Abstract
The National Institutes of Health (NIH) has required the inclusion of women in clinical studies since 1993, which has enhanced our understanding of how biological sex affects certain medical conditions and allowed the development of sex-specific treatment protocols. However, NIH’s policy did not previously apply to basic research and the NIH recently introduced a new policy requiring all new grant applications to explicitly address sex as a biological variable. The policy itself is grounded in the results of numerous investigations in animals and humans illustrating the existence of sex differences in the brain and behavior, and the importance of sex hormones, particularly estrogens, in regulating physiology and behavior. Here, we review findings from our laboratories and others, demonstrating how estrogens influence brain and behavior in adult females. Research from subjects throughout the adult lifespan on topics ranging from social behavior, learning and memory, to disease risk will be discussed to frame an understanding of why estrogens matter to behavioral neuroscience.
1. Introduction
Although sex differences exist in the risk, etiology, symptomatology, and progression of a wide variety of neuropsychiatric and neuropathological diseases, the vast majority of biomedical researchers rarely study sex differences in disease. As a result, North American and European granting agencies have made a concerted effort to compel basic and medical researchers to consider sex as a biological variable in studying brain disorders (Clayton & Collins, 2014; http://ec.europa.eu/research/swafs/index.cfm?pg=policy&lib=gender). Brain disorders such as stroke, Alzheimer Disease (AD), and depression display large sex differences in incidence as well as manifestation of symptoms (Angst et al., 2002; Barnes et al., 2005; Irvine et al., 2012). For example, women are more likely than men to be diagnosed with depression (Angst et al., 2002; Gutierrez-Lobos et al., 2002), show greater pathology and cognitive deficits related to AD (Barnes et al 2005; Irvine et al., 2012; Gao et al., 1998), and have poor functional outcomes and quality of life after stroke (Reeves et al., 2009).
When sex differences are observed, it is likely that sex chromosomes and/or sex hormones are involved (McCarthy et al., 2012; McCullough et al., 2014). In addition societal expectations undoubtedly influence gender differences in a number of outcomes that can affect diagnoses or management such as number of doctor visits, prescriptions, prescription use, and compliance (Bertakis, 2009; Leresche, 2011; Zeber et al., 2013). However, while gender is a psychosocial construct that determines what a given society may deem appropriate for men versus women this review is restricted to the biological differences between men and women, and in particular the effects of estrogens in females. The reader is directed to other reviews on sex and gender differences in contribution to disease (Mielke et al., 2014). Indeed, disease progression and manifestation in a number of brain disorders may be influenced by levels of sex steroids (Amiaz and Seidman, 2008; Baum, 2005; Bloch et al., 2003; Lv et al., 2015; McIntyre et al., 2006; Moffat et al., 2004; Rosario et al., 2011; Sankar & Hampson, 2012). For example, lower levels of 17β-estradiol in women are associated with an increased incidence of neurological and neuropsychiatric diseases (Baum, 2005; Rosario et al., 2011; Wieck, 2011). Similarly, exogenous 17β-estradiol given to aging women may reduce the risk of AD (Maki, 2013), improve cognition in older age (Hogervorst et al., 2000; Hogervorst et al., 2005), and provide relief from depression (Rubinow et al., 2015). Although one large clinical trial, the Women’s Health Initiative, did not find evidence of a beneficial effect of hormone therapy on cognition or AD risk in postmenopausal women (Shumaker et al., 2003), this study was widely criticized due to the older age of participants (most women were well past menopause), health of participants, and the particular choice of hormone therapy (Brinton, 2005; Maki, 2004; Resnick & Henderson, 2002). The concerns raised by this study, together with evidence for greater disease incidence in women that may be associated with estrogens, underscores the necessity for further research into the impact of estrogens on cognition, health and neuroprotection.
In younger adults, research attention is needed in human studies to assess the potential role of estrogens in mental health conditions that affect men and women unequally. New inroads into the sources of the sex disparity, as well as new insights into the basic mechanisms that underlie these poorly understood psychiatric disorders, could result from greater attention to the role of biological sex and the moderating effects of sex steroids. It could be argued that progress has been hampered in past research by the failure to take biological sex into consideration, and factors that covary with it such as differences in liver enzyme expression and lean-to-fat ratios (Waxman & Holloway, 2009), variations in circulating levels of estrogens, and sex differences in brain pathways. Thus, given the prominent sex and sex hormone interactions with a number of disorders, more research should be directed towards understanding the disease process in both males and females under different sex hormone conditions.
Here, we review literature surrounding the complex effects of estrogens in females from a variety of perspectives, beginning with basic science neurobehavioral and molecular biology investigations in young adult females, and building up to disease models in older adult females and human studies. These data were presented as a symposium at the annual meeting of the International Behavioral Neuroscience Society (IBNS) in June, 2015. In this review, we discuss how estrogens influence learning and memory in female rodents (sections 3 and 4) and women (section 7), the brain systems and cellular pathways through which estrogens may exert their effects (sections 3 and 4), the different effects of various types of estrogens on cognition and neuroplasticity (section 5), and findings indicating that the ability of estrogens to influence neuroprotection depends on age (section 6), preexisting pathology (section 6), and experience (section 5). We close by discussing why sex and hormonal status are important considerations in examining factors related to women’s brain health.
2. Estrogen receptor localization and function
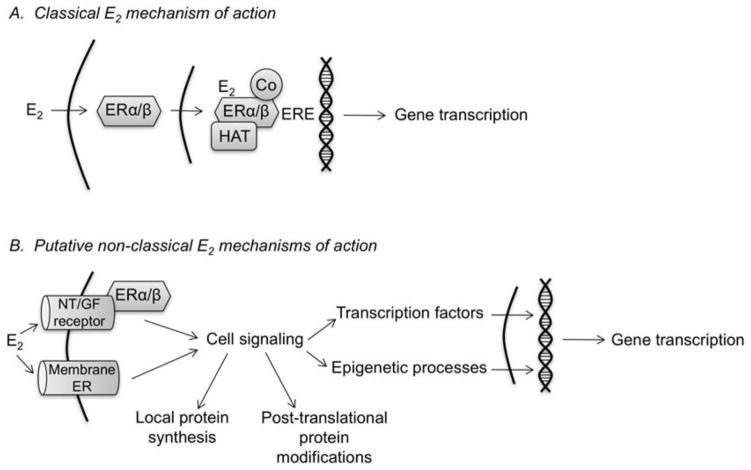
Like all steroid hormones, estrogens were once thought to act solely via straightforward actions at the genome. This classical “genomic” mechanism involves the binding of estrogens to the intracellular estrogen receptors ERα and ERβ, which form homo- or heterodimers that translocate to the nucleus and regulate expression of ER target genes by directly binding an estrogen response element (ERE) or forming protein-protein interactions to indirectly regulate other transcription factors (Figure 1A). However, intracellular ERs have been identified in numerous locations, including dendrites and axon terminals (Blaustein and Lehman, 1992). Intracellular ERs have been identified throughout the male and female rodent hippocampus in both pyramidal neurons and glia (Milner et al., 2001; Milner et al., 2005; Mitra et al., 2003; Mitterling et al., 2010; Shughrue et al., 1997a; Shughrue et al., 1997b; Shughrue and Merchenthaler, 2000). Within pyramidal neurons, ultrastructural analyses have localized ERα and ERβ to extranuclear sites including dendritic spines, axons, and axon terminals (Milner et al., 2005; Milner et al., 2001; Mitterling et al., 2010; Waters et al., 2011), suggesting functions for the ERs beyond classical ERE-dependent transcription. Such non-nuclear effects are supported by findings demonstrating that 17β-estradiol increases the localization of ERβ to dendritic spines and shafts in the adult female rat hippocampus, and promotes translocation of ERβ to the plasma membrane in hippocampal-derived cell lines (Sheldahl et al., 2008; Waters et al., 2011). These data indicate that 17β-estradiol mobilizes intracellular ERs to interact with membrane proteins in extranuclear cellular compartments. Such “non-classical” effects (Figure 1B) have been demonstrated in vitro and in vivo in the female rodent hippocampus, where ERα and ERβ interact at the plasma membrane with metabotropic glutamate receptors mGluR1 and mGluR2/3 to activate extracellular signal-regulated kinase/mitogen activated protein kinase (ERK/MAPK) signaling, which triggers increased cAMP response element-binding protein (CREB) phosphorylation and CREB-dependent gene transcription (Boulware et al., 2013; Boulware et al., 2005). In cultured neurons from female rat hippocampus, S-palmitoylation of the ERs regulates their interactions with mGluRs (Meitzen et al., 2013), thus providing a possible mechanism through which intracellular ERs can activate cell-signaling cascades like ERK.
Figure 1.
Schematic models illustrating mechanisms through which 17β-estradiol may modulate hippocampal memory. (A) In the classical mechanism, 17β-estradiol diffuses through the membrane to bind intracellular ERα and ERβ in the cytoplasm or nucleus. The 17β-estradiol-ER complex then binds to an estrogen response element (ERE) on the DNA with co-regulator proteins (Co) and histone acetyltransferases (HAT) to stimulate gene transcription. (B) Non-classical mechanisms reported thus far support a model in which 17β-estradiol activates neurotransmitter (NT) and/or growth factor (GF) receptors at the cell membrane or activates membrane ERs to trigger cell signaling cascades. Cell-signaling activation may lead to local protein synthesis, post-translational protein modifications in the cytoplasm or nucleus, and activation of transcription factors and epigenetic processes that increase gene transcription. Any of these 17β-estradiol-induced alterations could enhance hippocampal memory consolidation, although gene transcription is likely necessary for long-term memory storage.
In addition to the membrane-associated activity of intracellular ERs, several membrane-bound ERs have been hypothesized, including G-protein-coupled ER (GPER, formerly GPR30), Gq-ER, and ER-X. Of these, GPER is the most widely accepted as a membrane ER (Srivastava and Evans, 2013), and can be found throughout the hippocampus exclusively at extranuclear sites within glia, interneurons, and pyramidal neurons (Brailoiu et al., 2007; Waters et al., 2015). Notably, in pyramidal neurons, GPER can be found at or near the plasma membrane of dendrites, dendritic spines, soma, axons, and axon terminals in association with post-synaptic scaffolding proteins (Akama et al., 2013; Waters et al., 2015). As such, GPER is well positioned to mediate the rapid effects of estrogens on cell signaling and other cellular functions.
3. Rapid action of estrogens and their receptors: implications for learning and memory
As noted above, in addition to their delayed and long-lasting genomic actions, estrogens can also act very rapidly via non-genomic mechanisms that depend upon intracellular signaling (reviewed in Ervin et al., 2013 and Frick et al., 2015). The rapid effects have received research attention in recent years and have been shown to be involved in learning and memory. Investigations with treatments administered shortly after the acquisition phase of a learning task have demonstrated a crucial role for the rapid mechanisms of estrogens in the consolidation of a new memory (see Frick et al., 2015 and section 4 below in this review). Investigations showing an effect of estrogen administration prior to memory acquisition and with testing done shortly thereafter suggest the rapid effects of estrogen can enhance performance in learning tasks even at a time when memory consolidation is incomplete. This section focuses on rapid estrogenic effects during this early phase of the formation of a new memory.
Studies with systemic treatment with 17β-estradiol either 30 min before or immediately after exposure to objects (acquisition phase) improved object recognition in ovariectomized female rats when tested 4 h later (Luine et al., 2003). Studies in which systemic treatments of 17β-estradiol were given to ovariectomized mice have shown very rapid facilitation of various learning tasks including social recognition, object recognition, object placement and the social transmission of food preferences (Ervin et al., 2015; Phan et al., 2012). In these studies by the Choleris laboratory, mice were administered 17β-estradiol 15 min before a learning task, which was completed by 40 min post-treatment. Hence, both task acquisition and testing were completed within a timeframe that makes genomic effects of estrogens unlikely, and rather points towards rapid non-genomic mechanisms. Using systemic treatments with ER selective agonists, various investigations showed that ERα and GPER predominantly mediate these rapid effects of estrogens (Phan et al., 2011; Gabor, Lymer et al., 2015; Ervin et al., 2015). However, differences in specific ER-involvement in these tasks were also found. In particular, systemic treatment with the ERα agonist propyl pyrazole triol (PPT) (Phan et al., 2011) and GPER agonist G1 (Gabor, Lymer et al., 2015) rapidly enhanced social and object recognition in adult ovariectomized mice. Conversely, systemic treatment with the ERβ agonist diarylpropionitrile (DPN) may have rapidly impaired social recognition and had no effects on object recognition (Phan et al., 2011). Mice tested in the object placement task showed rapid enhanced performance with all 3 ER-selective agonists (Phan et al., 2011; Gabor et al., 2015). Together, these results suggest a generalized role for ERα and GPER in mediating the rapid enhancement of recognition and location memories by estrogens and a specific role for ERβ in location memory. The involvement of the 3 ERs in the rapid facilitation of social learning by estrogens, instead, appears different. The GPER agonist G1 rapidly enhanced social learning, while ERα agonist PPT and ERβ agonist DPN did not. Rather, the ERα agonist shortened a socially learned food preference (Ervin et al., 2015). The different and somewhat opposing effects of GPER and ERα agonists may explain why treatment with the GPER agonist resulted in a socially acquired food preference of longer duration than treatment with 17β-estradiol itself, which activates all ERs (Ervin et al., 2015). The results of the social learning test also point at a learning-specific role of the ERs in mediating the rapid effects of estradiol on memory encoding. In particular, the findings with the ERα agonist PPT are striking, as it strongly facilitated performance in social recognition, object recognition and object placement tasks, while it inhibited social learning (Ervin et al., 2015; Phan et al., 2011). These different results suggest that ERα may have specific effects on different types of memories mediated by different brain regions.
3.1 The hippocampus is implicated in the rapid learning enhancement of estrogens and their receptors
The brain responds to rapid effects of estrogens at different levels, from structural to functional (reviewed in Sellers et al., 2015; Woolley et al., 2007). In particular, dendritic spines, the site of most excitatory synapses, respond very rapidly to estrogen treatment. In the hippocampus, 17β-estradiol administration results in a transient increase in density and length (Jacome et al., 2016; MacLusky et al., 2005; Mukai et al., 2007; Murakami et al., 2006; Mendez et al., 2011; Srivastava, et al., 2008; Tuscher et al., 2016). In behaviorally naïve ovariectomized female mice, it was also found that systemic treatment with 17β-estradiol (Phan et al., 2012), ERα agonist PPT (Phan et al., 2011) and GPER agonist G1 (Gabor, Lymer et al., 2015) rapidly increased dendritic spine density in CA1 field of the hippocampus, while ERβ agonist DPN decreased it (Phan et al., 2011). Subsequently, it was shown that bath application of 17β-estradiol and ERα agonist PPT for 20–30 minutes to hippocampal sections from behaviorally naïve postnatal female (PND 20–32) mice enhanced dendritic spine density (Phan et al., 2015). Intriguingly, within the same timeframe as the dendritic spines and learning enhancements, 17β-estradiol also decreased CA1 hippocampal excitatory input, rapidly and transiently reducing AMPA responses, likely through AMPA receptor internalization (see Figure 2A; Phan et al., 2015). Hence, it appears new spines induced by estrogens are associated with silent or immature synapses. These synapses may become active when used in learning events as they are with induction of learning-associated long-term potentiation (LTP; Smejkalova & Woolley, 2010; Smith & McMahon, 2006). These effects confirmed the results of previous ex-vivo work (Srivastava et al., 2008) and prompted further investigations into the involvement of the hippocampus in the rapid effects of estrogens on learning.
Figure 2.
