Abstract
Purpose: To elucidate the performance of transcatheter aortic valve implantation (TAVI) in bicuspid aortic valve (BAV) patients through a systematic review and meta-analysis.
Methods: A systematic literature review was performed by searching eligible articles in PubMed, Medline, EMBASE, Google Scholar and CNKI. Meta-analysis of included case-control/cohort studies was further conducted. Relative risks (RRs) and the corresponding 95% confidence intervals (CIs) were used to compare clinical outcomes of BAV patients and non-BAV patients.
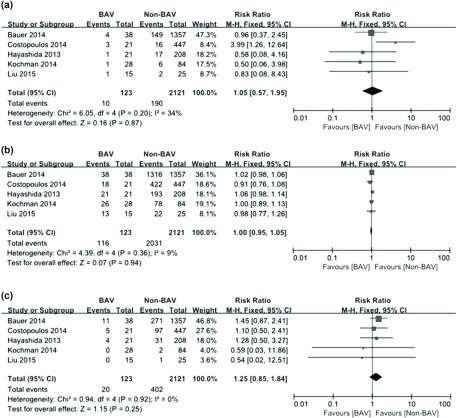
Results: A total of 17 articles including eight case reports, four case series and five case-control/cohort studies with 166 BAV patients were analyzed. Device success rate achieved for TAVI in this cohort of BAV patients was 95.2%. The 30-day mortality rate was 8.4%, and the medium-term (range from 6 months to 2 years) mortality rate reported was 17.9%. Overall, the performance of TAVI in BAV patients was comparable to that in non-BAV patients, as reported by the included case-control/cohort studies (30-day mortality rate: RR = 1.05, 95%CI 0.57–1.95, p = 0.87; Device success rate: RR = 1.00, 95%CI 0.95–1.05, p = 0.94; Incidence of moderate to severe paravalvular regurgitation: RR = 1.25, 95%CI 0.85–1.84, p = 0.25).
Conclusion: The present study suggested that TAVI may be a feasible and safe treatment modality for BAV patients.
Keywords: bicuspid aortic valve, transcatheter aortic valve implantation, aortic stenosis, systematic review, meta-analysis
Introduction
Bicuspid aortic valve (BAV), a condition in which two leaflets of the aortic valve fuse together during development, is one of the most frequently seen congenital heart abnormalities, which presents in 0.5%–2% of the population with men being 2 times more likely to be affected than women.1–3) This disease is not just anatomical variation of aortic valve and may be associated with other heart and aorta disorders including bacterial endocarditis, ventricular septal defect, coarctation of the aorta, ascending aortic aneurysms or aortic dissection.4–5) Although life-threatening aortic complications are the most feared, the so far largest population-based outcome study with a mean follow-up term of more than 16 years showed that severe aortic valve dysfunction driven by a much more rapidly progressed valvular stenosis is the most common morbidity for BAV patients, with more than 50% of patients requiring aortic valve replacement within 25 years of their initial diagnosis.6) Furthermore, on average, the aortic valve replacement occurs 18 years earlier in BAV patients than that in patient with a stenotic tricuspid aortic valve, usually within the productive years of life.7)
Transcatheter aortic valve implantation (TAVI), a minimally invasive procedure that entails insertion of a bioprosthetic valve into the stenotic aortic valve through a vascular access, is a newly developed catheter-based technology for delivering aortic valve replacement.8) And owing to the tremendous improvement in devices and growth in operator experience during the past few years, it is currently considered as an appealing alternative to surgical aortic valve replacement in severe aortic stenosis (AS) patients who are considered to be at high risk [expected mortality >20% with the European System for Cardiac Operative Risk Evaluation score (EuroSCORE) or >8% with the Society of Thoracic Surgeons score (STS score), or if surgery was expected to be risky due to serious comorbidities or advanced age] for conventional surgery, as long as life expectancy is more than one year.9) Although TAVI has already been accepted widely in treating AS, the existence of a BAV has long been regarded as a relative contraindication for TAVI since the unfavorable anatomy of BAV may impede the positioning and expansion of the prosthetic valve, and theoretically increase the incidence of procedural complications as well as decrease the efficacy and durability of the prosthetic valve.10,11) However, despite these concerns, we cannot ignore the fact that a portion of BAV patients will certainly benefit from TAVI.
So far, the clinical experience and evidence concerning TAVI in BAV patients were still limited. Nevertheless, BAV patients were increasingly referred for TAVI. Hence, a thorough evaluation of the feasibility and clinical outcomes of TAVI in BAV patients is warranted. The purpose of the present study was to analyze the efficacy and safety of TAVI in BAV patients by performing a systematic review and meta-analysis of all published literature to date.
Materials and Methods
Literature search strategy
Electronic databases of PubMed, Medline, EMBASE, Google Scholar and China National Knowledge Infrastructure (CNKI) were searched using the following keywords: “transcatheter aortic valve implantation”, “transcatheter aortic valve replacement”, “TAVI”, “TAVR” and “bicuspid aortic valve”. The initial search was conducted in April 2015 and the latest update was performed in December 2015. Besides, the reference lists of all relevant articles were reviewed manually for further identification of potentially eligible articles.
Inclusion criteria
The inclusion criteria for the present study were set prior to the literature search. Eligible studies met all of the following conditions: (1) article already published; (2) evaluation of the efficacy and safety of TAVI in BAV patient(s); (3) follow-up for at least 30 days (4) full text in English or Chinese available. Since relevant studies were extremely lacking, we included all available case reports, case series, and case-control/cohort studies to more comprehensively assess the performance of TAVI in BAV patients. If the report was duplicated or identical patients were enrolled in two studies, only the most recent and complete article was included. Abstracts, reviews, letters, editorials, conference presentations and expert opinions were intentionally excluded.
