Abstract
The immunoproteasome is an inducible host mechanism that aids in the clearance of damaged proteins. The immunoproteasome also influences immune function by enhancing peptide presentation by MHC class I and promotes inflammation via IκB degradation and activation of NF-κB. We used mouse adenovirus type 1 (MAV-1) to characterize the role of the immunoproteasome in adenovirus pathogenesis. Following intranasal infection of mice, immunoproteasome activity in the heart and lung was significantly increased in an IFN-γ-dependent manner. Absence of the β5i immunoproteasome subunit and pharmacological inhibition of β5i activity had minimal effects on viral replication, virus-induced cellular inflammation, or induction of cytokine expression. Likewise, the establishment of protective immunity following primary infection was not significantly altered by β5i deficiency. Thus, although immunoproteasome activity is robustly induced during acute infection with MAV-1, our data suggest that other mechanisms are capable of compensating for immunoproteasome activity to maintain antiviral immunity and appropriate inflammatory responses.
Introduction
The human adenoviruses (HAdVs) are common causes of disease. HAdV respiratory infections can present with syndromes that range from mild upper respiratory tract infections to more severe, sometimes life-threatening manifestations (Wold and Isom, 2013). Associations between myocarditis and the HAdVs are also well established (Bowles et al., 2003; Dennert et al., 2008; Kuhl et al., 2005; Martin et al., 1994; Pauschinger et al., 1999; Tatrai et al., 2011). HAdV infection can cause acute disease in the heart, and persistent HAdV infections of the myocardium have been implicated in the development of dilated cardiomyopathy and cardiac dysfunction (Kuhl et al., 2005; Pauschinger et al., 1999; Tatrai et al., 2011). The strict species specificity of the adenoviruses precludes detailed studies of HAdV pathogenesis in mouse models. We have used mouse adenovirus type 1 (MAV-1) to study the pathogenesis of adenovirus respiratory infection in its natural host (McCarthy et al., 2013; McCarthy et al., 2015b; McCarthy et al., 2014; Procario et al., 2012; Procario et al., 2016; Weinberg et al., 2005). Recently, we used MAV-1 to establish a mouse model of adenovirus myocarditis (McCarthy et al., 2015a) in which intranasal (i.n.) MAV-1 infection of neonatal C57BL/6 (B6) mice induces interferon gamma (IFN-γ) expression in the heart along with cellular inflammation, cardiac myocyte necrosis, and decreased cardiac contractility. Following acute infection, MAV-1 persistence in the heart is associated with long-term sequelae reminiscent of dilated cardiomyopathy, including cardiac remodeling and hypertrophy.
A variety of immune responses contribute to the clearance of virus but can also cause further damage, either directly or by autoimmune myocardial injury (Liu et al., 1996; Rose and Hill, 1996). Specifically, the actions of T cells and IFNs are intimately linked by the immunoproteasome, an inducible component of the ubiquitin proteasome system, which contributes to the degradation of misfolded and damaged proteins as well as processing of proteins to generate peptides presented by MHC class I (McCarthy and Weinberg, 2015). Stimulation by IFN-γ and other cytokines induces the replacement of constitutively active proteasome subunits (β1, β2, and β5) by immuno subunits (β1i, β2i, and β5i), and the incorporation of the proteasome activator 11S/PA28α/β, a large regulatory complex that binds the ends of the proteasome core to further enhance the ability of the proteasome to degrade short peptide substrates (Dubiel et al., 1992; Ma et al., 1992). These changes lead to the formation of the immunoproteasome complex, which is more efficient in generating MHC class I epitopes for recognition by CD8 T cells. Immunoproteasome activity may also contribute to host responses via degradation of IκB and activation of nuclear factor (NF)-κB-mediated pathways (Ebstein et al., 2012). Immunoproteasome activity is induced in the heart during coxsackievirus B3 (CVB3) myocarditis (Jakel et al., 2009; Opitz et al., 2011; Szalay et al., 2006), and enhanced CVB3-induced inflammation without differences in viral replication is observed in mice deficient in the β5i (also known as LMP7) immuno subunit (Opitz et al., 2011). No data are available regarding the role of immunoproteasome activity in adenovirus myocarditis or other adenovirus disease.
Although IFN-γ does not exert a critical antiviral function during MAV-1 respiratory infection (Anderson et al., 2009; McCarthy et al., 2015b) or myocarditis (McCarthy et al., 2015a), we demonstrated that it is necessary for virus-induced inflammatory responses in the hearts of MAV-1-infected mice (McCarthy et al., 2015a). In the present study, we tested the hypothesis that IFN-γ-induced immunoproteasome activity is an important contributor to virus-induced inflammation. We found that MAV-1 infection substantially upregulated the expression of immunoproteasome subunits and increased immunoproteasome activity in the heart in an IFN-γ-dependent manner. However, deficiency of the β5i subunit (in LMP7−/− mice) or pharmacological inhibition of β5i activity had minimal effect on heart viral loads, virus-induced cardiac inflammation and cardiac damage. MAV-1 infection also induced immunoproteasome subunit expression in the lungs of B6 mice, but not IFN-γ deficient mice. As in the heart, β5i deficiency had little effect on lung viral loads or virus-induced pulmonary inflammation. Our data also indicate that the establishment of protective immunity was not compromised by β5i deficiency.
Results
Induction of Immunoproteasome Activity during Acute MAV-1 Infection
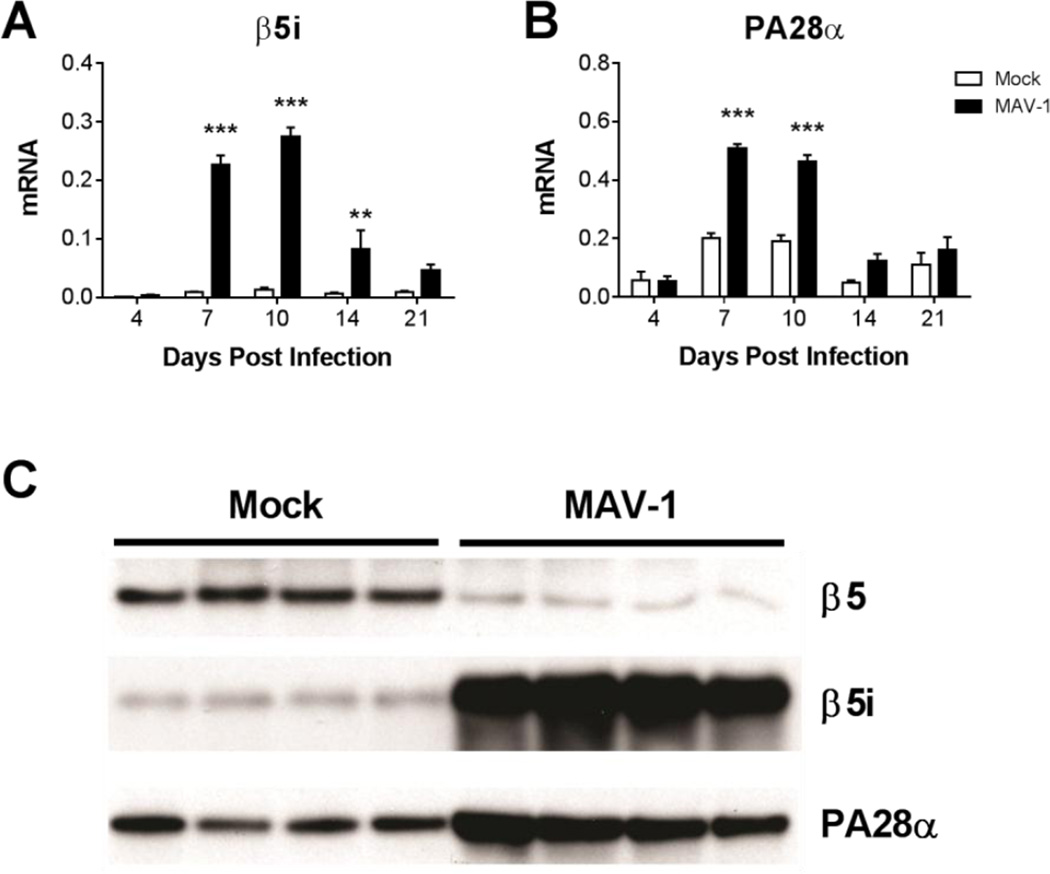
Acute MAV-1 infection induces production of IFN-γ and other cytokines in the heart (McCarthy et al., 2015a). To determine whether MAV-1 infection also induces immunoproteasome activity in the heart, we infected neonatal C57BL/6J (B6) mice i.n. with MAV-1 and used reverse transcriptase real-time quantitative PCR (RT-qPCR) to measure expression of the β5i immunoproteasome subunit and the PA28α immunoproteasome regulatory complex subunit. The expression of both β5i (Fig. 1A) and PA28α (Fig. 1B) was significantly higher in hearts of infected mice compared to mock infected mice beginning at 7 dpi. Higher expression of β5i and PA28α was still detected at 10 dpi and then decreased over time. To determine whether the increases in immunoproteasome subunit mRNA expression between 7 and 14 dpi correlated with increased immunoproteasome subunit protein production, we used Western blots to detect β5, β5i, and PA28α protein in heart lysate at 11 dpi. We observed a substantial increase in β5i protein and a corresponding decrease in β5 protein in heart lysates of infected mice compared to mock infected mice (Fig. 1C). PA28a protein was also increased in heart lysates of infected mice compared to mock infected mice.
Fig. 1.
Immunoproteasome activity in hearts of MAV-1-infected mice. Neonatal B6 mice were infected with MAV-1 or mock infected with conditioned media. Hearts were harvested at the indicated time points. RT-qPCR was used to quantify expression of A) β5i and B) PA28α, shown relative to GAPDH in arbitrary units. Combined data from 3 to 6 mice per group are presented as means ± S.E.M. **P<0.01, ***P<0.001. C) Western blot was used to detect β5, β5i, and PA28α protein in lysates of hearts harvested at 11 dpi. Each lane represents a sample from an individual mouse.
