Abstract
Introduction
Glycemic control is important in diabetes mellitus to minimize the progression of the disease and the risk of potentially devastating complications. Inhibition of the sodium–glucose cotransporter SGLT2 induces glucosuria and has been established as a new anti-hyperglycemic strategy. SGLT1 plays a distinct and complementing role to SGLT2 in glucose homeostasis and, therefore, SGLT1 inhibition may also have therapeutic potential.
Areas covered
This review focuses on the physiology of SGLT1 in the small intestine and kidney and its pathophysiological role in diabetes. The therapeutic potential of SGLT1 inhibition, alone as well as in combination with SGLT2 inhibition, for anti-hyperglycemic therapy are discussed. Additionally, this review considers the effects on other SGLT1-expressing organs like the heart.
Expert opinion
SGLT1 inhibition improves glucose homeostasis by reducing dietary glucose absorption in the intestine and by increasing the release of gastrointestinal incretins like glucagon-like peptide-1. SGLT1 inhibition has a small glucosuric effect in the normal kidney and this effect is increased in diabetes and during inhibition of SGLT2, which deliver more glucose to SGLT1 in late proximal tubule. In short-term studies, inhibition of SGLT1 and combined SGLT1/SGLT2 inhibition appeared to be safe. More data is needed on long-term safety and cardiovascular consequences of SGLT1 inhibition.
Keywords: Diabetes mellitus, hyperglycemia, sodium glucose cotransporter, glucose transport, kidney, intestine, heart, LX4211, GSK-1614235
1. Introduction
The cellular uptake and metabolism of D-glucose serves as an important energy source in many organisms including human [1, 2]. As a consequence, its homeostasis is finely regulated by various hormones, including insulin and glucagon. These hormones affect glucose uptake into target cells (e.g. skeletal muscle) and, by regulating glucose storage and endogenous glucose production, maintain blood glucose in a range of 4-9 mmol/L [1, 2]. The latter is particularly critical for organs that primarily run on glucose and depend on its continuous uptake like the brain, which alone requires ~125 grams of glucose every day. Two main classes of glucose transporters have been identified that mediate glucose transport into and out of cells: the sodium-dependent glucose cotransporters (SGLTs) and the facilitative glucose transporters (GLUTs).
GLUT proteins are encoded by the SLC2A gene family which codifies 12 GLUT proteins and the HMIT1 protein in human. Glucose flux through these transporters depends on the transmembrane glucose gradient. Key examples include GLUT1-mediated glucose transport across the endothelial cells of the blood–brain barrier, GLUT2-mediated glucose exit into the interstitium/blood compartment from small intestinal epithelia, renal tubular epithelia, and hepatocytes, and GLUT4-mediated and insulin-stimulated glucose uptake into skeletal muscle [3, 4].
The SGLT family is the subgroup SL5 of the solute carriers groups, SLC5, which currently includes six members containing transporters and a channel that vary in their preferences for sugar binding [5]. The glucose transporters of the SGLT family use the electrochemical gradient of sodium to transport sugar molecules against a chemical gradient into cells. Among them, SGLT1 and SGLT2, encoded by the genes SLC5A1 and SLC5A2, respectively, are the best studied members. These two transporters are of primary importance for glucose homeostasis by absorbing glucose from the diet in the small intestine (via SGLT1) and by reabsorbing the filtered glucose in the tubular system of the kidney (primarily SGLT2; to smaller extent via SGLT1); the latter process returns glucose into the blood stream and prevents urinary glucose loss [5].
Diabetes mellitus is a growing public health and economic problem worldwide. Good blood glucose control is vital in diabetic patients to attenuate the progression of the underlying metabolic dysfunction and to reduce the risk of complications including nephropathy and cardiovascular disease [6]. In recent years, SGLTs have drawn much attention due to their therapeutic potential in diabetes mellitus. Most of the attention has focused on the SGLT2 subtype, which is expressed primarily in early renal proximal tubules, where it is responsible for >90% of renal reabsorption of the filtered 160-180 grams of glucose/day [5, 7, 8]. Diabetes increases the renal SGLT2 expression and the glucose transport maximum (from ~500 g/day to 600 g/day), thereby further enhancing renal glucose reabsorption [5, 7, 8]. SGLT2 inhibitors improve glycemic control by increasing urinary glucose excretion as shown in experimental models and patients with hyperglycemia, and multiple compounds have recently been approved for use in patients with type 2 diabetes mellitus [7-11]. SGLT2 inhibitors work independent of insulin and have little hypoglycemia risk. The compounds induce a modest diuresis/natriuresis, urinary calorie loss, and activation of lipolysis, which are associated with a modest reduction in body fat, body weight, and blood pressure [12]. Ongoing long-term clinical trials explore the safety and the clinical efficacy of SGLT2 inhibitors in the prevention of diabetic renal and cardiovascular complications [7-9, 11, 13]. The first study recently reported beneficial effects of SGLT2 inhibition on cardiovascular mortality in T2DM [14] and preliminary data using the same compound indicated a nephroprotective potential (ASN meeting 2015, late breaking trials). Thus, SGLT2 inhibitors are a prime example how inhibition of a glucose transporter can have beneficial therapeutic effects in hyperglycemia and diabetes mellitus.
Whereas glucose reabsorption in the kidney is predominantly mediated by SGLT2, it is SGLT1 that is of primary importance for glucose absorption from the lumen into the epithelial cells of the small intestine [15], an organ that does not express SGLT2. In addition, SGLT1 is expressed in the late renal proximal tubules, where it reabsorbs the glucose that has escaped the upstream SGLT2. As a consequence, SGLT1-mediated glucose reabsorption in the kidney is increased when the tubular glucose load overwhelms the capacity of SGLT2 or when the latter is inhibited, as observed in hyperglycemia and with the use of SGLT2 inhibitors or Sglt2 gene knockout [16-18], respectively. The role of SGLT1 in intestinal and renal glucose transport makes the transporter a potential target for anti-hyperglycemic therapy. Relevant for the safety aspect of such an approach is the question to which extent such drugs induce unwanted side effects like diarrhea and/or by inhibiting the SGLT1 that is expressed beyond the intestine and kidney. In this review we summarize recent developments and our current understanding of the physiology and pathophysiology of SGLT1 with a focus on the kidney, the small intestine, and the heart. We discuss the potential of SGLT1 inhibition as a new therapeutic strategy, alone or in combination with SGLT2 inhibition, to treat hyperglycemia and diabetes mellitus.
2. Molecular nature of SGLT1
Studies on the molecular understanding of SGLTs have been largely pioneered by the laboratory of Wright and colleagues (for review see [5]). In the early 1980s, Semenza and Wright described the existence of phlorizin inhibitable sodium glucose cotransporter as a 73 kDa protein through a series of experiments using azido-phlorizin-photoaffinity labeling and antibodies [19, 20]. In 1987 Wright and coworkers cloned SGLT1 from rabbit [21]. Human SGLT1, encoded by the SLC5A1 gene on chromosome 22q13.1, is a 664-amino-acid protein, which contains 14 transmembrane α-helical domains [5, 22]. The glucose-binding domain of human SGLT1 is supposed to include amino acid residues 457-460 [23]. SGLT1 has a high-affinity for glucose (K0.5 of 0.5-2 mM) and transports sodium and glucose with a 2:1 stoichiometry which enhances its concentrating power [5, 23-25]. The affinity of SGLT1 is similar for glucose and galactose, whereas fructose is not transported [5]. In comparison, SGLT2 does not transport galactose, the affinity of SGLT2 for glucose is somewhat lower (K0.5 of 2-5 mM for glucose), and transport occurs with a 1:1 stoichiometry [5, 24, 25].
3. Localization of SGLT1 expression
The tissue-specific expression of SGLT1 is important for potential therapeutic effects of inhibitors but can also induce unwanted side effects by interfering with SGLT1 in these tissues. SGLT1 mRNA expression has been detected by RT-PCR in various tissues in humans including the small intestine, kidney, skeletal muscle, liver, lung, heart, trachea, prostate, cervix of the uterus, stomach, mesenteric adipose tissue, pancreatic alpha cells, and brain [26-29] (see Table 1). In two publications SGLT1 mRNA could not be detected in human brain [26, 28].
Table 1.
SGLT1 expression in human and animal tissue
| Species | Localization | Method |
|---|---|---|
| Human | Small intestine | RT-PCR [26, 28, 162]; Western Blotting [28, 163]; Immunostaining [28, 126]; Northern blot [164] |
| Kidney | RT-PCR [26, 28, 127]; Western Blotting [28]; Immunostaining [28, 126] | |
| Heart | RT-PCR [26, 125, 127]; Western Blotting [28, 125, 126]; Immunostaining [28, 126] | |
| Pancreatic alpha cells | RT-PCR [27], Western Blotting [27], Immunostaining [27] | |
| Liver | RT-PCR [28]; Western Blotting [28]; Immunostaining [28] | |
| Lung | RT-PCR [28]; Western Blotting [28]; Immunostaining [28] | |
| Skeletal muscle | RT-PCR [26] | |
| Trachea | RT-PCR [26] | |
| Prostate | RT-PCR [26] | |
| Cervix | RT-PCR [26] | |
| Mesenteric adipose tissue | RT-PCR [26] | |
| Brain | RT-PCR [29] | |
| Mouse | Small intestine | RT-PCR [162, 165]; Immunostaining [15] |
| Kidney | RT-PCR [15, 107, 113, 166]; Western Blotting [107, 113]; Immunostaining [15] | |
| Heart | Western Blotting [125, 126]; RT-PCR [125]; Immunostaining [125] | |
| Rat | Small intestine | RT-PCR [162]; High stringency Northern analysis [31]; Northern blot [164], Immunostaining [36, 41, 167]; Western Blotting [36] |
| Kidney | RT-PCR [36, 168]; High stringency Northern analysis [31]; Western Blotting [36, 168, 169]; Immunostaining [36, 42, 167, 168, 170] | |
| Liver | RT-PCR [171]; High stringency Northern analysis [31, 36]; Western Blotting [171] | |
| Lung | RT-PCR [172]; High stringency Northern analysis [31]; Western Blotting [173]; Immunostaining [173] | |
| Brain | RT-PCR [33]; Immunostaining [32-34, 36, 37] | |
| Salivary glands | Immunostaining [30, 36] | |
| Capillaries of heart and skeletal muscle | Immunostaining [128] | |
| Rabbit | Small intestine | Situ hybridization [39]; Northern blot [21, 164]; Western Blotting [40, 163]; Immunostaining [39] |
| Kidney | Northern blot[163, 164]; Western blot [163] | |
| Brain | RT-PCR [29], Situ hybridization [29, 40] | |
| Heart | Northern blot [21] | |
| Pleura | Western Blotting [174] | |
| Pig | Brain | RT-PCR[29]; Situ hybridization [29]; Western Blotting [29]; Immunostaining [29, 37] |
| Kidney | Western Blotting [169] |
On the protein level, human SGLT1 expression has been localized to the apical brush border of the small intestine and the late proximal tubule; SGLT1 protein was also detected in human parotid and submandibular salivary glands, liver, lung, skeletal muscle, heart and pancreatic alpha cells [21, 27, 30, 31]. More recently, Vrhovac and colleagues generated and used a novel affinity-purified antibody against human SGLT1 and confirmed the protein expression in the brush border membrane and subapical vesicles of the small intestine, in brush border membrane of late proximal tubule S3 segments, and also localized SGLT1 in biliary duct cells of the liver, and in alveolar epithelial type 2 cells and bronchiolar Clara cells of the lung. SGLT1 protein expression was detected in capillaries of the human heart rather than in myocyte sarcolemma (see below for further discussion) [28].
It is worth noting that brain expression of SGLT1 has been proposed in other species, like rats, mice, and pigs, using in situ hybridization and immunological and RT-PCR techniques [29, 32-37]. Expression of SGLT1 in neurons was described consistently [29, 32-37]. SGLT1 expression in blood vessels was described in rat brain following brain ischemia and in isolated microvessels of pig brain [37]. Using a SGLT-specific molecular imaging probe, alpha-methyl-4-deoxy-4-[(18)F]fluoro-D-glucopyranoside (Me-4-FDG), together with ex vivo autoradiography and immunohistochemistry in rats, Yu and colleagues localized SGLT activity in various brain regions that also expressed SGLT1, and proposed a potential role of SGLT-mediated cellular glucose uptake in rat brain when extracellular concentrations are low, such as in ischemia [34].
4. Role of intestinal SGLT1 in glucose homeostasis
4.1 SGLT1 mediates intestinal glucose absorption
According to data from the National Health and Nutrition Examination Survey, 2005–2010, men consumed a daily average of 83 grams from added sugars compared with 60 grams for women [38]. In the small intestine, SGLT1 is expressed in the apical cell membrane constituting the brush border [28, 31, 39-42], and is responsible for the transport of glucose and galactose from the lumen into the epithelial cells; whereas facilitative glucose transporters, especially GLUT2, subsequently mediate glucose transport across the basolateral membrane into the interstitium and thereby into the circulation [15, 43-45](see Figure 1). SGLT1 is electrogenic and luminal K+ secretion serves to stabilize the membrane potential and thereby the driving force for SGLT1 [46]. Wright and colleagues first described that genetic defects in SGLT1 were associated with intestinal malabsorption of glucose and galactose in humans [47, 48]. The phenotype includes neonatal onset of osmotic diarrhea caused by the non-absorbed excess of glucose and galactose in the intestinal lumen, leading to dehydration, metabolic acidosis, and death [47, 48]. Those symptoms have also been observed in mice lacking Sglt1 [15], consistent with the primary importance of SGLT1 in the absorption of glucose and galactose in the intestinal tract. Importantly and somewhat unexpectedly, pharmacological inhibition of SGLT1 appears not to induce severe diarrhea [49, 50], as discussed below.
Figure 1. SGLT1 inhibition reduces intestinal glucose absorption and enhances the sustained GLP-1 release from the distal intestine.
