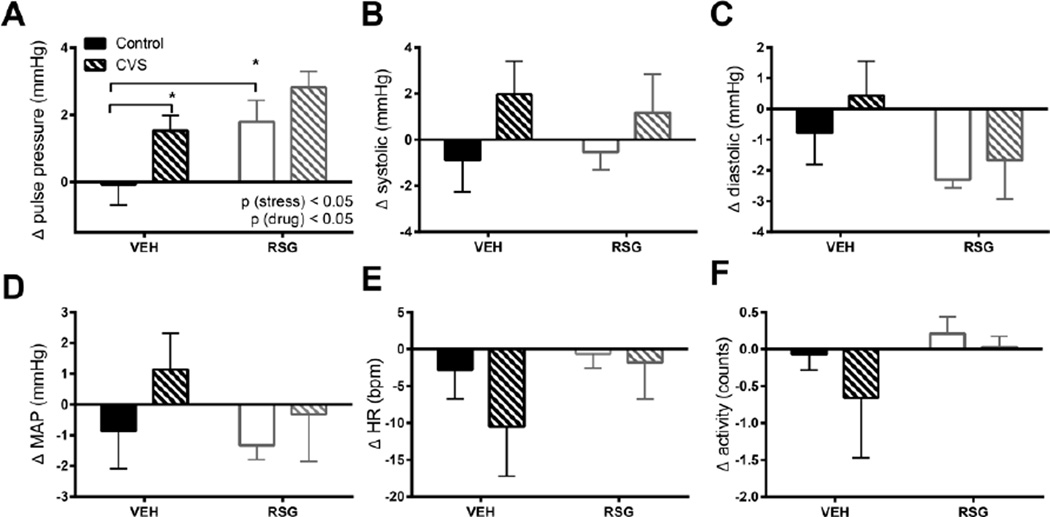
3.3 Changes in cardiovascular parameters during chronic stress (PM)
Next we measured the change in blood pressures and heart rate of these subjects during their active (dark) period. CVS induced an increase in pulse pressure [2-way ANOVA, p (stress) < 0.01] and this was further enhanced by RSG treatment [p (drug) < 0.05 (Fig 3A)], with no significant interaction [p (stress × drug), ns]. Tukey’s posthoc test indicated that VEH-CVS rats exhibited significantly greater pulse pressure compared to VEH-CON rats [p< 0.05] and that the RSG-CON rats exhibited significantly greater pulse pressure compared to VEH-CON rats [p< 0.05].
Figure 3. Cardiovascular response to chronic variable stress (CVS) and Rosiglitazone (RSG) during the active period.
Both CVS and RSG (10 mg/kg body weight, once daily) elicited a significant increase in pulse pressure (A). There were no significant effects of either CVS or RSG on systolic (B), diastolic (C), mean arterial pressure (MAP) (D), heart rate (E), or activity (F). Data were collected between 18:00–06:00 h (in rats maintained on an 06:00–18:00 h light-dark cycle) and averaged over 14 days of CVS and RSG treatment. Bars represent mean ± SEM. *p<0.05 by Tukey’s posthoc test; n = 7–8 rats per group.
We observed no significant effects of CVS or RSG on heart rate, activity, or on systolic, diastolic, or MAP (Fig 3 B–E).
