Abstract
The Achilles tendon is the most commonly ruptured tendon in the human body. Numerous studies have reported incidence of these injuries to be upwards of five times as common in men than women. Therefore, the objective of this study was to investigate the sex- and hormone-specific differences between Achilles tendon and muscle between female, ovariectomized female (ovarian hormone deficient), and male rats. Uninjured tissues were collected from all groups for mechanical, structural, and histological analysis. Our results showed that while cross-sectional area and failure load were increased in male tendons, female tendons exhibited superior tendon material properties and decreased muscle fiber size. Specifically, linear and dynamic moduli were increased while viscoelastic properties (e.g., hysteresis, percent relaxation) were decreased in female tendons, suggesting greater resistance to deformation under load and more efficient energy transfer, respectively. No differences were identified in tendon organization, cell shape, cellularity, or proteoglycan content. Additionally, no differences in muscle fiber type distribution were observed between groups. In conclusion, inferior tendon mechanical properties and increased muscle fiber size may explain the increased susceptibility for Achilles tendon injury observed clinically in men compared to women.
Keywords: Mechanics, Injury, Fatigue, Gender, Ankle, Skeletal Muscle, Sex Characteristics, Tensile Strength, Elasticity, Orthopaedics
INTRODUCTION
Achilles tendon ruptures are common and devastating injuries that result in significant pain, disability, and healthcare costs. The overall incidence of Achilles tendon ruptures has risen 10-fold over the last three decades, with similar annual increases across sexes14. Epidemiological studies currently estimate rupture rates between 7 and 40 per 100,000 person-years10. It has been suggested that this considerable surge in rupture rate is at least in part due to increased sports participation by the middle-aged population16. However, although men and women both experience Achilles tendon ruptures at similar ages and sport participation times, it is surprising that as many as 84% of all ruptures occur in men25.
The mechanism underlying this apparent predisposition to Achilles tendon rupture in men – including whether it is the role of tendon, of muscle, or of both that is most important in mediating this increased injury risk – remains unclear. Estrogen receptors have been identified in lower limb tendons and ligaments2, 15, and reduced fibroblast proliferation and type I procollagen synthesis have been observed with increased estrogen concentration30. Recent clinical data in humans further suggest that sex hormones may directly influence tendon mechanical properties3. Long-term attenuation of estrogen in women was associated with increased Achilles tendon stiffness, which may be explained by increased collagen synthesis in response to mechanical loading3. In muscle, sex hormones have also been shown to play a role. Orchiectomy (in males), as well as estrogen administration (in females), have been associated with decreases in muscle fiber diameter, which may decrease force potential, without changes in fiber type11. Furthermore, it has been suggested that the typically larger muscle mass and greater contraction force potential in men compared to women may mean that the male gastrocnemius muscle is more likely to exceed the ultimate strength of the Achilles tendon and thereby increase the risk of spontaneous rupture25.
Therefore, the objective of this study was to determine the mechanical, structural, and histological properties of the uninjured Achilles tendon-muscle complex in female, ovariectomized female (OVX), and male rats in order to better understand the sex-related difference in Achilles tendon injury risk. We hypothesized that female rats would exhibit equal tendon mechanical, structural, and histological properties, but decreased muscle fiber size compared to OVX and male rats.
MATERIALS AND METHODS
Study Design
Male (N=16), female (N=16), and ovariectomized female (OVX) (N=16) Sprague Dawley rats were euthanized at approximately five months of age (six weeks after OVX performed in relevant subgroup) (IACUC approved). Previous studies in female rats demonstrated significant alterations in bone morphology and muscle mass as early as two and four weeks, respectively, following ovariectomy6, 13.
Tendon and Muscle Sample Preparation
At the time of sacrifice, left and right Achilles tendon-foot complexes were harvested en bloc and either fixed for histological analysis in 4% paraformaldehyde or frozen until preparation for high-frequency ultrasound (HFUS) analysis and mechanical testing, as described19. Briefly, tendons were thawed and fine dissected to remove any non-tendinous tissue. Stain dots were then applied to the tendon for optical strain analysis, and cross-sectional areas (CSA) of tendons were measured using a custom laser-based device5, 7. Specifically, the tendon area was measured at 0, 3, 6, 9, and 12mm from the calcaneal insertion to construct an interpolated mesh representing the specimen volume. Next, the average local cross-sectional area from 3–9mm was determined and used to calculate all material properties. The calcaneus-foot complex was embedded in PMMA, and the free end of the tendon was glued between two pieces of sandpaper, leaving a gauge length of 12mm. Tendons from all groups were randomized to blind a single dissector at the time of fine dissection and subsequent testing and analysis. No two tendons from a single animal were assigned to the same assay.