Data extraction and quality assessment
From each included study, the following data was extracted: name of first author, year of publication, the number of patients with bicuspid aortic valve, baseline characteristics, interventional characteristics as well as post-TAVI outcomes of BAV patients. In addition, the Newcastle‐ottawa quality assessment scale (NOS), a classical rating tool which evaluates the quality of non-randomized studies from three perspectives: selection, comparability and exposure, was used to assess the validity of all case-control/cohort studies included. This rating system has a score range of 0 to 9. Studies with a score of more than 7 were assumed to be of high quality. Two reviewers (XCX and XHS) performed the data extraction and quality assessment independently. When necessary, the reviewers wrote to the authors for extra information or raw data. Any discrepancies between two reviewers were resolved by discussion until reaching a consensus. The final results were reviewed by a senior reviewer (LR).
Statistical analysis
All data analyses in this study were performed with Review Manager Version 5.3.3 (The Cochrane Collaboration, Software Update, Oxford, United Kingdom). Quantitative syntheses for included case-control/cohort studies regarding 30-day mortality rate, device success rate as well as the incidence of moderate to severe paravalvular regurgitation were performed. Relative risks (RRs) and the corresponding 95% confidence intervals (CIs) were used to compare post-TAVI clinical outcomes of BAV patients and non-BAV patients. Besides, Q test and I2 statistic were employed to evaluate heterogeneity between studies. If the probability value (p value) was less than 0.1 or I2 was greater than 50%, between-study heterogeneity was considered to be significant, and the random-effect model (REM) would be adopted for analyses. Otherwise, if studies were considered to be homogenous, the fixed-effect model (FEM) would be applied for analyses. Sensitivity analyses were performed to test the stability of quantitative synthetic results. Publication bias was further assessed with funnel plots. And a p value of 0.05 or less was regarded as statistically significant in comparisons between BAV patients and non-BAV patients.
Results
Included studies
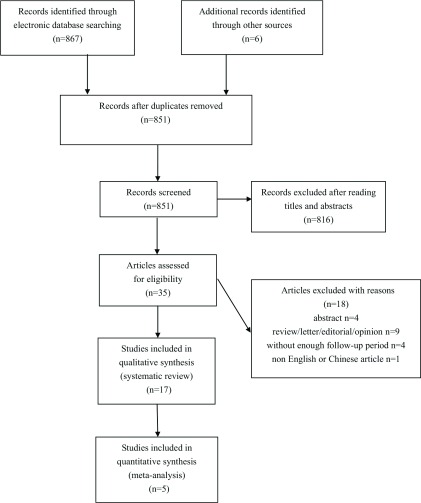
The literature search generated 873 results. After exclusion of irrelevant or duplicate articles by reading titles and abstracts, 35 articles were selected for further evaluation. Of these, a total of 17 articles including 166 BAV patients met our inclusion criteria12–28) (see Fig. 1). The basic characteristics of included studies are summarized in Tables 1a and 1b (name of first author, year of publication, NOS score and baseline characteristics of BAV patients).
Fig. 1.
Flowchart of study selection for the present systematic review and meta-analysis.
Table 1a.
Basic characteristics of included studies
| Liu | Bauer | Kochman | Costopoulos | Hayashida | Kosek | Himbert | Wijesinghe | |
|---|---|---|---|---|---|---|---|---|
| Year of publication | 2015 | 2014 | 2014 | 2014 | 2013 | 2015 | 2012 | 2010 |
| Number of patients | 15 | 38 | 28 | 21 | 21 | 7 | 15 | 11 |
| NOS score | 8 | 7 | 8 | 8 | 7 | – | – | – |
| Background | ||||||||
| Age (years) | 75.4 ± 5.7 | 80.7 ± 6.6 | 77.6 ± 5.5 | 76.7 ± 7.1 | 82.0 ± 7.0 | 77.7 ± 5.3 | 80 ± 10 | 73.2 ± 12.5 |
