Abstract
Rationale
Few studies have systematically assessed the influence of gut microbiota on cardiovascular disease (CVD) risk.
Objective
To examine the association between gut microbiota and lifetime CVD risk profile among 55 Bogalusa Heart Study (BHS) participants with the highest and 57 with the lowest lifetime burdens of CVD risk factors.
Methods and Results
16S rRNA sequencing was conducted on microbial DNA extracted from stool samples of the BHS participants. Alpha diversity, including measures of richness and evenness, and individual genera were tested for associations with lifetime CVD risk profile. Multivariable regression techniques were employed to adjust for age, gender, and race (Model 1), along with body mass index (BMI) (Model 2) and both BMI and diet (Model 3). In Model 1, odds ratios (95% confidence intervals) for each standard deviation increase in richness, measured by the number of observed operational taxonomic units, Chao 1 index, and abundance-based coverage estimator, were 0.62 (0.39, 0.99), 0.61 (0.38, 0.98), and 0.63 (0.39, 0.99), respectively. Associations were consistent in Models 2 and 3. Four genera were enriched among those with high versus low CVD risk profile in all models. Model 1 p-values were: 2.12×10−3, 7.95×10−5, 4.39×10−4, and 1.51×10−4 for Prevotella 2, Prevotella 7, Tyzzerella and Tyzzerella 4, respectively. Two genera were depleted among those with high versus low CVD risk profile in all models. Model 1 P-values were: 2.96×10−6 and 1.82×10−4 for Alloprevotella and Catenibacterium, respectively.
Conclusions
The current study identified associations of overall microbial richness and six microbial genera with lifetime CVD risk.
Keywords: Microbiota, cardiovascular disease risk factors, lipids, blood pressure, blood glucose
Subject Terms: Risk Factors, Cardiovascular Disease, Epidemiology, Genetics
INTRODUCTION
The human gut hosts a vast array of microbes collectively known as the microbiota. Although influences of the gut microbiota on human health have been hypothesized for some time, only in the past decade have advances in technology made it feasible to comprehensively characterize host microbial communities and examine their associations with disease states.1, 2 With growing evidence for a critical role of the gut microbiome in energy harvest3, glucose and lipid metabolism4, 5, and systemic inflammation5, a potential influence of this commensal supraorganism on cardiovascular disease (CVD) development has been hypothesized. Indeed, the cross-sectional relationships of the gut microbiota with certain CVD risk factors including obesity6, 7, type 2 diabetes8, 9, and dyslipidemia10, 11 have already been documented. Gut microbiota have also been shown to be integral in the derivation of the trimethylamine (TMA) metabolite from certain marine fish, as well as foodstuffs or supplements rich in phosphatidylcholine (lecithin), free choline and carnitine.12–14 TMA is the dietary-derived precursor to trimethylamine-N-oxide (TMAO)15, a metabolite that was recently linked prospectively to CVD.14 These data suggest a potentially important role of the human gut microbiome in CVD development. However, few studies have systematically assessed the influence of gut microbiota composition on overall CVD risk.
The purpose of the current study was to comprehensively explore the relation between composition of the gut microbiome and lifetime CVD risk profile among a subsample of 55 Bogalusa Heart Study (BHS) participants with the highest and 57 with the lowest lifetime burdens of traditional CVD risk factors. This study leveraged carefully collected data from an average of nine participant visits obtained over approximately 33 years of follow-up to profile CVD risk from childhood through adulthood. Furthermore, potential confounders such as age, gender, race, body mass index (BMI), and diet were controlled in the analyses.
METHODS
Study participants
The BHS is a long-term epidemiologic study investigating the natural history of atherosclerosis among a biracial sample (35% black and 65% white) of residents from Bogalusa, Louisiana begun in 1973 by Dr. Gerald Berenson. From 1973 to 2016, seven surveys of children aged 4 to 17 and ten surveys of adults aged 18 to 50 who had been examined previously as children were conducted. The current BHS cohort includes 1,298 participants born between 1959 and 1979 who were screened at least two times during childhood and two times during adulthood for CVD risk factors. A subsample of 203 participants from this cohort who were at highest and lowest estimated lifetime risk of CVD were contacted to determine eligibility for participation in the microbiome study. After excluding 42 participants who had taken antibiotics or probiotics in the past two months, 161 met the eligibility criteria for enrollment. One-hundred twelve of these participants (37% black and 63% white), including 55 with high lifetime CVD risk profile and 57 with low lifetime CVD risk profile, agreed to participate and returned stool specimens for subsequent microbiome characterization and analyses (response rate of 70%).
Informed consents were obtained from all the Bogalusa Heart Study participants after detailed explanation of the study. The study was approved by the Institutional Review Boards at all the participating institutions.