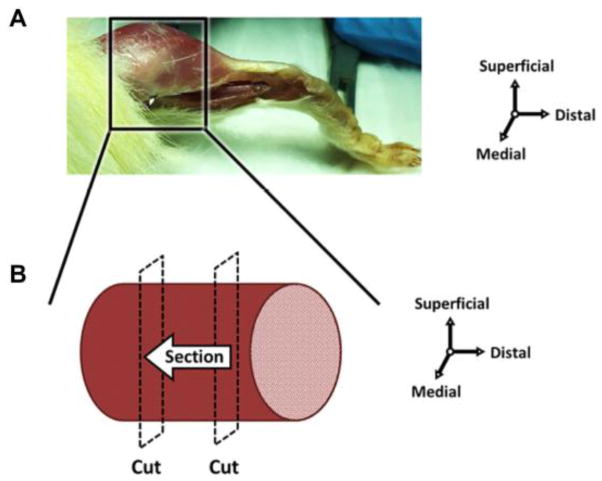
Muscle tissue from the posterior hindlimb was excised at time of sacrifice, embedded in optimal cutting temperature (OCT) compound, and flash frozen with N-methyl butane in liquid nitrogen for histological analysis. Prior to sectioning, distal and proximal portions of the muscle tissue were removed, effectively isolating the muscle belly (Figure 1). Muscle tissue samples (N=8/group) were stored at −80°C until cryo-sectioned axially at 10μm for immunofluorescence analysis.
Figure 1. Schematic of muscle harvest for immunohistochemistry.
(A) Muscle tissue was excised posterior to the tibia and proximal to the myotendinous junction, and then flash frozen. (B) Prior to sectioning, distal and proximal portions of the muscle tissue were removed, effectively isolating the muscle belly. The muscle was sectioned perpendicular to the long axis of the fibers from the distal to proximal direction.
High Frequency Ultrasound (HFUS) Assessment of Tissue Structure
Tendons (n=12/group) were loaded at 1N in a 1X PBS bath while a series of sagittal B-mode images were acquired using a 40MHz scanner (MS550D; VisualSonics, CA). Mean fiber alignment, a measure of extracellular matrix organization, was quantified by computing the circular standard deviation (CSD) across 3–4 of the centermost HFUS images (each spaced 0.5mm) using a custom MATLAB (Mathworks, Natick, MA) program19. Briefly, intensity versus angle data was fit to a power series function to determine the angle with the maximum intensity (fiber direction). Circular standard deviation was calculated for local fiber directions and averaged to obtain a representative value of tendon organization (increased CSD indicates increased disorganization).
Mechanical Testing
Bone-tendon units were gripped using custom fixtures to maintain a 90° angle between the foot and Achilles tendon, similar to in vivo physiological loading. The fixtures were attached to a testing frame (Electropuls E3000, Instron, Norwood, MA) using a 250 N load cell, and all specimens remained submerged in a 1X PBS bath maintained at 37°C. Tendons were then subjected to one of two mechanical testing protocols:
Quasi-static: Preconditioning and ramp to failure at 0.1%strain/sec (N=8/group).
Fatigue: Preconditioning, stress relaxation for 10 minutes at 6% strain, a low-load dynamic frequency sweep (at 0.1, 1, 5, and 10Hz, for 10 cycles each), and fatigue testing at 2 Hz using a sinusoidal waveform under load control (from ~7–40% of ultimate stress) until failure (N=12/group).
Force and displacement data were acquired between 100–1000 Hz using the WaveMatrix (Instron, Norwood, MA) data acquisition software and analyzed using custom MATLAB code (Mathworks; Natick, MA). Images for optical strain measures were captured using a Basler a102F camera (Basler; Exton, PA) and Nikon AF Micro-NIKKOR 200mm lens (Nikon; Melville, NY). From these tests, both quasi-static mechanical properties (stiffness and modulus, percent relaxation, failure load and stress) and dynamic mechanical properties (dynamic modulus, tan(δ), peak strain, hysteresis, laxity, and secant modulus) were computed. Note that laxity is defined as the percent change in nonrecoverable length relative to the tendon’s original length and has been suggested as a potential metric of tendon damage during fatigue loading9.
Tendon Histology
Achilles tendons (N=8/group) were processed using standard paraffin techniques, sectioned sagittally at 7 μm, and stained with Hematoxylin-Eosin (H&E) or Safranin-O/Fast Green1. Three blinded investigators (provided with previously prepared standard images) independently graded 100X images on a scale of 1–3 for cell shape (1, spindle shaped; 2, mixed; 3, rounded), cellularity (1, low; 2, moderate; 3, high), and proteoglycan staining intensity (1, no red; 2, light red; 3, light to dark red)22.
