Abstract
Background: Pulmonary metastasectomy has come to be recognized as an effective treatment for selected patients with some malignancies. On the other hand, the role of pulmonary metastasectomy for gastric cancer is still unknown. Metastasectomy is rarely indicated in cases of pulmonary metastasis from gastric cancer, because in most cases, the metastasis occurs in the form of lymphangitic carcinomatosis and the lesions are numerous. The purpose of this study was to determine the surgical outcomes and prognostic factors for survival after pulmonary metastasectomy.
Methods: From 1985 to 2012, 10 patients underwent pulmonary metastasectomy for gastric cancer at Saitama Cancer Center, Japan. The overall survival rate was examined by the Kaplan-Meier method and univariate analysis was carried out to identify prognostic factors.
Results: The overall 3-year survival rate was 30.0%. The median follow-up period was 26.8 months (range, 6.5–96.6) after the pulmonary metastasectomy. Univariate analysis revealed an advanced pathological stage of the gastric cancer and occurrence of extrapulmonary metastasis before the pulmonary metastasectomy as unfavorable prognostic factors.
Conclusion: Pulmonary metastasectomy should be considered in selected patients with lung metastasis from gastric cancer. An advanced pathological stage of gastric cancer and occurrence of extrapulmonary metastasis before the pulmonary metastasectomy are unfavorable prognostic factors.
Keywords: metastatic gastric cancer, pulmonary metastasis, pulmonary metastasectomy
Introduction
Pulmonary metastasectomy has come to be recognized as an effective treatment for selected patients with colorectal cancer, renal cancer and other malignancies, and in recent years, long-term survival after pulmonary metastasectomy has been reported in patients with several malignancies.1–3) On the other hand, the role of pulmonary metastasectomy in gastric cancer patients with pulmonary metastasis is still unclear.
The prognosis of patients with metastatic gastric cancer is poor. The median survival after diagnosis of pulmonary metastasis is 4 months, and even in patients treated by chemotherapy, the reported 5-year survival is only 2%–4%.4–6) Recently, several studies have revealed relatively good surgical outcomes, and the role of pulmonary metastasectomy is becoming clearer.7–9)
In this study, we reviewed the clinicopathological features of 10 patients who underwent pulmonary metastasectomy for gastric cancer, and examined the outcomes and prognostic factors affecting the survival of the patients after the pulmonary metastasectomy.
Methods
Patient cohort
From January 1985 to December 2012, 10 patients underwent pulmonary metastasectomy for metastatic gastric cancer at the Saitama Cancer Center Hospital; a total of 822 patients underwent pulmonary metastasectomy for metastatic lung tumors and 4898 patients underwent gastrectomy for gastric cancer during this period. We basically used Thomford’s criteria as a guideline for performing resection of the pulmonary metastases.10) The inclusion criteria were as follows: (1) the patient must be a good risk for pulmonary resction, (2) controlled disease at the primary site, (3) no other extrapulmonary metastases, and (4) pulmonary metastasis limited to one lung.
We performed pulmonary metastasectomy for the patients with bilateral tumors or extrapulmonary metastases, only if the tumor at the primary site was controlled or there is no effective therapy other than surgery.
In this study, we reviewed 10 patients with metastatic gastric cancer who underwent pulmonary resection, including nine who had undergone the gastrectomy for the primary gastric cancer at our institution, and one at another institution. The patients were aged 54–71 years (mean age, 64.1 years) at the time of the gastrectomy, and 54–73 years (mean age, 66.4 years) at the time of the pulmonary metastasectomy. All were males.
Patient follow-up and evaluation of the outcomes
Data on the outcomes, including the morbidity, mortality and survival, were obtained from the patient records. All patients were followed up until death after the pulmonary resection: the death was cancer-related in 8 of the 10 patients and not related to the cancer in one patient, while one patient is still alive and cancer-free. The median follow-up period of the patients after the pulmonary metastasectomy was 26.8 months (range, 6.5–96.6).