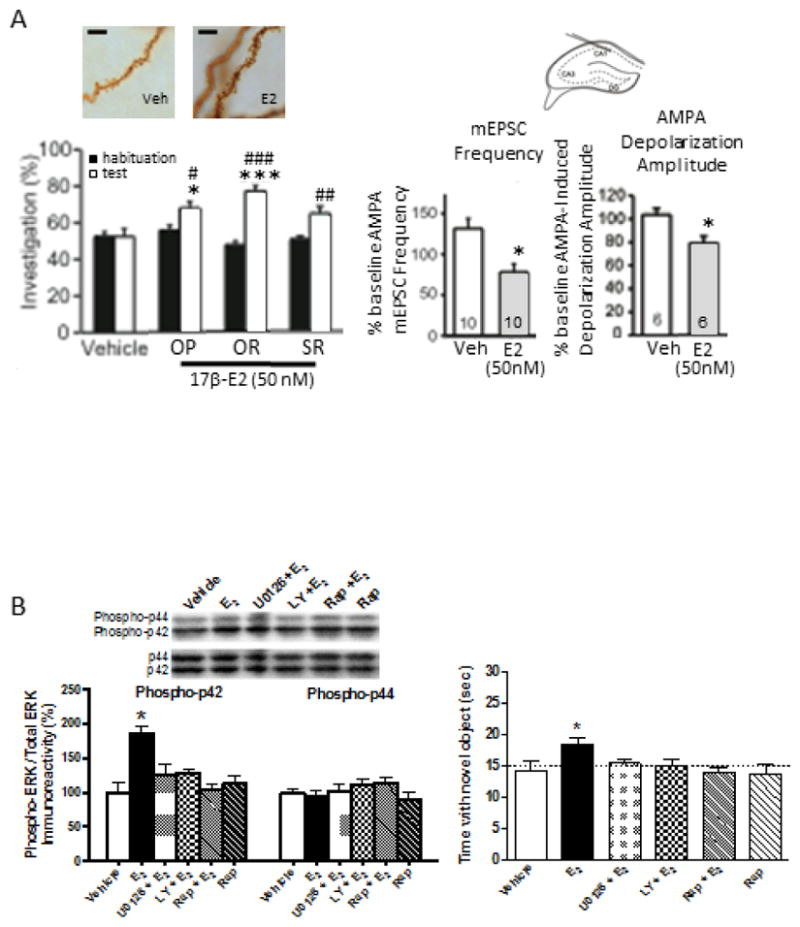
(A) Treatment (20–30 min) of hippocampal sections with 50 nM 17b-Estradiol (E2) increased dendritic spine density in the stratum radiatum (shown) and stratum oriens (not shown) of the CA1 subregion of the dorsal hippocampus and shown in the biocytin filled pyramidal neurons (scale bar, 100mm). In parallel, infusion of 50 nM 17-b estradiol (E2) in the dorsal hippocampus of ovariectomized female mice 15 min prior to learning (habituation) and testing rapidly enhanced performance in the Object Placement (OP), Object Recognition (OR), and Social Recognition (SR) task within 40 min of treatments. In this specific paradigm vehicle control mice (only control for OP task is shown here) do not show learning while E2 treated mice did, as indicated by a significant increase from habituation (black bars) to test (white bars) in the percent of time spent investigating a novel or displaced stimulus vs a familiar stimulus. Within the same timeframe, patch clamping recordings from CA1 pyramidal neurons of learning-naïve mice showed a significant inhibitory effect of the same 50nM dose of E2 on the frequency of miniature Excitatory Postsynaptic Current (mEPSC) and on the amplitude of AMPA-induced membrane depolarization. This effects is consistent with the notion that E2 rapidly induces the formation of silent/immature synapses. * P<0.05, *** P<0.001 in comparison to vehicle control; # P<0.05, ## P<0.01, ### P<0.001 in comparison to habituation phase. Modified from Phan et al., PNAS 112: 16018–16023.
(B) Dorsal hippocampal mTOR activation is necessary for E2 to enhance object recognition memory consolidation. Levels of phospho-p42 ERK, but not phsopho-p44 ERK, are increased in ovariectomized mice 5 min after bilateral dorsal hippocampal infusion of 5 μg/hemisphere E2 (*p < 0.05 relative to vehicle). This effect was blocked by the ERK inhibitor U0126 (0.5 μg/hemisphere), the PI3K inhibitor LY298002 (0.005 μg/hemisphere), or the mTOR inhibitor rapamycin (0.25 μg/hemisphere). Each inhibitor also prevented E2 from enhancing object recognition memory; only mice infused with E2 alone spent more time than chance (15 s) with the novel object (*p < 0.05). Error bars=mean ± standard error of the mean (SEM). Phosphorylated ERK normalized to total ERK. Insets are representative Western blots of phosphorylated and total ERK. Adapted with permission from Fortress et al., 2013.
Infusion of 17β-estradiol or the ERα agonist PPT (Phan et al., 2015) into the dorsal hippocampus rapidly improved performance in social recognition, object recognition, and object placement tasks, while the ERβ agonist DPN was ineffective. Similarly, infusion of the GPER agonist G1 enhanced social and object recognition but did not affect object location recognition in the object placement task (Lymer et al., submitted). Conversely, infusion of the same doses of 17β-estradiol in the dorsal hippocampus did not enhance the social transmission of food preferences (Ervin et al., unpublished results). These results suggest that ERα and the GPER are involved in most of the rapid enhancing effects of hippocampal estradiol on learning and also support the notion that estrogens in the dorsal hippocampus can promote social, object and location recognition. The lack of effects on social learning in initial investigations, suggest the improvement seen with systemic treatment (Ervin et al., 2015) was mediated by estrogenic action in brain regions other than the dorsal hippocampus. Further research is needed in order to identify those other regions.
Because the hippocampus is known to process spatial information and enhance spatial learning, it was further hypothesized that improvement in social and object recognition by estrogens in the dorsal hippocampus may be due to enhanced processing of spatial cues associated with the social and object stimuli used in the learning tasks. Using a Y-maze with high walls that minimize access to spatial information, it was found that dorsal hippocampal infusion of 17β-estradiol rapidly enhanced object recognition but not social recognition (Phan et al., unpublished results). Hence, dorsal hippocampal estrogens rapidly and directly improve performance in the object recognition and placement tasks, while they enhance social recognition via associated spatial information processing, likely in interplay with an extra hippocampal brain region. Recent results suggest the latter may be the medial amygdala, as infusion of 17β-estradiol here enhanced social recognition (Sheppard et al., unpublished results). Overall, these findings illustrate that an interconnected network of brain regions is being identified that are specifically implicated in the very rapid effects of estrogens and ERs on various learning tasks in females.
4. Cell-signaling pathways involved in 17β-estradiol-induced memory enhancement
The rapid activation of cell-signaling pathways by membrane receptors such as neurotransmitter and growth factor receptors allows for intracellular communication that need not depend on gene transcription. As such, cell-signaling mechanisms may allow estrogens to swiftly modulate cellular functions in response to a learning event (e.g., local protein synthesis to build new dendritic spines). Numerous cell-signaling cascades linked to glutamatergic receptor activation are involved in hippocampal memory formation, including ERK/MAPK, phosphatidylinositol 3-kinase (PI3K), protein kinase A (PKA), calcium-calmodulin kinase II (CaMKII), and the mammalian target of rapamycin (mTOR) (e.g., (Adams and Sweatt, 2002; Atkins et al., 1998; Guzowski and McGaugh, 1997; Hoeffer and Klann, 2010; Horwood et al., 2006; Impey et al., 1998a; Impey et al., 1998b; Lee and Silva, 2009; Selcher et al., 1999; Silva et al., 1992)). 17β-estradiol can activate many of these cell-signaling cascades in vivo and in vitro within minutes as discussed below.
Among the earliest examples of such activation came from in vitro studies of various cell types, including hippocampal neurons. This work shows that ERK phosphorylation was increased within 15 minutes of exposure to 17β-estradiol or a membrane-impermeable form of 17β-estradiol for which 17β-estradiol was conjugated to bovine serum albumin (BSA-17β-estradiol) (Wade and Dorsa, 2003; Wade et al., 2001; Watters et al., 1997; Yokomaku et al., 2003). In adult male rats and female mice, 17β-estradiol or BSA-17β-estradiol infused into the dorsal hippocampus or cerebral ventricles (ICV) significantly increases dorsal hippocampal ERK phosphorylation within 5 minutes, demonstrating very rapid effects of 17β-estradiol on ERK signaling in vivo (Boulware et al., 2013; Fernandez et al., 2008; Fortress et al., 2013; Kuroki et al., 2000; Pereira et al., 2014; Zhao et al., 2012; Zhao et al., 2010),. Accordingly, inhibitors of MAPK kinase (MEK), which exclusively phosphorylates ERK, block 17β-estradiol-induced ERK phosphorylation both in vitro and in vivo (Fernandez et al., 2008; Fortress et al., 2013; Nilsen and Brinton, 2003; Yokomaku et al., 2003; Zhao et al., 2010). Importantly, inhibition of MEK in the dorsal hippocampus prevents 17β-estradiol or BSA-17β-estradiol infused into the dorsal 3rd ventricle from enhancing the consolidation of hippocampal-dependent object recognition and spatial memories in ovariectomized mice (see Figure 2B; Boulware et al., 2013; Fernandez et al., 2008; Fortress et al., 2013; Pereira et al., 2014; Zhao et al., 2012; Zhao et al., 2010). These findings demonstrate two important points. The first is that the memory-enhancing effects of 17β-estradiol are dependent on cell signaling. Second, the effects of 17β-estradiol on ERK and memory consolidation can be mimicked by a membrane-impermeable form of 17β-estradiol, thus illustrating a key role for membrane proteins in the memory-enhancing effects of 17β-estradiol. These two points will be discussed in more detail below.
In support of the first point, ERK is one of many cell-signaling molecules whose activity is regulated by 17β-estradiol, and this estrogenic modulation of cell signaling has important consequences for neural functioning. For example, inhibitors of ERK, PI3K, PKA, protein kinase C (PKC), and CaMKII phosphorylation block spinogenesis and LTP in hippocampal slices from adult male rats (Hasegawa et al., 2015; Mukai et al., 2007; Murakami et al., 2014). Similarly, inhibition of ERK prevents 17β-estradiol from increasing dendritic spine density in cultured embryonic rat (sex not specified) cortical neurons (Srivastava et al., 2008). Although increased spine density does not necessarily translate into increased synaptic plasticity, the 17β-estradiol-induced facilitation of signaling in the RhoA/RhoA protein kinase (ROCK) pathway has been associated with enhanced LTP in male rat hippocampal slices (Kramár et al., 2009). ROCK activates LIM kinase, which then phosphorylates cofilin, thereby leading to actin polymerization and stabilization of the spine cytoskeleton (Gungabissoon and Bamburg, 2003; Thirone et al., 2009). 17β-estradiol increases filamentous actin levels and actin polymerization in dendritic spines via Rho/ROCK activation, whereas ovariectomy disrupts LTP stabilization and actin filament assembly in spines (Kramár et al., 2009). Moreover, the beneficial effects of 17β-estradiol on LTP in slices from the male rat hippocampus are completely blocked by the toxin latrunculin, which disrupts actin filament assembly (Kramár et al., 2009). Thus, 17β-estradiol appears to regulate spinogenesis and LTP in the male rat hippocampus by altering actin polymerization. Although a direct link between 17β-estradiol-induced alterations in ERK and cofilin signaling has yet to be reported in the hippocampus, inhibitors of ERK or the downstream mTOR protein synthesis pathway prevent dorsal hippocampus-infused 17β-estradiol from increasing dendritic spine density in the dorsal hippocampus of ovariectomized mice (Tuscher et al., 2016). This novel finding suggests the intriguing possibility that multiple cell-signaling pathways regulate the effects of 17β-estradiol on hippocampal morphology and synaptic plasticity.
Accordingly, numerous cell-signaling pathways are also involved in the memory- enhancing effects of 17β-estradiol. In addition to ERK, activation of PKA, PI3K, and mTOR are necessary for 17β-estradiol to enhance memory consolidation in hippocampal-dependent object recognition and object location tasks in ovariectomized mice (Fan et al., 2010; Fortress et al., 2013; Lewis et al., 2008). As with ERK, inhibitors of PKA, PI3K, or mTOR prevent 17β-estradiol from enhancing memory consolidation (Fan et al., 2010; Fortress et al., 2013; Lewis et al., 2008). Several of these signaling pathways work together to mediate the effects of 17β-estradiol in young and middle-aged ovariectomized mice. In particular, 17β-estradiol first activates PI3K, followed by ERK, and then mTOR (Fan et al., 2010; Fortress et al., 2013). Given the important role of the mTOR pathway in local protein synthesis and memory formation (Bekinschtein et al., 2007; Dash et al., 2006; Hoeffer and Klann, 2010; Myskiw et al., 2008; Parsons et al., 2006), the order of kinase activation induced by 17β-estradiol is consistent with the hypothesis that rapid effects of 17β-estradiol on cell signaling increase dendritic spine density, which then leads to increased synaptic plasticity and enhanced memory.
4.2. 17β-estradiol-induced cell-signaling and receptor interactions
If activation of cell-signaling pathways is critical for 17β-estradiol to facilitate memory formation, then how does 17β-estradiol trigger cell signaling? 17β-estradiol likely initiates cell signaling via interactions with membrane receptors. This notion is supported by evidence that the membrane impermeable BSA-17β-estradiol mimics the effects of 17β-estradiol on ERK activation and object recognition memory (Fernandez et al., 2008; Kuroki et al., 2000; Wade and Dorsa, 2003; Watters et al., 1997). Furthermore, these effects are not blocked by administration of the intracellular ER antagonist ICI 182,780 to the hippocampus (Fernandez et al., 2008; Kuroki et al., 2000). This finding suggests that activation of membrane ERs is sufficient for 17β-estradiol to increase ERK signaling and facilitate memory formation. However, the identity of these receptors remains unclear, as there is scant evidence that ERα and ERβ are integral membrane proteins (Bondar et al., 2009).
One possible mechanism links intracellular ERs with mGluR1 receptors. As mentioned above, 17β-estradiol activates ERK and phosphorylates CREB in hippocampal slices from neonatal female rats (Boulware et al., 2005). In slices, this effect is mediated by interactions between mGluR1 and ERα, but not ERβ (Boulware et al., 2005). In ovariectomized adult C57BL/6 mice however, both ERα and ERβ play key roles. When infused directly into the dorsal hippocampus of ovariectomized C57BL/6 mice, both the ERα agonist PPT and ERβ agonist DPN increase dorsal hippocampal ERK phosphorylation and enhance object recognition and object location memory consolidation (Boulware et al., 2013). The effects of 17β-estradiol and these agonists are blocked by either the ERK inhibitor U0126 or the mGluR1 antagonist LY367385 (Boulware et al., 2013; Zhao et al., 2010), suggesting direct interactions between the ERs and mGluR1. Supporting the existence of these interactions, fractionation and co-immunoprecipitation experiments indicate physical interactions between both ERs and mGluR1 at the membrane (Boulware et al., 2013). Although it is unknown how BSA-17β-estradiol might gain access to intracellular ERα and ERβ to facilitate the interactions with mGluR1, these data illustrate one way in which intracellular ERs may initiate cell signaling. Given the plethora of cell-signaling pathways activated by 17β-estradiol, numerous other membrane receptors are likely to be involved in mediating the effects of 17β-estradiol on memory. NMDA receptors have already been implicated in 17β-estradiol’s effects on ERK and object recognition in ovariectomized female mice (Lewis et al., 2008), and many more receptors are apt to play key roles. As such, this area is particularly ripe for future investigation.