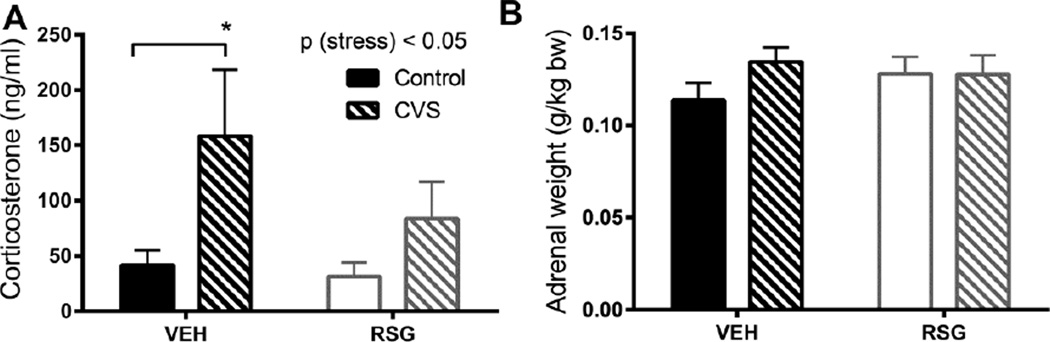
3.4 HPA response to chronic variable stress
In a second cohort, we repeated the chronic stress and drug treatments but without the use of telemetry devices, to facilitate dissection of the descending aorta at sacrifice. We also collected blood from this cohort on day 8 of CVS to measure basal AM corticosterone.
Previous work indicates that RSG blunts HPA responses to acute stress [6], with no effect on basal HPA axis tone, in lean, unstressed Long Evans [6] or Wistar rats [12]. RSG-treated fatty Zucker rats had higher corticosterone compared to both lean and vehicle-treated controls, likely reflecting an interaction between RSG and this obese leptin-receptor deficient phenotype [12].
In agreement with this literature, RSG had no effect on basal HPA axis tone in lean, unstressed Long-Evans rats. However, as expected, we observed a main effect of CVS to increase basal AM corticosterone [2-way ANOVA, p (stress) < 0.05 (Fig 4A)]. Tukey’s posthoc tests indicated CON and CVS groups were significantly different only among VEH treated rats [p< 0.05]. Frequent stimulation of the adrenal gland by adrenocorticotrophic hormone during CVS is typically [13–15], but not always [16] associated with adrenal hypertrophy. However we did not observe any significant effects of CVS or RSG on adrenal weight in this study (Fig 4B).
Figure 4. HPA response to chronic variable stress (CVS) and Rosiglitazone (RSG).
CVS elicited a significant increase in basal (resting) corticosterone (A). There were no significant effects of CVS or RSG on adrenal weight (B). Bars represent mean ± SEM ; *p<0.05 by Tukey’s posthoc test; n =10–11 rats per group (A); n =14–19 rats per group (B).
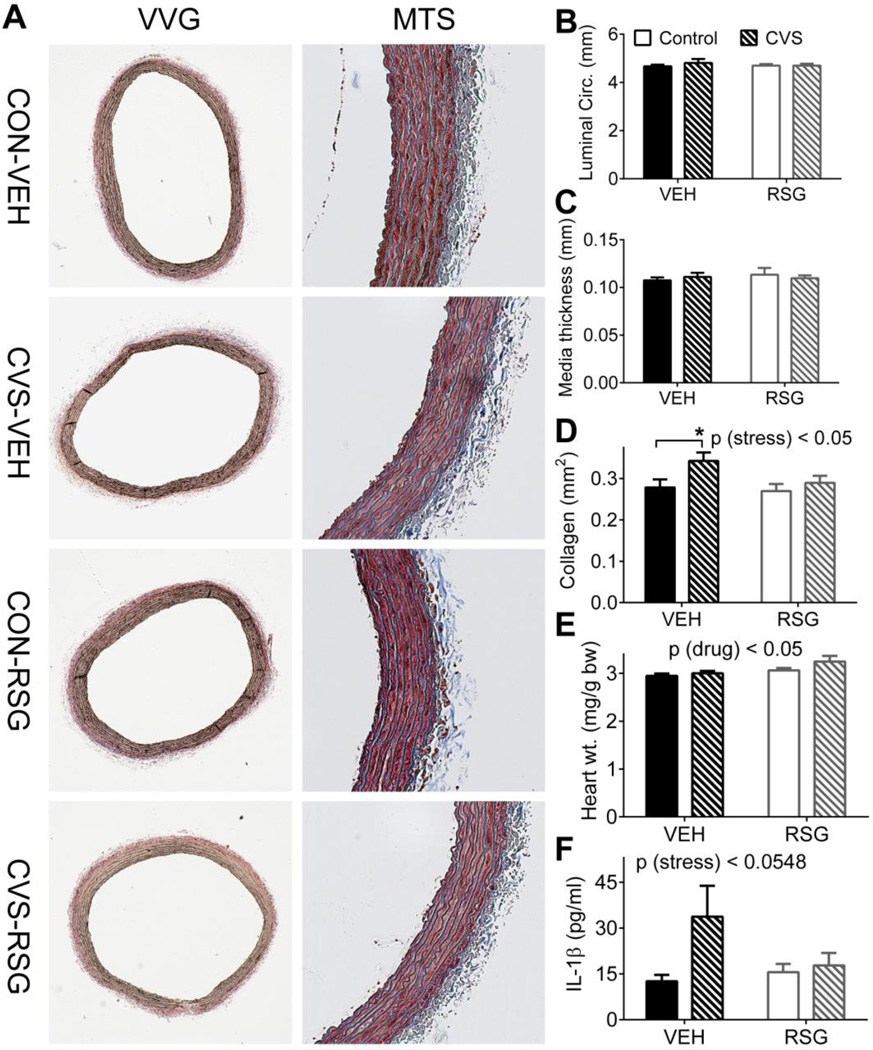
3.5 Cardiovascular tissues