Muscle Histology
Sections were blocked with 4% BSA and incubated 1:100 in antibodies against laminin (L9393, Sigma Aldrich; St. Louis, MO) and myosin heavy chain (MyHC) types 1, 2a, and 2b (type 1: BA-D5; type 2a: SC-71; type 2b: BF-F3). The anti-MyHC antibodies developed by Stefano Schiaffino were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242. MyHC2x expression was presumed from unstained fibers. Fluorescence conjugated secondary antibodies (Goat anti-mouse IgG2b 1:100 DyLight 405, IgG1 1:400 Alexa 488, and IgM 1:500 Alexa 546) were used for MyHC types 1, 2a, and 2b fiber type visualization, respectively (ThermoFisher; Waltham, MA). Anti-rabbit IgG (Alexa 488) was used for laminin staining. Slides were mounted with ProLong Gold anti-fade without DAPI. Three images were taken from both the superficial and deep regions of each muscle. Images were subsequently analyzed for fiber size (minimum Feret diameter) and fiber type distribution using the SMASH application21.
Statistics
One-way ANOVAs were used to compare groups for all assays except tendon histology. Significant relationships were further evaluated using post hoc t-tests with Bonferroni corrections (α=0.05/3). Non-parametric Kruskal-Wallis tests were used to evaluate differences in tendon histological properties between groups (α=0.05). All data are presented as mean ± standard deviation except for tendon histology, which is reported as median (interquartile range).
RESULTS
Male rats (541±31 g) were significantly heavier than female (305±25 g) and OVX rats (349±19 g) (p<0.001). Six weeks post-ovariectomy, OVX rats were significantly heavier than age-matched female rats (p<0.001).
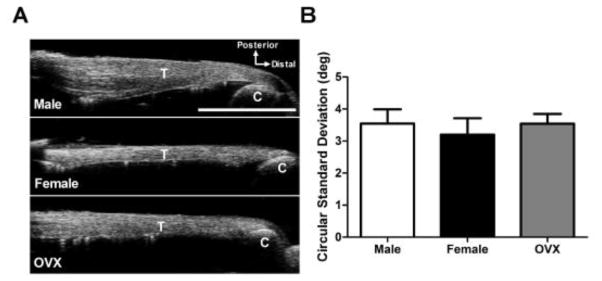
HFUS revealed no differences in extracellular matrix organization between groups (Figure 2). All tendons exhibited collagen fibers that were well-aligned along the long axis of the tissue.
Figure 2 . High Frequency Ultrasound (HFUS) Assessment of Tendon Structure.
(A) Representative HFUS images of uninjured male, female, and OVX Achilles tendons. (B) Extracellular matrix organization was similar between groups according to the circular standard deviation of collagen fiber orientation (measure of alignment). Key: T – tendon; C – calcaneus.
*Scale bar represents 5mm. Data represented as means and error bars indicating standard deviation.
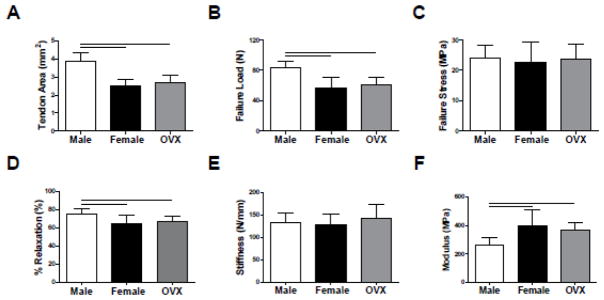
The CSA of Achilles tendons from male rats was 56% and 44% larger than female and OVX tendons, respectively (p<0.001) (Figure 3A). Male tendons also had increased failure loads (p<0.001), although there was no difference in failure stress between groups (Figure 3B–C). Percent relaxation was decreased in both female and OVX tendons compared to male tendons (p<0.01) (Figure 3D). Tendon linear stiffness was similar across groups, but linear modulus was greater in female and OVX tendons (p<0.01) (Figure 3E–F).
Figure 3. Achilles Tendon Quasi-Static and Viscoelastic Mechanical Properties.
Compared to female and OVX tendons, male tendons demonstrated increased (A) cross-sectional area and (B) failure load, but not (C) failure stress. (D) Percent relaxation was increased in male tendons, suggesting less of an elastic response to mechanical load than in female and OVX tendons. Despite differences in cross-sectional area, all groups exhibited similar (E) tendon stiffness. However, (F) modulus was inferior in male tendons.
*Data represented as means and error bars indicating standard deviation. Solid lines indicate significant differences (p<0.05/3).
