Abstract
Objective:
People living with sickle cell disease (SCD) experience severe episodic and chronic pain and frequently report poor interpersonal treatment within health-care settings. In this particularly relevant context, we examined the relationship between perceived discrimination and both clinical and laboratory pain.
Methods:
Seventy-one individuals with SCD provided self-reports of experiences with discrimination in health-care settings and clinical pain severity, and completed a psychophysical pain testing battery in the laboratory.
Results:
Discrimination in health-care settings was correlated with greater clinical pain severity and enhanced sensitivity to multiple laboratory-induced pain measures, as well as stress, depression, and sleep. After controlling for relevant covariates, discrimination remained a significant predictor of mechanical temporal summation (a marker of central pain facilitation), but not clinical pain severity or suprathreshold heat pain response. Furthermore, a significant interaction between experience with discrimination and clinical pain severity was associated with mechanical temporal summation; increased experience with discrimination was associated with an increased correlation between clinical pain severity and temporal summation of pain.
Discussion:
Perceived discrimination within health-care settings was associated with pain facilitation. These findings suggest that discrimination may be related to increased central sensitization among SCD patients, and more broadly that health-care social environments may interact with pain pathophysiology.
Key Words: clinical pain, quantitative sensory testing, patient-provider interaction, temporal summation, racial discrimination
People living with sickle cell disease (SCD) experience severe episodic and chronic pain1 and psychosocial sequelae associated with the disease.2 Although they report high levels of daily pain that is frequently managed at home,1 the experience of severe pain often leads to frequent engagement with the health-care system.3 Unfortunately, despite the availability of SCD pain treatment guidelines,4 individuals with SCD report undertreatment of pain and poor interpersonal treatment in health-care settings.5,6
SCD patients are often perceived as “difficult patients,”6 and may be disproportionately exposed to biased and discriminatory treatment in health-care settings because of a number of historical, cultural, and social factors. Although SCD affects people from various ethnic backgrounds worldwide, in the United States it is largely associated with, and perceived to only affect, African Americans.7 A recent survey found that many patients and providers at an SCD clinic believed that patient race affected treatment and pain management.8 Moreover, because of severe and undermanaged pain, SCD patients are often perceived as drug-seekers or addicts9 and might display behaviors in interactions with providers that are misperceived as being characteristic of substance abuse.10
Discrimination in health-care settings may directly affect multiple health outcomes, including pain management, clinical pain, and pain sensitivity. Several researchers have suggested that discrimination plays a role in the inadequate treatment and pain management in SCD.7,11,12 Negative interpersonal experiences contribute to frequent at-home management of vaso-occlusive crisis1 and self-discharge from hospitals,13 suggesting that interpersonal treatment factors, such as discrimination, impede adequate pain management for SCD patients.14 A few studies have assessed the relationship between discrimination and clinical pain in SCD15,16 and reveal that SCD patients experience more race-based discrimination in health-care settings than African Americans in general, and additionally experience disease-based discrimination that is associated with increased clinical pain.16 If also associated with increased pain sensitivity, discrimination within health-care settings may increase the burden on SCD patients several-fold.
Here, for the first time, we examine the relationship between perceived racial discrimination within health-care settings and both clinical pain and laboratory pain sensitivity among adults with SCD. Although clinical pain and laboratory pain sensitivity are related,17 examining both may provide valuable insight in characterizing the pathophysiology and sensory dimensions of pain because of SCD. Specifically, we include tests of central sensitization (eg, temporal summation), as this has been proposed as a mechanism and marker of pain chronification.18 Central sensitization (CS) is an amplification of central nociceptive processes that leads to altered pain responses after repeated exposure to pain.19 The majority of investigations on SCD pain have focused on the acute pain of vaso-occlusive crisis.20 However, adults with SCD also typically experience chronic pain that occurs every day in about one third of patients1 and is independent of vaso-occlusion.21 The problem of chronic pain in individuals with SCD remains largely unexplored and undertreated.20 To date, despite suggestion that CS may lead to chronic pain and hyperalgesia among SCD patients,4 no clinical studies have tested pain sensitization among adults with SCD.22 We hypothesized that discrimination would be associated with increased clinical pain and facilitation of laboratory pain.
MATERIALS AND METHODS
Participants
Individuals with SCD were recruited for participation from the Sickle Cell Center for Adults at Johns Hopkins and through posted advertisements. Seventy-one volunteers (68 African American/black, 3 multiracial) with SCD participated in this study (see Table 1 for demographic data), which is part of an ongoing larger study on pain in SCD (n=82). Major inclusion criteria included age 18 years and above, formal diagnosis of SCD (hemoglobinopathy genotype [Hb SS, Hb SC, Hb S/β-thalassemia]), and on a stable dose (if any) of NSAIDs, acetaminophen, or opioids 1 month before pain testing. Exclusion criteria included current alcohol or substance abuse/dependence; delirium, dementia, or cognitive impairment; and unstable psychiatric illness. All participants who provided responses to the discrimination subscale of the Interpersonal Processes of Care Survey (IPC-18)23 were included in the present analyses.
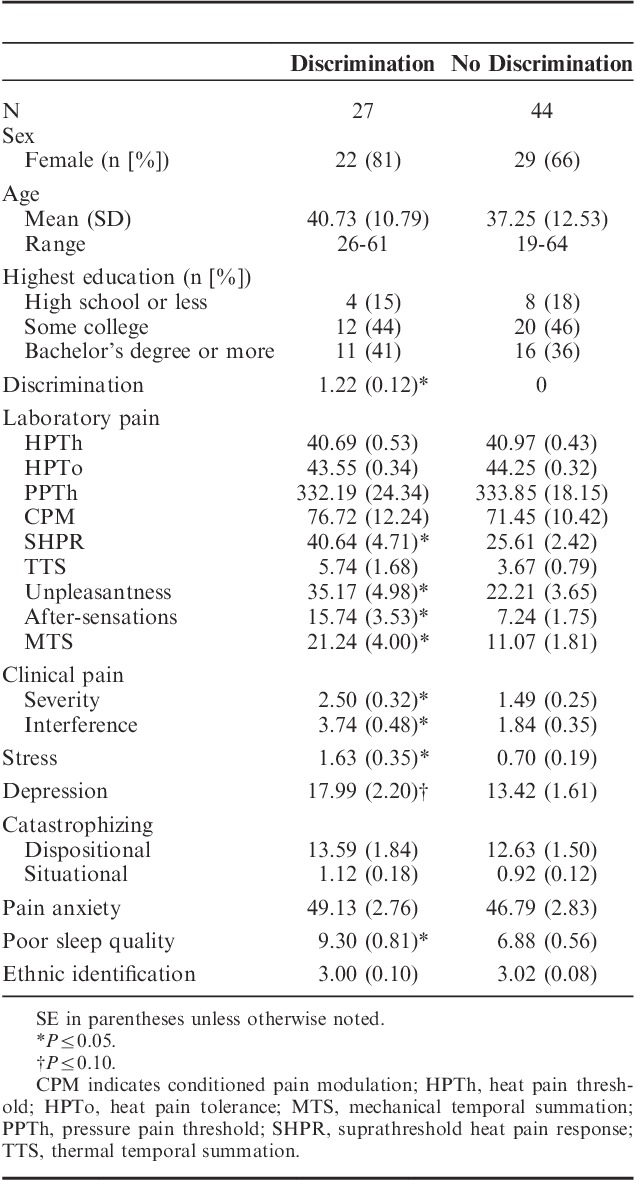
TABLE 1.