Elevated pulse pressure is an indirect index of vascular stiffness [17,18] and an independent risk factor for cardiovascular mortality [19–22]. To investigate the possibility that changes in arterial structure contribute to increased pulse pressure following CVS and RSG, we examined the descending aortas. We did not observe any significant main effects or interactions among the groups with respect to either luminal circumference or medial thickness (Fig 5A,B), but CVS was associated with a greater deposition of collagen fibers in the tunica adventitia [2-way ANOVA, p (stress) < 0.05 (Fig 5A,D)]. Tukey’s posthoc test indicated that VEH-CVS rats exhibited significantly greater collagen deposition compared to VEH-CON rats [p< 0.05].
Figure 5. Chronic variable stress (CVS), Rosiglitazone (RSG), and cardiovascular morphology.
CVS elicited a significant increase in the deposition of collagen in the aortic adventitia (A, D). RSG elicited a significant increase in heart weight (E). There were no significant effects of CVS or RSG on luminal circumference (B), medial thickness (C) or IL-1β (F). VVG = Verhoeff–Van Gieson stain; MTS = Masson's trichrome stain. Bars represent mean ± SEM; *p<0.05 by Tukey’s posthoc test; n =10–11 rats per group (A–D) and 17–19 rats per group (E).
Consistent with the known effects of both vascular stiffness [23,24] and RSG-treatment [25,26] on the heart, RSG elicited a mild cardiac hypertrophy [2-way ANOVA, p (drug) < 0.05 (Fig 5E)]. There were no significant posthoc comparisons.
3.6 Plasma inflammatory mediators
Increased inflammation and higher circulating levels of proinflammatory cytokines are associated with increased indices of vascular stiffness [27–30] and with exposure to psychological stress [31]. To determine if markers of inflammation were altered in response to CVS and RSG, we measured the proinflammatory cytokines TNFα, IL-1β, IL-6 and CXCL1 from plasma collected on day 8 of CVS. We did not observe any significant main effects or interactions for any of the cytokines tested, though there was a trend toward increased plasma IL-1β in response to stress [2-way ANOVA, p (stress) < 0.0548 (Fig 5F)].
