Summary
The vertebrate posterior body is formed by a combination of the gastrulation movements that shape the head and anterior trunk and posterior specific cell behaviors. Here, we investigated whether genes that regulate cell movements during gastrulation [no tail (ntl)/brachyury, knypek (kny) and pipetail (ppt)/wnt5] interact to regulate posterior body morphogenesis. Both kny;ntl and ppt;ntl double mutant embryos exhibit synergistic trunk and tail shortening by early segmentation. Gene expression analysis in the compound mutants indicates that anteroposterior germ-layer patterning is largely normal and that the tail elongation defects are not due to failure to specify or maintain posterior tissues. Moreover, ntl interacts with ppt and kny to synergistically regulate the posterior expression of the gene encoding bone morphogenetic protein 4 (bmp4) but not of other known T-box genes, fibroblast growth factor genes or caudal genes. Examination of mitotic and apoptotic cells indicates that impaired tail elongation is not simply due to decreased cell proliferation or increased cell death. Cell tracing in ppt;ntl and kny;ntl mutants demonstrates that the ventral derived posterior tailbud progenitors move into the tailbud. However, gastrulation-like convergence and extension movements and cell movements within the posterior tailbud are impaired. Furthermore, subduction movements of cells into the mesendoderm are reduced in kny;ntl and ppt;ntl mutants. We propose that Ntl and the non-canonical Wnt pathway components Ppt and Kny function in parallel, partially redundant pathways to regulate posterior body development. Our work initiates the genetic dissection of posterior body morphogenesis and links genes to specific tail-forming movements. Moreover, we provide genetic evidence for the notion that tail development entails a continuation of mechanisms regulating gastrulation together with mechanisms unique to the posterior body.
Keywords: Convergence, Extension, Gastrulation, knypek, silberblick (wnt11), pipetail (wnt5), subduction
Introduction
The rostral vertebrate body, including the head and anterior trunk, is generated by a set of gastrulation movements largely conserved among vertebrates (Schoenwolf and Smith, 2000). The germ layers form through the internalization of prospective mesendodermal cells beneath the ectoderm, and epiboly enlarges their surface. Convergence and extension movements narrow the germ layers from belly to back and lengthen them rostrocaudally. Formation of the posterior body uses the mechanisms that shape the anterior body during gastrulation as well as posterior-specific behaviors (Griffith et al., 1992; Kanki and Ho, 1997; Schoenwolf, 1984). In zebrafish, chick and mouse embryos, a cell aggregate (the tailbud, composed of dorsal and ventral derived cells) contributes to the tail and posterior trunk tissues (Hamburger and Hamilton, 1992; Hogan et al., 1994; Kanki and Ho, 1997; Tam, 1984; Tam, 1986). The zebrafish tailbud forms at late gastrulation; cells originating from dorsal and ventral gastrula regions arrive at the vegetal pole and close over the ventral yolk plug (Fig. 1) (Kimmel et al., 1995; Westerfield, 1995). Within the tailbud, the cells are spatially restricted according to their gastrula origin, such that the ventrally originating cells constitute the posterior half, whereas the dorsal derived cells occupy the anterior tailbud (Fig. 1) (Kanki and Ho, 1997). Cell fates and gene expression patterns are restricted within the tailbud of chick, frog, and zebrafish embryos, indicating that tail formation is a conserved process that is not simply mediated by the addition of cells to the posterior end (Beck and Slack, 1998; Catala et al., 1995; Gaertner, 1949; Gont et al., 1993; Hammerschmidt and Nüsslein-Volhard, 1993; Joly et al., 1992; Joly et al., 1993b; Kanki and Ho, 1997; Schoenwolf, 1977; Schulte-Merker et al., 1992; Talbot et al., 1995; Tucker, 1995). However, elevated cell proliferation in dorsal medial regions might contribute to tail extension in zebrafish (Kanki and Ho, 1997).
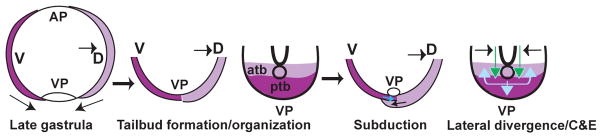
Fig. 1.
Gastrulation movements position posterior body progenitors in the tailbud region. As gastrulation ends ventral (dark purple) and dorsal (light purple) derived marginal cell layers close over the yolk to form the tailbud. The ventral derived cells contribute to the posterior tailbud and are spatially separated from the dorsal derived anterior bud cells with Kupffer’s vesicle as a morphological boundary (open circle). As tail-specific movements begin, the posterior bud cells subduct beneath the anterior bud cells. Gastrulation-like convergence and axial extension contribute to tailbud elongation; anterior tailbud cells (light green arrows) advance posteriorly and posterior tailbud cells (light blue arrows) move within the posterior flow and then laterally, avoiding the midline and anterior bud cells.