Pathological diagnosis
The Japanese classification of gastric carcinoma (3rd English edition) was used for the tumor staging, and the tumor depth and presence/absence of node metastasis was considered.11) Histology was defined as papillary (pap), tubular (tub), poorly differentiated (por) adenocarcinoma, or others. Both the gastrectomy and lymph node dissection were carried out based on the Japanese Gastric Cancer Treatment Guidelines 2010 (version 3).12)
Statistical analyses
Statistical calculations were carried out using a statistical software program (IBM SPSS Statistics version 21; IBM SPSS, New York, USA). The overall survival (OS) was statistically analyzed using the Kaplan-Meier estimated survivavl curves. The differences between the survival curves were analyzed using the log-rank test. Hazard ratios (HR) in the patient subsets were calculated using the Cox proportional hazards model. Differences were considered significant when the p-value was <0.05.
Results
Clinicopathological features of the patients with metastatic gastric cancer
The clinicopathological features of the patients with metastatic gastric cancer are shown in Table 1. Nine had undergone total gastrectomy and one had undergone distal gastrectomy for the primary gastric cancer. The distribution of the pathological stage of the primary gastric cancer was as follows: stage I, one patient; stage II, five patients; stage III, one patient; stage IV, three patients. The stage IV patients included two with synchronous liver metastasis and one with metastasis to sites other than the regional lymph nodes (distant metastasis). Complete resection at gastrectomy had been achieved in all the cases, in particular, in the three patients with stage IV disease. The histological type of the primary gastric cancer was distributed as follows: papillary adenocarcinoma in one patient, tubular adenocarcinoma in five patients, and poorly differentiated adenocarcinoma in four patients. The median disease-free interval (DFI) after gastrectomy was 16.2 months (range, 2.3–52.4 months). In seven patients, the first site of recurrence was the lung. The median interval between the gastrectomy and pulmonary metastasectomy was 29.5 months (range, 5.1–53.2 months).
Table 1.
Clinical characteristics of gastric cancer
| No. | Age at gastrectomy | Gender | Type of gastrectomy | Pathological stage | Histological type | DFI following gastrectomy (months) | First site of recurrence | Interval between gastrectomy and pulmonary metastasectomy (months) |
|---|---|---|---|---|---|---|---|---|
| 1 | 59 | Male | TG | IIIA | pap | 14 | Lung | 31 |
| 2 | 62 | Male | TG | IIB | por | 11 | Liver | 25 |
| 3 | 54 | Male | TG | IV | tub | 2 | LN | 5 |
| 4 | 66 | Male | TG | IIB | tub | 52 | Lung | 53 |
| 5 | 65 | Male | TG | IIA | tub | 25 | Lung | 26 |
| 6 | 70 | Male | TG | IV | por | 13 | Liver | 28 |
| 7 | 71 | Male | TG | IIA | tub | 29 | Lung | 33 |
| 8 | 60 | Male | TG | IIA | tub | 17 | Lung | 14 |
| 9 | 68 | Male | DG | IB | por | 18 | Lung | 35 |
| 10 | 66 | Male | TG | IV | por | 30 | Lung | 49 |
DFI: disease-free interval; TG: total gastrectomy; LN: lymph nodes; DG: distal gastrectomy
Pulmonary metastasis and pulmonary metastasectomy
The pulmonary lesions were solitary in 5 of the 10 cases, and multiple in the remaining five cases (Table 2). The median largest tumor size was 19 mm (range, 6–55 mm). The median tumor doubling time (TDT) was 46 days (range, 19–289 days).
Table 2.