Descriptive Statistics by Level of Experience With Discrimination in Health-Care Settings
Procedure
Before study participation, participants completed an initial phone screen in which they provided a brief medical history to ensure that the study criteria were met. All study-related procedures were approved by the Johns Hopkins University School of Medicine Institutional Review Board, and informed written consent was obtained from each participant. The in-person visit was scheduled on days when participants were experiencing typical SCD pain at the level of 5 or lower on a 0 to 10 pain rating scale and had not experienced a vaso-occlusive crisis in at least the previous 3 weeks. After the consent process, participants completed the surveys described below, as well as a psychophysical pain testing battery lasting approximately 1 hour. Participants also completed questions related to their situational responses to, and evaluations of, the pain. Participants were allowed to stop or refuse any procedure at any time.
Survey Measures
Participants completed a number of surveys before commencing pain testing, including a demographic and health history questionnaire, and the following previously validated surveys.
Discrimination
The discrimination subscale of the short form of the Interpersonal Processes of Care Survey (IPC-18)23 was used to assess experience with discrimination in health-care settings that is attributed to race or ethnicity. The discrimination subscale consists of 2 questions (How often did doctors pay less attention to you because of your race or ethnicity? How often did you feel discriminated against by doctors because of your race or ethnicity?). Participants answered each item using a 5-point scale (1, Never to 5, Always). For conceptual clarity, the scale was transformed to a 0 to 4 scale so that a response of Never corresponds to a score of 0. This discrimination subscale has been validated across diverse racial groups within clinical populations23 and has previously been shown to be associated with decreased quality of life24 and physiological assessments of disease severity25 in different patient populations.
Clinical Pain
Self-reported clinical pain severity was assessed as the average of patients’ current pain, as well as their worst, least, and average pain over the previous week using an 11-point scale (0, No Pain to 10, Pain as bad as it could be). The 10 items from the extended26,27 Brief Pain Inventory28 Pain Interference subscale assessed functional interference caused by pain during the previous week in the areas of mood, sleep, relationships with others, and various daily activities, and were scored on an 11-point scale (0, Does not interfere to 10, Completely interferes).
Potential Covariates
To more fully characterize the independent relationship between discrimination and pain, we include several well-validated measures of various behavioral and psychological constructs known to also be associated with pain sensitivity.
Stress: Baseline stress level (How much stress do you feel right now?) was assessed using an 11-point scale (0, none to 10, extreme), with higher scores representing a higher degree of stress. Stress is associated with altered pain sensitivity29 and discrimination,30 and it was collected as a potential mediator of the relationship between discrimination and pain.
Depression: The Center for Epidemiological Studies Depression Scale31 measures depressive symptomatology. In the current study, we asked participants to respond on the basis of the frequency of feelings and experiences during the last week on a 5-point scale (0, rarely/<1 d to 4, most of the time/5 to 7 d). Depression is consistently associated with clinical pain32 and modulates sensitivity to laboratory pain.33
Catastrophizing: The Pain Catastrophizing Scale34 assesses exaggerated negative cognitive and affective response to pain, and it is a powerful predictor of clinical pain across chronic pain populations.35 This standard version of the scale assesses trait-like responses to pain in general and consists of 13 items rated on a 5-point scale (0, not at all to 4, all the time), with higher scores indicating greater pain catastrophizing. Situational catastrophizing (sitCAT),36 which is predictive of laboratory pain,37 was assessed during the pain testing session, and it was scored on the same 5-point scale as the Pain Catastrophizing Scale.
Pain Anxiety: The short form of the Pain Anxiety Symptoms Scale38 measures fear and anxiety responses to pain in general, is related to enhanced clinical pain, and consists of 20 items rated on a 6-point scale (0, never to 5, always), with higher scores indicating greater pain anxiety.
Poor Sleep Quality: The Pittsburgh Sleep Quality Index39 assesses subjective sleep quality and continuity for the previous month. The global score takes into account sleep quality, latency, duration, efficiency, disturbance, medication, and daytime dysfunction. Possible scores range from 0 to 21, with higher scores indicating poorer sleep quality. Poor sleep quality was examined as a potential covariate because of its relationship with clinical pain40 and discrimination.41,42
Ethnic Identification: The Multigroup Ethnic Identity Measure43 measures degree of identification with one’s own ethnic group using a 4-point scale (1, strongly disagree to 4, strongly agree), with higher scores corresponding to greater identification. Ethnic identification has previously been shown to be associated with laboratory pain among healthy African Americans44 and was therefore included as a potential mediator of the relationship between discrimination and pain.
Psychophysical Pain Testing
Participants completed at least 1 trial of each of the below described procedures. All available data are included in subsequent analyses.
Pain Ratings
A numerical rating scale ranging from 0 (no pain) to 100 (worst pain imaginable) was used for each of the pain testing procedures.
Thermal Stimuli
All contact heat stimuli were delivered using a Contact Heat-Evoked Potential Stimulator (CHEPS; Medoc Ltd., Ramat Yishai, Israel) system, a peltier element–based stimulator with a 9 cm2 rapidly heating/cooling probe.