Tracing cell movements within the zebrafish tailbud revealed a mechanism for posterior body elongation that combines gastrulation (convergence and extension) and novel tailbud-specific movements (subduction and laterad divergence) (Fig. 1) (Kanki and Ho, 1997). Within the extending tail, dorsal convergence of paraxial cells and anteroposterior (AP) tissue extension as observed during gastrulation continues until the tail everts (Kanki and Ho, 1997). Concurrently, posterior tailbud cells move anteriorly and laterally (laterad divergence) to avoid the midline (Kanki and Ho, 1997). Posterior tailbud cells undergo subduction, movement underneath the anterior tailbud cells, at the boundary between the ventral-derived posterior and dorsal-derived anterior tailbud. This type of movement is not observed in the gastrula, where the ventral and dorsal blastopore lips do not meet (Kanki and Ho, 1997).
Several genes required for tail formation have been identified and recent studies suggest that a combination of high levels of Bmp, canonical Wnt and Nodal signaling activity in the ventral region of the zebrafish gastrula specify a tail-organizer region that can induce ectopic tails upon transplantation (Agathon et al., 2003). Little, however, is known about how the diverse cell movements underlying posterior body morphogenesis are regulated. Fibroblast growth factor (Fgf) signaling is important for normal gastrulation and trunk and tail formation, upstream of Tbx genes (which encode T-box transcription factors) (Amaya et al., 1991; Amaya et al., 1993; Griffin et al., 1995; Griffin et al., 1998; Isaacs et al., 1994; Isaacs et al., 1992; Schulte-Merker and Smith, 1995). Heterozygous brachyury mutant mice and mutants in its zebrafish homologue, no tail (ntl), exhibit tail truncations (Chesley, 1935; Halpern et al., 1993). Although homozygous mutant mice have more severe caudal mesoderm and gastrulation defects than zebrafish ntl mutants, this is probably due to compensation by partially redundant Tbx genes that act downstream of Fgf signaling during gastrulation and trunk development in zebrafish (Chesley, 1935; Griffin et al., 1995; Griffin et al., 1998; Halpern et al., 1993; Wilson et al., 1995). Fgf and Tbx are proposed to regulate cell adhesion during gastrulation, but it is not clear which/whether tail-specific cell movements require their function (Griffin et al., 1995; Ho and Kane, 1990; Isaacs et al., 1992; Wilson et al., 1995; Yamamoto et al., 1998).
Zebrafish Nodal-related signaling is essential for endoderm and dorsolateral mesoderm induction. Yet elimination of Nodal signaling in cyclops;squint compound mutants and in maternal-zygotic mutants for the Nodal cofactor one-eyed pinhead (MZoep), permits relatively normal posterior mesoderm formation and has relatively little effect on tail extension (Feldman et al., 1998; Griffin and Kimelman, 2002; Schier and Shen, 2000). The role of Nodal signaling in posterior body development has been revealed by genetic interactions; oep cooperates with the Tbx genes ntl and spadetail (spt) to regulate posterior tissue specification (Griffin and Kimelman, 2002; Schier et al., 1997). Although Spt has also been implicated in modulating cell adhesion and motility as a positive regulator of paraxial protocadherin expression (Yamamoto et al., 1998), its interaction with Zoep affects presomitic mesoderm progenitor differentiation rather than motility (Griffin and Kimelman, 2002). These studies suggest that several pathways coordinate tissue specification and morphogenesis within the posterior body.
Mutational analyses in zebrafish identified many genes regulating cell movements and behaviors required for normal convergence and extension during gastrulation (Hammerschmidt et al., 1996; Solnica-Krezel et al., 1996), including those that encode components of a non-canonical Wnt signaling pathway (Heisenberg et al., 2000; Kilian et al., 2003; Topczewski et al., 2001). Altered activity of non-canonical Wnt ligand genes silberblick (slb)/wnt11 and pipetail (ppt)/wnt5, results in morphogenesis defects without affecting cell fates (Heisenberg et al., 2000; Makita et al., 1998; Rauch et al., 1997). Similarly, inactivation of the putative Wnt signaling modulator glypican Knypek (Kny) impairs mediolateral (ML) cell elongation underlying convergence and extension movements (Topczewski et al., 2001). Zebrafish ppt and kny but not slb mutant embryos exhibit shorter tails, suggesting a role for these genes in tail morphogenesis (Hammerschmidt et al., 1996; Heisenberg et al., 2000; Rauch et al., 1997; Solnica-Krezel et al., 1996; Topczewski et al., 2001).
To investigate whether non-canonical Wnt signaling regulates cell movements within the developing tail and to identify genes that it might cooperate with during this process, we generated double mutants for loss of ntl and non-canonical Wnt signaling components, slb, ppt and kny. Here, we show that ppt;ntl and kny;ntl compound mutants exhibit synergistic posterior body shortening. These defects are not due to impaired posterior mesoderm specification and patterning, nor decreased proliferation or excess apoptosis. Cell tracing during tail development reveals that both the gastrulation-like movements and tailbud unique movements are impaired in double mutants. Specifically, ntl interacts genetically with both ppt and kny to regulate convergence and extension movements within the posterior tailbud, and to promote normal subduction movements. We demonstrate that these genes co-operate to regulate specific cell movements during posterior body morphogenesis through a mechanism parallel to Fgf signaling, cad1 or known Tbx genes.