Measurement of CVD risk factors in the BHS cohort
All BHS examinations have followed nearly identical study protocols.16, 17 During each study visit, blood pressure (BP) was measured in the morning in triplicate by each of two trained observers using a mercury sphygmomanometer with the participant in a relaxed, sitting position. Systolic and diastolic BP levels were measured as the first and fourth (in children) or fifth (in adults) Korotkoff sounds, respectively. The mean of the six BP values were used to estimate BP at each study visit. Anthropometric measures were obtained by trained staff with BHS participants in light clothing without shoes. Height and weight were measured in duplicate to the nearest 0.1 cm and 0.1 kg, respectively. The mean of height and weight were used to estimate BMI at each study visit. Diet information was collected at two time points in young adulthood using a validated food frequency questionnaire.18
Participants were instructed to fast for 12 hours prior to blood sample collection. During 1973–1986, cholesterol and triglyceride levels were measured by the use of chemical procedures with a Technicon AutoAnalyzer II (Technicon Instrument Corporation, Tarrytown, New York) according to the Laboratory Manual of the Lipid Research Clinics Program. Since 1987, these variables were determined using the Abbott VP instrument (Abbott Laboratories, North Chicago, Illinois) and on the Hitachi 902 Automatic Analyzer (Roche Diagnostics, Indianapolis, Indiana) afterward by enzymatic procedures.19 Serum lipoprotein cholesterols were analyzed using a combination of heparincalcium precipitation and agar–agarose gel electrophoresis procedures.20 Plasma glucose was measured initially by a glucose oxidase method using a Beckman Instant Glucose Analyzer (Beckman Instruments, Palo Alto, California). After 1991, glucose was measured in adults using a multichemistry (SMA20) profile by enzymatic procedures using the multichannel Olympus Au-5000 Analyzer (Olympus, Lake Success, New York).
Estimation of lifetime CVD risk profile
Lifetime CVD risk profile was estimated based on the average of fasting plasma glucose, systolic BP (SBP), and low-density lipoprotein (LDL) cholesterol values from at least four BHS study visits conducted from childhood to adulthood. Lifetime CVD risk groups were identified using a z-score based data reduction technique. For each participant, z-scores for mean lifetime measures of glucose, SBP, and LDL cholesterol were generated. Z-scores for each risk factor were then summed to create an overall lifetime CVD risk score for each participant. Participants were ranked according to risk score, and those with the highest and lowest scores were contacted for participation in the current study.
Microbiome sequencing and analysis
Fecal samples were collected at the homes of study participants and returned to BHS investigators within 24 hours of collection, at which time they were stored at −80°C until microbiome characterization. Using methods developed for the Human Microbiome Project, DNA isolation and microbiome sequencing were conducted at the Baylor College of Medicine’s Alkek Center for Metagenomics and Microbiome Research.21, 22 Briefly, genomic bacterial DNA was extracted from fecal samples using the MO BIO PowerSoil DNA Isolation Kit (MO BIO Laboratories, Inc., Carlsbad, CA). The 16S rDNA V4 hypervariable region was then amplified by PCR and sequenced on the MiSeq platform using the 2×250 bp paired-end protocol (Illumina®, San Diego, CA).23 The read pairs were demultiplexed based on their unique molecular barcodes and overlapping reads were merged using USEARCH v7.0.1001 software.24 Operational taxonomic unit (OTU) picking was conducted using the QIIME (Quantitative Insights Into Microbial Ecology) software package.25 16S rRNA gene sequences were clustered at a similarity cutoff value of 97% using UCLUST.24 Matching of OTUs to bacteria was then conducted using the SILVA reference database.26 Abundances were recovered by mapping the demultiplexed reads to the identified OTUs.
Since sequencing depth ranged from 4,118 to 13,146 reads per sample, library size was rarefied to the minimum read depth. Subsequently, several alpha diversity measures were estimated based on identified OTUs.27 Microbial richness, which measures the number of taxa in each sample (or the abundance of microbes), was examined by calculating the number of observed OTUs, Chao 1 index and abundance-based coverage estimator (ACE).28 Evenness, which measures the relative number of taxa in samples (accounting for the number of times each taxon was observed in a sample), was additionally assessed using the Shannon, Simpson and inverse Simpson indices.27 An additional quality control step was then conducted to restrict analyses to only those 116 OTUs which comprised ≥0.05% of reads and were present in at least 5% of samples. These OTUs accounted for an average of 95% of total reads per sample. The 116 OTUs were assigned to 75 genera which were examined in further analyses. The distribution of microbiome data prior to and post rarefying and quality filtering are shown in Supplementary Figure II. Processing of microbiome sequence data was conducted using the phyloseq package (V1.15.14) in R.28
Statistical analysis
Lifetime CVD risk groups were compared on pertinent characteristics with the use of x2 tests for categorical variables and t-tests for continuous variables. All alpha diversity measures were examined for normality and log-transformed as needed.
Logistic regression models were employed to examine the associations between each measure of alpha diversity and lifetime CVD risk profile after adjustment for important covariables. The association between individual microbial genera and lifetime CVD risk profile employed a two-step ‘partialling out’ approach.29 To accommodate the excessive zero counts and overdispersion of microbiome data, in step 1 a zero-inflated negative binomial model was employed to model each genus.30 This model assumes that microbiome data for each genus are from a mixture of two separate distributions, one from a negative binomial distribution and the other from a constant distribution generating only zero counts. We model both negative binomial data and the zero count data (the probability of being a zero count) simultaneously with important covariables treated as predictors. The log transformed total genus counts, which varied slightly between participants due to the additional quality control steps subsequent to rarefication, were included in the model as the offset variable. In step 2, the raw residuals from these models, representing the independent effects of each genus, were then compared between CVD risk groups using the Wilcoxon rank sum test. A Bonferroni correction for the 75 genera tested was employed using an alpha threshold of 6.67×10−4 to determine statistical significance.
Three multivariable adjustments were used to test associations of both alpha diversity estimates and individual genera with lifetime CVD risk profile. Model 1 adjusted for age, gender and race. To examine the potentially mediating influence of BMI, a second model adjusting for age, gender, race and BMI was also assessed (Model 2). To determine whether identified associations were consistent after adjustment for dietary factors in this relatively small sample, a third model adjusting for age, gender, race, BMI, total energy intake, and energy-adjusted31 intakes of dietary protein, animal fat, and fiber was examined.