Another way in which 17β-estradiol may trigger cell signaling is via binding to membrane ERs. As mentioned above, GPER is generally considered to be a membrane ER, although not all researchers agree (Langer et al., 2010; Levin, 1999). The GPER agonist G-1 increases hippocampal CA1 dendritic spine density in ovariectomized mice (Gabor et al., 2015), and enhances several forms of hippocampal memory in ovariectomized rats or mice including spatial working memory, social transmission of food preferences, social recognition, object recognition, and spatial recognition (Ervin et al., 2015; Gabor et al., 2015; Hammond et al., 2009; Hawley et al., 2014). In contrast, the GPER antagonist G-15 impairs spatial working memory in ovariectomized rats (Hammond et al., 2012). Consistent with these findings, post-training dorsal hippocampus or ICV infusion of G-1 enhances, whereas G-15 impairs, object recognition and object location memory consolidation (Kim et al., 2016). Unlike 17β-estradiol, however, G-1 does not increase ERK, PI3K, or Akt phosphorylation in the dorsal hippocampus, but rather increases activation of the c-Jun N-terminal Kinase (JNK) signaling pathway (Kim et al., 2016). Moreover, the memory-enhancing effects of G-1 are dependent on activation of JNK and not ERK, whereas the reverse is true for 17β-estradiol (Kim et al., 2016). These data suggest that GPER in the dorsal hippocampus is not involved in the memory-enhancing effects of 17β-estradiol. In accordance with this hypothesis, the GPER agonist G-15 does not prevent 17β-estradiol from enhancing object recognition or object location memory consolidation (Kim et al., 2016), suggesting that GPER may not function as an ER in the dorsal hippocampus. Additional evidence for disparate effects of 17β-estradiol and G-1 comes from a recent neurogenesis study, in which 17β-estradiol increased, but G-1 decreased, cell proliferation in the dorsal hippocampus of ovariectomized rats (Duarte-Guterman et al., 2015). However, other findings support similar roles for 17β-estradiol and G-1, as dorsal hippocampus infusion of G-1 reportedly increases dorsal hippocampus ERK activation in ovariectomized mice (Hart et al., 2014) and G-15 prevents 17β-estradiol from activating ERK in hippocampal slices from ovariectomized mice (Kumar et al., 2015). Therefore, the role of GPER in mediating the effects of 17β-estradiol on memory remains unresolved and will need to be clarified in future studies.
4.3. 17β-estradiol-induced cell-signaling and epigenetic interactions
Cell-signaling pathways have myriad effects within the cell, including altering gene transcription through post-translational modifications of one or more transcription factors such as CREB. As mentioned earlier, 17β-estradiol phosphorylates CREB via phosphorylation of ERK. Another way in which 17β-estradiol-induced cell-signaling alterations may affect gene transcription is by regulating the epigenetic mechanisms histone acetylation and DNA methylation. Although 17β-estradiol-induced enhancements of object recognition memory consolidation are dependent on DNA methylation (Zhao et al., 2010), this section will focus on histone acetylation because estrogenic regulation of histone acetylation is dependent on cell signaling (see (Fortress and Frick, 2014) for a more detailed review of estrogenic regulation of DNA methylation and histone acetylation).
Acetylation of the four core histones (H2A, H2B, H3, H4) around which DNA is coiled is a primary mechanism of increasing gene expression. Acetylation relaxes the bond between the histones and DNA, thereby allowing transcription factors access to DNA. Of the four core histones, acetylation of H3 has been particularly associated with hippocampal ERK activation and facilitation of memory formation in rodents (Chwang et al., 2006; Gräff and Tsai, 2013; Levenson et al., 2004). Consistent with its ability to phosphorylate ERK and enhance memory, dorsal hippocampus infusion of 17β-estradiol increases H3 acetylation in the dorsal hippocampus of ovariectomized mice within 30 minutes (Fortress and Frick, 2014; Zhao et al., 2010). This effect is blocked by dorsal hippocampus infusion of U0126 (Zhao et al., 2010), demonstrating that dorsal hippocampal ERK activation is necessary for 17β-estradiol to increase H3 acetylation. The 17β-estradiol-induced acetylation of H3 regulates the expression of memory-promoting genes such as brain derived neurotrophic factor (Bdnf), as illustrated by data showing that 17β-estradiol increases H3 acetylation of Bdnf promoters II and IV in the dorsal hippocampus of ovariectomized mice 30 minutes after infusion, and increases levels of pro-BDNF and BDNF protein in the dorsal hippocampus 4 and 6 hours after infusion (Fortress and Frick, 2014). Thus, estrogenic regulation of histone acetylation appears to promote the expression of genes that facilitate memory formation.
How might 17β-estradiol regulate histone acetylation? One possibility is by altering levels of histone deacetylases (HDACs), which are enzymes that remove acetyl groups from histones. In particular, expression of HDAC2 and HDAC3 is associated with impaired hippocampal plasticity and memory in rodents (Guan et al., 2009; McQuown et al., 2011). Dorsal hippocampus infusion of 17β-estradiol decreases levels of HDAC2 and HDAC3 protein in the dorsal hippocampus of ovariectomized mice (Fortress and Frick, 2014; Zhao et al., 2010). However, the rapid effects of 17β-estradiol on H3 acetylation are not likely due to regulation of HDAC2 or HDAC3 because changes in these proteins are not observed until 4 hours after infusion (Fortress and Frick, 2014; Zhao et al., 2010). Rather, the immediate effects of 17β-estradiol on histone acetylation may result from regulating the activity of histone acetyltransferases (HATs), the enzymes that acetylate histones. In ovariectomized mice, dorsal hippocampus infusion of 17β-estradiol increases HAT activity in the dorsal hippocampus within 30 minutes, and blocking histone acetylation with a dorsal hippocampus infusion of the HAT inhibitor garcinol prevents 17β-estradiol from enhancing object recognition memory consolidation (Zhao et al., 2012). These findings demonstrate that histone acetylation is necessary for 17β-estradiol to enhance memory formation in female mice. Together with the aforementioned findings that 17β-estradiol-induced histone acetylation depends on ERK activation, these data suggest a central importance of cell signaling in the estrogenic regulation of epigenetic processes that regulate gene expression and memory formation. Given the complexity of cell-signaling mechanisms involved in memory processes, much more work will be needed to fully understand the contributions of various cell-signaling pathways to epigenetic regulation of estrogen-dependent memory.
Research from the past decade has greatly expanded our understanding of how 17β-estradiol regulates hippocampal function beyond classical genomic mechanisms. Classical mechanisms certainly play an important role in the effects of estrogens on long-term memory, as well as the cytoarchitecture and plasticity that supports long-term memory formation and retention. However, recent studies suggest that rapid activation of cell-signaling processes may underlie short-term memory and/or the initial stages of long-term memory acquisition and consolidation in the hippocampus. Cellular events that may contribute to acute estrogenic regulation of acquisition and consolidation are summarized in Fig. 1B (see (Frick, 2015) for a more detailed description of this model). Although these findings have provided exciting new insights, they surely represent just the tip of the non-classical iceberg. Research in the coming years should melt away the ice until the full complexity of non-classical mechanisms underlying estrogenic regulation of memory is revealed.
5. How do different estrogens influence cognition and neurogenesis in the hippocampus?
Up until now, evidence has been presented outlining the epigenetic and cell signaling mechanisms by which 17β-estradiol influences novel object recognition, novel object placement, social learning and social recognition via ERs primarily in the dorsal hippocampus. In this section, the effects of other estrogens will be described in relation to 17β-estradiol. In addition, it is important to recognize that experience and environment matter to the effects of estrogens on brain and behavior and evidence demonstrating these effects will also be described.
There are three main forms of estrogens: estrone, estradiol and estriol. The most potent of the estrogens is 17β-estradiol. Estriol is at highest concentrations during pregnancy, but is not widely studied. Although limited, research suggests that estriol reduces relapses in women with multiple sclerosis (Voskuhl et al., 2015) perhaps via its effects to reduce inflammation and rescue synaptic dysfunction during an automimmune response in the hippocampus (Zeihn et al., 2010). Although estrone and 17β-estradiol both decline with aging in women, there is a shift in the ratio of estradiol to estrone such that there is more estrone relative to estradiol after menopause (Rannevik, et al., 1986). Estrone is a weaker estrogen than estradiol, binding with less affinity to the intracellular ERs. Nonetheless, a widely prescribed hormone therapy (HT) called Premarin, used for the relief of menopausal symptoms, is composed of approximately 50% sulphated estrone and 1% estradiol.
Premarin was used in the Women’s Health Initiative Memory Study (WHIMS) that found that Premarin (plus a synthetic progesterone) was associated with increased risk for dementia and reduced cognitive functioning (Shumaker et al., 2003). This study was criticized based on a number of issues relating to the healthy cell bias (Brinton, 2005), critical window hypothesis (Resnick & Henderson, 2002; Sherwin, 2005), and type of HT (Maki, 2004; Hogervorst et al., 2000; Ryan et al., 2008). Briefly, the healthy cell bias put forward by Roberta Brinton’s group suggested that estrogens will be neuroprotective in a healthy environment but not in a diseased environment. In the WHIMS study, patients were included even if they had a variety of health disorders and this may at least partially explain the negative findings on cognition. The critical window hypothesis refers to the idea that HT will only be effective when initiated early in menopause or just prior to menopause (Resnick & Henderson, 2002) whereas the women in the WHIMS study were on average 15 years past menopause (Resnick & Henderson, 2002; Sherwin, 2005). Meta-analyses indicate that HT therapies were more likely to have cognitive enhancing effects if given right after menopause and not 15 years later (Hogervorst et al., 2000; Ryan et al., 2008). The type of HT is another criticism of the WHIMS findings (Maki, 2004). Premarin, as mentioned above, contains 50% estrone but only 1% estradiol, and these estrogens have different influences on brain and behavior. 17β-estradiol has more positive effects on cognition, whereas estrone has more negative effects on cognition (Barha and Galea, 2009; Hogervorst et al., 2000). Indeed, in a meta-analysis, Hogervorst and colleagues (Hogervorst et al., 2000) found that the majority of studies using estradiol-based therapies were much more likely to find cognitive enhancing effects than studies using estrone-based therapies such as Premarin.
Studies from the Galea laboratory examined the ability of different estrogens to affect hippocampus learning and memory and neurogenesis in adult female rats. Neurogenesis is a form of neuroplasticity that is seen at robust levels in the dentate gyrus of the hippocampus of all mammalian species examined, including humans (see Kempermann et al., 2015 for review). Neurogenesis in the dentate gyrus has a number of stages: cell proliferation (the production of new cells); cell differentiation (into neurons or glia); cell migration (the migration of new neurons into the granule cell layer); and the survival of new neurons. Estrogens are associated with alterations in both cell proliferation and survival of new neurons in the dentate gyrus of adult female rodents (for review see Galea et al., 2013).
Galea and her colleagues found that acute 17β-estradiol and estrone upregulated cell proliferation in a dose-dependent manner in the dentate gyrus of adult female rats (Barha et al., 2009). However, although 17β-estradiol was associated with enhanced contextual fear conditioning at a lower dose, estrone was only associated with impaired contextual fear conditioning at a medium dose (Barha et al., 2011). The authors of those studies do not believe the differential effects of estrone versus 17β-estradiol are solely based due to differences in potency as three different doses of each were used in these experiments (Barha et al., 2009; 2011). Only the low dose of 17β-estradiol improved fear memory but none of the doses of estrone improved fear memory, while both the low and high doses of 17-β estradiol and estrone increased cell proliferation (Barha et al., 2009). It is important to note that although 17-β estradiol and estrone can be bi-directionally converted, the preferential pathway is from estradiol to estrone (Milewich et al., 1985). Furthermore chronic 17β-estradiol increased neurogenesis and activation of new neurons in response to spatial memory retrieval in the dentate gyrus, whereas chronic estrone decreased neurogenesis in the dentate gyrus in adult female rats (see Figure 3A; McClure et al., 2013). Indeed, in this study only 17β-estradiol had a positive correlation between activation of new neurons and spatial memory retrieval (McClure et al., 2013). These findings indicated that only 17β-estradiol was associated with positive effects on spatial memory in the Morris Water Maze, whereas estrone was not. These studies collectively provide evidence for differential effects between 17β-estradiol and estrone with 17β-estradiol facilitating cognition and neuroplasticity but estrone impairing cognition and neurogenesis at certain doses.
Figure 3.
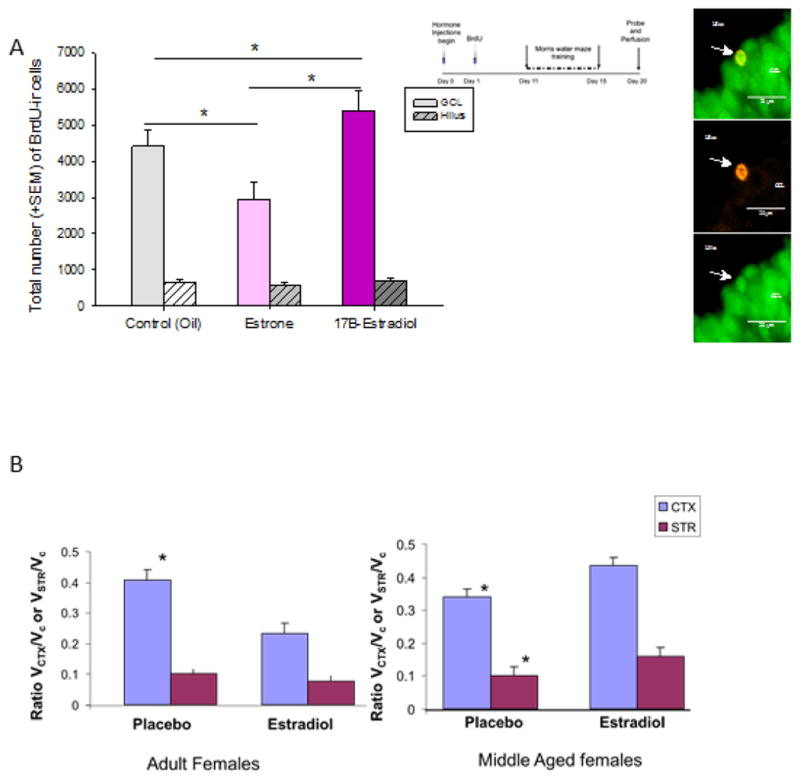
(A) Chronic estrone decreased the number of BrdU-immunoreactive (ir) cells whereas chronic 17β-estradiol increased the total number of BrdU-ir cells. Asterisks (*) indicate significant difference between groups (controls vs 17β-estradiol p=0.046; controls vs estrone p=0.005; 17β-estradiol vs estrone p=0.0002). Photomicrographs indicate a BrdU(red)-ir cell colablled with NeuN(green), indicating that the newly synthesized cell expressed a mature neuronal marker. NeuN-neuronal nuclei; BrdU- bromodeoxyuridine. Modified and reprinted with permission from McClure et al., 2013, Hormones and Behavior, 63: 144–157.
(B) Effect of estrogen treatment on infarct volume is dependent on “reproductive” age: Estrogen treatment to ovariectomized adult females decreased MCAo-induced infarct volume in the cortex and striatum, while the same dose of estrogen treatment increased stroke-induced infarction in middle aged females. CTX: cortex, STR: striatum, *:p<0.05 (modified from Selvamani and Sohrabji, 2010, Neurobiology of Aging, 31, 1618–1628.
Low doses of Premarin were associated with impaired reference and working memory in a spatial working/reference memory version of the radial arm maze (Barha and Galea, 2013). Paradoxically, Premarin also increased neurogenesis in the dentate gyrus (Barha and Galea, 2013). This paradoxical finding may be explained if the new neurons surviving under Premarin either did not make appropriate connections or are not involved in learning or memory per se. The former explanation seemed the most likely explanation, as activation of new neurons was not associated with learning under Premarin treatment but was associated with improved learning under vehicle treatment. Other studies from the Bimonte-Nelson laboratory have found that whereas low doses of Premarin impaired spatial reference acquisition, medium and high doses of Premarin improved spatial working memory (Engler-Chiurrazzi et al., 2011). These studies collectively show that estradiol and estrone have very different influences on cognition and plasticity within the hippocampus, with estradiol having greater dose-dependent facilitatory effects but estrone having more detrimental effects on hippocampus structure and function.