Materials and methods
Zebrafish maintenance, embryo generation, staging and genotyping
The pptta98+/−, knym119+/− and ntlb195+/− and double mutant zebrafish strains were maintained as described previously (Solnica-Krezel et al., 1994). Embryos were obtained from natural spawnings and morphologically staged as described previously (Kimmel et al., 1995). Embryos were genotyped using linked z markers for ntlb195, by PCR amplification followed by restriction digest for pptta98 (primers: forward, GATTACTGCCTGCGCAATGAAAC; reverse, GGTCTTG-AACTGGTCGTACACGCGG; followed by SacII digestion, giving 29 bp and 100 bp products for the mutant allele), slbtz216 (primers: forward, GTGAGGCAGCGTTTGTGGTT; reverse, CGTAGTAGCG-AAGGTTATCTCCACATT; followed by MseI digestion, giving 27 bp and 141 bp products for the mutant allele) and knym119 (primers: forward, TCCATGTTAGGTCTCGCTGA; reverse, CCTCAGGGCT-GTAGGGTCTA; followed by EcoRV digestion, giving a 310 bp fragment for the wild type and 230 and 80 bp fragments for the mutant allele).
In situ hybridization
Whole mount in situ hybridization was performed according to Thisse and Thisse (Thisse and Thisse, 1998). Embryos were photographed with a Zeiss Axiophot using either Axiocam digital camera or a 35 mm camera.
Cell labeling/movement analysis
Photoactivation of caged fluorescein in cells at the ventral blastoderm margin was performed as described in Myers et al. (Myers et al., 2002a), except that embryos were fixed at bud and six-somite stages. For tailbud uncaging, embryos were loaded with caged dye as described by Sepich et al. (Sepich et al., 2000) and then posterior tailbud cells were uncaged at the bud to two-somite stages and fixed at 20 somites. For cell transplantations, donor embryos were labeled with Rhodamine dextran (Molecular Probes) and transplanted to unlabeled hosts at blastula stages as described by Westerfield (Westerfield, 1996).
Cell proliferation
Embryos were fixed and stained as described previously (Topczewski et al., 2001) except that, in situ hybridization with digoxigenin-labeled paraxial protocadherin (papc), detection with fast red preceded immunohistochemistry. The primary antibody was a monoclonal mouse anti-phosphohistone (Sigma) and the secondary antibody was a CY2 anti-mouse IgG (Jackson Immuno). Images were acquired using the Zeiss LSM 510 laser scanning inverted microscope.
Results
ntl interacts with kny and ppt during tail and trunk formation
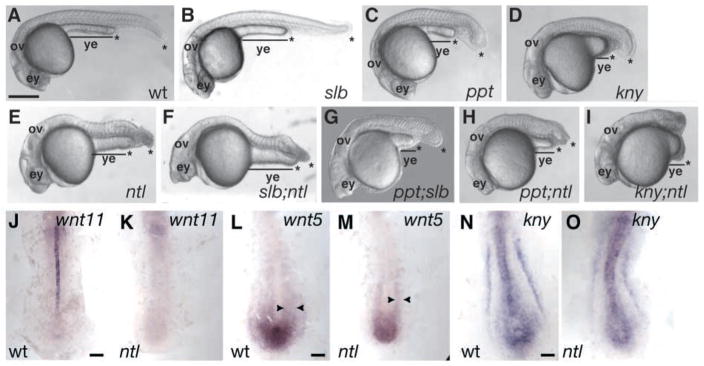
In mouse and frog, interference with Brachyury function impairs gastrulation and results in posterior truncations (Beddington et al., 1992; Chesley, 1935; Conlon and Smith, 1999; Wilson et al., 1995). By contrast, zebrafish null ntl mutants lack notochord and posterior tail tissues but exhibit a milder gastrulation defect characterized by impaired convergence but normal extension (Amacher et al., 2002; Glickman et al., 2003; Griffin et al., 1995; Griffin et al., 1998; Halpern et al., 1993). Therefore, other genes might compensate for loss of ntl in zebrafish gastrula. Although zebrafish embryos harboring mutations in non-canonical Wnt signaling components also exhibit shortened AP axes by early segmentation stages, they form the normal number of somites (Rauch et al., 1997; Solnica-Krezel et al., 1996). To investigate whether ntl interacts with non-canonical Wnt signaling we constructed ppt;ntl, kny;ntl and slb;ntl double mutants (Fig. 2). At 26 hours post-fertilization (hpf), compared to the individual mutants (Fig. 2A–E), the slb;ntl (Fig. 2F) mutants exhibited only the combined deficiencies expected for loss of ntl (Fig. 2E) and slb (Fig. 2B) function: they lacked differentiated notochord and displayed tail truncations and variable synopthalmia and cyclopia (Halpern et al., 1993; Heisenberg and Nusslein-Volhard, 1997). By contrast, ppt;ntl (Fig. 2H) and kny;ntl (Fig. 2I) double mutants showed a synergistic phenotype, characterized by a dramatically shortened axis and yolk extension, such that only head and anterior trunk are clearly distinguishable. The additive slb;ntl phenotype was not surprising, because posterior wnt11 expression has previously been shown to require ntl (Fig. 2J,K) (Makita et al., 1998). By contrast, compared to the wild type (Fig. 2L), tailbud and lateral aspects of wnt5 expression were only slightly reduced in ntl mutants (Fig. 2M), indicating that posterior wnt5 expression does not absolutely require ntl. Similarly, compared with the wild type (Fig. 2N), kny tailbud expression was mildly reduced in ntl (Fig. 2O) mutants, indicating that it does not entirely depend on ntl function. The persistence of ppt and kny expression in ntl mutants is consistent with these genes functioning in parallel pathways during posterior morphogenesis.