3.6 Food intake and body weight
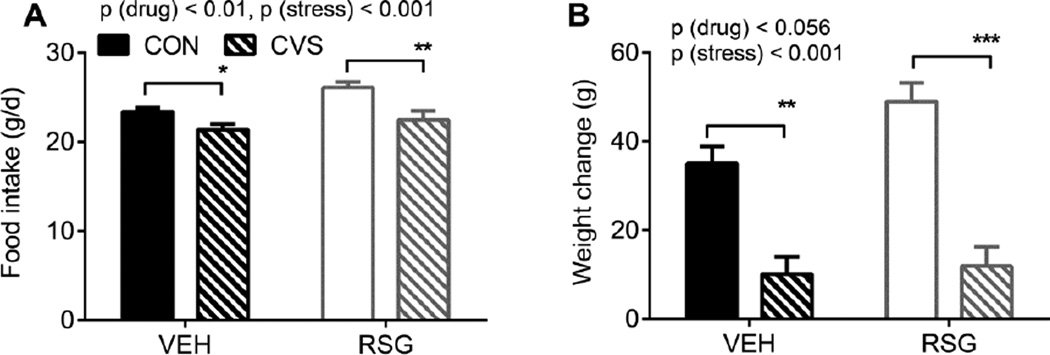
In agreement with the obesogenic effects of RSG [32–34] RSG-treated rats consumed more chow compared to vehicle-treated controls [2-way ANOVA p (drug) < 0.05 (Fig 6A)]. Also as expected from the literature [35], rats undergoing CVS consumed less chow [2-way ANOVA p (stress) < 0.05 (Fig 6A)] and gained less weight [2-way ANOVA p (stress) < 0.05 (Fig 6B)] compared to unstressed controls. Tukey’s posthoc tests indicated that VEH-CVS rats consumed less chow and gained less weight compared to VEH-CON rats [p< 0.05]. Likewise RSG-CVS rats consumed less chow and gained less weight compared to RSG-CON rats [p< 0.01]. Thus concurrent treatment with RSG did not blunt the stress-induced anorexia and weight loss.
Figure 6. Chronic variable stress (CVS), Rosigliatzone (RSG), and energy balance.
CVS significantly decreases, and RSG significantly increases, food intake (A). CVS significantly decreases body weight (B). Data represent mean ± SEM; *p<0.05, **p<0.01, ***p<0.001 by Tukey’s posthoc test; n = 17–19 rats per group.
4. DISCUSSION
The present findings support the hypothesis that chronic stress increases cardiovascular dysfunction. Specifically, we found that just two weeks of chronic variable stress induces an increase in pulse pressure, both during the active phase of the light-dark cycle and during the early morning when rats are at rest (Figs 2 and 3). This is significant since elevated pulse pressure is an independent risk factor for cardiovascular morbidity, including coronary heart disease [36,37], atrial fibrillation [38], myocardial infarction [39,40], congestive heart failure [41], and for cardiovascular mortality [21], independent of mean arterial pressure.
The arterial system acts to dampen pressure oscillations caused by intermittent ventricular ejection [17,42,43]. The efficiency of this dampening function depends on both elasticity and distensibility of arterial walls, as well as the geometry of the arteries themselves, including luminal area. In the present study, we observed significantly greater collagen deposition in the adventitial layer of the descending aorta in chronically stressed rats compared to unstressed controls, but no changes in luminal circumference (Fig 5). Such structural changes would result in lesser compliance of the aorta [18], leading to increased pulse pressure.
In contrast to our prediction, concurrent treatment with the PPARγ agonist RSG was not protective against CVS-induced cardiovascular dysfunction, despite that it blunted the corticosterone response to chronic stress (Fig 4). To the contrary, RSG exacerbated the adverse cardiovascular effects of CVS. Specifically we found that RSG itself elicited an increase in pulse pressure, and this began as early as the first stressor. Following the initial restraint challenge, RSG-treated rats exhibited a delayed recovery, characterized by significantly greater pulse pressure more than an hour after the onset of stress (Fig 1). Elevated pulse pressure persisted among RSG-treated rats throughout the experiment, during both the active and inactive phases of the light-dark cycle (Figs 2 and 3). Among rats receiving both RSG and undergoing CVS, the effects on pulse pressure were additive. Thus exposure to chronic stress significantly enhanced the negative consequences of RSG and vice versa, particularly during the early morning when animals were resting.