We measured proteasome and immunoproteasome activity in hearts of infected and mock infected mice at 11 dpi using the proteasome constitutive immuno-subunit (active site) ELISA (ProCISE) assay to quantify levels of β5 and β5i active sites (Kirk et al., 2014) and verify that increases in immunoproteasome subunit gene expression and protein production led to a corresponding increase in immunoproteasome activity. Activity of the β5 constitutive proteasome subunit was significantly lower in lysates of infected hearts compared to mock infected hearts, while β5i activity was substantially higher (Table 1). The β5i:β5 activity ratio was significantly higher in infected hearts compared to mock-infected hearts (Table 1), indicating that the immunoproteasome accounts for the majority of total proteasome activity in hearts at 11 dpi. Collectively, these results demonstrate that immunoproteasome expression and activity are induced in the hearts of mice infected with MAV-1. Because immunoproteasomes exhibit a substantially shorter half-life than proteasomes (Heink et al., 2005), the kinetics of immunoproteasome gene expression (Figs. 1A and 1B) suggest that immunoproteasome activity returns to pre-infection levels following resolution of acute MAV-1 infection.
Table 1.
Proteasome and Immunoproteasome Subunit Activity in the Heart at 11 dpi
| Active Subunits | Activity (% control ± SEM) | |||
|---|---|---|---|---|
| Subunit | Mock | MAV-1 | P | |
| β5 | 100.0 ± 2.3 | 64.8 ± 1.7 | <0.001 | |
| β5i | 100.0 ± 4.0 | 266.0 ± 15.9 | <0.001 | |
| Activity Ratio | Ratio | Mock | MAV-1 | P |
| β5i:β5 | 0.36 ± 0.02 | 1.48 ± 0.08 | <0.001 | |
MAV-1-Induced Immunoproteasome Activity Depends on IFN-γ
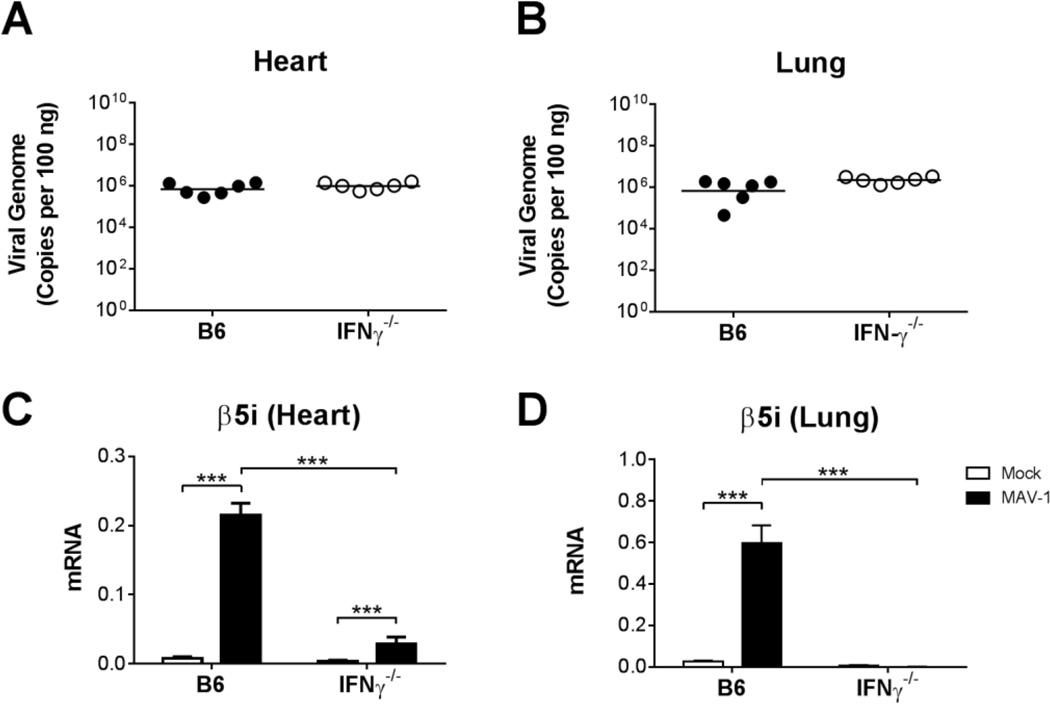
IFN-γ is a predominant host factor involved in the induction of immunoproteasome activity (McCarthy and Weinberg, 2015). We detected increased expression of genes in the heart encoding immunoproteasome subunits (Fig. 1) with kinetics similar to those that we reported for MAV-1-induced IFN-γ expression in the heart (McCarthy et al., 2015a). To determine the extent to which IFN-γ was responsible for the induction of immunoproteasome activity following MAV-1 infection, we infected neonatal B6 and IFN-γ−/− mice with MAV-1. As we previously observed with IFN-γ immunoneutralization (McCarthy et al., 2015a), IFN-γ deficiency was associated with increased mortality following infection, such that the majority of IFN-γ−/− mice, but not B6 mice, were moribund by 8 dpi (data not shown). This was not due to substantially greater viral replication in the absence of IFN-γ, because there were no differences in viral loads measured in hearts or lungs of B6 and IFN-γ−/− mice at 8 dpi (Figs. 2A,B). To determine whether IFN-γ deficiency had an effect on immunoproteasome induction following MAV-1 infection, we used RT-qPCR to quantify β5i and β1i expression in B6 and IFN-γ−/− mice. We detected markedly less β5i (Fig. 2C,D) and β1i (data not shown) expression in hearts and lungs of infected IFN-γ−/− mice compared to infected B6 mice at 8 dpi. Taken together, these data suggest that the induction of immunoproteasome activity during MAV-1 infection is largely dependent on IFN-γ signaling.
Fig. 2.
MAV-1 infection in IFN-γ−/− mice. Neonatal B6 and IFN-γ−/− mice were infected with MAV-1 or mock infected with conditioned media, and hearts and lungs were harvested at 8 dpi. A,B) qPCR was used to quantify MAV-1 viral loads in hearts of infected mice. DNA viral loads are expressed as copies of MAV-1 genome per 100 ng of input DNA. Individual circles represent values for individual mice and horizontal bars represent means for each group. C,D) RT-qPCR was used to quantify expression of β5i, shown relative to GAPDH in arbitrary units. Combined data from n=4–6 mice per group in C and D are presented as means ± S.E.M. *P<0.05, ***P<0.001.
Effects of LMP7 Deficiency on MAV-1 Myocarditis
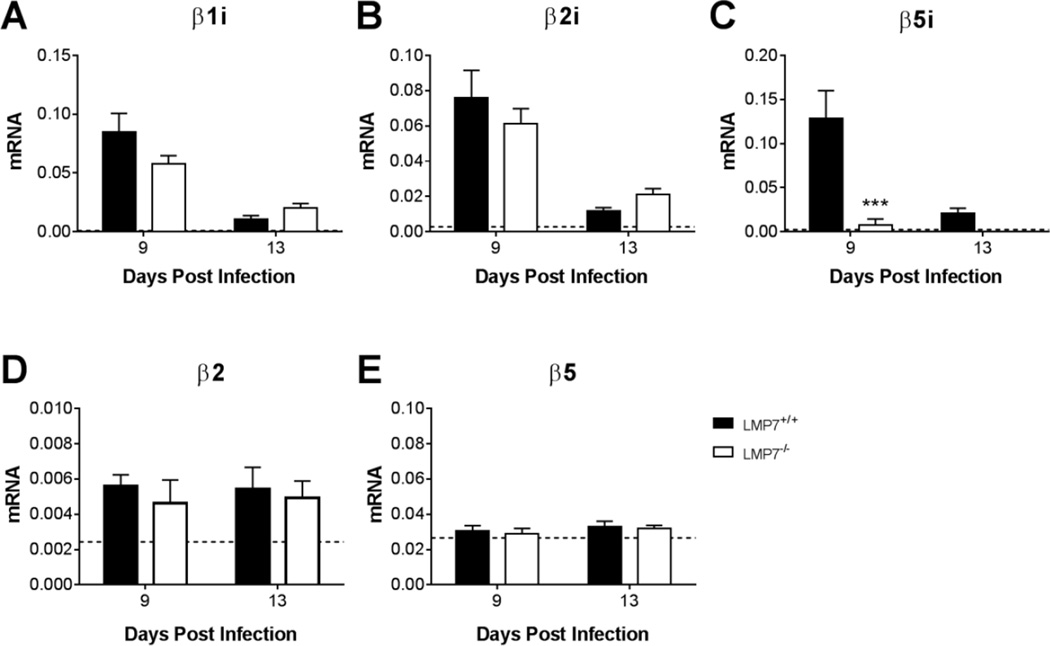
Immunoproteasome activity contributes to innate inflammatory responses in ways that may be separate from its role in peptide processing for presentation by MHC class I (McCarthy and Weinberg, 2015). To assess the possibility that immunoproteasome activity contributes to MAV-1-induced inflammation, we used neonatal LMP7−/− mice, which are deficient in the immunoproteasome subunit β5i (also known as LMP7). In addition to β5i deficiency, incorporation of β1i and β2i into cardiac proteasomes is impaired in LMP7−/− mice (Opitz et al., 2011). For these experiments, we used LMP7+/+ littermates as controls. We detected substantial upregulation of β1i, β2i, and β5i gene expression in the hearts of infected LMP7+/+ mice above levels seen in mock-infected mice (Fig. 3A–C). As expected, β5i mRNA was absent in infected LMP7−/− mice. Expression of β1i and β2i mRNA was not significantly different in hearts of LMP7−/− mice compared to LMP7+/+ mice, suggesting that there was no compensatory upregulation in the expression of those immuno subunits in the absence of β5i. Expression of the β2 (Fig. 3D) and β5 (Fig. 3E) constitutive proteasome subunits was similar in infected LMP7+/+ and LMP7−/− mice.
Fig. 3.
Expression of immuno and constitutive proteasome subunits in hearts of infected LMP7−/− mice. Neonatal LMP7−/− mice and LMP7+/+ littermate controls were infected with MAV-1 or mock infected with conditioned media. Hearts were harvested at 9 and 13 dpi and RT-qPCR was used to quantify expression of the indicated genes, shown relative to GAPDH in arbitrary units. Horizontal dotted lines corresponds to expression in mock-infected LMP7+/+ mice (n=3; lines fall very close to the X-axis where not readily visible) at 9 dpi. Combined data from n=4–8 mice per group are presented as means ± S.E.M. ***P<0.001.