Heat Pain Threshold (HPTh)/Heat Pain Tolerance (HPTo)
HPTh and HPTo were calculated as the average of 2 corresponding trials administered to participants’ dominant ventral forearm using an ascending method of limits paradigm. On each trial, the contact thermode gradually increased in temperature, from a baseline of 30°C at a 0.5°C/second rate of increase, until the participant indicated through button press that the stimulus first felt painful (HPTh) or when the stimulus became intolerable (HPTo). Between trials, the thermode was moved up the arm slightly to avoid overlapping stimulation sites.
Pressure Pain Threshold (PPTh)
An electronic algometer (Somedic, Sollentuna, Sweden) was used to assess PPTh using a 1-cm2 probe covered with a 1-mm polypropylene material.45 Pressure was applied to the muscle belly and increased steadily at a rate of 30 kPa/s until the patient verbally indicated that the pressure first felt painful (PPTh). PPThs were assessed twice at each of 4 body sites, bilaterally (trapezius muscle, interphalangeal joint of the thumb, the proximal third of the brachioradialis muscle [forearm], and middle of the quadriceps insertion point), for a total of 16 PPTh assessments. A minimum of 1-minute interval was maintained between applications at the same site. The final PPTh was calculated as the average across all sites and repetitions (Table 1). (The pattern of response was similar across all sites and repetitions. Results remain similar when site-specific PPThs were used in primary analyses).
Conditioned Pain Modulation (CPM)
CPM was assessed using pressure applied to the trapezius as the test stimulus, and hot water bath as the conditioning stimulus. First, PPTh was again assessed (separate from PPTh above) twice at the nondominant trapezius. The dominant hand was then submerged in a hot water bath for 20 seconds, at which time PPTh was reassessed. If participants removed their hands before 20 seconds, PPTh was assessed immediately upon withdrawal. The hot water temperature was determined early in the pain testing session as the temperature at which participants rate their pain as a 60 to 70 out of 100 after 20 seconds of hand submersion. Hot water temperature was first tested at 40°C. Subsequent tests with increasing temperatures were conducted as needed until the target pain intensity was achieved. CPM was calculated as the difference between the PPThs during and before water submersion. This procedure was repeated a second time, and final scores reflect an average of the difference score obtained during each trial. Participants revealed a significant increase in PPTh in the presence of the conditioning stimulus (Mbaseline=228.81 kPa, Mhot water=302.23 kPa, t69=9.29, P<0.001), indicating that our procedure successfully elicited CPM.
Suprathreshold Heat Pain Response (SHPR), Thermal Temporal Summation (TTS), Pain Unpleasantness, and After-Sensations
Ten repetitive thermal stimuli were applied rapidly, to participants’ dominant ventral forearm, in a series of identical pulses. A pain rating was obtained for each pulse. The thermode remained in a fixed position during administration of each sequence of 10 heat pulses (0.5 s each, with a 2.5 s interpulse interval). A practice trial with pulses at participants’ warmth detection threshold was conducted to familiarize participants with the procedure. Experimental trials were conducted at tailored temperatures (HPTh −2°C, HPTh, HPTh +2°C), and at a standard temperature of 45°C. The thermode was moved slightly between trials to avoid overlapping stimulation sites.
The pain rating on the fifth pulse was used as a measure of SHPR as has been used by others.46 Participants rated the fifth pulse in each series as painful (suprathreshold) across temperatures (MHPTh-2=17.12, SEHPTh-2=2.50; MHPTh=24.88, SEHPTh=2.86; MHPTh+2=34.52, SEHPTh+2=3.33; M45=46.82, SE45=3.51), and the pattern of response was similar across temperatures. An average of the SHPR at the 4 experimental temperatures was used for all analyses. (Results remain similar when the response at HPTh-2 was excluded from the average composite score).
TTS was calculated as the difference between maximum pain rating within each trial and the pain rating on the first pulse. An average TTS across 4 four experimental temperatures was used for all analyses.
Pain unpleasantness (0 to 100) was assessed immediately after each trial. Pain unpleasantness was averaged across the 4 experimental temperatures.
Residual pain was queried after each trial, and these “after-sensations” were rated 15 seconds after the final “pulse” of each trial. After-sensations were similarly averaged across the 4 temperatures and used in all analyses.
Mechanical Temporal Summation (MTS)
MTS was calculated as the difference between pain ratings in response to a single punctuate stimulus compared with a sequence of 10 identical punctuate stimuli. Weighted pinprick stimulators with a flat contact area of 0.2 mm diameter were used to deliver stimuli at a 1/second rate to the middle phalange of the middle finger. A practice trial was conducted with a stimulator that produced 32 mN force. Experimental trials were conducted at 128 and 256 mN. An average MTS at the 2 experimental weights was used for all analyses.
Data Analysis
The goal of the first level of analysis was to assess the relationship between discrimination and clinical and laboratory pain, as well as related behavioral, psychological, and physiological variables. Descriptive statistics were evaluated and guided first-level inferential statistical analyses.
The goal of the second level of analysis was to determine the statistical effect of current clinical pain and discrimination on laboratory pain sensitivity. Discrimination was included as a continuous, not dichotomous, variable in all multivariate models. Second-level analyses were not conducted on factors that were not significantly correlated with individual differences on the discrimination scale. We conducted hierarchical multiple regression to determine the relationship between discrimination and pain after controlling for demographic data and correlated covariates (constructs that were correlated with dependent variables of interest). When covariates were highly correlated and overlapping with each other (ie, depression and anxiety, dispositional and situational catastrophizing), we included the variable that was more strongly correlated with the dependent variable in the models. When clinical pain was correlated with laboratory pain–dependent variables, potential clinical pain×discrimination interactions were also examined. Finally, we probed significant interactions using moderation models. The Johnson-Neyman technique was used to identify the region of significance of the moderator.47 All data analyses were conducted using SPSS (version 21; IBM Corp., Armonk, NY), and moderation was tested using Hayes’ PROCESS macro47 implemented in SPSS.
Missing Data
Participants were not excluded because of partially missing data, and the majority of participants (N=65, 91.5%) completed all procedures. Although some participants did not complete every trial of each psychophysical pain testing procedure because of voluntary discontinuance or rating the maximum (100) before the completion of a procedure, average ratings are available for all participants on each procedure with the exception of CPM (missing, N=1) and SPTH/TTS (missing, N=2). All participants (N=71) completed the discrimination, clinical pain, stress, pain catastrophizing, pain anxiety, and sleep surveys; however, a few participants chose not to respond to the depression (missing, N=3) and ethnic identification (missing, N=1) surveys.