Despite being contrary to our initial hypothesis, the association between RSG and adverse cardiovascular endpoints is not entirely surprising. RSG was once widely prescribed for the treatment of diabetes, until a 2007 meta-analysis concluded that its use was associated with a significant increase in the risk of myocardial infarction, as well as a tendency to increase the risk of death from cardiovascular causes [44]. Those results led quickly to a black box warning and restrictions on its use [45]. Although these and subsequent (e.g. [46]) analyses have been extremely controversial [44,46–48] and FDA restrictions were subsequently relaxed [49], the cardiovascular safety profile of this drug remains a concern. This work suggests that chronic psychological stress may exacerbate any cardiovascular risk associated with RSG.
Frequent and/or persistent exposure to sympathetic and HPA stress responses during exposure to chronic stress is thought to create wear and tear on organs and systems, predisposing to disease [5]. Thus exposure to chronic psychological stress is associated with poor cardiovascular outcomes in numerous studies (reviewed in [1–3]). Here we find that exposure to just two weeks of chronic variable stress increases collagen deposition in the aortic adventitia of male rats, and that this is further associated with increased pulse pressure—itself a significant risk factor for cardiovascular morbidity and mortality [18,50–52]. Thus CVS may represent a useful rodent model for stress-associated cardiovascular disease, particularly in middle aged and older populations for which widening pulse pressure is a well-known risk factor. Moreover, we find that despite blunting the tachycardic response to acute stress, as well as the acute [6] and chronic glucocorticoid responses to stress, the TZD RSG is not protective against these adverse cardiovascular outcomes. Rather RSG itself is associated with increased pulse pressure and the effects of stress are additive to this, highlighting that chronic stress may represent an additional contributor to RSG-associated cardiovascular risk.
HIGHLIGHTS.
Chronic variable stress is associated with increased in pulse pressure.
Chronic variable stress is associated with increased collagen deposition in the aortic adventitia.
Treatment with Rosiglitazone during chronic variable stress additionally increases pulse pressure.
Acknowledgments
This work was supported by the NIH: R00HL111319 to KKR, F32DK102334 & T32DK059803 to AEBP, K99HL122454 to BM, and P30DK078392 (Integrative Morphology Core) of the Digestive Disease Research Core of Cincinnati Children’s Hospital. We thank James Graham (UC Davis) for performing the corticosterone and inflammation assays and William Li and Sri Ghosal (University of Cincinnati) for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ryan KK. Stress and Metabolic Disease. In: Weinstein M, Lane MA, editors. Soc. Hierarchy, Heal. Comp. Biodemography A Collect. Pap. US: National Academies Press; 2014. [accessed May 4, 2015]. pp. 247–267. http://www.ncbi.nlm.nih.gov/books/NBK242443/ [Google Scholar]
- 2.Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nat. Rev. Cardiol. 2012;9:360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- 3.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–2217. doi: 10.1161/01.cir.99.16.2192. http://www.ncbi.nlm.nih.gov/pubmed/10217662. [DOI] [PubMed] [Google Scholar]
- 4.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 1993;153:2093–2101. http://www.ncbi.nlm.nih.gov/pubmed/8379800. [PubMed] [Google Scholar]