| Male, n | 9 | 17 | 13 | 12 | 12 | 3 | 12 | 6 |
| BMI | 23.6 ± 4.8 | 26.0 ± 5.0 | NR | 26.6 ± 4.4 | 24.7 ± 4.1 | NR | NR | NR |
| DM, n (%) | 0 | 14 (36.8) | 11 (39.2) | 6 (28.6) | 1 (4.8) | 5 (71.4) | NR | NR |
| Dyslipidaemia, n (%) | NR | NR | NR | NR | 9 (42.9) | NR | NR | NR |
| Hypertension, n (%) | 5 (33.3) | NR | 17 (60.7) | 14 (66.7) | 12 (57.1) | NR | NR | NR |
| Smoker, n (%) | NR | NR | NR | NR | 1 (4.8) | NR | NR | NR |
| NYHA (3–4), n (%) | 13 (86.7) | 32 (84.2) | 20 (71.4) | 15 (71.4) | 19 (90.5) | 4 (57.1) | 15 (100) | NR |
| CAD, n (%) | 3 (20.0) | 26 (68.4) | 14 (50.0) | NR | 10 (47.6) | NR | NR | NR |
| MI, n (%) | 0 | NR | 11 (39.3) | 4 (19.0) | 1 (4.8) | 3 (42.9) | NR | NR |
| PCI, n (%) | 3 (20.0) | 13 (34.2) | 6 (21.4) | 6 (28.6) | 4 (19.0) | 0 | NR | NR |
| CABG, n (%) | 0 | 5 (13.2) | 4 (14.3) | 3 (14.3) | 2 (9.5) | 2 (28.6) | NR | NR |
| Valve Sx, n (%) | 0 | 1 (2.6) | NR | NR | NR | NR | NR | NR |
| Valvuloplasty, n (%) | 0 | 7 (18.4) | NR | NR | NR | NR | NR | NR |
| CVA, n (%) | 0 | 5 (13.2) | 8 (28.6) | 4 (19.0) | 1 (4.8) | 1 (14.3) | NR | NR |
| PAD, n (%) | 2 (13.3) | 4 (10.5) | 6 (21.4) | 7 (33.3) | 5 (23.8) | NR | NR | NR |
| COPD, n (%) | 4 (26.7) | 8 (21.1) | 6 (21.4) | 7 (33.3) | 5 (23.8) | NR | NR | NR |
| eGFR <60, n (%) | 4 (26.7) | NR | 12 (42.9) | 11 (52.3) | 12 (57.1) | NR | 5 (33.3) | NR |
| STS Score | 5.6 ± 4.1 | NR | NR | 7.6 ± 4.2 | NR | NR | 8.0 ± 5.0 | 4.4 ± 2.6 |
| log EuroSCORE | 16.1 ± 11.1 | 18.0 ± 10.0 | 19.2 ± 9.0 | 23.9 ± 12.0 | 19.9 ± 11.9 | 19.8 ± 10.9 | 17.0 ± 11.0 | NR |
| Pre-TAVI parameters | ||||||||
| Mean AVA cm2 | 0.47 ± 0.13 | 0.68 ± 0.22 | 0.6 ± 0.1 | 0.7 ± 0.23 | 0.67 ± 0.11 | 0.57 ± 0.10 | 0.8 ± 0.3 | 0.65 ± 0.17 |
| MAVPG mmHg | 64.1 ± 19.5 | 47.1 ± 19.6 | 55.5 ± 17.6 | 54.4 ± 17.9 | 47.8 ± 18.6 | 74.6 ± 10.9 | 60.0 ± 19.0 | 41.0 ± 22.2 |
| LVEF % | 51.1 ± 12.6 | 50.0 ± 16.0 | 48.1 ± 13.1 | 50.1 ± 12.4 | 47.5 ± 14.5 | 59.0 ± 8.5 | 52.0 ± 13.0 | 46.7 ± 17.1 |
| EF <40, n (%) | 3 (20.0) | NR | 7 (25.0) | NR | 6 (28.6) | 0 | NR | NR |
| Low gradient AS, n (%) | NR | 4 (10.5) | NR | NR | NR | NR | NR | NR |
| AR >2, n (%) | 2 (13.3) | 11 (28.9) | NR | NR | NR | NR | NR | 0 |
| MR >2, n (%) | NR | 14 (36.8) | NR | NR | NR | NR | NR | 1 (9.1) |
| Annular diameter (mm) | 24.7 ± 1.8 | 21 ± 7 | 24.8 ± 2.4 | 25 ± 1.8 | 23.4 ± 2.7 | 22.6 ± 2.4 | 24.0 ± 2.0 | 24.0 ± 2.3 |
| AR (0–4) | 1.8 ± 0.9 | NR | 1.3 ± 1.1 | 1.05 ± 0.94 | 0.95 ± 0.74 | NR | NR | NR |
| MR (0–4) | 2.3 ± 0.7 | NR | 1.4 ± 1.0 | 1.29 ± 1.0 | 0.74 ± 0.87 | NR | NR | NR |
| Asc Aorta Size (mm) | 40.4 ± 4.4 | NR | 38.9 ± 6.2 | NR | 38.3 ± 3.4 | 36.6 ± 7.1 | 37.0 ± 4.0 | NR |
NOS: Newcastle-ottawa quality assessment scale; BMI: body mass index; NR: not reported; DM: diabetes mellitus; NYHA: New York Heart Association; CAD: coronary artery disease; MI: myocardial infarction; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; Valve Sx: valve surgery; CVA: cerebrovascular accident; PAD: peripheral arterial disease; COPD: chronic obstructive pulmonary disease; eGFR: glomerular filtration rate; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation; AVA: aortic valve area; MAVPG: mean aortic valve pressure gradient; LVEF: left ventricular ejection fraction; EF: ejection fraction; AS: aortic stenosis; AR: aortic regurgitation; MR: mitral regurgitation; Asc Aorta size: ascending aorta size
Table 1b.