Fig. 2.
The ntl gene interacts with kny and ppt, but not slb. At 26 hpf, the tail extends beyond the yolk extension (ye) in wild-type embryos (A). Embryos with the slb mutation (B) have normal tails, whereas ppt (C), kny (D) and ntl (E) embryos have shorter tails (*). Embryos with the slb;ntl double mutation (F) have cyclopic eyes (ey) and tail defects like individual mutants, and slb;ppt (G) double mutants are shorter than individual mutants. The tail does not extend beyond the yolk tube in ppt;ntl (H) and kny;ntl (I) double-mutant embryos. At the ten-somite stage, slb/wnt11 is expressed in the notochord of wild-type (J) embryos but is absent in ntl mutants (K). Posterior ppt/wnt5 expression is mediolaterally broader in wild-type (L) but persists in ntl (M) embryos, and kny expression is comparable between the wild type (N) and ntl mutants (O). (A–I) Lateral views. (J–O) Dorsal posterior views. Scale bar=50 μm (A–I) and 100 μm (J–O).
Given its abundant expression in posterior tissues, ppt(wnt5) could compensate for slb function in posterior body extension. Indeed, recent studies of ppt(wnt5);slb(wnt11) double mutants revealed that the two genes display some functional redundancy in the anterior mesendoderm movements (Kilian et al., 2003). Morphological analysis of ppt(wnt5);slb(wnt11) double mutants at 1 day post-fertilization (dpf) revealed more pronounced shortening of the posterior body in the double mutants (Fig. 2G), compared with single mutants (Fig. 2B,C) (Westfall et al., 2003). Therefore, whereas slb(wnt11) largely compensates for ppt(wnt5) function in more anterior parts of the gastrula (Kilian et al., 2003), the reverse is true in the posterior region, where ppt(wnt5) activity suffices for normal posterior body extension in the absence of slb(wnt11).
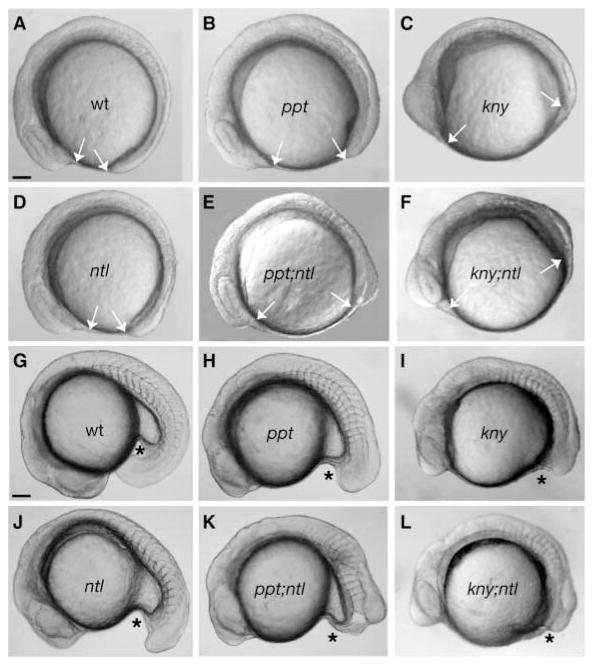
By the six-somite stage, wild-type (Fig. 3A), ppt (Fig. 3B), kny (Fig. 3C) and ntl (Fig. 3D) embryos were morphologically distinct from the shorter ppt;ntl (Fig. 3E) and kny;ntl (Fig. 3F) double mutants. At this stage, the differences between ppt;ntl (Fig. 3E) embryos were greater than those between kny;ntl (Fig. 3F) and the individual mutants. At the 16-somite stage, wild-type (Fig. 3G), ppt (Fig. 3H) and kny (Fig. 3I) somites were separated by the midline chordamesoderm tissue. Although anterior somites were normal in ntl (Fig. 3J), ppt;ntl (Fig. 3K) and kny;ntl (Fig. 3L), the caudal somites were medially fused as previously reported for ntl (Halpern et al., 1993). In the wild type (Fig. 3G) and ppt (Fig. 3H) and ntl (Fig. 3J) individual mutants, caudal tissues extended beyond the posterior limit of the yolk tube extension, whereas posterior tissue ended abruptly in ppt;ntl embryos (Fig. 3K). Although the yolk tube had not extended in both kny (Fig. 3I) and kny;ntl (Fig. 3L) mutant embryos, the double mutants exhibited a shorter axis.
Fig. 3.
The ppt;ntl and kny;ntl double mutants exhibit synergistic tail elongation defects. At the eight-somite stage, the AP axis of the wild-type embryo (A) is elongated such that the head and tail almost touch. This distance is greater (arrows) in ppt (B), and kny (C) mutants exhibiting shortened AP axes. In ntl (D) mutants, the AP axis is only slightly shorter at this stage, whereas ppt;ntl (E) and kny;ntl (F) double mutants are shorter than the individual mutants. At 18 somites, the tail extends beyond the yolk tube (*) in wild-type (G) embryos and is shorter in ppt (H), kny (I) and ntl (J) mutants. At this stage, the tail has not extended in ppt;ntl (K) and kny;ntl (L) double mutants. (A–L) Lateral views. Scale bars=100 μm.
