Abstract
Macrophages play a role in innate immunity within the body, are located in muscle tissue, and can release inflammatory cytokines that sensitize local nociceptors. Here we investigate the role of resident macrophages in the non-inflammatory muscle pain model induced by 2 pH 4.0 injections 5 days apart in the gastrocnemius muscle. We demonstrate that injecting 2 pH 4.0 injections into the gastrocnemius muscle increased the number of local muscle macrophages, and depleting muscle macrophages with clodronate liposomes prior to acid injections attenuated the hyperalgesia produced by this model. To further examine the contribution of local macrophages to this hyperalgesia, we injected mice intramuscularly with C34, a TLR4 receptor antagonist. When given before the first pH 4.0 injection, C34 attenuated the muscle and tactile hyperalgesia produced by the model. However, when given before the second injection C34 had no effect on the development of hyperalgesia. Then to test whether activation of local macrophages sensitizes nociceptors to normally non-nociceptive stimuli we replaced either the first or second acid injection with the immune cell activator lipopolysaccharide (LPS), or the inflammatory cytokine interleukin-6 (IL-6). Injecting LPS or IL-6 instead of the either the first or second pH 4.0 injection resulted in a dose-dependent increase in paw withdrawal responses and decrease in muscle withdrawal thresholds. The highest doses of LPS and IL-6 resulted in development of hyperalgesia bilaterally. The present study shows that resident macrophages in muscle are key to development of chronic muscle pain.
Keywords: macrophages, hyperalgesia, pain, proton, cytokine
Introduction
Macrophages play a role in innate immunity within the body, protect the organism against disease, and are located in nearly every tissue type including muscle [4;29;33]. Classical macrophages (M1) are involved in host defense, can arise in response to stress, and secrete pro-inflammatory cytokines that subsequently activate nociceptors to produce hyperalgesia and pain [3;6;45;47]. Prior studies show mixed results for the role of macrophages in development of hyperalgesia after injury. In inflammatory models, systemic blockade or depletion of macrophages respectively, prolongs hyperalgesia to intraplantar interleukin-1β (IL-1β) or carrageenan [62] suggesting macrophages contribute to resolution of inflammatory pain. On the other hand, Ulmann and colleagues [57] show that P2X4−/− mice have reduced hyperalgesia induced by intraplantar injection of formalin, carrageenan or Complete Freund’s Adjuvant (CFA). They further show that P2X4 is expressed on resident macrophages in the injected paw, and thus together these data suggest that resident macrophages are important in the generation of inflammatory pain.
Chronic muscle pain conditions, like fibromyalgia, affect between 11–24% of the population [7]. However, the pathophysiology of chronic non-inflammatory muscle pain is virtually unknown. While there are clear clinical manifestations of pain and disability, chronic muscle pain conditions like fibromyalgia can persist in the absence of overt tissue injury or inflammation. To mimic this non-inflammatory pain, we developed an animal model that results in widespread hyperalgesia without tissue damage or inflammation that is induced by 2 intramuscular acid injections 5 days apart [50]. The long-lasting hyperalgesia does not develop in those with a single injection, and is prevented by blockade of acid-sensing ion channels (ASICs) in muscle during the first or second injection [25;52]. Muscle releases a number of factors that could directly activate macrophages including protons, lactate and ATP through ASICs and purinergic receptors which are expressed on macrophages [11;27;31;56]. In addition, exercise increases plasma and muscle interleukin-6 (IL-6) concentrations and increases mRNA levels of IL-6 in exercising muscle [20;42]. Interestingly, a prior injection of IL-6 in muscle can enhance hyperalgesia to a subsequent noxious stimuli [12]. Moreover, IL-6 is released from macrophages after LPS stimulation, IL-6 receptors are located on macrophages, and IL-6 increases infiltration of macrophages after injection [33;67].
Together these data led to the hypothesis that resident macrophages in muscle are critical for the development of chronic muscle pain after repeated acid injection. Specifically we tested 1) if depletion or blockade of macrophages in muscle prevents the development of hyperalgesia to repeated acid injections, 2) if there are changes in expression of macrophages in muscle after repeated acid injections and after activation of macrophages, 3) if activation of macrophages by lipopolysaccharide (LPS) or IL-6 mimics the effects of acidic saline, and 4) if macrophages release inflammatory cytokines to LPS or acid injection.
Materials and methods
All experiments were approved by the Animal Care and Use Committee at the University of Iowa and are in accordance with the guidelines of the National Institute of Health policies on the use of laboratory animals. A total of 146 C57BL/6J mice (73 male, 73 female) (19–22 g) were used in this study. Mice were housed in transparent plastic cages with free access to food and water and a 12 h light–dark cycle. We used an equal number of male and female mice in each experiment. No differences between male and female mice were observed for any of the groups tested and therefore all data were combined and analyzed by condition.
Induction of Muscle Hyperalgesia
To induce chronic muscle hyperalgesia, acid saline was injected twice into the left gastrocnemius muscle as described previously [50]. Before injection, mice were anesthetized briefly with 4% isoflurane (Piramal Healthcare Limited, Andhra Pradesh, India) in oxygen at a flow rate of 2L/min. The left gastrocnemius muscle was injected with 20 µl of pH 4.0 preservative-free sterile saline (Day 0). Five days later, the same muscle was re-injected with the same pH and volume (Day 5). The pH was adjusted with HCl to within 0.1 pH.
Behavioral assessments
Animals were acclimated to the testing room and procedures 2 times per day for 2 days. After transport to the laboratory from the animal care facility, animals were acclimated to the testing room for 30 minutes, and then were placed in transparent Lucite cubicles (3 × 5 × 9 cm3) on an elevated mesh platform for 20 min to acclimate (paw withdrawal response test) and in a gardener’s glove for 5 min to acclimate (muscle withdrawal threshold test).
Behavioral tests were done with the examiner blinded to group. Paw withdrawal responses were tested using a von Frey filaments with 0.4 mN applied to the plantar surface of the paw as previously described [61]. The number of withdrawals out of 5 applications was recorded in 10 trials, and an average of all 10 trials was determined for each time period. The number of responses was measured bilaterally. An increase in the number of responses was interpreted as cutaneous hyperalgesia.
Muscle withdrawal threshold was tested using a pair of calibrated forceps applied to the gastrocnemius muscle as previously described [61]. The force at which the animal withdrew the hindlimb was recorded as the muscle withdrawal threshold in mN. Three trials, spaced 5 min apart, were averaged to obtain 1 reading at each time point. Withdrawal thresholds were measured bilaterally. A decrease in muscle withdrawal threshold is interpreted as muscle hyperalgesia.
Depletion of local macrophages
To deplete local macrophages in the injected gastrocnemius muscle, clodronate liposomes (Clod) (clodronateliposomes.com, Haarlem, The Netherlands) were injected twice into the muscle. This was compared to 2 injections of PBS-containing liposomes (PBS-Lipo). Before injection, mice were deeply anesthetized briefly with 4% isoflurane in oxygen at a flow rate of 2 L/min. Clod or PBS-Lipo were injected into 4 sites evenly spaced across the muscle belly (5µl per site) of the ipsilateral gastrocnemius muscle 2 days before the first injection of acidic saline (Day 2). Three days later, the same muscle was re-injected with the same drug, volume and position (Day 1). Liposomes were generated as described previously using phosphatidylcholine (LIPOID E PC; Lipoid GmbH, Ludwigshafen, Germany) and cholesterol (Sigma, St. Louis, MO) [58;59]. Paw withdrawal threshold and muscle withdrawal threshold were assessed before the first injection of pH 4.0 saline (Baseline), before the second injection of pH 4.0 saline (Pre) and 24 h after the second injection of pH 4.0 saline (Post). Twelve mice were randomly divided into 2 groups (n=6/group, 3 males, 3 females): Clod-Lipo and control PBS-Lipo (Fig. 1). Depletion of macrophages in muscle was confirmed using immunohistochemistry for F4/80 and demonstrated a 62% reduction in total macrophages when comparing the 2 groups.
