Abstract
A 24 year old male presented with a history of recurrent bronchopulmonal infections. Chest computed tomography was performed, revealing a right central mass. In the following bronchoscopy and ultrasound guided needle aspiration of the tumour no specific diagnosis could be obtained. Due to the central location of the tumour thoracotomy and middle lobe resection was performed. Histopathological analysis revealed an intrapulmonary, subpleural located Morbus Castleman of the hyaline-vascular type.
Castleman’s disease is a very rare disorder of the lymphatic tissue that is differentiated into two clinical subtypes. The localized type presents histologically almost always as the hyaline-vascular form. Findings have been reported in mediastinal lymph nodes, the abdomen and peripheral lymphnodes. Intrapulmonary development is very rare and only 9 cases have previously been described in literature.
On the other hand the multicentric type accounts for approximately 10%–15% of cases and histologically usually presents as the plasma cell variant. It is accompanied by fatigue and general weakness and often requires systemic steroid or chemotherapy.
The localized type develops less clinical symptoms and is curable by complete surgical resection.
Keywords: lung cancer, lymph node, hyaline-vascular form, lung resection
Introduction
Castleman’s disease (CD) is a rare disorder of the lymphatic tissue. It was first described in 1956 by Benjamin Castleman and is also called angiofollicular lymph node hyperplasia. There are two main types described: a localized variant, which is more common form with 85%–90% of all CD and a multicentric form. Histologically, two distinct patterns exist, the hyaline vascular and the plasma cell type, of which the former is represented in the vast majority of localized CD.
The localized type can be found in different locations, the most common are mediastinal, pleural, chest wall and even extra thoracic.1) Intrapulmonary findings are very rare and previously only 9 cases have been described in literature.1–6) Usually findings are incidentally as part of the work up in a history of cough or pulmonary infections. We present a case of intrapulmonary CD and its surgical treatment.
Case Presentation
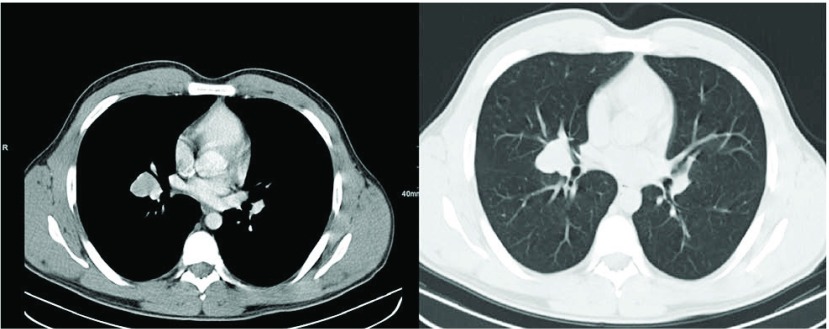
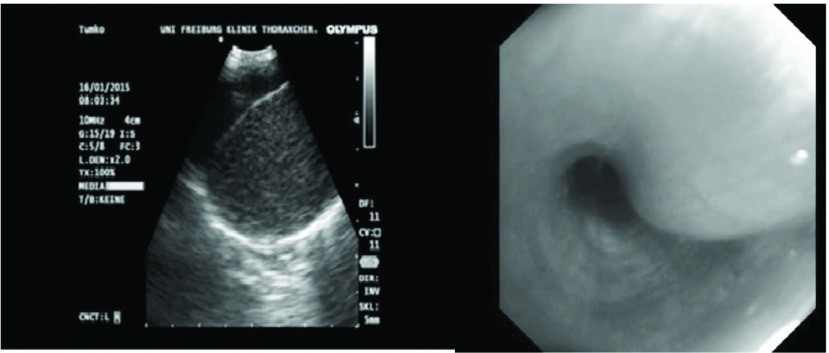
A 24 year old male presented with history of bronchopulmonal infection. After a routine Chest X-ray revealing a central mass, chest computed tomography was performed. A 3 cm × 2.4 cm tumour was seen on the right side in the bifurcation of the middle and lower lobe bronchi (Fig. 1). The physical examination was without pathological findings and routine blood cell count and biochemical markers within normal limits. Initially, to further specify the central mass and obtain tissue for histopathological examination a bronchoscopy and endobronchial ultrasound (EBUS) was performed. An endobronchial swelling at the beginning of the middle lobe bronchus was seen and EBUS showed a tumorous mass with homogenous echo texture (Fig. 2). Ultrasound guided transbronchial needle aspiration of the tumour was performed. Histopathological analysis of the specimen revealed no specific diagnosis. Because of the uncertain dignity and the central location of the tumour, open middle lobe resection was performed.