Pulmonary metastasis and post-operative course following pulmonary metastasectomy
| No. | Number of lesions | Largest tumor size on CT (cm) | TDT (days) | Surgical procedure | Post lung metastasectomy course | DFI following pulmonary metastasectomy (months) | OS following metastasectomy (months) | OS following gastrectomy (months) | Status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 28 | 135 | Lobectomy | Lung recurrence | 18 | 28 | 59 | Dead |
| 2 | 1 | 13 | 21 | Wedge | Liver dysfunction | 19 | 19 | 44 | Dead |
| 3 | 11 | 15 | 19 | Wedge | Liver recurrence | 2 | 7 | 12 | Dead |
| 4 | 3 | 55 | NA | Lobectomy | Lung recurrence | 7 | 29 | 82 | Dead |
| 5 | 1 | 50 | 57 | Wedge | Para-aortic LN recurrence | 35 | 48 | 73 | Dead |
| 6 | 2 | 22 | NA | Wedge | Radiation for brain metastasis | 1 | 7 | 35 | Dead |
| 7 | 3 | 6 | 36 | Wedge | Lung recurrence | 18 | 37 | 70 | Dead |
| 8 | 1 | 7 | 56 | Wedge | Disease free | 97 | 97 | 110 | Alive |
| 9 | 1 | 34 | 36 | Wedge | Para-aortic LN recurrence | 19 | 26 | 60 | Dead |
| 10 | 4 | 16 | 289 | Wedge | Did not resect contralateral nodule | 0 | 17 | 67 | Dead |
CT: computed tomography; TDT: tumor doubling time; DFI: disease-free interval; OS: overall survival; Wedge: wedge resection; NA: not available;LN: lymph node
The surgical procedure and clinical course after pulmonary metastasectomy in the 10 patients of our study are shown in Table 2. Eight patients were diagnosed as having metastatic gastric cancer, while in the remaining two, primary lung cancer could not be ruled out. An intraoperative frozen section diagnosis was performed in 8 patients, and all eight were confirmed as having metastatic gastric cancer. Wedge resection was performed in eight patients, and lobectomy in two. The reasons for performing lobectomy were as follows: difficulty in distinguishing metastatic gastric carcinoma from primary lung adenocarcinoma in the intraoperative frozen section diagnosis, and large tumor size. No postoperative complications were observed. The median DFI after the pulmonary metastasectomy was 17.8 months (range, 0–96.6 months).
Results of univariate analysis carried out to identify predictors of the OS following pulmonary metastasectomy and the survival curves
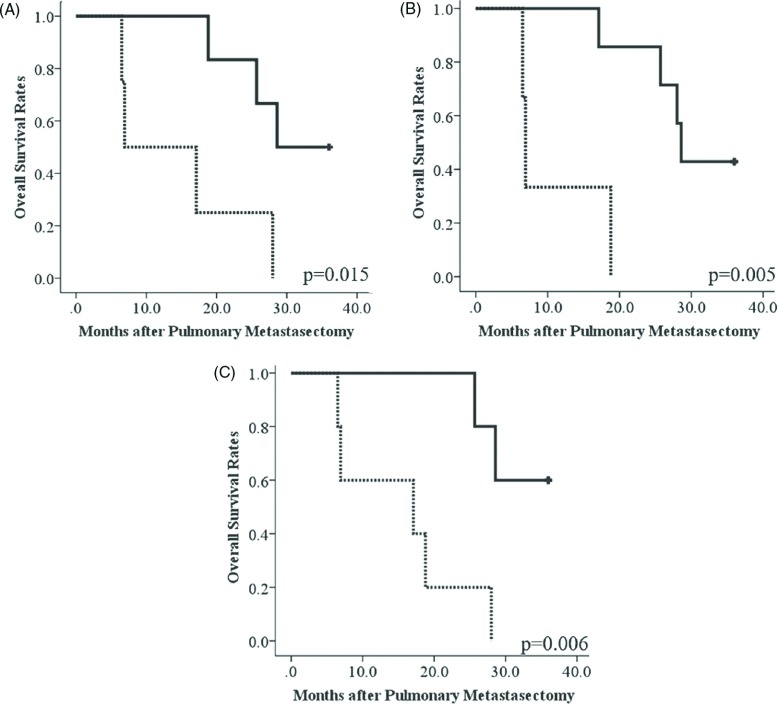
The results of the univariate analysis are shown in Table 3, and the Kaplan-Meier curves for survival are shown in Fig. 1. The overall 3-year survival rate after pulmonary metastasectomy was 30.0%, and the median survival time following pulmonary resection was 26.8 months. Univariate analysis identified early gastric cancer as being significantly associated with a favorable survival. The HR in stage III/IV patients as compared to stage I/II patients was 6.68 (95% confidence interval 1.17–38.03; p = 0.032). The cumulative 3-year survival rate in patients with stage I/II gastric cancer was 50.0%, whereas that in patients with stage III/IV gastric cancer was 0% (p = 0.015, Fig. 1A). The first site of recurrence also significantly influenced the survival. The HR in patients with a history of extrapulmonary metastasis prior to the pulmonary metastasectomy as compared to patients without a history of extrapulmonary