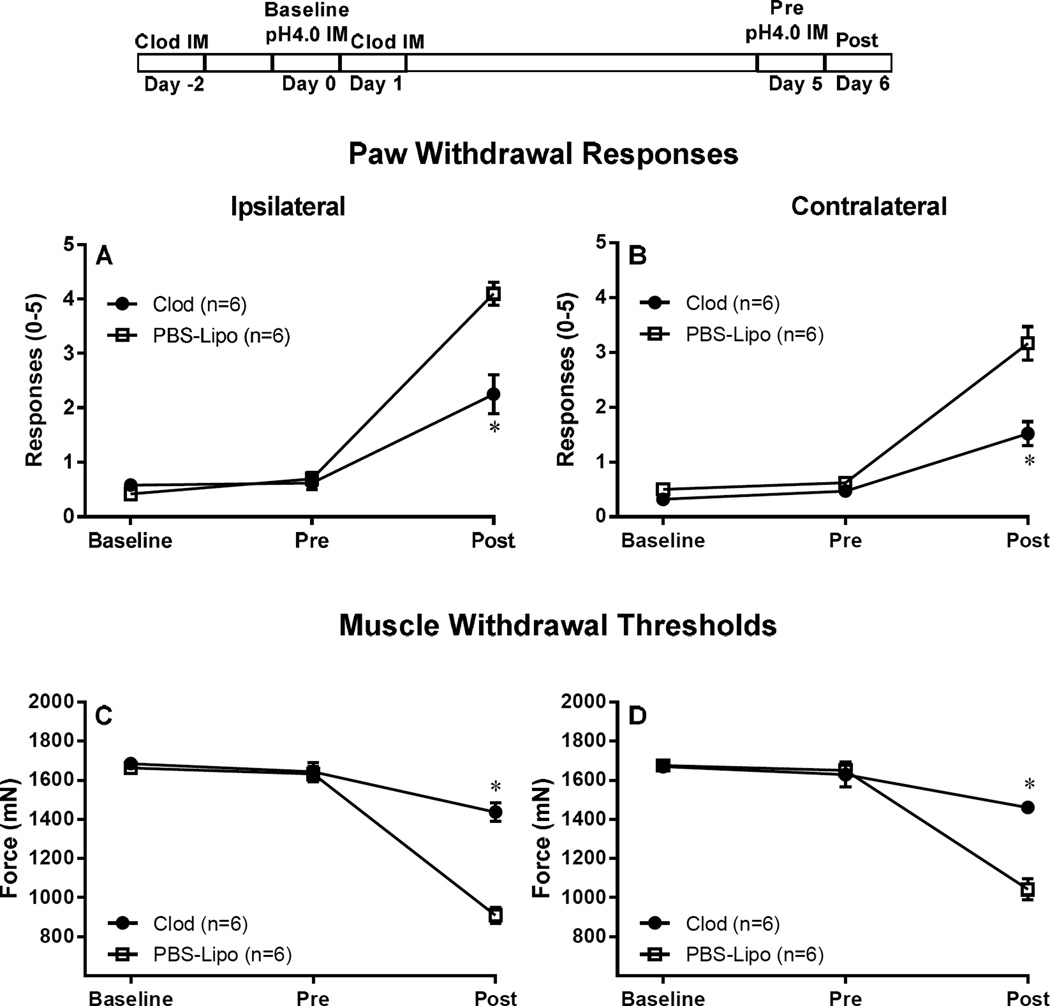
Figure 1. Depletion of resident muscle macrophages attenuates hyperalgesia induced by repeated acid injections.
Resident tissue macrophages, in the left gastrocnemius, were depleted by injecting 2 injections of Clod-Lipo. The injections were administered 2 days before, and 1 day after the first acid injection. Control mice were injected with PBS-Lipo. Acid induced hyperalgesia was induced by two pH 4 injections in the left gastrocnemius muscle, given 5 days apart (experimental protocol is demonstrated on top of figure). Depletion of resident macrophages resulted in attenuation of both, the primary muscle hyperalgesia, and secondary tactile allodynia, as demonstrated by the bilateral increase in muscle withdrawal thresholds (C, D) and decrease in paw withdrawal response when compared to control mice (A, B). *P<0.001, significantly lower in Paw Withdrawal Responses and significant higher in Muscle Withdrawal Threshold in mice treated with Clod-Lipo when compared to mice treated with PBS-Lipo at 24 h after the second acid injection. Data are presented as means ± SEM, n = 6. Clod-lipo: Clodronate-containing liposomes; PBS-Lipo: PBS-containing liposomes; IM: intramuscular injection; pH 4.0: pH 4.0 preservative-free sterile saline;
Blockade of macrophage activation
To attenuate local macrophage activity in the gastrocnemius muscle, C34 (Tocris, Minneapolis, MN) was injected in the gastrocnemius muscle. Control mice were injected with saline. C34 was used to either block TLR4 activation before the first or second acid injection. To block activation before the first injection, 1 mg/ml of 20 µl C34 [40] was injected i.m. 30–45 minutes before the first acid injection and 24h after the first injection. Whereas, to block activation before the second injection, C34 was injected i.m. 30–45 minutes before the second injection of acid. Paw withdrawal threshold and muscle withdrawal threshold were assessed before the first injection of pH 4.0 saline (Baseline) and 24 h after the second injection of pH 4.0 saline (Post). Mice were randomly divided into groups with n=6/group (3 males, 3 females)(Fig. 2,3).
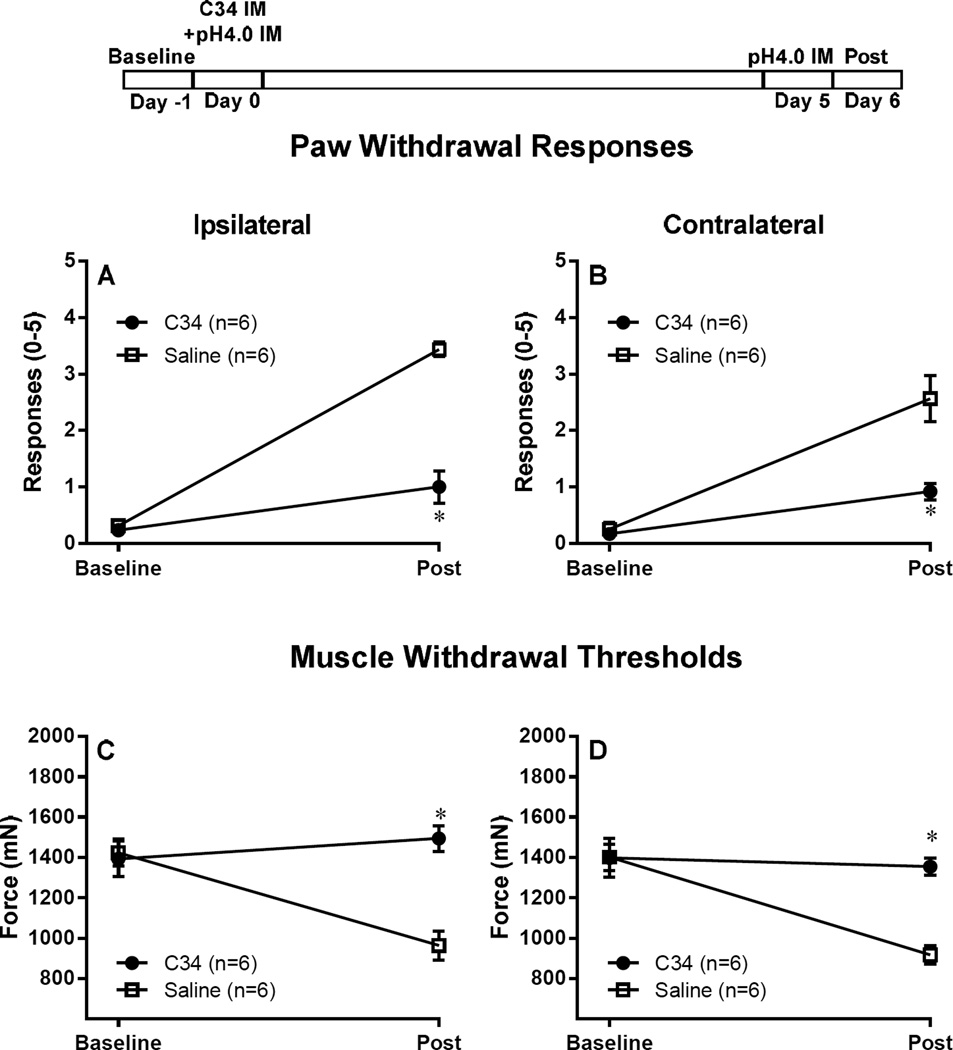
Figure 2. Blocking macrophage activation before initial acid insult, using a TLR4 antagonist, prevents hyperalgesia induced by repeated acid injections.
C34 (1 mg/ml of 20 µl C34 [40]) a TLR4 antagonist was injected i.m. into the left gastrocnemius muscle 30–45 minutes before and 24h after the first injection (experimental protocol is demonstrated on top of figure). Control mice were injected with saline. Blocking macrophage activation prevented both, the primary muscle hyperalgesia, and secondary tactile allodynia, as demonstrated by the bilateral increase in muscle withdrawal thresholds (C, D) and decrease in paw withdrawal response when compared to control mice (A, B). *P<0.001, significantly lower in Paw Withdrawal Responses and significant higher in Muscle Withdrawal Threshold in mice injected with C34 when compared to mice treated with Saline at 24 h after the second acid injection. Data are presented as means ± SEM, n = 6.