Basic characteristics of included studies
| Segev | Colkesen | Kassaian | Urena | Maluenda | Raja | Jilaihawi | Chiam | Delgado | |
|---|---|---|---|---|---|---|---|---|---|
| Year of publication | 2013 | 2015 | 2015 | 2014 | 2013 | 2011 | 2010 | 2010 | 2009 |
| Number of patients | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| NOS score | – | – | – | – | – | – | – | – | – |
| Background | |||||||||
| Age (years) | 70.5 | 51 | 68 | 71 | 81 | 61 | 80 | 77 | 54 |
| Male, n | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| BMI | NR | NR | NR | NR | 21.2 | 42 | NR | NR | NR |
| DM, n (%) | NR | NR | NR | 1 | 1 | NR | NR | NR | NR |
| Dyslipidaemia, n (%) | NR | NR | NR | NR | 1 | NR | NR | NR | 1 |
| Hypertension, n (%) | NR | NR | NR | 1 | 1 | NR | NR | NR | 1 |
| Smoker, n (%) | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| NYHA (3–4), n (%) | NR | NR | NR | 1 | 1 | NR | 1 | NR | NR |
| CAD, n (%) | 1 (50) | 1 | 1 | 1 | NR | NR | NR | NR | NR |
| MI, n (%) | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| PCI, n (%) | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| CABG, n (%) | 1 (50) | 1 | 1 | 1 | NR | NR | NR | NR | NR |
| Valve Sx, n (%) | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Valvuloplasty, n (%) | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| CVA, n (%) | NR | NR | NR | NR | NR | NR | NR | NR | 1 |
| PAD, n (%) | NR | NR | NR | 1 | NR | NR | NR | NR | 1 |
| COPD, n (%) | NR | NR | NR | NR | NR | NR | 1 | NR | NR |
| eGFR <60, n (%) | NR | NR | NR | 1 | 0 | NR | NR | NR | NR |
| STS Score | NR | NR | NR | 6 | 9.4 | NR | NR | NR | NR |
| log EuroSCORE | 33.2 | NR | NR | 21 | NR | NR | 29.7 | NR | NR |
| Pre-TAVI parameters | |||||||||
| Mean AVA cm2 | NR | 0.9 | NR | 0.54 | 0.8 | 0.9 | 0.37 | 0.6 | 0.9 |
| MAVPG mmHg | NR | 41 | 43 | 41 | 51 | 55 | 68 | 57 | 65 |
| LVEF % | NR | 64 | NR | 60 | 65 | NR | 25 | 40 | NR |
| EF <40, n (%) | NR | 0 | NR | 0 | 0 | NR | 80 | 0 | NR |
| Low gradient AS, n (%) | NR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AR > 2, n (%) | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| MR > 2, n (%) | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Annular diameter (mm) | NR | 27 | 23 | NR | 27.1 | 24 | NR | 20 | 26 |
| AR (0–4) | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| MR (0–4) | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Asc Aorta Size (mm) | NR | 42 | 39 | NR | 42 | 42 | 1 | NR | NR |
NOS: Newcastle-ottawa quality assessment scale; BMI: body mass index; NR: not reported; DM: diabetes mellitus; NYHA: New York Heart Association; CAD: coronary artery disease; MI: myocardial infarction; PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft; Valve Sx: valve surgery; CVA: cerebrovascular accident; PAD: peripheral arterial disease; COPD: chronic obstructive pulmonary disease; eGFR: glomerular filtration rate; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation; AVA: aortic valve area; MAVPG: mean aortic valve pressure gradient; LVEF: left ventricular ejection fraction; EF: ejection fraction; AS: aortic stenosis; AR: aortic regurgitation; MR: mitral regurgitation; Asc Aorta size: ascending aorta size
Risk of bias in included studies
Of the 17 eligible studies, eight were case reports, four were case series, and five were case-control/cohort studies. For case reports and case series, there were no available evaluation systems, and although these studies met the inclusion criteria of the present study, we still could not rule out the possibility of potential selection bias and publication bias. For case-control/cohort studies, however, we assessed the validity of the results with the NOS rating system. And as shown in Tables 1a and 1b, the average NOS score of eligible studies was 7.6 (range from 7 to 8), suggesting that all included case-control/cohort studies were of relatively high quality. Among case-control/cohort studies, the improper selection of controls was the major source of bias.
Baseline characteristics
The baseline characteristics of BAV patients are summarized in Tables 1a and 1b. The mean age and body mass index (BMI) of BAV patients ranged from 51 to 82 years, and 21.2 to 42, respectively. STS score ranged from 4.4% to 9.4%, while EuroSCORE ranged from 16.1% to 33.2%. As expected, BAV patients had elevated mean aortic valve pressure gradients (41–74.6 mmHg) and reduced mean aortic valve areas (0.37–0.9 cm2). The left ventricular ejection fraction (LVEF) and annular diameter of BAV patients ranged from 25% to 64%, and 20 to 27.1 mm, respectively.
Interventional characteristics
Interventional characteristics of TAVI for BAV patients are summarized in Tables 2a and 2b. The self-expandable Medtronic CoreValve was used in 59% of BAV patients while the rest had the balloon-expandable Edwards SAPIEN/ SAPIEN XT valve implanted. The femoral access was the most frequently taken access route, with 76.5% of the procedures performed via the transfemoral approach.
Table 2a.
Interventional characteristics of TAVI for BAV patients
| Liu | Bauer | Kochman | Costopoulos | Hayashida | Kosek | Himbert | Wijesinghe | |
|---|---|---|---|---|---|---|---|---|
| Number of patients | 15 | 38 | 28 | 21 | 21 | 7 | 15 | 11 |
| Type of valve | ||||||||
| Edwards, n (%) | 10 (66.7) | 12 (31.6) | 5 (17.9) | 8 (38.1) | 11 (52.4) | 2 (28.6) | 0 | 11 (100) |
| Corevalve, n (%) | 5 (33.3) | 26 (68.4) | 23 (82.1) | 13 (61.9) | 10 (47.6) | 5 (71.4) | 15 (100) | 0 |
| Acess route | ||||||||
| Transfemoral, n (%) | 13 (86.7) | 31 (81.6) | 22 (78.6) | 15 (71.4) | 13 (61.9) | 5 (71.4) | 14 (93.3) | 7 (63.6) |
| Transapical, n (%) | 0 | 6 (15.8) | 3 (10.7) | 1 (4.8) | 3 (14.3) | 1 (14.3) | 0 | 4 (36.4) |
| Others, n (%) | 2 (13.3) | 1 (2.6) | 3 (10.7) | 5 (23.8) | 5 (23.8) | 1 (14.3) | 1 (6.7) | 0 |
| Valve size | ||||||||
| Edwards | ||||||||
| 23 mm, n (%) | 0 | NR | 3 (10.7) | 2 (9.5) | 5 (23.8) | 2 (28.6) | 0 | 1 (9.1) |
| 26 mm, n (%) | 1 (6.7) | NR | 1 (3.6) | 5 (23.8) | 2 (9.6) | 0 | 0 | 10 (90.1) |
| 29 mm, n (%) | 9 (60.0) | NR | 1 (3.6) | 1 (4.8) | 4 (19.0) | 0 | 0 | 0 |
| Corevalve | ||||||||
| 26 mm, n (%) | 0 | NR | 2 (7.1) | 2 (9.5) | 0 | 2 (28.6) | 2 (13.3) | 0 |
| 29 mm, n (%) | 2 (13.3) | NR | 18 (64.3) | 10 (47.6) | 5 (23.8) | 3 (42.8) | 13 (86.7) | 0 |
| 31 mm, n (%) | 3 (20.0) | NR | 3 (10.7) | 1 (4.8) | 5 (23.8) | 0 | 0 | 0 |
TAVI: transcatheter aortic valve implantation; BAV: bicuspid aortic valve; NR: not reported
Table 2b.