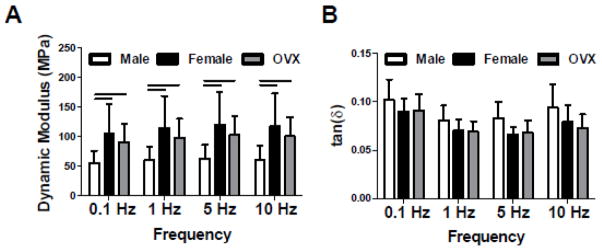
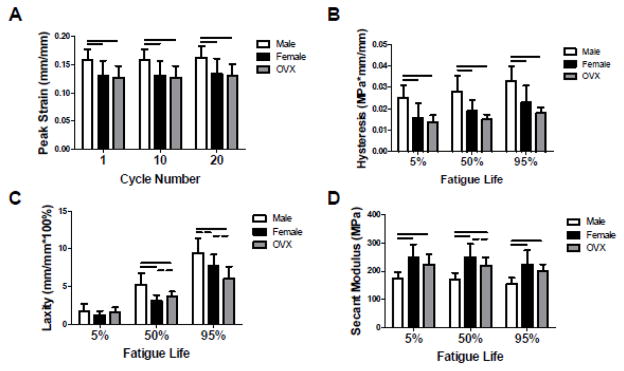
Similar to the linear modulus results, female and OVX tendons exhibited nearly double the dynamic modulus of male tendons at all frequencies tested (p<0.01) (Figure 4A). However, there were no differences in tan(δ) between groups at any frequency (Figure 4B). Male tendons demonstrated increased peak strain (p<0.013) and hysteresis (p<0.001) compared to female and OVX tendons throughout fatigue loading, as well as increased laxity at 50% (p<0.01) and 95% (p<0.05) fatigue life (Figure 5A–C). Secant modulus was superior in female and OVX tendons compared to male tendons throughout fatigue life (p<0.001) (Figure 5D). Furthermore, the secant modulus of female tendons trended higher than OVX tendons at 50% fatigue life (p<0.10).
Figure 4 . Achilles Tendon Dynamic Mechanical Properties.
At all frequencies tested, male tendons had decreased (A) dynamic modulus compared to female and OVX tendons. (B) Tan(δ), a measure of energy dissipation, was not different between groups at any frequency.
*Data represented as means and error bars indicating standard deviation. Solid lines indicate significant differences (p<0.05/3).
Figure 5 . Achilles Tendon Fatigue Mechanical Properties.
Early in fatigue testing, (A) peak strain was elevated in male tendons. (B) Hysteresis was increased in male tendons throughout fatigue life. At 50% and 95% fatigue life, (C) laxity (nonrecoverable length) was increased in male tendons compared to female and OVX tendons. Male tendons also had inferior (D) modulus throughout fatigue life. There was a trend toward increased modulus in female compared to OVX tendons at 50% fatigue life, indicating a potential hormone-specific difference in tendon mechanical integrity.
*Data represented as means and error bars indicating standard deviation. Solid lines indicate significant differences (p<0.05/3) and dashed lines indicate trends (p<0.10).
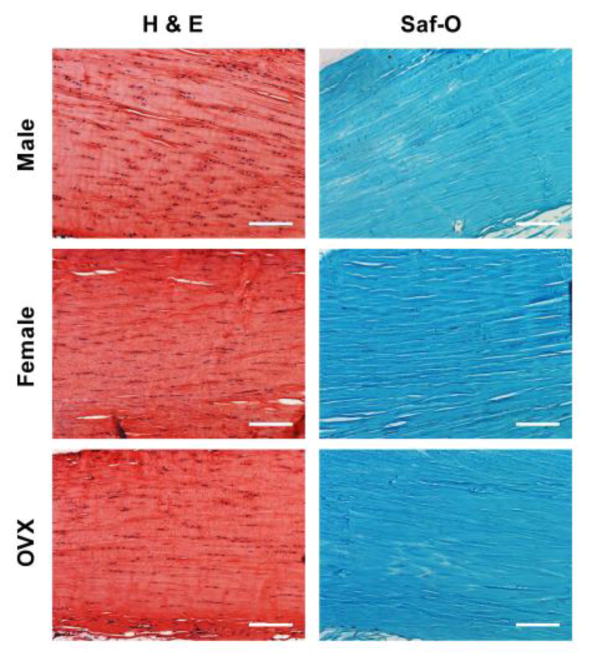
There were no differences in tendon histology parameters between groups (Table 1). Each group exhibited cells with a high aspect ratio along the long axis of the tendon (spindle-shaped), along with low/moderate cellularity and proteoglycan content (Figure 6).
Table 1 . Tendon Histology Semi-Quantitative Analysis.
No differences between experimental groups in tendon cell shape, cellularity, or proteoglycan content were observed by blinded grading of Hematoxylin-Eosin (H&E) or Safranin-O/Fast Green stained tissue sections.
*Data presented as median (interquartile range).
| Cell Shape | Cellularity | Proteoglycan Content | |
|---|---|---|---|
| Male | 1.8 (1.0–2.1) | 2.0 (1.0–3.0) | 2.5 (1.6–3.0) |
| Female | 2.0 (1.0–3.0) | 2.0 (2.0–3.0) | 2.0 (1.5–2.0) |
| OVX | 2.0 (1.5–2.0) | 2.0 (2.0–3.0) | 2.0 (2.0–2.0) |
| p-value | 0.55 | 0.81 | 0.32 |
Figure 6 . Achilles Tendon Histology.
Representative images from semi-quantitative analysis of tendon cellularity and cell shape (H&E), and proteoglycan content (Saf-O).