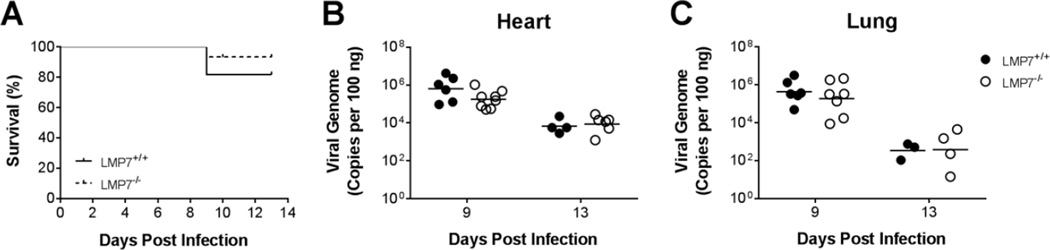
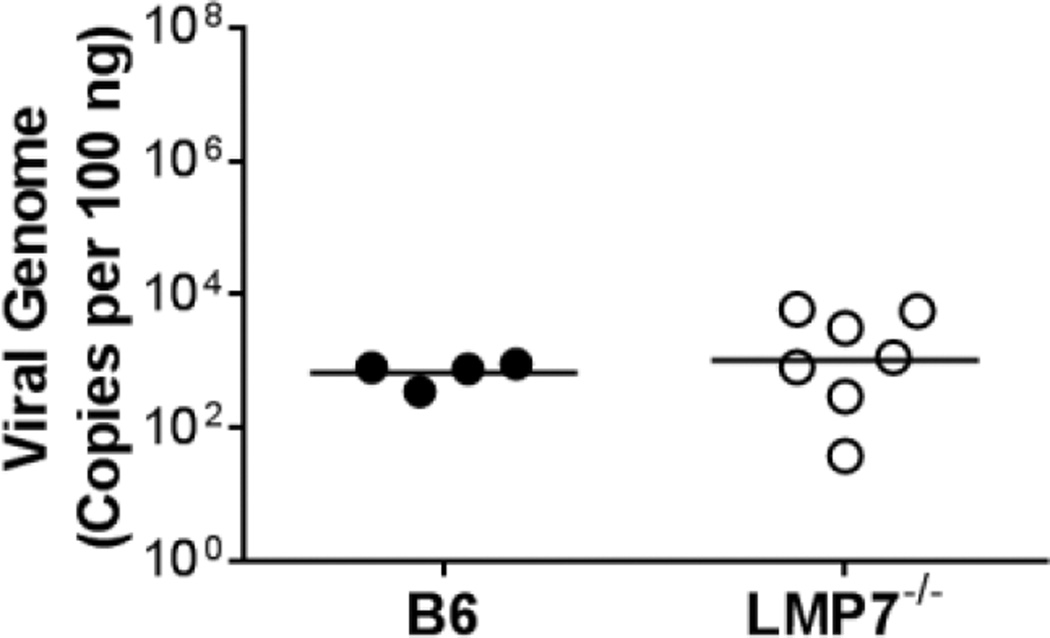
To determine whether immunoproteasome deficiency had an effect on MAV-1 pathogenesis, we assessed survival in LMP7+/+ and LMP7−/− mice following MAV-1 infection. We did not observe substantial mortality overall, and there was no significant difference in survival of LMP7+/+ and LMP7−/− mice during acute MAV-1 infection (Fig. 4A). To determine the extent to which immunoproteasome deficiency affected viral replication we measured heart viral loads in LMP7+/+ and LMP7−/− mice at 9 dpi, near the peak of β5i expression in the hearts of infected mice (Fig. 1A), and then later in the acute infection at 13 dpi. We detected no statistically significant difference between viral loads measured in hearts of LMP7+/+ and LMP7−/− mice at 9 or 13 dpi (Fig. 4B). Likewise, there was no statistically significant difference between viral loads measured in the lungs of LMP7+/+ and LMP7−/− mice at either time point (Fig. 4C), suggesting that there were no organ-specific effects of β5i deficiency on viral replication.
Fig. 4.
Survival and viral replication in infected LMP7−/− mice. Neonatal LMP7−/− mice and LMP7+/+ littermate controls were infected with MAV-1. A) Survival was monitored (n=11–15 per group). B) Hearts and C) lungs were harvested at the indicated time points and qPCR was used to quantify viral loads in each organ. DNA viral loads are expressed as copies of MAV-1 genome per 100 ng of input DNA. Individual circles represent values for individual mice and horizontal bars represent means for each group. There were no statistically significant differences between groups in survival or viral loads.
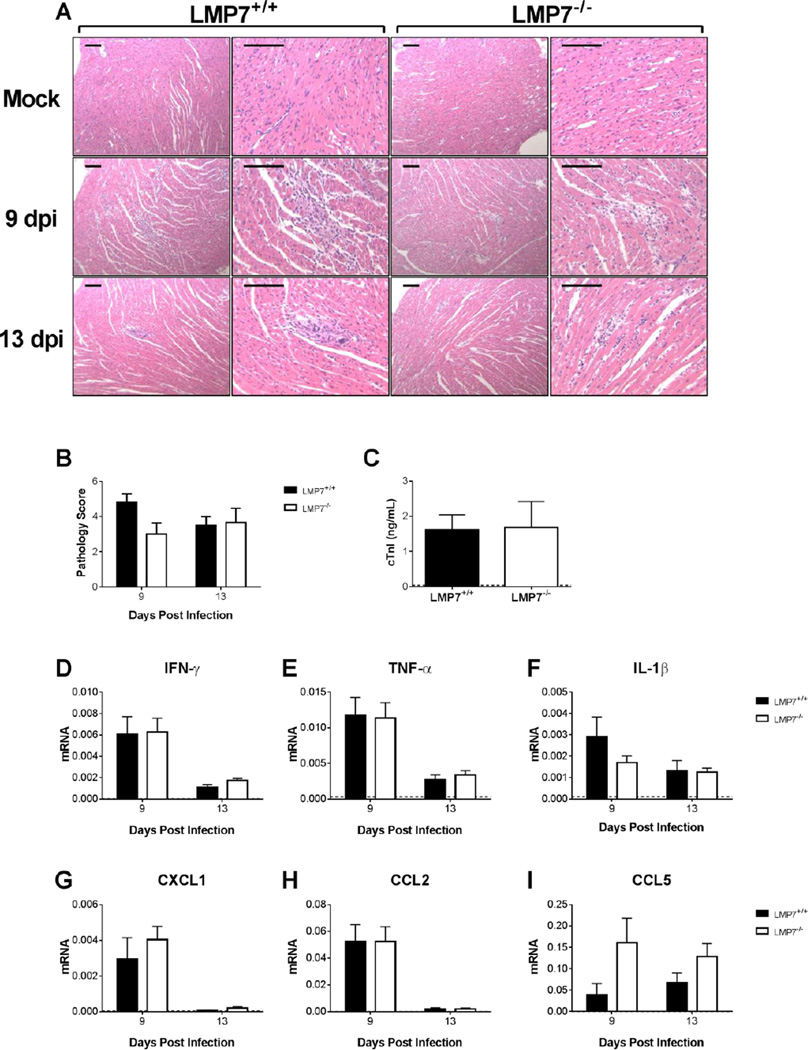
Acute MAV-1 infection causes cellular inflammation and tissue destruction in neonatal B6 mice (McCarthy et al., 2015a). Accordingly, we observed focal accumulations of inflammatory cells scattered throughout hearts of LMP7+/+ mice at 9 dpi (Fig. 5A). Inflammation was also present in the hearts of infected LMP7−/− mice at 9 dpi. There was histological evidence of cardiac inflammation at 13 dpi in both LMP7+/+ and LMP7−/− mice. We quantified the severity of MAV-1-induced myocarditis using blinded scoring of histological sections. There were no statistically significant differences between LMP7+/+ and LMP7−/− mice in cardiac pathology scores at 9 or 13 dpi (Fig. 5B). We detected cardiac troponin I (cTnI) in serum of infected LMP7+/+ and LMP7−/− mice at 9 dpi (Fig. 5C), while cTnI was undetectable in mock infected mice (data not shown). There was no difference between serum cTnI concentrations in infected LMP7+/+ and LMP7−/− mice at 9 dpi.
Fig. 5.
MAV-1-induced inflammation in hearts of LMP7−/− mice. Neonatal LMP7−/− mice and LMP7+/+ littermate controls were infected with MAV-1 or mock infected with conditioned media. Hearts and serum were harvested at 9 and 13 dpi. A) Hematoxylin-and-eosin-stained sections were prepared from paraffin-embedded hearts. Representative images are shown from mock-infected and infected mice. Adjacent images are from the same section at different magnifications. Scale bars, 100 µm. B) Pathology index scores were generated to quantify histological evidence of cellular inflammation in hearts. Combined data from n=4–6 mice per group are presented as means ± S.E.M. C) ELISA was used to measure concentrations of cardiac troponin I (cTnI) in serum at 9 dpi. Serum cTnI concentrations were below the limit of detection (horizontal dotted line) in mock-infected mice (data not shown). Combined data from n=3–5 mice per group are presented as means ± S.E.M. There were no statistically significant differences in pathology scores or cTnI concentrations between groups. D-I) RT-qPCR was used to quantify expression in the heart of the indicated cytokines and chemokines, shown relative to GAPDH in arbitrary units. Horizontal dotted lines correspond to expression in mock-infected LMP7+/+ mice at 9 dpi (n=3; lines fall very close to the X-axis where not readily visible). Combined data from n=4–6 mice per group are presented as means ± S.E.M. There were no statistically significant differences between groups.
Next, we used RT-qPCR to quantify cytokine and chemokine expression in hearts of mock infected and infected LMP7+/+ and LMP7−/− mice. Consistent with our previous report (McCarthy et al., 2015a), IFN-γ expression in the heart was upregulated by MAV-1 infection in both LMP7+/+ and LMP7−/− mice (Fig. 5D). The magnitude of virus-induced IFN-γ upregulation was similar in LMP7+/+ and LMP7−/− mice. MAV-1 infection also induced the expression of other pro-inflammatory cytokines and chemokines, including TNF-α, IL-1β, CXCL1, CCL2, and CCL5, but there were no significant differences in the magnitude of their expression in hearts of LMP7+/+ and LMP7−/− mice (Figs. 5E–H). Thus, although immunoproteasome activity was robustly increased in the heart during acute MAV-1 infection, the absence of β5i had minimal overall effects on virus-induced cardiac inflammation, control of viral replication in the heart, or survival during acute MAV-1 infection.