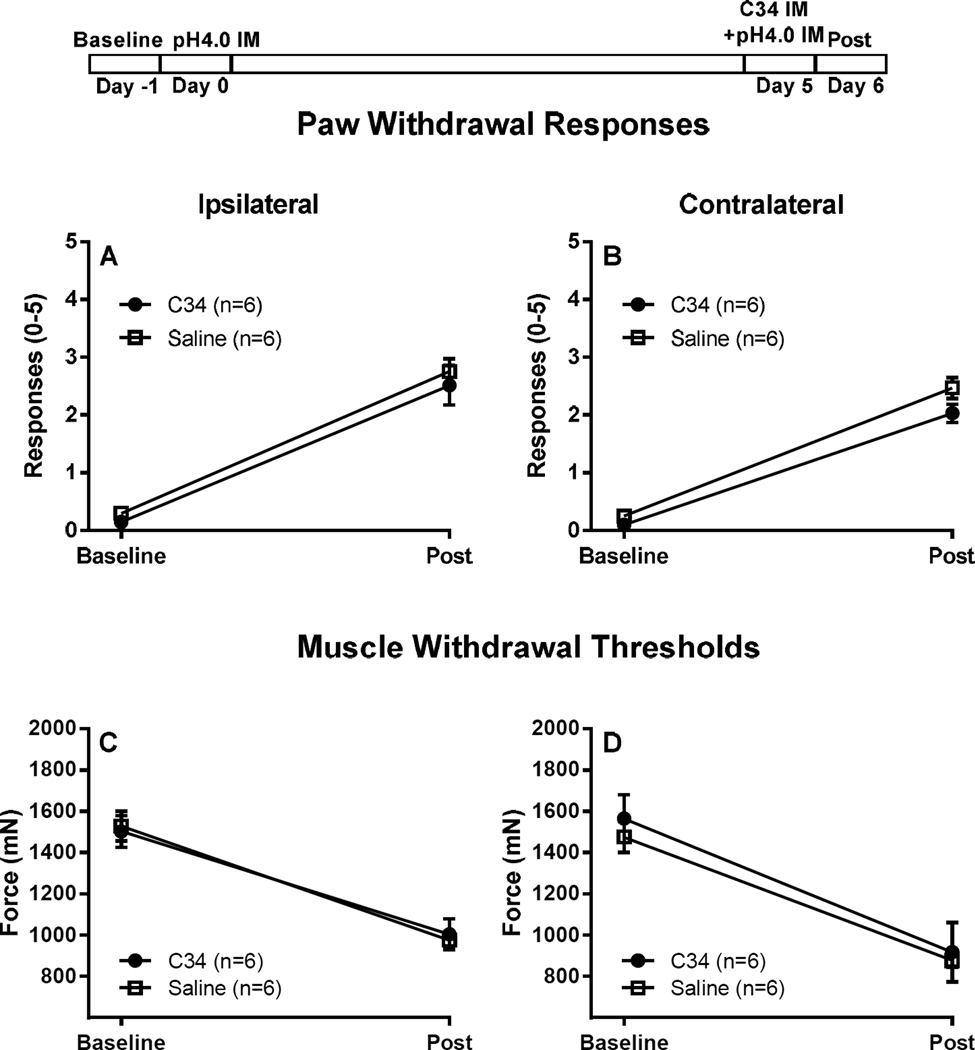
Figure 3. Blocking macrophage activation after first acid injection, but before second acid injection, has no effect on acid induced hyperalgesia.
C34 (1 mg/ml of 20 µl C34 [40]) was injected i.m. into the left gastrocnemius muscle 30–45 minutes after the second acid injection (experimental protocol is demonstrated on top of figure). Control mice were injected with saline. When injected after the second injection C34 had no effect on primary muscle hyperalgesia, and secondary tactile allodynia, as demonstrated by the muscle withdrawal thresholds (C, D) and paw withdrawal response (A, B). Data are presented as means ± SEM, n = 6.
Injection of LPS or IL-6
Before injection, mice were anesthetized briefly with 4% isoflurane in oxygen at a flow rate of 2 L/min. The left gastrocnemius muscle was injected with 20 µl of normal sterile saline, LPS (83–830 ng/20µl; Chondrex, Inc. Redmond, WA), or IL-6 (2–20 ng/20µl; Sigma-Aldrich) (Day 0) instead of the first pH 4.0 saline injection (Fig. 4). Five days later, the same muscle was injected with 20 µl of pH 4.0 saline (Day 5). For Figure 5, the left gastrocnemius muscle was injected with 20 µl of pH 4.0 saline (Day 0). Five days later, the same muscle was injected with 20 µl of normal sterile saline, LPS (83–830 ng/20µl), or IL-6 (2–20 ng/20µl) (Day 5) instead of the second pH 4.0 saline injection. Paw withdrawal threshold and muscle withdrawal threshold were assessed before the first injection of pH 4.0 saline (Baseline) and 24 h after the second injection of pH 4.0 saline. A total of 6 animals per group (3 male, 3 female) were treated with vehicle or drug under each condition; one dose of drug was delivered per animal.
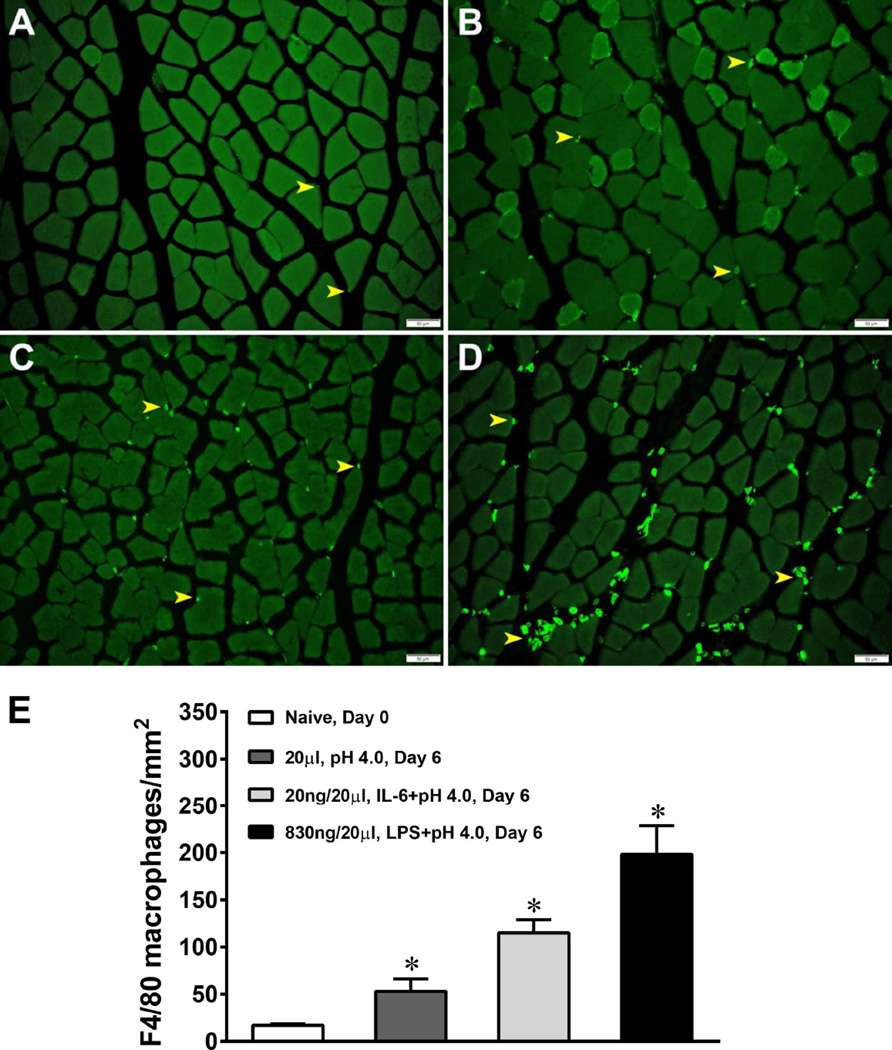
Figure 4. Acid, LPS and IL-6 increase the number of macrophages in the gastrocnemius muscle.
Mice were either injected with 20 µl of pH4.0 saline, 830 ng of LPS or 20ng of IL-6 into the left gastrocnemius muscle. Six days later the left gastrocnemius muscle was extracted and stained for F4/80+ positive cells (macrophages). A significant increase in the number of F4/80+ cells occurred in the muscle on Day 6 when compared to muscles from naïve mice on Day 0 following all 3 treatments. (A) demonstrates a muscle section from a naïve mouse; (B) demonstrates a muscle section from a pH4 injected mouse; (C) demonstrates a muscle section from an IL-6 injected mouse; (D) demonstrates a muscle section from an LPS injected mouse; and (E) demonstrates the average number of macrophages per mm2 of tissue following each treatment. *P<0.001, significantly more macrophages than the naïve mice.