*Scale bar represents 150 μm.
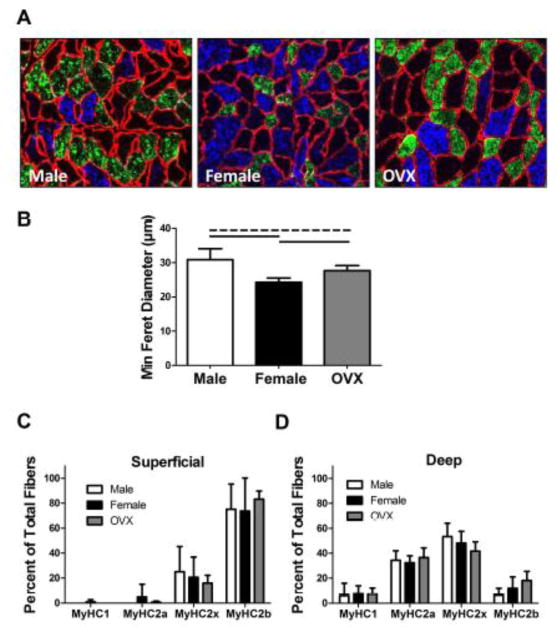
In muscle, there was decreased fiber size in female rats compared to both male and OVX rats (p<0.001) (Figure 7A–B). Additionally, male rats had larger muscle fibers than OVX rats (p=0.034). Muscle fiber type distribution did not differ between groups (Figure 7C–D). However, muscles from all animals revealed more type 2b (fast-twitch glycolytic) fibers in the superficial region and more type 2a (fast-twitch oxidative) fibers in the deep region.
Figure 7 . Muscle Fiber Size and Type Distribution.
(A) Representative images displaying muscle fiber size (fiber membrane shown in red by fluorescent tagging of laminin). (B) Fiber size was increased in males compared to females (significant) and OVX (trend). Additionally, OVX animals demonstrated greater fiber size than females. Fiber types were determined by fluorescent tagging of myosin heavy chain (MyHC) types 1, 2a, and 2b. MyHC type 2x expression was presumed from unstained fibers. Fiber type distribution was similar between groups in both the (C) superficial and (D) deep regions of the muscle. However, more type 2b (fast-twitch glycolytic) fibers were evident in the superficial region and more type 2a (fast-twitch oxidative) fibers were evident in the deep region for all groups.
*Data represented as means and error bars indicating standard deviation. Solid lines indicate significant differences (p<0.05/3) and dashed lines indicate trends (p<0.10).
DISCUSSION
The Achilles tendon is the most frequently ruptured tendon in humans25. The incidence of Achilles tendon ruptures continues to climb, which is likely influenced by increased levels of physical activity in an aging population16. These injuries are substantially more common in men, although the reason for this apparent susceptibility has yet to be elucidated. Previous studies have demonstrated that sex hormones can alter tendon and muscle mechanobiology, yet their specific role in modulating Achilles tendon injury risk remains unclear20, 23. In order to better understand the predisposition of men to Achilles tendon ruptures, we investigated sex- and hormone-specific differences in Achilles tendon and muscle properties.
Contrary to our hypothesis, female rats exhibited superior Achilles tendon quasi-static, dynamic, and fatigue mechanical properties compared to male, but not OVX rats. The increased modulus of female Achilles tendons indicates that they are more resistant to deformation under stress, which is supported further by the decreases detected in peak strain and laxity. Interestingly, the differences identified in peak strain conflict with a previous study which showed increased Achilles tendon strain (but no differences in tendon thickness or calf maximum plantarflexion force) in females with normal versus attenuated estrogen levels3. In the previous study, strain was measured at a given force across groups, whereas in our study, fatigue testing was stress-controlled (i.e. male tendons were cycled at a higher maximum load than female tendons). Distinct from this contrast in testing protocol, decreases in viscoelastic properties (percent relaxation, hysteresis) suggest that female tendons more efficiently store and release elastic energy following deformation, which is critical given the repeated loading endured by the Achilles tendon in vivo. This is also supported by the lack of difference in tendon laxity at 5% fatigue life, but increased laxity in male tendons compared to female tendons at 50% and 95% fatigue life, indicating that female tendons appear to remain more elastic during persistent mechanical loading. Overall, these results may partially explain why females are less likely to rupture their Achilles tendon despite their inferior failure load compared to males.
Muscle fiber size was decreased in female rats compared to OVX and male rats, which supports our hypothesis. This is consistent with previous work that demonstrated increased rat soleus and caudofemoralis muscle fiber size following ovariectomy and decreased fiber size following estrogen administration11. Muscle fiber size is positively associated with (though not the sole determinant of) muscle strength, which suggests that female Achilles tendons may be “protected” from overloading by a weaker gastrocnemius muscle24. Such a theory is challenged, however, by a previous study that did not find changes in maximal specific isometric soleus muscle force as a result of ovariectomy, despite seeing a reduction in muscle fiber size in response to estrogen treatment17. However, this study did not include male rats, which we showed to have larger muscles than OVX and female rats. Taken in context with our study, it is possible that males have greater maximum muscle force potential than females, and this should be investigated in future studies.