metastasis prior to pulmonary metastasectomy was 13.55 (95% CI 1.36–135.16; p = 0.026). The cumulative 3-year survival rate in patients without a history of extrapulmonary metastasis prior to the pulmonary metastasectomy was 42.9%, whereas that in patients with a history of extrapulmonary metastasis prior to the pulmonary metastasectomy was 0% (p = 0.005, Fig. 1B). The 3-year cumulative survival in the patients without either of the aforementioned adverse prognostic factors was 60.0%, while that in the patients with one or more of the aforementioned adverse prognostic factors was 0% (p = 0.006, Fig. 1C).
Table 3.
Results of the univariate analysis for the OS following pulmonary metastasectomy
| Baseline and clinical features | Patients | OS | ||
|---|---|---|---|---|
| HR | 95% CI | p-value | ||
| Initial stage of gastric cancer | ||||
| I/II | 6 | 1.00 | ||
| III/IV | 4 | 6.68 | 1.17–38.03 | 0.03 |
| Histological type of gastric cancer | ||||
| por | 4 | 1.00 | ||
| pap/tub | 6 | 0.11 | 0.012–1.053 | 0.06 |
| DFI following gastrectomy | ||||
| <Median (16.2 months) | 5 | 1.00 | ||
| >Median (16.2 months) | 5 | 0.47 | 0.10–2.15 | 0.33 |
| History of extrapulmonary metastasis | ||||
| without a history | 3 | 1.00 | ||
| with a history | 7 | 13.55 | 1.36–135.16 | 0.03 |
| Number of Lesions | ||||
| One | 5 | 1.00 | ||
| Two or more | 5 | 1.99 | 0.44–9.05 | 0.35 |
| TDT (n = 8) | ||||
| <Median (46 days) | 4 | 1.00 | ||
| >Median (46 days) | 4 | 0.46 | 0.074–2.86 | 0.41 |
| Surgical procedure | ||||
| Wedge resection | 8 | 1.00 | ||
| Lobectomy | 2 | 1.04 | 0.20–5.47 | 0.96 |
OS: overall survival; HR: hazard ratios; 95% CI: 95% confidence interval; DFI: disease-free interval; TDT: tumor doubling time
Fig. 1.
Overall survival rates after pulmonary metastasectomy. (A) Survival curves for patients with early-stage gastric cancer (bold line) and patients with advanced-stage gastric cancer (dashed line). (Log-rank test, p = 0.015) (B) Survival curves for the patients without a history of extrapulmonary metastasis prior to the pulmonary metastasectomy (bold line) and those with a history of extrapulmonary metastasis prior to the pulmonary metastasectomy (dashed line). (Log-rank test, p = 0.005) (C) Survival curves for patients without poor prognostic factors (bold line) and those with eith
Discussion
In the present study, we reviewed the data of 10 patients with metastatic gastric cancer who underwent pulmonary metastasectomy at a single institution. While gastric cancer is one of the most commonly encountered malignancies in clinical practice, solitary pulmonary metastases are rare in patients with gastric cancer. Metastatic gastric cancer is often unresectable because it often occurs in the form of lymphangitic carcinomatosis or is associated with numerous lesions.13,14) The reported incidence of pulmonary resection for metastatic gastric cancer is 0.4%–3.5% in patients undergoing pulmonary metastasectomy and 0.1%–0.26% in patients undergoing gastrectomy for gastric cancer.9,15,16) Our data were consistent with these reported values (1.2% in patients undergoing pulmonary metastasectomy and 0.2% in patients undergoing gastrectomy for gastric cancer.).
