Abstract
Neovascularization is a hallmark of physiological and pathological tissue remodelling that is regulated in part by the extracellular matrix (ECM). Collagen I hydrogels or Matrigel are frequently used to study vascular network formation; however, in isolation these materials do not typically mimic the integrated effects of ECM structure and composition that may influence endothelial cells in vivo. Here, we have utilized microfabricated 3D culture models to control collagen I microstructure in the presence and absence of Matrigel and tested the effect of these variations on vascular network formation by human cerebral microvascular endothelial cells (hCMECs). Varied collagen microarchitecture was achieved by adjusting the gelation temperature and subsequently confirmed by structural analysis. Casting at colder temperature increased collagen fiber thickness and length, and inclusion of Matrigel further pronounced these differences. Interestingly, presence of Matrigel affected vascular network formation by modulating hCMEC growth, whereas altered collagen fiber structure impacted the morphology and maturity of the developed vascular network. These differences were related to substrate-dependent changes in interleukin-8 (IL-8) secretion and were functionally relevant as vascular networks preformed in more fibrillar, Matrigel-containing hydrogels promoted angiogenic sprouting. Our studies indicate that collagen hydrogel microstructure and composition conjointly regulate vascular network formation with implications for translational and basic science approaches.
Keywords: Vascularization, Extracellular Matrix, Endothelial cells, Microenvironment, Interleukin-8
Graphical Abstract
1. Introduction
Hydrogel-based 3D cultures are widely used to study the formation of new blood vessels from naïve endothelial cells (vasculogenesis) or the preexisting vasculature (angiogenesis) in health and disease [1], [2], and [3]. Much focus has been placed on utilizing endothelial cell-embedded hydrogels to delineate the role of soluble factors including growth factors and cytokines as well as oxygen and nutrient gradients in microvascular network assembly [4] and [5]. Yet the extracellular matrix (ECM) plays a similarly important role in regulating endothelial cell behavior, but our understanding of the underlying cellular and molecular mechanisms remains relatively limited. Indeed, ECM composition and structure have been independently shown to influence vascularization in the native microenvironment [6]. However, few hydrogel-based model systems currently exist that allow systematic investigations into the combined and individual effects of ECM composition and structure on microvascular network assembly.
Instructive signals from the ECM can both drive and suppress vascular development and remodeling [7] and [8]. The basement membrane, a highly porous and organized network of proteins, surrounds the endothelium in vivo and typically contributes to vessel stabilization [9]. Loss of contact with the basement membrane either through tissue insult or proteolytic degradation initiates endothelial cell migration and sprouting [10]. Induction of endothelial tip cells defines the path of neovascularization and the following stalk cells establish a nascent lumen and remodel basement membrane [11] and [12]. In addition to directly regulating endothelial cell polarization, migration, adhesion, and function, the basement membrane also physically anchors the endothelium to the interstitial matrix, typically collagen I [13].
Collagen I is the primary ECM component of most connective tissues, easily remodeled by cells, and also represents one of the main structural proteins of blood vessels [14]. The importance of collagen I in angiogenesis is evident as inhibition of collagen crosslinking decreases angiogenesis, while collagen degradation releases sequestered proangiogenic morphogens such as growth factors and ECM fragments [15] and [16]. Consequently, collagen I is an attractive material for mimicking ECM-related aspects of the microenvironment. However, most current studies disregard that the microscale structure of collagen I can vary significantly, and these structural differences may significantly affect microvascular network assembly [17]. For example, fibrotic ECM remodeling during obesity is associated with both differences in collagen fiber length and thickness and abnormal vasculature, but whether these two phenomena are functionally linked remains unclear [18].
Many current hydrogel-based approaches to investigate microvascular network assembly as a function of the above-described ECM-mediated phenomena rely on the use of either Matrigel or collagen I [19] and [20]. However, Matrigel, a tumor-derived ECM cocktail, differs from the native basement membrane by lacking physiologically relevant matrix microstructure [21] and [22]. Inversely, collagen I hydrogels can be fabricated reliably with controlled microstructure depending on the utilized isolation and gelation protocols but lacks key compositional elements of the ECM that influence endothelial cell behavior in vivo [23] and [24]. Hence, exclusive use of collagen I or Matrigel is likely insufficient in mimicking the complex cell-ECM and ECM-ECM interactions that influence endothelial cell behavior during vascularization.
Here, we describe a method for evaluating 3D microvascular network formation as a function of ECM composition and microstructure. We have created hydrogels composed of both collagen I and Matrigel and adjusted their microstructure through varying the hydrogel casting temperature. Subsequently, we tested whether the resulting differences in hydrogel microarchitecture and composition altered endothelial cell vasculogenic and angiogenic potential. Lastly, we examined matrix-dependent changes of endothelial cell-secreted interleukin-8 (IL-8) as a potential molecular mechanism underlying the detected changes in endothelial cell response.
2. Materials and Methods
2.1 Cell culture
Immortalized human cerebral microvascular endothelial cells (hCMECs) were provided by Dr. Babette Weksler (Weill Cornell Medical College, New York, NY, [25]). Cells were routinely cultured in Endothelial Growth Media-2 (EGM-2, Lonza) containing 2 % FBS, growth factors, and 1% penicillin/streptomycin on bovine collagen I-coated (26.35 mg/mL, BD Biosciences) cell culture flasks, at 37°C, and 5% CO2. For experiments, hCMECs were used between passages p13 and 19. Fluorescently labeled hCMECs were generated by transfecting the cells with a lentiviral vector containing the mCherry gene. Prior to experiments, mCherry labeled hCMECs were FACS-sorted using a FASCAria III (BD Biosciences) to enrich for highly fluorescent mCherry cells. Human umbilical vein endothelial cells (HUVECs, p2-6) (Lonza) were cultured in Bio-Whittaker medium 199 (M199) supplemented with 20% FBS, endothelial cell growth supplement (ECGS, Millipore), 1% penicillin/streptomycin, 2 mM Glutamax, and 5 units/mL heparin.