As hypothesized, all groups exhibited similar structural and histological properties. In an uninjured state, tendons are primarily composed of highly organized type-1 collagen fibers that bundle tightly along the tissue’s longitudinal axis12. Combined with small matrix molecules including proteoglycans, these fibers form a hierarchical structure that contributes to efficient and effective mechanical performance8. Previous work has reported that estrogen reduces fibroblast proliferation30; however, it is challenging to directly compare their results to our study, as exogenous estrogen treatment of isolated fibroblasts in vitro is quite different than evaluating cellularity in female tendons following ovariectomy. Furthermore, our semi-quantitative method does not directly investigate cell proliferation, but rather, overall cell density within a tissue. We have used this method extensively and thus believe we have the sensitivity to measure meaningful differences in tendon cellularity. Our histological images and HFUS structural findings are consistent with previous characterization of the relatively hypocellular and well-aligned collagen structure of healthy tendon4.
Achilles tendon ruptures are one of the few musculoskeletal injuries that are less prevalent in females25. Anterior cruciate ligament (ACL) rupture, for example, is disproportionately more common in female athletes18. Furthermore, studies have demonstrated an increased risk of ACL rupture in women during the ovulatory phase of the menstrual cycle26 and decreased risk in women using oral contraceptives28, 29. While these findings suggest a critical role for sex hormones in modulating ACL injury risk, the direct effect of estrogen treatment on matrix production24 and mechanical properties26, 30 remains controversial. Similarly, rotator cuff pathology is more common in women than men, with 90 cases per 100,000 person-years in women and 83 per 100,000 person-years in men27. These data, taken together with the current work, suggest that the role of sex hormones in tendon/ligament physiology and injury risk may be tissue-specific.
There are limitations to this study. First, it should be acknowledged that this study utilizes an animal model and thus cannot perfectly replicate the human condition. Several studies have suggested that Achilles tendon degeneration may be a predisposing factor for rupture16, 25, which was not investigated here. The use of generally healthy rats for this work allowed for a highly controlled and consistent cohort in order to focus on biomechanical differences in uninjured Achilles tendons between sexes. The study of normal tendon properties is also useful for understanding the manner in which tendon injuries may develop initially. For example, the increased laxity observed in male tendons (which may be considered a metric of tendon damage9) may indicate that male Achilles tendons are more susceptible to degeneration in response to physical activity. Next, we investigated hormone-specific differences between female and OVX rats only six weeks after ovariectomy. Although this time point was appropriate given previous studies in ovariectomized rodents12, it is possible that a longer time point would reveal more differences between these two groups (as was observed between female and male rats). This concept of a long-term (but not short-term) effect of estrogen is supported by a human study that did not find changes in Achilles tendon strain in response to acute estrogen level changes (throughout a single menstrual cycle), but did observe differences between females who used oral birth control for at least 12 months compared to those who did not3. Despite studying tendon and muscle only six weeks after ovariectomy, our current study does demonstrate decreased muscle fiber size and increased secant modulus at 50% fatigue life in female tendons compared to OVX tendons; this suggests that sex hormones do affect Achilles tendon and muscle homeostasis, even in the short-term. Additionally, although the muscle harvested in this study was predominantly from the gastrocnemius, it cannot be excluded that a minor portion of the soleus may have also been included in the deep region analyzed. Lastly, some studies have reported that women tend to be slightly older than men at the time of Achilles tendon rupture16, 25. Although this age discrepancy was not the focus of the current work, future studies could investigate the effect of aging on Achilles tendon injury risk.
In conclusion, this study begins to define the mechanobiological basis for the disproportionate incidence of Achilles tendon rupture across sexes. Specifically, we evaluated the mechanical, structural, and histological properties of healthy Achilles tendon and muscle from female, OVX, and male rats. This study identified inferior tendon mechanical properties and increased muscle fiber size as a potential explanation for the increased susceptibility for Achilles tendon damage observed clinically in men compared to women. It is possible that poorer tendon properties lead to worse degeneration over time, which coupled with greater muscle strength, are responsible for the increased rates of spontaneous rupture in middle-aged men. Future studies should incorporate additional molecular characterization, which may provide a biological mechanism to explain the sex- and hormone-related differences in tendon and muscle properties identified here.
Acknowledgments
Grant Support: This study was funded by NIH/NIAMS R01AR064216S1, the NIH/NIAMS supported Penn Center for Musculoskeletal Disorders (P30 AR050950), the NIH/NCATS (TL1TR000138), the NIH/NIAMS T32AR007132, and NSF GRFP.