- 6.Ryan KK, Grayson BE, Jones KR, Schneider AL, Woods SC, Seeley RJ, et al. Physiological responses to acute psychological stress are reduced by the PPARγ agonist rosiglitazone. Endocrinology. 2012;153:1279–1287. doi: 10.1210/en.2011-1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickavance LC, Tadayyon M, Widdowson PS, Buckingham RE, Wilding JP. Therapeutic index for rosiglitazone in dietary obese rats: separation of efficacy and haemodilution. Br. J. Pharmacol. 1999;128:1570–1576. doi: 10.1038/sj.bjp.0702932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egan AE, Ulrich-Lai YM. Activation of physiological stress responses by a natural reward: Novel vs. repeated sucrose intake. Physiol. Behav. 2015;150:30–38. doi: 10.1016/j.physbeh.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J, Thabet SR, Kirabo A, Trott DW, Saleh Ma, Xiao L, et al. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ. Res. 2014;114:616–625. doi: 10.1161/CIRCRESAHA.114.302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins TJ. ImageJ for microscopy. Biotechniques. 2007;43:25–30. doi: 10.2144/000112517. [DOI] [PubMed] [Google Scholar]
- 11.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Q, Dryden S, Frankish HM, Bing C, Pickavance L, Hopkins D, et al. Increased feeding in fatty Zucker rats by the thiazolidinedione BRL 49653 (rosiglitazone) and the possible involvement of leptin and hypothalamic neuropeptide Y. Br. J. Pharmacol. 1997;122:1405–1410. doi: 10.1038/sj.bjp.0701535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am. J. Physiol. Endocrinol. Metab. 2006;291:E965–E973. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- 14.Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. [accessed August 6, 2015];Neuroendocrinology. 1995 61:180–190. doi: 10.1159/000126839. http://www.ncbi.nlm.nih.gov/pubmed/7753337. [DOI] [PubMed] [Google Scholar]
- 15.Gómez F, Lahmame A, de Kloet ER, Armario A. Hypothalamic-pituitary-adrenal response to chronic stress in five inbred rat strains: differential responses are mainly located at the adrenocortical level. Neuroendocrinology. 1996;63:327–337. doi: 10.1159/000126973. [DOI] [PubMed] [Google Scholar]
- 16.Marin MT, Cruz FC, Planeta CS. Chronic restraint or variable stresses differently affect the behavior, corticosterone secretion and body weight in rats. Physiol. Behav. 2007;90:29–35. doi: 10.1016/j.physbeh.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 17.Safar ME. Pulse pressure, arterial stiffness and wave reflections (augmentation index) as cardiovascular risk factors in hypertension. Ther. Adv. Cardiovasc. Dis. 2008;2:13–24. doi: 10.1177/1753944707086652. [DOI] [PubMed] [Google Scholar]
- 18.Safar ME, Levy BI, Struijker-Boudier H. Current Perspectives on Arterial Stiffness and Pulse Pressure in Hypertension and Cardiovascular Diseases. Circulation. 2003;107:2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 19.Blacher J, Staessen Ja, Girerd X, Gasowski J, Thijs L, Liu L, et al. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch. Intern. Med. 2000;160:1085–1089. doi: 10.1001/archinte.160.8.1085. [DOI] [PubMed] [Google Scholar]
- 20.Vaccarino V, Holford TR, Krumholz HM. Pulse pressure and risk for myocardial infarction and heart failure in the elderly. J. Am. Coll. Cardiol. 2000;36:130–138. doi: 10.1016/s0735-1097(00)00687-2. doi:S0735-1097(00)00687-2 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Benetos A, Safar M, Rudnichi A, Smulyan H, Richard JL, Ducimetieère P, et al. Pulse pressure: a predictor of long-term cardiovascular mortality in a French male population. Hypertension. 1997;30:1410–1415. doi: 10.1161/01.hyp.30.6.1410. http://www.modern-psychiatry.com/pulse_pressure.htm\ nhttp://www.modern-psychiatry.com/pulsepressure.htm. [DOI] [PubMed] [Google Scholar]