Effects of Immunoproteasome Inhibition on MAV-1 Myocarditis
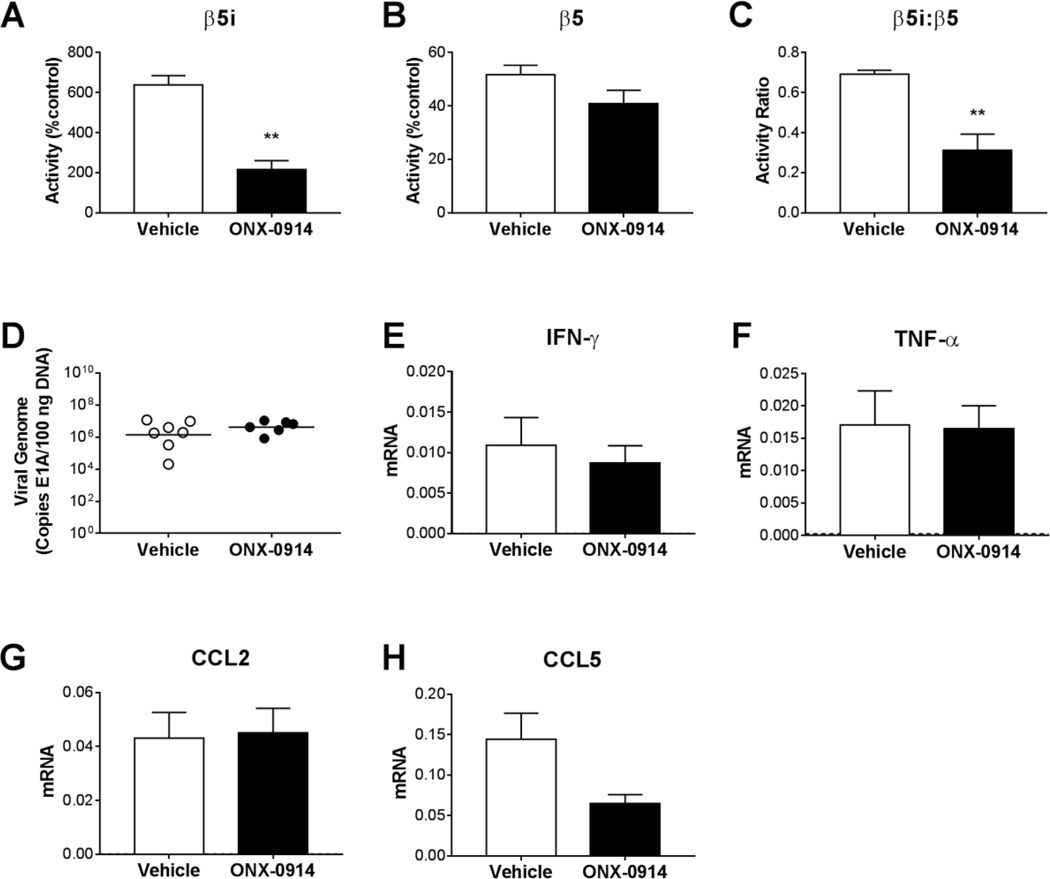
We used ONX 0914, a derivative of epoxomicin with increased selectivity for β5i compared to β5 (Muchamuel et al., 2009), to provide complementary data regarding the role of immunoproteasome activity during MAV-1 infection of B6 mice. At 9 dpi, near the peak of immunoproteasome activity in the heart during acute MAV-1 infection (Fig. 1), β5i activity (Fig. 6A), but not β5 activity (Fig. 6B), was lower in hearts of infected mice treated with ONX 0914 compared to infected control mice, such that the β5i:β5 activity ratio was significantly lower (Fig. 6C). Treatment with ONX 0914 had no effect on heart viral loads at 9 dpi (Fig. 6D). Likewise, treatment with ONX 0914 had no effect on histological evidence of cardiac pathology (data not shown), and there were no statistically significant differences between ONX 0914-treated mice and controls in virus-induced expression of IFN-γ, TNF-α, CCL2 and CCL5 (Figs. 6E–H). Thus, selective inhibition of β5i with ONX 0914, like β5i deficiency in LMP7−/− mice, did not substantially affect viral replication or host inflammatory responses to acute infection in the heart.
Fig. 6.
Effects of immunoproteasome inhibition on MAV-1 viral loads and virus-induced inflammation in the heart. Neonatal B6 mice were infected with MAV-1. Mice were treated with ONX 0914 (5 mg/kg/dose administered s.c.) every other day beginning at 1 day post infection (d.p.i.). Control mice were treated with vehicle. Hearts were harvested at 9 dpi. ProCISE assay was used to measure A) β5i and B) β5 subunit activity in the heart, expressed as the percentage of activity measured in mock-infected, vehicle-treated control mice. C) Values for β5i and β5 subunit activity were used to calculate the β5i:β5 activity ratio. D) qPCR was used to quantify MAV-1 genome copies in heart DNA. DNA viral loads are expressed as copies of MAV-1 genome per 100 ng of input DNA. Individual circles represent values for individual mice and horizontal bars represent means for each group. E-I) RT-qPCR was used to quantify expression in the heart of the indicated cytokines and chemokines, shown relative to GAPDH in arbitrary units. For comparison, horizontal dotted lines correspond to expression in mock-infected, vehicle-treated control mice (n=3; lines fall very close to the X-axis where not readily visible). Combined data from n=6–7 mice per group are presented as means ± S.E.M. **P<0.01.
Effects of β5i Deficiency on MAV-1 Respiratory Infection
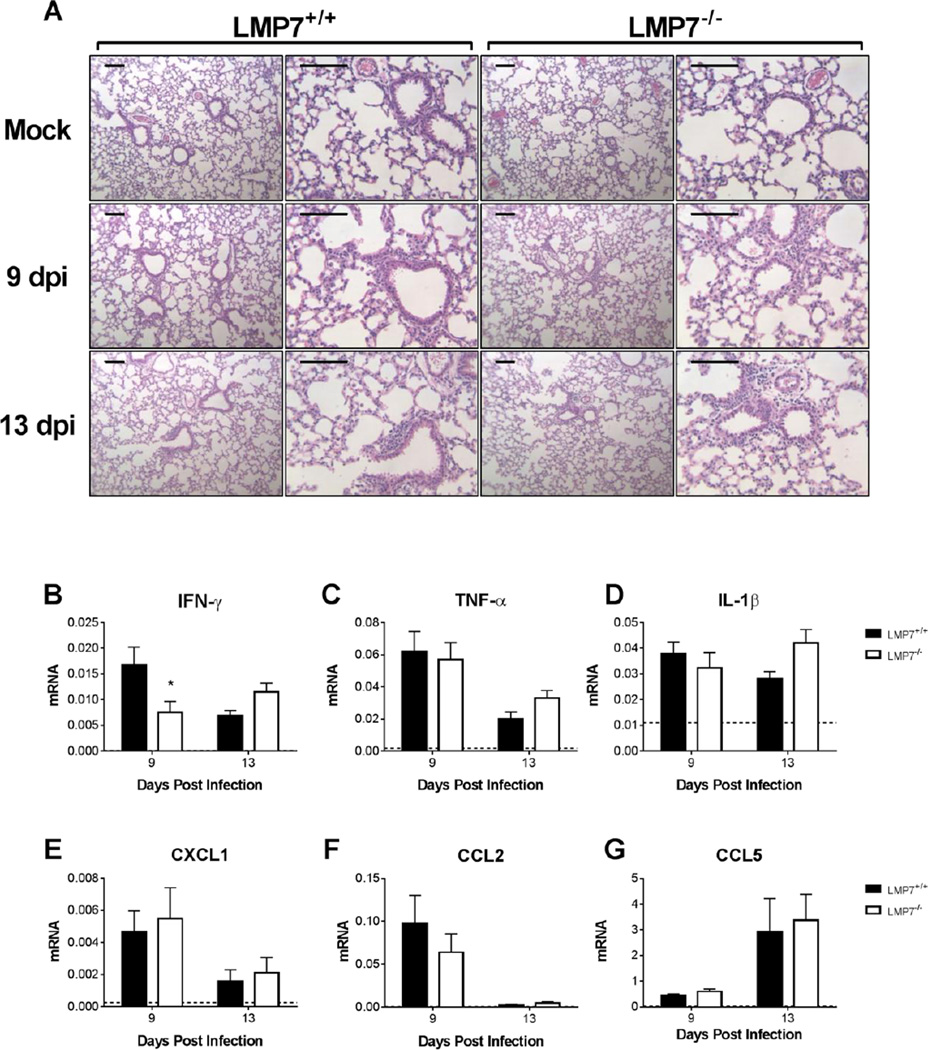
MAV-1 is detected in multiple organs following i.n. or i.p. infection (Kajon et al., 1998), and acute MAV-1 infection of neonatal mice causes both myocarditis (McCarthy and Weinberg, 2015) and lung disease (Procario et al., 2012). Consistent with our findings in the heart, β5i deficiency in LMP7−/− mice had no effect on lung viral loads (Fig. 4C). We examined inflammatory responses in the lungs of LMP7−/− mice to determine whether there were organ-specific differences in immunoproteasome induction and function during MAV-1 infection. We detected increases in β5i expression in the lungs of infected B6 mice (Fig. 2D). Histological evidence of virus-induced lung inflammation was similar in LMP7+/+ and LMP7−/− mice (Fig. 7A). Virus-induced IFN-γ expression was significantly lower in the lungs of LMP7−/− mice compared to LMP7+/+ mice at 9 dpi, but not at 13 dpi (Fig. 7B). There were no significant differences in the magnitude of expression of other cytokines and chemokines in the lungs of LMP7+/+ and LMP7−/− mice, including TNF-α, IL-1β, CXCL1, CCL2, and CCL5 (Figs. 7C–G). Thus, as in the heart, β5i deficiency had little effect on virus-induced inflammation in the lung during acute MAV-1 infection.
Fig. 7.