RESULTS
Discrimination in Health-Care Settings
Participants responded similarly to both discrimination items; the mean scores were 0.51 (doctors paid less attention) and 0.42 (patients felt discriminated). Thirty-eight percent (n=27) of participants reported some experience with discrimination in health-care settings. Most of these (n=21) reported that doctors paid less attention to them because of their race (range of reports from “rarely” to “always”) and (n=22) that they felt discriminated against by doctors because of their race or ethnicity (range of reports from “rarely” to “sometimes”). Items were correlated (R=0.58, P<0.001), and the subscale was reliable (Spearman-Brown Coefficient=0.73) within our sample.
Descriptive statistics revealed 2 distinguishable groups of patients—those who reported experiences of discrimination in health-care settings and those who reported no experience with discrimination in health-care settings. (The split was identical whether a mean split, median split, or all-or-nothing split was chosen). To evaluate the differences in pain sensitivity between participants reporting no discrimination and those reporting any discrimination, these groups were compared on all study variables of interest using independent t tests (Table 1). There were no group differences in age, sex, or education level, pain catastrophizing, pain anxiety, ethnic identification, nor in HPTh, HPTo, PPTh, CPM, or TTS. Participants who reported experience with discrimination in health-care settings reported greater clinical pain severity and interference (Table 1); these patients also demonstrated greater suprathreshold pain ratings (SHPR), MTS, and after-sensations, all of which are indicators of CS.18 Participants who experienced discrimination within health-care settings also reported more pain unpleasantness, greater stress, marginally more depressive symptomatology, and worse sleep quality.
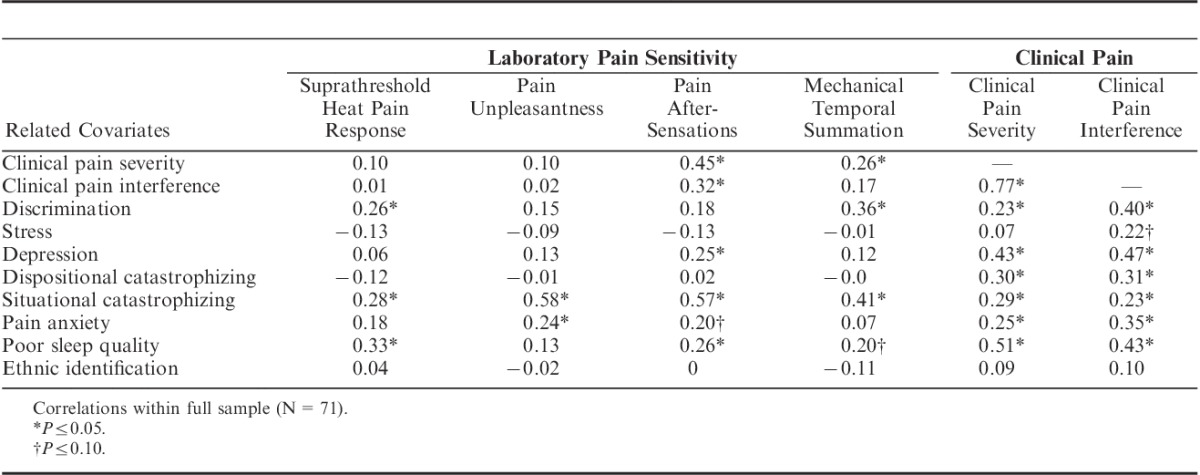
To identify potential covariates for multivariate analyses, correlations were examined between potential covariates and pain measures (both clinical pain and markers of CS) that showed a difference across discrimination groups. Across all participants, individual differences in experiences of discrimination in health-care settings were associated with greater SHPR, MTS, and clinical pain severity and interference but not with pain unpleasantness or after-sensations (Table 2). Pain after-sensations and MTS correlated with clinical pain severity. As expected, situational catastrophizing correlated with all psychophysical pain measures, and clinical pain severity was related to stress, depression, catastrophizing, pain anxiety, and poor sleep quality.
TABLE 2.
Correlations Between Laboratory Pain Sensitivity, Clinical Pain, and Related Covariates
Discrimination, Clinical Pain, and Laboratory Pain Sensitivity
Regression models were used to probe the relationships between discrimination and clinical pain, and discrimination and laboratory pain sensitivity, focusing on the 2 markers of central sensitization—SHPR and MTS—that were consistently associated with individual differences in experiences of discrimination in health-care settings.
Clinical Pain
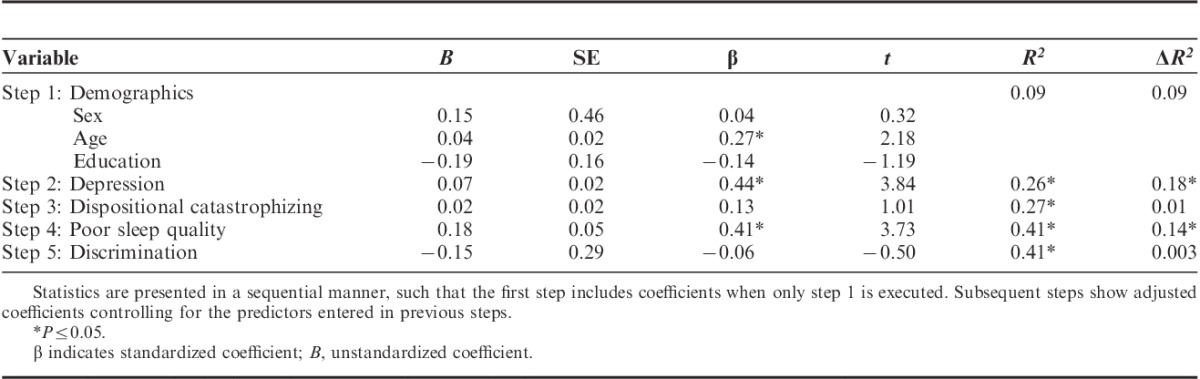
Hierarchical multiple regression revealed that discrimination did not predict clinical pain severity over and above other factors. Age, depression, and poor sleep quality were significant predictors of clinical pain severity (Table 3).
TABLE 3.
Results of Regression Model Predicting Clinical Pain Severity
Laboratory Pain
Suprathreshold Heat Pain Response: Discrimination did not remain a significant predictor of SHPR over and above situational catastrophizing and poor sleep quality (Table 4).
TABLE 4.