In the small intestine, SGLT1 mediates glucose and galactose absorption across the apical cell membrane. Membrane SGLT1 expression is upregulated i) by intracellular glucose which blocks the inhibitory effect of RS1 and ii) by luminal nutrients/glucose that activate luminal taste sensors. This adapts glucose absorption to dietary glucose intake. SGLT1 expression is regulated by multiple signaling cascades and is upregulated in diabetes. SGLT1 in L cells in proximal intestine sense dietary glucose, which subsequently triggers the “acute” release of GLP-1. SGLT1 inhibition in early intestine reduces glucose absorption and thereby increases the glucose delivery to the more distal gut, where glucose is used by the microbiome to form short-chain fatty acids (SCFAs) which enter the distal L-cells through FFAR2 and FFAR3 and trigger a “sustained” release in GLP-1. SGLT1: sodium glucose cotransporter 1; GLP-1: glucagon- like peptide 1; SCFAs: short-chain fatty acids; FFAR2: G-protein-coupled free fatty acid receptor 2; FFAR3: G-protein-coupled free fatty acid receptor 3; GLUT2: glucose transporter 2. T1R3: sweet taste receptor subunit 3;a-gustducin: a-transducin-like Gprotein a-subunit; PKA: protein kinase A; AMPK: AMP-activated protein kinase; SGK1: serum and glucocorticoid inducible kinase 1; SGK3: serum and glucocorticoid inducible kinase 3;PKC: protein kinase C; RS1: gene product of RSC1A1 that blocks release of SGLT1 containing vesicles from the Golgi at low intracellular glucose concentrations; SPAK: ‘with-no-K[Lys] kinases’/STE20/SPS1-related proline/alanine-rich kinase.
4.2 Direct and indirect effects of SGLT1 on intestinal incretin secretion
Intestinal glucose uptake via SGLT1 modulates the secretion of intestinal hormones that regulate glucose homeostasis. In addition to protein and fat, the ingestion of glucose induces the secretion of the incretins, glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic peptide (GIP), from intestinal L cells and K cells, respectively [51, 52]. These hormones then act on pancreatic β cells to increase insulin release in a glucose-dependent manner; GLP-1 also decreases pancreatic glucagon secretion and appetite and was found to increase β cell mass in preclinical studies; all these effects contribute to the anti-hyperglycemic effect of GLP-1 receptor agonist, which are used as antidiabetic drugs [53]. Oral administration of glucose to mice induced incretin secretion within 5 min, followed by the secretion of insulin after 15 min [54]. Studies using the mixed SGLT1/SGLT2 inhibitors, phlorizin or LX4211 as well as studies in Sglt1−/− mice indicated that SGLT1 in the L and K cells of the “proximal” small intestine serves as the intestinal glucose sensor for the acute glucose-induced incretin secretion [15, 44, 54, 55]. In addition, alpha-gustducin-coupled T1R3 taste receptors in the luminal membrane of duodenal L-cells have been implicated in glucose-induced secretion of GLP-1 [56] (Figure 1).
Notably, GLP-1 secretion after a meal is biphasic and the second more sustained phase appears to involve the L-cells in the more “distal” gut and colon, where GLP-1 secretion is insensitive to glucose itself [54]. Studies with knockout or inhibition of SGLT1 or dual SGLT1/SGLT2 inhibition provided evidence in rodents and healthy humans that inhibition of SGLT1 in the small intestine increases the delivery of glucose to the more distal gut and colon where the maneuver induces a sustained increase in circulating GLP-1 for up to 6 hours [49, 50, 55, 57]. It has been proposed that bacterial fermentation of luminal glucose to short-chain-fatty-acids (SCFAs), which have been shown to trigger GLP-1 secretion by L-cells through G-protein coupled receptors in mice [58], mediates this indirect effect of SGLT1 inhibition [55](Figure 1). Thus, intestinal SGLT1 inhibition can improve glucose homeostasis through inhibiting intestinal glucose uptake as well as inducing a sustained intestinal release of incretins.
5. Luminal nutrients upregulate intestinal SGLT1 expression
The level of SGLT1 expression in the small intestine determines the capacity for glucose absorption, shows a circadian periodicity, and undergoes long-term and short-term regulation in response to luminal nutrients [15, 59-62]. For example, a high glucose diet and high sodium diet can increase the expression of intestinal SGLT1 on the transcriptional level [60, 61, 63]. The sweet taste receptor subunit T1R3 and the taste G protein gustducin, expressed in enteroendocrine L-cells, are involved in intestinal sensing of dietary sugar and artificial sweeteners which subsequently up-regulates intestinal SGLT1 mRNA and protein expression [64, 65]. Short-term upregulation of SGLT1 in the brush-border membrane within minutes after glucose ingestion includes the activation and release of SGLT1 containing vesicles from the trans-Golgi network (TGN) [66-70]. The release of SGLT1 containing vesicles from the TGN is steered in a glucose-dependent manner by a highly phosphorylated regulatory domain of the intracellular protein RS1 (gene RSc1A1) [70]. An increase in luminal glucose concentrations can also induce GLUT2 translocation to the brush border membrane, however, the quantitative contribution to glucose absorption remains a matter of debate [15, 44, 71-73].
A better understanding of SGLT1 regulation may provide opportunities for modulation beyond transporter inhibition and is therefore discussed in the following. Whereas studies in heterologous expressions systems and rodent models provided insights on the molecular regulation of SGLT1, the in vivo relevance and the detailed mechanisms in which identified intracellular signaling cascades are involved remains to be fully defined.
5.1 Factors that are involved in upregulation of SGLT1
Short-term regulation has been elucidated in oocytes in which SGLT1 was expressed by cRNA injection and in cell culture where the amount of SGLT2 mRNA has been changed. Long-term regulation has been determined in tissue. Wright and coworkers identified and characterized the human SGLT1 minimal promoter and showed a critical role of hepatocyte nuclear factor-1 (HNF-1) and the Sp1-family of transcription factors in enhancing the basal level of SGLT1 transcription in Caco-2 cells [74]. Studies in Xenopus oocytes and Chinese hamster ovary cells expressing rabbit or human SGLT1 showed that activation of protein kinase A (PKA) increased SGLT1 membrane expression, and the authors hypothesized associated changes in the conformation of the empty carrier and the glucose carrier complex [75, 76]. Studies in Madin-Darby canine kidney (MDCK) cells confirmed that SGLT1 protein is up-regulated by a cAMP-PKA dependent pathway, a response that required intact actin filaments, suggesting involvement of vesicle trafficking, probably the exocytotic pathway [77]. The in vivo relevance of PKA-regulated SGLT1 regulation remains to be determined. Studies with SGLT1 expression in Xenopus oocytes and in Caco2 cells revealed that the plasma membrane protein caveolin-1 can also enhance SGLT1 activity by increasing carrier protein abundance in the cell membrane [78]. Studies using a similar approach indicated that activation of AMP-activated protein kinase (AMPK) increases SGLT1 membrane expression and activity [79]. AMPK has been implicated in the upregulation of intestinal glucose absorption in piglets in response to feeding long-chain n-3 polyunsaturated fatty acids, which was associated with increased GLUT2 and SGLT1 expression, indicating long-term regulation involving changes in transcription [80]. In contrast, studies in murine jejunum indicated that AMPK activation (by AICAR and the anti-diabetic drug metformin) may up-regulate the luminal membrane expression of non-energy requiring glucose uptake by GLUT2 and induce a concurrent down-regulation of sodium-dependent SGLT1, which may reflect the role of AMPK as a key sensor of energy status within the cell [81].
Xenopus oocytes studies showed that SGLT1-mediated glucose transport is up-regulated by the serum and glucocorticoid-inducible kinase 1 (SGK1), and that this interaction involves the ubiquitin ligase Nedd4-2 [82] as well as the phosphatidylinositol-3-phosphate-5-kinase PIKfyve [83]. Electrogenic glucose transport in the intestine was not significantly different between Sgk1−/− mice and their wild-type littermates under basal conditions but was significantly stimulated by the glucocorticoid dexamethasone only in the latter [84]. Thus, SGK1 seems dispensable with regard to basal intestinal glucose absorption but can mediate a glucocorticoid-induced stimulation. Excessive extracellular glucose concentrations can stimulate the transcription of SGK1 [85] and mutations in SGK1 have been associated with obesity, which may relate to long-term regulation of intestinal SGLT1 activity [82]. Notably, SGLT1 was also found to be upregulated by the SGK3 isoform in Xenopus oocytes [82]. Moreover, studies in Sgk3−/− mice revealed convincing evidence for a physiological, long-term role of SGK3 in maintaining SGLT1-mediated intestinal glucose absorption, whereas intestinal electrogenic transport of phenylalanine, cysteine, glutamine and proline were not affected in Sgk3−/− mice [86] (Figure 1).
5.2 Factors that are involved in downregulation of SGLT1
Studies in heterologous expression systems indicated that protein kinase C (PKC) is involved in posttranscriptional short term regulation of SGLT1 comprising effects on membrane trafficking and on transport function [67, 76, 87, 88]. In Xenopus oocytes, expressed human SGLT1 was upregulated posttranscriptionally, whereas expressed rabbit and rat SGLT1 were downregulated by stimulation of PKC [67, 76]. The short term effects of PKC may be influenced by the coexpression of other proteins. For example, whereas stimulation of PKC induced short term upregulation of human SGLT1-mediated glucose uptake in oocytes it induced short term downregulation after coexpression of the regulatory protein RS1 which only occurs in mammals [67]. RS1 which is located at the trans-Golgi network (TGN) [68] binds to a receptor at the TGN containing a glucose binding site that blocks the release of SGLT1 containing vesicles from the TGN at low intracellular glucose concentrations [70]. At high intracellular glucose concentrations the RS1-induced blockade of vesicle release is blunted and, as a consequence, vesicle translocation to the plasma membrane is increased leading to a 40-50% increase of SGLT1 expression in the plasma membrane. In RS1 knockout mice the amount of SGLT1 in the plasma membrane is increased at low intracellular glucose and no further increase is observed at high intracellular glucose [70, 89]. Thus, upregulation of SGLT1 expression in small intestine after glucose ingestion is promoted by glucose dependent disinhibition of the RS1-mediated blockade of SGLT1 exocytosis (Figure 1).
RS1 is also involved in glucose dependent down-regulation of SGLT1 transcription. Employing the porcine renal epithelial cell line LLC-PK1 expressing SGLT1 and RS1 it was observed that transcriptional downregulation of SGLT1 after cultivation with 25 mM glucose versus 5 mM glucose was dependent on the presence of RS1 [90]. This effect of RS1 requires migration of RS1 into the nucleus which is steered by PKC dependent phosphorylation of a nuclear shuttling domain in RS1 [91].
Another powerful negative regulator of SGLT1 protein abundance in the cell membrane that decreased SGLT1-mediated glucose transport in Xenopus oocytes and mouse intestine is ‘with-no-K[Lys] kinases’/STE20/SPS1-related proline/alanine-rich kinase (SPAK)[92]. Leptin, an adipocyte-derived “satiety hormone”, has also been implicated in the regulation of intestinal glucose absorption [93], and leptin administration to rats, especially from the lumen, rapidly reduced luminal SGLT1 expression and glucose uptake [94](Figure 1).
The KATP channel has also been implicated in SGLT1 regulation, inasmuch as SGLT1 mRNA was increased in the duodenum (but not the jejunum) of mice lacking this channel, which was associated with increased glucose absorption [95]. The KATP channel, however, was not critical for the increase in duodenal SGLT1 mRNA expression in response to high-sucrose diet for 5 weeks or streptozotocin (STZ)-induced diabetes mellitus [95](see below).
6. Upregulation of intestinal SGLT1 in diabetes mellitus
Diabetes has been proposed to increase the intestinal SGLT1 expression (Figure 1, Table 2), which may involve the response to greater dietary sugar intake (see above). Intestinal SGLT1 mRNA expression was increased in mice [95] and rats [43] with STZ-induced diabetes, a model of hyperphagic type I diabetes (T1DM). Similarly, Otsuka Long-Evans Tokushima Fatty rats, a model of type II diabetes (T2DM), presented increased intestinal mRNA expression of SGLT1; this was associated with impaired glucose tolerance and occurred before the development of insulin resistance and hyperinsulinemia [96]. Most importantly, small intestinal SGLT1 mRNA expression and brush border membrane SGLT1 protein expression and intestinal glucose uptake were also found to be increased in patients with noninsulin-dependent diabetes mellitus [97]. Notably, the latter patients were on a carbohydrate restricted diet compared with the non-diabetic controls indicating that factors other than increased luminal glucose delivery contributed to increased SGLT1 expression. The upregulation of SGLT1-mediated intestinal glucose uptake is expected to contribute to the rapid post-prandial rise in blood glucose levels observed in diabetes [49, 55]. High-carbohydrate diets (55–60% of energy) had been standard recommendations for management of T2DM for a long time but this has been questioned more recently, in particular in relation to protein intake [98]. Along these lines, we observed in preliminary studies that the application of an equicaloric glucose free/high protein diet significantly reduced hyperglycemia in Akita mice, a genetic T1DM model (Panai, Onishi, Fu, Vallon, unpublished observation), consistent with a prominent role of intestinal glucose uptake for hyperglycemia.
Table 2.
Diabetes-induced changes of SGLT1 expression in human and rodents
| Species | Localization | Method/change in diabetes |
|---|---|---|
| Human | Small intestine | RT-PCR [97] / up |
| Western Blotting [97] / up | ||
| Heart | RT-PCR [125] / up | |
| Mouse | Duodenum | RT-PCR [95] / up |
| Kidney | RT-PCR [113] / down | |
| Western Blotting [113] / up; [107] / down | ||
| Rat | Small intestine | RT-PCR [43] / up |
| Kidney | RT-PCR [110, 112] / up | |
| Western Blotting [111] / up; [114-116] / unchanged | ||
| Brain (ventromedial hypothalamus) | RT-PCR [33] / unchanged |
A major defect in T2DM is a reduction of the insulinotropic response to GLP-1 and GIP in the pancreas, which can be overcome by higher doses of GLP-1 [99]. In addition, the sustained post-prandial secretion of GLP-1 can be reduced in T2DM which could be secondary to the increased SGLT1 expression and activity in the proximal intestine thereby limiting glucose delivery to the more distal intestine (see above). Effects of SGLT1 inhibition on intestinal glucose absorption and secondary effects on incretin release have therapeutic potential in diabetes mellitus, as discussed below.