Double mutant phenotypes are not due to loss or failure to specify posterior tissues
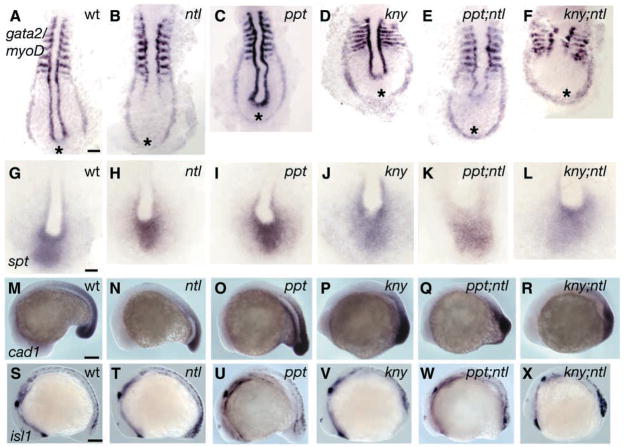
To determine whether ppt;ntl and kny;ntl mutants were shorter than single mutants owing to failure to specify or maintain posterior cell fates, we examined tissue specific marker expression. At the six-somite stage, gata2 (gta2) is expressed in bilateral stripes marking blood precursors (Dorfman et al., 1992). In wild-type (Fig. 4A), ppt (Fig. 4C) and kny (Fig. 4D) embryos, the stripes were separated in the posterior tailbud region. By contrast, the gta2 stripes were less well separated in ntl (Fig. 4B) and were fused caudally to form a horseshoe-shaped domain in ppt;ntl (Fig. 4E) and kny;ntl (Fig. 4F) embryos. The expression of pax2.1 in the optic stalks, otic vesicles and midbrain-hindbrain boundary (MHB) (Krauss et al., 1991) was normal in all individual and double mutant embryos. However, pronephric duct expression extended more caudomedially in ntl, ppt;ntl and kny;ntl double mutants, and, like gta1 and gta2 expression, it surrounded the tailbud (data not shown). Double mutant embryos lacked myoD expression in posterior adaxial cells and displayed broader somites (Fig. 4A–F) (Hammerschmidt et al., 1996; Solnica-Krezel et al., 1996; Topczewski et al., 2001; Weinberg et al., 1996).
Fig. 4.
Posterior tissues are specified in ppt;ntl and kny;ntl mutants. At nine somites, myoD is expressed in the somites and adaxial cells, and gta2 is expressed in two bilateral stripes of prospective blood in wild-type (A) embryos. In ntl mutants (B), adaxial cell expression of myoD is lost and the stripes of gta2 are less separated posteriorly. In ppt mutants (C), adaxial cell expression of myoD is kinked and gta2 is similar to the wild type. In kny mutants (D), somites are mediolaterally broader and the AP lengths of adaxial cells and the gta2 domain are shorter. The ppt;ntl (E) and kny;ntl (F) mutants exhibit myoD expression patterns as expected for the combined individual mutants, and gta2 stripes are fused posteriorly. (G–L) Expression of spt in the paraxial mesoderm of wild-type (G), ntl (H), ppt (I), kny (J), ppt;ntl (K) and kny;ntl (L) embryos. (M–R) Expression of Caudal in the tailbud of wild-type (M), ntl (N), ppt (O), kny (P), ppt;ntl (Q) and kny;ntl (R) embryos. (S–X) Expression of isl1 in developing neurons at the 16 somite stage. Ectopic isl1 expression in observed in ntl (T), ppt;ntl (W) and kny;ntl (X) mutants. (A–L) Dorsal posterior flat mounts. (M–X) Lateral views. Scale bars=100 μm (A–F,M–X), 50 μm (G–L).
Functional redundancy between ntl and spt Tbx genes during trunk and tail mesoderm formation has already been demonstrated (Amacher et al., 2002; Griffin et al., 1998). Therefore, we examined spt expression in double mutant clutches (Fig. 4G–L). Compared with the wild-type (Fig. 4G), ppt (Fig. 4I) and kny (Fig. 4J) embryos exhibited broader spt expression domains, consistent with their respective convergence and extension defects. Expression of spt was weaker in the double mutants (Fig. 4K,L) than in ntl (Fig. 4H) single mutants, suggesting that reduced spt might contribute to their posterior shortening. However, because ppt;ntl and kny;ntl mutants do not phenocopy spt;ntl double mutants and spt expression is not abolished in ppt;ntl and kny;ntl, the phenotypic differences between these double mutant classes probably reflect distinct underlying mechanisms.