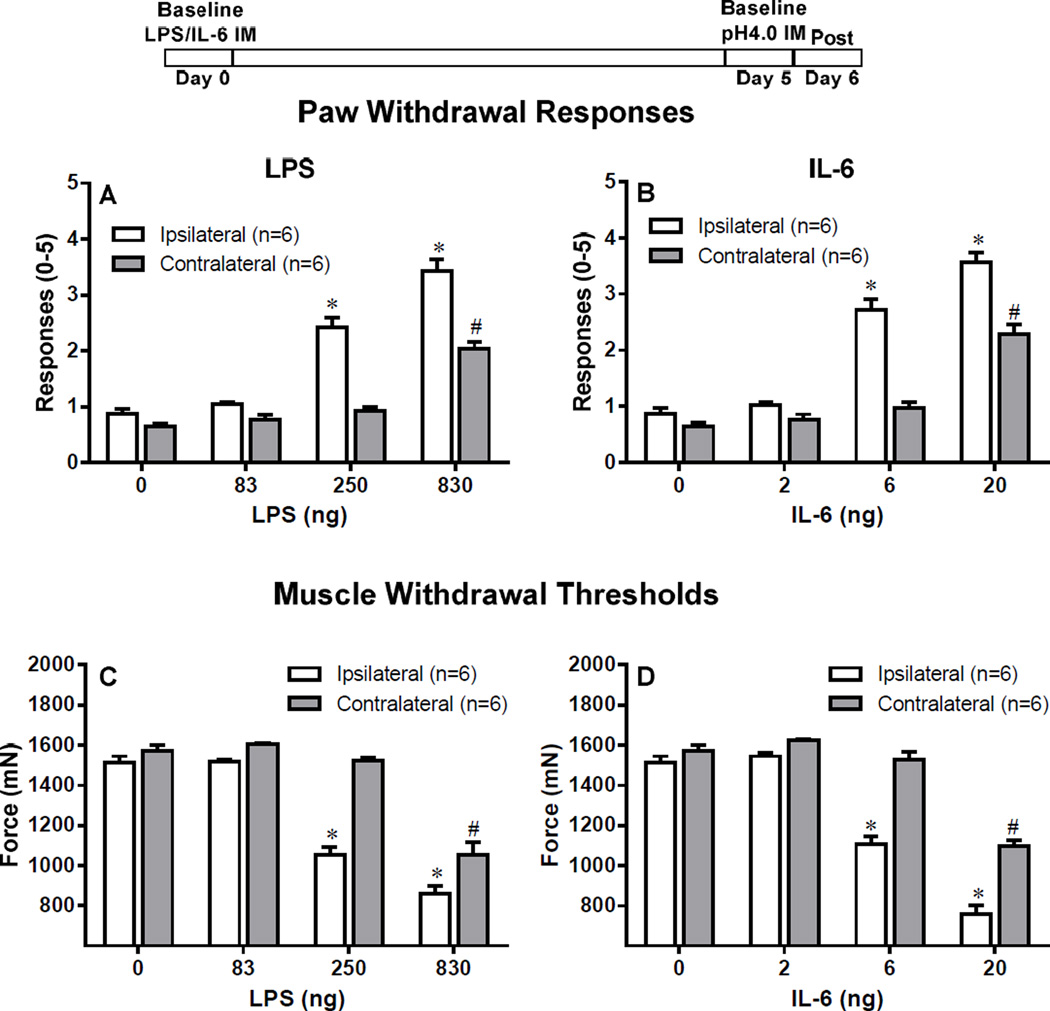
Figure 5. Hyperalgesia remains intact after replacement of the first acid injection with LPS or IL-6.
The first acid injection was either replaced by LPS i.m.(83, 250, 830 ng) or IL-6 (2, 6, 20 ng), and muscle hyperalgesia was recorded. Control mice were injected with saline. Replacing the first acid injection with LPS produced both primary and secondary hyperalgesia in the ipsilateral side with all three doses, however, only the highest dose of LPS produced bilateral hyperalgesia (A, C). Similarly, replacing the first acid injection with IL-6 produced both primary and secondary hyperalgesia in the ipsilateral side with all three doses, however, only the highest dose of IL-6 produced bilateral hyperalgesia (B, D). *P<0.001 (ipsilateral) and #P (contralateral), significantly lower in Paw Withdrawal Responses and significant higher in Muscle Withdrawal Threshold in mice injected with LPS or acid when compared to mice treated with Saline at 24 h after the second acid injection. Data are presented as means ± SEM, n = 6.
Immunohistochemistry of macrophages in muscle
Immunohistochemistry for macrophages was performed on cryopreserved sections of mouse muscle using rat monoclonal antibody recognizing F4/80 antigen (AbDSerotec, Raleigh, North Carolina), a glycoprotein expressed by mature murine macrophages using techniques we previously published [17;30]. Male and female C57BL/6J mice were anesthetized with an intraperitoneal injection of pentobarbital (50 mg/kg) and transcardially perfused with heparinized saline followed by either 1) 4% paraformaldehyde (clodronate quantification), or 2) PLP fixative (2% paraformaldehyde; 75mM Lysine; 10mM periodate; 0.05M Phosphate Buffer) (Fig. 6). The ipsilateral (Left) gastrocnemius muscle was dissected and post-fixed overnight with either 4% paraformaldehyde or PLP at 4°C. The next day the muscle was washed with 0.1M Sorenson’s buffer and sequentially incubated in 15% sucrose in PBS for 24 h at 4°C, 30% sucrose in PBS for 24 h at 4°C, and 1:1 mixture of 30% sucrose and OCT mounting media for 24 h at 4°C. Finally the tissues were snap frozen in OCT using gentle Jane and stored at −80°C until cryosectioned . Muscle tissues were cut at 20µm, placed on slides and immunostained for macrophages. Groups were stained simultaneously to minimize experimental variability.
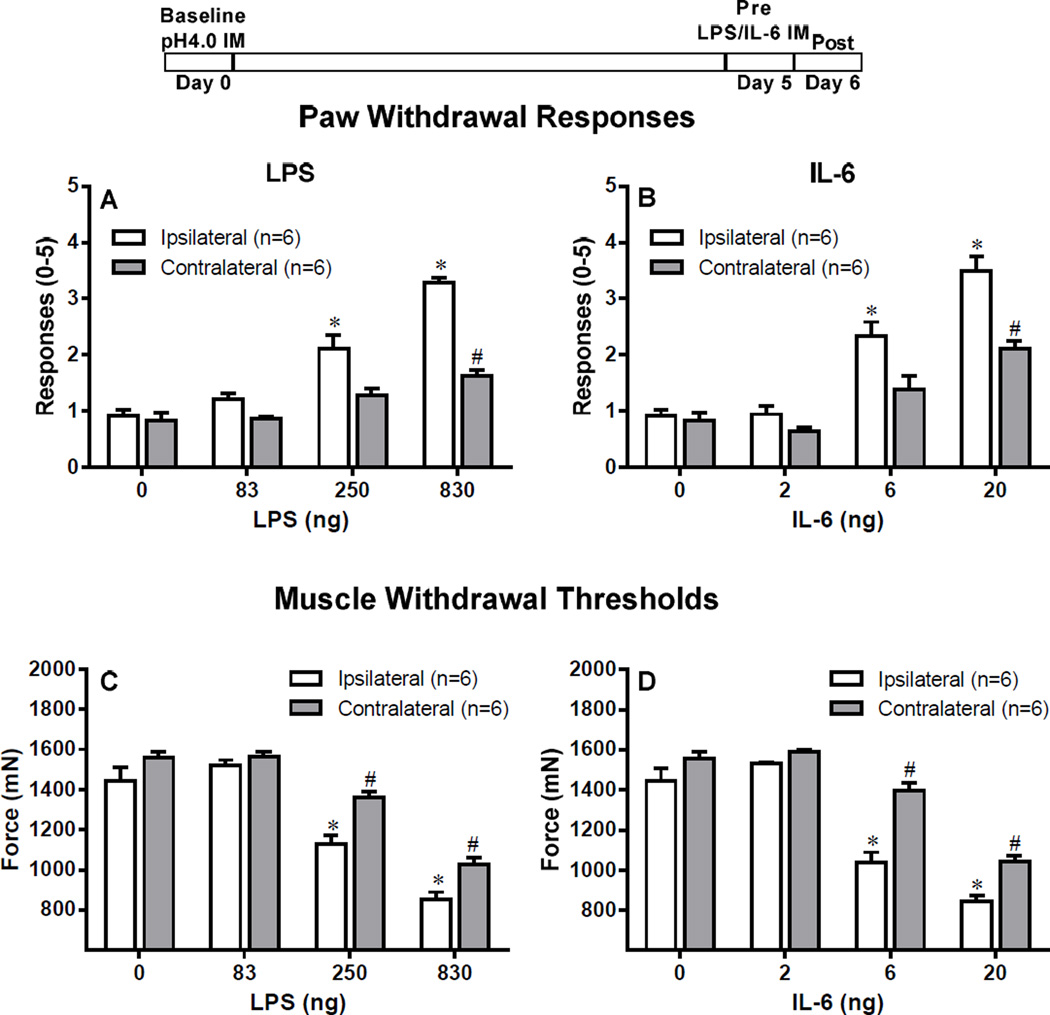
Figure 6. Hyperalgesia remains intact after replacement of the second acid injection with LPS or IL-6.
The second acid injection was either replaced by LPS i.m.(83, 250, 830 ng) or IL-6 (2, 6, 20 ng), and muscle hyperalgesia was recorded. Control mice were injected with saline. Replacing the second acid injection with LPS produced both primary and secondary hyperalgesia in the ipsilateral side with all three doses, however, only the highest dose of LPS produced bilateral hyperalgesia (A, C). Similarly, replacing the second acid injection with IL-6 produced both primary and secondary hyperalgesia in the ipsilateral side with all three doses, however, only the highest dose of IL-6 produced bilateral hyperalgesia (B, D). *P<0.001 (ipsilateral) and #P (contralateral), significantly lower in Paw Withdrawal Responses and significant higher in Muscle Withdrawal Threshold in mice injected with LPS or acid when compared to mice treated with Saline at 24 h after the second acid injection. Data are presented as means ± SEM, n = 6.
For Figure 6, the tissues were post-fixed in PLP for 5 minutes and then washed with PBST (PBS, 0.05% triton-100). Prior to primary antibody incubation sections were blocked using 5% normal goat serum, background buster and Fc receptor blocker (Innovex Biosciences, Richmond, California). Sections were incubated with 1:500 rat anti-F4/80 (AbDserotec, Bio-Rad Co) for 1h at room temperature followed by 1:500 goat anti rat-IgG Alexa 488 (Life Technologies, Grand Island, NY) for 1 h at room temperature. Slides were coverslipped with Prolong Diamond anti-fade mountant (Life Technologies, Grand Island, NY).