7. Physiology of SGLT1 in the kidney – unmasking its capacity by SGLT2 inhibition
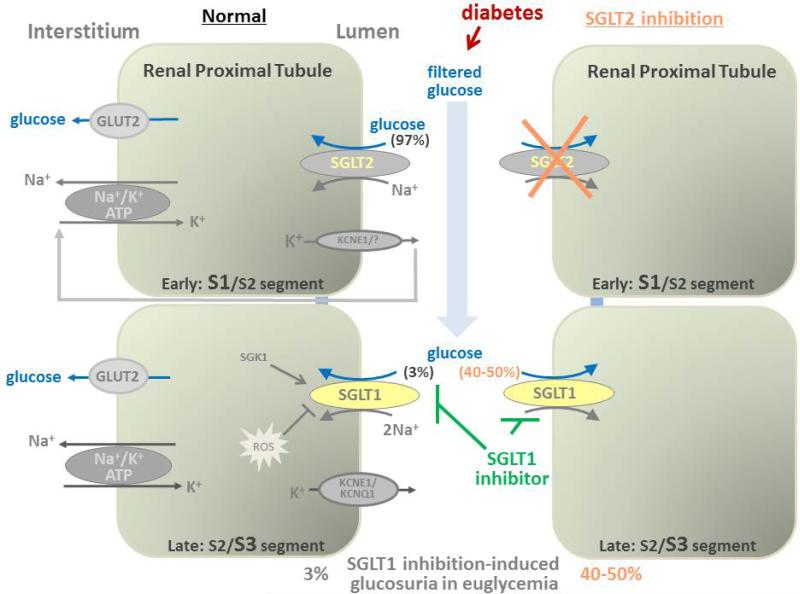
Under euglycemic conditions, the normal kidneys filter about 180 grams of glucose daily, which reflects about 30% of the daily calorie intake. The filtered glucose is subsequently reabsorbed by the proximal tubule and mostly returned to the circulation, consistent with the finding that proximal tubular cells are not utilizing glucose in relevant amounts for energy production [100]. Glucose is transported across the apical membrane of the proximal tubule by SGLT2 and SGLT1 and then exits through the basolateral membrane by the facilitative glucose transporters GLUT2 and GLUT1 [5, 101]. As demonstrated in mice, rats, and humans, SGLT2 is expressed in the early part of the proximal tubules (S1/S2 segments), whereas SGLT1 is expressed in the more distal part (S2/S3 segments) of the proximal tubules [17, 28, 36](Figure 2). SGLT2 is a high-capacity system that reabsorbs 95% or more of the filtered glucose in euglycemia, while the further downstream expressed SGLT1 transporter reabsorbs practically all of the remaining glucose or about 3% of the filtered glucose [15-18]. Whereas SGLT2 transports sodium and glucose in a 1:1 ratio, two sodium ions accompany one glucose molecule when transported through SGLT1, which enhances the concentrating power to reabsorb the small amounts of glucose delivered to the late proximal tubule [25]. Both cotransporters are electrogenic and luminal K+ secretion serves to stabilize the membrane potential and thereby the driving force for SGLTs [46, 102]. Importantly, when pharmacological SGLT2 inhibition enhanced the glucose load to the downstream late proximal tubule in mice, then the glucose transport capacity of renal SGLT1 was unmasked: this was evident by a doubling in maximal glucosuria in response to pharmacological SGLT2 inhibition in mice lacking SGLT1 versus wild-type mice, as well as a reduction in fractional renal glucose reabsorption in response to pharmacological SGLT2 inhibition from 50–60% in wild-type mice to zero net renal glucose reabsorption when SGLT2 was inhibited in mice lacking SGLT1; the latter phenotype was confirmed in mice lacking both SGLT2 and SGLT1 [16](Figure 2). This is consistent with observations in humans which also maintain fractional glucose reabsorption rates of ~40–50% following maximal effective doses of SGLT2 inhibitors [103-105]. A compensatory effect of SGLT1 is also supported by micropuncture studies along the late proximal tubule in Sglt2−/− mice [17], by data in Sglt1/Sglt2 double knockout mice [16, 18], and by studies using a potent dual SGLT2/SGLT1 inhibitor [106]. Based on renal glucose reabsorption studies in gene-targeted mice and considering the levels of renal SGLT2 and SGLT1 protein expression in these settings (e.g. ~50% down-regulation of SGLT1 expression in response to pharmacologic or genetic SGLT2 inhibition)[15-17, 107], we estimated that the basal overall capacities of the kidneys for glucose reabsorption in the non-diabetic setting for SGLT2 versus SGLT1 is in the range of 3:1 to 5:1 [8].
Figure 2. SGLT1 inhibition enhances glucosuria, particularly when upstream SGLT2 is overwhelmed by hyperglycemia or when SGLT2 is inhibited.
SGLT2 and SGLT1 are expressed in the luminal membrane of the early and late proximal tubule, respectively. They reabsorb ~97% and ~3% of filtered glucose in normoglycemia, respectively. Both cotransporters are electrogenic and luminal K+ secretion serves to stabilize the membrane potential and thereby the driving force for SGLTs. A significant capacity of SGLT1 to reabsorb glucose is unmasked by SGLT2 inhibition and during hyperglycemia, which both enhance glucose delivery to late proximal tubule. As a consequence, diabetes-induced hyperglycemia or SGLT2 inhibition increase the SGLT1-inhibition induced rise in glucose excretion, the latter providing the renal rationale for dual SGLT1/SGLT2 inhibition. Serum and glucocorticoid inducible kinase SGK1 enhance glucose reabsorption in the kidney, whereas the induction of oxidative stress (ROS) can reduce glucose reabsorption. SGLT1: sodium glucose cotransporter 1; SGLT2: sodium glucose cotransporter 2; SGK1: Serum and glucocorticoid inducible kinase 1; ROS: reactive oxygen species; GLUT2: glucose transporter 2.
8. Diabetes increases renal SGLT1-mediated glucose reabsorption
Poorly controlled hyperglycemia can overwhelm the transport capacity of early proximal SGLT2 (despite of its upregulation) thereby enhancing the glucose delivery to the late proximal tubule and increasing SGLT1-mediated glucose reabsorption (Figure 2). The cellular susceptibility to glucose-induced toxicity has been proposed to depend on the presence of cellular glucose uptake mechanisms and the ability of these cells to down-regulate glucose uptake in the setting of hyperglycemia [108]. Multiple studies in diabetic rodents, including genetic models of T1DM and T2DM, reported an increased renal protein expression of SGLT2, which is expected to contribute to the enhanced renal glucose reabsorption capacity and hyperglycemia in diabetes [7, 109]. In comparison, the reported data for SGLT1 appear more variable with evidence for increased, unchanged, or reduced renal SGLT1 expression in diabetes or under high glucose conditions [109](Table 2). The renal cortex of STZ-diabetic rats was found to contain increased mRNA expression for SGLT1 [110] and greater renal SGLT1 protein expression [111]. Likewise, renal SGLT1 mRNA levels in diabetic obese Zucker rats were higher than in age-matched non-diabetic lean rats [112]. BTBR ob/ob T2DM mice have been reported to have lower SGLT1 mRNA expression but increased renal membrane SGLT1 protein expression [113]. A dissociation of the response in SGLT1 mRNA and protein expression indicates a complex translational and post-translation regulation [107].
Other studies found unchanged renal SGLT1 protein expression [114-116] or reduced renal expression and activity of SGLT1 [117] in diabetic rodent models. Using Sglt1−/− mice as a critical negative control for the antibody, renal membrane protein expression of SGLT1 was found to be reduced in Akita mice, a genetic model of T1DM [107]. This response mimicked the reduced renal SGLT1 protein expression observed in response to Sglt2 gene knockout in mice [17] and in response to pharmacological SGLT2 inhibition in non-diabetic mice [107]. All 3 conditions share an increase in the glucose load to the late proximal tubule and as a consequence an increase in glucose reabsorption via SGLT1; albeit the increase in reabsorption is attenuated by the down-regulation of SGLT1 expression. Thus, down-regulation in renal SGLT1 expression may serve to limit renal glucose uptake and toxicity in the late proximal tubule, which is localized in the semi-hypoxic and vulnerable outer medulla, when glucose delivery and uptake are increased [17, 107].
As discussed above, SGLT2 and SGLT1 can account for all renal glucose reabsorption in normoglycemic mice [16]. It is unknown whether this is also true in the diabetic kidney. Studies in diabetic rats suggested translocation of GLUT2 to the luminal brush border of the proximal tubule associated with an increase in facilitative glucose absorption [118, 119]. Further studies are needed to define the relative relevance of GLUT translocation in the diabetic kidney.
The molecular mechanisms that regulate renal SGLT1 remain poorly understood. In vitro studies by Ghezzi and Wright reported that insulin markedly increased human SGLT2 activity, but human SGLT1 was relatively insensitive to insulin [120]. SGK1 has been proposed to be upregulated in the diabetic kidney in rodents and humans [121, 122]. Studies in T1DM Akita mice lacking SGK1 indicated that the kinase may stimulate electrogenic glucose transport in the diabetic proximal tubule [122]. On the other hand, in vitro studies in rabbit renal proximal tubule cells indicated that high glucose-induced oxidative stress can induce a reduction in SGLT2 and SGLT1 expression and activity [123]. In the porcine renal epithelial cell line LLC-PK1 transcriptional downregulation of SGLT1 occurred after cultivation with 25 mM glucose versus 5 mM glucose; this was dependent on the presence of RS1 [90]. Furthermore, diabetes has been shown to upregulate various PKC isoforms in the kidney; whether these can mediate an inhibitory influence on SGLT1 (as indicated in intestine, see above) has not been tested.
Thus, diabetes increases the glucose delivery to the late proximal tubule and thereby the reabsorption through SGLT1. SGLT2 inhibition will improve blood glucose control in diabetes but enhance the glucose delivery to SGLT1 and thereby increase its glucose reabsorption. The quantitative role of renal SGLT1 in diabetes-induced hyperglycemia and in renal injury has not yet been specifically determined.
9. SGLT1 is expressed in heart but its physiological function remains unclear
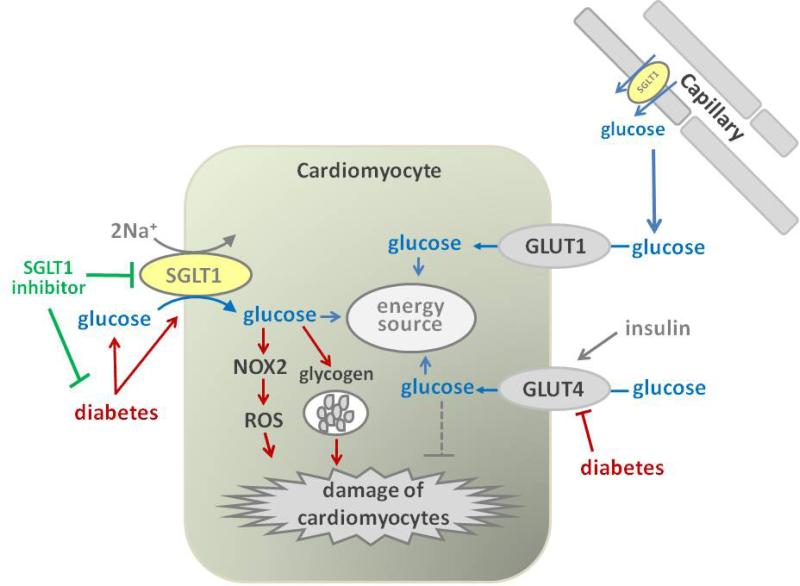
Cardiomyocytes utilize glucose and long-chain fatty acids as the major energy sources. Two types of glucose transporters, GLUT1 and GLUT4 play primary roles in cardiac glucose uptake: GLUT1 mediates basal glucose transport, and GLUT4 promotes insulin-induced glucose uptake and elevated contractile activity [124]. In addition to these GLUTs, SGLT1 was found to be strongly expressed in the heart, including in cell membranes of cardiomyocytes in mice and humans [26, 125-127](Figure 3, Table 1). Vrhovac and colleagues recently used double labeling for SGLT1 and aquaporin 1 in human hearts and proposed that SGLT1 protein is localized to heart capillaries rather than cardiomyocytes [28]. The localization of SGLT1 in capillaries of the heart (and skeletal muscle), but not in capillaries of the small intestine had previously been reported by immunohistochemistry in rats [128]. More recently, Kashiwagi et al. reported that SGLT1 was highly expressed in both human autopsied hearts and murine perfused hearts, including in cardiomyocytes, as assessed by immunostaining and immunoblotting with membrane fractionation [126]. Thus, cardiac SGLT1 could be involved in glucose transport from the capillaries into the cardiomyocytes. However, the physiological role of SGLT1 in the heart remains elusive. Under basal conditions, the perfusion of phlorizin, a dual SGLT1/SGLT2 inhibitor, into the mouse heart did not change cardiac function as determined in the Langendorff model [126]. Banerjee and colleagues provided first evidence that the increase in cardiac glucose uptake induced by insulin and leptin in cultured mouse cardiomyocytes is inhibited by phlorizin, suggesting a potential role of SGLT1 in the hormone regulated glucose uptake in the mouse heart, but the in vivo relevance was not tested [125].
Figure 3. Potential effects of SGLT1 inhibition on the heart – a two-edged sword?
In addition to fatty acids, cardiomyocytes use glucose as an energy source for glycolysis and oxidative phosphorylation. GLUT1 is responsible for basal glucose uptake and GLUT4 mediates insulin-induced glucose uptake. GLUT4 expression may be reduced in diabetes, which could impair energy supply and facilitate cardiac damage. Diabetes may upregulate SGLT1, which has been linked to NOX2 activation and ROS production and could facilitate cardiac damage. SGLT1 has been proposed to contribute to cardiac glycogen accumulation. Thus, SGLT1 inhibition may be cardioprotective in diabetes by inhibiting diabetes-induced ROS formation and glycogen accumulation. However, SGLT1-mediated glucose uptake may be important for cell function and viability in response to ischemia, making SGLT1 inhibition a two-edged sword. SGLT1: sodium glucose cotransporter 1; NOX2: NADPH oxidase 2; ROS: reactive oxygen species; GLUT2: glucose transporter 2; GLUT1: glucose transporter 1.
10. SGLT1 in the diabetic heart – a role for ROS formation and glycogen accumulation?
Diabetic cardiomyopathy is clinically defined as diastolic or systolic cardiac dysfunction occurring in a diabetic patient independent of cardiomyopathy due to coronary artery disease, hypertension, or valvular disease [129]. Diabetic cardiomyopathy contributes to the higher occurrence of heart failure in diabetic patients compared with the general population [130]. The involved pathophysiology remains poorly understood.