The lung is one of the most frequent sites of distant metastasis in patients with gastric carcinoma, and pulmonary metastasis is known to be associated with a poor prognosis in these patients. Recently, however, several case reports have been published of gastric cancer patients showing long-term survival after pulmonary metastasectomy,7,17) and also several reports of prognostic factors in patients with metastatic gastric cancer undergoing pulmonary metastasectomy.7–9) Kemp et al.7) summarized 21 studies and analyzed the data of 43 patients who underwent pulmonary metastasectomy for metastatic gastric cancer. They reported an overall 5-year survival rate of 33% and a median survival time of 29 months (range, 3–84 months). They also concluded that it is reasonable to consider pulmonary metastasectomy in patients with single, small lesions and a long DFI. Based on a review of 51 patients, Shiono et al.8) reported that the 5-year overall survival rate after pulmonary metastasectomy was 28% and that the survival in patients with a DFI of less than 12 months was significantly lower than that in patients with a DFI of 12 months or longer. Kobayashi et al.9) reviewed the data of 14 patients and reported that the 5-year overall survival rate was 58.4%, and that the TDT was a significant predictor of the disease-free survival (DFS).
Until date, patients with recurrent or advanced gastric cancer continue to have a poor prognosis. Systemic chemotherapy is widely accepted as palliative treatment for these patients, as it has been verified to improve the quality of life and prolong the survival time of these patients. According to the literature, however, the median survival time of advanced or recurrent gastric cancer patients treated with first-line chemotherapy is typically less than one year.18–22) The 3-year survival rate of gastric cancer patients after pulmonary metastasectomy at our institution was 30% and the median survival time was 26.8 months, worse than the values reported previously. In our study, however, univariate analysis revealed that early-stage (pathological stage I or II) gastric cancer and absence of extrapulmonary metastasis were associated with a significantly favorable prognosis at 3 years. Moreover, our series included a patient who is still alive more than 8 years after pulmonary metastasectomy and 11 years after gastrectomy. Although we could not reach a conclusion as to the indications for pulmonary metastasectomy in patients with metastatic gastric cancer because of the small number of cases, the result of this study suggest that pulmonary metastasectomy should be considered in selected patients with metastatic gastric cancer. Pulmonary metastasectomy is possibly associated with at least a favorable 3-year prognosis in patients with early gastric cancer and those without a history of extrapulmonary metastasis prior to the pulmonary metastasectomy.
Acknowledgement
We would like to express our gratitude to all the patients who participated in the study, and also to Drs. Tatsuya Yamada and Yoshiyuki Kawashima of the Division of Gastroenterological Surgery, Saitama Cancer Center, and Iwao Mikami of the Division of Thoracic Surgery, Ishikiriseiki Hospital for their important contributions to this study.