RESULTS
Lifetime CVD risk profiles were estimated using an average of 9 measures of glucose, SBP, and LDL cholesterol values over an average of 33 years of follow-up. Table 1 compares the characteristics of BHS microbiome study participants with high and low lifetime CVD risk profiles. Compared to those with low CVD risk profiles, those with high CVD risk profiles were more likely to be male and, on average, were older and had higher BMI. While total energy, protein and animal fat intakes appeared higher and dietary fiber intake lower among participants with high versus low CVD risk profiles, none of the nutrients were significantly different between groups. As expected, glucose, SBP, and LDL cholesterol levels in childhood and adulthood were higher in the high risk versus the low risk group.
Table 1.
Characteristics of 112 Bogalusa microbiome study participants according to lifetime CVD risk profile.
| High Lifetime CVD Risk Profile (N=55) | Low Lifetime CVD Risk Profile (N=57) | P-value | |
|---|---|---|---|
| Number of visits, mean (SD) | 8.56 (2.29) | 9.11 (2.17) | 0.20 |
| Male, % | 60.00 | 22.81 | <0.0001 |
| Black, % | 40.00 | 33.33 | 0.46 |
| Childhood | |||
| Age, y, mean (SD) | 13.66 (1.97) | 12.14 (2.06) | <0.0001 |
| BMI, kg/m2, mean (SD) | 22.21 (4.53) | 17.87 (3.01) | <0.0001 |
| Systolic BP, mmHg, mean (SD) | 110.00 (7.91) | 99.47 (6.42) | <0.0001 |
| LDL cholesterol, mmol/l, mean (SD) | 2.67 (0.73) | 2.11 (0.44) | <0.0001 |
| Glucose, mmol/l, mean (SD) | 4.89 (0.50) | 4.51 (0.32) | <0.0001 |
| Adulthood | |||
| Age, y, mean (SD) | 34.88 (2.46) | 32.29 (2.47) | <0.0001 |
| BMI, kg/m2, mean (SD) | 33.19 (7.13) | 25.11 (4.52) | <0.0001 |
| Systolic BP, mmHg, mean (SD) | 125.60 (11.08) | 105.90 (5.63) | <0.0001 |
| LDL cholesterol, mmol/l, mean (SD) | 3.73 (0.79) | 2.60 (0.56) | <0.0001 |
| Glucose, mmol/l, mean (SD) | 5.27 (0.76) | 4.35 (0.28) | <0.0001 |
| Total energy intake, kcal/d | 2334.7 ± 1424.6 | 2200.5 ± 959.9 | 0.57 |
| Protein, g/day* | 76.4 ± 11.0 | 74.7 ± 12.3 | 0.44 |
| Animal fat, g/day* | 38.4 ± 9.7 | 35.5 ± 7.5 | 0.08 |
| Total dietary fiber, g/day* | 15.3 ± 4.0 | 16.4 ± 4.0 | 0.17 |
BMI=Body mass index; BP=Blood pressure; CVD=Cardiovascular disease; LDL=Low-density lipoprotein; SD=Standard deviation.
Adjusted for total energy intake using the residual method.31
The association between estimates of alpha diversity and lifetime CVD risk profile are presented in Table 2. Increased microbial richness was consistently associated with decreased lifetime CVD risk profile. For example, for each one standard deviation increase in the number of observed OTUs, there was a 38% (95% CI: 1% to 61%) decreased odds of high lifetime CVD risk profile (P=0.04). Similarly, odds ratios (95% CI) for every standard deviation increase in the Chao 1 index and ACE were 0.62 (0.39, 0.99) and 0.61 (0.38, 0.98), respectively (P=0.04 and 0.05, respectively). Although the p-values were slightly attenuated, the magnitudes of associations remained consistent after adjustment for BMI (Model 2) and additionally dietary factors (Model 3). Alpha diversity estimates that also assessed evenness of microbial taxa were not significantly associated with CVD risk in any statistical model (all P>0.05).
Table 2.
Odds ratio for high compared to low lifetime CVD risk profile for each standard deviation increase in alpha diversity estimates among Bogalusa microbiome study participants (N=112).
| Model 1*
|
Model 2†
|
Model 3‡
|
||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Richness | ||||||
| Observed OTUs | 0.62 (0.39, 0.99) | 0.04 | 0.54 (0.28, 1.01) | 0.06 | 0.54 (0.27, 1.10) | 0.09 |
| Chao 1 | 0.61 (0.38, 0.98) | 0.04 | 0.54 (0.29, 1.01) | 0.05 | 0.54 (0.27, 1.07) | 0.08 |
| ACE | 0.63 (0.39, 0.99) | 0.05 | 0.53 (0.28, 1.01) | 0.05 | 0.55 (0.27, 1.10) | 0.09 |
| Richness and Evenness | ||||||
| Shannon index | 0.77 (0.49, 1.20) | 0.25 | 0.57 (0.29, 1.13) | 0.11 | 0.54 (0.26, 1.16) | 0.11 |
| Simpson’s index | 0.86 (0.56, 1.34) | 0.51 | 0.62 (0.32, 1.22) | 0.17 | 0.62 (0.30, 1.28) | 0.19 |
| Inverse Simpson index | 0.82 (0.52, 1.28) | 0.37 | 0.65 (0.33, 1.32) | 0.24 | 0.61 (0.28, 1.30) | 0.20 |
ACE=Abundance-based coverage estimator; CI=Confidence interval; OR=Odds ratio; OUT=Operational taxonomic unit.
Adjusted for age, gender and race.