In the absence of diabetes, cardiac-specific inactivation of GLUT4 abolished insulin-stimulated glucose uptake, but increased cardiac GLUT1 expression and basal glucose transport by 3-fold; this was associated with modest cardiac hypertrophy and compensated contractile performance [131]. In STZ-induced T1DM rats, GLUT4 mRNA and protein expression as well as glucose uptake in the heart were decreased, and restored by insulin administration, whereas GLUT1 mRNA expression was not significantly altered [129, 132]. Contractile dysfunction was evident in the hearts of T2DM db/db mice; this was associated with a 52% and 84% reduction in the rate of glycolysis and glucose oxidation, respectively, whereas palmitate oxidation was increased twofold in db/db hearts versus controls [133]. Both glucose metabolism and palmitate metabolism as well as contractile function were normalized in hearts of db/db mice by overexpressing the insulin-regulated GLUT4 [133]. Thus, whereas the role of GLUT1 in cardiac pathophysiology remains unclear [134-136], some experimental evidence suggested that diabetes reduced cardiac GLUT4 activity which may impair cardiac glucose uptake and contribute to the development of diabetic cardiomyopathy [129].
Reactive oxygen species (ROS) has been considered to play important roles in the pathogenesis of diabetic cardiomyopathy, through direct damage to proteins and DNA, induction of apoptosis, activation of matrix metalloproteinases, and modulation of signal transduction pathways, which lead to cardiac dysfunction and hypertrophy [137]. Balteau and colleagues reported that the addition of high glucose concentrations (21 mM versus 5 mM in controls) to cultured primary rat cardiomyocytes activated NADPH oxidase 2 (NOX2) through the activation of Rac1GTP and the translocation of p47phox to the plasma membrane, resulting in the production of ROS and subsequent cell death. Interestingly, the ROS production was strongly inhibited by the addition of 1 mM phlorizin, indicating that SGLT1 (SGLT2 is not expressed in heart) might contribute to the development of diabetes-induced cardiac damage by mediating glucose-induced ROS production (Figure 3), but the in vivo relevance has not been tested [138]. Notably, cardiac mRNA expression of SGLT1 has been reported to be increased in patients with T2DM and diabetic cardiomyopathy (Figure 3), as well as in patients with ischemic cardiomyopathy or after the implantation of left ventricular assist devices [125].
Exposure of cultured neonatal rat ventricular myocytes for 24 h to high concentrations of glucose (30 mM) in the presence of insulin (10 nM) resulted in glycogen accumulation [139]. The glycogen content was also increased by 35-50% in hearts of diabetic db/db mice compared with non-diabetic mice [140]. Furthermore, STZ-diabetic T1DM rats have increased cardiac expression of autophagy proteins that are specific for glycogen [139]. Interestingly in this regard, cardiac-specific knockdown of SGLT1 has been reported to prevent glycogen storage cardiomyopathy in a mouse model carrying a mutation in the gene for the gamma2 subunit of AMPK [141, 142]. The relevance of this finding for the diabetic heart has not been tested.
Thus, SGLT1 may be upregulated in the diabetic heart and might be involved in the progression of diabetic cardiomyopathy through triggering ROS and/or facilitating the accumulation of glycogen in cardiomyocytes (Figure 3). If reduced glucose uptake via GLUT4 and increased glucose uptake via SGLT1 activity both mediate deleterious effects in the diabetic heart, then one may speculate that their subcellular localization and the glucose-induced downstream intracellular signaling cascades differ thereby defining whether glucose uptake is beneficial or deleterious for cardiomyocytes.
11. Therapeutic potential of SGLT1 inhibition
Whereas the potential of selective SGLT2 inhibition as a new antihyperglycemic strategy has been well established, the efficacy and safety of selective SGLT1 inhibition or dual SGLT2/SGLT1 remain less clear. The pharmacological tools that have been used to determine the potential of SGLT1 inhibition include phlorizin (or phloridzin), canagliflozin, LX4211 (or sotagliflozin), LP-925219, KGA-2727, and GSK-1614235. Phlorizin, which was first isolated from the bark of the apple tree by French chemists in 1835 [143] is a non-specific inhibitor of both SGLT1 and SGLT2 (IC50 ratio of 1.5:1 [144]) and the forerunner of the development of more selective SGLT2 inhibitors [145]. Canagliflozin is an approved SGLT2 inhibitor that also is a low-potency SGLT1 inhibitor (IC50 ratio of 155:1). LX4211 is an orally available L-xyloside and dual inhibitor of SGLT1 and SGLT2 (IC50 ratio of 20:1)[49, 146, 147] that is currently in clinical trials. LP-925219 is a novel, orally available dual SGLT1/SGLT2 inhibitor with structure and preclinical pharmacokinetics very similar to that of sotagliflozin (IC50 ratio for mouse SGLT1:SGLT2 of 45:1) [148]. The pyrazole-O-glucoside KGA-2727 is the first selective SGLT1 inhibitor and has inhibition constant values (Ki) of 97 and 13,600 nM for human SGLT1 and SGLT2, respectively (~1:140)[57]. GSK-1614235 is an analog of KGA-2727 with Ki values of 27 and 8,170 nM for human SGLT1 and SGLT2, respectively (~1:300)[50]. The use of these compounds provided first insights on the potential efficacy of SGLT1 inhibition on glucose homeostasis as well as the safety of such an approach, as discussed in the following. Other studies also indicated the feasibility to develop molecules that inhibit SGLT1 but not SGLT2 [144, 149, 150]. The development of selective SGLT1 inhibitors needs to consider the potential metabolism to aglycone-derivatives and their potential effects on different epithelial transport mechanisms (e.g., see the below discussed conversion of phlorizin to its aglycone, phloretin).
11.1 Inhibition of SGLT1 in the intestine can lower hyperglycemia by inhibiting glucose absorption and increasing GLP-1 release
Postprandial hyperglycemia is a cardiovascular risk factor [151, 152]. As discussed above, glucose absorption from the small intestine is largely mediated via SGLT1. In accordance, oral application of phlorizin and other polyphenols from apple extracts inhibited intestinal glucose absorption and lowered blood glucose levels in rodents and humans [145, 153]. Notably, orally consumed phlorizin is nearly entirely converted into phloretin by hydrolytic enzymes in the small intestine; the absorbed phloretin does not inhibit SGLT1 or SGLT2 but can produce glycosuria by inhibiting GLUT2 in the kidney [145]. Oral application of canagliflozin likewise delayed the appearance of oral glucose in plasma thereby reducing postprandial plasma glucose and insulin levels in healthy subjects [154]. The inhibition of intestinal glucose uptake has also been shown by selective SGLT1 inhibition using KGA-2727 in rats and GSK-1614235 in healthy human subjects [50, 57]. Moreover, KGA-2727 improved postprandial hyperglycemia in STZ-induced diabetic rats, and chronic administration reduced glucose concentrations and glycated hemoglobin in Zucker diabetic fatty rats [57].
While the effect on intestinal glucose absorption was not directly tested, LX4211 stimulated intestinal GLP-1 release in T2DM patients, which would be consistent with partial inhibition of intestinal glucose absorption thereby enhancing the glucose delivery to the more distal gut (see discussion above) [49]. The promotion of GLP-1 secretion by transient inhibition of intestinal SGLT1 was also observed using phlorizin or canagliflozin in rodents [54, 155] as well as more recently with GSK-1614235 in healthy human subjects [50]. Chronic treatment of Zucker diabetic fatty rats with KGA-2727 also increased the level of GLP-1 in the portal vein [57]. In contrast, GSK-1614235 reduced postprandial GIP secretion in healthy human subjects [50]. With regard to the GLP-1 pathway, studies in normal and diabetic rodents indicated that inhibition of intestinal SGLT1 may have additive effects with GLP-1 receptor agonists as well as DPP4 inhibitors, which inhibit the endogenous GLP-1 degradation [155].
11.2 Inhibition of SGLT1 in the kidney enhances the effects of SGLT2 inhibition and can lower blood glucose levels
It has been known for decades that parenteral application of phlorizin (thereby preventing its intestinal effects and degradation) inhibits renal SGLT2 and SGLT1 and thereby renal glucose reabsorption and improves glucose homeostasis [156]. Recent studies using selective SGLT2 inhibition and mice lacking SGLT1 defined more precisely the renal transport capacity of SGLT1, and showed that the increase in SGLT1-mediated glucose absorption explains the finding that renal glucose reabsorption is maintained at ~50% when SGLT2 is inhibited under normal glucose conditions [16]. During modest hyperglycemia, selective renal SGLT1 inhibition alone would be expected to induce only a small increase in urinary glucose excretion because the glucose transport capacity of upstream SGLT2 becomes increasingly engaged under these conditions, such that small amounts of glucose are delivered to SGLT1. However, inhibition of SGLT2 under these conditions as well as more severe hyperglycemia that overwhelms the upstream SGLT2 activates the full renal transport capacity of renal SGLT1. As a consequence, dual renal SGLT2/SGLT1 inhibition is expected to induce a significantly greater glucosuria than inhibition of either SGLT2 or SGLT1 alone [16, 18, 148], and thus a stronger effect on blood glucose levels is observed in mice with dual inhibition not only during euglycemia but also during modest hyperglycemia [16, 18]. Thus, dual SGLT1/2 inhibition induces synergistic effects via combined effects on the early and late proximal tubule as well as the intestine. Whereas selective SGLT2 inhibitors as well as LX4211 alone have very little risk for hypoglycemia [7, 146], more complete dual SGLT2/SGLT1 inhibition may increase the risk of hypoglycemia as indicated by murine studies [16, 18]. This could be relevant, especially when other factors facilitating hypoglycemia are prevalent (e.g. low food intake), and potentially also applies to the combination of SGLT1 inhibition with other anti-hyperglycemic agents (intestinal SGLT1 inhibition may in addition delay the hypoglycemic rescue with oral glucose). Whether renal SGLT1 inhibition has renal protective effects, e.g. by lowering glucose uptake and glucotoxicity in the vulnerable late proximal tubule, remains to be determined.
11.3 Does pharmacological SGLT1 inhibition induce gastrointestinal side effects?
As discussed above, genetic mutations in the SGLT1 gene as well as SGLT1 knockout mice develop glucose-galactose malabsorption syndrome characterized by neonatal onset of severe diarrhea, which is fatal within a few weeks unless lactose (glucose and galactose) is removed from the diet [4, 15, 157]. To prevent these side effects, SGLT2 inhibitors were developed that are highly selective for SGLT2 over SGLT1 [10]. However and remarkably, the dual SGLT2/SGLT1 inhibitor LX4211 was reported to be well tolerated without evidence of increased gastrointestinal side effects when 36 patients with T2DM were given a once-daily oral dose of placebo or 150 or 300 mg of the compound for 28 days [49]. The findings indicate that LX4211 can be applied at a dose that induces therapeutic effects on the intestine and kidney without clinical relevant gastrointestinal side effects, most likely due to incomplete inhibition of intestinal SGLT1. Drug-related adverse events that occurred after a single dose of the SGLT1-selective inhibitor GSK-1614235 had been applied before breakfast to 12 healthy subjects included diarrhea (n=4), abdominal pain (n=1), and flatulence (n=1)[50]. All were reported as mild or moderate in intensity, and there were no serious adverse events. Plasma concentrations of GSK-1614235 indicated very low systemic exposure of the active parent molecule with rapid clearance and formation of two metabolites that lack inhibitory activity on SGLT1. No apparent drug-related trends and no clinically relevant changes in clinical laboratory results, ECG measures, heart rate, or blood pressure were observed [50]. In these studies, GSK-1614235 had reduced intestinal glucose uptake (measured by 3-OMG) by 50% relative to placebo. Our preliminary data show that a 50% downregulation of SGLT1 in small intestine of mice receiving standard diet does not lead to diarrhea (A. Friedrich, C. Otto and H. Koepsell, unpublished data). Whereas SGLT1 contributes to intestinal sodium absorption, in quantitative terms the latter is mainly mediated by sodium hydrogen exchangers [158]. As a consequence, no major intestinal sodium loss is expected.
A long-established approach to target postprandial hyperglycemia in patients with type 2 diabetes mellitus is the use of alpha-glucosidase inhibitors (AGIs), which prevent the absorption of most polysaccharides in the intestine. Their application, either alone or in combination with other oral anti-hyperglycemic agents and insulin, can provide overall glycemic control with transient mild gastrointestinal disorders due to the delivery of unabsorbed polysaccharides to the colon [159]. Notably, treatment with AGIs, acarbose in particular, was reported to have beneficial effects on lipid levels, blood pressure, carotid intima-media thickness and endothelial dysfunction as well as incretin hormones and the gut-microbiota [159]. In contrast to SGLT1 inhibition, AGIs do not alter the intestinal absorption of ingested monosaccharides. While oral monosaccharides can be used to rescue hypoglycemic episodes in AGI-treated patients, it has not been established to which extent oral hypoglycemic rescue is affected by pharmacological SGLT1 inhibition.
11.4 Potential effects of SGLT1 inhibition on the heart – a two-edged sword?
The pathophysiology of the diabetic heart outlined above indicated that cardiac inhibition of SGLT1 could have beneficial effects on cardiac ROS formation and glycogen accumulation. Intragastrical administration of phlorizin was reported to prevent the phenotype of diabetic cardiomyopathy, including hypertrophy and disarray of cardiomyocytes in T2DM db/db mice, through modulating cardiac lipid and energy metabolism and altering the expression of a set of proteins involved in cardiac damage; this was associated with a decrease in blood glucose, body weight, triglyceride, total cholesterol, and advanced glycation end products [160]. These findings suggest that phlorizin may be an effective therapeutic approach for the treatment of diabetic cardiomyopathy. The latter study did not discriminate, however, between effects of phlorizin on blood glucose versus direct inhibition of cardiac SGLT1, which considering the low oral bioavailability of phlorizin may have been minimal.