Results of Regression Models Predicting Laboratory Pain Sensitivity
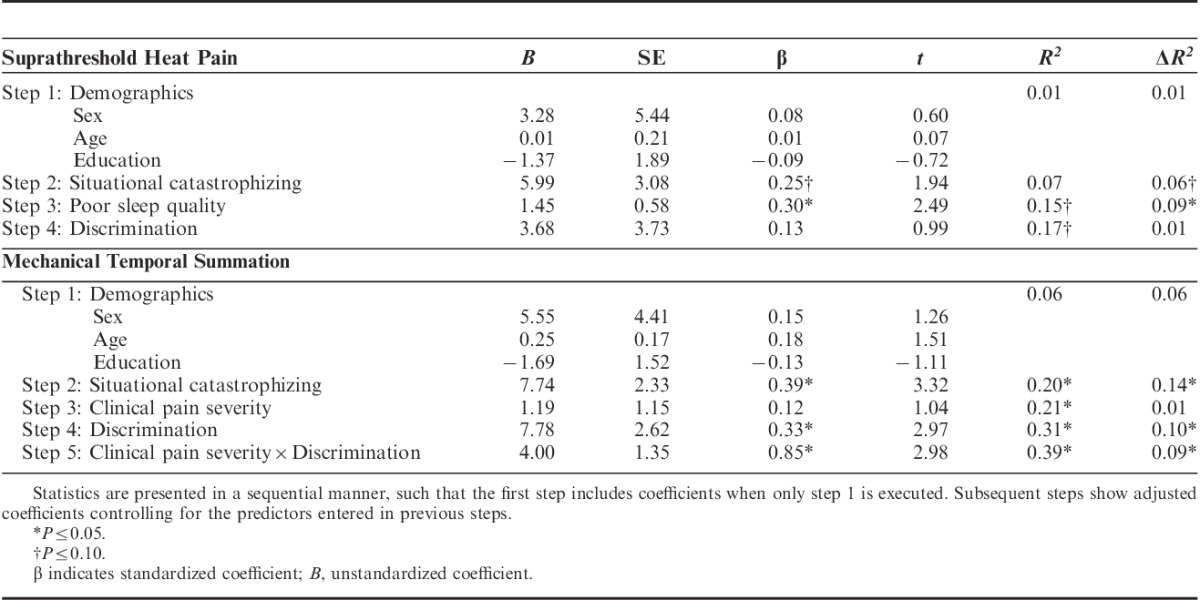
Mechanical Temporal Summation: Clinical pain severity was no longer a significant predictor of MTS after controlling for situational catastrophizing. Discrimination and the interaction between discrimination and clinical pain severity significantly predicted MTS, independently accounting for 10% and 9% of the variance in MTS, respectively, even after controlling for demographic variables and correlated covariates (Table 4).
Moderation analysis conducted to further probe the interaction between clinical pain severity and discrimination on MTS indicated that for participants experiencing greater racial discrimination, greater clinical pain severity was associated with significantly greater MTS. There was no significant relationship between clinical and laboratory pain among those with no experience with discrimination (Table 5). Further decomposition of the interaction using the Johnson-Neyman technique47 revealed that clinical pain severity was positively associated with MTS at discrimination frequencies >0.65 (Fig. 1). A discrimination score of 0.50 corresponds to any report of discrimination (a response above “never” on one of the items). Therefore, the moderating effect of discrimination on the relationship between clinical pain severity and MTS is significant for most participants reporting any discrimination at all. (The results of the moderation model remained significant, and the pattern unchanged, when discrimination was included as a dichotomous [discrimination vs. no discrimination], rather than a continuous, variable.)
TABLE 5.
Moderation Analysis: The Effect of Discrimination on the Relationship Between Clinical Pain and Mechanical Temporal Summation
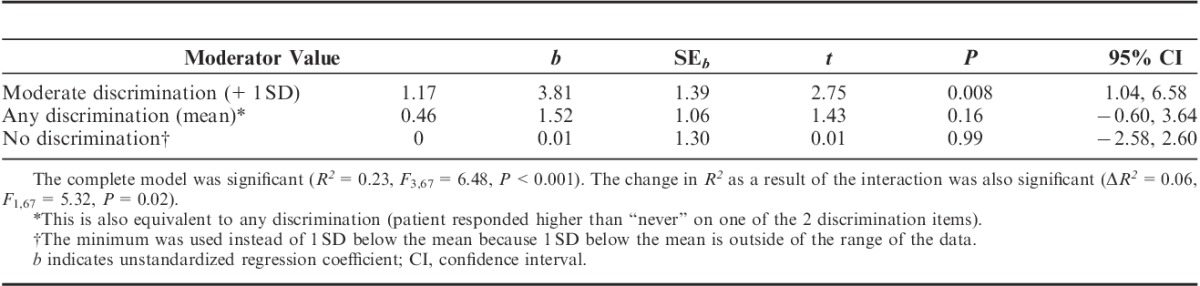
FIGURE 1.

Racial discrimination moderates the relationship between clinical pain severity and MTS. A, Regression lines for the association between clinical pain severity and MTS as moderated by experience with discrimination. For the purpose of demonstration, values are not adjusted for the covariate situational catastrophizing. B, Conditional effect of clinical pain severity on TS (θX→Y) as a function of perceived discrimination in health-care settings. CI indicates confidence interval; MTS, mechanical temporal summation.
DISCUSSION
SCD patients experience both severe and poorly managed pain, as well as the social harm of discriminatory interpersonal treatment. Compared with patients who report no discrimination within health-care settings, patients who experienced such discrimination show a profile of increased pain sensitivity that includes greater clinical pain severity, heightened sensitivity to suprathreshold thermal stimuli, increased after-sensations, greater MTS of pain, and greater pain unpleasantness. Although discrimination did not remain significantly associated with clinical pain severity when other pain-related covariates were included in the multivariate models, discrimination was independently associated with acute pain processing in the laboratory, particularly measures of pain facilitation. Health-care discrimination was also associated with a variety of symptoms of distress, including greater depressive symptomatology, poorer sleep, and higher stress ratings. Overall, these findings are consistent with the literature demonstrating positive associations between life-time discrimination and pain,48–50 stress,30 depression,51 and poor sleep.41,42 Importantly, discrimination among African American adults is associated with delays in seeking medical care and lower adherence to doctor recommendations.52 Our results extend these findings by demonstrating that discrimination within the health-care environments is independently correlated with increased pain and poorer psychological outcomes among SCD patients.