Adjusted for age, gender, race and body mass index.
Adjusted for age, gender, race, body mass index, dietary protein intake§, animal fat intake§, dietary fiber intake§, and total energy intake.
Adjusted for total energy intake using the residual method.
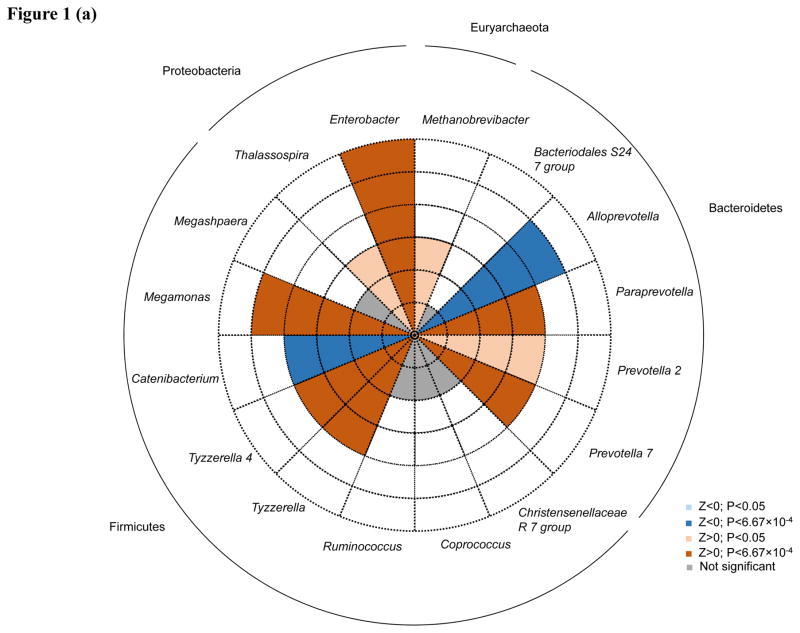
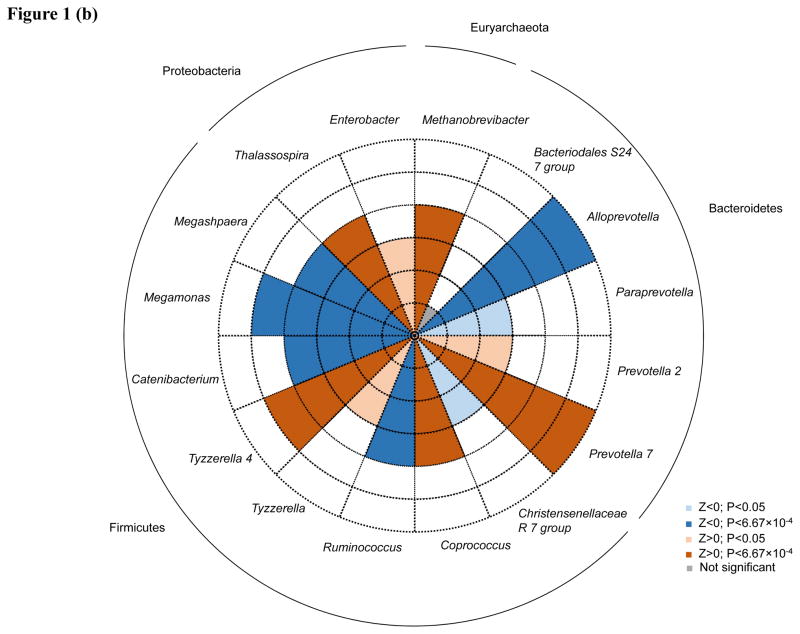
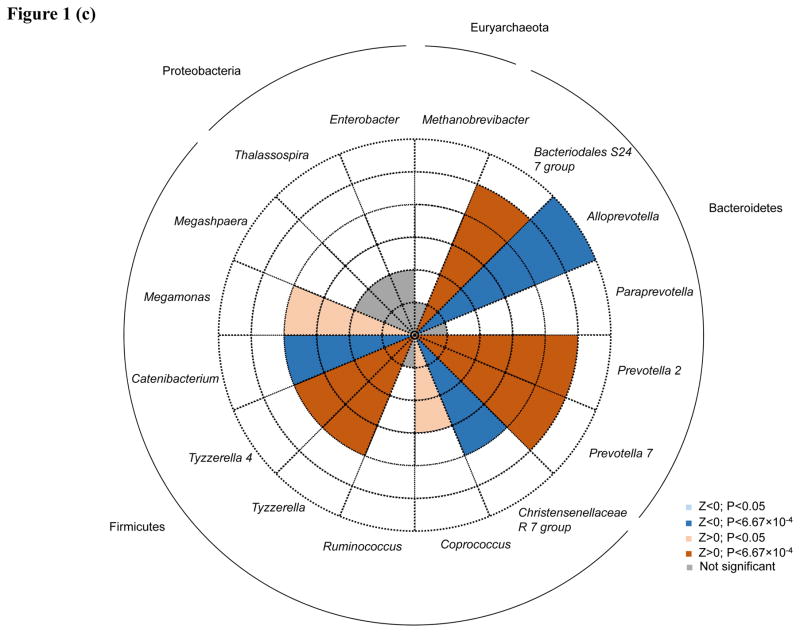
Associations between individual genera and lifetime CVD risk profile were generally consistent between models (Supplementary Table), with moderate pairwise correlation coefficient for z-statistics of 0.60 (P<0.0001) and 0.57 (P<0.0001), for Models 1 and 2 and Models 1 and 3, respectively. After adjustment for age, gender, and race (Model 1), eight taxa were significant after Bonferroni correction for multiple testing. Within the Bacteroidetes phylum, three genera from the family Prevotellaceae significantly associated with CVD risk, including Alloprevotella (P=2.96×10−6), Prevotella 7 (P=7.95×10−5) and Paraprevotella (P=6.48×10−4). Within the Firmicutes phylum, four taxa associated with lifetime CVD risk, including two genera from the Lachnospiraceae family, Tyzzerella 4 (P=1.51×10−4) and Tyzzerella (P=4.39×10−4), and two genera from families Veillonellaceae and Erysipelotrichaceae, Megamonas (P=4.18×10−5) and Catenibacterium (P=1.51×10−4), respectively. In addition, the Enterobacter genus of phylum Proteobacteria was also associated with lifetime CVD risk (P=6.41×10−8). After additional adjustment for BMI (Model 2), all 8 of these taxa remained nominally significant (P<0.05), including five which retained significance after Bonferroni correction (Alloprevotella, P=6.53×10−9; Prevotella 7, P=5.58×10−7; Tyzzerella, P=7.83×10−6; Megamonas, P=3.41×10−6; and Catenibacterium, P=1.87×10−4). After further adjustment for diet, all genera remained at least nominally significant, except for Paraprevotella and Enterobacter, which no longer associated with CVD risk. Five additional genera attained significance after Bonferroni correction in Model 2, including three from the Firmicutes phylum (Coprococcus 2, P=1.38×10−4; Megasphaera, P=1.38×10−4; and Ruminococcus, P=5.01×10−4), one from the phylum Euryarchaeota (Methanobrevibacter, P=1.20×10−4) and one from the phylum Proteobacteria (Thalassospira, P=3.44×10−4). Among these, two were nominally significant prior to BMI adjustment (Methanobrevibacter, P=5.12×10−3 and Thalassospira, P=3.61×10−3). In Model 3, three additional genera were significant including two from the Bacteroidetes genera (Bacteriodales S24-7, P=2.77×10−6 and Prevotella 2, P=5.67×10−5) and one from the Firmicutes phylum (Christensenellaceae R-7 group, P= P=5.69×10−4). Only Prevotella 2 attained nominal significance in the prior models.
Direction of associations for all 16 taxa achieving significance after Bonferroni correction for multiple testing in any model are shown in Figure 1A–C. Among the taxa significantly associated with lifetime CVD risk profile in Model 1, Alloprevotella and Catenibacterium were associated with decreased lifetime CVD risk profile (Z=−4.67 and −3.74, respectively) and Paraprevotella, Prevotella 7, Megamonas, Tyzzerella, Tyzzerella 4, and Enterobacter were associated with increased lifetime CVD risk profile (Z=3.41, 3.95, 4.10, 3.52, 3.79, and 5.41, respectively). After additional adjustment for BMI, effect directions for these genera remained consistent except for Megamonas and Paraprevotella, which were significantly and nominally associated with decreased lifetime CVD risk profile, respectively (Z=−4.64 and −2.19, respectively). Aside from Paraprevotella and Enterobacter, which were no longer associated