Muscle sections were imaged with an Olympus BX-51 (clodronate experiments) or BX-61 (Fig. 6) fluorescence microscope with a spot camera in the Central Microscopy Facility at the University of Iowa. All images were taken under the same conditions on the same microscope and stored for later analysis. For clodronate experiments, 5 muscle sections per animal were imaged, and 5 images per section were taken. The total number of macrophages per animal were counted using Image J software (National Institutes of Health). For quantification of macrophage content in Figure 6, muscles were serially sectioned saving 1 section every 200 µm throughout each muscle. We then examined every 4th section of muscle for macrophage numbers. Six images were taken representing an even spread across the muscle section. The total number of labeled macrophages was counted for each muscle section, and the area counted was calculated using Image J software. The number of macrophages per mm2 of tissue is represented in Fig. 6E. A total of 6 animals (3 male and 3 female) per group were analyzed for the clodronate experiments, and 6 animals (3 male and 3 female) per group were analyzed for data in Figure 6.
Macrophage cytokine release
Peritoneal macrophages were extracted using peritoneal lavage of C57Bl/6 mice after mice were euthanized using CO2. Briefly, 5 ml of ice cold, sterile, endotoxin-free PBS was injected into the peritoneal cavity using a 27g needle, the belly was then gently massaged to dislodge macrophages, and then cells were collected using a sterile Pasteur pipette or a 25g needle. The recovered cells were pelleted by centrifugation at 1500 rpm for 10 min at 4°C and then plated on a 96 well plate at a concentration of 1×106 cells/ml in DMEM containing 10% FBS, and Pen/Strep. The next day the cell media was changed and macrophages were treated with 100 ng/ml of LPS for 4h or pH 6.0 for 4h. Afterwards, the supernatant media was extracted and cytokines were measured using a multiplex system (Mouse Cytokine Magnetic 10-Plex Panel from Invitrogen Life Technologies). Macrophages were isolated from 4 mice (2 male, 2 female) for these studies, and macrophages from each mouse were treated with pH 7, pH 6, or LPS (Fig. 7).
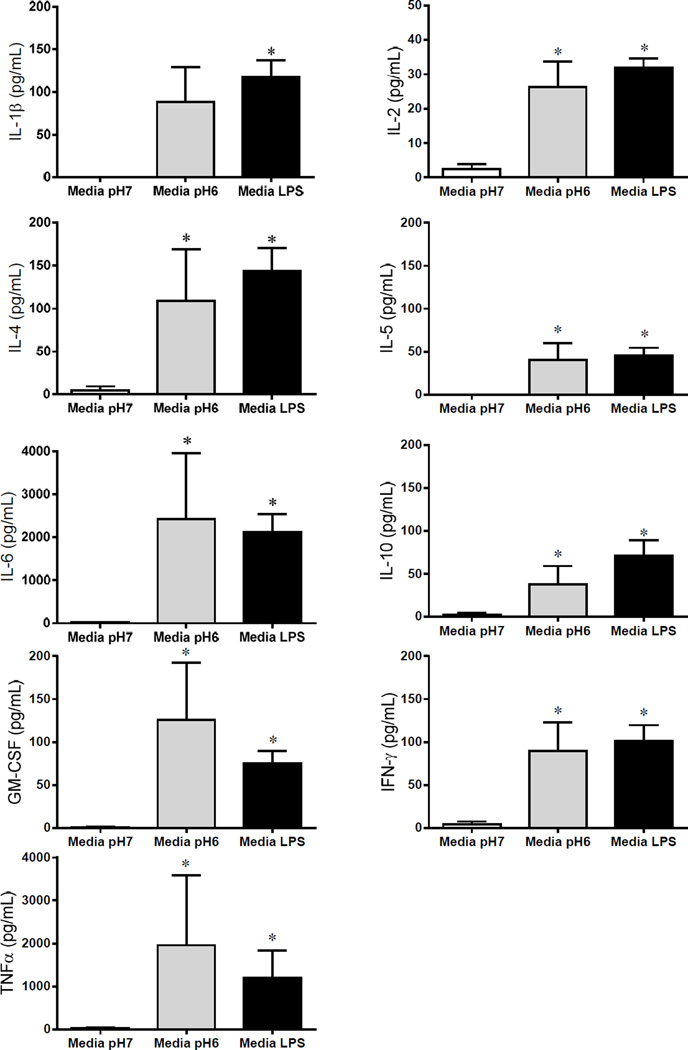
Figure 7. LPS and acid induce release of cytokines from peritoneal macrophages.
Peritoneal macrophages were harvested and plated on 96 well plates, at a concentration of 1 × 106 cells/ml. The cells were treated with either LPS (100ng/ml) or pH6 and compared to controls incubated in the same media. LPS significantly increased release of all 9 cytokines analyzed: IL-1β (p=.004) (A), IL-4 (p=.01) (B), IL-6 (p=.01) (C), GM-CSF (p=.01) (D), TNFα (p=0.01) (E), IL-2 (p=.0002) (F), IL-5 (p=0.01) (G), IL-10 (p=0.01) (H), and IFNγ (p=.001) (I). Acidic pH significantly increased release of 8 of the 9 cytokines measured: IL-4 (p=.03) (B), IL-6 (p=.01) (C), GM-CSF (p=.01) (D), TNFγ (p=0.01) (E), IL-2 (p=.02) (F), IL-5 (p=0.01) (G), IL-10 (p=0.01) (H), and IFNγ (p=.04) (I).
Data presentation and statistics
Analysis of the data was performed using SPSS Version 22.0 (SPSS Inc, Chicago, IL) or GraphPad Prism version 6.00 for Windows, GraphPad Software, La Jolla California USA. Data were tested for normal distribution using the Shapiro Wilk test. All data were normally distributed except cytokine release for GMCSF, IL-10, Il-5, Il-6, and TNF. No differences were found for sex and thus all comparisons were made with both male and female included in each group. For behavior tests, differences across time were analyzed with a repeated-measures analysis of variance, and differences between groups were analyzed post hoc with a Tukey test. For normally distributed cytokine release values for Il-1b, IFNy and IL-2 data were analyzed by repeated measures ANOVA with differences between groups analyzed with a paired t-test. For non-normally distributed cytokine release data, Kruskall Wallis test followed by a Mann Whitney test for differences between group. Differences in the average number of macrophages between control and experimental groups were analyzed using a t-test. P value <0.05 was considered significant. Data was presented as mean ± S.E.M.
Results
Depletion of resident macrophages attenuates hyperalgesia induced by repeated acid injections
To test if the hyperalgesia induced by repeated injections of pH 4.0 saline requires macrophage activation in muscle, we injected clodronate liposomes into muscle ipsilaterally to deplete macrophages and compared to injections of PBS-Liposomes. Quantitation of F4/80-stained muscle macrophages showed that the average number of macrophages was approximately 20.83 +/− 2.80 in 5 sections in gastrocnemius muscles treated with Clod and was significantly less than the number from PBS-Lipo which averaged 82.33 +/− 9.79 (P<0.0001), similar to prior data by us [17]. Twenty-four hours after the second injection of acidic saline, responses to repeated mechanical stimulation of the paw increased and muscle withdrawal thresholds significantly decreased bilaterally in control animals treated with PBS-Lipo (p<0.0001). The increases in paw withdrawal responses were attenuated in the animals treated with Clod and were significantly less than animals that received PBS-Lipo (P<0.0001) (Fig 1A and B). Similarly, the decrease in muscle withdrawal thresholds did not occur in animals treated with Clod and were significantly less than animals treated with PBS-Lipo (P<0.0001) (Fig 1C and D).
Repeated acid hyperalgesia is attenuated by blocking macrophage activation using TLR4 antagonist
We next tested if blockade of macrophage activation prevented the development of chronic muscle hyperalgesia by repeated acid injections. Since TLR4 is located on macrophages [2] and activation of macrophage-TLR4 receptors enhances release of inflammatory cytokines [41;66], we tested if blockade of TLR4 receptors, with C34, in muscle prevented development of hyperalgesia to repeated acid injections. C34, when given before the first pH4.0 injection (Fig 2), significantly attenuated paw withdrawal responses and muscle withdrawal threshold. However, when C34 was given before the second injection, it had no effect of acid induced hyperalgesia (Fig 3).
Hyperalgesia can be induced by replacement of one acid injection with LPS
To test if activation of macrophages reproduces the effects of intramuscular acid injection, we replaced either the first pH 4.0 saline or the second pH 4.0 saline injection with LPS, a TLR4 agonist. When the first acid injection was replaced by LPS, there was a significant increase in the number of responses to mechanical stimulation ipsilaterally for the 250 ng and 830 ng doses, and contralaterally for the 830 ng dose when compared to vehicle (P<0.001, Fig 4A). Similarly, there was a significant decrease in the muscle withdrawal threshold ipsilaterally at the 250 ng and 830 ng doses and contralaterally for the 830 ng dose when compared to vehicle than vehicle (P<0.001, Fig 4C).