2.2 Microwell and collagen hydrogel fabrication
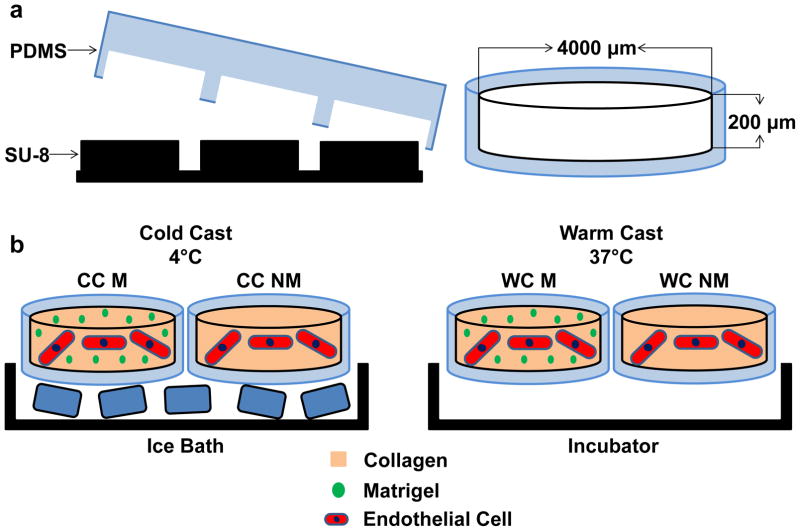
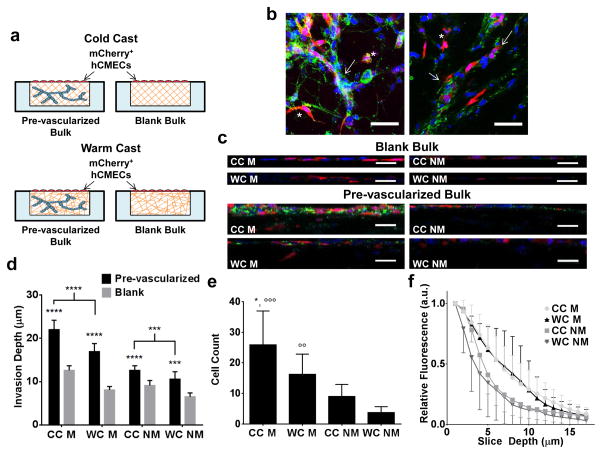
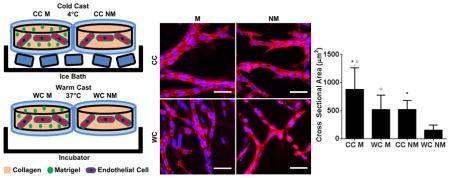
Poly(dimethylsiloxane) (PDMS, Dow Corning) microwells (4 mm in diameter, 250 μm in depth) were fabricated as described previously [26]. Briefly, PDMS was cast onto a silicon master coated with a SU-8 negative photoresist pattern and demolded after curing at 65°C for 3 hours. PDMS microwells were punched out using an 8 mm biopsy punch and transferred individually into 24-well plates. To covalently bond collagen hydrogels, microwell surfaces were activated by treatment with 1% polyethylimine (PEI, Sigma-Aldrich) and 0.1% glutaraldehyde (GA, Fisher Scientific). Collagen type I was prepared from rat tails as described previously [23]. Prior to casting, collagen stock solution (1.5% [wt/V] in 0.1% acetic acid) was diluted to 0.6% [wt/V], osmotically balanced with concentrated (10×) EGM-2 media, and titrated with 1N NaOH to a pH of 7.2–7.4. Cells were suspended in the final gel solution at a density of 1 × 106 cells/mL. For conditions containing Matrigel (BD Biosciences) collagen solutions were mixed with growth factor reduced (GFR) Matrigel (2% V/V) prior to neutralization with NaOH. Hydrogels were cast into the PDMS microwells and allowed to crosslink for 40 minutes, at either 4°C or 37°C (cold and warm cast, respectively) (Fig. 1).
Figure 1. Experimental design.
a) Microwells were fabricated by polymerizing poly(dimethylsiloxane) (PDMS) on photolithographically etched wafers and were individually punched out and transferred into conventional 24-well culture plates. b) Type-1 collagen was cast into the microwells with (M) and without (NM) Matrigel and allowed to crosslink either in an ice bath (cold cast [CC], 4°C) or in an incubator (warm cast [WC], 37°C). For all cell experiments, human endothelial cells were suspended in the different hydrogels prior to gelation, while characterization experiments were performed with cell-free gels.
2.3 Endothelial vasculogenesis and anastomosis assays
Cell-seeded microwell cultures were maintained in their respective growth media for 11 days with media changed every 2 days. For anastomosis studies, microwell cultures were pre-vascularized for 11 days. Subsequently, a confluent monolayer of mCherry-labeled hCMECs (0.5 × 106 cells/mL) was seeded on top of these cultures and maintained for an additional 3 days. To quantify differences in IL-8 secretion, fresh media was added and collected after 24 hours of incubation. IL-8 levels in the collected media were quantified using an enzyme-linked immunosorbent assay according to manufacturer’s instructions (ELISA, R&D Systems) and normalized to DNA content measured using Quantifluor dsDNA System (Promega). To assess the effects of IL-8 suppression on vasculogenesis, a function-blocking HuMax-IL-8 antibody (20 μg/mL, Cormorant Pharmaceuticals) was added to the cell culture media. To analyze differences in endothelial cell behavior, cultures were fixed with 0.4% paraformaldehyde. Collagen IV was labeled with a rabbit anti-human collagen IV antibody (1:200 dilution, ABCAM) followed by incubation with a donkey anti-rabbit IgG (1:500 dilution, Alexafluor 488, Life Technologies). To exclusively detect extracellular collagen IV, staining was performed without prior permeabilization. Subsequently, cells were permeabilized and nuclei and f-actin were stained with 4′,6-diamidino-2-phenylindole (DAPI, 1:5000 dilution, Invitrogen) and phalloidin (1:100 dilution, Alexafluor 546 or 647, Life Technologies), respectively.
2.4 Structural analysis of collagen gels
Quantitative microstructural analysis of the different hydrogels was performed on micrographs captured with scanning electron microscopy (SEM). Blank hydrogels prepared as described above were dehydrated using an ethanol gradient. Samples were then placed into hexamethyldisilazane for 10 minutes to preserve collagen microstructure and air dried in a chemical hood. Dry samples were attached to SEM specimen mounts and coated with gold/palladium alloy in a sputter coater (SCD 500, Baltec). All SEM images were taken at 40,500 fold magnification (Mira3FESEM, Tescan). For SEM image analysis, fiber length and diameter were measured utilizing ImageJ (NIH). For each condition, 3 samples were analyzed, images from 5 randomly selected areas were taken and 7 fibers from each image were analyzed. Fiber diameter and length were measured utilizing ImageJ (NIH) following manual tracing of single surface-associated fibers (Supplementary Figure 1).