Diabetes is often associated with ischemic cardiac disease. In this regard, very recent studies using phlorizin in the murine Langendorff preparation indicated that cardiac SGLTs, possibly SGLT1, may provide an important protective mechanism against schemia reperfusion injury by replenishing ATP stores in ischemic cardiac tissues via enhancing the availability of glucose [126]. Thus, SGLT1 inhibition could be a two-edged sword when used to treat the diabetic heart. Clearly, further studies are needed to define the pathophysiology of the diabetic heart and the mechanism and relevance of glucose transport and glycogen accumulation [139, 140] as well as the in vivo physiological and pathophysiological role of cardiac glucose transporters including SGLT1.
11.5 Potential additional effects of SGLT1 inhibition in diabetes
Electrogenic sugar entry via SGLTs may regulate the activity of glucose-sensing neurons through depolarizing the neurons, i.e., in a metabolism-independent way [161]. A recent study in rats used bilateral microinjections into the ventromedial hypothalamus (VMH) of an adenoassociated viral vector containing the SGLT1 short hairpin RNA (shRNA) [33]. The results indicated that SGLT1 in the VMH plays a significant role in the detection and activation of counterregulatory responses to hypoglycemia, including improved glucagon and epinephrine responses in response to hypoglycemia as well as restoration of the impaired epinephrine response to hypoglycemia in STZ-diabetic rats. As a consequence, the authors proposed that inhibition of SGLT1 may offer a potential therapeutic target to diminish the risk of hypoglycemia in diabetes [33]. Whether this translates to humans and to the potential combination of SGLT1 inhibitors with other antihyperglycemic and, thus, potentially hypoglycemic drugs remains to be determined.
12. Conclusions and perspectives
Effective glycemic control in diabetes is important to minimize the progression of the disease and the risk of potentially devastating complications. Intestinal SGLT1 is of primary importance for glucose absorption. As a consequence, inhibition of SGLT1 delays and attenuates intestinal glucose absorption and the rapid postprandial increase in blood glucose levels. In addition, inhibition of glucose absorption via SGLT1 in the early small intestine can enhance the sustained release of the antihyperglycemic integrin, GLP-1, in more distal aspects of the intestine, which is expected to further improve glucose metabolism. While SGLT1 mediates ~3% of fractional glucose reabsorption in the kidney in euglycemic conditions, its quantitative contribution increases whenever glucose delivery is increased to the late proximal tubule, which occurs with increasing hyperglycemia and, in particular, when the upstream SGLT2 is inhibited. As a consequence, inhibition of renal SGLT1 can enhance urinary glucose excretion in diabetes and when combined with SGLT2 inhibition. The distinct roles of SGLT1 and SGLT2 in intestine and kidney provide the rationale for dual SGLT1/SGLT2 inhibitors. Certainly, the potential for intestinal malabsorption, strong blood-glucose lowering effects, and effects in other SGLT1-expressing organs, including the heart, lung and brain, need to be carefully addressed and understood. Long-term clinical studies are needed to determine whether SGLT1 and/or dual SGLT1/SGLT2 inhibitors have a safety profile and exert cardiovascular benefits that are superior to SGLT2 inhibitors and more traditional agents, and will help to further define their role in antidiabetic therapy.
13. Expert opinion
Diabetes mellitus affects more than 380 million individuals worldwide and is the leading cause of end-stage kidney disease. Despite reductions in blood glucose to almost normal levels, using traditional therapies such as metformin, sulphonylureas and insulin, it is difficult to avoid the potential for hypoglycemia and weight gain, and existing therapies may not always reduce cardiovascular complications. Thus, there is a need for new anti-hyperglycemic strategies. In recent years, much attention has focused on SGLT2, which is responsible for the majority of glucose reabsorption in the kidney. SGLT2 inhibitors improve glycemic control by increasing urinary glucose excretion with little risk for hypoglycemia. Beneficial effects also include lowering of blood pressure, body weight, and blood levels of uric acid, and a recent study showed that SGLT2 inhibition can lower the risk of death from cardiovascular disease, hospitalization for heart failure, and death from any cause (relative risk reductions of 38, 35, and 32%, respectively) in patients with T2DM and high cardiovascular risk [14]. Thus, SGLT2 inhibition illustrates how blockade of a glucose transporter can have beneficial therapeutic effects in hyperglycemia and diabetes mellitus. Whereas glucose reabsorption in the kidney is predominantly mediated by SGLT2, it is SGLT1 that is of primary importance for glucose absorption from the lumen into the epithelial cells of the small intestine [15], an organ that does not express SGLT2. Inhibition of intestinal SGLT1 also induces a sustained release of GLP-1, which facilitates glucose-dependent insulin release from the pancreas. In addition, SGLT1 is expressed in the late renal proximal tubules, where it reabsorbs the glucose that has escaped the upstream SGLT2 [16]. As a consequence, SGLT1-mediated glucose reabsorption in the kidney is strongly increased when the tubular glucose load overwhelms the capacity of SGLT2 or when the latter is inhibited, as observed in hyperglycemia and with the use of SGLT2 inhibitors, respectively. All this makes SGLT1 a very attractive target for anti-hyperglycemic therapy, alone or in combination with SGLT2 inhibition. In combination therapy, SGLT1 inhibition has the potential to further lower blood glucose levels and thereby enhance many of the beneficial effects induced by lowering blood glucose by SGLT2 inhibition, including lower glucose toxicity (e.g., preservation of pancreatic beta cells) and lower body weight and blood pressure, although this remains to be specifically tested. In the kidney, SGLT1 is expressed in the vulnerable late proximal tubule of the outer medulla. Whether SGLT1-mediated glucose uptake in the kidney contributes to the renal toxicity of hyperglycemia and diabetic nephropathy has not been specifically tested. Currently, most evidence from clinical trials is based on the dual inhibitor LX4211 but a first study with the SGLT1-selective inhibitor GSK-1614235 has just recently been published [50]. The concern has been that an oral SGLT1 inhibitor would induce diarrhea, as observed in patients with mutations in SGLT1 and in SGLT1 knockout mice. Somewhat unexpected, LX4211 and GSK-1614235 can be applied at doses that inhibit glucose transport in the intestine without severe gastrointestinal side effects. While these promising findings indicated the presence of a therapeutic window (partial SGLT1 inhibition), long-term studies are needed to better document the intestinal responses and safety. Specific caution also requires the potential for ketoacidosis as documented for SGLT2 inhibitors in diabetic patients (http://www.fda.gov/Drugs/DrugSafety/ucm446845.htm), and which likely applies at least to the same extent to dual SGLT2/SGLT1 inhibition. Moreover, studies in rodents indicate that SGLT1 inhibition could be a two-edged sword with regard to consequences on heart function and integrity. Similarly, SGLT1 expression has been consistently documented in the brain of many species and one study reported SGLT1 expression in human brain, which in part may be localized to the cerebral vasculature. It is clear that we need a much more comprehensive understanding of the function of SGLT1 including the heart and brain. Nevertheless, SGLT1 inhibitors and dual SGLT1/SGLT2 inhibitors merit further evaluation for their potential in patients with diabetes to achieve better glycemic control and cardiovascular outcome than is currently achieved.
Article highlights box.
The sodium glucose cotransporter SGLT1 is strongly expressed in the apical brush border of the small intestine and the late proximal tubule of the kidney, where it is critical for absorption/reabsorption of glucose into the blood stream.
Inhibition of SGLT1 and combined inhibition of SGLT1/SGLT2 is an interesting new anti-hyperglycemic concept.
SGLT1 inhibition improves glucose homeostasis in diabetic patients by reducing dietary glucose absorption in the intestine and by increasing the release of gastrointestinal incretins like glucagon-like peptide-1.
SGLT1 inhibition has a small glucosuric effect in the normal kidney and this effect is increased when more glucose is delivered to SGLT1 in the late proximal tubule, as shown in mice during inhibition of SGLT2 and as can occur in diabetes.
In short-term studies, inhibition of SGLT1 and combined SGLT1/SGLT2 inhibition appeared to be safe and not associated with increased rates of hypoglycemia or clinical relevant gastrointestinal side effects.
More data is needed on long-term clinical efficacy and outcome, and to determine whether SGLT1 and/or dual SGLT1/SGLT2 inhibitors are safe, with particular focus on intestine, brain, lung, and heart.
Acknowledgments
P Song's work was supported by China Scholarship Council (CSC); A Onishi's work was supported by Japan Heart Foundation and the Bayer Yakuhin Research Grant Abroad; V Vallon's work was supported by the National Institutes of Health (R01DK56248, R01HL094728, P30DK079337) and the Department of Veterans Affairs. V Vallon has served as a consultant and received honoraria from Boehringer Ingelheim, Janssen Pharmaceutical, and Intarcia Therapeutics. V Vallon's work was supported by investigator-initiated research grants by Bristol-Myers Squibb, Astra-Zeneca, and Boehringer Ingelheim, Biberach.
Abbreviations
- AMPK
AMP-activated protein kinase
- a-gustducin
a-transducin-like Gprotein a-subunit
- DPP4
dipeptidyl peptidase-4
- FFAR2
G-protein-coupled free fatty acid receptor 2
- FFAR3
G-protein-coupled free fatty acid receptor 3
- GLUT1
glucose transporter 1
- GLUT2
glucose transporter 2
- GLUT4
glucose transporter 4
- GLP-1
glucagon- like peptide 1
- GIP
glucose-dependent insulinotropic peptide
- MDCK
Madin-Darby canine kidney cells
- Me-4-FDG
alpha-methyl-4-deoxy-4-[(18)F]fluoro-D-glucopyranoside
- NOX2
NADPH oxidase 2
- PKA
protein kinase A
- PKC
protein kinase C
- RS1
regulator of SGLT1, gene product of RSC1A1
- ROS
reactive oxygen species
- T1DM
type I diabetes
- T2DM
type II diabetes
- T1R3
sweet taste receptor subunit 3
- TGN
trans-Golgi network
- SLC5A1
solute carrier family 5, member 1
- SLC5A2
solute carrier family 5, member 2
- SGLT1
sodium-dependent glucose cotransporter 1
- SGLT2
sodium-dependent glucose cotransporter 2
- SCFAs
short-chain fatty acids
- SGK1
serum and glucocorticoid inducible kinase 1
- SGK3
serum and glucocorticoid inducible kinase 3
Footnotes
Declaration of interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reference List
* Of interest
** Of considerable interest
- 1.Zierler K. Whole body glucose metabolism. Am J Physiol. 1999;276:E409–E426. doi: 10.1152/ajpendo.1999.276.3.E409. [DOI] [PubMed] [Google Scholar]
- 2.Kowalski GM, Bruce CR. The regulation of glucose metabolism: implications and considerations for the assessment of glucose homeostasis in rodents. Am J Physiol Endocrinol Metab. 2014;307:E859–E871. doi: 10.1152/ajpendo.00165.2014. [DOI] [PubMed] [Google Scholar]
- 3.Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34:121–38. doi: 10.1016/j.mam.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007;261:32–43. doi: 10.1111/j.1365-2796.2006.01746.x. [DOI] [PubMed] [Google Scholar]
- 5.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–94. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial Research Group The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 7.Vallon V. The mechanisms and therapeutic potential of SGLT2 inhibitors in diabetes mellitus. Annu Rev Med. 2015;66:255–70. doi: 10.1146/annurev-med-051013-110046. [DOI] [PubMed] [Google Scholar]
- 8.Gallo LA, Wright EM, Vallon V. Probing SGLT2 as a therapeutic target for diabetes: Basic physiology and consequences. Diab Vasc Dis Res. 2015;12:78–89. doi: 10.1177/1479164114561992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75:33–59. doi: 10.1007/s40265-014-0337-y. [DOI] [PubMed] [Google Scholar]
- 10.Washburn WN. Development of the renal glucose reabsorption inhibitors: a new mechanism for the pharmacotherapy of diabetes mellitus type 2. J Med Chem. 2009;52:1785–94. doi: 10.1021/jm8013019. [DOI] [PubMed] [Google Scholar]