When the second acid injection was replaced by LPS, there was a significant increase in the number of withdrawal to mechanical stimulation ipsilaterally for the 250 ng and 830 ng doses and contralaterally for the 830 ng dose when compared to vehicle (P<0.001, Fig 5A). Similarly, there was a significant decrease in the muscle withdrawal threshold ipsilaterally at the 250 ng and 830 ng doses and contralaterally for the 830 ng dose when compared to vehicle (P<0.001, Fig 5C).
Hyperalgesia can be induced by replacement of one acid injection with IL-6
Since IL-6 is released from macrophages and can activate nociceptors [12;33;67], we tested if IL-6 reproduces the effects of intramuscular acid injection by replacing either the first or the second pH 4.0 saline injection with IL-6 (2, 6 and 20 ng). When the first acid injection was replaced by IL-6, there was a significant increase in the number of responses to mechanical stimulation ipsilaterally for the 6 ng and 20 ng doses, and contralaterally for the 20 ng dose when compared to vehicle (P<0.001, Fig 4B). Similarly, there was a significant decrease in the muscle withdrawal threshold ipsilaterally at the 6 ng and 20 ng doses and contralaterally for the 20 ng dose when compared to vehicle (P<0.001, Fig 4D).
When the second acid injection was replaced by IL-6, there was a significant increase in the number of withdrawal to mechanical stimulation ipsilaterally for the 6 ng and 20 ng doses and contralaterally for the 20 ng dose when compared to vehicle (P<0.001, Fig 5B). Similarly, there was a significant decrease in the muscle withdrawal threshold ipsilaterally at the 6 ng and 20 ng doses and contralaterally for the 6 ng (P<0.01) and 20 ng (P<0.001) doses when compared to vehicle (Fig 5D)
Acid, LPS and IL-6 increase the number of macrophages in the gastrocnemius muscle
To examine the effect of acidic saline injections on macrophage recruitment to local tissue, mice were injected twice with pH4.0 saline and the number of macrophages F4/80+ in the injected muscle was counted 24h after the second injection. A significant increase in the number of F4/80+ cells occurred in the muscle on Day 6 when compared to muscles from naïve mice on Day 0 (Fig. 6A,B,E). Injecting 20µL of pH4.0 saline into the left gastrocnemius muscle significantly increased local macrophage concentration when compared to saline injected mice (P<0.05) for up to 6 days after injection. We next tested if the number of macrophages was altered after injection of LPS or IL-6. Injecting 20µL of 20ng IL-6 or 20µL of 830ng LPS significantly increased the total number of F4/80+ macrophage (P<0.001) when compared to naïve mice (Figure 6C,D,E).
LPS and acid induce release of cytokines from peritoneal macrophages
To test if activation of macrophages with LPS and decreased pH induce release of cytokines from macrophages, we incubated peritoneal macrophages in either LPS or pH 6.0 and compared to controls incubated with media. Both LPS and pH 6.0 significantly increased release of cytokines when compared to values after incubation in media (Fig. 7). LPS significantly increased release of all 9 cytokines analyzed: IL-1β (p=.004), IL-4 (p=.01), IL-6 (p=.01), GM-CSF (p=.01), TNFα (p=0.01), IL-2 (p=.0002), IL-5 (p=0.01), IL-10 (p=0.01), and IFNγ (p=.001). Acidic pH significantly increased release of 8 of the 9 cytokines measured: IL-4 (p=.03), IL-6 (p=.01), GM-CSF (p=.01), TNFα (p=0.01), IL-2 (p=.02), IL-5 (p=0.01), IL-10 (p=0.01), and IFNγ (p=.04).
Discussion
The present study supports our hypothesis that resident macrophages in muscle are a critical component to the pathogenesis of chronic non-inflammatory muscle pain. Specifically we show the following findings: (1) depletion of resident macrophages and blockade of TLR4 receptors in muscle attenuated the development of bilateral mechanical hyperalgesia induced by repeated acid saline injection; (2) replacement of a one acid injection by LPS to directly activate resident macrophages, or the inflammatory cytokine IL-6 to mimic macrophage activation, induces bilateral mechanical hyperalgesia in a dose-dependent manner mimicking the effect of repeated acid injections; (3) Acidic pH, LPS and IL-6 increase the number of macrophages in muscle tissue, and acidic pH and LPS induce release of cytokines from peritoneal macrophages. Thus, these data extend prior studies which macrophages are important in fatigue-induced and inflammatory muscle pain [10;18], and suggest that acidic saline in muscle activates macrophages involving TLR4 receptors, and induces release of inflammatory cytokines to produce hyperalgesia.
We propose that resident macrophages, but not hematogenous macrophages, are responsible for generation of acid-induced muscle pain. Macrophages play a homeostatic role that is independent of their immune responses [36]. The present study provided the evidence that local depletion of macrophages in muscle prevents the hyperalgesia induced by repeated acid saline injection and activation of macrophages in muscle reproduces the effect. Indeed, microglial cells in the central nervous system, the possible counterparts of resident macrophages, respond rapidly to a wide variety of pathological stimuli [28] and seem to be the primary local cells involved in immunosurveillance [44]. To discriminate between infiltrating hematogenous and resident macrophages, Mueller and colleagues [37;38], used bone marrow chimeras, which combine a transgene with macrophage markers, to identify resident macrophages in nerve. In animals with nerve injury, they show activation of resident macrophages early before the influx of hematogenous macrophages, proliferation of resident macrophages throughout a 28-day sampling period, and a slow turnover of resident macrophages in nerve suggesting resident macrophages play an active role in the cellular events after peripheral insult. Further, activation and proliferation of resident macrophages occurs in mild pathophysiological conditions, and hematogenous macrophages are supplemented only in more severe pathological processes [37–39]. Our study showed that acid injection produced a small increase in the number of macrophages in muscle. Since the repeated acid model used in the current study is not associated with pathological changes in the injected muscle [50], it is likely that there are changes in resident macrophages that are not reflected systemically.
Interestingly, immune cells, including macrophages, respond to decreases in pH and express the pH-sensor ASIC3 [27;55]. The current study showed an increased release of inflammatory cytokines from peritoneal macrophages by acidic pH, in a similar magnitude to LPS. Classically activated macrophages release pro-inflammatory cytokines that are known to sensitize nociceptors and are the predominant immune cell type in muscle tissue [3;6;36;47]. Pro-inflammatory mediators including IL-1β, tumor necrosis factor-α (TNF-α), IL-6 and MIP-1α are directly implicated in pain hypersensitivity [26;53]. For example, injection of TNF-α and IL-1β into the sciatic nerve, to mimic release of endogenous cytokines locally after nerve injury, produces mechanical and thermal hyperalgesia [65]. Inflammatory cytokines also play a key role in muscle pain [12;13;22;33]. Injection of IL-6 into muscle activates nociceptors and evokes a time- and dose-dependent mechanical hyperalgesia [22;33]. Injection of TNF-α into muscle induces mechanical hyperalgesia and evokes intramuscular upregulation of calcitonin gene-related peptide (CGRP) and nerve growth factor (NGF), and interestingly TNF-α does not induce histopathological muscle damage [46]. Similarly, the current study shows injection of LPS into muscle to activate resident macrophages and injection of IL-6 into muscle induce the bilateral mechanical hyperalgesia in a dose-dependent manner and mimics the effect of repeated acid injections. On the other hand blockade of TLR4 receptors, which are located on macrophages prevents the development of hyperalgesia [2;41;63;66]. Thus, resident macrophages and IL-6 play an important role in development of chronic muscle pain.
TLR4 receptors are expressed on primary afferent neurons, particularly those expressing TRPV1 [19;32;60], and thus we cannot rule out the possibility that TLR on primary afferent fibers are responsible for the induction of hyperalgesia. While centrally blockade of TLR4 receptors produces analgesia in animal models of pain [24;32], it is unclear if peripheral blockade of TLR4 has a similar effect. The current study, interestingly, showed that blockade of TLR4 receptors during the first, but not the second, injection of acidic saline prevents the development of hyperalgesia. Similarly, Jasper and MacNeil [23] show that the non-selective immune blocker minocycline prevents development of hyperalgesia when given systemically before the first, but not the second injection in this chronic muscle pain model. They similarly show that replacement of the first acid injection with the TLR4 agonist, LPS, systemically mimics the development of hyperalgesia to repeated acid injection [23]. These data suggests that the initial injection activates immune cells, but that other mechanisms are responsible for the subsequent development of hyperalgesia.
The present results also show that depletion of resident macrophages attenuates, but does not abolish the hyperalgesia induced by repeated acid injections. We also show that the contralateral hyperalgesia is not as robust when 1 of the 2 acid injections is replaced by LPS or IL-6 (to mimic macrophage activation) when compared to the ipsilateral side. Note the repeated acid injection model produces bilateral hyperalgesia of a similar magnitude as shown in Fig 1. Thus, other complementary mechanisms are likely involved in the development of chronic widespread muscle pain in this animal model. Indeed, we previously show a strong central component including reduced central inhibition and enhanced central excitation play an important role in the generation and maintenance of hyperalgesia in this chronic muscle pain model [8;9;21;43;49;54]. Specifically, there is sensitization of neurons in the spinal cord and the rostral ventromedial medulla (RVM) contributes to both the development and maintenance of hyperalgesia after repeated acid injection [52;54]. After the second acid saline injection, both spinally and supraspinally, there is an increase in release of glutamate [43;49], increase in phosphorylation of the N-methyl-d-aspartate (NMDA) receptor [5;51], and blockade or downregulation of NMDA receptors attenuates the development of hyperalgesia [8;9;48]. Thus, central mechanisms are also involved in the development of chronic muscle pain and thus a combination of both peripheral and central factors are critical to the full manifestation of the pain-behaviors in this chronic muscle pain model.