5.1 The effects of estrogens on neurogenesis are dependent on experience
The effects of estrogens on neurogenesis within the hippocampus are altered by various forms of experience such as reproductive experience, spatial training, and/or food restriction. Galea and colleagues found that estrogens do not influence cell proliferation in nulliparous (never mothered) middle-aged rats, but all three estrogens tested (17α-estradiol, 17β-estradiol and estrone) increased cell proliferation in multiparous (pregnant and mothered at least four times) middle-aged rats (Barha and Galea, 2011). Indeed, the effects of the selective serotonin reuptake inhibitor, fluoxetine, on neurogenesis were altered by parity as fluoxetine increased the number of immature neurons in nulliparous animals but not in primiparous animals (Workman et al., in press). These studies among others suggest that reproductive experience influences the ability of certain factors to promote neurogenesis in the hippocampus of adult female rodents. In addition, Barha and Galea (2013) showed that chronic Premarin (1 and 2μg doses) for 33 days increased neurogenesis in the dentate gyrus in females but only in those undergoing a spatial task and food restriction (to 85%). However, the same doses of Premarin did not influence neurogenesis in cage controls that neither underwent spatial training or food restriction. This finding indicates that either food restriction and/or spatial training influences the effects of estrogens on neurogenesis and more studies examining the factors that influence estrogens ability to alter hippocampal parameters need to be considered in the future.
6. Is estradiol neuroprotective? It depends on age and pathology
Estradiol has been shown to be neuroprotective in several experimental models of disease, such as stroke, Parkinson’s disease, and multiple sclerosis (reviewed in Sohrabji et al. 2015). The mechanisms underlying estradiol’s actions are likely pleitropic, involving a combination of anti-apoptotic and anti-inflammatory actions (Simpkins et al., 2010; Suzuki et al., 2009. In the case of ischemic stroke, which is usually modeled by middle cerebral artery occlusion (MCAo), 17β-estradiol was first implicated as a neuroprotectant because of the dramatic sex differences in stroke outcomes. Specifically, females were found to have smaller infarct volumes and better cerebral blood flow than age-matched males both in normoglycemic (Alkayed et al., 1998) and diabetic (Toung et al., 2000) animals, and this sex difference was eliminated when females were bilaterally ovariectomized.
Several other pieces of evidence also support the role of estradiol as neuroprotectant. Stroke injury to females in proestrus (high estradiol levels) result in smaller infarcts than those in metestrus (low estradiol state) and the extent of ischemic damage was inversely related to circulating levels of estradiol (Liao et al., 2011). Bilateral ovariectomy worsens infarct volume and longer periods of estradiol deprivation (1 week versus 4 week of ovariectomy) further increase the size of the infarct (Fukuda et al., 2000). Direct evidence of 17β-estradiol’s role was shown in studies where estradiol treatment to ovariectomized females reduced infarct volume and mortality (Simpkins et al., 1997). Exogenous 17β-estradiol replacement is neuroprotective when given prior (Dubal et al, 1998) or subsequent to the injury (Liu et al., 2007; Yang et al., 2003), and is also effective in males (Toung et al., 1998). Remarkably, activating GPER30 via the G1 ligand is neuroprotective in ovariectomized females, but paradoxically increases infarct volume in males (Broughton et al., 2014), indicating sex specific effects of activating estrogen receptors.
However, 17β-estradiol’s actions are not uniformly neuroprotective in females and, in specific conditions, may have deleterious actions. 17β-estradiol replacement to the Wistar-Kyoto rat strain (Carswell et al., 2004), Lister Hooded and Sprague–Dawley rats (Bingham et al., 2005; Gordon et al., 2005), reportedly increases infarct volume and has no protective effect on infarct size in the ovariectomized stroke-prone spontaneously hypertensive rat (SHRSP) (Carswell et al., 2004). In a model of severe ischemic injury, where cerebral vessels (single middle cerebral artery [MCA] and both common carotids) were occluded for 3h, there were no sex differences in infarct size and no reduction of the infarct due to intravenous or subcutaneous 17β-estradiol (Vergouwen et al., 2000). Based on these studies, Macrae and Carswell (2006) have suggested that the neuroprotective effect of 17β-estradiol may be less effective in permanent ischemic models.
Age also affects the neuroprotective capacity of 17β-estradiol. Relatively few studies have assessed older females in experimental stroke models, and while most studies agree that stroke damage is worse in older females as compared to younger females (see Figure 3B; Takaba et al., 2004; Liu et al., 2009, Selvamani and Sohrabji, 2010a), they differ in their conclusions regarding the efficacy of 17β-estradiol treatment to older females. Chronic 17β-estradiol replacement is neuroprotective to middle-aged females in the MCAo suture stroke model (Alkayed et al., 2000; Dubal and Wise 2001) as well as a single injection of estradiol administered ICV or systemically following a four vessel occlusion model (Lebesgue et al., 2010), although 17β-estradiol failed to attenuate hippocampal cell death in a bilateral carotid artery occlusion model in middle-aged gerbils (De Butte-Smith et al., 2007). In middle-aged female rats, characterized as reproductively senescent by daily vaginal smears and with virtually undetectable levels of estradiol (constant diestrus), 17β-estradiol treatment increased infarct volume and worsened sensory motor performance in an endothelin-1 vasoconstriction model, although 17β-estradiol treatment to multiparous young females was neuroprotective in this model (Selvamani and Sohrabji, 2010a; 2010b). The lack of protective response to 17β-estradiol treatment to older females may be due to an extended period of estrogen deficiency, making their response to subsequent 17β-estradiol treatment less favorable. Some support for this hypothesis comes from a study by Wise and colleagues where ovariectomized females replaced with chronic 17β-estradiol immediately had reduced infarct volume, while those replaced with 17β-estradiol 10 weeks later had no improvement in stroke outcomes (Suzuki et al., 2007).
Another explanation for the ineffectiveness of 17β-estradiol treatment to older animals with stroke comes from observations that estradiol typically collaborates with peptide growth factors to promote neuronal growth and proliferation. Thus in aging females, the loss of estrogens is also accompanied by decreasing levels of other hormones, including IGF-1. In fact, post-stroke IGF-1 replacement to middle-aged females pretreated with 17β-estradiol abrogates the neurotoxic effects of 17β-estradiol in this group (Selvamani and Sohrabji, 2010b). Reciprocally, treatment of young females with the IGF receptor antagonist JB-1 attenuates the protective effect of estradiol typically seen in this group. Together, these data suggest that the collaborative actions of 17β-estradiol and IGF-1 are critical for neuroprotection at any age, but are likely to be unmasked in aging when both estradiol and IGF-1 levels fall.
Both 17β-estradiol and IGF-1 regulate common second messenger systems that are critical for disease process such as cell survival and angiogenesis (Sohrabji, 2014). 17β-estradiol and IGF-1 are critical mitogens, and the PI3K-AKT-mTOR pathway appears to be the principal transducer of their actions. 17β-estradiol proliferative actions on MCF-7 cells, for example, are mediated via IGF-1, and 17β-estradiol increases IRS-1 (IGF-1 substrate) as well as p85 (the active subunit of the PI3K). Conversely, IGF-1 is proliferative only in steroid-treated cells (Bernard et al., 2006). Similarly, a second common pathway for 17β-estradiol and IGF-1 is the MAP kinase signaling family. In 17β-estradiol treatment in vivo results in a dose-dependent activation of ERK and Akt and has a synergistic effect on IGF-1 mediated activation of the pAkt/PKB pathway (Cardona-Gomez et al., 2002). In a medial forebrain bundle injury that models Parkinson’s disease, IGF-1 attenuates lesion effects and also mediates the neuroprotective effects of estrogen (Quesada et al., 2004). IGF-1 and 17β-estradiol both signal through MAPK and Akt pathways and Akt inhibitors blocked the survival effects of both 17β-estradiol and IGF-1 (Quesada et al., 2008), implicating the Akt survival pathway in 17β-estradiol and IGF-1 mediated neuroprotection.
7. Estrogens in the human brain
It should be abundantly clear from the foregoing discussion that estrogens are a significant influence on brain and behavior in various rodent species. Are estrogens similarly important in the human central nervous system (CNS)? The question has received much less study in humans than in laboratory animals, for 2 reasons. One is conceptual: sex differences in humans, to the extent that they are biologically-based, are usually assumed to be driven by exposure of the CNS to androgens during fetal development, not to adult estrogens (Berenbaum & Beltz, 2011; McCarthy & Konkle, 2005). This view derives from animal models, where the organizational effects of androgens (with or without aromatization) during pre- or perinatal development have been a dominant focus of study for decades among those interested in how sex differences arise (Breedlove & Hampson, 2002). At present, it is not widely recognized among human researchers that circulating estrogens can be important for engendering sex differences. The second reason is practical: human researchers can less easily conduct the sort of blinded controlled administration of estradiol vs placebo that is typically done when studying other species. Such manipulations are permissible in some forms of clinical research (or in postmenopausal women whose gonads are inactive), but ethical and logistical complexities are introduced when studying young women of reproductive age where the endogenous ovarian cycle and fertility issues must be taken into consideration.
Animal studies have revealed the surprising breadth of estradiol’s modulatory effects on the cholinergic, serotonergic, dopaminergic, noradrenergic, and other brain pathways (for a review see McEwen & Alves, 1999). To be clear, these effects are dependent on changes in estradiol levels in adult animals, not estrogens that act during early brain development. If such effects do occur in humans, they potentially could have far-reaching implications for a number of mental health conditions. Indeed, symptom expression in some clinical conditions does vary as a function of the ovarian cycle. Women with schizophrenia, for example, show increased symptom severity and more hospital admissions during times when circulating estrogens are low (e.g., Riecher-Rössler et al., 1994; Bergemann et al., 2002). Several randomized trials have now suggested that estradiol treatment administered in conjunction with antipsychotics can improve drug control and perhaps improve positive symptoms in particular (Kulkarni et al., 2015), although conflicting evidence does exist (Bergemann et al., 2005). It is very plausible that variations in circulating estrogen levels bring about these changes, but the role of estrogen is ill defined and because of the lack of dedicated research inquiry is still largely a matter of conjecture.
The role played by estrogens in major depression also is uncertain, including forms of depression closely tied to reproduction, such as antepartum or postpartum depression, but an influence of estradiol levels is suggested by a small body of findings. Major depressive disorder is twice as prevalent in women as men, and the sex difference arises at puberty (Tanner stage III) and declines after menopause (Steiner & Young, 2008). It is possible that a modulatory influence of ovarian hormones on the neurochemistry of major depression might explain the sex difference in prevalence rates. Collaborative work by Hampson and colleagues has found a candidate genetic polymorphism in ESR1 (the gene coding for ERα) that was linked statistically to the occurrence of postpartum depression (Pinsonneault et al., 2013; see also Costas et al., 2010) and a possible interaction with the serotonin transporter was identified (Pinsonneault et al., 2013). Hampson and colleagues also found lower serum estradiol (but increased cortisol) in women suffering from antepartum depression during pregnancy compared with healthy controls assessed at the same gestational timepoint (Hampson et al., 2015). Emerging animal data supports the idea that steroids may play a role in postpartum depression (for a review see Brummelte & Galea, 2015).
So how do we study estrogen-related effects in humans, and do they really matter? Although we can’t always manipulate hormones exogenously, it is still possible to design intelligent studies that probe the potential role of biological sex and the role of estrogens. A review of ‘best practices’ when doing sex differences research was published by Endocrinology in 2005 (Becker et al., 2005) and contains a wealth of useful tips for novices (see also Becker et al., 2008). Certain issues peculiar to humans need to be taken into account. For instance, estrogen levels differ greatly pre- and post-menopause, or as a function of the use of exogenous estrogens in menopausal women who opt to use hormone replacement therapy (HT). In young women, it is necessary for researchers to attend to whether or not hormonal contraception is used. Oral contraceptives contain ethinyl estradiol combined with one of at least 13 different progestins. Oral contraceptives suppress the endogenous changes in sex steroids associated with the menstrual cycle and the synthetic steroids contained in the pills themselves exert a combination of estrogenic, progestogenic, and androgenic effects (Hampson & Young, 2008).
Although active in the CNS, ethinyl estradiol is not detected by most routine immunoassays available commercially (data available from the manufacturers), so simply measuring women’s estradiol levels is not an effective method to deal with oral contraceptive use when designing a study. Although it is beyond the scope of this review, it is often necessary when studying sex differences in brain and behavior to limit participation to women who have naturally-occurring cycles, or to consider oral contraceptive users separately in statistical analyses, or to take advantage of the steroidal milieu offered by the pills to harness their effects in a meaningful way that is useful to the research question (for a discussion of issues related to oral contraceptives and their effects see Hampson & Young, 2008; Beltz et al., 2015). One common method for studying the effects of estrogens (and progestins) in neuroscience studies involving human participants is to carefully time the behavioral testing on an individualized basis to coincide with specific phases of the menstrual cycle when estradiol levels are expected to be either high or low (Hampson & Young, 2008; Becker et al., 2005), a method used in many studies in the Hampson laboratory (Hampson et al., 2005; cf. Hampson & Morley, 2013) and also adopted by others (e.g. Maki et al., 2002). In effect, targeted timing affords researchers a naturalistic method to compare the differences in behavioral outcomes that result under low versus high estradiol conditions.
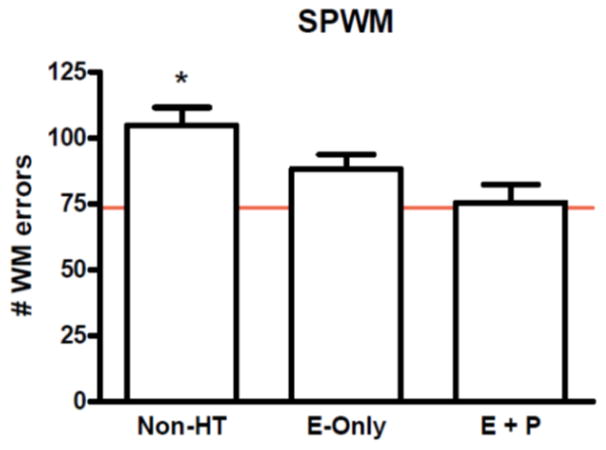
An illustration of these approaches is useful here, not simply to illustrate how estrogens can be considered in human research studies, but also to illustrate why it matters conceptually. Working memory is a form of short-term memory that is well known to decline during normal aging and also in certain medical conditions. The executive components of working memory depend upon activity in the prefrontal cortex in humans and other primates (Owen, 1997). Duff and Hampson (2000) showed, in healthy postmenopausal women, that the use of estrogen replacement therapy (taken in the form of conjugated equine estrogens), whether or not it was combined with a progestin, was associated with significantly better accuracy on a test of spatial working memory (the SPWM task; Duff & Hampson, 2000) compared with women not using HT. Two other working memory tasks showed the same effect. Of note, all women in the Duff and Hampson study were in good general health and began treatment at the onset of menopause. This finding has since been replicated and extended by others using either observational designs (e.g., Keenan et al., 2001) or randomized trials of estradiol or placebo (e.g., Krug et al., 2006), and is supported by work in rhesus monkeys using a delayed response task (Rapp et al., 2003) where superior performance under estradiol treatment was found. In the study by Duff and Hampson (2000), the difference in the numbers of working memory errors between non-users and women treated with estrogen-progestin therapy was relatively large (approximately 20–40% greater errors among non-users on the SPWM task, see Figure 4), and women using replacement estrogens in fact performed within the range of scores typically observed in much younger control women (Hampson & Moffat, 2004; Hampson & Morley, 2013). Taking estrogen status into account thus advanced our understanding of memory changes during aging, suggesting that at least some of the change is not, in fact, age-related as commonly assumed, but may be attributable instead to endocrine changes that accompany aging.
Figure 4.
Total number of working memory (WM) errors committed by 3 groups of postmenopausal women (mean age 55.6 yrs) on the SPWM test of working memory. WM errors were significantly more frequent among women not receiving hormone therapy (Non-HT, n = 35), compared with women who were receiving estrogens only (E-Only, n = 38) or women receiving estrogens plus a progestin (E + P, n = 23) at the time of testing. Difference between the 2 treated groups (E-Only, E + P) was not significant. Bars represent standard errors. (Data redrawn from Duff & Hampson, 2000, Horm Behav, 38, 262–276). Horizontal line shows mean level of performance on the same task in a group of young women not using oral contraceptives (n = 39, mean age 21.6 yrs) (first 2 trials only; for details see Hampson & Morley, 2013, Psychoneuroendocrinology, 38, 2897–2904).