with CVD risk profile after adjusting for diet, association directions for all remaining Model 1 findings were similar in Model 3. In Model 2, Megasphaera and Ruminococcus appeared to be associated with a decreased lifetime CVD risk profile (Z=−3.81 and −3.48, respectively), while Thalassospira, Coprococcus 2, and Methanobrevibacter with an increased lifetime CVD risk profile (Z=3.58, 3.81, and 3.58, respectively). In Model 3, Bacteriodales S24-7 and Prevotella 2 (Z=4.69 and 4.03, respectively) were enriched among those with a high lifetime CVD risk profile, while Christensenellaceae R-7 (Z=−3.45) was depleted among those with a high lifetime risk profile.
Figure 1.
Associations of 16 taxa achieving P<6.67×10−4 in genus-level analyses. Direction of associations for all 16 genera are presented for (a.) age, gender and race; (b.) age, gender, race and BMI; and (c.) age, gender, race, BMI, total energy intake, and energy-adjusted intake of protein§, animal fat§, and fiber§ adjusted analyses. Red shading indicate increased odds of high cardiovascular disease risk profiles and blue shading indicates decreased odds of high cardiovascular disease risk profiles. Darker shading indicates significance after Bonferroni correction for multiple testing. Dashed circles indicate the scale of (absolute) Z values ranging from 1 to 6. Phyla are indicated next to the outer solid circle. § Adjusted for total energy intake using the residual method.
DISCUSSION
In the current study, we identified composite measures of alpha diversity and individual microbial genera associated with lifetime CVD risk profile among BHS participants. We observed consistent inverse associations of measures of microbial richness, but not evenness, with lifetime CVD risk. While previous studies have reported beneficial associations of the Bacteroidetes phylum and deleterious association of the Firmicutes phylum with CVD risk factors3, 32, findings of other studies have been conflicting.7 At the genera level, we identified 16 taxa significantly associated with lifetime CVD risk profile, including 6 which showed consistent associations in all models examined, including Alloprevatella, Prevotella 2, Prevotella 7, Tyzzerella, Tyzzerella 4, and Catenibacterium. Of note, these findings included genera with opposite effects within the same phyla. For example, among Bacteroidetes, Alloprevotella was associated with decreased lifetime CVD risk while Prevotella 2 and Prevotella 7 were associated with increased lifetime CVD risk in our study. Among the Firmicutes, Tyzzerella and Tyzzerella 4 were enriched among those with high CVD risk profile while Catenibacterium was depleted in this group. These data may help to explain the inconsistency of findings when assessing associations of higher order taxa. While Methanobrevibacter, Enterobacter, and Thalassospira were enriched among those with high CVD risk profiles in Models 1 and 2, these associations were completely attenuated after adjustment for dietary factors, suggesting that diet could confound the observed associations. Several findings appeared relatively inconsistent across models and should be considered with caution in this small sample. For example, both Megamonas and Paraprevotella appeared enriched among those with high CVD risk profile in Model 1, depleted in Model 2 and unassociated in Model 3. Furthermore, Ruminoococcus, Coprococcus, and Megasphaera only achieved significance in Model 2, while Bacteroidales S24-7 and Christensenellaceae R-7 only achieved significance in Model 3. In aggregate, these data add to the accumulating evidence suggesting an important role of the gut microbiota on CVD risk.