Interventional characteristics of TAVI for BAV patients
| Segev | Colkesen | Kassaian | Urena | Maluenda | Raja | Jilaihawi | Chiam | Delgado | |
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Type of valve | |||||||||
| Edwards, n (%) | 2 (100) | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| Corevalve, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Acess route | |||||||||
| Transfemoral, n (%) | 1 (50) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 |
| Transapical, n (%) | 1 (50) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
| Others, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Valve size | |||||||||
| Edwards | |||||||||
| 23 mm, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 26 mm, n (%) | 2 (100) | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 |
| 29 mm, n (%) | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Corevalve | |||||||||
| 26 mm, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 29 mm, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 31 mm, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
TAVI: transcatheter aortic valve implantation; BAV: bicuspid aortic valve; NR: not reported
Post-TAVI outcomes
Post-TAVI outcomes of BAV patients are summarized in Tables 3a and 3b. For the evaluation of the efficacy, device success rate achieved for TAVI in BAV patients was 95.2%. BAV patients demonstrated remarkable hemodynamic and symptomatic improvements after receiving TAVI. The mean aortic valve pressure gradients improved from 41–74.6 mmHg to 6–16 mmHg. In addition, 79.7% of the patients reported achieving NYHA class I–II during follow-up. For the evaluation of the safety, the 30-day mortality rate post-TAVI was 8.4% (14 patients). Of these, two patients died following emergency surgery for aortic dissection and severe aortic regurgitation, two patients died due to multisystem organ failure following an apical approach, one due to cardiac tamponade resulting from annular rupture, one due to infectious endocarditis following a pacemaker implantation, and one because of non-cardiac etiologies. The causes of the other seven cases were not reported. The medium-term (range from 6 months to 2 years) mortality rate reported was 17.9%. Of these, 13 additional deaths occurred beyond 30 days post-TAVI, 2 patients died due to device failure, one due to aortic dissection and five because of non-cardiac etiologies. The details of the other five cases were unavailable. As for complications, 19.3% of the patients required a pacemaker implantation, 18.5% had major vascular or access site complications, 9.5% suffered from major bleeding, and 4.9% got AKI. Later conversion to open heart surgery occurred in three patients, and there were only single one reported case of stroke. However, it should be emphasized that 66.7% of the patients developed paravalvular leak post-TAVI, although the majority of which (76.9%) were reported as only trivial to mild.
Table 3a.
Post-TAVI outcomes of BAV patients
| Sample size | Liu | Bauer | Kochman | Costopoulos | Hayashida | Kosek | Himbert | Wijesinghe |
|---|---|---|---|---|---|---|---|---|
| 15 | 38 | 28 | 21 | 21 | 7 | 15 | 11 | |
| Device success, n (%) | 13 (86.7) | 38 (100) | 26 (92.9) | 18 (85.7) | 21 (100) | 7 (100) | 14 (93.3) | 11 (100) |
| Post TAVI MAVPG mmHg | 9.6 ± 3.1 | 10.5 ± 7.1 | 11.5 ± 6.4 | 10.3 ± 5.7 | 10.0 ± 3.4 | 13.1 ± 7.7 | 11.0 ± 4.0 | 13.9 ± 5.7 |
| 30-day Mortality, n (%) | 1 (6.7) | 4 (10.5) | 1 (3.6) | 3 (14.3) | 1 (4.8) | 1 (14.3) | 1 (6.7) | 2 (18.2) |
| Paravalvular leak | ||||||||
| AR <2, n (%) | NR | NR | 19 (67.9) | NR | NR | 5 (71.4) | 13 (86.7) | NR |
| AR ≥−2, n (%) | 0 | 9 (23.7) | 9 (32.1) | 5 (23.8) | 4 (19.0) | 2 (28.6) | 1 (6.7) | 2 (18.2) |
| Stroke, n (%) | 1 (6.7) | 0 | 0 | 0 | 0 | 0 | NR | NR |
| AKI, n (%) | 0 | NR | 0 | 4 (19.0) | 1 (4.8) | 0 | NR | NR |
| Pacemaker implantation, n (%) | 2 (13.3) | 6 (15.8) | 8 (28.6) | 3 (14.3) | 3 (14.3) | 2 (28.6) | 6 (40.0%) | NR |
| Bleed-life threatening, n (%) | 0 | NR | 3 (10.7) | 1 (4.8) | 2 (9.5) | 0 | NR | NR |
| Bleed-major, n (%) | 1 (6.7) | NR | NR | 4 (19.0) | 1 (4.8) | 1 (14.3) | NR | NR |
| Coronary occlusion, n (%) | 0 | NR | NR | 0 | 1 (4.8) | 0 | NR | NR |
| Conversion to open heart, n (%) | 0 | 0 | 1 (3.6) | 1 (4.8) | 0 | 0 | 1 (6.7) | 0 |
| Major vascular complication, n (%) | 1 (6.7) | 1 (2.6) | 0 | 2 (9.5) | 1 (4.8) | 0 | NR | NR |
| Access site complication, n (%) | 0 | 9 (23.7) | 3 (10.7) | NR | NR | 1 (14.3) | NR | NR |
| Follow up period | 30 days | 1 year | 1 year | 1 year | 1 year | 6 months | 8 months | 208 days |
| NYHA (1–2), n | 14 | NR | 21 | NR | NR | 6 | 12 | 7 |
| Death, n (%) | 1 (6.7) | 5 (13.2) | 5 (17.9) | 6 (28.6) | 3 (14.3) | 1 (14.3) | 2 (13.3) | 4 (36.4) |
| Device failure, n (%) | 0 | 1 (2.6) | NR | NR | NR | 0 | NR | 1 (9.1) |
TAVI: transcatheter aortic valve implantation; BAV: bicuspid aortic valve; MAVPG: mean aortic valve pressure gradient; NR: not reported; AR: aortic regurgitation; AKI: acute kidney injury; NYHA: New York Heart Association
Table 3b.