In the non-inflammatory muscle pain model used in the current study, morphological analysis of injected muscle reveals no obvious muscle damage or inflammation [50]. Despite the lack of damage or inflammation, repeated intramuscular acid injections results in bilateral hyperalgesia of paw, muscle and the viscera that persist for weeks [34;50;64]. Similarly, in human subjects infusion of acidic solutions into muscle produces pain and hyperalgesia both locally at the site of infusion but also in a referred pain site [15]. It is well known that ASICs are sensitive to H+ and involved in acute and chronic muscle hyperalgesia [1;17;52;61]. In general, they are widely expressed nervous systems, including sensory nerves that innervate the skeletal muscle (muscle afferents)[1;35]. Evidence show native ASICs in muscle afferents are predominantly heteromeric channels comprising ASIC1a, ASIC2 and ASIC3 subunits [16] and the pH of half-maximal activation (pH0.5) of homomeric ASIC channels differs: 6.2–6.8 for ASIC1a, 4.1–5 for ASIC2a and 6.2–6.7 for ASIC3 [14]. In the present animal model, the pH of tissue reached an average of pH 6.5 (pH 6.0 in individual animals) after intramuscular acid injection of pH 4.0 [50], a pH that could activate the ASIC1a and ASIC3. Our previous work shows activation of ASIC3 on muscle afferents is required for development of mechanical hyperalgesia and central sensitization that normally occurs in response to repeated intramuscular acid, but not ASIC1 [52]. ASICs also are expressed on macrophages [27] and thus could be directly activated by decreases in pH. Indeed, the current study showed that pH 6.0 induced release of inflammatory cytokines from macrophages. These data suggest that decreases in pH induce release of inflammatory cytokines possibly through activation of ASIC3 on macrophages to result in development of hyperalgesia.
In conclusion, we propose that resident macrophages in muscle are key to the development of chronic muscle pain. We propose that muscle metabolites, like decreased pH, lactate or ATP, directly activate macrophages which subsequently release inflammatory cytokines to enhance nociceptor sensitivity.
Highlights.
Removal of macrophages from muscle prevents development of chronic muscle hyperalgesia induced by repeated acid injections
Blockade of TLR4 or activation prevents development of chronic muscle pain (repeated acid injection).
Replacement of one acid injection with either LPS or IL-6 to mimic macrophage activation results in a similar development of hyperalgesia.
Acid, LPS, and IL-6 increase the number of macrophages in muscle and induce release of cytokines from cultured macrophages.
Thus, macrophages play a critical role in the development of chronic muscle pain induced by repeated acid injection.
Perspective.
This article presents evidence for the role of macrophages in the development of chronic muscle pain using a mouse model. These data suggest that macrophages could be a potential therapeutic target to prevent transition of acute to chronic muscle pain particularly under tissue acidosis conditions.
Acknowledgments
The authors thank Lynn Rasmussen for technical assistance with behavior tests, and Sandra Kolker and Jessica Danielson for technical assistance with immunohistochemistry of macrophages. This work was supported by NIH grant AR061371.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare no conflicts of interest
Reference List
- 1.Abdelhamid RE, Sluka KA. ASICs Mediate Pain and Inflammation in Musculoskeletal Diseases. Physiology (Bethesda) 2015;30:449–459. doi: 10.1152/physiol.00030.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand RJ, Kohler JW, Cavallo JA, Li J, Dubowski T, Hackam DJ. Toll-like receptor 4 plays a role in macrophage phagocytosis during peritoneal sepsis. J Pediatr Surg. 2007;42:927–932. doi: 10.1016/j.jpedsurg.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 3.Andratsch M, Mair N, Constantin CE, Scherbakov N, Benetti C, Quarta S, Vogl C, Sailer CA, Uceyler N, Brockhaus J, Martini R, Sommer C, Zeilhofer HU, Muller W, Kuner R, Davis JB, Rose-John S, Kress M. A key role for gp130 expressed on peripheral sensory nerves in pathological pain. J NEUROSCI. 2009;29:13473–13483. doi: 10.1523/JNEUROSCI.1822-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold L, Henry A, Poron F, Baba-Amer Y, van RN, Plonquet A, Gherardi RK, Chazaud B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bement MK, Sluka KA. Co-localization of p-CREB and p-NR1 in spinothalamic neurons in a chronic muscle pain model. Neurosci Lett. 2007;418:22–27. doi: 10.1016/j.neulet.2007.02.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, Brenner GJ, Ji RR, Bean BP, Woolf CJ, Samad TA. Nociceptors are interleukin-1beta sensors. J NEUROSCI. 2008;28:14062–14073. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cimmino MA, Ferrone C, Cutolo M. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol. 2011;25:173–183. doi: 10.1016/j.berh.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 8.da Silva LFS, DeSantana JM, Sluka KA. Activation of NMDA receptors in the brainstem, rostral ventromedial medulla, and nucleus reticularis gigantocellularis mediates mechanical hyperalgesia produced by repeated intramuscular injections of acidic saline in rats. Pain. 2010;11:378–387. doi: 10.1016/j.jpain.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Silva LFS, Walder RY, Davidson BL, Wilson SP, Sluka KA. Changes in expression of NMDA-NR1 receptor subunits in the rostral ventromedial medulla modulates pain behaviors. Pain. 2010;151:155–161. doi: 10.1016/j.pain.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Silva MD, Bobinski F, Sato KL, Kolker SJ, Sluka KA, Santos AR. IL-10 cytokine released from M2 macrophages is crucial for analgesic and anti-inflammatory effects of acupuncture in a model of inflammatory muscle pain. Mol Neurobiol. 2015;51:19–31. doi: 10.1007/s12035-014-8790-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dessem D, Ambalavanar R, Evancho M, Moutanni A, Yallampalli C, Bai G. Eccentric muscle contraction and stretching evoke mechanical hyperalgesia and modulate CGRP and P2X(3) expression in a functionally relevant manner. Pain. 2010;149:284–295. doi: 10.1016/j.pain.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. N S. 2008;152:521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dina OA, Levine JD, Green PG. Enhanced cytokine-induced mechanical hyperalgesia in skeletal muscle produced by a novel mechanism in rats exposed to unpredictable sound stress. Eur J Pain. 2011;15:796–800. doi: 10.1016/j.ejpain.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diochot S, Salinas M, Baron A, Escoubas P, Lazdunski M. Peptides inhibitors of acid-sensing ion channels. Toxicon. 2007;49:271–284. doi: 10.1016/j.toxicon.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 15.Frey Law LA, Sluka KA, McMullen T, Lee J, rendt-Nielsen L, Graven-Nielsen T. Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. Pain. 2008;140:254–264. doi: 10.1016/j.pain.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gautam M, Benson CJ. Acid-sensing ion channels (ASICs) in mouse skeletal muscle afferents are heteromers composed of ASIC1a, ASIC2, and ASIC3 subunits. FASEB J. 2013;27:793–802. doi: 10.1096/fj.12-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory NS, Brito R, Fusaro MCGO, Sluka KA. ASIC3 is required for development of fatigue-induced hyperalgesia. Mol Neurobiol. 2015 doi: 10.1007/s12035-014-9055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregory NS, Gibson-Corley K, Frey-Law L, Sluka KA. Fatigue-enhanced hyperalgesia in response to muscle insult: Induction and development occur in a sex-dependent manner. Pain. 2013;154:2668–2676. doi: 10.1016/j.pain.2013.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helley MP, Abate W, Jackson SK, Bennett JH, Thompson SW. The expression of Toll-like receptor 4, 7 and co-receptors in neurochemical sub-populations of rat trigeminal ganglion sensory neurons. N S. 2015;310:686–698. doi: 10.1016/j.neuroscience.2015.09.069. [DOI] [PubMed] [Google Scholar]
- 20.Hiscock N, Chan MH, Bisucci T, Darby IA, Febbraio MA. Skeletal myocytes are a source of interleukin-6 mRNA expression and protein release during contraction: evidence of fiber type specificity. FASEB J. 2004;18:992–994. doi: 10.1096/fj.03-1259fje. [DOI] [PubMed] [Google Scholar]