These studies collectively implicate the prefrontal cortex as a novel site of estrogen action in the human female brain, a fact not previously appreciated. The possibility that the frontal cortex might be modulated by estrogens in women is a new idea that is beginning to receive support from investigations using functional MRI. Regional changes in frontal activation are observed during working memory tasks when women are receiving estradiol compared with placebo (e.g., Smith et al., 2006; Joffe et al., 2006). Recent work suggests that estradiol levels are positively correlated with working memory performance in younger women of reproductive age (Hampson & Morley, 2013), corroborating earlier observations showing a modest sex difference on the same memory task (SPWM) in young adults (Duff & Hampson, 2001; Lejbak et al., 2009). These are exciting findings that have a potential to transform our present understanding of the prefrontal cortex, and perhaps shed new light on the clinical syndromes described earlier (schizophrenia, major depression), in which the frontal cortex plays a significant but inadequately understood role (Koenigs & Grafman, 2009).
8. The importance of including females in studies of behavioral neuroscience
The studies reviewed above, from basic science and animal models of neuropathology, to investigations in women, demonstrate the prominent role played by estrogens in female brain function and behavior. Such estrogenic effects highlight the need for the inclusion of females in neurobiological investigations and are one important facet to our understanding of biological sex differences. Despite a scientific climate in which understanding sex differences is increasingly emphasized, a survey of the literature from 2009 found a strong bias towards studying only males in biomedical research (Beery and Zucker, 2011). In this regard, the recent policy to include sex as a variable in NIH-funded research is welcome. However, the careful study of sex differences requires more than the simple inclusion of males and females. Currently the general practice has been to use sex as a covariate in analyses to “control for sex differences” or to combine males and females together in the analysis. For example Beery and Zucker (2011) found in their review that studies that included both sexes analyzed sex as an independent factor only 20% of the time. Thus, unfortunately the vast majority of studies that included both sexes did not analyze the effect of biological sex.
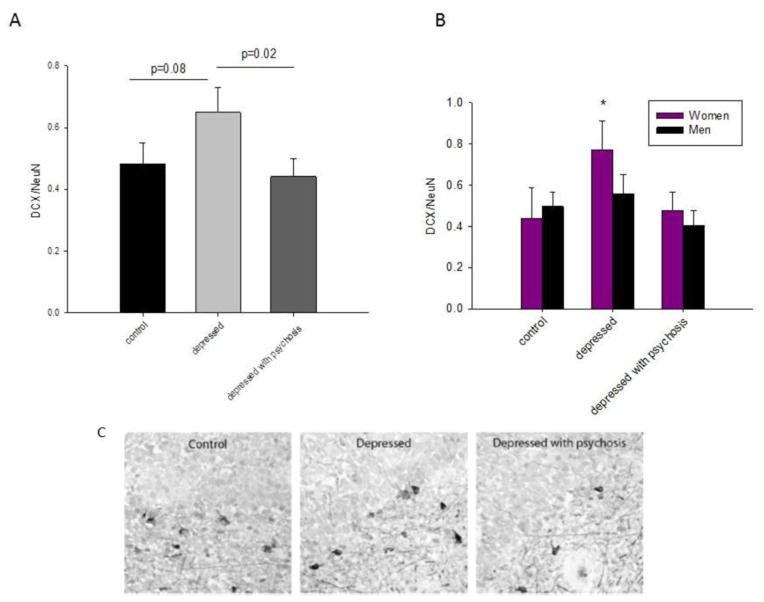
Here we give three general recommendations for studies examining sex differences: 1) use sex as an independent factor in the analysis (and not as a covariate, see explanation below); 2) design studies with sufficient statistical power; and 3) include a thoughtful evaluation of factors such as reproductive and hormonal status, which can affect brain and behavior. First, we think it is important to consider sex as an independent factor in the analysis and not simply as a covariate, because the latter in part precludes the detection of interactions with sex and other independent variables and/or non-linear variations with sex (Mefford and Witte, 2012). For example, when the authors used sex as a factor in their a priori analysis in a study of men and women, it found that only depressed women prescribed antidepressants showed an increase in immature neuron ratio in the hippocampus (Epp et al., 2013). However, analyzing these data using sex as a covariate yielded no significant effect of antidepressants to increase immature neuron ratio (see Figure 5). Had the authors only used sex as a covariate, they would have lost important information about female specific response to prescribed antidepressants. Similar sex-dependent effects have been found in other studies (Brody et al., 2011; Wright et al., 2007) and support the importance of assessing sex as a variable. Second, it is critical that sufficient numbers of males and females are included in each study, as statistical underpowering has been identified as a major problem in neuroscience. Lack of statistical power leads to a lack of reproducibility due to either a low probability of finding a true effect or an overestimate of the size of a true effect (Button et al., 2013). Third, when hormonal effects have been identified for a certain biological system, it is critical that they are taken into account when both sexes are investigated. For example, studies on oxytocin have seen an upsurge in publications recently, and many have featured only men or males (e.g. Auyeung et al., 2015; Ma et al., 2015). However, sex differences and estrogenic regulation of oxytocin are established (e.g. in rodents and humans, Feng et al., 2015; Rilling et al., 2014). Thus, the exclusion of females may lead to conclusions that cannot be extrapolated to girls or women (Dhakar et al 2013).
Figure 5.
The density of doublecortin (DCX)/NeuN expression in the dentate gyrus is increased in depressed patients. A) The density of DCX/NeuN expression was increased in depressed patients compared to depressed patients with psychosis (p=.02) and with a strong trend in controls (p=0.08). B) The density of DCX/NeuN expression was significantly greater in depressed women compared to all other groups. C) Representative DCX-expression is shown from depressed patients, depressed patients with psychosis and controls. Data shown is mean + SEM (standard error of the mean). Reprinted with permission from Epp JR, Beasley CL, Galea LAM. (2013). Neuropsychopharmacology, 38:2297–2306.
The lack of inclusion of sex as a statistical factor could become problematic in clinical trials. For example there is no planned assessment of sex differences as either a primary or secondary outcome in an ongoing clinical trial examining the use of intranasal oxytocin as a therapy in autism (ClinicalTrials.gov Identifier: NCT01944046). Thus, potential sex differences in response to oxytocin will not be examined and although autism has a greater incidence in boys compared to girls, the lack of attention to sex differences may lead to erroneous conclusions regarding the effectiveness of oxytocin for the treatment of girls with autism. Lastly, when examining females, care must be taken to record and analyze variables such as estrous or menstrual cycle phase that cause within-sex or within-subject variability as seen in the review of the literature above. This may be particularly important when examining measures that involve the hippocampus, as discussed below.
9. Studying females: when to track the menstrual/estrous cycle
A meta-analysis indicated that there is no significant difference in variance between male and female mice across a wide variety of behavioral, molecular, morphological and physiological traits (Pendergast et al., 2011). It has been suggested that these findings indicate that the estrous cycle phase does not need to be monitored (McCarthy, 2015). However, it should be noted that the lack of significant difference in variability between males and females does not exclude the possibility that estrous phase produces variability in females. To be fair, it has been argued that studying females is more complicated than studying males because of the estrous cycle and the authors of the meta-analysis (Pendergast et al., 2013) undoubtedly wanted to assuage researcher’s fears that estrous cycle collection is needed (McCarthy, 2015). Others have argued against the importance of tracking estrous cycle and suggest that environmental factors such as single versus pair housing are more important to behavioral variability (Richardson et al., 2015). Arguing that the variability is larger within one context (housing variability > variability within females) does not preclude the possibility that estrous cycle contributes to the variability seen in females. Indeed, many factors such as chronic high corticosterone (Brummelte and Galea, 2010), housing parameters (Baker and Bielajew, 2007), and aging (Rubin, 2000) can alter cyclicity in the female. In general, we think it is important to acknowledge that estrous cycle phase does indeed influence some factors quite dramatically (see below).
Although there are equivocal findings as to the effects of estrous cycle phase on some measures such as cell proliferation in the hippocampus (Tanapat et al., 21999; Rummel et al., 2010 compared to Lagace et al., 2007), it is important to know that estradiol and progesterone levels can vary dramatically within a few hours of the day (Tada et al., 2015). Thus, timing of testing is very important to observing an effect of estrous cycle phase (Tada et al., 2015) and may lead to variable findings in the literature. This is not to say that the estrous cycle will be important to monitor for every variable that a researcher may be interested in testing, but that estrous cycle has been demonstrated repeatedly to influence a wide variety of measures. For example, there are estrous cycle effects on hippocampal CA1 spine density (Woolley et al., 1990), LTP parameters (Warren et al., 1997), spatial cognition (Warren and Juraska, 1995), depressive-like behaviors (Kokras et al., 2015), antinociceptive potency of opioids (Terner et al., 2005), dopamine signaling (Perez et al., 2014) and cell proliferation (Tanapat et al., 1999). Indeed, the menstrual cycle has been reported to influence cognitive and electrophysiological measures in women as well, such as seizure frequency (Herzog et al., 2014), mental rotation (Hampson et al., 2014; Maki et al., 2002), and fMRI activity patterns (Dietrich et al., 2001; Schöning et al., 2007). However, care needs to be taken in measuring menstrual cycle phase as age (Klein et al., 1996) and parity (Barrett et al., 2014) can affect both the length of menstrual cycle and ovarian hormone levels across each stage.
Conclusions
In conclusion, estrogens impact specific cognitive and social behaviors (e.g., working memory, spatial memory, novel object recognition and social behavior) and influence neuroprotection in models of stroke. However, a number of experiential and genetic factors (e.g., age, reproductive factors, estrogen type, genotype, disease background) can moderate cognitive outcome and neuroprotection with estrogens, and these factors are not currently fully appreciated. There is a growing realization of the importance of studying sex differences in neuroscience, and our review has presented accumulating evidence that estrogens matter for hippocampus and prefrontal cortex-related cognition, neuroplasticity, and neuroprotection. Only by studying the role of sex hormones on brain and behavior in both males and females will we begin to develop better more effective therapeutic advances to promote brain health.
Highlights.
17β-estradiol impacts specific cognitive and social behaviors in adult females
17β-estradiol influences neuroprotection in models of stroke dependent on age
Estrone and 17β-estradiol differentially influence cognition and neuroplasticity
Experiential factors moderate cognitive outcome and neuroprotection with estrogens
Acknowledgments
Empirical work described from LAMG’s laboratory was supported by Canadian Institutes for Health Research (MOP102568), Natural Sciences and Engineering Research Council of Canada (203596-13), Pacific Alzheimer Research Foundation and Alzheimer Society of Canada (13–27). KMF would like to thank The University of Wisconsin-Milwaukee and R01DA038042 for supporting the writing of this manuscript and travel to the IBNS meeting. Empirical work described from KMF’s laboratory was supported by R01AG022525, R03MH065460, the American Federation for Aging Research, a University of Wisconsin-Milwaukee Research Growth Initiative Award, the University of Wisconsin-Milwaukee, and Yale University. Empirical work from EC laboratory was supported by Natural Sciences and Engineering Research Council of Canada (400212). Empirical work described from EH’s laboratory was supported by the Natural Sciences and Engineering Research Council of Canada (138017), the Ontario Mental Health Foundation (T37789), and the Society for Women’s Health Research through the Isis Fund Network on Sex, Gender, Drugs and the Brain. Empirical work from the FS laboratory was supported by AG042189 and NS074895.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JP, Sweatt JD. Molecular psychology: Roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- Akama KT, Thompson LI, Milner TA, McEwen BS. Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled Receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines. J Biol Chem. 2013;288:6438–6450. doi: 10.1074/jbc.M112.412478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiaz R, Seidman SN. Testosterone and depression in men. Curr Opin Endocrinol Diabetes Obes. 2008;15:278–83. doi: 10.1097/MED.0b013e3282fc27eb. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–165. doi: 10.1161/01.str.29.1.159. discussion 166. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31:161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- Angst J, Gamma A, Gastpar M, Lépine JP, Mendlewicz J, Tylee A. Gender differences in depression. Epidemiological findings from the European DEPRES I and II studies. Eur Arch Psychiatry Clin Neurosci. 2002;252:201–209. doi: 10.1007/s00406-002-0381-6. [DOI] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Auyeung B, Lombardo MV, Heinrichs M, Chakrabarti B, Sule A, Deakin JB, Bethlehem RA, Dickens L, Mooney N, Sipple JA, Thiemann P, Baron-Cohen S. Oxytocin increases eye contact during a real-time, naturalistic social interaction in males with and without autism. Transl Psychiatry. 2015;5:e507. doi: 10.1038/tp.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S, Bielajew C. Influence of housing on the consequences of chronic mild stress in female rats. Stress. 2007 Aug;10(3):283–93. doi: 10.1080/10253890701265362. [DOI] [PubMed] [Google Scholar]
- Barha CK, Dalton GL, Galea LAM. Low doses of 17α-estradiol and 17β-estradiol facilitate, while higher doses of estrone and 17α- and 17β-estradiol impair, contextual fear conditioning in adult female rats. Neuropsychopharmacology. 2010;35:547–559. doi: 10.1038/npp.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Galea LAM. The hormone therapy, Premarin, impairs hippocampus-dependent spatial learning and memory and reduces activation of new granule neurons in response to memory in female rats. Neurobiology of Aging. 2013;34:986–1004. doi: 10.1016/j.neurobiolaging.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Barha CK, Galea LAM. Motherhood alters the cellular response to estrogens in the hippocampus later in life. Neurobiology of Aging. 2011;32:2091–2095. doi: 10.1016/j.neurobiolaging.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Barha CK, Lieblich SE, Galea LAM. Different forms of oestrogens rapidly upregulate hippocampal cell proliferation in the dentate gyrus of young female rats. Journal of Neuroendocrinology. 2009;21:155–166. doi: 10.1111/j.1365-2826.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005 Jun;62(6):685–91. doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- Barrett ES, Parlett LE, Windham GC, Swan SH. Differences in ovarian hormones in relation to parity and time since last birth. Fertil Steril. 2014 Jun;101(6):1773–80.e1. doi: 10.1016/j.fertnstert.2014.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, Vicens V, Hollander E. Oxytocin can hinder trust and cooperation in borderline personality disorder. Soc Cogn Affect Neurosci. 2011 Oct;6(5):556–63. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum LW. Sex, hormones, and Alzheimer’s disease. J Gerontol A Biol Sci Med Sci. 2005;60(6):736–43. doi: 10.1093/gerona/60.6.736. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young EA. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young EA. Sex Differences in the Brain: From Genes to Behavior. New York: Oxford University Press; 2008. [Google Scholar]