This study was funded by NIH/NIAMS R01AR064216S1, the NIH/NIAMS supported Penn Center for Musculoskeletal Disorders (P30 AR050950), the NIH/NCATS (TL1TR000138), the NIH/NIAMS T32AR007132, and NSF GRFP. The authors thank Dan Choi and Cori Riggin for their contributions.
Footnotes
Study approved by: University of Pennsylvania IACUC.
Author Contributions: All authors were fully involved in the study and preparation of the manuscript.
The manuscript has been read and approved by all of the authors.
References
- 1.Archambault JM, Jelinsky SA, Lake SP, Hill AA, Glaser DL, Soslowsky LJ. Rat supraspinatus tendon expresses cartilage markers with overuse. Journal of Orthopaedic Research. 2007;25:617–624. doi: 10.1002/jor.20347. [DOI] [PubMed] [Google Scholar]
- 2.Bridgeman JT, Zhang Y, Donahue H, Wade AM, Juliano PJ. Estrogen receptor expression in posterior tibial tendon dysfunction: a pilot study. Foot and Ankle International. 2010;31:1081–1084. doi: 10.3113/FAI.2010.1081. [DOI] [PubMed] [Google Scholar]
- 3.Bryant AL, Clark RA, Bartold S, Murphy A, Bennell KL, Hohmann E, Marshall-Gradisnik S, Payne C, Crossley KM. Effects of estrogen on the mechanical behavior of the human Achilles tendon in vivo. J Appl Physiol (1985) 2008;105:1035–1043. doi: 10.1152/japplphysiol.01281.2007. [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain CS, Duenwald-Kuehl SE, Okotie G, Brounts SH, Baer GS, Vanderby R. Temporal healing in rat achilles tendon: ultrasound correlations. Annals of Biomedical Engineering. 2013;41:477–487. doi: 10.1007/s10439-012-0689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Favata M. Bioengineering. Philadelphia: University of Pennsylvania; 2006. Scarless healing in the fetus: implications and strategies for postnatal tendon repair. [Google Scholar]
- 6.Fisher JS, Kohrt WM, Brown M. Food restriction suppresses muscle growth and augments osteopenia in ovariectomized rats. J Appl Physiol (1985) 2000;88:265–271. doi: 10.1152/jappl.2000.88.1.265. [DOI] [PubMed] [Google Scholar]
- 7.Freedman BR, Gordon JA, Bhatt PB, Pardes AM, Thomas SJ, Sarver JJ, Riggin CN, Tucker JJ, Williams AW, Zanes RC, Hast MW, Farber DC, Silbernagel KG, Soslowsky LJ. Nonsurgical treatment and early return to activity leads to improved Achilles tendon fatigue mechanics and functional outcomes during early healing in an animal model. Journal of Orthopaedic Research. 2016 doi: 10.1002/jor.23253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman BR, Gordon JA, Soslowsky LJ. The Achilles tendon: fundamental properties and mechanisms governing healing. Muscles Ligaments Tendons J. 2014;4:245–255. [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman BR, Zuskov A, Sarver JJ, Buckley MR, Soslowsky LJ. Evaluating changes in tendon crimp with fatigue loading as an ex vivo structural assessment of tendon damage. Journal of Orthopaedic Research. 2015;33:904–910. doi: 10.1002/jor.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huttunen TT, Kannus P, Rolf C, Fellander-Tsai L, Mattila VM. Acute achilles tendon ruptures: incidence of injury and surgery in Sweden between 2001 and 2012. American Journal of Sports Medicine. 2014;42:2419–2423. doi: 10.1177/0363546514540599. [DOI] [PubMed] [Google Scholar]
- 11.Kobori M, Yamamuro T. Effects of gonadectomy and estrogen administration on rat skeletal muscle. Clin Orthop Relat Res. 1989:306–311. [PubMed] [Google Scholar]
- 12.Lake SP, Miller KS, Elliott DM, Soslowsky LJ. Effect of fiber distribution and realignment on the nonlinear and inhomogeneous mechanical properties of human supraspinatus tendon under longitudinal tensile loading. Journal of Orthopaedic Research. 2009;27:1596–1602. doi: 10.1002/jor.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambers FM, Kuhn G, Schulte FA, Koch K, Muller R. Longitudinal assessment of in vivo bone dynamics in a mouse tail model of postmenopausal osteoporosis. Calcified Tissue International. 2012;90:108–119. doi: 10.1007/s00223-011-9553-6. [DOI] [PubMed] [Google Scholar]
- 14.Lantto I, Heikkinen J, Flinkkila T, Ohtonen P, Leppilahti J. Epidemiology of Achilles tendon ruptures: increasing incidence over a 33-year period. Scandinavian Journal of Medicine and Science in Sports. 2015;25:e133–138. doi: 10.1111/sms.12253. [DOI] [PubMed] [Google Scholar]