- 21.Hoeger-Bement MK, Sluka KA. Phosphorylation of CREB and mechanical hyperalgesia is reversed by blockade of the cAMP pathway in a time-dependent manner after repeated intramuscular acid injections. J Neurosci. 2003;23:5437–5445. doi: 10.1523/JNEUROSCI.23-13-05437.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoheisel U, Unger T, Mense S. Excitatory and modulatory effects of inflammatory cytokines and neurotrophins on mechanosensitive group IV muscle afferents in the rat. Pain. 2005;114:168–176. doi: 10.1016/j.pain.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 23.Jasper LL, MacNeil BJ. Diverse sensory inputs permit priming in the acidic saline model of hyperalgesia. Eur J Pain. 2012;16:966–973. doi: 10.1002/j.1532-2149.2011.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jurga AM, Rojewska E, Piotrowska A, Makuch W, Pilat D, Przewlocka B, Mika J. Blockade of Toll-Like Receptors (TLR2, TLR4) Attenuates Pain and Potentiates Buprenorphine Analgesia in a Rat Neuropathic Pain Model. Neural Plast. 2016;2016:5238730. doi: 10.1155/2016/5238730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karczewski J, Spencer RH, Garsky VM, Liang A, Leitl MD, Cato MJ, Cook SP, Kane S, Urban MO. Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br J Pharmacol. 2010;161:950–960. doi: 10.1111/j.1476-5381.2010.00918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kiguchi N, Kobayashi Y, Maeda T, Saika F, Kishioka S. CC-chemokine MIP-1alpha in the spinal cord contributes to nerve injury-induced neuropathic pain. Neurosci Lett. 2010;484:17–21. doi: 10.1016/j.neulet.2010.07.085. [DOI] [PubMed] [Google Scholar]
- 27.Kong X, Tang X, Du W, Tong J, Yan Y, Zheng F, Fang M, Gong F, Tan Z. Extracellular acidosis modulates the endocytosis and maturation of macrophages. Cell Immunol. 2013;281:44–50. doi: 10.1016/j.cellimm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. TRENDS NEUROSCI. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 30.Leung A, Gregory NS, Allen LA, Sluka KA. Regular physical activity prevents chronic pain by alatering resident muscle macrophage phenotype and increasing IL-10 in mice. Pain. 2015 doi: 10.1097/j.pain.0000000000000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu T, Gao YJ, Ji RR. Emerging role of Toll-like receptors in the control of pain and itch. Neurosci Bull. 2012;28:131–144. doi: 10.1007/s12264-012-1219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manjavachi MN, Motta EM, Marotta DM, Leite DF, Calixto JB. Mechanisms involved in IL-6-induced muscular mechanical hyperalgesia in mice. Pain. 2010;151:345–355. doi: 10.1016/j.pain.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 34.Miranda A, Peles S, Rudolph C, Shaker R, Sengupta JN. Altered visceral sensation in response to somatic pain in the rat. Gastroenterology. 2004;126:1082–1089. doi: 10.1053/j.gastro.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Molliver DC, Immke DC, Fierro L, Pare M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Molecular Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mueller M, Leonhard C, Wacker K, Ringelstein EB, Okabe M, Hickey WF, Kiefer R. Macrophage response to peripheral nerve injury: the quantitative contribution of resident and hematogenous macrophages. Lab Invest. 2003;83:175–185. doi: 10.1097/01.lab.0000056993.28149.bf. [DOI] [PubMed] [Google Scholar]
- 38.Mueller M, Wacker K, Ringelstein EB, Hickey WF, Imai Y, Kiefer R. Rapid response of identified resident endoneurial macrophages to nerve injury. Am J Pathol. 2001;159:2187–2197. doi: 10.1016/S0002-9440(10)63070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller M, Wacker K, Getts D, Ringelstein EB, Kiefer R. Further evidence for a crucial role of resident endoneurial macrophages in peripheral nerve disorders: lessons from acrylamide-induced neuropathy. Glia. 2008;56:1005–1016. doi: 10.1002/glia.20674. [DOI] [PubMed] [Google Scholar]
- 40.Neal MD, Jia H, Eyer B, Good M, Guerriero CJ, Sodhi CP, Afrazi A, Prindle T, Jr, Ma C, Branca M, Ozolek J, Brodsky JL, Wipf P, Hackam DJ. Discovery and validation of a new class of small molecule Toll-like receptor 4 (TLR4) inhibitors. PLoS One. 2013;8:e65779. doi: 10.1371/journal.pone.0065779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, Nakanishi K, Kimoto M, Miyake K, Takeda K, Akira S. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000;164:3476–3479. doi: 10.4049/jimmunol.164.7.3476. [DOI] [PubMed] [Google Scholar]
- 42.Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. J Physiol. 1998;508(Pt 3):949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radhakrishnan R, Sluka KA. Increased glutamate and decreased glycine release in the rostral ventromedial medulla during induction of a pre-clinical model of chronic widespread muscle pain. Neurosci Lett. 2009;457:141–145. doi: 10.1016/j.neulet.2009.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raivich G, Bohatschek M, Kloss CU, Werner A, Jones LL, Kreutzberg GW. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res Brain Res Rev. 1999;30:77–105. doi: 10.1016/s0165-0173(99)00007-7. [DOI] [PubMed] [Google Scholar]
- 45.Richter F, Natura G, Loser S, Schmidt K, Viisanen H, Schaible HG. Tumor necrosis factor causes persistent sensitization of joint nociceptors to mechanical stimuli in rats. Arthritis Rheum. 2010;62:3806–3814. doi: 10.1002/art.27715. [DOI] [PubMed] [Google Scholar]
- 46.Schafers M, Sorkin LS, Sommer C. Intramuscular injection of tumor necrosis factor-alpha induces muscle hyperalgesia in rats. Pain. 2003;104:579–588. doi: 10.1016/S0304-3959(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 47.Schaible HG, Von Banchet GS, Boettger MK, Brauer R, Gajda M, Richter F, Hensellek S, Brenn D, Natura G. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann N Y Acad Sci. 2010;1193:60–69. doi: 10.1111/j.1749-6632.2009.05301.x. [DOI] [PubMed] [Google Scholar]
- 48.Skyba DA, King EW, Sluka KA. Effects of NMDA and non-NMDA ionotropic glutamate receptor antagonists on the development and maintenance of hyperalgesia induced by repeated intramuscular injection of acidic saline. Pain. 2002;98:69–78. doi: 10.1016/s0304-3959(01)00471-7. [DOI] [PubMed] [Google Scholar]
- 49.Skyba DA, Lisi TL, Sluka KA. Excitatory amino acid concentrations increase in the spinal cord dorsal horn after repeated intramuscular injection of acidic saline. Pain. 2005;119:142–149. doi: 10.1016/j.pain.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 50.Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle & Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 51.Sluka KA, O’Donnell JM, Danielson J, Rasmussen LA. Regular physical activity prevents development of chronic pain and activation of central neurons. J Appl Physiol. 2013;114:725–733. doi: 10.1152/japplphysiol.01317.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 53.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 54.Tillu DV, Gebhart GF, Sluka KA. Descending facilitatory pathways from the RVM initiate and maintain bilateral hyperalgesia after muscle insult. Pain. 2008;136:331–339. doi: 10.1016/j.pain.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tong J, Wu WN, Kong X, Wu PF, Tian L, Du W, Fang M, Zheng F, Chen JG, Tan Z, Gong F. Acid-sensing ion channels contribute to the effect of acidosis on the function of dendritic cells. J Immunol. 2011;186:3686–3692. doi: 10.4049/jimmunol.1001346. [DOI] [PubMed] [Google Scholar]
- 56.Tu J, Lu L, Cai W, Ballard HJ. cAMP/protein kinase A activates cystic fibrosis transmembrane conductance regulator for ATP release from rat skeletal muscle during low pH or contractions. PLoS One. 2012;7:e50157. doi: 10.1371/journal.pone.0050157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ulmann L, Hirbec H, Rassendren F. P2X4 receptors mediate PGE2 release by tissue-resident macrophages and initiate inflammatory pain. EMBO J. 2010;29:2290–2300. doi: 10.1038/emboj.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van RN. The liposome-mediated macrophage ‘suicide’ technique. J Immunol Methods. 1989;124:1–6. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
- 59.van RN, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol. 2010;605:189–203. doi: 10.1007/978-1-60327-360-2_13. [DOI] [PubMed] [Google Scholar]
- 60.Wadachi R, Hargreaves KM. Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J Dent Res. 2006;85:49–53. doi: 10.1177/154405910608500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 Play Different Roles in the Development of Hyperalgesia After Inflammatory Muscle Injury. J Pain. 2010;11:210–218. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Willemen HL, Eijkelkamp N, Garza CA, Wang H, Mack M, Zijlstra J, Heijnen CJ, Kavelaars A. Monocytes/Macrophages control resolution of transient inflammatory pain. J Pain. 2014;15:496–506. doi: 10.1016/j.jpain.2014.01.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang S, Lu H, Wang J, Qi X, Liu X, Zhang X. The effect of toll-like receptor 4 on macrophage cytokines during endotoxin induced uveitis. Int J Mol Sci. 2012;13:7508–7520. doi: 10.3390/ijms13067508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yokoyama T, Audette KM, Sluka KA. Pregabalin reduces muscle and cutaneous hyperalgesia in two models of chronic muscle pain in rats. J Pain. 2007;8:422–429. doi: 10.1016/j.jpain.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Zelenka M, Schafers M, Sommer C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain. 2005;116:257–263. doi: 10.1016/j.pain.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 66.Zhang S, Yu M, Guo Q, Li R, Li G, Tan S, Li X, Wei Y, Wu M. Annexin A2 binds to endosomes and negatively regulates TLR4-triggered inflammatory responses via the TRAM-TRIF pathway. Sci Rep. 2015;5:15859. doi: 10.1038/srep15859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Chirmule N, Gao GP, Qian R, Croyle M, Joshi B, Tazelaar J, Wilson JM. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol Ther. 2001;3:697–707. doi: 10.1006/mthe.2001.0329. [DOI] [PubMed] [Google Scholar]