- Bellott DW, Hughes JF, Skaletsky H, Brown LG, Pyntikova T, Cho TJ, Koutseva N, Zaghlul S, Graves T, Rock S, Kremitzki C, Fulton RS, Dugan S, Ding Y, Morton D, Khan Z, Lewis L, Buhay C, Wang Q, Watt J, Holder M, Lee S, Nazareth L, Alföldi J, Rozen S, Muzny DM, Warren WC, Gibbs RA, Wilson RK, Page DC. Mammalian Y chromosomes retain widely expressed dosage-sensitive regulators. Nature. 2014 Apr 24;508(7497):494–9. doi: 10.1038/nature13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltz AM, Hampson E, Berenbaum SA. Oral contraceptives and cognition: A role for ethinyl estradiol. Hormones and Behavior. 2015;74:209–217. doi: 10.1016/j.yhbeh.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011 Jan;35(3):565–72. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Katche C, Slipczuk LN, Igaz LM, Cammarota M, Izquierdo I, Medina JH. mTOR signaling in the hippocampus is necessary for memory formation. Neurobiol Learn Mem. 2007;87:303–307. doi: 10.1016/j.nlm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Beltz A. Sexual differentiation of human behavior: Effects of prenatal and pubertal organizational hormones. Frontiers in Neuroendocrinology. 2011;32:183–200. doi: 10.1016/j.yfrne.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Bergemann N, Mundt C, Parzer P, Pakrasi M, Eckstein-Mannsperger U, Haisch S, Salbach B, Klinga K, Runnebaum B, Resch F. Estrogen as an adjuvant therapy to antipsychotics does not prevent relapse in women suffering from schizophrenia: Results of a placebo-controlled double-blind study. Schizophrenia Research. 2005;74:125–134. doi: 10.1016/j.schres.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Bergemann N, Parzer P, Nagl I, Salbach B, Runnebaum B, Mundt C, Resch F. Acute psychiatric admission and menstrual cycle phase in women with schizophrenia. Archives of Women’s Mental Health. 2002;5:119–126. doi: 10.1007/s00737-002-0004-2. [DOI] [PubMed] [Google Scholar]
- Bernard L, Legay C, Adriaenssens E, Mougel A, Ricort JM. Estradiol regulates the insulin-like growth factor-I (IGF-I) signalling pathway: A crucial role of phosphatidylinositol 3-kinase (PI 3-kinase) in estrogens requirement for growth of MCF-7 human breast carcinoma cells. Biochem and Biophys Res Commun. 2006;350:916–921. doi: 10.1016/j.bbrc.2006.09.116. [DOI] [PubMed] [Google Scholar]
- Bertakis KD. The influence of gender on the doctor-patient interaction. Patient Educ Couns. 2009;76:356–360. doi: 10.1016/j.pec.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Bingham D, Macrae IM, Carswell HV. Detrimental effects of 17beta-oestradiol after permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2005;25:414–420. doi: 10.1038/sj.jcbfm.9600031. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Lehman MN, Turcotte JC, Greene G. Estrogen receptors in dendrites and axon terminals in the guinea pig hypothalamus. Endocrinology. 1991;131:281–90. doi: 10.1210/endo.131.1.1612006. [DOI] [PubMed] [Google Scholar]
- Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. 2003;44(3):234–46. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-α trafficking. J Neurosci. 2009;29:15323–15330. doi: 10.1523/JNEUROSCI.2107-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. J Neurosci. 2013;33:15184–15194. doi: 10.1523/JNEUROSCI.1716-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Hampson E. Sexual differentiation of the brain and behavior. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral endocrinology. MIT Press; Cambridge MA: 2002. pp. 75–114. [Google Scholar]
- Brinton RD. Investigative models for determining hormone therapy-induced outcomes in brain: evidence in support of a healthy cell bias of estrogen action. Ann N Y Acad Sci. 2005;1052:57–74. doi: 10.1196/annals.1347.005. [DOI] [PubMed] [Google Scholar]
- Brody GH, Beach SR, Chen YF, Obasi E, Philibert RA, Kogan SM, Simons RL. Perceived discrimination, serotonin transporter linked polymorphic region status, and the development of conduct problems. Dev Psychopathol. 2011;23:617–627. doi: 10.1017/S0954579411000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton BR, Brait VH, Kim HA, Lee S, Chu HX, Gardiner-Mann CV, Guida E, Evans MA, Miller AA, Arumugam TV, Drummond GR, Sobey CG. Sex-dependent effects of G protein-coupled estrogen receptor activity on outcome after ischemic stroke. Stroke. 2014;45:835–841. doi: 10.1161/STROKEAHA.113.001499. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LAM. Postpartum depression: Etiology, treatment and consequences for maternal care. Hormones and Behavior. 2015 doi: 10.1016/j.yhbeh.2015.08.008. *** [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafò MR. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Cardona-Gomez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogen and insulin-like growth factor-I in the brain: molecular mechanisms and functional implications. J Steroid Biochem Mol Biol. 2002;83:211–217. doi: 10.1016/s0960-0760(02)00261-3. [DOI] [PubMed] [Google Scholar]
- Carswell HV, Bingham D, Wallace K, Nilsen M, Graham DI, Dominiczak AF, Macrae IM. Differential effects of 17beta-estradiol upon stroke damage in stroke prone and normotensive rats. J Cereb Blood Flow Metab. 2004;24:298–304. doi: 10.1097/01.WCB.0000112322.75217.FD. [DOI] [PubMed] [Google Scholar]
- Chwang WB, O’Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Gray DG, Diaz-Gonzalez S, Welsman RG. Differential effects of dopamine receptor D1-type and D2-type antagonists and phase of the estrous cycle on social learning of food preferences, feeding and social interactions in mice. Neuropsychopharmacology. 2011;36:1689–1702. doi: 10.1038/npp.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014 May 15;509(7500):282–3. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costas J, Gratacos M, Escaramis G, Martin-Santos R, de Diego Y, Baca-Garcia E, Canellas F, Estivill X, Guillamat R, Guitart M, Gutierrez-Zotes A, Garcia-Esteve L, Mayoral F, Molto MD, Phillips C, Roca M, Carracedo A, Vilella E, Sanjuan J. Association study of 44 canadidate genes with depressive and anxiety symptoms in post-partum women. Journal of Psychiatry Research. 2013;44:717–724. doi: 10.1016/j.jpsychires.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Dash PK, Orsi SA, Moore AN. Spatial memory formation and memory-enhancing effect of glucose involves activation of the tuberous sclerosis complex-Mammalian target of rapamycin pathway. J Neurosci. 2006;26:8048–8056. doi: 10.1523/JNEUROSCI.0671-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakar MB, Stevenson EL, Caldwell HK. Oxytocin, vasopressin, and their interplay with gonadal steroids. In: Choleris E, Pfaff D, Kavaliers M, editors. Oxytocin, Vasopressin and Related Peptides in the Regulation of Behavior. Cambridge University Press; 2013. pp. 3–26. [Google Scholar]
- Dietrich T, Krings T, Neulen J, Willmes K, Erberich S, Thron A, Sturm W. Effects of blood estrogen level on cortical activation patterns during cognitive activation as measured by functional MRI. Neuroimage. 2001;13:425–432. doi: 10.1006/nimg.2001.0703. [DOI] [PubMed] [Google Scholar]
- Diver MJ, Imtiaz KE, Ahmad AM, Vora JP, Fraser WD. Diurnal rhythms of serum total, free and bioavailable testosterone and of SHBG in middle-aged men compared with those in young men. Clin Endocrinol (Oxf) 2003 Jun;58(6):710–7. doi: 10.1046/j.1365-2265.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- Duarte-Guterman P, Leiblich SE, Chow C, Galea LA. Estradiol and GPER activation differentially affect cell proliferation but not GPER expression in the hippocampus of adult female rats. PLoS ONE. 2015;10:e0129880. doi: 10.1371/journal.pone.0129880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal D, Kashon M, Pettigrew L, Ren J, Finklestein S, Rau S, Wise P. Estradiol protects against ischemic injury. J Cereb Blood Flow Metab. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Wise PM. Neuroprotective effects of estradiol in middle-aged female rats. Endocrinology. 2001;142:43–48. doi: 10.1210/endo.142.1.7911. [DOI] [PubMed] [Google Scholar]
- Duff SJ, Hampson E. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Hormones and Behavior. 2000;38:262–276. doi: 10.1006/hbeh.2000.1625. [DOI] [PubMed] [Google Scholar]
- Duff SJ, Hampson E. A sex difference on a novel spatial working memory task in humans. Brain and Cognition. 2001;47:470–493. doi: 10.1006/brcg.2001.1326. [DOI] [PubMed] [Google Scholar]
- Ellegren H, Parsch J. The evolution of sex-biased genes and sex-biased gene expression. Nat Rev Genet. 2007 Sep;8(9):689–98. doi: 10.1038/nrg2167. [DOI] [PubMed] [Google Scholar]
- Epp JR, Beasley CL, Galea LAM. Increased Hippocampal Neurogenesis and p21 Expression in Depression: Dependent on Antidepressants, Sex, Age, and Antipsychotic Exposure. Neuropsychopharmacology. 2013;38:2297–2306. doi: 10.1038/npp.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervin SJK, Lymer J, Matta R, Clipperton-Allen AE, Kavaliers M, Choleris E. Estrogen involvement in social behavior in rodents: rapid and long-term actions. Horm Behav. 2015;74:53–76. doi: 10.1016/j.yhbeh.2015.05.023. [DOI] [PubMed] [Google Scholar]
- Ervin SJK, Mulvale E, Gallagher N, Roussel V, Choleris E. Activation of the G protein-coupled estrogen receptor, but not estrogen receptor α or β, rapidly enhances social learning. Psychoneuroendocrinology. 2015;58:51–66. doi: 10.1016/j.psyneuen.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Ervin SJK, Phan A, Gabor CS, Choleris E. Rapid oestrogenic regulation of social and non-social learning. J Neuroendocrinol. 2013;25:1116–1132. doi: 10.1111/jne.12079. [DOI] [PubMed] [Google Scholar]
- Fan L, Zhao Z, Orr PT, Chambers CH, Lewis MC, Frick KM. Estradiol-induced object memory consolidation in middle-aged female mice requires dorsal hippocampal extracellular signal-regulated kinase and phosphatidylinositol 3-kinase activation. J Neurosci. 2010;30:4390–4400. doi: 10.1523/JNEUROSCI.4333-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Hackett PD, DeMarco AC, Chen X, Stair S, Haroon E, Ditzen B, Pagnoni G, Rilling JK. Oxytocin and vasopressin effects on the neural response to social cooperation are modulated by sex in humans. Brain Imaging Behav. 2015 Dec;9(4):754–64. doi: 10.1007/s11682-014-9333-9. [DOI] [PubMed] [Google Scholar]
- Fernandez SM, Lewis MC, Pechenino AS, Harburger LL, Orr PT, Gresack JE, Schafe GE, Frick KM. Estradiol-induced enhancement of object memory consolidation involves hippocampal ERK activation and membrane-bound estrogen receptors. J Neurosci. 2008;28:8660–8667. doi: 10.1523/JNEUROSCI.1968-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. Estradiol-induced object recognition memory consolidation is dependent on activation on mTOR signaling in the dorsal hippocampus. Learn Mem. 2013;20:147–155. doi: 10.1101/lm.026732.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortress AM, Frick KM. Epigenetic regulation of estrogen-dependent memory. Front Neuroendocrinol. 2014;35:530–549. doi: 10.1016/j.yfrne.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav. 2015;74:4–18. doi: 10.1016/j.yhbeh.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Kim J, Tuscher JJ, Fortress AM. Sex steroid hormones matter for learning and memory: estrogenic regulation of hippocampal function in male and female rodents. Learn Mem. 2015;22:472–493. doi: 10.1101/lm.037267.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Yao H, Ibayashi S, Nakahara T, Uchimura H, Fujishima M, Hall ED. Ovariectomy exacerbates and estrogen replacement attenuates photothrombotic focal ischemic brain injury in rats. Stroke. 2000;31:155–160. doi: 10.1161/01.str.31.1.155. [DOI] [PubMed] [Google Scholar]
- Gabor CS, Lymer J, Phan A, Choleris E. Rapid effects of the G-Protein Coupled Estrogen Receptor (GPER) on learning and dorsal hippocampus dendritic spines in female mice. Physiol Behav. 2015;149:53–60. doi: 10.1016/j.physbeh.2015.05.017. [DOI] [PubMed] [Google Scholar]
- Gabor CS, Phan A, Clipperton-Allen AE, Kavaliers M, Choleris E. Interplay of oxytocin, vasopressin and sex hormones in the regulation of social recognition. Behav Neurosci. 2012;126:97–109. doi: 10.1037/a0026464. [DOI] [PubMed] [Google Scholar]
- Galea LAM, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK. Sex, hormones, and neurogenesis in the hippocampus: Hormonal modulation of neurogenesis and potential functional implications. Journal of Neuroendocrinology. 2014;25:1039–1061. doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch Gen Psychiatry. 1998 Sep;55(9):809–15. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- Gordon KB, Macrae IM, Carswell HV. Effects of 17beta-oestradiol on cerebral ischaemic damage and lipid peroxidation. Brain Res. 2005;1036:155–162. doi: 10.1016/j.brainres.2004.12.052. [DOI] [PubMed] [Google Scholar]
- Gräff J, Tsai LH. Histone acetylation: Molecular mnemonics on the chromatin. Nat Rev Neurosci. 2013;14:97–111. doi: 10.1038/nrn3427. [DOI] [PubMed] [Google Scholar]
- Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Lobos K, Scherer M, Anderer P, Katschnig H. Theinfluence of age on the female/male ratio of treated incidence rates in depression. BMC Psychiatry. 2002;2:3. doi: 10.1186/1471-244X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungabissoon RA, Bamburg JR. Regulation of growth cone actin dynamics by ADF/cofilin. J Histochem Cytochem. 2003;51:411–420. doi: 10.1177/002215540305100402. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McGaugh JL. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci USA. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Horm Behav. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Kline E, Gibbs RB. Chronic treatment with a GPR30 antagonist impairs acquisition of a spatial learning task in young female rats. Horm Behav. 2012;62:367–374. doi: 10.1016/j.yhbeh.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson E, Levy-Cooperman N, Korman JM. Estradiol and mental rotation: Relation to dimensionality, difficulty, or angular disparity? Horm Behav. 2014;65:238–248. doi: 10.1016/j.yhbeh.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Hampson E, Moffat SD. The psychobiology of gender: Cognitive effects of reproductive hormones in the adult nervous system. In: Eagly AH, Beall AE, Sternberg RJ, editors. The Psychology of Gender. 2. New York: Guilford Press; 2004. pp. 38–64. [Google Scholar]
- Hampson E, Morley EE. Estradiol concentrations and working memory performance in women of reproductive age. Psychoneuroendocrinology. 2013;38:2897–2904. doi: 10.1016/j.psyneuen.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Hampson E, Phillips SD, Duff-Canning SJ, Evans KL, Merrill M, Pinsonneault JK, Sadee W, Soares CN, Steiner M. Working memory in pregnant women: Relation to estrogen and antepartum depression. Hormones and Behavior. 2015;74:218–227. doi: 10.1016/j.yhbeh.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson E, Young EA. Methodological issues in the study of hormone-behavior relations in humans: Understanding and monitoring the menstrual cycle. In: Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young EA, editors. Sex Differences in the Brain: From Genes to Behavior. New York: Oxford University Press; 2008. pp. 63–78. [Google Scholar]
- Hart D, Nilges M, Pollard K, Lynn T, Patsos O, Shiel C, Clark SM, Vasudevan N. Activation of the G-protein coupled receptor 30 (GPR30) has different effects on anxiety in male and female mice. Steroids. 2014;81:49–56. doi: 10.1016/j.steroids.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Hojo Y, Kojima H, Ikeda M, Hotta K, Sato R, Ooishi Y, Yoshiya M, Chung BC, Yamazaki T, Kawato S. Estradiol rapidly modulates synaptic plasticity of hippocampal neurons: Involvement of kinase networks. Brain Res. 2015;1621:147–161. doi: 10.1016/j.brainres.2014.12.056. [DOI] [PubMed] [Google Scholar]
- Hawley WR, Grissom EM, Moody NM, Dohanich GP, Vasudevan N. Activation of G-protein-coupled receptor 30 is sufficient to enhance spatial recognition memory in ovariectomized rats. Behav Brain Res. 2014;262:68–73. doi: 10.1016/j.bbr.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Fowler KM, Sperling MR, Massaro JM Progesterone Trial Study Group. Distribution of seizures across the menstrual cycle in women with epilepsy. Epilepsia. 2015 May;56(5):e58–62. doi: 10.1111/epi.12969. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: At the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E1, Bandelow S, Moffat SD. Increasing testosterone levels and effects on cognitive functions in elderly men and women: a review. Curr Drug Targets CNS Neurol Disord. 2005 Oct;4(5):531–40. doi: 10.2174/156800705774322049. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Williams J, Budge M, Riedel W, Jolles J. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post-menopausal women: a meta-analysis. Neuroscience. 2000;101(3):485–512. doi: 10.1016/s0306-4522(00)00410-3. [DOI] [PubMed] [Google Scholar]