Alpha diversity, measured as microbial richness by the number of observed OTUs, Chao 1 index and ACE, was consistently and inversely associated with lifetime CVD risk in our analyses. These data are in line with previous studies examining other CVD risk factors such as BMI7, 11 and lipid levels.11 In contrast, lifetime CVD risk was not associated with alpha diversity measures incorporating evenness of microbial taxa, such as Shannon, Simpson and inverse Simpson indices. Similarly, previous studies examining the relation of Shannon Index with CVD risk factors have generally showed null results.33, 34 These data suggest that the abundance of distinct microbial taxa may be more important than their relative frequency in determining CVD risk.
In the Bacteroidetes phylum, four genera of the family Prevotelleceae were identified by the current study. Previous research has linked this group to CVD risk factors including obesity35, 36 and diabetes37–39, but the directions of reported associations have been inconsistent. For example, Furet and colleagues identified a decreased frequency of the Prevotella group in obese versus non-obese individuals.35 This is in contrast to findings by Zhang and colleagues, who reported enrichment of the Prevotelleceae family in obese compared to non-obese.36 Similarly, Alkanani and Larsen identified an increase in Prevotelleceae in diabetes patients versus controls38, 39, while Fugmann and colleagues identified an overrepresentation of Prevotelleceae in women with gestational diabetes versus controls.37 The current study identified genera within the family Prevotelleceae that had different effects; some were associated with an increased and others with a decreased CVD risk profile. Given the presence of both beneficial and deleterious genera within this phylogenetic family, our data may again suggest that past inconsistencies could have resulted from grouping disparate genera at higher taxonomic levels. Furthermore, our findings indicate that the relation between Prevotelleceae and lifetime burden of CVD risk factors is independent of BMI. While the previous studies have also identified associations with diabetes, we are not aware of previous reports suggesting that the relationship between Prevotelleceae and CVD risk may be independent BMI. Whether this relationship remains after adjustment for measures of central adiposity is unclear. An additional genera, unidentified bacteria form the Bacteriodales S24-7 family, was also identified from the Bacteroidetes phylum. To the knowledge of the authors, this is the first study to link this genus to CVD risk.
Our results show that eight genera from the phylum Firmicutes associated with lifetime CVD risk in the current study. Within the family Lachnospiraceae, we identified genera which were enriched among those with high lifetime CVD risk profile, such as Tyzzerella, Tyzzerella 4, and Coprococcus 2, as well as those which were enriched among the low CVD risk profile group, including Ruminococcus. Similar to findings reported here, Zhang and colleagues identified certain taxa from the family Lachnospiraceae which were enriched in type 2 diabetes patients and others which were enriched in normal controls.40 From the family Veillonellaceae, genera Megamonas and Megasphaera associated with lifetime CVD risk. Megamonas has been reported previously to inversely associate with type 2 diabetes.40 After adjustment for BMI, we observed a similar inverse association with lifetime CVD risk profile. The genus Catenibacterium of the Erysipelotrichaceae family was also associated with decreased lifetime CVD risk, which is similar to findings reported by Fu and colleagues of an inverse relation of a genus from this family with BMI.11 Furthermore, the genus Christensenellaceae R-7 from the family Christensenellaceae, which was not previously associated with CVD-risk related phenotypes was also identified. Further studies to confirm this association are needed.
We found two genera of the phylum Proteobacteria, Thalassospira and Enterobacter, and one of the phylum Euryarchaeota, Methanobrevibactor, to be associated with increased lifetime CVD risk in the current study. These findings are consistent with reports of genera of the phylum Proteobacteria being associated with numerous cardiometabolic traits in human and mouse studies.38, 41 For example, Alkanani and colleagues showed an inverse association between Thalassospira and type 1 diabetes38, while Enterobacter was experimentally shown to increase obesity risk in mouse models.41 Methanobrevibactor, the only genus in the domain Archaea assessed in the current study, has also previously been implicated to influence CVD risk, primarily through its association with obesity.36, 42 Researchers have hypothesized that this taxon may increase obesity risk via its role in increasing polysaccharide fermentation leading to increased adiposity and have found support in animal models.42 The current study found an increased frequency of Methanobrevibacter in participants with high lifetime CVD risk profiles compared to their low risk counterparts. While these findings were independent of BMI, the associations were completely attenuated when accounting for diet in the analysis. These data suggest that associations of Thalassospira, Enterobacter, and Methanobrevibacter may be confounded by dietary factors. To the knowledge of the authors, this is the first study to adjust for dietary factors in the analysis.