Mutation of the zebrafish caudal (cad1) homeobox gene results in a shortened trunk and loss of the tail (Golling et al., 2002), prompting us to investigate whether Tbx and Wnt proteins interact to regulate cad1 expression. Zebrafish cad1 was expressed in the neuroectoderm and mesoderm of the caudal mass in wild-type (Fig. 4M), ppt (Fig. 4O) and kny (Fig. 4P) embryos during segmentation stages (Joly et al., 1992). Expression of cad1 was reduced in the mesoderm of ntl (Fig. 4N) embryos and was similarly reduced in the caudal mass of ppt;ntl (Fig. 4Q) and kny;ntl (Fig. 4R) mutants. Therefore, Ntl and non-canonical Wnt components interact to mediate posterior development via a mechanism that does not involve regulation of cad1 expression.
Murine T and zebrafish ntl mutants exhibit excess neuronal markers in the posterior body (Nguyen et al., 2000). We detected an increase relative to the wild type of isl1 neural marker expression in ppt;ntl and kny;ntl mutants that was comparable to that observed in single ntl mutants (Fig. 4S–X). We concluded that ntl and non-canonical Wnt components do not co-operate to limit neural cell fates in the posterior body. Together, these expression data indicate that gross AP patterning of the embryo is intact in the double mutants. Furthermore, the persistence of posterior mesodermal and neuroectodermal markers indicates that defective specification or maintenance of posterior tissues does not underlie the compound mutant phenotypes.
Tailbud expression of bmp4 but not Fgf genes requires both Ntl and non-canonical Wnt signaling
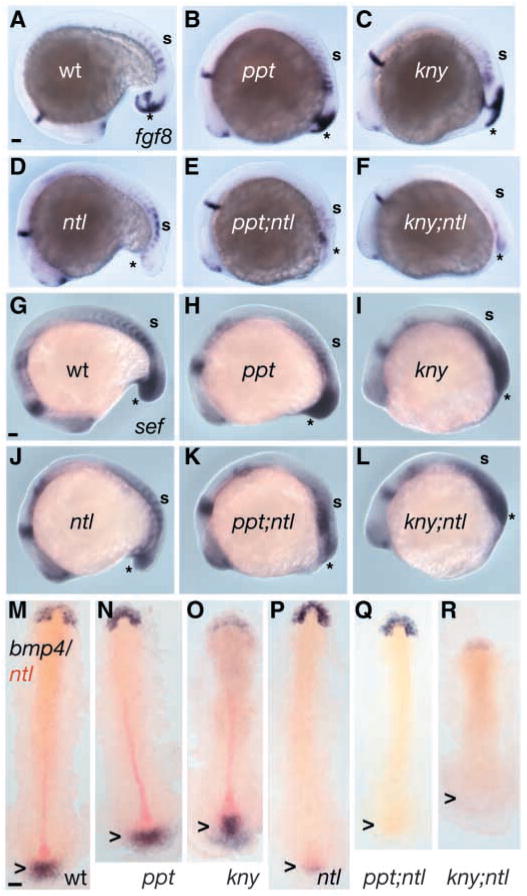
Fgf signaling is important for mesoderm induction and patterning, gastrulation movements, and maintenance of Tbx gene expression through a mutually dependent feedback mechanism (Gerhart, 1989; Griffin et al., 1995; Isaacs et al., 1992; Kimelman and Kirschner, 1987; Slack et al., 1987; Smith et al., 1991). Furthermore, dominant negative Fgf receptor overexpression results in more severe defects than observed for ntl mutants, including loss of trunk and tail in Xenopus (Amaya et al., 1991) and zebrafish (Griffin et al., 1995). However, inactivation of the zebrafish acerebellar (ace)/fgf8 gene does not produce posterior body deficiencies, possibly owing to overlapping and redundant Fgf genes (Reifers et al., 1998). Thus, the ppt;ntl and kny;ntl posterior defects might be a consequence of further impairment of Fgf activity owing to synergistic regulation by Ntl and non-canonical Wnts. To test this possibility, we analysed the expression of fgf8, fgf3 and the Fgf-induced feedback antagonist sef (Furthauer et al., 2002; Kiefer et al., 1996; Tsang et al., 2002) during gastrulation and somitogenesis (Fig. 5A–L, and data not shown). At the 18-somite stage, fgf8 was expressed in the forebrain, MHB, somites and tailbud (Reifers et al., 1998) of wild-type (Fig. 5A), ppt (Fig. 5B) and kny (Fig. 5C) embryos. Despite normal fgf8 expression in all rostral tissues and the trunk somites, its tailbud expression was reduced to a similar degree in ntl (Fig. 5D), ppt;ntl (Fig. 5E) and kny;ntl (Fig. 5F) mutants. As for fgf8, expression of fgf3 was similarly reduced in ntl and double mutants (data not shown). At the 16-somite stage, sef transcripts were detected in the forebrain, MHB, somites and ectoderm and mesoderm of the caudal mass of wild-type (Fig. 5G), ppt (Fig. 5H) and kny (Fig. 5I) embryos. In ntl (Fig. 5J), ppt;ntl (Fig. 5K) and kny;ntl (Fig. 5L) mutants sef expression was normal in rostral tissues and somites, but was equally reduced in the caudal mass, particularly in the ectoderm. Together, these data suggest that ntl and non-canonical Wnt components do not interact to regulate Fgf activity, so reduced Fgf signaling is an unlikely cause for the posterior defects of double-mutant embryos.
Fig. 5.