- 22.Assmann G, Cullen P, Evers T, Petzinna D, Schulte H. Importance of arterial pulse pressure as a predictor of coronary heart disease risk in PROCAM. Eur. Heart J. 2005;26:2120–2126. doi: 10.1093/eurheartj/ehi467. [DOI] [PubMed] [Google Scholar]
- 23.Girerd X, Laurent S, Pannier B, Asmar R, Safar ME. Arterial distensibility and left ventricular hypertrophy in patients with sustained essential hypertension. Am. Heart J. 1991;122:1210–1214. doi: 10.1016/0002-8703(91)90941-a. http://www.ncbi.nlm.nih.gov/pubmed/1833966. [DOI] [PubMed] [Google Scholar]
- 24.Saba PS, Roman MJ, Pini R, Spitzer M, Ganau A, Devereux RB. Relation of arterial pressure waveform to left ventricular and carotid anatomy in normotensive subjects. J. Am. Coll. Cardiol. 1993;22:1873–1880. doi: 10.1016/0735-1097(93)90772-s. [DOI] [PubMed] [Google Scholar]
- 25.Arakawa K, Ishihara T, Aoto M, Inamasu M, Kitamura K, Saito A. An antidiabetic thiazolidinedione induces eccentric cardiac hypertrophy by cardiac volume overload in rats. Clin. Exp. Pharmacol. Physiol. 2004;31:8–13. doi: 10.1111/j.1440-1681.2004.03954.x. [DOI] [PubMed] [Google Scholar]
- 26.Duan SZ, Ivashchenko CY, Russell MW, Milstone DS, Mortensen RM. Cardiomyocyte-specific knockout and agonist of peroxisome proliferator-activated receptor-gamma both induce cardiac hypertrophy in mice. Circ. Res. 2005;97:372–379. doi: 10.1161/01.RES.0000179226.34112.6d. [DOI] [PubMed] [Google Scholar]
- 27.Barbaro NR, Fontana V, Modolo R, De Faria AP, Sabbatini AR, Fonseca FH, et al. Increased arterial stiffness in resistant hypertension is associated with inflammatory biomarkers. Blood Press. 2015;24:7–13. doi: 10.3109/08037051.2014.940710. [DOI] [PubMed] [Google Scholar]
- 28.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. http://www.ncbi.nlm.nih.gov/pubmed/11566912. [DOI] [PubMed] [Google Scholar]
- 29.Yasmin CM, McEniery CM, Wallace S, Mackenzie IS, Cockcroft JR, Wilkinson IB. C-reactive protein is associated with arterial stiffness in apparently healthy individuals. Arterioscler. Thromb. Vasc. Biol. 2004;24:969–974. doi: 10.1161/01.ATV.zhq0504.0173. [DOI] [PubMed] [Google Scholar]
- 30.Kampus P, Kals J, Ristimäe T, Fischer K, Zilmer M, Teesalu R. High-sensitivity C-reactive protein affects central haemodynamics and augmentation index in apparently healthy persons. J. Hypertens. 2004;22:1133–1139. doi: 10.1097/00004872-200406000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain. Behav. Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Ryan KK, Li B, Grayson BE, Matter EK, Woods SC, Seeley RJ. A role for central nervous system PPAR-γ in the regulation of energy balance. Nat. Med. 2011;17:623–626. doi: 10.1038/nm.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eldershaw TP, Rattigan S, Cawthorne MA, Buckingham RE, Colquhoun EQ, Clark MG. Treatment with the thiazolidinedione (BRL 49653) decreases insulin resistance in obese Zucker hindlimb. Horm. Metab. Res. = Horm. Und Stoffwechselforsch. = Horm. Métabolisme. 1995;27:169–172. doi: 10.1055/s-2007-979932. [DOI] [PubMed] [Google Scholar]
- 34.Patel J, Anderson RJ, Rappaport EB. Rosiglitazone monotherapy improves glycaemic control in patients with type 2 diabetes: a twelve-week, randomized, placebo-controlled study. Diabetes. Obes. Metab. 1999;1:165–172. doi: 10.1046/j.1463-1326.1999.00020.x. http://www.ncbi.nlm.nih.gov/pubmed/11220295. [DOI] [PubMed] [Google Scholar]
- 35.Martí O, Martí J. a Armario, Effects of chronic stress on food intake in rats: influence of stressor intensity and duration of daily exposure. [accessed July 16, 2015];Physiol. Behav. 1994 55:747–753. doi: 10.1016/0031-9384(94)90055-8. http://www.ncbi.nlm.nih.gov/pubmed/8190805. [DOI] [PubMed] [Google Scholar]