The current study has several important strengths. The longitudinal BHS design provided a unique opportunity to examine the association between microbiome and lifetime burden of CVD risk factors. Estimation of CVD risk profile was made using carefully collected measures of SBP, LDL cholesterol, and glucose from an average of 9 study visits conducted over approximately 33 years of follow-up. Furthermore, stool specimens were collected using a stringent protocol, with sequencing and downstream analyses following guidelines set forth by the Human Microbiome Project.21 However, certain limitations should also be addressed. The study was conducted among a relatively small subsample of BHS participants. The small sample size may have limited our statistical power to detect other important microbiota and may be responsible for the modestly significant associations of alpha-diversity measures with CVD risk. Furthermore, since the collection of stool specimens occurred after determination of lifetime CVD risk profile, it is not possible to delineate the temporal relation between the observed microbiota-lifetime CVD risk associations. Given the somewhat provisional nature of these findings, replication of the results in independent samples is warranted. In addition, prospective studies are needed to confirm whether the presence of identified microbiota precede alterations in CVD risk profiles. Finally, 16S rRNA sequencing does not generally provide sequencing resolution beyond the genus level and does not allow for direct functional profiling. Therefore, the current study was limited in its ability to identify specific microbial species and biological pathways that may influence CVD risk. Future research, taking advantage of shotgun metagenomic DNA sequencing, could contribute important information to this area.
In summary, the current study identified consistent associations of overall microbiota composition and six microbial taxa with lifetime CVD risk profile. Microbial richness was depleted among those with a high lifetime burden of CVD risk factors compared to those with a low lifetime burden. Identified genera included those with differing effects, some being enriched and others depleted among participants with high lifetime CVD risk profiles, from phyla including Bacteroidetes and Firmicutes. These cross-sectional data add to the accumulating evidence that microbiota may play an important role in CVD risk. However, further studies examining the prospective relation of the microbiome with CVD health are still needed.
Supplementary Material
Novelty and Significance.
What Is Known?
Gut microbes are associated with cardiovascular disease (CVD) risk factors including obesity, type 2 diabetes, and plasma lipid levels.
The trimethylamine-oxide metabolite, which can be formed in the liver from a gut microbiota derived precursor, has been linked to CVD development.
What New Information Does This Article Contribute?
The findings show differential gut microbial composition between those with high versus low lifetime CVD risk profile.
Within the Bacteroidetes phylum, two microbial genera, Prevotella 2 and Prevotella 7 were enriched, while one microbial genera, Alloprevotella, was depleted between those with high compared to low CVD risk profile.
Similarly, within the Firmicutes phylum, two microbial genera, Tyzzerella and Tyzzerella 4, were enriched, while one microbial genera, Catenibacterium, was depleted between those with high compared to low CVD risk profile.
Few studies have systematically assessed the influence of gut microbes on CVD risk. The current study leveraged carefully collected data on traditional CVD risk factors obtained over approximately 33 years of follow-up to estimate CVD risk among Bogalusa Heart Study participants. Associations of gut microbiota with lifetime CVD risk profile were then assessed after adjusting for important confounders including age, gender, race, body mass index and diet. Gut microbial composition differed between those with high compared to low CVD risk profiles. At the genus level, six taxa were associated with lifetime burden of CVD risk factors. Within the Bacteroidetes phylum, Alloprevotella was associated with decreased lifetime CVD risk while Prevotella 2 and Prevotella 7 were associated with increased lifetime CVD risk. Within the Firmicutes phylum, Tyzzerella and Tyzzerella 4 were enriched among those with high CVD risk profile while Catenibacterium was depleted in this group. These data add to the accumulating evidence that microbiota may play an important role in CVD risk. If a causal association is established, these findings suggest that strategies aimed at altering gut microbial ecology, including changes to diet or supplementation with probiotics, could be employed to help decrease CVD risk.
Acknowledgments
SOURCES OF FUNDING
Research reported in this publication was partially supported by the National Institute of General Medical Sciences of the NIH under Award Number P20GM109036 and the National Institute of Aging of the NIH under Award Number R01AG041200. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Nonstandard Abbreviations and Acronyms
- ACE
Abundance-based coverage estimator
- BMI
Body mass index
- BP
Blood pressure
- BHS
Boglusa Heart Study
- CVD
Cardiovascular disease
- LDL
Low-density lipoprotein
- OTU
Operational taxonomic unit
- QIIME
Quantitative Insights Into Microbial Ecology
- SBP
Systolic blood pressure
Footnotes
DISCLOSURES
None.