Ntl and non-canonical Wnts synergistically regulate the expression of bmp4, but not Fgf genes. At 16 somites, fgf8 is expressed in the brain and somites, and posteriorly in ectoderm and mesoderm in the wild type (A) and in the ppt (B) and kny (C) mutants. Despite normal anterior fgf8 expression in ntl mutants (D), tail expression is extremely reduced but is not further reduced in ppt;ntl (E) or kny;ntl (F) double mutants. At 16 somites, sef expression in the brain, somites and tail is comparable in wild-type (G), ppt (H) and kny (I) embryos, but is reduced in ntl (J) mutants. Expression of sef is not further reduced in ppt;ntl (K) and kny;ntl (L) mutants. At five somites, bmp4 is expressed in the prechordal plate and tailbud of wild-type (M), ppt (N) and kny (O) embryos. In ntl mutants (P), tailbud expression of bmp4 is severely reduced and is absent in ppt;ntl (Q) and kny;ntl (R) double mutants. (A–L) Lateral views. (M–R) Dorsal flat mounts; arrowheads indicate tailbud. Scale bars=100 μm.
Bmp4 signaling was proposed to act upstream of Notch to promote outgrowth and patterning of the developing tailbud in Xenopus embryos (Beck et al., 2001). In zebrafish and mice, null mutations in Bmp pathway components result in ventral tail tissue deficiencies and patterning defects (Connors et al., 1999; Kishimoto et al., 1997; Winnier et al., 1995; Yang et al., 1999) and are associated with decreased tail progenitor migration into the tailbud in the zebrafish (Myers et al., 2002a). Hence, we examined bmp4 expression at gastrula and tailbud stages. During early gastrulation, individual and double mutants could not be distinguished from wild-type gastrulae (data not shown). By contrast, at the five-somite stage, bmp4 was expressed in the anterior prechordal plate of wild-type, single- and double-mutant embryos (Fig. 5M–R), whereas tailbud expression was severely reduced in ntl (Fig. 5P) embryos and was absent in ppt;ntl (Fig. 5Q) and kny;ntl (Fig. 5R) mutants. These data indicate that ntl and non-canonical Wnt components act together to promote bmp4 expression in the developing tailbud, which might contribute to the synergistic double mutant phenotype.
Cell proliferation and death cannot account for the tail extension defects in kny;ntl and ppt;ntl double mutants
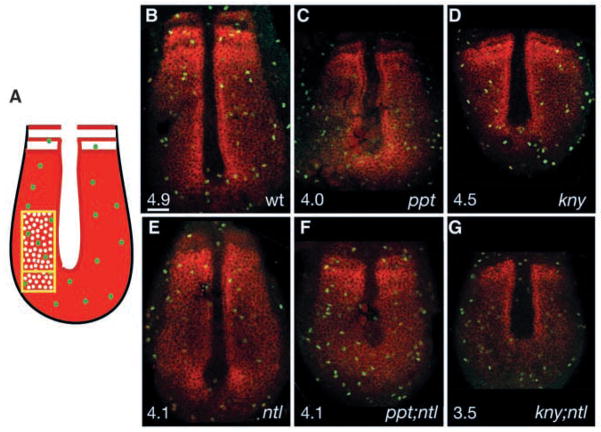
During vertebrate development, Bmp signaling regulates both cell proliferation and cell death (Ashique et al., 2002; Hogan, 1996a; Hogan, 1996b). To investigate whether loss of bmp4 expression in the developing tail was associated with decreased cell proliferation or enhanced cell death in compound mutant embryos, we analysed cell proliferation using an anti-phosphohistone antibody (pH3) that recognizes M-phase cells (Ajiro et al., 1996; Chadee et al., 1995; Mahadevan et al., 1991). Given the altered double mutant morphology, we used paraxial protocadherin (papc) expression in presomitic mesoderm (PSM) (Yamamoto et al., 1998) as a landmark to ensure that equivalent cell populations were evaluated. We determined the incidence of pH3-positive cells within the paraxial mesoderm adjacent to the notochord, previously shown to exhibit the highest mitotic indices during zebrafish tailbud development (Fig. 6A) (Kanki and Ho, 1997). At the five-somite stage, the papc expression domain included the forming somites, adaxial cells and undifferentiated paraxial mesoderm in wild-type (Fig. 6B), ppt (Fig. 6C) and kny (Fig. 6D) embryos, but was broader mediolaterally and shortened anteroposteriorly in ppt and kny, as expected for their convergence and extension defects (Topczewski et al., 2001; Rauch et al., 1997). In ntl mutants, papc expression was weaker in paraxial mesoderm and absent in adaxial cells (Fig. 6E) (Odenthal et al., 1996). In ppt;ntl (Fig. 6F) and kny;ntl (Fig. 6G) embryos, papc expression was altered as predicted for the combined individual mutant phenotypes. The mitotic indices (MIs) for wild-type (MI=4.9; n=13 embryos; 5568 cells), ppt (MI=4.0; n=6 embryos; 2875 cells; p >0.3), ppt;ntl (MI=4.1; n=4 embryos; 1706 cells; P>0.1), ntl (MI=4.1; n=5 embryos; 2366 cells; P>0.1), kny (MI=4.5; n=7 embryos; 2737 cells; P>0.5) and kny;ntl (MI=3.5; n=5 embryos; 2262 cells; P>0.1) embryos were not significantly different. Furthermore; the MI for double mutants did not statistically differ from the individual mutants (ppt vs ppt;ntl, P>0.8; kny vs kny;ntl, P>0.2; ntl vs ppt;ntl, P>0.9; ntl vs kny;ntl, P>0.3); therefore, reduced cell proliferation in the paraxial mesoderm cannot account for the severe tail extension defects in compound mutants.