- 11.Hasan FM, Alsahli M, Gerich JE. SGLT2 inhibitors in the treatment of type 2 diabetes. Diabetes Res Clin Pract. 2014;104:297–322. doi: 10.1016/j.diabres.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Novikov A, Vallon V. Sodium glucose cotransporter 2 inhibition in the diabetic kidney: an update. Curr Opin Nephrol Hypertens. 2016;25:50–8. doi: 10.1097/MNH.0000000000000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inzucchi SE, Zinman B, Wanner C, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12:90–100. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 15*.Gorboulev V, Schurmann A, Vallon V, et al. Na(+)-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes. 2012;61:187–96. doi: 10.2337/db11-1029. [Description of the pivotal role of SGLT1 in intestinal glucose absorption and the glucose-induced secretion of GIP and GLP-1.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.Rieg T, Masuda T, Gerasimova M, et al. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Renal Physiol. 2014;306:F188–F193. doi: 10.1152/ajprenal.00518.2013. [The study makes use of gene-targeted mouse models to show that SGLT2 and SGLT1 account for renal glucose reabsorption in euglycemia. When SGLT2 is fully inhibited, the increase in SGLT1-mediated glucose reabsorption explains why only 50-60% of filtered glucose is excreted.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vallon V, Platt KA, Cunard R, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol. 2011;22:104–12. doi: 10.1681/ASN.2010030246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powell DR, DaCosta CM, Gay J, et al. Improved glycemic control in mice lacking Sglt1 and Sglt2. Am J Physiol Endocrinol Metab. 2013;304:E117–E130. doi: 10.1152/ajpendo.00439.2012. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt UM, Eddy B, Fraser CM, et al. Isolation of (a subunit of) the Na+/D-glucose cotransporter(s) of rabbit intestinal brush border membranes using monoclonal antibodies. FEBS Lett. 1983;161:279–83. doi: 10.1016/0014-5793(83)81025-4. [DOI] [PubMed] [Google Scholar]
- 20.Peerce BE, Wright EM. Conformational changes in the intestinal brush border sodium-glucose cotransporter labeled with fluorescein isothiocyanate. Proc Natl Acad Sci U S A. 1984;81:2223–6. doi: 10.1073/pnas.81.7.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hediger MA, Coady MJ, Ikeda TS, Wright EM. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. Nature. 1987;330:379–81. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- 22.Turk E, Wright EM. Membrane topology motifs in the SGLT cotransporter family. J Membr Biol. 1997;159:1–20. doi: 10.1007/s002329900264. [DOI] [PubMed] [Google Scholar]
- 23.Tyagi NK, Kumar A, Goyal P, et al. D-Glucose-recognition and phlorizin-binding sites in human sodium/D-glucose cotransporter 1 (hSGLT1): a tryptophan scanning study. Biochemistry. 2007;46:13616–28. doi: 10.1021/bi701193x. [DOI] [PubMed] [Google Scholar]
- 24.Hediger MA, Rhoads DB. Molecular physiology of sodium-glucose cotransporters. Physiol Rev. 1994;74:993–1026. doi: 10.1152/physrev.1994.74.4.993. [DOI] [PubMed] [Google Scholar]
- 25.Hummel CS, Lu C, Loo DD, et al. Glucose transport by human renal Na+/D-glucose cotransporters SGLT1 and SGLT2. Am J Physiol Cell Physiol. 2011;300:C14–C21. doi: 10.1152/ajpcell.00388.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Williams S, Ho S, et al. Quantitative PCR tissue expression profiling of the human SGLT2 gene and related family members. Diabetes Ther. 2010;1:57–92. doi: 10.1007/s13300-010-0006-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonner C, Kerr-Conte J, Gmyr V, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med. 2015;21:512–7. doi: 10.1038/nm.3828. [DOI] [PubMed] [Google Scholar]
- 28*.Vrhovac I, Balen ED, Klessen D, et al. Localizations of Na-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch. 2015;467:1881–98. doi: 10.1007/s00424-014-1619-7. [This study uses novel affinity-purified antibodies against human SGLT1 (hSGLT1) and SGLT2 (hSGLT2) to localize hSGLT2 in human kidney and hSGLT1 in human kidney, small intestine, liver, lung, and heart.] [DOI] [PubMed] [Google Scholar]
- 29.Poppe R, Karbach U, Gambaryan S, et al. Expression of the Na+-D-glucose cotransporter SGLT1 in neurons. J Neurochem. 1997;69:84–94. doi: 10.1046/j.1471-4159.1997.69010084.x. [DOI] [PubMed] [Google Scholar]
- 30.Sabino-Silva R, Freitas HS, Lamers ML, et al. Na+-glucose cotransporter SGLT1 protein in salivary glands: potential involvement in the diabetes-induced decrease in salivary flow. J Membr Biol. 2009;228:63–9. doi: 10.1007/s00232-009-9159-3. [DOI] [PubMed] [Google Scholar]
- 31.Lee WS, Kanai Y, Wells RG, Hediger MA. The high affinity Na+/glucose cotransporter. Re-evaluation of function and distribution of expression. J Biol Chem. 1994;269:12032–9. [PubMed] [Google Scholar]
- 32.Yu AS, Hirayama BA, Timbol G, et al. Functional expression of SGLTs in rat brain. Am J Physiol Cell Physiol. 2010;299:C1277–C1284. doi: 10.1152/ajpcell.00296.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Fan X, Chan O, Ding Y, et al. Reduction in SGLT1 mRNA Expression in the Ventromedial Hypothalamus Improves the Counterregulatory Responses to Hypoglycemia in Recurrently Hypoglycemic and Diabetic Rats. Diabetes. 2015;64:3564–72. doi: 10.2337/db15-0022. [This study in hypoglycemic and diabetic rats indicated that inhibition of SGLT1 in the ventrolateral hypothalamus may diminish the risk of hypoglycemia in diabetes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu AS, Hirayama BA, Timbol G, et al. Regional distribution of SGLT activity in rat brain in vivo. Am J Physiol Cell Physiol. 2013;304:C240–C247. doi: 10.1152/ajpcell.00317.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 36.Balen D, Ljubojevic M, Breljak D, et al. Revised immunolocalization of the Na+-D-glucose cotransporter SGLT1 in rat organs with an improved antibody. Am J Physiol Cell Physiol. 2008;295:C475–C489. doi: 10.1152/ajpcell.00180.2008. [DOI] [PubMed] [Google Scholar]
- 37.Elfeber K, Kohler A, Lutzenburg M, et al. Localization of the Na+-D-glucose cotransporter SGLT1 in the blood-brain barrier. Histochem Cell Biol. 2004;121:201–7. doi: 10.1007/s00418-004-0633-9. [DOI] [PubMed] [Google Scholar]
- 38.Ervin RB, Ogden CL. Consumption of added sugars among U.S. adults, 2005-2010. National Center for Health Statistics; Hyattsville, MD: 2013. [NCHS data brief, no 122.] [Google Scholar]
- 39.Hwang ES, Hirayama BA, Wright EM. Distribution of the SGLT1 Na+/glucose cotransporter and mRNA along the crypt-villus axis of rabbit small intestine. Biochem Biophys Res Commun. 1991;181:1208–17. doi: 10.1016/0006-291x(91)92067-t. [DOI] [PubMed] [Google Scholar]
- 40.Hirayama BA, Wong HC, Smith CD, et al. Intestinal and renal Na+/glucose cotransporters share common structures. Am J Physiol. 1991;261:C296–C304. doi: 10.1152/ajpcell.1991.261.2.C296. [DOI] [PubMed] [Google Scholar]
- 41.Takata K, Kasahara T, Kasahara M, et al. Immunohistochemical localization of Na(+)-dependent glucose transporter in rat jejunum. Cell Tissue Res. 1992;267:3–9. doi: 10.1007/BF00318685. [DOI] [PubMed] [Google Scholar]
- 42.Takata K, Kasahara T, Kasahara M, et al. Localization of Na(+)-dependent active type and erythrocyte/HepG2-type glucose transporters in rat kidney: immunofluorescence and immunogold study. J Histochem Cytochem. 1991;39:287–98. doi: 10.1177/39.3.1993828. [DOI] [PubMed] [Google Scholar]
- 43.Miyamoto K, Hase K, Taketani Y, et al. Diabetes and glucose transporter gene expression in rat small intestine. Biochem Biophys Res Commun. 1991;181:1110–7. doi: 10.1016/0006-291x(91)92053-m. [DOI] [PubMed] [Google Scholar]
- 44.Roder PV, Geillinger KE, Zietek TS, et al. The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS One. 2014;9:e89977. doi: 10.1371/journal.pone.0089977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gouyon F, Caillaud L, Carriere V, et al. Simple-sugar meals target GLUT2 at enterocyte apical membranes to improve sugar absorption: a study in GLUT2-null mice. J Physiol. 2003;552:823–32. doi: 10.1113/jphysiol.2003.049247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallon V, Grahammer F, Volkl H, et al. KCNQ1-dependent transport in renal and gastrointestinal epithelia. Proc Natl Acad Sci U S A. 2005;102:17864–9. doi: 10.1073/pnas.0505860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin MG, Turk E, Lostao MP, et al. Defects in Na+/glucose cotransporter (SGLT1) trafficking and function cause glucose-galactose malabsorption. Nat Genet. 1996;12:216–20. doi: 10.1038/ng0296-216. [DOI] [PubMed] [Google Scholar]
- 48.Turk E, Zabel B, Mundlos S, et al. Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter. Nature. 1991;350:354–6. doi: 10.1038/350354a0. [DOI] [PubMed] [Google Scholar]
- 49*.Zambrowicz B, Freiman J, Brown PM, et al. LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial. Clin Pharmacol Ther. 2012;92:158–69. doi: 10.1038/clpt.2012.58. [This clinical study indicated that LX4211 can improve glycemic control in patients with type 2 diabetes without increasing gastrointestinal side effects. The data support further study of LX4211-mediated dual SGLT1/SGLT2 inhibition as a novel mechanism of action in the treatment of T2DM.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Dobbins RL, Greenway FL, Chen L, et al. Selective sodium-dependent glucose transporter 1 inhibitors block glucose absorption and impair glucose-dependent insulinotropic peptide release. Am J Physiol Gastrointest Liver Physiol. 2015;308:G946–G954. doi: 10.1152/ajpgi.00286.2014. [The study uses the first selective SGLT1 inhibitors and shows that they inhibit intestinal glucose absorption in rats and humans. Moreover, they show that selective SGLT1 inhibition in humans emhances postprandial plasma GLP-1 levels.] [DOI] [PubMed] [Google Scholar]
- 51.Gribble FM. The gut endocrine system as a coordinator of postprandial nutrient homoeostasis. Proc Nutr Soc. 2012;71:456–62. doi: 10.1017/S0029665112000705. [DOI] [PubMed] [Google Scholar]
- 52.Deacon CF, Ahren B. Physiology of incretins in health and disease. Rev Diabet Stud. 2011;8:293–306. doi: 10.1900/RDS.2011.8.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drucker DJ. Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls. Diabetes. 2013;62:3316–23. doi: 10.2337/db13-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moriya R, Shirakura T, Ito J, et al. Activation of sodium-glucose cotransporter 1 ameliorates hyperglycemia by mediating incretin secretion in mice. Am J Physiol Endocrinol Metab. 2009;297:E1358–E1365. doi: 10.1152/ajpendo.00412.2009. [DOI] [PubMed] [Google Scholar]
- 55*.Powell DR, Smith M, Greer J, et al. LX4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)-mediated absorption of intestinal glucose. J Pharmacol Exp Ther. 2013;345:250–9. doi: 10.1124/jpet.113.203364. [The study shows that LX4211, a dual sodium/glucose cotransporter 1 (SGLT1) and SGLT2 inhibitor, reduces intestinal glucose absorption and increases GLP-1 and PYY release, associated with a decrease in blood glucose excursions.] [DOI] [PubMed] [Google Scholar]
- 56.Kokrashvili Z, Mosinger B, Margolskee RF. T1r3 and alpha-gustducin in gut regulate secretion of glucagon-like peptide-1. Ann N Y Acad Sci. 2009;1170:91–4. doi: 10.1111/j.1749-6632.2009.04485.x. [DOI] [PubMed] [Google Scholar]
- 57.Shibazaki T, Tomae M, Ishikawa-Takemura Y, et al. KGA-2727, a novel selective inhibitor of a high-affinity sodium glucose cotransporter (SGLT1), exhibits antidiabetic efficacy in rodent models. J Pharmacol Exp Ther. 2012;342:288–96. doi: 10.1124/jpet.112.193045. [DOI] [PubMed] [Google Scholar]
- 58.Tolhurst G, Heffron H, Lam YS, et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–71. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tavakkolizadeh A, Berger UV, Shen KR, et al. Diurnal rhythmicity in intestinal SGLT-1 function, V(max), and mRNA expression topography. Am J Physiol Gastrointest Liver Physiol. 2001;280:G209–G215. doi: 10.1152/ajpgi.2001.280.2.G209. [DOI] [PubMed] [Google Scholar]
- 60.Ferraris RP, Diamond J. Regulation of intestinal sugar transport. Physiol Rev. 1997;77:257–302. doi: 10.1152/physrev.1997.77.1.257. [DOI] [PubMed] [Google Scholar]
- 61.Dyer J, Hosie KB, Shirazi-Beechey SP. Nutrient regulation of human intestinal sugar transporter (SGLT1) expression. Gut. 1997;41:56–9. doi: 10.1136/gut.41.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rhoads DB, Rosenbaum DH, Unsal H, et al. Circadian periodicity of intestinal Na+/glucose cotransporter 1 mRNA levels is transcriptionally regulated. J Biol Chem. 1998;273:9510–6. doi: 10.1074/jbc.273.16.9510. [DOI] [PubMed] [Google Scholar]
- 63.Barfull A, Garriga C, Tauler A, Planas JM. Regulation of SGLT1 expression in response to Na(+) intake. Am J Physiol Regul Integr Comp Physiol. 2002;282:R738–R743. doi: 10.1152/ajpregu.00263.2001. [DOI] [PubMed] [Google Scholar]
- 64.Dyer J, Daly K, Salmon KS, et al. Intestinal glucose sensing and regulation of intestinal glucose absorption. Biochem Soc Trans. 2007;35:1191–4. doi: 10.1042/BST0351191. [DOI] [PubMed] [Google Scholar]
- 65*.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–80. doi: 10.1073/pnas.0706678104. [This study shows that the sweet taste receptor subunit T1R3 and the taste G protein gustducin in enteroendocrine cells sense luminal glucose, which induces an up regulation of intestinal SGLT1 expression. This molecular pathway may provide novel therapeutic targets for modulating the gut's capacity to absorb sugars.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Veyhl M, Keller T, Gorboulev V, et al. RS1 (RSC1A1) regulates the exocytotic pathway of Na+-D-glucose cotransporter SGLT1. Am J Physiol Renal Physiol. 2006;291:F1213–F1223. doi: 10.1152/ajprenal.00068.2006. [DOI] [PubMed] [Google Scholar]