2.5 Confocal reflectance and immunofluorescence analysis
Samples were imaged using a confocal microscope (Zeiss 710 LSM) in 1.1 μm step sizes and up to 150 μm in depth. For all analyses, 3 samples from each condition were imaged. Confocal image analysis of endothelial cell and network parameters was performed using ImageJ. To quantify cell numbers, 3 randomly selected 212.55 μm × 212.55 μm × 100 μm optical volumes (400× magnification) per microwell were flattened into a single z-stack and DAPI-stained nuclei were counted using the cell counter tool in ImageJ. Each condition was repeated in triplicate. Cross sectional areas of vascular branches were assessed using the same optical volumes and the orthographic projection tool in ImageJ; boundaries of branches were circumscribed and the area measured with the freehand selection function; 10 different branches were measured for each condition. Vascular branching was also determined through image analysis of similarly flattened z-stack. The largest vascular ‘cords’ were used as the base branch and endothelial sprouts consisting of 3 or more endothelial cells and extending outwards beyond 50 μm were counted. Collagen IV deposition was measured by determining the total pixel intensity of collagen IV immunofluorescence at a depth of 20 μm in each hydrogel. Images were transformed into a tab-delimited matrix, imported into MatLab (Mathworks, R2013b), and graphed using the colored surface function. For monolayer invasion experiments, the monolayer was established to be z-slice with the highest mCherry signal. To analyze invasion depth, fluorescence intensity of individual z-stacks was recorded as the raw integrated density and normalized to the fluorescence intensity of the corresponding monolayer. For endothelial cell invasion analysis, mCherry-labeled cells that had invaded 10 μm beyond the monolayer were analyzed
2.6 Statistical Analysis
Three independent experiments were performed for each experimental condition with 3 replicates per experiment (n=3) and results are presented as the mean +/− standard deviation. All statistical tests were conducted in Graphpad PRISM. In conditions comparing matrix composition and structure, a two-way ANOVA with Tukey’s post-hoc test was used. In conditions comparing the effect of pre-vascularization or IL-8 inhibition relative to controls, student’s t-test was used, P-values less than 0.05, 0.01, 0.001, 0.0001 were considered statistically significant and were labeled with *, **, ***, **** and °, °°, °°°, °°°° where * indicates comparisons between cold and warm cast hydrogels while ° indicates comparisons between hydrogels with and without Matrigel unless otherwise indicated.
3. Results and Discussion
3.1 Collagen hydrogel casting temperature and composition alter fiber microstructure
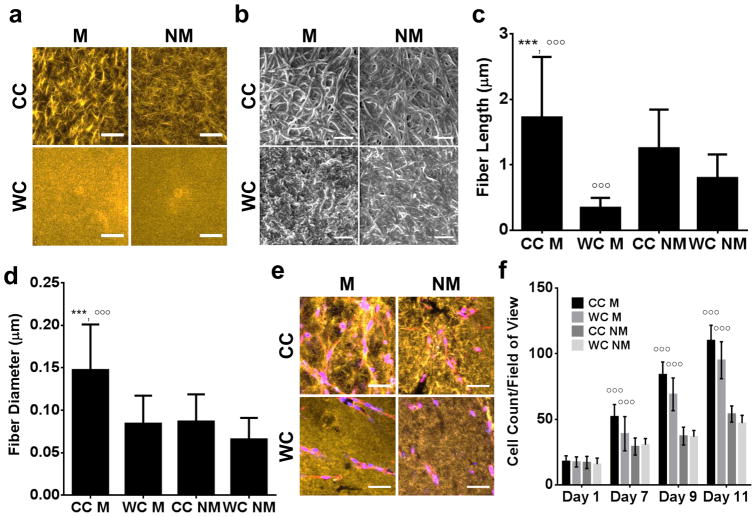
To investigate the individual and combined effects of crosslinking temperature and inclusion of Matrigel on type I collagen microarchitecture, acellular hydrogels were cast in microfabricated PDMS wells (Fig. 1) and analyzed using Confocal Reflectance Microscopy (CRM) and Scanning Electron Microscopy (SEM). Consistent with previous findings, CRM micrographs suggested that cold cast collagen hydrogels contained larger fibers relative to warm cast hydrogels (Fig. 2a) [27], [28], and [29]. Interestingly, the addition of Matrigel appeared to increase fiber size in cold cast collagen hydrogels, while appearing to have no effect under warm cast conditions. To confirm these qualitative results, lyophilized hydrogels were subjected to SEM for a more detailed structural analysis of individual fibers. Indeed, image analysis of SEM micrographs confirmed that cold cast hydrogels were comprised of significantly longer and thicker collagen fibers relative to warm cast hydrogels (Fig. 2b–d). The inclusion of Matrigel pronounced these differences and increased both fiber length and diameter in cold cast hydrogels. In contrast, addition of Matrigel reduced fiber length in warm cast hydrogels, but did not impact fiber thickness (Fig. 2b–d).
Figure 2. Hydrogel composition and casting conditions impact collagen fibrillar structure.
a) Confocal Reflectance and b) Scanning Electron Microscopy micrographs of cell-free hydrogels prepared by cold (CC) or warm (WC) casting in the presence (M) or absence (NM) of Matrigel. Scale bars are 50 μm and 1 μm, respectively. Image analysis of SEM micrographs indicated that collagen fiber c) length and d) diameter increases with decreased casting temperature; effects were enhanced in Matrigel-containing hydrogels. e) Changes in matrix architecture due to varied hydrogel composition and casting conditions were maintained in the presence of hCMECs. Blue is DAPI and red is phalloidin. Scale bars are 50 μm. f) Quantification of cell number by image analysis revealed that presence of Matrigel enhances hCMEC growth in hydrogels more prominently than altered microstructure. P-values less than 0.001 were labeled with *** when comparing CC vs. WC or °°° when comparing M vs. NM.
The observed temperature-dependent differences in collagen microstructure can be explained by the mechanism that underlies collagen I fiber formation in solution, i.e. entropy-driven self-assembly of collagen I monomers into collagen fibrils that grow laterally [30]. Lower temperatures limit nucleation of new fibers via decreasing entropy, which promotes thickening and elongation of already existing fibers. The modulating effects of Matrigel on collagen microstructure on the other hand, are likely related to composition [31]. Fibronectin, laminin, entactin/nidogen-1, and perlecan found in Matrigel interact in collagen fibrillogenesis by providing collagen nucleation sites. In addition, these components regulate the elongation of existing collagen fibers by functioning as linkers between individual fibers collagen IV [31] and [32]. Furthermore, laminin and perlecan support the self-assembly of collagen IV to produce mesh-like networks [21] and [33]. This spontaneous assembly is sensitive to temperature; higher temperatures accelerate collagen IV network formation, which may explain the compromised fibrillar structure of warm cast Matrigel-containing hydrogels. It is therefore likely that lowering the casting temperature of collagen I and Matrigel allows for prolonged interactions between collagens fibrils and other Matrigel-associated proteins, promoting formation of thicker and longer collagen fibers as found in our experiments.