- Holland J, Bandelow S, Hogervorst E. Testosterone levels and cognition in elderly men: a review. Maturitas. 2011 Aug;69(4):322–37. doi: 10.1016/j.maturitas.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Horwood JM, Dufour F, Laroche S, Davis S. Signaling mechanisms mediated by the phosphoinositide 3-kinase/Akt cascade in synaptic plasticity and memory in the rat. Eur J Neurosci. 2006;23:3375–3384. doi: 10.1111/j.1460-9568.2006.04859.x. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998a;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci. 1998b;1:595–601. doi: 10.1038/2830. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine K, Laws KR, Gale TM, Kondel TK. Greater cognitive deterioration in women than men with Alzheimer’s disease: a meta analysis. J Clin Exp Neuropsychol. 2012;34:989–98. doi: 10.1080/13803395.2012.712676. [DOI] [PubMed] [Google Scholar]
- Jacome LF, Barateli K, Buitrago D, Lema F, Frankfurt M, Luine VN. Gonadal hormones rapidly enhance spatial memory and increase hippocampal spine density in male rats. Endocrinology 2016. 2016 Feb 4;:en20151959. doi: 10.1210/en.2015-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe H, Hall JE, Gruber S, Sarmiento IA, Cohen LS, Yurgelun-Todd D, Martin KA. Estrogen therapy selectively enhances prefrontal cognitive processes: A randomized, double-blind, placebo-controlled study with functional magnetic resonance imaging in perimenopausal and recently postmenopausal women. Menopause. 2006;13:411–422. doi: 10.1097/01.gme.0000189618.48774.7b. [DOI] [PubMed] [Google Scholar]
- Keenan PA, Ezzat WH, Ginsburg K, Moore GJ. Prefrontal cortex as the site of estrogen’s effect on cognition. Psychoneuroendocrinology. 2001;26:577–590. doi: 10.1016/s0306-4530(01)00013-0. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Song H, Gage FH. Neurogenesis in the Adult Hippocampus. Cold Spring Harb Perspect Biol. 2015;7(9):a018812. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Szinte JS, Boulware MI, Frick KM. 17β-estradiol and agonism of G-protein Coupled Estrogen Receptor (GPER) enhance hippocampal memory consolidation via different cell-signaling mechanisms. Journal of Neuroscience. 2016;36(11) doi: 10.1523/JNEUROSCI.0257-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. The Journal of Clinical Endocrinology & Metabolism. 1996;81:1038–1045. doi: 10.1210/jcem.81.3.8772573. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. The functional neuroanatomy of depression: Distinct roles for ventromedial and dorsolateral prefrontal cortex. Behavioral Brain Research. 2009;201:239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug R, Born J, Rasch B. A 3-day estrogen treatment improves prefrontal cortex-dependent cognitive function in postmenopausal women. Psychoneuroendocrinology. 2006;31:965–975. doi: 10.1016/j.psyneuen.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Kokras N, Antoniou K, Mikail HG, Kafetzopoulos V, Papadopoulou-Daifoti Z, Dalla C. Forced swim test: What about females? Neuropharmacology. 2015 Dec;99:408–21. doi: 10.1016/j.neuropharm.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Kramár EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G. Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J Neurosci. 2009;29:12982–12993. doi: 10.1523/JNEUROSCI.3059-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni J, Gavrilidis E, Wang W, Worsley R, Fitzgerald PB, Gurvich C, Van Rheenen T, Berk M, Burger H. Estradiol for treatment-resistant schizophrenia: A large-scale randomized controlled trial in women of child-bearing age. Molecular Psychiatry. 2015;20:695–702. doi: 10.1038/mp.2014.33. [DOI] [PubMed] [Google Scholar]
- Kumar A, Bean LA, Rani A, Jackson T, Foster TC. Contribution of estrogen receptor subtypes, ERα, ERβ, and GPER1 in rapid estradiol-mediated enhancement of hippocampal synaptic transmission in mice. Hippocampus. 2015 doi: 10.1002/hipo.22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki Y, Fukushima K, Kanda Y, Mizuno K, Watanabe Y. Putative membrane-bound estrogen receptors possibly stimulate mitogen-activated protein kinase in the rat hippocampus. Eur J Pharmacol. 2000;400:205–209. doi: 10.1016/s0014-2999(00)00425-8. [DOI] [PubMed] [Google Scholar]
- Lagace DC, Fischer SJ, Eisch AJ. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007;17(3):175–80. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- Langer G, Bader B, Meoli L, Isensee J, Delbeck M, Noppinger PR, Otto C. A critical review of fundamental controversies in the field of GPR30 research. Steroids. 2010;75:603–610. doi: 10.1016/j.steroids.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Lebesgue D, Traub M, De Butte-Smith M, Chen C, Zukin RS, Kelly MJ, Etgen AM. Acute Administration of Non-Classical Estrogen Receptor Agonists Attenuates Ischemia-Induced Hippocampal Neuron Loss in Middle-Aged Female Rats. PLoS One. 2010;5:e8642. doi: 10.1371/journal.pone.0008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Silva AJ. The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci. 2009;10:126–140. doi: 10.1038/nrn2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lejbak L, Vrbancic M, Crossley M. The female advantage in object location memory is robust to verbalizability and mode of presentation of test stimuli. Brain and Cognition. 2009;69:148–153. doi: 10.1016/j.bandc.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Leresche L. Defining gender disparities in pain management. Clin Orthop Relat Res. 2011;469:1871–1877. doi: 10.1007/s11999-010-1759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004;279:40545–40559. doi: 10.1074/jbc.M402229200. [DOI] [PubMed] [Google Scholar]
- Levin ER. Cellular functions of the plasma membrane estrogen receptor. Trends Endocrinol Metab. 1999;10:374–377. doi: 10.1016/s1043-2760(99)00192-7. [DOI] [PubMed] [Google Scholar]
- Lewis MC, Kerr KM, Orr PT, Frick KM. Estradiol-induced enhancement of object memory consolidation involves NMDA receptors and protein kinase A in the dorsal hippocampus of female C57BL/6 mice. Behav Neurosci. 2008;122:716–721. doi: 10.1037/0735-7044.122.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Chen W, Kuo J, Chen C. Association of serum estrogen level and ischemic neuroprotection in female rats. Neurosci Lett. 2001;297:159–162. doi: 10.1016/s0304-3940(00)01704-3. [DOI] [PubMed] [Google Scholar]
- Liu F, Yuan R, Benashski SE, McCullough LD. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab. 2009;29:792–802. doi: 10.1038/jcbfm.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Wang X, Liu Q, Yang SH, Simpkins JW. Dose dependence and therapeutic window for the neuroprotective effects of 17beta-estradiol when administered after cerebral ischemia. Neurosci Lett. 2007;415:237–241. doi: 10.1016/j.neulet.2007.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Lv W, Du N, Liu Y, Fan X, Wang Y, Jia X, Hou X, Wang B. Low Testosterone Level and Risk of Alzheimer’s Disease in the Elderly Men: a Systematic Review and Meta-Analysis. Mol Neurobiol. 2015 Jul 8; doi: 10.1007/s12035-015-9315-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Ma Y, Liu Y, Rand DG, Heatherton TF, Han S. Opposing Oxytocin Effects on Intergroup Cooperative Behavior in Intuitive and Reflective Minds. Neuropsychopharmacology. 2015;40(10):2379–87. doi: 10.1038/npp.2015.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Hajszan T, Leranth C. The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the CA1 hippocampal subfield of ovariectomized female rats. Endocrinology. 2005;146:287–293. doi: 10.1210/en.2004-0730. [DOI] [PubMed] [Google Scholar]
- MacRae IM, Carswell HV. Oestrogen and stroke: the potential for harm as well as benefit. Biochem Soc Trans. 2006;34:1362–1365. doi: 10.1042/BST0341362. Erratum in: Biochem Soc Trans. 2007 Feb;35(Pt 1):166. [DOI] [PubMed] [Google Scholar]
- Maki PM. Hormone therapy and risk for dementia: where do we go from here? Gynecol Endocrinol. 2004;19(6):354–359. doi: 10.1080/09513590400018207. [DOI] [PubMed] [Google Scholar]
- Maki PM. The critical window hypothesis of hormone therapy and cognition: A scientific update on clinical studies. Menopause. 2013;20:695–709. doi: 10.1097/GME.0b013e3182960cf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Rich JB, Rosenbaum RS. Implicit memory varies across the menstrual cycle: Estrogen effects in young women. Neuropsychologia. 2002;40:518–529. doi: 10.1016/s0028-3932(01)00126-9. [DOI] [PubMed] [Google Scholar]
- McCarthy MM. Incorporating Sex as a Variable in Preclinical Neuropsychiatric Research. Schizophr Bull. 2015 Sep;41(5):1016–20. doi: 10.1093/schbul/sbv077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP, Ball GF, Blaustein JD, De Vries GJ. 2012Sex differences in the brain: the not so inconvenient truth. J Neurosci. 32:2241–2247. doi: 10.1523/JNEUROSCI.5372-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Konkle ATM. When is a sex difference not a sex difference? Frontiers in Neuroendocrinology. 2005;26:85–102. doi: 10.1016/j.yfrne.2005.06.001. [DOI] [PubMed] [Google Scholar]
- McClure RE, Barha CK, Galea LAM. 17β-Estradiol, but not estrone, increases the survival and activation of new neurons in the hippocampus in response to spatial memory in adult female rats. Hormones and Behavior. 2013;63:144–157. doi: 10.1016/j.yhbeh.2012.09.011. [DOI] [PubMed] [Google Scholar]
- McCullough LD, de Vries GJ, Miller VM, Becker JB, Sandberg K, McCarthy MM. NIH initiative to balance sex of animals in preclinical studies: generative questions to guide policy, implementation, and metrics. Biol Sex Differ. 2014 Oct 3;5:15. doi: 10.1186/s13293-014-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocrine Reviews. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- McIntyre RS, Mancini D, Eisfeld BS, Soczynska JK, Grupp L, Konarski JZ, Kennedy SH. Calculated bioavailable testosterone levels and depression in middle-aged men. Psychoneuroendocrinology. 2006 Oct;31(9):1029–35. doi: 10.1016/j.psyneuen.2006.06.005. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MA, Wood MA. HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci. 2011;31:764–747. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mefford J, Witte JS. The Covariate’s Dilemma. PLoS Genet. 2012;8(11):e1003096. doi: 10.1371/journal.pgen.1003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meitzen J, Luoma JI, Boulware MI, Hedges VL, Peterson BM, Tuomela K, Britson KA, Mermelstein PG. Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Neuroendocrinology. 2013;154:4293–4304. doi: 10.1210/en.2013-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez P, Garcia-Segura LM, Muller D. Estradiol promotes spine growth and synapse formation without affecting pre-established networks. Hippocampus. 2011;21:1263–1267. doi: 10.1002/hipo.20875. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milewich L, Garcia RL, Gerrity LW. 17β-Hydroxysteroid oxidoreductase: A ubiquitous enzyme. Interconversion of estrone and estradiol-17β in BALBc mouse tissues. Metabolism. 1985;34:938–944. doi: 10.1016/0026-0495(85)90142-8. [DOI] [PubMed] [Google Scholar]
- Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- Mirowska-Guzel D, Gromadzka G, Mendel T, Janus-Laszuk B, Dzierka J, Sarzynska-Dlugosz I, Czlonkowski A, Czlonkowska A. Impact of BDNF -196 G>A and BDNF -270 C>T polymorphisms on stroke rehabilitation outcome: sex and age differences. Top Stroke Rehabil. 2014;21(Suppl 1):S33–41. doi: 10.1310/tsr21S1-S33. [DOI] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor β in the mouse brain: Comparison with estrogen receptor α. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Mitterling KL, Spencer JL, Dziedzic N, Shenoy S, McCarthy K, Waters EM, McEwen BS, Milner TA. Cellular and subcellular localization of estrogen and progestin receptor immunoreactivities in the mouse hippocampus. J Comp Neurol. 2010;518:2729–2743. doi: 10.1002/cne.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat SD, Hampson E. Salivary testosterone concentrations in left-handers: An association with cerebral language lateralization. Neuropsychology. 2000;14:71–81. [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004 Jan 27;62(2):188–93. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue-Ikeda M, Takata N, Tanabe N, Furukawa A, Hojo Y, Ooishi Y, Morrison JH, Janssen WG, Rose JA, Chambon P, Kato S, Izumi S, Yamazaki T, Kimoto T, Kawato S. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem. 2007;100:950–967. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- Murakami G, Hojo Y, Ogiue-Ikeda M, Mukai H, Chambon P, Nakajima K, Ooishi Y, Kimoto T, Kawato S. Estrogen receptor KO mice study on rapid modulation of spines and long-term depression in the hippocampus. Brain Res. 2014;1621:133–146. doi: 10.1016/j.brainres.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Murakami G, Tsurugizawa T, Hatanaka Y, Komatsuzaki Y, Tanabe N, Mukai H, Hojo Y, Kominami S, Yamazaki T, Kimoto T, Kawato S. Comparison between basal and apical dendritic spines in estrogeninduced rapid spinogenesis of CA1 principal neurons in the adult hippocampus. Biochem Biophys Res Commun. 2006;351:553–558. doi: 10.1016/j.bbrc.2006.10.066. [DOI] [PubMed] [Google Scholar]
- Myskiw JC, Rossato JI, Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. On the participation of mTOR in recognition memory. Neurobiol Learn Mem. 2008;89:338–351. doi: 10.1016/j.nlm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci USA. 2003;100:10506–10511. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM. The functional organization of working memory processes with human lateral frontal cortex: The contribution of functional neuroimaging. European Journal of Neuroscience. 1997;9:1329–1339. doi: 10.1111/j.1460-9568.1997.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira LM, Bastos CP, de Souza JM, Ribeiro FM, Pereira GS. Estradiol enhances object recognition memory in Swiss female mice by activating hippocampal estrogen receptor α. Neurobiol Learn Mem. 2014;114:1–9. doi: 10.1016/j.nlm.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Perez SM, Chen L, Lodge DJ. Alterations in dopamine system function across the estrous cycle of the MAM rodent model of schizophrenia. Psychoneuroendocrinology. 2014;47:88–97. doi: 10.1016/j.psyneuen.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan A, Gabor CS, Favaro KJ, Kaschak SL, Armstrong JN, MacLusky NJ, Choleris E. Low doses of 17β-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology. 2012;37(10):2299–2309. doi: 10.1038/npp.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan A, Lancaster KE, Armstrong JN, MacLusky N, Choleris E. Rapid effects of estrogen receptor α and β selective agonists on learning and dendritic spines in female mice. Endocrinology. 2011;152(4):1492–1502. doi: 10.1210/en.2010-1273. [DOI] [PubMed] [Google Scholar]
- Phan A, Suchkov S, Molinaro L, Reynolds K, Lymer JM, Bailey CDC, Kow L-M, MacLusky NJ, Pfaff DW, Choleris E. Rapid increases in immature synapses parallel estrogen-induced hippocampal learning enhancements. Proc Natl Acad Sci USA. 2015 doi: 10.1073/pnas.1522150112. pii: 201522150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsonneault JK, Sullivan D, Sadee W, Soares CN, Hampson E, Steiner M. Association study of the estrogen receptor gene ESR1 with postpartum depression--A pilot study. Archives of Women’s Mental Health. 2013;16:499–509. doi: 10.1007/s00737-013-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Onishi KG, Zucker I. Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014 Mar;40:1–5. doi: 10.1016/j.neubiorev.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Quesada A, Micevych PE. Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxydopamine lesions. J Neurosci Res. 2004;75:107–116. doi: 10.1002/jnr.10833. [DOI] [PubMed] [Google Scholar]