Fig. 1.
Computed tomography revealing a 3.0 cm × 2.4 cm lesion located in the middle lobe.
Fig. 2.
Endoscopic ultrasound showing the tumour at the middle lobe bronchus, bronchoscopy shows a swelling at the start of the bronchus.
Intraoperatively, a central tumour was seen, in vicinity of the middle lobe bronchus and extending towards segment 6. The tumour was dissected from the middle lobe vein as well as from the lower lobe and middle lobe resection was performed. In frozen section suspicion for lymphoma or bronchopulmonary carcinoid was stated.
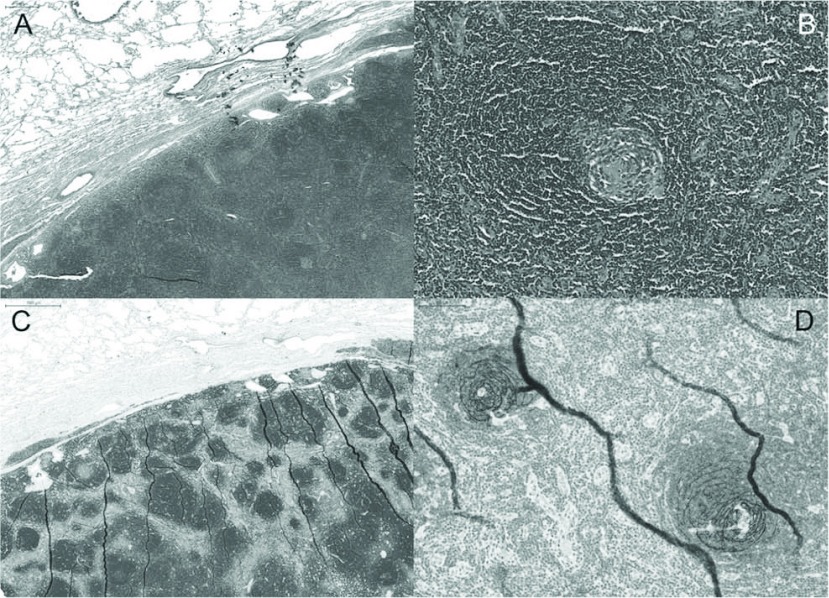
After formalin fixation, thorough grossing and embedding procedures, histopathological examination revealed a nodular lymphocytic tumour with follicular architecture. In the germinal centres prominent dendritic cells were noted with an onion ring like growth pattern. Immunohistochemical stains confirmed the follicular arrangement of CD20 positive B-lymphocytes and the prominent CD21 positive dendritic cells resulting in the diagnosis of localized hyaline vascular CD (Fig. 3).
Fig. 3.
Sharp demarcated lymphatic lesion with follicular architecture (A), H&E, 5× and (C) CD20, 5× in higher magnification “onion ring” formation of the follicular dendritic cells is visible (B) H&E, 20× and (D) CD21, 20× and the morphological hallmark of hyaline vascular Castleman’s disease.
Discussion
CD is a rare disorder of the lymphatic tissue. We present a very rare case of localized intrapulmonary CD in a young patient. The localized type of the disease presents with a slow growing, progressive and painless lymph node enlargement and is situated cervical (42%), mediastinal (31%), abdominal (18%) and retroperitoneal (5%).7) Other authors reported that the majority of CD is detected mediastinal (46%–70%), only 3%–39% intraabdominal and 10%–15% is seen in the periphery.8) These differences are due to different diagnostic algorithms and to the low incidence of the disease. Intrapulmonary manifestation of CD is even less common, as only 6 cases have been reported in the literature.1–6)
CD histologically presenting as the plasma cell type (95%) is associated with severe systemic symptoms such as weakness (81%), fever (71%), weight loss (58%), anorexia and nausea (42%) and signs as splenomegaly (79%), hepatomegaly (63%), oedema and effusion, hypergammaglobulinemia, anaemia and multicentric lymphadenopathy.9)
The localized variant mostly presents as the hyaline-vascular type with typical onion-skin arrangement of the lymphatic tissue. It is very common, that the diagnosis of CD is unclear until the histopathological examination of the resected lymph node. To date there are no diagnostic criteria to obtain the specific diagnosis of CD ahead of surgical resection. In some cases Positron emission tomography–computed tomography (PET CT) or technetium 99m whole body bone scan has been performed ahead of resection10) with no specific standardized uptake value (SUV) to rule out malignancy. In agreement with previous reports, bronchoscopy and ultrasound guided transbronchial needle aspiration could also not provide a definitve diagnosis.