3.2 Collagen hydrogel casting temperature and composition alter endothelial cell growth
Matrix structure and composition are known to influence endothelial cell behavior and morphology [34]. We next tested whether the detected structural differences of the varied hydrogels affected the behavior of human cerebral microvascular endothelial cells (hCMECs). Initial experiments confirmed that incorporating 1 × 106 cells/mL prior to gelation yielded the same casting induced changes in hydrogel structure as described above (Fig. 2e). Subsequent analysis of cell growth further indicated that hCMECs were able to sense and respond to differences in hydrogel formulation. While casting temperature-induced changes of collagen fiber size appeared to play a minimal role in influencing cell growth, addition of Matrigel promoted cell growth in both warm cast and cold cast gels (Fig. 2f). These results are consistent with previous findings suggesting that endothelial cell adhesion to Matrigel-contained ECM proteins (such as laminin and collagen IV) promotes endothelial cell morphogenesis and proliferation via altered integrin engagement [35]. In addition, the presence of sequestered growth factors in Matrigel (even at low concentrations) is likely contributing to increased endothelial cell growth as well. For example, epidermal growth factor (EGF) is contained in growth factor-reduced Matrigel and may influence hCMEC proliferation [36].
3.3 Collagen microstructure and composition influence microvascular network formation
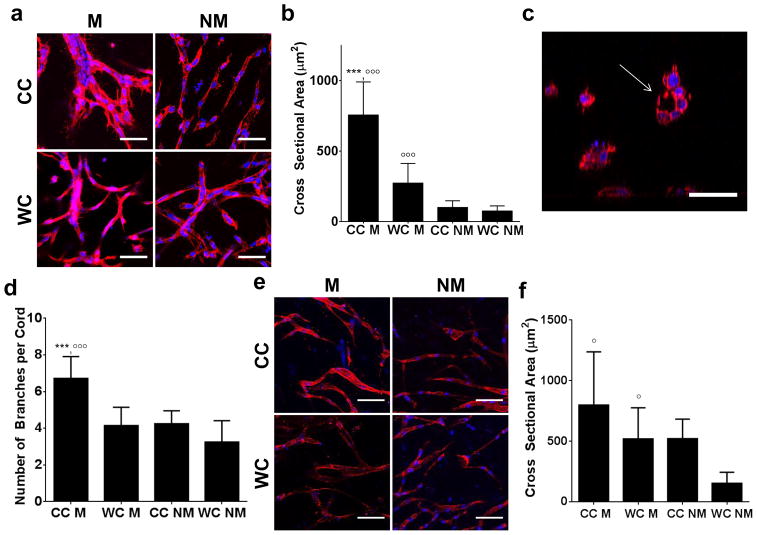
While differences in cell growth can serve as initial indicators for substrate-dependent alterations of cell behavior, they do not reliably predict endothelial cell behavior in the context of neovascularization. Accordingly, varied casting temperatures did not affect hCMEC growth in the different hydrogels (Fig. 2f), but markedly impacted the quality of the vascular network that formed (Fig. 3a). More specifically, confocal image analysis not only revealed that Matrigel promoted the formation of thicker endothelial branches, but that this difference was significantly more pronounced in cold cast gels with thicker and longer collagen fibrils (Fig. 3a, b). Importantly, lumen formation was only observed in large vascular branches that formed in cold cast Matrigel hydrogels (Fig. 3c). Furthermore, vascular networks formed in Matrigel-containing cold cast hydrogels were more branched relative to all other conditions (Fig. 3d). These data indicate that the structure of endothelial networks within the ECM is dependent on both the intrinsic microarchitecture of the collagen as well as the presence of basement membrane components. Importantly, these findings were not limited to hCMECs, but a similar trend was noted for HUVECs suggesting that our findings may be broadly relevant to endothelial cells and not an artifact of the hCMEC cell line (Fig. 3e, f). Our data complement results from others who have previously shown that longer and thicker collagen fibers promote cell elongation, a phenomenon that is critical for endothelial cell alignment during vascular assembly [14]. Differences in collagen fiber size likely contribute to enhanced endothelial cell contractility, which is a key characteristic for organized matrix remodeling, cell-cell interactions, and vascular assembly. Importantly, we show that concomitant control of ECM composition may potentiate endothelial cell responses to collagen microarchitecture and ultimately promote vascular assembly and maturation.
Figure 3. Collagen hydrogel structure and composition influence 3D endothelial network assembly.
a) Confocal micrographs of hCMEC cultures suggest that hydrogel composition and casting conditions influence vasculogenic network assembly. Blue is DAPI and red is phalloidin. Scale bars are 50 μm. b) Image analysis of confocal micrographs indicates that vascular branches are thicker in Matrigel-containing hydrogels and that gels prepared by cold-casting further increase this effect. c) Orthographic re-slicing of confocal micrographs demonstrates that thick vascular branches contain lumens. Blue is DAPI and red is phalloidin. Scale bar is 50μm. d) Image analysis reveals that vascular cords in Matrigel-containing, cold-cast hydrogels are more branched than in all other conditions. e) Similar differences in morphology and f) vascular branch cross sectional area were observed with HUVECs. Blue is DAPI and red is phalloidin. Scale bars are 50 μm. P-values less than 0.05 and 0.001 were labeled with * and *** when comparing CC vs. WC and p-values less than 0.05 and 0.001 were labeled with ° and °°° when comparing M vs. NM.
3.4 Matrix structure and composition influence basement membrane deposition
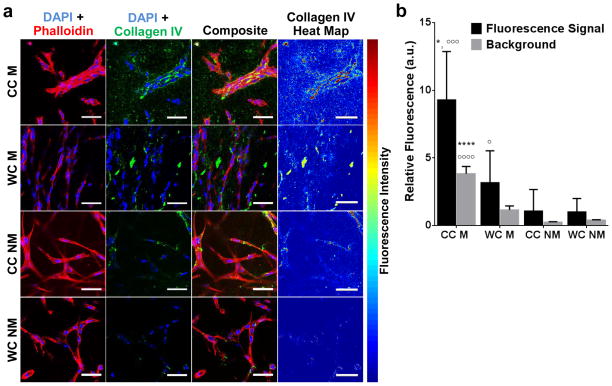
In vivo, a hallmark of vessel maturation and stability is the development of a tightly associated basement membrane surrounding the endothelium [37]. To test whether the detected, hydrogel-dependent differences in microvascular network development were associated with varied levels of vascular maturation, we analyzed deposition of the basement membrane component collagen IV. Confocal-based immunofluorescence analysis of collagen IV and f-actin alongside with fluorescence intensity mapping indicated increased deposition of collagen IV at cell-cell junctions, particularly when endothelial cells had assembled into vascular networks (Fig. 4a). Collagen IV deposition was significantly increased in Matrigel-containing hydrogels, a trend that was more pronounced in cold vs. warm cast hydrogels (Fig. 4b). Conversely, collagen IV deposition was barely detectable in warm cast hydrogels lacking Matrigel. Our results were likely due to both new deposition and remodeling of Matrigel-contained collagen IV, but depended on direct cell-cell contact as the background signal from Matrigel-containing gels was significantly reduced relative to collagen IV associated with cell-cell junctions. The heightened effect of Matrigel on collagen IV deposition is likely related to the various growth factors and cytokines contained in Matrigel. For example, transforming growth factor β (TGF-β), which is contained in growth factor-reduced Matrigel plays a role in vascular maturation and promotes vessel stability via changes in basement membrane deposition [38].