- Quesada A, Lee BY, Micevych PE. PI3 kinase/Akt activation mediates estrogen and IGF-1 nigral DA neuronal neuroprotection against a unilateral rat model of Parkinson’s disease. Dev Neurobiol. 2008;68:632–644. doi: 10.1002/dneu.20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. Journal of Neuroscience. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannevik G, Carlström K, Jeppsson S, Bjerre B, Svanberg L. A prospective long-term study in women from pre-menopause to post-menopause: changing profiles of gonadotrophins, oestrogens and androgens. Maturitas. 1986;8:297–307. doi: 10.1016/0378-5122(86)90038-1. [DOI] [PubMed] [Google Scholar]
- Reeves M, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L. Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 2008;7:915–926. doi: 10.1016/S1474-4422(08)70193-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Henderson VW. Hormone therapy and risk of Alzheimer disease: A critical time. JAMA. 2002;288:2170–2172. doi: 10.1001/jama.288.17.2170. [DOI] [PubMed] [Google Scholar]
- Riecher-Rössler A, Häfner H, Stumbaum M, Maurer K, Schmidt R. Can estradiol modulate schizophrenic symptomatology? Schizophrenia Bulletin. 1994;20:203–214. doi: 10.1093/schbul/20.1.203. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Demarco AC, Hackett PD, Chen X, Gautam P, Stair S, Haroon E, Thompson R, Ditzen B, Patel R, Pagnoni G. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014 Jan;39:237–48. doi: 10.1016/j.psyneuen.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SS, Reiches M, Shattuck-Heidorn H, LaBonte ML, Consoli T. Opinion: Focus on preclinical sex differences will not address women’s and men’s health disparities. Proc Natl Acad Sci U S A. 2015 Nov 3;112(44):13419–20. doi: 10.1073/pnas.1516958112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Head EH, Stanczyk FZ, Pike CJ. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol Aging. 2011 Apr;32(4):604–13. doi: 10.1016/j.neurobiolaging.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin BS. Hypothalamic alterations and reproductive aging in female rats: evidence of altered luteinizing hormone-releasing hormone neuronal function. Biol Reprod. 2000 Oct;63(4):968–76. doi: 10.1095/biolreprod63.4.968. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Johnson SL, Schmidt PJ, Girdler S, Gaynes B. Efficacy of estradiol in perimenopausal depression: so much promise and so few answers. Depress Anxiety. 2015 Aug;32(8):539–49. doi: 10.1002/da.22391. Epub 2015 Jun 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel J+, Epp JR+, Galea LAM. Estradiol does not influence strategy choice but place strategy choice is associated with increased cell proliferation in the hippocampus of female rats. Hormones and Behavior. 2010;58(4):582–90. doi: 10.1016/j.yhbeh.2010.07.009. [NSERC] IF: 4.142+ both authors contributed equally. [DOI] [PubMed] [Google Scholar]
- Ryan J, Scali J, Carriere I, Ritchie K, Ancelin ML. Hormonal treatment, mild cognitive impairment and Alzheimer’s disease. Int Psychogeriatr. 2008;20(1):47–56. doi: 10.1017/S1041610207006485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar JS, Hampson E. Testosterone levels and androgen receptor gene polymorphism predict specific symptoms of depression in young men. Gender Medicine. 2012;9:232–243. doi: 10.1016/j.genm.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Schöning S, Engelien A, Kugel H, Schäfer S, Schiffbauer H, Zwitserlood P, Pletziger E, Beizai P, Kersting A, Ohrmann P, Greb RR, Lehmann W, Heindel W, Arolt V, Konrad C. Functional anatomy of visuo-spatial working memory during mental rotation is influenced by sex, menstrual cycle, and sex steroid hormones. Neuropsychologia. 2007 Nov 5;45(14):3203–14. doi: 10.1016/j.neuropsychologia.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Selcher JC, Atkins CM, Trzaskos JM, Paylor R, Sweatt JD. A necessity for MAP kinase activation in mammalian spatial learning. Learn Mem. 1999;6:478–490. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers K, Raval P, Srivastava DP. Molecular signature of rapid estrogen regulation of synaptic connectivity and cognition. Front Neuroendocrinol. 2015;36:72–89. doi: 10.1016/j.yfrne.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging. 2010a;31:1618–28. doi: 10.1016/j.neurobiolaging.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J Neurosci. 2010b;30:6852–61. doi: 10.1523/JNEUROSCI.0761-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw ND, Srouji SS, Welt CK, Cox KH, Fox JH, Adams JM, Sluss PM, Hall JE. Evidence that increased ovarian aromatase activity and expression account for higher estradiol levels in African American compared with Caucasian women. J Clin Endocrinol Metab. 2014 Apr;99(4):1384–92. doi: 10.1210/jc.2013-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldahl LC, Shapiro RA, Bryant DN, Koerner IP, Dorsa DM. Estrogen induced rapid translocation of estrogen receptor β, but not estrogen receptor α, to the neuronal plasma membrane. Neuroscience. 2008;153:751–761. doi: 10.1016/j.neuroscience.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen and memory in women: how can we reconcile the findings? Horm Behav. 47(3):371–375. doi: 10.1016/j.yhbeh.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Shughrue P, Scrimo P, Lane M, Askew R, Merchenthaler I. The distribution of estrogen receptor-β mRNA in forebrain regions of the estrogen receptor-α knockout mouse. Endocrinology. 1997a;138:5649–5652. doi: 10.1210/endo.138.12.5712. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997b;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience. 2000;99:605–612. doi: 10.1016/s0306-4522(00)00242-6. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J WHIMS Investigators. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003. 2003 May 28;289(20):2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Paylor R, Wehner JM, Tonegawa S. Impaired spatial learning in α-calcium- calmodulin kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Rajakumar G, Zhang YQ, Simpkins CE, Greenwald D, Yu CJ, Bodor N, Day AL. Estrogens may reduce mortality and ischemic damage caused by middle cerebral artery occlusion in the female rat. J Neurosurg. 1997;87:724–730. doi: 10.3171/jns.1997.87.5.0724. [DOI] [PubMed] [Google Scholar]
- Simpkins JW, Yi KD, Yang S-H. Role of protein phosphatases and mitochondria in the neuroprotective effects of estrogens. Front Neuroendocrinol. 2009;30:93–105. doi: 10.1016/j.yfrne.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30:16137–16148. doi: 10.1523/JNEUROSCI.4161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith YR, Love T, Persad CC, Tkaczyk A, Nichols TE, Zubieta JK. Impact of combined estradiol and norethindrone therapy on visuospatial working memory assessed by functional magnetic resonance imaging. Journal of Clinical Endocrinology & Metabolism. 2006;91:4476–4481. doi: 10.1210/jc.2006-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, McMahon LL. Estradiol-induced increase in the magnitude of long-term potentiation is prevented by blocking NR2B-containing receptors. J Neurosci. 2006;26:8517–8522. doi: 10.1523/JNEUROSCI.5279-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F. Estrogen-IGF-1 interactions in neuroprotection: Ischemic stroke as a case study. Front Neuroendocrinol. 2014;36:1–14. doi: 10.1016/j.yfrne.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Welsh CJ, Reddy DS. Sex differences in neurological diseases. In: Shansky Rebecca M., editor. Chapter 12 in Sex differences in the central nervous system. Academic Press; London UK: 2015. pp. 297–323. [Google Scholar]
- Spiteri T, Musatov S, Ogawa S, Ribeiro A, Pfaff DW, Ågmo A. The role of the estrogen receptor alpha in the medial amygdala and ventromedial nucleus of the hypothalamus in social recognition, anxiety and aggression. Behav Brain Res. 2012;210:211–220. doi: 10.1016/j.bbr.2010.02.033. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Evans PD. G-protein oestrogen receptor 1: Trials and tribulations of a membrane oestrogen receptor. J Neuroendocrinol. 2013;25:1219–1230. doi: 10.1111/jne.12071. [DOI] [PubMed] [Google Scholar]
- Srivastava DP, Woolfrey KM, Jones KA, Shum CY, Lash LL, Swanson GT, Penzesm P. Rapid enhancement of two-step wiring plasticity by estrogen and NMDA receptor activity. Proc Natl Acad Sci USA. 2008;105:14650–14655. doi: 10.1073/pnas.0801581105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M, Young EA. Hormones and mood. In: Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young EA, editors. Sex Differences in the Brain: From Genes to Behavior. New York: Oxford University Press; 2008. pp. 405–426. [Google Scholar]
- Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A. 2007;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30:201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H, Koide M, Ara W, Shibata Y, Funabashi T, Suyama K, Goto T, Takahashi T. Estrous Cycle-Dependent Phasic Changes in the Stoichiometry of Hippocampal Synaptic AMPA Receptors in Rats. PLoS One. 2015 Jun 29;10(6):e0131359. doi: 10.1371/journal.pone.0131359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terner JM, Lomas LM, Picker MJ. Influence of estrous cycle and gonadal hormone depletion on nociception and opioid antinociception in female rats of four strains. J Pain. 2005;6:372–383. doi: 10.1016/j.jpain.2005.01.354. [DOI] [PubMed] [Google Scholar]
- Thirone AC, Speight P, Zulys M, Rotstein OD, KSs, Pedersen SF, Kapus A. Hyperosmotic stress induces Rho/Rho kinase/LIM kinase- mediated cofilin phosphorylation in tubular cells: key role in the osmotically triggered F-actin response. Am J Physiol Cell Physiol. 2009;296:C463–475. doi: 10.1152/ajpcell.00467.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toung TK, Hurn PD, Traystman RJ, Sieber FE. Estrogen decreases infarct size after temporary focal ischemia in a genetic model of type 1 diabetes mellitus. Stroke. 2000;31:2701–2706. doi: 10.1161/01.str.31.11.2701. [DOI] [PubMed] [Google Scholar]
- Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–1670. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- Tuscher JJ, Luine V, Frankfurt M, Frick KM. Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus. J Neurosci. 2016;36(5):1483–1489. doi: 10.1523/JNEUROSCI.3135-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl RR, Wang H, Wu TC, Sicotte NL, Nakamura K, Kurth F, Itoh N, Bardens J, Bernard JT, Corboy JR, Cross AH, Dhib-Jalbut S, Ford CC, Frohman EM, Giesser B, Jacobs D, Kasper LH, Lynch S, Parry G, Racke MK, Reder AT, Rose J, Wingerchuk DM, MacKenzie-Graham AJ, Arnold DL, Tseng CH, Elashoff R. Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2015 Nov 24; doi: 10.1016/S1474-4422(15)00322-1. pii: S1474-4422(15)00322-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Wade CB, Dorsa DM. Estrogen activation of cyclic adenosine 5′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)α and ERβ exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- Walf AA, Rhodes ME, Frye CA. Ovarian steroids enhance object recognition in naturally cycling and ovariectomized, hormone-primed rats. Neurobiol Learn Mem. 2008;86:35–46. doi: 10.1016/j.nlm.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SG, Humphreys AG, Juraska JM, Greenough WT. LTP varies across the estrous cycle: enhanced synaptic plasticity in proestrus rats. Brain research. 1995;703:26–30. doi: 10.1016/0006-8993(95)01059-9. [DOI] [PubMed] [Google Scholar]
- Warren SG, Juraska JM. Spatial and nonspatial learning across the rat estrous cycle. Behav Neurosci. 1997;111:259–266. doi: 10.1037//0735-7044.111.2.259. [DOI] [PubMed] [Google Scholar]
- Waters EM, Thompson LI, Patel P, Gonzales AD, Ye HZ, Filardo EJ, Clegg DJ, Gorecka J, Akama KT, McEwen BS, Milner TA. G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. J Neurosci. 2015;35:2384–2397. doi: 10.1523/JNEUROSCI.1298-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters EM, Yildirim M, Janssen WG, Lou WY, McEwen BS, Morrison JH, Milner TA. Estrogen and aging affect the synaptic distribution of estrogen receptor beta-immunoreactivity in the CA1 region of female rat hippocampus. Brain Res. 2011;1379:86–97. doi: 10.1016/j.brainres.2010.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters JJ, Campbell JS, Cunningham MJ, Krebs EG, Dorsa DM. Rapid membrane effects of steroids in neuroblastoma cells: Effects of estrogen on mitogen activated protein kinase signalling cascade and c-fos immediate early gene transcription. Endocrinology. 1997;138:4030–4033. doi: 10.1210/endo.138.9.5489. [DOI] [PubMed] [Google Scholar]
- Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Molecular Pharmacology. 2009;76:215–228. doi: 10.1124/mol.109.056705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SM, Eisenberg DP, Kohn PD, Kippenhan JS, Kolachana BS, Weinberger DR, Berman KF. Brain-derived neurotrophic factor Val66Met polymorphism affects resting regional cerebral blood flow and functional connectivity differentially in women versus men. J Neurosci. 2012 May 16;32(20):7074–81. doi: 10.1523/JNEUROSCI.5375-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieck A. Oestradiol and psychosis: clinical findings and biological mechanisms. Curr Top Behav Neurosci. 2011;8:173–187. doi: 10.1007/7854_2011_127. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–80. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990 Dec;10(12):4035–9. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman JL, Gobinath AR, Brummelte S, Galea LAM. Parity modifies the effects of fluoxetine and corticosterone on HPA axis responses and hippocampal neurogenesis. Neuropharmacology. 2015 Nov 25; doi: 10.1016/j.neuropharm.2015.11.027. in press. [DOI] [PubMed] [Google Scholar]
- Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS, Lowery DW, Patel MM, Denson DD, Gordon AB, Wald MM, Gupta S, Hoffman SW, Stein DG. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007 Apr;49(4):391–402. 402.e1–2. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- Wright DW, Yeatts SD, Silbergleit R, Palesch YY, Hertzberg VS, Frankel M, Goldstein FC, Caveney AF, Howlett-Smith H, Bengelink EM, Manley GT, Merck LH, Janis LS, Barsan WG NETT Investigators. Very early administration of progesterone for acute traumatic brain injury. N Engl J Med. 2014 Dec 25;371(26):2457–66. doi: 10.1056/NEJMoa1404304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Liu R, Wu SS, Simpkins JW. The use of estrogens and related compounds in the treatment of damage from cerebral ischemia. Ann N Y Acad Sci. 2003;1007:101–107. doi: 10.1196/annals.1286.010. [DOI] [PubMed] [Google Scholar]
- Yokomaku D, Numakawa T, Numakawa Y, Suzuki S, Matsumoto T, Adachi N, Nishio C, Taguchi T, Hatanaka H. Estrogen enhances depolarization-induced glutamate release through activation of phosphatidylinositol 3-kinase and mitogen-activated protein kinase in cultured hippocampal neurons. Mol Endocrinol. 2003;17:831–844. doi: 10.1210/me.2002-0314. [DOI] [PubMed] [Google Scholar]
- Zarrouf FA, Artz S, Griffith J, Sirbu C, Kommor M. Testosterone and depression: systematic review and meta-analysis. J Psychiatr Pract. 2009;15:289–305. doi: 10.1097/01.pra.0000358315.88931.fc. [DOI] [PubMed] [Google Scholar]
- Zeber JE, Manias E, Williams AF, Hutchins D, Udezi WA, Roberts CS, Peterson AM. A systematic literature review of psychosocial and behavioral factors associated with initial medication adherence: a report of the ISPOR medication adherence & persistence special interest group. Value Health. 2013;16:891–900. doi: 10.1016/j.jval.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM. Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci. 2012;32:2344–2351. doi: 10.1523/JNEUROSCI.5819-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Fan L, Frick KM. Epigenetic alterations regulate the estradiol-induced enhancement of memory consolidation. Proc Natl Acad Sci USA. 2010;107:5605–5610. doi: 10.1073/pnas.0910578107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziehn MO, Avedisian AA, Dervin SM, O’Dell TJ, Voskuhl RR. Estriol preserves synaptic transmission in the hippocampus during autoimmune demyelinating disease. Lab Invest. 2012;92:1234–1245. doi: 10.1038/labinvest.2012.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirkin BR, Tenover JL. Aging and Declining Testosterone: Past, Present, and Hopes for the Future. J Androl. 2012;33:1111–1118. doi: 10.2164/jandrol.112.017160. [DOI] [PMC free article] [PubMed] [Google Scholar]