MAV-1-induced pulmonary inflammation in LMP7−/− mice. Neonatal LMP7−/− mice and LMP7+/+ littermate controls were infected with MAV-1 or mock infected with conditioned media, and lungs were harvested at 9 and 13 dpi. A) Hematoxylin-and-eosin-stained sections were prepared from paraffin-embedded lungs. Representative images are shown from mock-infected and infected mice. Adjacent images are from the same section at different magnifications. Scale bars, 100 µm. B–G) RT-qPCR was used to quantify cytokine expression in the lung, shown relative to GAPDH in arbitrary units. Horizontal dotted lines correspond to expression in mock-infected LMP7+/+ mice at 9 dpi (n=3; lines fall very close to the X-axis where not readily visible). Combined data from n=4–8 mice per group are presented as means ± S.E.M. *P<0.05.
Effects of β5i Deficiency on Protective Immunity to MAV-1
To determine whether immunoproteasome activity was essential for adaptive immune responses that develop following primary infection, we infected neonatal LMP7−/− mice at 7 days of age and then rechallenged them with virus at 29 dpi. In these experiments, neonatal B6 mice were infected similarly as controls. Protective immunity to MAV-1 measured using this assay is established in neonatal and adult B6 mice (Procario et al., 2012). Of note, there was no difference in survival beyond acute infection to this time point between B6 and LMP7−/− mice (data not shown). To evaluate the degree of protective immunity established following primary infection, we measured lung viral loads 7 days following rechallenge. Lung viral loads were low in rechallenged B6 and LMP7−/− mice, comparable to values measured at a late time point during primary infection (compare viral loads in Fig. 8 to viral loads at 13 dpi in Figure 4C). There was no significant difference between lung viral loads in rechallenged B6 and LMP7−/− mice. Consistent with the lack of effect of β5i deficiency on inflammatory responses during acute MAV-1 infection, the establishment of protective immunity was not substantially affected by β5i deficiency.
Fig. 8.
Protective immunity in LMP7−/− mice. Neonatal LMP7−/− mice and B6 controls were infected with MAV-1. At 29 dpi (36 days of age), mice were reinfected with the same dose of MAV-1. Lungs were harvested 7 days following rechallenge. qPCR was used to quantify MAV-1 lung viral loads. DNA viral loads are expressed as copies of MAV-1 genome per 100 ng of input DNA. Individual circles represent values for individual mice and horizontal bars represent means for each group. There were no statistically significant differences between groups.
Discussion
Proteasomes are responsible for the regulated degradation of almost all cellular proteins, and proteasome activity is required for cell viability (Rock et al., 1994; Tanaka, 1995). Proteasomes also play a primary role in generation of antigenic peptides for presentation on MHC class I molecules (Craiu et al., 1997; Groettrup et al., 1996; Rock et al., 1994). IFN-γ stimulation induces formation of the immunoproteasome, resulting in altered generation of MHC class I epitopes for recognition by CD8 T cells (McCarthy and Weinberg, 2015) as well as effects on protein homeostasis and proinflammatory cytokine production via NF-κB-mediated signaling and other pathways (Ebstein et al., 2012). Many immunoproteasome functions are relevant for viral pathogenesis, but no data are available regarding contributions of the immunoproteasome to adenovirus pathogenesis. Using MAV-1, which provides the advantage of studying an adenovirus in its natural host, we demonstrated that acute MAV-1 infection of neonatal mice upregulated immunoproteasome activity in the heart and lungs. Immunoproteasome upregulation was largely dependent on IFN-γ, because MAV-1-induced increases in immunoproteasome subunit expression were essentially absent in IFN-γ−/− mice, as is the case in models of infection with lymphocytic choriomeningitis virus (LCMV), Listeria monocytogenes, and Histoplasma capsulatum infection (Barton et al., 2002; Khan et al., 2001). β5i deficiency (in LMP7−/− mice) had no effect on viral replication or virus-induced inflammation in heart and lung during acute infection, or on the establishment of protective immunity. Thus, although immunoproteasome activity was robustly induced during acute infection, intact immunoproteasome activity was largely dispensable for innate and adaptive immune responses that are critical for MAV-1 pathogenesis.
CD8 T cells are recruited to the lungs, hearts, and brains of mice infected with MAV-1 (McCarthy et al., 2015a; McCarthy et al., 2015b; McCarthy et al., 2014; Procario et al., 2012; Weinberg et al., 2007). Compared to MAV-1 viral loads in wild type mice, viral loads are higher in brains of MHC class I-deficient mice following i.p. inoculation (Moore et al., 2003), in the lungs of CD8-deficient mice (McCarthy et al., 2015b), and in hearts of CD8-depleted mice (McCarthy and Weinberg, unpublished data) following i.n. inoculation. CD8 T cell dysfunction in mice following allogeneic bone marrow transplantation is associated with delayed clearance of MAV-1 from the lungs (McCarthy et al., 2015b). We therefore anticipated that immunoproteasome deficiency would impair viral clearance if it was associated with effects on virus-specific CD8 T cell function due to altered presentation of virus-specific epitopes by MHC class I. However, we observed no significant impairment in control of viral replication in LMP7−/− mice. Likewise, deficiency of β5i in LMP7−/− mice does not affect control of CVB3 replication in the heart (Opitz et al., 2011), and deficiency of β1i and β2i in β1i−/−/ β2i−/− mice does not affect LCMV viral replication in the brain (Basler et al., 2011). Although we did not extend our experiments to examine further clearance of virus at later time points, virus clearance by 13 dpi (indicated by lower viral loads) was comparable in the hearts and lungs of LMP7+/+ and LMP7−/− mice. It therefore seems unlikely that CD8 T cell function was substantially impaired by immunoproteasome deficiency. It is possible that β5i deficiency had an effect on the overall profile of MAV-1-specific epitopes presented by MHC class I to CD8 T cells, as has been demonstrated for influenza virus (Chen et al., 2001; Pang et al., 2006) and murine cytomegalovirus (Hutchinson et al., 2011). MAV-1-specific MHC class I epitopes have not yet been defined, so we were not able to directly assess this possibility. However, even if there were such differences between LMP7+/+ and LMP7−/− mice, our results suggest that would not have substantial implications for MAV-1 pathogenesis.
The immunoproteasome may promote inflammation via mechanisms such as degradation of IκB and subsequent activation of NF-κB-mediated signaling (Ebstein et al., 2012). Thus, we hypothesized that there might be less MAV-1-induced inflammation in LMP7−/− mice than in LMP7+/+ mice. We observed minimal differences in MAV-1-induced myocarditis in LMP7−/− mice compared to LMP7+/+ mice or in ONX 0914-treated mice compared to controls. It is possible that there were differences in cytokine protein levels that did not correlate with the mRNA levels that we measured in heart and lungs. Although we did not directly measure it in this study, it is also possible that MAV-1-induced NF-κB activation was impaired in LMP7−/− mice without a substantial effect on the inflammatory responses that we assayed. However, because there were no overall differences between LMP7−/− and LMP7+/+ mice in other measures of inflammation, viral replication, or survival, any such differences in cytokine expression or NF-κB activation are unlikely to be substantial or relevant to MAV-1 pathogenesis.
Our results contrast with those in studies that show enhanced manifestations of CVB3 myocarditis in β5i-deficient mice (Opitz et al., 2011) or decreased evidence of central nervous system inflammation induced by LCMV in β1i−/−/ β2i−/−-deficient mice (Basler et al., 2011). Even though some manifestations of CVB3 myocarditis are greater in β5i-deficient mice than in wild type mice, CVB3 myocarditis in β5i-deficient mice is not associated with altered expression of proinflammatory cytokines (Opitz et al., 2011). Instead, β5i deficiency is linked to impaired clearance of poly-ubiquitinated proteins, increased oxidative damage, and enhanced apoptosis in hearts following CVB3 infection. In vitro, HAdV infection induces reactive oxygen species production, which contributes to inflammasome activation (Barlan et al., 2011; McGuire et al., 2011). We have not studied potential contributions of oxidative stress or apoptosis to MAV-1 pathogenesis. It is possible that MAV-1, despite causing cardiac and pulmonary inflammation, does so without the induction of substantial oxidative damage or apoptosis, thereby potentially minimizing potential effects of immunoproteasome deficiency on MAV-1 pathogenesis. It is also possible that virus inhibition of immunoproteasome activity could account for the lack of substantial differences between MAV-1-infected LMP7+/+ and LMP7−/− mice. For instance, the HAdV early region 1A (E1A) protein inhibits immunoproteasome activity in vitro (Berhane et al., 2011; Vertegaal et al., 2003), downregulating IFN-γ-induced expression of β1i, β2i, and β5i and physically binding to β2i (Berhane et al., 2011). The MAV-1 E1A protein shares some functional roles with HAdV E1A (Ball et al., 1988; Fang and Spindler, 2005; Smith et al., 1996), but direct interactions between MAV-1 E1A and the immunoproteasome have not been defined.
Immunoproteasome subunit deficiency has a variety of effects on viral pathogenesis that depend on the subunit or subunits that are deficient along with the virus and organ that is studied. Immunoproteasome activity is increased in the lungs of mice infected with LCMV and murine gammaherpesvirus 68 (Keller et al., 2015; Kremer et al., 2010). Immunoproteasome deficiency (in β1i−/−, β2i−/−, or β5i/ β2i−/− mice) alters the profile of influenza virus-specific epitopes presented by MHC class I during respiratory infection (Chen et al., 2001; Zanker et al., 2013), but very little information is available regarding the effects of immunoproteasome deficiency on viral replication in the lungs or effects on virus-induced pulmonary inflammation. Our results suggest that acute MAV-1 infection upregulates immunoproteasome activity in the lungs in an IFN-γ-dependent manner. We observed greater mortality in IFN-γ−/− mice than in B6 controls, but this was not due to increased viral replication (Fig. 2) or differences in virus-induced inflammation (data not shown) in hearts or lungs. Our experiments with LMP7−/− mice indicate that increased mortality in IFN-γ−/− mice was not directly related to a lack of IFN-γ-induced β5i activity.