- 67.Veyhl M, Wagner CA, Gorboulev V, et al. Downregulation of the Na(+)- D-glucose cotransporter SGLT1 by protein RS1 (RSC1A1) is dependent on dynamin and protein kinase C. J Membr Biol. 2003;196:71–81. doi: 10.1007/s00232-003-0626-y. [DOI] [PubMed] [Google Scholar]
- 68.Kroiss M, Leyerer M, Gorboulev V, et al. Transporter regulator RS1 (RSC1A1) coats the trans-Golgi network and migrates into the nucleus. Am J Physiol Renal Physiol. 2006;291:F1201–F1212. doi: 10.1152/ajprenal.00067.2006. [DOI] [PubMed] [Google Scholar]
- 69.Vernaleken A, Veyhl M, Gorboulev V, et al. Tripeptides of RS1 (RSC1A1) inhibit a monosaccharide-dependent exocytotic pathway of Na+-D-glucose cotransporter SGLT1 with high affinity. J Biol Chem. 2007;282:28501–13. doi: 10.1074/jbc.M705416200. [DOI] [PubMed] [Google Scholar]
- 70.Veyhl-Wichmann M, Friedrich A, Vernaleken A, et al. Phosphorylation of RS1 (RSC1A1) Steers Inhibition of Different Exocytotic Pathways for Glucose Transporter SGLT1 and Nucleoside Transporter CNT1 and a RS1 Derived Peptide Inhibits Glucose Absorption. Mol Pharmacol. 2016;89:118–32. doi: 10.1124/mol.115.101162. [DOI] [PubMed] [Google Scholar]
- 71.Kellett GL, Brot-Laroche E, Mace OJ, Leturque A. Sugar absorption in the intestine: the role of GLUT2. Annu Rev Nutr. 2008;28:35–54. doi: 10.1146/annurev.nutr.28.061807.155518. [DOI] [PubMed] [Google Scholar]
- 72.Naftalin RJ. Does apical membrane GLUT2 have a role in intestinal glucose uptake? F1000Res. 2014;3:304. doi: 10.12688/f1000research.5934.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chaudhry RM, Scow JS, Madhavan S, et al. Acute enterocyte adaptation to luminal glucose: a posttranslational mechanism for rapid apical recruitment of the transporter GLUT2. J Gastrointest Surg. 2012;16:312–9. doi: 10.1007/s11605-011-1752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Martin MG, Wang J, Solorzano-Vargas RS, et al. Regulation of the human Na(+)-glucose cotransporter gene, SGLT1, by HNF-1 and Sp1. Am J Physiol Gastrointest Liver Physiol. 2000;278:G591–G603. doi: 10.1152/ajpgi.2000.278.4.G591. [DOI] [PubMed] [Google Scholar]
- 75.Subramanian S, Glitz P, Kipp H, et al. Protein kinase-A affects sorting and conformation of the sodium-dependent glucose co-transporter SGLT1. J Cell Biochem. 2009;106:444–52. doi: 10.1002/jcb.22025. [DOI] [PubMed] [Google Scholar]
- 76.Hirsch JR, Loo DD, Wright EM. Regulation of Na+/glucose cotransporter expression by protein kinases in Xenopus laevis oocytes. J Biol Chem. 1996;271:14740–6. doi: 10.1074/jbc.271.25.14740. [DOI] [PubMed] [Google Scholar]
- 77.Ikari A, Harada H, Takagi K. Role of actin in the cAMP-dependent activation of sodium/glucose cotransporter in renal epithelial cells. Biochim Biophys Acta. 2005;1711:20–4. doi: 10.1016/j.bbamem.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 78.Elvira B, Honisch S, Almilaji A, et al. Up-regulation of Na(+)-coupled glucose transporter SGLT1 by caveolin-1. Biochim Biophys Acta. 2013;1828:2394–8. doi: 10.1016/j.bbamem.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 79.Sopjani M, Bhavsar SK, Fraser S, et al. Regulation of Na+-coupled glucose carrier SGLT1 by AMP-activated protein kinase. Mol Membr Biol. 2010;27:137–44. doi: 10.3109/09687681003616870. [DOI] [PubMed] [Google Scholar]
- 80.Gabler NK, Radcliffe JS, Spencer JD, et al. Feeding long-chain n-3 polyunsaturated fatty acids during gestation increases intestinal glucose absorption potentially via the acute activation of AMPK. J Nutr Biochem. 2009;20:17–25. doi: 10.1016/j.jnutbio.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 81.Walker J, Jijon HB, Diaz H, et al. 5-aminoimidazole-4-carboxamide riboside (AICAR) enhances GLUT2-dependent jejunal glucose transport: a possible role for AMPK. Biochem J. 2005;385:485–91. doi: 10.1042/BJ20040694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dieter M, Palmada M, Rajamanickam J, et al. Regulation of glucose transporter SGLT1 by ubiquitin ligase Nedd4-2 and kinases SGK1, SGK3, and PKB. Obes Res. 2004;12:862–70. doi: 10.1038/oby.2004.104. [DOI] [PubMed] [Google Scholar]
- 83.Shojaiefard M, Strutz-Seebohm N, Tavare JM, et al. Regulation of the Na(+), glucose cotransporter by PIKfyve and the serum and glucocorticoid inducible kinase SGK1. Biochem Biophys Res Commun. 2007;359:843–7. doi: 10.1016/j.bbrc.2007.05.111. [DOI] [PubMed] [Google Scholar]
- 84.Grahammer F, Henke G, Sandu C, et al. Intestinal function of gene-targeted mice lacking serum- and glucocorticoid-inducible kinase 1. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1114–G1123. doi: 10.1152/ajpgi.00231.2005. [DOI] [PubMed] [Google Scholar]
- 85.Lang F, Klingel K, Wagner CA, et al. Deranged transcriptional regulation of cell-volume-sensitive kinase hSGK in diabetic nephropathy. Proc Natl Acad Sci U S A. 2000;97:8157–62. doi: 10.1073/pnas.97.14.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sandu C, Rexhepaj R, Grahammer F, et al. Decreased intestinal glucose transport in the sgk3-knockout mouse. Pflugers Arch. 2005;451:437–44. doi: 10.1007/s00424-005-1474-7. [DOI] [PubMed] [Google Scholar]
- 87.Vayro S, Silverman M. PKC regulates turnover rate of rabbit intestinal Na+-glucose transporter expressed in COS-7 cells. Am J Physiol. 1999;276:C1053–C1060. doi: 10.1152/ajpcell.1999.276.5.C1053. [DOI] [PubMed] [Google Scholar]
- 88.Castaneda-Sceppa C, Subramanian S, Castaneda F. Protein kinase C mediated intracellular signaling pathways are involved in the regulation of sodium-dependent glucose co-transporter SGLT1 activity. J Cell Biochem. 2010;109:1109–17. doi: 10.1002/jcb.22489. [DOI] [PubMed] [Google Scholar]
- 89.Osswald C, Baumgarten K, Stumpel F, et al. Mice without the regulator gene Rsc1A1 exhibit increased Na+-D-glucose cotransport in small intestine and develop obesity. Mol Cell Biol. 2005;25:78–87. doi: 10.1128/MCB.25.1.78-87.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Korn T, Kuhlkamp T, Track C, et al. The plasma membrane-associated protein RS1 decreases transcription of the transporter SGLT1 in confluent LLC-PK1 cells. J Biol Chem. 2001;276:45330–40. doi: 10.1074/jbc.M105975200. [DOI] [PubMed] [Google Scholar]
- 91.Filatova A, Leyerer M, Gorboulev V, et al. Novel shuttling domain in a regulator (RSC1A1) of transporter SGLT1 steers cell cycle-dependent nuclear location. Traffic. 2009;10:1599–618. doi: 10.1111/j.1600-0854.2009.00982.x. [DOI] [PubMed] [Google Scholar]
- 92.Elvira B, Blecua M, Luo D, et al. SPAK-sensitive regulation of glucose transporter SGLT1. J Membr Biol. 2014;247:1191–7. doi: 10.1007/s00232-014-9719-z. [DOI] [PubMed] [Google Scholar]
- 93.Yarandi SS, Hebbar G, Sauer CG, et al. Diverse roles of leptin in the gastrointestinal tract: modulation of motility, absorption, growth, and inflammation. Nutrition. 2011;27:269–75. doi: 10.1016/j.nut.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ducroc R, Guilmeau S, Akasbi K, et al. Luminal leptin induces rapid inhibition of active intestinal absorption of glucose mediated by sodium-glucose cotransporter 1. Diabetes. 2005;54:348–54. doi: 10.2337/diabetes.54.2.348. [DOI] [PubMed] [Google Scholar]
- 95.Ogata H, Seino Y, Harada N, et al. KATP channel as well as SGLT1 participates in GIP secretion in the diabetic state. J Endocrinol. 2014;222:191–200. doi: 10.1530/JOE-14-0161. [DOI] [PubMed] [Google Scholar]
- 96.Fujita Y, Kojima H, Hidaka H, et al. Increased intestinal glucose absorption and postprandial hyperglycaemia at the early step of glucose intolerance in Otsuka Long-Evans Tokushima Fatty rats. Diabetologia. 1998;41:1459–66. doi: 10.1007/s001250051092. [DOI] [PubMed] [Google Scholar]
- 97.Dyer J, Wood IS, Palejwala A, et al. Expression of monosaccharide transporters in intestine of diabetic humans. Am J Physiol Gastrointest Liver Physiol. 2002;282:G241–G248. doi: 10.1152/ajpgi.00310.2001. [DOI] [PubMed] [Google Scholar]
- 98.Layman DK, Clifton P, Gannon MC, et al. Protein in optimal health: heart disease and type 2 diabetes. Am J Clin Nutr. 2008;87:1571S–5S. doi: 10.1093/ajcn/87.5.1571S. [DOI] [PubMed] [Google Scholar]
- 99.Holst JJ. Enteroendocrine secretion of gut hormones in diabetes, obesity and after bariatric surgery. Curr Opin Pharmacol. 2013;13:983–8. doi: 10.1016/j.coph.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 100.Mandel LJ. Metabolic substrates, cellular energy production, and the regulation of proximal tubular transport. Annu Rev Physiol. 1985;47:85–101. doi: 10.1146/annurev.ph.47.030185.000505. [DOI] [PubMed] [Google Scholar]
- 101.Vallon V, Sharma K. Sodium-glucose transport: role in diabetes mellitus and potential clinical implications. Curr Opin Nephrol Hypertens. 2010;19:425–31. doi: 10.1097/MNH.0b013e32833bec06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Vallon V, Grahammer F, Richter K, et al. Role of KCNE1-dependent K+ fluxes in mouse proximal tubule. J Am Soc Nephrol. 2001;12:2003–11. doi: 10.1681/ASN.V12102003. [DOI] [PubMed] [Google Scholar]
- 103.Komoroski B, Vachharajani N, Boulton D, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther. 2009;85:520–6. doi: 10.1038/clpt.2008.251. [DOI] [PubMed] [Google Scholar]
- 104.Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:613–21. doi: 10.1111/dom.12073. [DOI] [PubMed] [Google Scholar]
- 105.Sha S, Devineni D, Ghosh A, et al. Canagliflozin, a novel inhibitor of sodium glucose co-transporter 2, dose dependently reduces calculated renal threshold for glucose excretion and increases urinary glucose excretion in healthy subjects. Diabetes Obes Metab. 2011;13:669–72. doi: 10.1111/j.1463-1326.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 106*.Powell DR, DaCosta CM, Smith M, et al. Effect of LX4211 on glucose homeostasis and body composition in preclinical models. J Pharmacol Exp Ther. 2014;350:232–42. doi: 10.1124/jpet.114.214304. [These preclinical studies show that LX4211 improves glycemic control and promotes weight loss by dual SGLT1/SGLT2 inhibition.] [DOI] [PubMed] [Google Scholar]
- 107.Vallon V, Gerasimova M, Rose MA, et al. SGLT2 inhibitor empagliflozin reduces renal growth and albuminuria in proportion to hyperglycemia and prevents glomerular hyperfiltration in diabetic Akita mice. Am J Physiol Renal Physiol. 2014;306:F194–F204. doi: 10.1152/ajprenal.00520.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–25. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 109.Vallon V, Thomson SC. Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol. 2012;74:351–75. doi: 10.1146/annurev-physiol-020911-153333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vestri S, Okamoto MM, De Freitas HS, et al. Changes in sodium or glucose filtration rate modulate expression of glucose transporters in renal proximal tubular cells of rat. J Membr Biol. 2001;182:105–12. doi: 10.1007/s00232-001-0036-y. [DOI] [PubMed] [Google Scholar]
- 111.Vidotti DB, Arnoni CP, Maquigussa E, Boim MA. Effect of long-term type 1 diabetes on renal sodium and water transporters in rats. Am J Nephrol. 2008;28:107–14. doi: 10.1159/000109967. [DOI] [PubMed] [Google Scholar]
- 112.Tabatabai NM, Sharma M, Blumenthal SS, Petering DH. Enhanced expressions of sodium-glucose cotransporters in the kidneys of diabetic Zucker rats. Diabetes Res Clin Pract. 2009;83:e27–e30. doi: 10.1016/j.diabres.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gembardt F, Bartaun C, Jarzebska N, et al. The SGLT2 inhibitor empagliflozin ameliorates early features of diabetic nephropathy in BTBR ob/ob type 2 diabetic mice with and without hypertension. Am J Physiol Renal Physiol. 2014;307:F317–F325. doi: 10.1152/ajprenal.00145.2014. [DOI] [PubMed] [Google Scholar]
- 114.Adachi T, Yasuda K, Okamoto Y, et al. T-1095, a renal Na+-glucose transporter inhibitor, improves hyperglycemia in streptozotocin-induced diabetic rats. Metabolism. 2000;49:990–5. doi: 10.1053/meta.2000.7729. [DOI] [PubMed] [Google Scholar]
- 115.Kamran M, Peterson RG, Dominguez JH. Overexpression of GLUT2 gene in renal proximal tubules of diabetic Zucker rats. J Am Soc Nephrol. 1997;8:943–8. doi: 10.1681/ASN.V86943. [DOI] [PubMed] [Google Scholar]
- 116.Dominguez JH, Camp K, Maianu L, et al. Molecular adaptations of GLUT1 and GLUT2 in renal proximal tubules of diabetic rats. Am J Physiol. 1994;266:F283–F290. doi: 10.1152/ajprenal.1994.266.2.F283. [DOI] [PubMed] [Google Scholar]
- 117.Albertoni Borghese MF, Majowicz MP, Ortiz MC, et al. Expression and activity of SGLT2 in diabetes induced by streptozotocin: relationship with the lipid environment. Nephron Physiol. 2009;112:45–52. doi: 10.1159/000214214. [DOI] [PubMed] [Google Scholar]
- 118.Marks J, Carvou NJ, Debnam ES, et al. Diabetes increases facilitative glucose uptake and GLUT2 expression at the rat proximal tubule brush border membrane. J Physiol. 2003;553:137–45. doi: 10.1113/jphysiol.2003.046268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goestemeyer AK, Marks J, Srai SK, et al. GLUT2 protein at the rat proximal tubule brush border membrane correlates with protein kinase C (PKC)-betal and plasma glucose concentration. Diabetologia. 2007;50:2209–17. doi: 10.1007/s00125-007-0778-x. [DOI] [PubMed] [Google Scholar]