Figure 4. Collagen hydrogel structure and composition affect collagen IV deposition.
a) Confocal micrographs and corresponding heat maps as well as b) image analysis suggest that vascular structures formed in cold-cast, Matrigel-containing hydrogels deposit more type-IV collagen relative to all other conditions. Scale bars are 50 μm. P-values less than 0.05 and 0.0001 were labeled with * and **** when comparing CC vs. WC and p-values less than 0.05, 0.001, and 0.0001 were labeled with or °, °°°, and °°°° when comparing M vs. NM.
3.5 ECM-dependent vascular network characteristics influence invasion
Vascular network formation in vivo can occur through anastomosis, i.e. the induction and joining of adjacent vascular structures that originate from either vasculogenesis or angiogenesis and ultimately permit perfusion [39]. In order to assess whether the detected hydrogel-dependent differences in vasculogenesis were functionally relevant to subsequent anastomosis with an adjacent preexisting endothelium, hCMECs were cultured in cold and warm cast collagen hydrogels with and without Matrigel for 11 days. Subsequently, a monolayer of mCherry-labeled hCMECs was seeded on top and angiogenic invasions were analyzed as previously described (Fig. 5a) [40]. Blank, non-pre-vascularized hydrogels were used as controls to assess the isolated effects of collagen hydrogel structure and composition on endothelial cell invasion.
Figure 5. Hydrogel-dependent changes of vasculogenesis impact subsequent endothelial cell invasion and anastomosis.
a) Schematic depicting the experimental design of invasion experiments in blank and pre-vascularized collagen hydrogels. A monolayer of mCherry-labeled hCMECs was seeded on top of hydrogels that were either pre-vascularized with unlabeled hCMECs for 11 days or were left blank. b) Confocal micrographs show both individually invading endothelial cells (labeled with *) and anastomosis between labeled and unlabeled endothelial cells (labeled with arrow). Blue is DAPI, red is mCherry, and green is phalloidin. Scale bars are 50 μm. c) Confocal re-slices suggest that invasion of mCherry+ endothelial cells into the pre-vascularized vs. blank bulk is significantly enhanced in the presence of Matrigel. Blue is DAPI, red is mCherry, and green is type-IV collagen. Scale bar is 50 μm. d) Confocal image analysis confirmed that invasion from the endothelial monolayer was increased in pre-vascularized cultures, irrespective of condition. P-values less than 0.001 and 0.0001 were labeled with *** and ****. e) Quantification of the number of invaded mCherry+ endothelial cells and f) mCherry+ fluorescence intensity distribution supports these findings and further suggests that vascular invasion into pre-vascularized cultures is enhanced in cold-cast gels. P-values less than 0.05 were labeled with * when comparing CC vs. WC and p-values less than 0.01 and 0.001 were labeled with °° and °°° when comparing M vs. NM.
Pre-vascularized cultures exhibited extensive angiogenic invasion from the hCMEC monolayer that both integrated into the pre-existing vascular networks in the bulk and invaded as single cells (Fig. 5b). Confocal micrographs confirmed that pre-vascularized hydrogels exhibited increased invasion of mCherry-labeled hCMECs into the bulk hydrogel when compared to their blank counterparts (Fig. 5c). To more directly evaluate differences in endothelial cell invasion between pre-vascularized culture systems, we determined endothelial cell invasion depth and number via fluorescence intensity measurements. When compared to their blank counterparts, pre-vascularized hydrogels revealed significantly increased cell invasion independent of structure and composition (Fig. 5d). In addition, vascular networks preformed in cold cast gels appeared to exhibit a more pronounced effect as compared to their counterparts that had formed in warm cast gels. Perhaps not surprisingly, Matrigel-containing, pre-vascularized hydrogels exhibited increased invasion of endothelial cells from the top monolayer when compared to Matrigel-deficient, pre-vascularized hydrogels. Interestingly, this effect was further pronounced in pre-vascularized hydrogels that were generated with Matrigel-containing, cold-cast hydrogels (Fig. 5e, f). Collectively, these data suggest that endothelial cell invasion and anastomosis are mediated in part by matrix structure and composition and that endothelial cell remodeling of the underlying tissue plays a role in this process. These findings were consistent with previous reports suggesting that vascular network morphogenesis promotes blood vessel assembly by driving endothelial cell migration and anastomosis through vascular guidance tunnels [41]. Moreover, the generation of morphogen gradients likely plays a key role in the noted difference between blank and pre-vascularized hydrogels [42]. These differences in paracrine signaling may be further accentuated through the barrier function of the top endothelial monolayer, which can impact diffusion of nutrients, oxygen, and signaling molecules.