References
- 1.Weinstock GM. Genomic approaches to studying the human microbiota. Nature. 2012;489:250–256. doi: 10.1038/nature11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuczynski J, Lauber CL, Walters WA, Parfrey LW, Clemente JC, Gevers D, Knight R. Experimental and analytical tools for studying the human microbiome. Nat Rev Genet. 2012;13:47–58. doi: 10.1038/nrg3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 4.Joyce S, MacSharry J, Casey P, Kinsella M, Murphy E, Shanahan F, Hill C, Gahan C. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci U S A. 2014;111:7421–7426. doi: 10.1073/pnas.1323599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 6.Moran CP, Shanahan F. Gut microbiota and obesity: Role in aetiology and potential therapeutic target. Baillieres Best Pract Res Clin Gastroenterol. 2014;28:585–597. doi: 10.1016/j.bpg.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tilg H, Moschen AR. Microbiota and diabetes: An evolving relationship. Gut. 2014;63:1513–1521. doi: 10.1136/gutjnl-2014-306928. [DOI] [PubMed] [Google Scholar]
- 9.Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 10.Le Chatelier E, Nielsen T, Qin J, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 11.Fu J, Bonder MJ, Cenit MC, et al. The gut microbiome contributes to a substantial proportion of the variation in blood lipids. Circ Res. 2015;117:817–824. doi: 10.1161/CIRCRESAHA.115.306807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang AQ, Mitchell SC, Smith RL. Dietary precursors to trimethylamine in man: a pilot study. Food Chem Toxicol. 1999;37:515–520. doi: 10.1016/s0278-6915(99)00028-9. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell SC, Zhang AQ, Smith RL. Chemical and biological liberation of trimethylamine from foods. J Food Comp Anal. 2002;15:277–282. [Google Scholar]
- 14.Tang WHW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolphin CT, Janmohamed A, Smith RL, Shephard EA, Phillips IR. Missense mutation in the flavin-containing mono-oxygenase 3 gene, FMO3, underlies fish-odour syndrome. Nat Genet. 1997;17:491–494. doi: 10.1038/ng1297-491. [DOI] [PubMed] [Google Scholar]
- 16.Foster TA, Berenson GS. Measurement error and reliability in four pediatric cross-sectional surveys of cardiovascular disease risk factor variables--the bogalusa heart study. J Chronic Dis. 1987;40:13–21. doi: 10.1016/0021-9681(87)90092-0. [DOI] [PubMed] [Google Scholar]
- 17.Foster TA, Webber LS, Srinivasan SR, Voors AW, Berenson GS. Measurement error of risk factor variables in an epidemiologic study of children-the bogalusa heart study. J Chronic Dis. 1980;33:661–672. doi: 10.1016/0021-9681(80)90009-0. [DOI] [PubMed] [Google Scholar]
- 18.Rockett HR, Breitenbach M, Frazier AL, Witschi J, Wolf AM, Field AE, Colditz GA. Validation of a youth/adolescent food frequency questionnaire. Prev Med. 1997;26:808–816. doi: 10.1006/pmed.1997.0200. [DOI] [PubMed] [Google Scholar]
- 19.Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973;19:476–482. [PubMed] [Google Scholar]
- 20.Srinivasan SR, Berenson GS. Serum lipoproteins in children and methods for study. In: Lewis LA, editor. CRC handbook of electrophoresis. Vol. 3. Lipoprotein methodology and human studies. Boca Raton, Fla: CRC Press; 1983. pp. 185–203. [Google Scholar]
- 21.Human Microbiome Project. A framework for human microbiome research. Nature. 2012;486:215–221. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Human Microbiome Project. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Huntley J, Fierer N, Owens SM, Betley J, Fraser L, Bauer M, Gormley N, Gilbert JA, Smith G, Knight R. Ultra-high-throughput microbial community analysis on the illumina hiseq and miseq platforms. ISME J. 2012;6:1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edgar RC. Search and clustering orders of magnitude faster than blast. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 25.Caporaso JG, Kuczynski J, Stombaugh J, et al. Qiime allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glockner FO. The silva ribosomal rna gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013;41:D590–596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lozupone CA, Knight R. Species divergence and the measurement of microbial diversity. FEMS Microbiol Rev. 2008;32:557–578. doi: 10.1111/j.1574-6976.2008.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McMurdie PJ, Holmes S. Phyloseq: An r package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wooldridge J. Introductory econometrics: A modern approach. Nelson Education; 2015. [Google Scholar]
- 30.Xu L, Paterson AD, Turpin W, Xu W. Assessment and selection of competing models for zero-inflated microbiome data. PLoS ONE. 2015;10:e0129606. doi: 10.1371/journal.pone.0129606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willet WC, Howe GR, Kushi LH. Adjustment of total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 32.Ley R, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 33.Lambeth SM, Carson T, Lowe J, Ramaraj T, Leff JW, Luo L, Bell CJ, Shah VO. Composition, diversity, and abundance of gut microbiome in prediabetes and type 2 diabetes. J Diabetes Obes. 2015;2:1–7. doi: 10.15436/2376-0949.15.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sepp E, Kolk H, Loivukene K, Mikelsaar M. Higher blood glucose level associated with body mass index and gut microbiota in elderly people. Microb Ecol Health Dis. 2014;25 doi: 10.3402/mehd.v25.22857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Furet J, Kong L-C, Tap J, Poitou C, Basdevant A, Bouillot J-L, Mariat D, Corthier G, Dore J, Henegar C, Rizkalla S, Clement K. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, Krajmalnik-Brown R. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fugmann M, Breier M, Rottenkolber M, Banning F, Ferrari U, Sacco V, Grallert H, Parhofer KG, Seissler J, Clavel T, Lechner A. The stool microbiota of insulin resistant women with recent gestational diabetes, a high risk group for type 2 diabetes. Sci Rep. 2015;5:13212. doi: 10.1038/srep13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alkanani AK, Hara N, Gottlieb PA, Ir D, Robertson CE, Wagner BD, Frank DN, Zipris D. Alterations in intestinal microbiota correlate with susceptibility to type 1 diabetes. Diabetes. 2015;64:3510–3520. doi: 10.2337/db14-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larsen N, Vogensen FK, van den Berg FWJ, Nielsen DS, Andreasen AS, Pedersen BK, Al-Soud WA, Sorensen SJ, Hansen LH, Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, Chen Y, Ji L. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE. 2013;8:e71108. doi: 10.1371/journal.pone.0071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 2013;7:880–884. doi: 10.1038/ismej.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Million M, Lagier JC, Yahav D, Paul M. Gut bacterial microbiota and obesity. Clin Microbiol Infect. 2013;19:305–313. doi: 10.1111/1469-0691.12172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.