As in the heart, we detected minimal differences in viral replication or virus-induced inflammation in the lungs of LMP7−/− and LMP7+/+ mice. We did detect less IFN-γ expression in the lungs of LMP7−/− mice than in LMP7+/+ mice at 9 dpi. To our knowledge, direct effects of immunoproteasome activity on IFN-γ expression have not been described. A subtle effect of β5i deficiency on T cell function in the lungs, although not sufficient to alter control of viral replication, may have contributed to this effect on IFN-γ expression. In general, both the induction of immunoproteasome activity during MAV-1 infection and the overall effects of the immunoproteasome on MAV-1 pathogenesis are therefore generalized rather than organ-specific. In addition to the potential effects of the immunoproteasome on CD8 T cell function via MHC class I presentation and on NF-κB-mediated inflammatory responses, it may influence functions of other immune cells, including B cell number, differentiation, and antibody production; and macrophage and DC maturation and cytokine production (Hensley et al., 2010; Reis et al., 2011). It remains to be seen whether any of these factors are influenced by immunoproteasome activity during MAV-1 infection.
We chose LMP7−/− mice for these experiments with MAV-1 based on the likelihood that they are largely deficient in immunoproteasome function, since incorporation of β5i is required for the maturation of β1i and β2i, removing propeptides that would otherwise prevent their catalytic activity (Griffin et al., 1998; Groettrup et al., 1997). Although it is theoretically possible that changes have developed in LMP7−/− mice that could compensate for the lack of β5i, our data demonstrating equivalent β1i, β2i, β2, and β5 mRNA levels in LMP7−/− and LMP+/+ mice suggest that this was not the case during MAV-1 infection. Consistent with this possibility, incorporation of β1i and β2i into cardiac proteasomes is substantially impaired in LMP7−/− mice infected with CVB3 (Opitz et al., 2011), and β5i activity (measured by ProCISE assay) was absent without compensatory changes in β1i, β1, or β5 activity in LMP7−/− mice in a myocardial infarction model (Weinberg and Day, unpublished data). Our results from experiments in which MAV-1-infected B6 mice were treated with ONX 0914 are largely consistent with results from experiments using LMP7−/− mice, further supporting the validity of those experiments. It remains possible that even a small amount of residual β1i and/or β2i activity in LMP7−/− mice is sufficient compensate for the lack of β5i activity.
Targeting an inducible host factor, such as the immunoproteasome, that is predominantly active during an inflammatory state such as infection would be an appealing strategy if it could facilitate modulation of detrimental inflammatory responses with minimal impact on constitutively active host processes. Our results suggest that selective inhibitors of β5i activity such as ONX 0914 are unlikely to provide substantial benefit during adenovirus infection. It would be of interest to study MAV-1 pathogenesis in mice deficient in all immuno subunits (triply deficient in β1i, β2i, and β5i), which exhibit defects in antigen presentation that are broader than those in mice deficient in single subunits (Kincaid et al., 2012). If complete immunoproteasome deficiency affected MAV-1 pathogenesis in a manner different than isolated β5i deficiency, particularly if complete deficiency suppressed host inflammatory response to MAV-1 infection, then combined therapy using inhibitors of more than one immunoproteasome subunit may ultimately prove useful for patients infected with adenoviruses or other pathogens.
Materials and Methods
Mice
All work was approved by the University of Michigan Committee on Use and Care of Animals. β5i (LMP7) heterozygote knockout (LMP7+/−) mice on a B6 background were purchased from The Jackson Laboratory and bred at the University of Michigan to produce homozygote knockout (LMP7−/−) mice and homozygote wild type (LMP7+/+) littermates. We confirmed the genotypes of all LMP7−/− and LMP+/+ mice used in the experiments. IFN-γ knockout (IFN-γ−/−) mice on a B6 background (originally from The Jackson Lab) were generously provided by Dr. Benjamin Segal (University of Michigan) and were bred at the University of Michigan. B6 mothers with litters of neonatal mice were obtained from The Jackson Laboratory. For the rechallenge experiments, B6 neonates were bred at the University of Michigan. All mice were maintained under specific pathogen-free conditions.
Virus and Infections
MAV-1 was grown and titered on NIH 3T6 fibroblasts as previously described (Cauthen et al., 2007). Neonatal mice (7 days old) were manually restrained and infected i.n. with 105 plaque-forming units (pfu) in 10 µl of sterile phosphate-buffered saline (PBS). Control mice were mock infected i.n. with conditioned media at an equivalent dilution in sterile PBS. Mice were euthanized by pentobarbital overdose at the indicated time points. Organs were harvested, snap frozen in dry ice, and stored at −80°C until processed further. RNA and DNA were isolated from organs as previously described (McCarthy et al., 2015a; Procario et al., 2012). In some experiments, mice were infected at 7 days of age and then rechallenged i.n. with 105 pfu MAV-1 at 29 days post infection (dpi).
Inhibition of Immunoproteasome Activity
ONX 0914 was purchased from Selleckchem. ONX 0914 (5 mg/kg/dose) was administered subcutaneously (s.c.) beginning at 1 day post infection (d.p.i.) and continuing every other day. Control mice received equivalent amounts of 10% captisol (Selleckchem), the vehicle used for ONX 0914.
SDS-PAGE and Western Blot Analysis
Tissues were lysed using 1x loading buffer (50 mM Tris-Cl at pH 6.8, 2% SDS, and 10% glycerol) for the preparation of total proteins (Li et al., 2011). Western blots were performed as previously described (Wang et al., 2014), loading equivalent microgram amounts of lysate from each sample and using primary antibodies against proteasome subunit β5 (BML-PW8895, Enzo Life Sciences), immunoproteasome subunit β5i (BML-PW8845, Enzo Life Sciences), and the regulatory subunit PA28α (PW 8185; Affiniti Research Products, Devon, UK).
Measurement of β5 and β5i Subunit Activity
ProCISE assay was performed as previously described to quantify levels of individual proteasome subunit active sites and differentiate between activity of β5 and β5i active sites (Kirk et al., 2014). Fresh lysate was prepared by homogenizing frozen heart tissue in lysis buffer (20 mMTris-HCl, pH 8 + 5 mM EDTA in water) to yield lysate protein concentration of >4 µg/µl. Two sets of triplicate samples of lysate (4 µg/µl) were incubated with the biotinylated active site labeling probe, PR-584 (5 µM, Onyx Pharmaceuticals, Inc.), in a microcentrifuge tube at 25°C for 2 h (“sample + probe”). Two additional sets of triplicate samples of lysate were incubated without the PR-584 at 25°C for 2 h (“sample - probe”).
Streptavidin sepharose high performance beads (GE Healthcare) were washed in ELISA buffer (1X PBS with 0.1% Tween-20, 1% BSA) and then centrifuged at 2,000 RPM for 2 minutes. After the final wash, beads were resuspended in ELISA buffer and placed on ice. Filter membranes [Millipore 96-well MultiScreenHTS DV, 0.65 µm, opaque, non-sterile filter plates (#MSDVN6B50)] were hydrated and blocked by adding ELISA buffer to each well for 30 minutes. ELISA buffer was removed and the membranes blotted dry. Wells were loaded with 20 µl washed streptavidin beads (containing 4.6 µl bead suspension/well); 70 µl of 8 M guanidine HCL in assay buffer (20 mMTris-HCl, pH 8 + 0.5 mM EDTA in MilliQ water); and 10 µl of either “sample + probe” or “sample - probe.” The plate was then rocked for 1 hour to ensure that all samples mixed well with denaturant. Unbound material was removed by filtration and wells were washed with ELISA buffer.
Next, 100 µl anti-β5 polyclonal antibody (1:2000 in ELISA buffer, provided by Onyx Pharmaceuticals, Inc.) to one set of wells (± probe). Anti-LMP7 (β5i) monoclonal antibody (1:2000 in ELISA buffer, Biomol #BML-PW8845) was added to the other set of wells (± probe). The plate was covered and incubated with the antibodies, shaking overnight at 4°C. Primary antibody was removed and wells were washed with ELISA buffer. Wells were then incubated for 2 h at room temperature with secondary antibody [β5: HRP-goat anti-rabbit 1:5000, Jackson ImmunoResearch #111-035-003; LMP7 (β5i): HRP-goat anti-mouse 1:5000, Jackson ImmunoResearch #115-035-003]. Secondary antibody was then removed and wells were washed with ELISA buffer. Chemiluminescence was developed using the SuperSignal ELISA Pico Chemiluminescent Substrate kit and read for 10 min on a multilabel plate reader (Victor X3, Perkin Elmer). Final luminescence was calculated by subtracting values for “sample - probe” from values for “sample + probe.”
Analysis of Viral Loads
MAV-1 viral loads were measured in hearts using quantitative real-time polymerase chain reaction (qPCR) as previously described (McCarthy et al., 2015a; Procario et al., 2012). MAV-1 viral loads were measured in lungs in a similar manner in reactions using the same primers with Power SYBR Green PCR Mix (Applied Biosystems). Melt curves confirmed the specificity of those reactions (data not shown). Results were standardized to the nanogram (ng) amount of input DNA. Each sample was assayed in triplicate.
Real-time PCR Analysis of Gene Expression
Cytokine gene expression was quantified using reverse transcriptase (RT)-qPCR as previously described (McCarthy et al., 2015a; Procario et al., 2012). cDNA was amplified using duplexed gene expression assays for mouse CCL5, CXCL1, CCL2, and GAPDH (Applied Biosystems). Primers used with SYBR to detect other targets are listed in Table 2. Primers used to detect IL-1β (PrimerBank ID 6680415a1) were identified using the Harvard PrimerBank (Spandidos et al., 2010). Quantification of target gene mRNA was normalized to GAPDH and expressed in arbitrary units as 2−ΔCt, where Ct is the threshold cycle and ΔCt = Ct(target)-Ct(GAPDH).
Table 2.