Disclosure Statement
Y. Iijima and his co-authors have no conflicts of interests to declare.
References
- 1).Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997; 113: 37-49. [DOI] [PubMed] [Google Scholar]
- 2).Lin BR, Chang TC, Lee YC, et al. Pulmonary resection for colorectal cancer metastases: duration between cancer onset and lung metastasis as an important prognostic factor. Ann Surg Oncol 2009; 16: 1026-32. [DOI] [PubMed] [Google Scholar]
- 3).Bölükbas S, Kudelin N, Eberlein M, et al. The influence of the primary tumor on the long-term results of pulmonary metastasectomy for metastatic renal cell carcinoma. Thorac Cardiovasc Surg 2012; 60: 390-7. [DOI] [PubMed] [Google Scholar]
- 4).Kong JH, Lee J, Yi CA, et al. Lung metastases in metastatic gastric cancer: pattern of lung metastases and clinical outcome. Gastric Cancer 2012; 15: 292-8. [DOI] [PubMed] [Google Scholar]
- 5).Ohkuwa M, Ohtsu A, Boku N, et al. Long-term results for patients with unresectable gastric cancer who received chemotherapy in the Japan Clinical Oncology Group (JCOG) trials. Gastric Cancer 2000; 3: 145-50. [DOI] [PubMed] [Google Scholar]
- 6).Yoshida M, Ohtsu A, Boku N, et al. Long-term survival and prognostic factors in patients with metastatic gastric cancers treated with chemotherapy in the Japan Clinical Oncology Group (JCOG) study. Jpn J Clin Oncol 2004; 34: 654-9. [DOI] [PubMed] [Google Scholar]
- 7).Kemp CD, Kitano M, Kerkar S, et al. Pulmonary resection for metastatic gastric cancer. J Thorac Oncol 2010; 5: 1796-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Shiono S, Sato T, Horio H, et al. Outcomes and prognostic factors of survival after pulmonary resection for metastatic gastric cancer. Eur J Cardiothorac Surg 2013; 43: e13-6. [DOI] [PubMed] [Google Scholar]
- 9).Kobayashi Y, Fukui T, Ito S, et al. Pulmonary metastasectomy for gastric cancer: a 13-year single-institution experience. Surg Today 2013; 43: 1382-9. [DOI] [PubMed] [Google Scholar]
- 10).Thomford NR, Woolner LB, Clagett OT. The surgical treatment of metastatic tumors in the lungs. J Thorac Cardiovasc Surg 1965; 49: 357-63. [PubMed] [Google Scholar]
- 11).Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14: 101-12. [DOI] [PubMed] [Google Scholar]
- 12).Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 2011; 14: 113-23. [DOI] [PubMed] [Google Scholar]
- 13).Hirakata K, Nakata H, Nakagawa T. CT of pulmonary metastases with pathological correlation. Semin Ultrasound CT MR 1995; 16: 379-94. [DOI] [PubMed] [Google Scholar]
- 14).Kong JH, Lee J, Yi CA, et al. Lung metastases in metastatic gastric cancer: pattern of lung metastases and clinical outcome. Gastric Cancer 2012; 15: 292-8. [DOI] [PubMed] [Google Scholar]
- 15).Sakaguchi K, Yamamoto M, Horio H. Resection of solitary pulmonary metastasis from gastric cancer. Jpn J Lung Cancer 2007; 47: 323-6. (in Japanese) [Google Scholar]
- 16).Kanemitsu Y, Kondo H, Katai H, et al. Surgical resection of pulmonary metastases from gastric cancer. J Surg Oncol 1998; 69: 147-50. [DOI] [PubMed] [Google Scholar]
- 17).Tanai C, Hamaguchi T, Watanabe S, et al. A case of long-term survival after surgical resection of solitary pulmonary metastasis from gastric cancer. Jpn J Clin Oncol 2010; 40: 85-9. [DOI] [PubMed] [Google Scholar]
- 18).Yuan M, Yang Y, Lv W, et al. Paclitaxel combined with capecitabine as first-line chemotherapy for advanced or recurrent gastric cancer. Oncol Lett 2014; 8: 351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Kang HJ, Chang HM, Kim TW, et al. A phase II study of paclitaxel and capecitabine as a first-line combination chemotherapy for advanced gastric cancer. Br J Cancer 2008; 98: 316-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006; 24: 4991-7. [DOI] [PubMed] [Google Scholar]
- 21).Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008; 358: 36-46. [DOI] [PubMed] [Google Scholar]
- 22).Webb A, Cunningham D, Scarffe JH, et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin, and methotrexate in advanced esophagogastric cancer. J Clin Oncol 1997; 15: 261-7. [DOI] [PubMed] [Google Scholar]