- 15.Liu SH, al-Shaikh R, Panossian V, Yang RS, Nelson SD, Soleiman N, Finerman GA, Lane JM. Primary immunolocalization of estrogen and progesterone target cells in the human anterior cruciate ligament. Journal of Orthopaedic Research. 1996;14:526–533. doi: 10.1002/jor.1100140405. [DOI] [PubMed] [Google Scholar]
- 16.Maffulli N, Waterston SW, Squair J, Reaper J, Douglas AS. Changing incidence of Achilles tendon rupture in Scotland: a 15-year study. Clinical Journal of Sport Medicine. 1999;9:157–160. doi: 10.1097/00042752-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 17.McCormick KM, Burns KL, Piccone CM, Gosselin LE, Brazeau GA. Effects of ovariectomy and estrogen on skeletal muscle function in growing rats. Journal of Muscle Research and Cell Motility. 2004;25:21–27. doi: 10.1023/b:jure.0000021398.78327.39. [DOI] [PubMed] [Google Scholar]
- 18.Murshed KA, Cicekcibasi AE, Karabacakoglu A, Seker M, Ziylan T. Distal femur morphometry: a gender and bilateral comparative study using magnetic resonance imaging. Surgical and Radiologic Anatomy. 2005;27:108–112. doi: 10.1007/s00276-004-0295-2. [DOI] [PubMed] [Google Scholar]
- 19.Riggin CN, Sarver JJ, Freedman BR, Thomas SJ, Soslowsky LJ. Analysis of collagen organization in mouse achilles tendon using high-frequency ultrasound imaging. Journal of Biomechanical Engineering. 2014;136:021029. doi: 10.1115/1.4026285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romani WA, Russ DW. Acute effects of sex-specific sex hormones on heat shock proteins in fast muscle of male and female rats. European Journal of Applied Physiology and Occupational Physiology. 2013;113:2503–2510. doi: 10.1007/s00421-013-2686-8. [DOI] [PubMed] [Google Scholar]
- 21.Smith LR, Barton ER. SMASH - semi-automatic muscle analysis using segmentation of histology: a MATLAB application. Skelet Muscle. 2014;4:21. doi: 10.1186/2044-5040-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soslowsky LJ, Thomopoulos S, Tun S, Flanagan CL, Keefer CC, Mastaw J, Carpenter JE. Neer Award 1999 Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. Journal of Shoulder and Elbow Surgery. 2000;9:79–84. [PubMed] [Google Scholar]
- 23.Torricelli P, Veronesi F, Pagani S, Maffulli N, Masiero S, Frizziero A, Fini M. In vitro tenocyte metabolism in aging and oestrogen deficiency. Age (Dordr) 2013;35:2125–2136. doi: 10.1007/s11357-012-9500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Wessel T, de Haan A, van der Laarse WJ, Jaspers RT. The muscle fiber type-fiber size paradox: hypertrophy or oxidative metabolism? European Journal of Applied Physiology and Occupational Physiology. 2010;110:665–694. doi: 10.1007/s00421-010-1545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vosseller JT, Ellis SJ, Levine DS, Kennedy JG, Elliott AJ, Deland JT, Roberts MM, O’Malley MJ. Achilles tendon rupture in women. Foot and Ankle International. 2013;34:49–53. doi: 10.1177/1071100712460223. [DOI] [PubMed] [Google Scholar]
- 26.Wentorf FA, Sudoh K, Moses C, Arendt EA, Carlson CS. The effects of estrogen on material and mechanical properties of the intra- and extra-articular knee structures. American Journal of Sports Medicine. 2006;34:1948–1952. doi: 10.1177/0363546506290060. [DOI] [PubMed] [Google Scholar]
- 27.White JJ, Titchener AG, Fakis A, Tambe AA, Hubbard RB, Clark DI. An epidemiological study of rotator cuff pathology using The Health Improvement Network database. Bone Joint J. 2014;96-B:350–353. doi: 10.1302/0301-620X.96B3.32336. [DOI] [PubMed] [Google Scholar]
- 28.Wojtys EM, Huston LJ, Boynton MD, Spindler KP, Lindenfeld TN. The effect of the menstrual cycle on anterior cruciate ligament injuries in women as determined by hormone levels. American Journal of Sports Medicine. 2002;30:182–188. doi: 10.1177/03635465020300020601. [DOI] [PubMed] [Google Scholar]
- 29.Wojtys EM, Huston LJ, Lindenfeld TN, Hewett TE, Greenfield ML. Association between the menstrual cycle and anterior cruciate ligament injuries in female athletes. American Journal of Sports Medicine. 1998;26:614–619. doi: 10.1177/03635465980260050301. [DOI] [PubMed] [Google Scholar]
- 30.Yu WD, Panossian V, Hatch JD, Liu SH, Finerman GA. Combined effects of estrogen and progesterone on the anterior cruciate ligament. Clin Orthop Relat Res. 2001:268–281. doi: 10.1097/00003086-200102000-00031. [DOI] [PubMed] [Google Scholar]