Post-TAVI outcomes of BAV patients
| Sample size | Segev | Colkesen | Kassaian | Urena | Maluenda | Raja | Jilaihawi | Chiam | Delgado |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| Device success, n (%) | 2 (100) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Post TAVI MAVPG mmHg | 11.5 | 7 | 6 | NR | NR | NR | 16 | 20 | 10 |
| 30-day Mortality, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Paravalvular leak | |||||||||
| AR <2, n (%) | 1 (50) | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| AR ≥−2, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stroke, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AKI, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pacemaker implantation, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bleed-life threatening, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bleed-major, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coronary occlusion, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Conversion to open heart, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Major vascular complication, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Access site complication, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Follow up period | 30 days | 30 days | 30 days | 9 months | 30 days | 10 months | 2 years | 6 months | 30 days |
| NYHA (1–2), n | NR | NR | NR | NR | 1 | 1 | 1 | NR | NR |
| Death, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Device failure, n (%) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
TAVI: transcatheter aortic valve implantation; BAV: bicuspid aortic valve; MAVPG: mean aortic valve pressure gradient; NR: not reported; AR: aortic regurgitation; AKI: acute kidney injury; NYHA: New York Heart Association
For the five included case-control/cohort studies, quantitative syntheses of results in regard to 30-day mortality rate, device success rate as well as the incidence of moderate to severe paravalvular regurgitation were conducted, and no significant differences were detected between BAV patients and non-BAV patients [30-day mortality rate: RR = 1.05, 95%CI 0.57–1.95, p = 0.87; Device success rate: RR = 1.00, 95%CI 0.95–1.05, p = 0.94; Incidence of moderate to severe paravalvular regurgitation: RR = 1.25, 95%CI 0.85–1.84, p = 0.25] (see Figs. 2a–2c).
Fig. 2.
Forest plots on post-TAVI clinical outcomes of BAV and non-BAV patients. (a) Forest plot on 30-day mortality rate post-TAVI in BAV and non-BAV patients is shown. (b) Forest plot on device success rate for TAVI in BAV and non-BAV patients is shown. (c) Forest plot on incidence of moderate to severe paravalvular regurgitation post-TAVI in BAV and non-BAV patients is shown. TAVI: transcatheter aortic valve implantation; BAV: bicuspid aortic valve; 95%CI: 95% confidence interval
Sensitivity analyses
Sensitivity analyses were performed by removing one individual study each time. For 30-day mortality rate, device success rate and the incidence of moderate to severe paravalvular regurgitation, removing any study did not impact the overall results, suggesting that quantitative synthetic results of the present study were quite reliable and stable.
Publication bias
Potential publication bias was evaluated with funnel plots. Visual inspection of funnel plots revealed no apparent asymmetry for 30-day mortality rate, device success rate and the incidence of moderate to severe paravalvular regurgitation. And these results suggested that significant publication bias was unlikely in included case-control/cohort studies. For case reports and case series, however, we could not eliminate the possibility of potential publication bias since positive results are more likely to be reported.
Discussion
BAV is a common congenital cardiac defect and just as its name implies, a BAV contains 2 cusps, usually of unequal size, instead of 3 cusps of similar size as seen in the tricuspid aortic valve.1–3) Despite being identified as a congenital abnormality, it is not uncommon to see patients remain asymptomatic early in life, yet develop severe valvular dysfunction later in life.4–5) It is estimated that more than half of BAV patients will require aortic valve replacement within 25 years of initial diagnosis.6) Besides, over 50% of resected aortic valves during surgical aortic valve replacement procedures for severe AS were found to be bicuspid.29)
A substantial proportion of severe AS patients present with BAV, however, according to current guidelines, the presence of a BAV remains to be a relative contraindication for TAVI.9,30) And several concerns may explain the reason why BAV patients are precluded from TAVI. Firstly, the asymmetrical configuration and heavy calcification of BAV annulus may hinder valve positioning and expansion, which can subsequently impair the normal functioning and long-term durability of the prosthetic valve as well as increase the risk of paravalvular leak, a complication which has already been regarded as the major drawback of TAVI. Secondly, due to the elliptical shape and relatively larger size of BAV annulus, the current available prosthetic valves may not be suitable for BAV patients. Although larger sized prosthetic valves have already become available recently, the non-circular unsymmetrical form of BAV still means a higher risk of paravalvular leak and valve dysfunction. Thirdly, BAV is not just a valvular abnormality and may be associated with other aortic diseases, and TAVI procedure may increase the risk of aortic dissections or ruptures in patients with aortopathy.31) So to conclude, the unfavorable anatomical features of BAV bring even higher procedural risk for these patients, and this may justify the hesitation for practitioners to generalize about the use of TAVI into BAV patients.