- 36.Glasser SP, Halberg DL, Sands C, Gamboa CM, Muntner P, Safford M. Is pulse pressure an independent risk factor for incident acute coronary heart disease events? The REGARDS study. Am. J. Hypertens. 2014;27:555–563. doi: 10.1093/ajh/hpt168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franklin SS, Khan SA, Wong ND, Larson MG, Levy D. Is pulse pressure useful in predicting risk for coronary heart Disease? The Framingham heart study. Circulation. 1999;100:354–360. doi: 10.1161/01.cir.100.4.354. [DOI] [PubMed] [Google Scholar]
- 38.Valbusa F, Bonapace S, Bertolini L, Zenari L, Arcaro G, Targher G. Increased pulse pressure independently predicts incident atrial fibrillation in patients with type 2 diabetes. Diabetes Care. 2012;35:2337–2339. doi: 10.2337/dc12-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darne B, Girerd X, Safar M, Cambien F, Guize L. Pulsatile versus steady component of blood pressure: a cross-sectional analysis and a prospective analysis on cardiovascular mortality. Hypertension. 1989;13:392–400. doi: 10.1161/01.hyp.13.4.392. http://www.ncbi.nlm.nih.gov/pubmed/2522417. [DOI] [PubMed] [Google Scholar]
- 40.Madhavan S, Ooi WL, Cohen H, Alderman MH. Relation of pulse pressure and blood pressure reduction to the incidence of myocardial infarction. Hypertension. 1994;23:395–401. doi: 10.1161/01.hyp.23.3.395. http://www.ncbi.nlm.nih.gov/pubmed/7993447. [DOI] [PubMed] [Google Scholar]
- Chae CU, Pfeffer Ma, Glynn RJ, Mitchell GF, Taylor JO, Hennekens CH. Increased pulse pressure and risk of heart failure in the elderly. JAMA. 1999;281:634–639. doi: 10.1001/jama.281.7.634. http://www.ncbi.nlm.nih.gov/pubmed/10029125. [DOI] [PubMed] [Google Scholar]
- 42.Safar ME, Nilsson PM, Blacher J, Mimran A. Pulse pressure, arterial stiffness, and end-organ damage. Curr. Hypertens. Rep. 2012;14:339–344. doi: 10.1007/s11906-012-0272-9. [DOI] [PubMed] [Google Scholar]
- 43.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 44.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N. Engl. J. Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 45.Woodcock J, Sharfstein JM, Hamburg M. Regulatory action on rosiglitazone by the U.S. Food and Drug Administration. N. Engl. J. Med. 2010;363:1489–1491. doi: 10.1056/NEJMp1010788. [DOI] [PubMed] [Google Scholar]
- 46.Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007;298:1189–1195. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- 47.Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, et al. Rosiglitazone evaluated for cardiovascular outcomes--an interim analysis. N. Engl. J. Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 48.Home PD, Pocock SJ, Beck-Nielsen H, Curtis PS, Gomis R, Hanefeld M, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet (London, England) 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 49.Tucker ME. FDA panel advises easing restrictions on rosiglitazone. BMJ. 2013;346:f3769. doi: 10.1136/bmj.f3769. [DOI] [PubMed] [Google Scholar]
- 50.Schlatmann TJM, Becker AE. Histologic changes in the normal aging aorta: implications for dissecting aortic aneurysm. Am. J. Cardiol. 1977;39:13–20. doi: 10.1016/s0002-9149(77)80004-0. [DOI] [PubMed] [Google Scholar]
- 51.Tsamis A, Krawiec JT, Vorp Da. Elastin and collagen fibre microstructure of the human aorta in ageing and disease: a review. J. R. Soc. Interface. 2013;10:20121004. doi: 10.1098/rsif.2012.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steppan J, Barodka V, Berkowitz DE, Nyhan D. Vascular stiffness and increased pulse pressure in the aging cardiovascular system. Cardiol. Res. Pract. 2011;2011:263585. doi: 10.4061/2011/263585. [DOI] [PMC free article] [PubMed] [Google Scholar]