Differential diagnosis of intrapulmonary CD includes all types of tumours that can present as such, like lung carcinoma, carcinoid tumours, hamartochondroma, lymphoma or lymph node metastases. The cause of CD is unknown; it is discussed that the localized type is started by an antigen stimulus of an abnormal plasmacytoid monocyte population in a lymph node.7) The multicentric form is believed to be induced by chronic infection.
The treatment of choice for the localized form of CD is complete surgical resection including systematic lymph node dissection, resulting in cure in all reported cases. To resect intrapulmonary findings of CD a lobectomy was performed by most authors. However, if not centrally located, lung sparing, margin negative resection seems to be an adequate procedure based on the knowledge we have to date on this rare tumour entity. But central location of the tumour and adhesions to adjacent bronchi or vessels often lead to lobectomy. In case of technical inoperability radiotherapy could provide an alternative. De Vries reported of a complete response rate of 43.8% and a partial response of 43.8% reviewing a series of 32 patients with CD. However, in 12.4% there was no response.11) In cases of multicentric CD radiotherapy or steroid treatment is the first choice; chemotherapy is only indicated in steroid-refractory cases.12)
The prognosis of Castleman’s disease differs between the different types. The hyaline-vascular type has a very good prognosis after complete resection, whereas the plasma cell type is at greater risk of progression and deterioration with a median survival of 6–36 months. Infection is a common cause of death.11)
Conclusions
Intrapulmonary localized CD is a differential diagnosis for central tumours of unknown dignity. To date there are no criteria that could provide a definitive diagnosis based on tissue biopsy or radiological findings. Complete surgical excision is the treatment of choice in diagnostic as well as curative considerations. The prognosis of the disease after complete resection is excellent as it allows full recovery and cure in all cases.
Disclosure Statement
The author has no conflicts of interest.
References
- 1).Mohanna S, Sanchez J, Ferrufino JC, et al. Characteristics of Castleman’s disease in Peru. Eur J Intern Med 2006; 17: 170-4. [DOI] [PubMed] [Google Scholar]
- 2).Keller AR, Hochholzer L, Castleman B. Hyaline-vascular and plasma-cell types of giant lymph node hyperplasia of the mediastinum and other locations. Cancer 1972; 29: 670-83. [DOI] [PubMed] [Google Scholar]
- 3).Yeh CM, Chou CM, Wong LC. Castleman’s disease mimicking intrapulmonary malignancy. Ann Thorac Surg 2007; 84: e6-7. [DOI] [PubMed] [Google Scholar]
- 4).Nadir A, Colak N, Koktener A, et al. Isolated intrapulmonary Castleman’s disease: a case report, review of the literature. Ann Thorac Cardiovasc Surg 2014; 20 Suppl: 689-91. [DOI] [PubMed] [Google Scholar]
- 5).Racil H, Cheikh Rouhou S, Ismail O, et al. Castleman’s disease: an intrapulmonary form with intrafissural development. ScientificWorldJournal 2009; 9: 940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Ota H, Kawai H, Matsuo T. Unicentric Castleman’s Disease Arising from an Intrapulmonary Lymph Node. Case Rep Surg 2013; 2013: 289089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Danon AD, Krishnan J, Frizzera G. Morpho-immunophenotypic diversity of Castleman’s disease, hyaline-vascular type: with emphasis on a stroma-rich variant and a new pathogenetic hypothesis. Virchows Arch A Pathol Anat Histopathol 1993; 423: 369-82. [DOI] [PubMed] [Google Scholar]
- 8).Gangopadhyay K, Mahasin ZZ, Kfoury H. Pathologic quiz case 2. Castleman disease (giant lymph node hyperplasia). Arch Otolaryngol Head Neck Surg 1997; 123: 1137-9. [PubMed] [Google Scholar]
- 9).Peterson BA, Frizzera G. Multicentric Castleman’s disease. Semin Oncol 1993; 20: 636-47. [PubMed] [Google Scholar]
- 10).Gunluoglu G, Olcmen A, Sokucu SN, et al. Intrapulmonary-located Castleman’s disease, which was surgically resected without pulmonary resection. Ann Thorac Cardiovasc Surg 2011; 17: 580-3. [DOI] [PubMed] [Google Scholar]
- 11).de Vries IA, van Acht MM, Demeyere T, et al. Neoadjuvant radiotherapy of primary irresectable unicentric Castleman’s disease: a case report and review of the literature. Radiat Oncol 2010; 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Gaba AR, Stein RS, Sweet DL, et al. Multicentric giant lymph node hyperplasia. Am J Clin Pathol 1978; 69: 86-90. [DOI] [PubMed] [Google Scholar]