Health-care discrimination showed a fairly large association with MTS, accounting for an additional 10% of variance even after controlling for numerous factors known to be associated with pain. These results indicate that measures of discrimination should be included in future studies of pain sensitivity in SCD and more broadly suggest that interpersonal experiences may influence physiological processes underlying pain processing and central sensitization. We also find a significant interaction between discrimination and clinical pain severity that is independently associated with MTS. Participants who report any discrimination in health care show increased clinical pain severity–related mechanical sensitization, a relationship absent in participants who do not report discrimination. The mechanisms underlying this interaction are unclear; however, 1 plausible explanation is that discrimination alters the physiological environment such that heightened clinical pain facilitates central sensitization. This may occur through neuroendocrine responses to discrimination53,54 or other mechanisms related to social exclusion55 (discussed in more detail below). Furthermore, greater central sensitization may have a bidirectional effect with clinical pain, maintaining and even worsening pain over time.
Discrimination is one type of social stressor, and future studies should directly compare discrimination with other stressors, including other social stressors. Current evidence suggests that discrimination may have unique effects on pain processing, over and above that of stress broadly defined. In the experimental social laboratory, direct comparisons of performance stress and discrimination suggest that discrimination produces significantly more risky health behaviors than performance stress or control conditions.56 Prior research has not found an effect of cognitive stress inductions on temporal summation of pain among healthy or chronic pain populations.57,58 Social exclusion in the lab increases the unpleasantness of acute heat pain,55 and social support decreases pain intensity ratings in response to cold pressor59 and heat60 pain among healthy volunteers. Taken together, our results and the experimental literature among healthy volunteers suggest that negative social experience contributes to enhanced pain sensitization. The ways in which experiences with discrimination are similar, and different from that of other stressors, and the influence of discrimination on the neuroendocrine system, have yet to be fully explored (see initial examinations of the relationship between discrimination and functioning of the HPA system53,54). Future research should examine whether intervening at the level of interpersonal interactions may potentially lessen some of the deleterious effects of discrimination.
This is the first study to find that the social experience of discrimination is associated with pain facilitation processes. Prior findings have demonstrated that cognitive-affective psychological processes such as pain catastrophizing61,62 and fear of movement63 are associated with temporal summation and other indices of CS within other chronic pain conditions. The current findings extend this evidence and suggest that social factors may also contribute to pain facilitation and perhaps CS above the influence of clinical pain on CS. Importantly, this relationship is also independent of the influence of previously identified cognitive-affective processes such as situational catastrophizing. Thus, it will be important in future research to investigate how and when these social experiences translate into increased CS to pain. Our pattern of findings do not suggest an overall heightened sensitivity resulting from the experience of health-care discrimination, as increased discrimination did not correlate with all pain outcomes (eg, HPTh or PPTh). Furthermore, this demonstration of the relationship between discrimination and pain sensitization has important broader implications for the study of pain disparities. Numerous studies have demonstrated heightened pain sensitization among African Americans in the laboratory relative to white Americans, but the mechanisms underlying this disparity are not understood.64,65 Our results suggest that social mechanisms, such as the influence of discrimination on pain sensitization, should be investigated in future studies of pain disparities.
While we propose that the social experience of discrimination modulates pain sensitivity and facilitates CS, we have considered a number of alternative hypotheses that warrant further exploration in future research. One plausible alternative explanation is that discrimination decreases health-care utilization, treatment seeking, and/or adherence to medical advice, which all may increase pain. Among people with SCD, who are already resistant to engaging the medical system and prefer to manage their pain at home when possible,1 experiences of discrimination may provide further discouragement from seeking medical care. Being distrusted by hospital staff and having difficulty convincing providers of one’s pain have also been associated with self-discharge from the hospital, an indicator of dissatisfaction with pain management.13 However, the relationship between discrimination and health-care utilization depends on SCD patient optimism,15 suggesting that this relationship may not be simply a function of discrimination-evoked reduction in health-care utilization.
Another plausible explanation of our findings is that the participants who experience heightened sensitization may also be engaging the health-care system more, and therefore may have more opportunity to experience discrimination within these settings. More health-care visits might increase exposure to specific settings, providers, or both who may be biased or have negative attitudes about SCD patients. Controlled laboratory studies have demonstrated that perceiver bias and patient factors such as race and medication status can alter pain perception, empathy, and treatment decisions.66–68 Negative attitudes among providers toward SCD patients are a consistent and significant barrier to SCD treatment and pain management across studies.14 Future studies should seek to examine the effect of discrimination, particularly within health-care settings, on treatment seeking and health-care utilization over time.
A final consideration is that some participants may have a response bias to report greater sensitivity to a wide variety of challenges and insults, including laboratory pain and discrimination. However, the lack of association between discrimination and some measures of pain sensitivity (eg, HPTh, HPTo, and PPTh) does not suggest a consistent response bias. One might expect that if these results are because of response bias, controlling for other similar constructs, such as catastrophizing, might nullify the relationship. Nonetheless, longitudinal studies that examine the impact of insults over time will likely provide additional insight into the progression and cause of this finding.
Limitations of the current study include our use of a single subscale to assess discrimination. Discrimination is a complex construct, and patients with SCD are likely to experience other forms of discrimination, including disease-based discrimination.16 However, by examining discrimination within health-care settings, a specifically relevant context to patients with a chronic and complicated illness, this study importantly advances current knowledge about the relationship between discrimination and health outcomes. The inclusion of multidimensional discrimination measures will enable future studies to directly compare the predictive value of the various dimensions of discrimination. In addition, discrimination was not associated with static markers of sensitization (eg, pain thresholds) or pain inhibition, and it did not remain significantly associated with clinical pain severity, over and above highly correlated covariates, which suggests that discrimination may be most influential in affecting central pain sensitizing mechanisms. Future studies are needed to further parse these findings. Finally, our sample size may have limited our ability to detect smaller effects of discrimination on pain.
Given the prevalence and severity of both pain and discrimination experienced by people living with SCD, and the importance of avoiding additional pain and barriers to treatment embedded in patient care, we suggest that future studies test whether interventions that reduce discrimination within health-care settings also reduce clinical pain, and whether this reduction occurs independently or through mediating effects of other variables, such as depression. Prior studies have demonstrated the effectiveness of brief interventions on provider attitudes toward SCD patients,69 but whether these interventions also reduce perceptions of discrimination on the part of patients receiving care from these providers after such interventions needs to be established. Other research shows that brief training in cognitive coping skills reduces laboratory-induced pain, increases coping attempts, and decreases negative thinking in SCD patients.70 Future studies should examine the potential effects of such interventions on the relationship between perceived discrimination and pain sensitivity and severity.