- 120.Ghezzi C, Wright EM. Regulation of the human Na+ dependent glucose cotransporter hSGLT2. Am J Physiol Cell Physiol. 2012;303:C348–C354. doi: 10.1152/ajpcell.00115.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lang F, Bohmer C, Palmada M, et al. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–78. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 122.Ackermann TF, Boini KM, Volkl H, et al. SGK1-sensitive renal tubular glucose reabsorption in diabetes. Am J Physiol Renal Physiol. 2009;296:F859–F866. doi: 10.1152/ajprenal.90238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Han HJ, Lee YJ, Park SH, et al. High glucose-induced oxidative stress inhibits Na+/glucose cotransporter activity in renal proximal tubule cells. Am J Physiol Renal Physiol. 2005;288:F988–F996. doi: 10.1152/ajprenal.00327.2004. [DOI] [PubMed] [Google Scholar]
- 124.Schwenk RW, Luiken JJ, Bonen A, Glatz JF. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res. 2008;79:249–58. doi: 10.1093/cvr/cvn116. [DOI] [PubMed] [Google Scholar]
- 125.Banerjee SK, McGaffin KR, Pastor-Soler NM, Ahmad F. SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states. Cardiovasc Res. 2009;84:111–8. doi: 10.1093/cvr/cvp190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126*.Kashiwagi Y, Nagoshi T, Yoshino T, et al. Expression of SGLT1 in Human Hearts and Impairment of Cardiac Glucose Uptake by Phlorizin during Ischemia-Reperfusion Injury in Mice. PLoS One. 2015;10:e0130605. doi: 10.1371/journal.pone.0130605. [The study provides evidence that SGLT1 may provide an important protective mechanism against schemia reperfusion injury by replenishing ATP stores in ischemic cardiac tissues via enhancing the availability of glucose, and provide new insights into the significant role of SGLTs in optimizing cardiac energy metabolism, at least during the acute phase of IRI.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou L, Cryan EV, D'Andrea MR, et al. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1). J Cell Biochem. 2003;90:339–46. doi: 10.1002/jcb.10631. [DOI] [PubMed] [Google Scholar]
- 128.Elfeber K, Stumpel F, Gorboulev V, et al. Na(+)-D-glucose cotransporter in muscle capillaries increases glucose permeability. Biochem Biophys Res Commun. 2004;314:301–5. doi: 10.1016/j.bbrc.2003.12.090. [DOI] [PubMed] [Google Scholar]
- 129.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 130.Asghar O, Al-Sunni A, Khavandi K, et al. Diabetic cardiomyopathy. Clin Sci (Lond) 2009;116:741–60. doi: 10.1042/CS20080500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Abel ED, Kaulbach HC, Tian R, et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. J Clin Invest. 1999;104:1703–14. doi: 10.1172/JCI7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garvey WT, Hardin D, Juhaszova M, Dominguez JH. Effects of diabetes on myocardial glucose transport system in rats: implications for diabetic cardiomyopathy. Am J Physiol. 1993;264:H837–H844. doi: 10.1152/ajpheart.1993.264.3.H837. [DOI] [PubMed] [Google Scholar]
- 133.Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–E1113. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- 134.Liao R, Jain M, Cui L, et al. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002;106:2125–31. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- 135.Pereira RO, Wende AR, Olsen C, et al. GLUT1 deficiency in cardiomyocytes does not accelerate the transition from compensated hypertrophy to heart failure. J Mol Cell Cardiol. 2014;72:95–103. doi: 10.1016/j.yjmcc.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pereira RO, Wende AR, Olsen C, et al. Inducible overexpression of GLUT1 prevents mitochondrial dysfunction and attenuates structural remodeling in pressure overload but does not prevent left ventricular dysfunction. J Am Heart Assoc. 2013;2:e000301. doi: 10.1161/JAHA.113.000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu Q, Wang S, Cai L. Diabetic cardiomyopathy and its mechanisms: Role of oxidative stress and damage. J Diabetes Investig. 2014;5:623–34. doi: 10.1111/jdi.12250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Balteau M, Tajeddine N, de MC, et al. NADPH oxidase activation by hyperglycaemia in cardiomyocytes is independent of glucose metabolism but requires SGLT1. Cardiovasc Res. 2011;92:237–46. doi: 10.1093/cvr/cvr230. [DOI] [PubMed] [Google Scholar]
- 139.Mellor KM, Varma U, Stapleton DI, Delbridge LM. Cardiomyocyte glycophagy is regulated by insulin and exposure to high extracellular glucose. Am J Physiol Heart Circ Physiol. 2014;306:H1240–H1245. doi: 10.1152/ajpheart.00059.2014. [DOI] [PubMed] [Google Scholar]
- 140.Shearer J, Ross KD, Hughey CC, et al. Exercise training does not correct abnormal cardiac glycogen accumulation in the db/db mouse model of type 2 diabetes. Am J Physiol Endocrinol Metab. 2011;301:E31–E39. doi: 10.1152/ajpendo.00525.2010. [DOI] [PubMed] [Google Scholar]
- 141.Ramratnam M, Sharma RK, D'Auria S, et al. Transgenic knockdown of cardiac sodium/glucose cotransporter 1 (SGLT1) attenuates PRKAG2 cardiomyopathy, whereas transgenic overexpression of cardiac SGLT1 causes pathologic hypertrophy and dysfunction in mice. J Am Heart Assoc. 2014:3. doi: 10.1161/JAHA.114.000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Banerjee SK, Wang DW, Alzamora R, et al. SGLT1, a novel cardiac glucose transporter, mediates increased glucose uptake in PRKAG2 cardiomyopathy. J Mol Cell Cardiol. 2010;49:683–92. doi: 10.1016/j.yjmcc.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Petersen C. Analyse des Phloridzins. Annales Academie Science Francaise. 1835;15:178. [Google Scholar]
- 144.Castaneda F, Burse A, Boland W, Kinne RK. Thioglycosides as inhibitors of hSGLT1 and hSGLT2: potential therapeutic agents for the control of hyperglycemia in diabetes. Int J Med Sci. 2007;4:131–9. doi: 10.7150/ijms.4.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21:31–8. doi: 10.1002/dmrr.532. [DOI] [PubMed] [Google Scholar]
- 146.Sands AT, Zambrowicz BP, Rosenstock J, et al. Sotagliflozin, a Dual SGLT1 and SGLT2 Inhibitor, as Adjunct Therapy to Insulin in Type 1 Diabetes. Diabetes Care. 2015;38:1181–8. doi: 10.2337/dc14-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Goodwin NC, Mabon R, Harrison BA, et al. Novel L-xylose derivatives as selective sodium-dependent glucose cotransporter 2 (SGLT2) inhibitors for the treatment of type 2 diabetes. J Med Chem. 2009;52:6201–4. doi: 10.1021/jm900951n. [DOI] [PubMed] [Google Scholar]
- 148.Powell DR, Smith MG, Doree DD, et al. LP-925219 maximizes urinary glucose excretion in mice by inhibiting both renal SGLT1 and SGLT2. Pharmacol Res Perspect. 2015;3:e00129. doi: 10.1002/prp2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fushimi N, Teranishi H, Shimizu K, et al. Design, synthesis, and structure-activity relationships of a series of 4-benzyl-5-isopropyl-1H-pyrazol-3-yl beta-D-glycopyranosides substituted with novel hydrophilic groups as highly potent inhibitors of sodium glucose co-transporter 1 (SGLT1). Bioorg Med Chem. 2013;21:748–65. doi: 10.1016/j.bmc.2012.11.041. [DOI] [PubMed] [Google Scholar]
- 150.Fushimi N, Fujikura H, Shiohara H, et al. Structure-activity relationship studies of 4-benzyl-1H-pyrazol-3-yl beta-d-glucopyranoside derivatives as potent and selective sodium glucose co-transporter 1 (SGLT1) inhibitors with therapeutic activity on postprandial hyperglycemia. Bioorg Med Chem. 2012;20:6598–612. doi: 10.1016/j.bmc.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 151.Qiao Q, Tuomilehto J, Borch-Johnsen K. Post-challenge hyperglycaemia is associated with premature death and macrovascular complications. Diabetologia. 2003;46(Suppl 1):M17–M21. doi: 10.1007/s00125-002-0932-4. [DOI] [PubMed] [Google Scholar]
- 152.O'Keefe JH, Bell DS. Postprandial hyperglycemia/hyperlipidemia (postprandial dysmetabolism) is a cardiovascular risk factor. Am J Cardiol. 2007;100:899–904. doi: 10.1016/j.amjcard.2007.03.107. [DOI] [PubMed] [Google Scholar]
- 153.Schulze C, Bangert A, Kottra G, et al. Inhibition of the intestinal sodium-coupled glucose transporter 1 (SGLT1) by extracts and polyphenols from apple reduces postprandial blood glucose levels in mice and humans. Mol Nutr Food Res. 2014;58:1795–808. doi: 10.1002/mnfr.201400016. [DOI] [PubMed] [Google Scholar]
- 154.Polidori D, Sha S, Mudaliar S, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care. 2013;36:2154–61. doi: 10.2337/dc12-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oguma T, Nakayama K, Kuriyama C, et al. Intestinal Sodium Glucose Cotransporter 1 Inhibition Enhances Glucagon-Like Peptide-1 Secretion in Normal and Diabetic Rodents. J Pharmacol Exp Ther. 2015;354:279–89. doi: 10.1124/jpet.115.225508. [DOI] [PubMed] [Google Scholar]
- 156.Rossetti L, Smith D, Shulman GI, et al. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79:1510–5. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wright EM. I. Glucose galactose malabsorption. Am J Physiol. 1998;275:G879–G882. doi: 10.1152/ajpgi.1998.275.5.G879. [DOI] [PubMed] [Google Scholar]
- 158.Kiela PR, Xu H, Ghishan FK. Apical NA+/H+ exchangers in the mammalian gastrointestinal tract. J Physiol Pharmacol. 2006;57(Suppl 7):51–79. [PubMed] [Google Scholar]
- 159.Joshi SR, Standl E, Tong N, et al. Therapeutic potential of alpha-glucosidase inhibitors in type 2 diabetes mellitus: an evidence-based review. Expert Opin Pharmacother. 2015;16:1959–81. doi: 10.1517/14656566.2015.1070827. [DOI] [PubMed] [Google Scholar]
- 160.Cai Q, Li B, Yu F, et al. Investigation of the Protective Effects of Phlorizin on Diabetic Cardiomyopathy in db/db Mice by Quantitative Proteomics. J Diabetes Res. 2013;2013:263845. doi: 10.1155/2013/263845. [This paper reported that intragastrical administration of phlorizin can prevent the phenotype of diabetic cardiomyopathy, including hypertrophy and disarray of cardiomyocytes in T2DM db/db mice, through modulating cardiac lipid and energy metabolism and altering the expression of a set of proteins involved in cardiac damage.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gribble FM, Williams L, Simpson AK, Reimann F. A novel glucose-sensing mechanism contributing to glucagon-like peptide-1 secretion from the GLUTag cell line. Diabetes. 2003;52:1147–54. doi: 10.2337/diabetes.52.5.1147. [DOI] [PubMed] [Google Scholar]
- 162.Kim HR, Park SW, Cho HJ, et al. Comparative gene expression profiles of intestinal transporters in mice, rats and humans. Pharmacol Res. 2007;56:224–36. doi: 10.1016/j.phrs.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 163.Morrison AI, Panayotova-Heiermann M, Feigl G, et al. Sequence comparison of the sodium-D-glucose cotransport systems in rabbit renal and intestinal epithelia. Biochim Biophys Acta. 1991;1089:121–3. doi: 10.1016/0167-4781(91)90093-2. [DOI] [PubMed] [Google Scholar]
- 164.Coady MJ, Pajor AM, Wright EM. Sequence homologies among intestinal and renal Na+/glucose cotransporters. Am J Physiol. 1990;259:C605–C610. doi: 10.1152/ajpcell.1990.259.4.C605. [DOI] [PubMed] [Google Scholar]
- 165.Yoshikawa T, Inoue R, Matsumoto M, et al. Comparative expression of hexose transporters (SGLT1, GLUT1, GLUT2 and GLUT5) throughout the mouse gastrointestinal tract. Histochem Cell Biol. 2011;135:183–94. doi: 10.1007/s00418-011-0779-1. [DOI] [PubMed] [Google Scholar]
- 166.Tabatabai NM, Blumenthal SS, Lewand DL, Petering DH. Differential regulation of mouse kidney sodium-dependent transporters mRNA by cadmium. Toxicol Appl Pharmacol. 2001;177:163–73. doi: 10.1006/taap.2001.9321. [DOI] [PubMed] [Google Scholar]
- 167.Haase W, Heitmann K, Friese W, et al. Characterization and histochemical localization of the rat intestinal Na(+)-D-glucose cotransporter by monoclonal antibodies. Eur J Cell Biol. 1990;52:297–309. [PubMed] [Google Scholar]
- 168.Sabolic I, Skarica M, Gorboulev V, et al. Rat renal glucose transporter SGLT1 exhibits zonal distribution and androgen-dependent gender differences. Am J Physiol Renal Physiol. 2006;290:F913–F926. doi: 10.1152/ajprenal.00270.2005. [DOI] [PubMed] [Google Scholar]
- 169.Koepsell H, Korn K, Raszeja-Specht A, et al. Monoclonal antibodies against the renal Na+-D-glucose cotransporter. Identification of antigenic polypeptides and demonstration of functional coupling of different Na+-cotransport systems. J Biol Chem. 1988;263:18419–29. [PubMed] [Google Scholar]
- 170.Haase W, Koepsell H. Electron microscopic immunohistochemical localization of components of Na+-cotransporters along the rat nephron. Eur J Cell Biol. 1989;48:360–74. [PubMed] [Google Scholar]
- 171.Lazaridis KN, Pham L, Vroman B, et al. Kinetic and molecular identification of sodium-dependent glucose transporter in normal rat cholangiocytes. Am J Physiol. 1997;272:G1168–G1174. doi: 10.1152/ajpgi.1997.272.5.G1168. [DOI] [PubMed] [Google Scholar]
- 172.Mamchaoui K, Makhloufi Y, Saumon G. Glucose transporter gene expression in freshly isolated and cultured rat pneumocytes. Acta Physiol Scand. 2002;175:19–24. doi: 10.1046/j.1365-201X.2002.00970.x. [DOI] [PubMed] [Google Scholar]
- 173.Bodega F, Sironi C, Armilli M, et al. Evidence for Na+-glucose cotransporter in type I alveolar epithelium. Histochem Cell Biol. 2010;134:129–36. doi: 10.1007/s00418-010-0725-7. [DOI] [PubMed] [Google Scholar]
- 174.Sironi C, Bodega F, Porta C, et al. Expression of Na+-glucose cotransporter (SGLT1) in visceral and parietal mesothelium of rabbit pleura. Respir Physiol Neurobiol. 2007;159:68–75. doi: 10.1016/j.resp.2007.05.012. [DOI] [PubMed] [Google Scholar]