Primers and probes used for real-time PCR analysis
| Target | Oligonucleotide | Sequence (5′ to 3′) |
|---|---|---|
| MAV-1 E1A genomic | Forward primer | GCACTCCATGGCAGGATTCT |
| Reverse primer | GGTCGAAGCAGACGGTTCTTC | |
| Probe | TACTGCCACTTCTGC | |
| GAPDH | Forward primer | TGCACCACCAACTGCTTAG |
| Reverse primer | GGATGCAGGGATGATGTTC | |
| IFN-γ | Forward primer | AAAGAGATAATCTGGCTCTGC |
| Reverse primer | GCTCTGAGACAATGAACGCT | |
| TNF-α | Forward primer | CCACCACGCTCTTCTGTCTAC |
| Reverse primer | AGGGTCTGGGCCATAGAACT | |
| IL-1β | Forward primer | GCAACTGTTCCTGAACTCAACT |
| Reverse primer | ATCTTTTGGGGTCCGTCAACT | |
| β1i | Forward primer | ATGGCAGTGGAGTTTGACGG |
| Reverse primer | ATACCTGTCCCCCCTCACATTG | |
| β5i | Forward primer | CATTCCTGAGGTCCTTTGGTGG |
| Reverse primer | ATGCGTTCCCCATTCCGAAG | |
| β2 | Forward primer | CGCAAAAAAGGGGTTCAAGC |
| Reverse primer | AGATGCTGTAGAGATGAGGTCCAG | |
| β5 | Forward primer | CACAGCAGGTGCTTATATTGC |
| Reverse primer | CTGTTCCCCTCGCTGTCTACG | |
| PA28α | Forward primer | GTCAAAGAGAAAGAGAAGGAGGAGC |
| Reverse primer | GGTGTGAAGGTTGGTCATCAGC |
Histology
Hearts and lungs were fixed in 10% formalin and embedded in paraffin, and 5 µm sections were stained with hematoxylin and eosin to evaluate cellular infiltrates. Prior to fixation, lungs were gently inflated with PBS via the trachea to maintain lung architecture. Sectioning and staining were performed by the University of Michigan Unit for Laboratory Animal Medicine Pathology Cores for Animal Research. Slides were viewed through a Laborlux 12 microscope (Leitz). Digital images were obtained with an EC3 digital imaging system (Leica Microsystems) using Leica Acquisition Suite software (Leica Microsystems).
Determination of Serum Cardiac Troponin Levels
In some experiments, blood was collected from the inferior vena cava of euthanized neonates and incubated on ice for approximately 30 m. Samples were then centrifuged in a tabletop microcentrifuge at 13,000 × g for 15 min at 4°C. Serum was transferred to a new labeled microcentrifuge tube and stored at −80°C. Serum cTnI concentrations were measured using the Ultra Sensitive Mouse Cardiac Troponin-I ELISA Kit (Life Diagnostics) according to the manufacturer’s instructions. According to the manufacturer, the lower limit of detection of this assay is 0.039 ng/mL. Samples were assayed in duplicate.
Statistics
Analysis for statistical significance was conducted using Prism 6 for Macintosh (GraphPad Software, Inc.). Differences between two groups were analyzed using Mann-Whitney test or Student’s t test, as indicated. Differences between more than two groups were analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison tests. For viral load data, log-transformed values were used in all analyses. P values less than 0.05 were considered statistically significant.
Highlights.
Mouse adenovirus type 1 (MAV-1) infection increased immunoproteasome (IP) activity.
MAV-1-induced IP expression depended on IFN-γ.
IP activity was not essential for control of MAV-1 replication.
IP deficiency did not affect MAV-1-induced inflammation in heart or lung
Acknowledgments
The authors thank Kathy Spindler and Mike Imperiale for helpful review of the manuscript. This work was supported by T32 AI007528 (MKM), R21 AI103452 (JBW), a University of Michigan Charles Woodson Interdisciplinary Award in Children’s Health (JBW), The Taubman Medical Institute and the Protein Folding Diseases Initiative at the University of Michigan (SMD), NIH HL068936 (SRP) and AHA Founders Affiliate Grant in Aid 14GRNT20450069 (SRP), and a grant from Onyx Pharmaceuticals, Inc., an Amgen subsidiary (SRP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson VE, Nguyen Y, Weinberg JB. Effects of allergic airway disease on mouse adenovirus type 1 respiratory infection. Virology. 2009;391:25–32. doi: 10.1016/j.virol.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball AO, Williams ME, Spindler KR. Identification of mouse adenovirus type 1 early region 1: DNA sequence and a conserved transactivating function. J. Virol. 1988;62:3947–3957. doi: 10.1128/jvi.62.11.3947-3957.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlan AU, Griffin TM, McGuire KA, Wiethoff CM. Adenovirus membrane penetration activates the NLRP3 inflammasome. J Virol. 2011;85:146–155. doi: 10.1128/JVI.01265-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton LF, Cruz M, Rangwala R, Deepe GS, Jr, Monaco JJ. Regulation of immunoproteasome subunit expression in vivo following pathogenic fungal infection. J Immunol. 2002;169:3046–3052. doi: 10.4049/jimmunol.169.6.3046. [DOI] [PubMed] [Google Scholar]
- Basler M, Beck U, Kirk CJ, Groettrup M. The antiviral immune response in mice devoid of immunoproteasome activity. J Immunol. 2011;187:5548–5557. doi: 10.4049/jimmunol.1101064. [DOI] [PubMed] [Google Scholar]
- Berhane S, Areste C, Ablack JN, Ryan GB, Blackbourn DJ, Mymryk JS, Turnell AS, Steele JC, Grand RJ. Adenovirus E1A interacts directly with, and regulates the level of expression of the immunoproteasome component MECL1. Virology. 2011;421:149–158. doi: 10.1016/j.virol.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss HP, McCarthy R, Hare J, Bricker JT, Bowles KR, Towbin JA. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42:466–472. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- Cauthen AN, Welton AR, Spindler KR. Construction of mouse adenovirus type 1 mutants. Methods Mol Med. 2007;130:41–59. doi: 10.1385/1-59745-166-5:41. [DOI] [PubMed] [Google Scholar]
- Chen W, Norbury CC, Cho Y, Yewdell JW, Bennink JR. Immunoproteasomes shape immunodominance hierarchies of antiviral CD8(+) T cells at the levels of T cell repertoire and presentation of viral antigens. J Exp Med. 2001;193:1319–1326. doi: 10.1084/jem.193.11.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craiu A, Gaczynska M, Akopian T, Gramm CF, Fenteany G, Goldberg AL, Rock KL. Lactacystin and clasto-lactacystin beta-lactone modify multiple proteasome beta-subunits and inhibit intracellular protein degradation and major histocompatibility complex class I antigen presentation. J Biol Chem. 1997;272:13437–13445. doi: 10.1074/jbc.272.20.13437. [DOI] [PubMed] [Google Scholar]
- Dennert R, Crijns HJ, Heymans S. Acute viral myocarditis. Eur Heart J. 2008;29:2073–2082. doi: 10.1093/eurheartj/ehn296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubiel W, Pratt G, Ferrell K, Rechsteiner M. Purification of an 11 S regulator of the multicatalytic protease. J Biol Chem. 1992;267:22369–22377. [PubMed] [Google Scholar]
- Ebstein F, Kloetzel PM, Kruger E, Seifert U. Emerging roles of immunoproteasomes beyond MHC class I antigen processing. Cell Mol Life Sci. 2012;69:2543–2558. doi: 10.1007/s00018-012-0938-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Spindler KR. E1A-CR3 interaction-dependent and -independent functions of mSur2 in viral replication of early region 1A mutants of mouse adenovirus type 1. J. Virol. 2005;79:3267–3276. doi: 10.1128/JVI.79.6.3267-3276.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin TA, Nandi D, Cruz M, Fehling HJ, Kaer LV, Monaco JJ, Colbert RA. Immunoproteasome assembly: cooperative incorporation of interferon gamma (IFN-gamma)-inducible subunits. J Exp Med. 1998;187:97–104. doi: 10.1084/jem.187.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groettrup M, Soza A, Kuckelkorn U, Kloetzel PM. Peptide antigen production by the proteasome: complexity provides efficiency. Immunol Today. 1996;17:429–435. doi: 10.1016/0167-5699(96)10051-7. [DOI] [PubMed] [Google Scholar]
- Groettrup M, Standera S, Stohwasser R, Kloetzel PM. The subunits MECL-1 and LMP2 are mutually required for incorporation into the 20S proteasome. Proc Natl Acad Sci USA. 1997;94:8970–8975. doi: 10.1073/pnas.94.17.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heink S, Ludwig D, Kloetzel PM, Kruger E. IFN-gamma-induced immune adaptation of the proteasome system is an accelerated and transient response. Proc Natl Acad Sci U S A. 2005;102:9241–9246. doi: 10.1073/pnas.0501711102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley SE, Zanker D, Dolan BP, David A, Hickman HD, Embry AC, Skon CN, Grebe KM, Griffin TA, Chen W, Bennink JR, Yewdell JW. Unexpected role for the immunoproteasome subunit LMP2 in antiviral humoral and innate immune responses. J Immunol. 2010;184:4115–4122. doi: 10.4049/jimmunol.0903003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson S, Sims S, O'Hara G, Silk J, Gileadi U, Cerundolo V, Klenerman P. A dominant role for the immunoproteasome in CD8+ T cell responses to murine cytomegalovirus. PLoS ONE. 2011;6:e14646. doi: 10.1371/journal.pone.0014646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakel S, Kuckelkorn U, Szalay G, Plotz M, Textoris-Taube K, Opitz E, Klingel K, Stevanovic S, Kandolf R, Kotsch K, Stangl K, Kloetzel PM, Voigt A. Differential interferon responses enhance viral epitope generation by myocardial immunoproteasomes in murine enterovirus myocarditis. Am J Pathol. 2009;175:510–518. doi: 10.2353/ajpath.2009.090033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajon AE, Brown CC, Spindler KR. Distribution of mouse adenovirus type 1 in intraperitoneally and intranasally infected adult outbred mice. J. Virol. 1998;72:1219–1223. doi: 10.1128/jvi.72.2.1219-1223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller IE, Vosyka O, Takenaka S, Kloss A, Dahlmann B, Willems LI, Verdoes M, Overkleeft HS, Marcos E, Adnot S, Hauck SM, Ruppert C, Gunther A, Herold S, Ohno S, Adler H, Eickelberg O, Meiners S. Regulation of immunoproteasome function in the lung. Sci Rep. 2015;5:10230. doi: 10.1038/srep10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S, van den Broek M, Schwarz K, de Giuli R, Diener PA, Groettrup M. Immunoproteasomes largely replace constitutive proteasomes during an antiviral and antibacterial immune response in the liver. J Immunol. 2001;167:6859–6868. doi: 10.4049/jimmunol.167.12.6859. [DOI] [PubMed] [Google Scholar]