Footnotes
Supported by National Heart, Lung, and Blood Institute R01HL98110 (J.A.H.), National Institutes of Health Ruth L. Kirschstein National Research Service Award T32 NS070201 (V.A.M.), and Career Development Award from the National Heart, Lung, and Blood Institute 5K01HL108832-03 (C.H.), Bethesda, MD.
The authors declare no conflict of interest.
REFERENCES
- 1.Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. [DOI] [PubMed] [Google Scholar]
- 2.Bediako SM.Neville HA, Tynes BM, Utsey SO. Psychosocial aspects of sickle cell disease: A primer for African American psychologists. Handbook of African American Psychology. Thousand Oaks, CA: SAGE; 2009:417–427. [Google Scholar]
- 3.Brousseau DC, Owens PL, Mosso AL, et al. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303:1288–1294. [DOI] [PubMed] [Google Scholar]
- 4.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120:3647–3656. [DOI] [PubMed] [Google Scholar]
- 5.Maxwell K, Streetly A, Bevan D. Experiences of hospital care and treatment seeking for pain from sickle cell disease: qualitative study. BMJ. 1999;318:1585–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman EJ, Diamond NJ. Sickle cell disease and the “difficult patient” conundrum. Am J Bioeth. 2013;13:3–10. [DOI] [PubMed] [Google Scholar]
- 7.Bediako SM, Lavender AR, Yasin Z. Racial centrality and health care use among African American adults with sickle cell disease. J Black Psychol. 2007;33:422–438. [Google Scholar]
- 8.Nelson SC, Hackman HW. Race matters: perceptions of race and racism in a sickle cell center. Pediatr Blood Cancer. 2013;60:451–454. [DOI] [PubMed] [Google Scholar]
- 9.Elander J, Marczewska M, Amos R, et al. Factors affecting hospital staff judgments about sickle cell disease pain. J Behav Med. 2006;29:203–214. [DOI] [PubMed] [Google Scholar]
- 10.Elander J, Lusher J, Bevan D, et al. Pain management and symptoms of substance dependence among patients with sickle cell disease. Soc Sci Med. 2003;57:1683–1696. [DOI] [PubMed] [Google Scholar]
- 11.Hasan SP, Hashmi S, Alhassen M, et al. Depression in sickle cell disease. J Natl Med Assoc. 2003;95:533–537. [PMC free article] [PubMed] [Google Scholar]
- 12.Telfair J, Myers J, Drezner S. Does race influence the provision of care to persons with sickle cell disease?: perceptions of multidisciplinary providers. J Health Care Poor Underserved. 1998;9:184–195. [DOI] [PubMed] [Google Scholar]
- 13.Haywood C, Lanzkron S, Ratanawongsa N, et al. Hospital self-discharge among adults with sickle-cell disease (SCD): associations with trust and interpersonal experiences with care. J Hosp Med. 2010;5:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haywood C, Jr, Beach MC, Lanzkron S, et al. A systematic review of barriers and interventions to improve appropriate use of therapies for sickle cell disease. J Natl Med Assoc. 2009;101:1022–1033. [DOI] [PubMed] [Google Scholar]
- 15.Stanton MV, Jonassaint CR, Bartholomew FB, et al. The association of optimism and perceived discrimination with health care utilization in adults with sickle cell disease. J Natl Med Assoc. 2010;102:1056–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haywood C, Jr, Diener-West M, Strouse J, et al. Perceived discrimination in health care is associated with a greater burden of pain in sickle cell disease. J Pain Symptom Manage. 2014;48:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards RR, Sarlani E, Wesselmann U, et al. Quantitative assessment of experimental pain perception: multiple domains of clinical relevance. Pain. 2005;114:315–319. [DOI] [PubMed] [Google Scholar]
- 18.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor LEV, Stotts NA, Humphreys J, et al. A review of the literature on the multiple dimensions of chronic pain in adults with sickle cell disease. J Pain Symptom Manage. 2010;40:416–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballas SK. Current issues in sickle cell pain and its management. Hematology Am Soc Hematol Educ Program. 2007;1:97–105. [DOI] [PubMed] [Google Scholar]
- 22.Niscola P, Sorrentino F, Scaramucci L, et al. Pain syndromes in sickle cell disease: an update. Pain Med. 2009;10:470–480. [DOI] [PubMed] [Google Scholar]
- 23.Stewart AL, Nápoles-Springer AM, Gregorich SE, et al. Interpersonal processes of care survey: patient-reported measures for diverse groups. Health Serv Res. 2007;42:1235–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwan ML, Tam EK, Ergas IJ, et al. Patient–physician interaction and quality of life in recently diagnosed breast cancer patients. Breast Cancer Res Treat. 2013;139:581–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piette JD, Bibbins-Domingo K, Schillinger D. Health care discrimination, processes of care, and diabetes patients’ health status. Patient Educ Couns. 2006;60:41–48. [DOI] [PubMed] [Google Scholar]
- 26.Tyler EJ, Jensen MP, Engel JM, et al. The reliability and validity of pain interference measures in persons with cerebral palsy. Arch Phys Med Rehabil. 2002;83:236–239. [DOI] [PubMed] [Google Scholar]
- 27.Jensen MP, Ehde DM, Hoffman AJ, et al. Cognitions, coping and social environment predict adjustment to phantom limb pain. Pain. 2002;95:133–142. [DOI] [PubMed] [Google Scholar]
- 28.Cleeland C, Ryan K. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 29.Reinhardt T, Kleindienst N, Treede RD, et al. Individual modulation of pain sensitivity under stress. Pain Med. 2013;14:676–685. [DOI] [PubMed] [Google Scholar]
- 30.Pascoe EA, Smart Richman L. Perceived discrimination and health: a meta-analytic review. Psychol Bull. 2009;135:531–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 32.Kroenke K, Wu J, Bair MJ, et al. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain. 2011;12:964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickens C, McGowan L, Dale S. Impact of depression on experimental pain perception: a systematic review of the literature with meta-analysis. Psychosom Med. 2003;65:369–375. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan MJ, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 35.Sullivan MJ, Thorn B, Haythornthwaite JA, et al. Theoretical perspectives on the relation between catastrophizing and pain. Clin J Pain. 2001;17:52–64. [DOI] [PubMed] [Google Scholar]