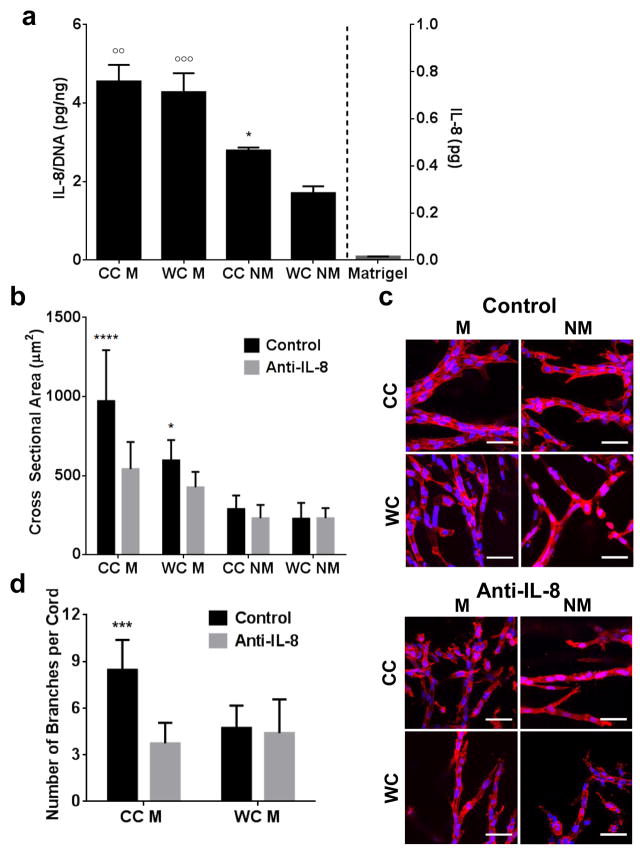
3.6 IL-8 regulates endothelial cell response to matrix structure and composition
Interleukin-8 (IL-8) is a potent pro-inflammatory chemokine that plays a key role during angiogenesis and is produced by a variety of cell types including endothelial cells [43] and [44]. Previous studies have associated increased levels of IL-8 with tumor progression and neovascularization, which may be due in part to IL-8’s ability to interfere with signaling of other growth factors including VEGF [45]. In order to assess whether or not IL-8 may be implicated in hydrogel-dependent differences in endothelial cell behavior, IL-8 secretion from the different cultures was measured and normalized to the DNA content in the microwell. The inclusion of Matrigel promoted endothelial cell secretion of IL-8 more dramatically than matrix structure, although cell-secreted IL-8 levels were also significantly enhanced in cold cast vs. warm cast hydrogels in the absence of Matrigel (Fig. 6a). These differences were due to varied cellular secretion rather than Matrigel-contained IL-8 as confirmed by ELISA of blank Matrigel (Fig. 6a). Importantly, these hydrogel-dependent differences in endothelial cell secretion of IL-8 were functionally relevant because inhibition of IL-8 using a function-blocking human antibody affected the quality of microvascular branches that formed in response to varied hydrogel composition and structure (Fig. 6b–d). More specifically, inhibition of IL-8 signaling reduced the cross sectional area of vascular sprouts in Matrigel-containing cold-cast collagen gels to levels that were comparable to vascular sprout thickness in Matrigel-containing warm-cast collagen gels (Fig. 6c). Interestingly, inhibition of IL-8 had no significant effect on vascular network structure under Matrigel-free conditions. However, IL-8 blockade in the presence of Matrigel reduced the branching of vascular networks in cold cast, but not warm cast hydrogels (Fig. 6d) suggesting that IL-8 regulates vascular responses to collagen structure in Matrigel-containing hydrogels. Collectively, these data support that Matrigel-induced IL-8 is not the sole regulator of vascular network formation in Matrigel-containing hydrogels, but that the physical effects mediated by altered collagen structure also play a role in this process.
Figure 6. Hydrogel structure and composition influence vasculogenesis and subsequent anastomosis through varying IL-8 secretion.
a) ELISA indicates that hCMECs secrete enhanced levels of IL-8 in the presence of Matrigel, and that cold-casting enhances IL-8 secretion in collagen gels without Matrigel. ELISA also confirms that Matrigel alone contains no significant levels of IL-8. P-values less than 0.05 were labeled with * when comparing CC vs. WC and p-values less than 0.01 and 0.001 were labeled with °° and °°° when comparing M vs. NM. b) Inhibition of IL-8 with a function-blocking antibody decreased vascular cross sectional area in Matrigel conditions. P-values less than 0.05 and 0.0001 were labeled with * and **** when comparing Control vs. Anti-IL-8. c) Confocal micrographs indicate that IL-8 inhibition completely disrupts vascular network assembly in Matrigel-containing, cold-cast hydrogels, while having a diminished effect in all other conditions. Blue is DAPI and red is phalloidin. Scale bars are 50 μm. d) Inhibition of IL-8 reduces vascular branching in Matrigel-containing, cold-cast hydrogels to similar levels as in Matrigel-containing, warm-cast hydrogels. P-values less than 0.001 were labeled with *** when comparing Control vs. Anti-IL-8.
Following investigation of matrix-mediated changes in IL-8 secretion and its role in vasculogenesis, we next tested whether vascular networks that had formed in the absence of functional IL-8 signaling differentially affected anastomosis. To exclude the independent effect of hydrogel structure on anastomosis, these experiments were performed in cold cast hydrogels that were formed in the presence and absence of Matrigel. Using the above-described experimental setup (Fig. 5a), a mCherry-labeled hCMEC monolayer was seeded on pre-vascularized and blank hydrogels with the exception that the hydrogels were treated with an IL-8 antibody during the first 11 days of culture. Inhibition of IL-8 during the pre-culture period significantly inhibited subsequent invasion and integration of adjacent endothelial cells into the preformed vascular structures regardless of the presence of Matrigel (Fig. 7a, b). These data highlight that IL-8 not only plays a role in de novo-formation of vascular networks via vasculogenesis, but that the morphological differences of the newly formed vascular networks impact subsequent angiogenic invasion and anastomosis with preexisting endothelial structures. Given that there exists a bidirectional cross-talk between IL-8 and other pro-angiogenic signaling pathways such as VEGF, NF-κB, and STAT3, our results suggest that changes in ECM remodeling modulate vascularization via altered biochemical signaling [44].
Figure 7. Inhibition of IL-8 decreases endothelial cell invasion and anastomosis.
a) Analysis of endothelial cell invasion according to Fig. 5a reveals that blockade of IL-8 during vasculogenic network assembly significantly reduces subsequent endothelial cell invasion from an adjacent endothelial monolayer; this effect was more pronounced in Matrigel-containing vs. Matrigel-free, cold-cast hydrogels. P-values less than 0.001 and 0.0001 were labeled with *** and **** when comparing Pre-vascularized, Control vs. Pre-vascularized, Anti-IL-8. b) IL-8 inhibition also diminishes integration of mCherry+ into the preformed vascular networks regardless of condition. Blue is DAPI, red is mCherry, and green is phalloidin. Scale bars are 100 μm.
4. Conclusion
Current in vitro approaches to study new blood vessel formation frequently lack the inherent structural and compositional complexity of the ECM in vivo. By varying the casting temperature and including Matrigel, we fabricated collagen I-based hydrogels of differing structure and composition. We demonstrate the individual and combined effects of these properties on both vasculogenesis and angiogenesis and suggest IL-8 as a molecular mechanism underlying ECM-induced changes of vascular assembly. A number of possible mechanisms could contribute to varying IL-8 secretion and responses in our studies. For example, IL-8 secretion has been linked to altered integrin engagement which, in turn, may differ as a function of varied hydrogel composition and structure [46] and [47]. Cellular responses to IL-8 likely also depend on altered ECM structure. More specifically, IL-8 activates phosphatidylinositol-3-kinase (PI3K), a signaling molecule that is central to ECM-induced changes of mechanotransduction [48]. As thicker collagen fibers are stiffer than their thinner counterparts and because both IL-8 secretion and PI3K activity are linked to integrin-mediated changes in mechanosignaling, it is likely that synergistic interactions between both pathways contribute to our results [49]. Future studies will need to confirm these possible links. Our results are of broad relevance to tissue engineering for reconstructive purposes, but also provide new insights into how blood vessels form during pathological tissue remodeling. For example, both wound healing and fibrotic/desmoplastic remodeling during tumorigenesis are characterized by increased deposition of fibrillar collagen I into otherwise basement membrane-rich microenvironments [50]. Furthermore, endothelial cell-secreted IL-8 has been implicated in regulating cancer stem cell behavior and glioblastoma induction/progression, highlighting a possible connection between these parameters and the ECM [43], [51], and [52]. Our results motivate future studies on how changes in the ECM affect vascularization in the presence of morphogen gradients, additional cell types (e.g. pericytes, fibroblasts, etc.), flow-induced phenomena (e.g. shear stress and cyclic perfusion), and pathological conditions. Integration of these design parameters into physiologically relevant tissue culture platforms will advance our understanding of physiological and pathological blood vessel formation.