While multi-center studies evaluating the performance of TAVI in BAV patients are unlikely in the nearby future, we performed the present systematic review and meta-analysis to better elucidate the efficacy and safety of TAVI in BAV patients. And our findings suggest that TAVI in BAV was feasible and safe. Besides, post-TAVI clinical outcomes of BAV patients were comparable to that of non-BAV patients, as reported by included case control/cohort studies. However, it is notable that paravalvular leak was reported in two-thirds of the patients, although the majority of which were graded as only trivial to mild, 23.1% were reported to be of moderate or even greater severity. Previous clinical trials and observational studies have demonstrated that even mild degree of paravalvular leak could lead to worse outcomes for patients receiving TAVI. Therefore, although the long-term influence of paravalvular leak on the prognosis of BAV patients is still unclear, it is rational to hypothesize that it may similarly deteriorate outcomes of these patients and remain to be the biggest obstacle for the wider application of TAVI in BAV patients.
Although our results demonstrated that TAVI may be an effective and safe therapeutic option for BAV patients, they do not mean that TAVI is the best treatment regime for all BAV patients. When conventional surgery is infeasible and TAVI becomes the only choice, careful patient evaluation and valve selection are important for achieving satisfactory outcomes. As for patient evaluation, since currently available risk assessment scales were mainly designed for conventional heart surgery rather than TAVI, a thorough evaluation of patients’ condition by a multi-disciplinary heart team which includes interventional cardiologists, cardiothoracic surgeons, anesthetists, clinical nurse specialists and primary care doctors is crucial for a more precise risk stratification of BAV patients. Besides, certain anatomical criteria should be emphasized since successful results are likely to be achieved in BAV patients with predominant aortic stenosis, while patients with bulky leaflets, enlarged aortic root, dilated ascending aorta, or significant aortic regurgitation are at high risk for procedure failure. As a result, imaging evaluation of a BAV and its associated aortopathy is extremely important for patient selection. And to get exact information on anatomy, apart from routine echocardiography examination, computed tomography or magnetic resonance imaging may also be needed.21–23) As for valve choice, despite that both the self-expandable Medtronic CoreValve and the balloon-expandable Edwards SAPIEN/SAPIEN XT valve were shown to be associated with desirable efficacy and safety profiles, the Medtronic CoreValve may be a better choice for BAV patients since it avoids potential injury resulting from balloon expansion during valve deployment. Besides, it is more compatible to the elliptical shape and relatively larger size of BAV annulus. However, it is worth noting that Medtronic CoreValves were associated with higher incidence of periprosthetic regurgitation as well as heart block, and therefore may require post-dilation or pacemaker implantation.23,25)
The present study certainly has several limitations. Firstly, data regarding the performance of TAVI in BAV patients is still limited, making it impossible for us to draw definitive conclusions. Secondly, our results are obtained from analyzing all of the relevant literature to date, a significant portion (12 articles covering 25.9% of the patients) of which are actually case reports or case series, therefore, publication bias may exist since poor outcomes are less likely to be reported. Thirdly, we did observe a significant variability in reported outcomes of included studies, for example, the 30-day mortality rate ranged from 3.6% to 18.2%, as did the incidence of pacemaker implantation (ranged from 13.3% to 40.0%). And these discrepancies in results are likely to be partly attributed to the heterogeneity in study population, types of prosthetic valve implanted, and different access route used. Thus, it would be especially meaningful to perform subgroup analysis accordingly. However, this was not possible since the sample size of eligible case-control/cohort studies was not sufficient. Fourth, included studies presented outcome data only up to 2-year follow-up, thus, we could not analyze the long-term efficacy and safety of TAVI in BAV patients. Fifth, it would be interesting to assess the performance of new generation prosthetic valves such as CoreValve Evolut or SAPIEN 3 in BAV patients. However, regrettably, the present systematic literature review did not get any relevant data. Sixthly, bicuspid aortic valves actually include truly congenital bicuspid valves which consist of only two leaflets and functional bicuspid aortic valves which are made up of three leaflets, two of which are partially fused together. Currently included studies fail to distinguish between these two groups, which may actually have different feasibility and outcome profiles in TAVI procedure. Therefore, it is difficult to tell whether our results are applicable to both truly congenital and functional bicuspid valves. Taken these limitations into consideration, the results reported by the present study should be interpreted with caution.
Conclusion
The present systematic review and meta-analysis suggested that TAVI may be feasible and safe in BAV patients. However, given that our results were based on limited number of preliminary observational studies with only short-term or mid-term follow-up, further multi-center studies with larger sample size and longer-term follow-up are warranted to more conclusively assess the performance of TAVI in BAV patients.
Disclosure Statement
The authors report no conflict of interest regarding the content herein.