- 36.Campbell CM, Kronfli T, Buenaver LF, et al. Situational versus dispositional measurement of catastrophizing: associations with pain responses in multiple samples. J Pain. 2010;11:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campbell CM, Quartana PJ, Buenaver LF, et al. Changes in situation-specific pain catastrophizing precede changes in pain report during capsaicin pain: a cross-lagged panel analysis among healthy, pain-free participants. J Pain. 2010;11:876–884. [DOI] [PubMed] [Google Scholar]
- 38.McCracken LM, Dhingra L. A short version of the Pain Anxiety Symptoms Scale (PASS-20): preliminary development and validity. Pain Res Manag. 2002;7:45–50. [DOI] [PubMed] [Google Scholar]
- 39.Buysse DJ, Reynolds CF, III, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 40.Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. 2013;14:1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas KS, Bardwell WA, Ancoli-Israel S, et al. The toll of ethnic discrimination on sleep architecture and fatigue. Health Psychol. 2006;25:635–642. [DOI] [PubMed] [Google Scholar]
- 42.Steffen PR, Bowden M. Sleep disturbance mediates the relationship between perceived racism and depressive symptoms. Ethnic Groups. 2006;5:8–11. [PubMed] [Google Scholar]
- 43.Phinney JS. The multigroup ethnic identity measure a new scale for use with diverse groups. J Adolesc Res. 1992;7:156–176. [Google Scholar]
- 44.Rahim-Williams FB, Riley JL, Herrera D, et al. Ethnic identity predicts experimental pain sensitivity in African Americans and Hispanics. Pain. 2007;129:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jensen K, Andersen HØ, Olesen J, et al. Pressure-pain threshold in human temporal region. Evaluation of a new pressure algometer. Pain. 1986;25:313–323. [DOI] [PubMed] [Google Scholar]
- 46.Valencia C, Fillingim RB, George SZ. Suprathreshold heat pain response is associated with clinical pain intensity for patients with shoulder pain. J Pain. 2011;12:133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayes AF. An Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- 48.Edwards RR. The association of perceived discrimination with low back pain. J Behav Med. 2008;31:379–389. [DOI] [PubMed] [Google Scholar]
- 49.Burgess DJ, Grill J, Noorbaloochi S, et al. The effect of perceived racial discrimination on bodily pain among older African American men. Pain Med. 2009;10:1341–1352. [DOI] [PubMed] [Google Scholar]
- 50.Goodin BR, Pham QT, Glover TL, et al. Perceived racial discrimination, but not mistrust of medical researchers, predicts the heat pain tolerance of African Americans with symptomatic knee osteoarthritis. Health Psychol. 2013;32:1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mickelson KD, Williams DR. The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. J Health Soc Behav. 1999;40:208–230. [PubMed] [Google Scholar]
- 52.Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richman LS, Jonassaint C. The effects of race-related stress on cortisol reactivity in the laboratory: implications of the Duke lacrosse scandal. Ann Behav Med. 2008;35:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fuller-Rowell TE, Doan SN, Eccles JS. Differential effects of perceived discrimination on the diurnal cortisol rhythm of African Americans and Whites. Psychoneuroendocrinology. 2012;37:107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eisenberger NI, Jarcho JM, Lieberman MD, et al. An experimental study of shared sensitivity to physical pain and social rejection. Pain. 2006;126:132–138. [DOI] [PubMed] [Google Scholar]
- 56.Stock ML, Gibbons FX, Walsh LA, et al. Racial identification, racial discrimination, and substance use vulnerability among African American young adults. Person Soc Psychol Bull. 2011;37:1349–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cathcart S, Winefield AH, Lushington K, et al. Noxious inhibition of temporal summation is impaired in chronic tension-type headache. Headache. 2010;50:403–412. [DOI] [PubMed] [Google Scholar]
- 58.Reinhardt T, Kleindienst N, Treede R, et al. Individual modulation of pain sensitivity under stress. Pain Med. 2013;14:676–685. [DOI] [PubMed] [Google Scholar]
- 59.Brown JL, Sheffield D, Leary MR, et al. Social support and experimental pain. Psychosom Med. 2003;65:276–283. [DOI] [PubMed] [Google Scholar]
- 60.Master SL, Eisenberger NI, Taylor SE, et al. A picture’s worth: partner photographs reduce experimentally induced pain. Psychol Sci. 2009;20:1316–1318. [DOI] [PubMed] [Google Scholar]
- 61.Edwards RR, Smith MT, Stonerock G, et al. Pain-related catastrophizing in healthy women is associated with greater temporal summation of and reduced habituation to thermal pain. Clin J Pain. 2006;22:730–737. [DOI] [PubMed] [Google Scholar]
- 62.Rhudy JL, Martin SL, Terry EL, et al. Pain catastrophizing is related to temporal summation of pain but not temporal summation of the nociceptive flexion reflex. Pain. 2011;152:794–801. [DOI] [PubMed] [Google Scholar]
- 63.Sullivan MJ, Thibault P, Andrikonyte J, et al. Psychological influences on repetition-induced summation of activity-related pain in patients with chronic low back pain. Pain. 2009;141:70–78. [DOI] [PubMed] [Google Scholar]
- 64.Rahim-Williams B, Riley JL, Williams AK, et al. A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter? Pain Med. 2012;13:522–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Campbell CM, Edwards RR. Ethnic differences in pain and pain management. Pain Manag. 2012;2:219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirsh AT, Alqudah AF, Stutts LA, et al. Virtual human technology: capturing sex, race, and age influences in individual pain decision policies. Pain. 2008;140:231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mathur VA, Richeson JA, Paice JA, et al. Racial bias in pain perception and response: experimental examination of automatic and deliberate processes. J Pain. 2014;15:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drwecki BB, Moore CF, Ward SE, et al. Reducing racial disparities in pain treatment: the role of empathy and perspective-taking. Pain. 2011;152:1001–1006. [DOI] [PubMed] [Google Scholar]
- 69.Haywood C, Jr, Sophie Lanzkron MDM, Hughes MT, et al. A video-intervention to improve clinician attitudes toward patients with sickle cell disease: the results of a randomized experiment. J Gen Intern Med. 2011;26:518–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gil KM, Wilson JJ, Edens JL, et al. Effects of cognitive coping skills training on coping strategies and experimental pain sensitivity in African American adults with sickle cell disease. Health Psychol. 1996;15:3–10. [DOI] [PubMed] [Google Scholar]