Supplementary Material
Individual fibers were randomly selected in SEM micrographs and were manually traced for analysis of fiber diameter and fiber length. Thick, red lines represent fiber length tracings whereas thin red lines represent fiber diameter tracings.
Statement of Significance.
Neovascularization is a hallmark of both tissue homeostasis and disease and is in part regulated by cell remodelling that occurs in the extracellular matrix (ECM). The use of bio-mimetic hydrogel cell culture systems has been used to study the effects of the ECM on cell behavior. Here, we employ a hydrogel system that enables control over both the structure and composition of the ECM and subsequently investigated the effects that these have on blood vessel dynamics. Finally, we linked these differences to changes in protein secretion and the implications that this may play in scientific translation.
Acknowledgments
We thank Katharina Wittmann, Peter DelNero, and Nora Springer for proofreading our manuscript. We also acknowledge assistance from the Imaging Facilities of Cornell’s Biotechnology Resource Center (BRC; funded through NIH 1S10RR025502-01) and Cornell’s Center for Materials Research (CCMR; a Materials Research Science and Engineering Center of the National Science Foundation funded through DMR-1120296). Additional financial support was provided by the NIH/NCI (the Cornell Center on the Microenvironment & Metastasis through Award Number U54CA143876 and R01CA185293). M.G. McCoy was supported by a NSF graduate research fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chwalek K, Bray LJ, Werner C. Tissue-engineered 3D tumor angiogenesis models: Potential technologies for anti-cancer drug discovery. Adv Drug Deliv Rev. 2014;79:30–39. doi: 10.1016/j.addr.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Kusuma S, Shen YI, Hanjaya-Putra D, Mali P, Cheng L, Gerecht S. Self-organized vascular networks from human pluripotent stem cells in a synthetic matrix. Proc Natl Acad Sci U S A. 2013;110:12601–6. doi: 10.1073/pnas.1306562110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kniazeva E, Putnam AJ. Endothelial cell traction and ECM density influence both capillary morphogenesis and maintenance in 3-D. AJP Cell Physiol. 2009;297:C179–C187. doi: 10.1152/ajpcell.00018.2009. [DOI] [PubMed] [Google Scholar]
- 4.Shin Y, Jeon JS, Han S, Jung GS, Shin S, Lee SH, Sudo R, Kamm R, Chung S. In vitro 3D collective sprouting angiogenesis under orchestrated ANG-1 and VEGF gradients. Lab Chip. 2011;11:2175–2181. doi: 10.1039/c1lc20039a. [DOI] [PubMed] [Google Scholar]
- 5.Verbridge SS, Chakrabarti A, Delnero P, Kwee B, Varner JD, Stroock AD, Fischbach C. Physicochemical regulation of endothelial sprouting in a 3D microfluidic angiogenesis model. J Biomed Mater Res - Part A. 2013;101:2948–2956. doi: 10.1002/jbm.a.34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis GE, Senger DR. Endothelial extracellular matrix: Biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res. 2005;97:1093–1107. doi: 10.1161/01.RES.0000191547.64391.e3. [DOI] [PubMed] [Google Scholar]
- 7.Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19:134–148. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rao RR, Peterson AW, Ceccarelli J, Putnam AJ, Stegemann JP. Matrix composition regulates three-dimensional network formation by endothelial cells and mesenchymal stem cells in collagen/fibrin materials. Angiogenesis. 2012;15:253–264. doi: 10.1007/s10456-012-9257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yurchenco PD. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3:1–27. doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu P, Takai K, Weaver VM, Werb Z. Extracellular Matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bentley K, Mariggi G, Gerhardt H, Bates PA. Tipping the balance: Robustness of tip cell selection, migration and fusion in angiogenesis. PLoS Comput Biol. 2009;5 doi: 10.1371/journal.pcbi.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ribatti D, Crivellato E. “Sprouting angiogenesis”, a reappraisal. Dev Biol. 2012;372:157–165. doi: 10.1016/j.ydbio.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Francis ME, Uriel S, Brey EM. Endothelial cell-matrix interactions in neovascularization. Tissue Eng Part B Rev. 2008;14:19–32. doi: 10.1089/teb.2007.0115. [DOI] [PubMed] [Google Scholar]
- 14.Critser PJ, Kreger ST, Voytik-Harbin SL, Yoder MC. Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc Res. 2010;80:23–30. doi: 10.1016/j.mvr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Hinsbergh VWM, Koolwijk P. Endothelial sprouting and angiogenesis: Matrix metalloproteinases in the lead. Cardiovasc Res. 2008;78:203–212. doi: 10.1093/cvr/cvm102. [DOI] [PubMed] [Google Scholar]
- 16.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 17.Lai ES, Huang NF, Cooke JP, Fuller GG. Aligned nanofibrillar collagen regulates endothelial organization and migration. Regen Med. 2012;7:649–61. doi: 10.2217/rme.12.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo BR, Bhardwaj P, Choi S, Gonzalez J, Andresen Eguiluz RC, Wang K, Mohanan S, Morris P, Du B, Zhou XK, Vahdat LT, Verma A, Elemento O, Hudis CA, Williams RM, Gourdon D, Dannenberg AJ, Fischbach C. Obesity-dependent changes in interstitial ECM mechanics promote breast tumorigenesis. Sci Transl Med. 2015;7:301ra130–301ra130. doi: 10.1126/scitranslmed.3010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoo CP, Micklem K, Watt SM. A comparison of methods for quantifying angiogenesis in the Matrigel assay in vitro. Tissue Eng Part C Methods. 2011;17:895–906. doi: 10.1089/ten.TEC.2011.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis GE, Black SM, Bayless KJ. Capillary morphogenesis during human endothelial cell invasion of three-dimensional collagen matrices. Vitr Cell Dev Biol Anim. 2000;36:513–9. doi: 10.1290/1071-2690(2000)036<0513:CMDHEC>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Kleinman HK, Martin GR. Matrigel: Basement membrane matrix with biological activity. Semin Cancer Biol. 2005;15:378–386. doi: 10.1016/j.semcancer.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Hughes CS, Postovit LM, Lajoie GA. Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics. 2010;10:1886–1890. doi: 10.1002/pmic.200900758. [DOI] [PubMed] [Google Scholar]