References
- 1).Mulder BJ. Epidemiology of adult congenital heart disease: demographic variations worldwide. Neth Heart J 2012; 20: 505‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Cedars A, Braverman AC. The many faces of bicuspid aortic valve disease. Prog Pediatr Cardiol 2012; 34: 91‐6. [Google Scholar]
- 3).Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39: 1890‐900. [DOI] [PubMed] [Google Scholar]
- 4).van Engelen K, Bartelings MM, Gittenberger-de Groot AC, et al. Bicuspid aortic valve morphology and associated cardiovascular abnormalities in fetal Turner syndrome: a pathomorphological study. Fetal Diagn Ther 2014; 36: 59‐68. [DOI] [PubMed] [Google Scholar]
- 5).Grewal N, DeRuiter MC, Jongbloed MR, et al. Normal and abnormal development of the aortic wall and valve: correlation with clinical entities. Neth Heart J 2014; 22: 363‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Michelena HI, Khanna AD, Mahoney D, et al. Incidence of aortic complications in patients with bicuspid aortic valves. JAMA 2011; 306: 1104‐12. [DOI] [PubMed] [Google Scholar]
- 7).Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117: 2776‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Holinski S, Staebe P, Geyer T, et al. Transfemoral versus conventional aortic valve implantation—early postoperative cognitive outcome. Ann Thorac Cardiovasc Surg 2013; 19: 195‐200. [DOI] [PubMed] [Google Scholar]
- 9).Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012; 42: S1‐44. [DOI] [PubMed] [Google Scholar]
- 10).Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010; 363: 1597‐607. [DOI] [PubMed] [Google Scholar]
- 11).Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011; 364: 2187‐98. [DOI] [PubMed] [Google Scholar]
- 12).Delgado V, Tops LF, Schuijf JD, et al. Successful deployment of a transcatheter aortic valve in bicuspid aortic stenosis: role of imaging with multislice computed tomography. Circ Cardiovasc Imaging 2009; 2: e12‐3. [DOI] [PubMed] [Google Scholar]
- 13).Chiam PT, Chao VT, Tan SY, et al. Percutaneous transcatheter heart valve implantation in a bicuspid aortic valve. JACC Cardiovasc Interv 2010; 3: 559‐61. [DOI] [PubMed] [Google Scholar]
- 14).Jilaihawi H, Asgar A, Bonan R. Good outcome and valve function despite Medtronic-corevalve underexpansion. Catheter Cardiovasc Interv 2010; 76: 1022‐5. [DOI] [PubMed] [Google Scholar]
- 15).Raja Y, Holloway B, Doshi SN. Symmetrical expansion of an Edwards Sapien valve in a congenitally bicuspid aortic valve. Heart 2011; 97: 1113. [DOI] [PubMed] [Google Scholar]
- 16).Maluenda G, Araya M, Ibañez-Arenas R. Successful transfemoral aortic valve replacement in a bicuspid aortic stenotic valve: requirements for a safe implant. Catheter Cardiovasc Interv 2013; 82: E826‐30. [DOI] [PubMed] [Google Scholar]
- 17).Urena M, Doyle D, Dumont E, et al. Transcatheter aortic valve replacement with a balloon-expandable valve for the treatment of noncalcified bicuspid aortic valve disease. Rev Esp Cardiol (Engl Ed) 2014; 67: 327‐9. [DOI] [PubMed] [Google Scholar]
- 18).Colkesen Y, Baykan O, Dagdelen S, et al. Transcatheter aortic valve implantation in a patient with bicuspid aortic stenosis and a borderline-sized annulus. Interact Cardiovasc Thorac Surg 2015; 21: 691‐3. [DOI] [PubMed] [Google Scholar]
- 19).Kassaian SE, Fallahi F, Shirzad M, et al. Percutaneous aortic valve implantation in bicuspid aortic valve: a case report. ARYA Atheroscler 2015; 11: 204‐7. [PMC free article] [PubMed] [Google Scholar]
- 20).Segev A, Spiegelstein D, Fefer P, et al. Trans-catheter aortic valve implantation for non-classical indications. Isr Med Assoc J 2013; 15: 399‐403. [PubMed] [Google Scholar]
- 21).Wijesinghe N, Ye J, Rodés-Cabau J, et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve stenosis. JACC Cardiovasc Interv 2010; 3: 1122‐5. [DOI] [PubMed] [Google Scholar]
- 22).Himbert D, Pontnau F, Messika-Zeitoun D, et al. Feasibility and outcomes of transcatheter aortic valve implantation in high-risk patients with stenotic bicuspid aortic valves. Am J Cardiol 2012; 110: 877‐83. [DOI] [PubMed] [Google Scholar]
- 23).Kosek M, Witkowski A, Da˛browski M, et al. Transcatheter aortic valve implantation in patients with bicuspid aortic valve: a series of cases. Kardiol Pol 2015; 73: 627‐36. [DOI] [PubMed] [Google Scholar]
- 24).Hayashida K, Bouvier E, Lefèvre T, et al. Transcatheter aortic valve implantation for patients with severe bicuspid aortic valve stenosis. Circ Cardiovasc Interv 2013; 6: 284‐91. [DOI] [PubMed] [Google Scholar]
- 25).Costopoulos C, Latib A, Maisano F, et al. Comparison of results of transcatheter aortic valve implantation in patients with severely stenotic bicuspid versus tricuspid or nonbicuspid valves. Am J Cardiol 2014; 113: 1390‐3. [DOI] [PubMed] [Google Scholar]
- 26).Bauer T, Linke A, Sievert H, et al. Comparison of the effectiveness of transcatheter aortic valve implantation in patients with stenotic bicuspid versus tricuspid aortic valves (from the German TAVI Registry). Am J Cardiol 2014; 113: 518‐21. [DOI] [PubMed] [Google Scholar]
- 27).Kochman J, Huczek Z, Scislo P, et al. Comparison of one- and 12-month outcomes of transcatheter aortic valve replacement in patients with severely stenotic bicuspid versus tricuspid aortic valves (results from a multicenter registry). Am J Cardiol 2014; 114: 757‐62. [DOI] [PubMed] [Google Scholar]
- 28).Liu XB, Jiang JB, Zhou QJ, et al. Evaluation of the safety and efficacy of transcatheter aortic valve implantation in patients with a severe stenotic bicuspid aortic valve in a Chinese population. J Zhejiang Univ Sci B 2015; 16: 208‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Roberts WC, Ko JM. Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, with or without associated aortic regurgitation. Circulation 2005; 111: 920‐5. [DOI] [PubMed] [Google Scholar]
- 30).Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 63: e57‐185. [DOI] [PubMed] [Google Scholar]
- 31).Furukawa H, Tanemoto K. Current topics on bicuspid aortic valve: clinical aspects and surgical management. Ann Thorac Cardiovasc Surg 2015; 21: 314‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]