- Kincaid EZ, Che JW, York I, Escobar H, Reyes-Vargas E, Delgado JC, Welsh RM, Karow ML, Murphy AJ, Valenzuela DM, Yancopoulos GD, Rock KL. Mice completely lacking immunoproteasomes show major changes in antigen presentation. Nat Immunol. 2012;13:129–135. doi: 10.1038/ni.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk CJ, Powell SR, Miller EJ. Assessment of cytokine-modulated proteasome activity. Methods Mol Biol. 2014;1172:147–162. doi: 10.1007/978-1-4939-0928-5_13. [DOI] [PubMed] [Google Scholar]
- Kremer M, Henn A, Kolb C, Basler M, Moebius J, Guillaume B, Leist M, Van den Eynde BJ, Groettrup M. Reduced immunoproteasome formation and accumulation of immunoproteasomal precursors in the brains of lymphocytic choriomeningitis virus-infected mice. J Immunol. 2010;185:5549–5560. doi: 10.4049/jimmunol.1001517. [DOI] [PubMed] [Google Scholar]
- Kuhl U, Pauschinger M, Seeberg B, Lassner D, Noutsias M, Poller W, Schultheiss HP. Viral persistence in the myocardium is associated with progressive cardiac dysfunction. Circulation. 2005;112:1965–1970. doi: 10.1161/CIRCULATIONAHA.105.548156. [DOI] [PubMed] [Google Scholar]
- Li J, Powell SR, Wang X. Enhancement of proteasome function by PA28α overexpression protects against oxidative stress. FASEB J. 2011;25:883–893. doi: 10.1096/fj.10-160895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Martino T, Opavsky MA, Penninger J. Viral myocarditis: balance between viral infection and immune response. Can J Cardiol. 1996;12:935–943. [PubMed] [Google Scholar]
- Ma CP, Slaughter CA, DeMartino GN. Identification, purification, and characterization of a protein activator (PA28) of the 20 S proteasome (macropain) J Biol Chem. 1992;267:10515–10523. [PubMed] [Google Scholar]
- Martin AB, Webber S, Fricker FJ, Jaffe R, Demmler G, Kearney D, Zhang YH, Bodurtha J, Gelb B, Ni J, et al. Acute myocarditis. Rapid diagnosis by PCR in children. Circulation. 1994;90:330–339. doi: 10.1161/01.cir.90.1.330. [DOI] [PubMed] [Google Scholar]
- McCarthy MK, Levine RE, Procario MC, McDonnell PJ, Zhu L, Mancuso P, Crofford LJ, Aronoff DM, Weinberg JB. Prostaglandin E2 induction during mouse adenovirus type 1 respiratory infection regulates inflammatory mediator generation but does not affect viral pathogenesis. PLoS ONE. 2013;8:e77628. doi: 10.1371/journal.pone.0077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MK, Procario MC, Twisselmann N, Wilkinson JE, Archambeau AJ, Michele DE, Day SM, Weinberg JB. Proinflammatory effects of interferon gamma in mouse adenovirus 1 myocarditis. J Virol. 2015a;89:468–479. doi: 10.1128/JVI.02077-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MK, Procario MC, Wilke CA, Moore BB, Weinberg JB. Prostaglandin E2 production and T cell function in mouse adenovirus type 1 Infection following allogeneic bone marrow rransplantation. PLoS ONE. 2015b;10:e0139235. doi: 10.1371/journal.pone.0139235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MK, Weinberg JB. The immunoproteasome and viral infection: a complex regulator of inflammation. Front Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MK, Zhu L, Procario MC, Weinberg JB. IL-17 contributes to neutrophil recruitment but not to control of viral replication during acute mouse adenovirus type 1 respiratory infection. Virology. 2014;456–457:259–267. doi: 10.1016/j.virol.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire KA, Barlan AU, Griffin TM, Wiethoff CM. Adenovirus type 5 rupture of lysosomes leads to cathepsin B-dependent mitochondrial stress and production of reactive oxygen species. J Virol. 2011;85:10806–10813. doi: 10.1128/JVI.00675-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ML, Brown CC, Spindler KR. T cells cause acute immunopathology and are required for long-term survival in mouse adenovirus type 1-induced encephalomyelitis. J Virol. 2003;77:10060–10070. doi: 10.1128/JVI.77.18.10060-10070.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchamuel T, Basler M, Aujay MA, Suzuki E, Kalim KW, Lauer C, Sylvain C, Ring ER, Shields J, Jiang J, Shwonek P, Parlati F, Demo SD, Bennett MK, Kirk CJ, Groettrup M. A selective inhibitor of the immunoproteasome subunit LMP7 blocks cytokine production and attenuates progression of experimental arthritis. Nat Med. 2009;15:781–787. doi: 10.1038/nm.1978. [DOI] [PubMed] [Google Scholar]
- Opitz E, Koch A, Klingel K, Schmidt F, Prokop S, Rahnefeld A, Sauter M, Heppner FL, Volker U, Kandolf R, Kuckelkorn U, Stangl K, Kruger E, Kloetzel PM, Voigt A. Impairment of immunoproteasome function by beta5i/LMP7 subunit deficiency results in severe enterovirus myocarditis. PLoS Pathog. 2011;7:e1002233. doi: 10.1371/journal.ppat.1002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang KC, Sanders MT, Monaco JJ, Doherty PC, Turner SJ, Chen W. Immunoproteasome subunit deficiencies impact differentially on two immunodominant influenza virus-specific CD8+ T cell responses. J Immunol. 2006;177:7680–7688. doi: 10.4049/jimmunol.177.11.7680. [DOI] [PubMed] [Google Scholar]
- Pauschinger M, Bowles NE, Fuentes-Garcia FJ, Pham V, Kuhl U, Schwimmbeck PL, Schultheiss HP, Towbin JA. Detection of adenoviral genome in the myocardium of adult patients with idiopathic left ventricular dysfunction. Circulation. 1999;99:1348–1354. doi: 10.1161/01.cir.99.10.1348. [DOI] [PubMed] [Google Scholar]
- Procario MC, Levine RE, McCarthy MK, Kim E, Zhu L, Chang CH, Hershenson MB, Weinberg JB. Susceptibility to acute mouse adenovirus type 1 respiratory infection and establishment of protective immunity in neonatal mice. J Virol. 2012;86:4194–4203. doi: 10.1128/JVI.06967-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procario MC, McCarthy MK, Levine RE, Molloy CT, Weinberg JB. Prostaglandin E production during neonatal respiratory infection with mouse adenovirus type 1. Virus Res. 2016;214:26–32. doi: 10.1016/j.virusres.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Hassan F, Guan XQ, Shen J, Monaco JJ, Papasian CJ, Qureshi AA, Van Way CW, 3rd, Vogel SN, Morrison DC, Qureshi N. The immunoproteasomes regulate LPS-induced TRIF/TRAM signaling pathway in murine macrophages. Cell Biochem Biophys. 2011;60:119–126. doi: 10.1007/s12013-011-9183-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell. 1994;78:761–771. doi: 10.1016/s0092-8674(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Rose NR, Hill SL. The pathogenesis of postinfectious myocarditis. Clin Immunol Immunopathol. 1996;80:S92–S99. doi: 10.1006/clin.1996.0146. [DOI] [PubMed] [Google Scholar]
- Smith K, Ying B, Ball AO, Beard CW, Spindler KR. Interaction of mouse adenovirus type 1 early region 1A protein with cellular proteins pRb and p107. Virology. 1996;224:184–197. doi: 10.1006/viro.1996.0520. [DOI] [PubMed] [Google Scholar]
- Spandidos A, Wang X, Wang H, Seed B. PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res. 2010;38:D792–D799. doi: 10.1093/nar/gkp1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalay G, Meiners S, Voigt A, Lauber J, Spieth C, Speer N, Sauter M, Kuckelkorn U, Zell A, Klingel K, Stangl K, Kandolf R. Ongoing coxsackievirus myocarditis is associated with increased formation and activity of myocardial immunoproteasomes. Am J Pathol. 2006;168:1542–1552. doi: 10.2353/ajpath.2006.050865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. Molecular biology of proteasomes. Mol Biol Rep. 1995;21:21–26. doi: 10.1007/BF00990966. [DOI] [PubMed] [Google Scholar]
- Tatrai E, Hartyanszky I, Jr, Laszik A, Acsady G, Sotonyi P, Hubay M. The role of viral infections in the development of dilated cardiomyopathy. Pathol Oncol Res. 2011;17:229–235. doi: 10.1007/s12253-010-9302-6. [DOI] [PubMed] [Google Scholar]
- Vertegaal AC, Kuiperij HB, Houweling A, Verlaan M, van der Eb AJ, Zantema A. Differential expression of tapasin and immunoproteasome subunits in adenovirus type 5-versus type 12-transformed cells. J Biol Chem. 2003;278:139–146. doi: 10.1074/jbc.M206267200. [DOI] [PubMed] [Google Scholar]
- Wang P, Calise J, Powell K, Divald A, Powell SR. Upregulation of proteasome activity rescues cardiomyocytes following pulse treatment with a proteasome inhibitor. Am J Cardiovasc Dis. 2014;4:6–13. [PMC free article] [PubMed] [Google Scholar]
- Weinberg JB, Jensen DR, Gralinski LE, Lake AR, Stempfle GS, Spindler KR. Contributions of E1A to mouse adenovirus type 1 pathogenesis following intranasal inoculation. Virology. 2007;357:54–67. doi: 10.1016/j.virol.2006.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg JB, Stempfle GS, Wilkinson JE, Younger JG, Spindler KR. Acute respiratory infection with mouse adenovirus type 1. Virology. 2005;340:245–254. doi: 10.1016/j.virol.2005.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold WSM, Isom MG. Adenoviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 6. Lippincott: Williams & Wilkins, Philadelphia; 2013. pp. 1732–1767. [Google Scholar]
- Zanker D, Waithman J, Yewdell JW, Chen W. Mixed proteasomes function to increase viral peptide diversity and broaden antiviral CD8+ T cell responses. J Immunol. 2013;191:52–59. doi: 10.4049/jimmunol.1300802. [DOI] [PMC free article] [PubMed] [Google Scholar]