- 23.Cross VL, Zheng Y, Won Choi N, Verbridge SS, Sutermaster BA, Bonassar LJ, Fischbach C, Stroock AD. Dense type I collagen matrices that support cellular remodeling and microfabrication for studies of tumor angiogenesis and vasculogenesis in vitro. Biomaterials. 2010;31:8596–8607. doi: 10.1016/j.biomaterials.2010.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dye J, Lawrence L, Linge C, Leach L, Firth J, Clark P. Distinct patterns of microvascular endothelial cell morphology are determined by extracellular matrix composition. Endothelium. 2004;11:151–67. doi: 10.1080/10623320490512093. [DOI] [PubMed] [Google Scholar]
- 25.Weksler B, Romero IA, Couraud PO. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS. 2013;10:16. doi: 10.1186/2045-8118-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verbridge SS, Choi NW, Zheng Y, Brooks DJ, Stroock AD, Fischbach C. Oxygen-controlled three-dimensional cultures to analyze tumor angiogenesis. Tissue Eng Part A. 2010;16:2133–41. doi: 10.1089/ten.TEA.2009.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raub CB, Suresh V, Krasieva T, Lyubovitsky J, Mih JD, Putnam AJ, Tromberg BJ, George SC. Noninvasive assessment of collagen gel microstructure and mechanics using multiphoton microscopy. Biophys J. 2007;92:2212–2222. doi: 10.1529/biophysj.106.097998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keech MK. the Formation of Fibrils From Collagen Solutions. J Biophys Biochem Cytol. 1961;9:193–209. doi: 10.1083/jcb.9.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silver FH, Birk DE. Molecular structure of collagen in solution: comparison of types I, II, III and V. Int J Biol Macromol. 1984;6:125–132. doi: 10.1016/0141-8130(84)90052-7. [DOI] [Google Scholar]
- 30.Birk DE, Silver FH. Collagen fibrillogenesis in vitro: comparison of types I, II, and III. Arch Biochem Biophys. 1984;235:178–185. doi: 10.1016/0003-9861(84)90266-2. [DOI] [PubMed] [Google Scholar]
- 31.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr Opin Cell Biol. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant DS, Leblond CP, Kleinman HK, Inoue S, Hassell JR. The incubation of laminin, collagen IV, and heparan sulfate proteoglycan at 35° C yields basement membrane-like structures. J Cell Biol. 1989;108:1567–1574. doi: 10.1083/jcb.108.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martin GR, Timpl R. Laminin and Other Basement Membrane Components. Ann Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- 34.Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol. 2003;200:423–8. doi: 10.1002/path.1437. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez AM, Gonzales M, Herron GS, Nagavarapu U, Hopkinson SB, Tsuruta D, Jones JCR. Complex interactions between the laminin alpha 4 subunit and integrins regulate endothelial cell behavior in vitro and angiogenesis in vivo. Proc Natl Acad Sci U S A. 2002;99:16075–16080. doi: 10.1073/pnas.252649399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehta VB, Besner GE. HB-EGF promotes angiogenesis in endothelial cells via PI3-kinase and MAPK signaling pathways. Growth Factors. 2007;25:253–263. doi: 10.1080/08977190701773070. 787646686 [pii]\n. [DOI] [PubMed] [Google Scholar]
- 37.Senger DR, Davis GE. Angiogenesis. Cold Spring Harb Perspect Biol. 2011;3:1–19. doi: 10.1101/cshperspect.a005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walshe TE, Saint-Geniez M, Maharaj ASR, Sekiyama E, Maldonado AE, D’Amore PA. TGF-β is required for vascular barrier function, endothelial survival and homeostasis of the adult microvasculature. PLoS One. 2009;4:1–16. doi: 10.1371/journal.pone.0005149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1146/annurev.physiol.49.1.453. [DOI] [PubMed] [Google Scholar]
- 40.Diaz-Santana A, Shan M, Stroock AD. Endothelial cell dynamics during anastomosis in vitro. Integr Biol (Camb) 2015;7:454–66. doi: 10.1039/c5ib00052a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stratman AN, Saunders WB, Sacharidou A, Koh W, Fisher KE, Zawieja DC, Davis MJ, Davis GE. Endothelial cell lumen and vascular guidance tunnel formation requires MT1-MMP-dependent proteolysis in 3-dimensional collagen matrices. Blood. 2009;114:237–247. doi: 10.1182/blood-2008-12-196451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fleury ME, Boardman KC, Swartz Ma. Autologous morphogen gradients by subtle interstitial flow and matrix interactions. Biophys J. 2006;91:113–21. doi: 10.1529/biophysj.105.080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Infanger DW, Cho Y, Lopez BS, Mohanan S, Liu SC, Gursel D, Boockvar JA, Fischbach C. Glioblastoma stem cells are regulated by interleukin-8 signaling in a tumoral perivascular niche. Cancer Res. 2013;73:7079–7089. doi: 10.1158/0008-5472.CAN-13-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–76. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 45.DelNero P, Lane M, Verbridge SS, Kwee B, Kermani P, Hempstead B, Stroock A, Fischbach C. 3D culture broadly regulates tumor cell hypoxia response and angiogenesis via pro-inflammatory pathways. Biomaterials. 2015;55:110–118. doi: 10.1016/j.biomaterials.2015.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischbach C, Kong HJ, Hsiong SX, Evangelista MB, Yuen W, Mooney DJ. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci U S A. 2009;106:399–404. doi: 10.1073/pnas.0808932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lowrie aG, Salter DM, Ross Ja. Latent effects of fibronectin, alpha5beta1 integrin, alphaVbeta5 integrin and the cytoskeleton regulate pancreatic carcinoma cell IL-8 secretion. Br J Cancer. 2004;91:1327–34. doi: 10.1038/sj.bjc.6602132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SFT, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix Crosslinking Forces Tumor Progression by Enhancing Integrin Signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christiansen DL, Huang EK, Silver FH. Assembly of type I collagen: Fusion of fibril subunits and the influence of fibril diameter on mechanical properties. Matrix Biol. 2000;19:409–420. doi: 10.1016/S0945-053X(00)00089-5. [DOI] [PubMed] [Google Scholar]
- 50.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, Guan JL, Dontu G, Wicha MS. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de la Iglesia N, Konopka G, Lim KL, Nutt CL, Bromberg JF, Frank DA, Mischel PS, Louis DN, Bonni A. Deregulation of a STAT3-interleukin 8 signaling pathway promotes human glioblastoma cell proliferation and invasiveness. J Neurosci. 2008;28:5870–8. doi: 10.1523/JNEUROSCI.5385-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Individual fibers were randomly selected in SEM micrographs and were manually traced for analysis of fiber diameter and fiber length. Thick, red lines represent fiber length tracings whereas thin red lines represent fiber diameter tracings.