Fig. 6.
Abnormal cell proliferation cannot account for the tail elongation defects in double mutants. Schematic representation of the dorsal medial paraxial region, where the ratio of phosphorylated-histone-positive cells (green) to papc-expressing cells (red) was determined (A). Confocal images of dorsal posterior section of the wild type (B), ppt (C), kny (D) and ntl (E) mutants, and ppt;ntl (F) and kny;ntl (G) double mutants at five somites, phosphorylated-histone-positive cells (green) and papc-expressing cells (red). (B–G) Scale bar=50 μm.
Next, we tested whether increased cell death might underlie the double-mutant phenotypes. TUNEL staining, performed at the 5-, 13-, 16- and 22-somite stages, revealed apoptotic cells along the entire rostro-caudal axis with more cell death in the head than the tail of all embryos, consistent with previous reports (Cole and Ross, 2001) (data not shown). Compared with the wild type and individual mutants, ppt;ntl and kny;ntl embryos did not exhibit increased cell death in the tail region during early tail formation (data not shown). By contrast, increased cell death was observed in ntl and double mutants at the 22-somite stage (data not shown), when apoptosis of the tail was morphologically apparent in ntl and double mutants. However, this was well after the double-mutant tail extension defects were morphologically manifested (Fig. 3). Therefore, increased cell death probably does not underlie the early tail extension defects, although it contributes to later truncation of the tail.
Impaired movements of posterior tailbud cells underlie defective tail extension
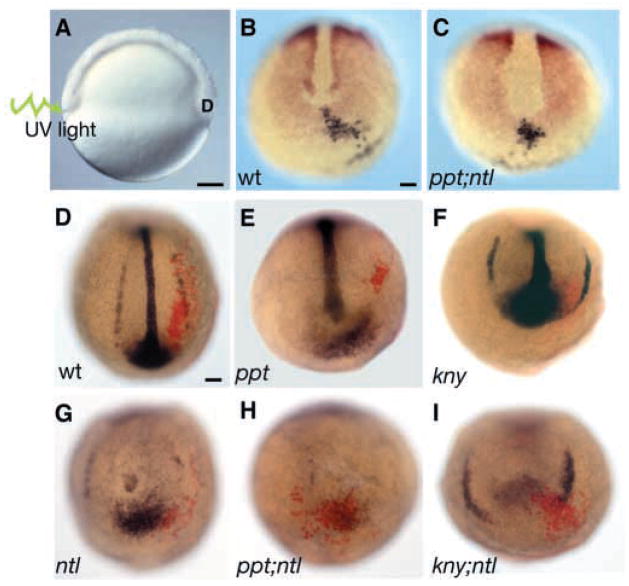
Tailbud formation is initiated when blastoderm margin cells arrive at the ventral yolk plug forming the ‘core’ of cells that will mostly contribute to the posterior body (Kimmel et al., 1995; Westerfield, 1995). Fate mapping studies in the zebrafish have shown that posterior tailbud cells are ventral derived (Kanki and Ho, 1997; Kimmel and Law, 1985; Myers et al., 2002a; Warga and Nüsslein-Volhard, 1998). One possible explanation for the posterior defects in the double mutants is failure of progenitor cells to reach the posterior bud. To investigate whether double mutants enter tail extension stage with a deficit in contributing cells, we labeled cells in the ventral blastoderm margin during early gastrulation and monitored their position at bud stage and before the tailbud extension stage (Fig. 7A) (Myers et al., 2002a). Because gene expression analysis revealed gta1-positive cells in posterior medial regions of the compound mutants’ tailbuds (Fig. 4E,F), we also asked whether these ectopic cells arose because of impaired cell movements or altered fate. In the wild type and in all mutants, the labeled cell groups moved from the ventral blastoderm margin into the posterior tailbud region (Fig. 7B,C). Subsequently, during early segmentation, they became displaced laterally and extended anteroposteriorly (Fig. 7D; n=49). By the six-somite stage, the labeled cell groups formed an elongated array positioned laterally, overlapping with gta1 expression. In ppt (Fig. 7E) (n=4) and kny (Fig. 7F) (n=16), the labeled cells overlapped with lateral gta1 expression, but their (and gta1 cells’) convergence and extension were reduced compared with the wild type (Fig. 7D) and the ntl mutant (Fig. 7G) (n=9). Notably, in ppt;ntl (Fig. 7H) (n=6) and kny;ntl (Fig. 7I) (n=3), the labeled cells remained in the posterior bud, overlapping the ectopic gta1 expression domain. These data indicate that the movements that bring the posterior body precursors to the tailbud region are normal in the double mutants. However, the movements that shift cells from the posterior tailbud into paraxial positions and extend the posterior body are impaired in double mutants.
Fig. 7.
Cell movements are impaired in double mutants before the elongation stage of tail morphogenesis. Prospective posterior tailbud cells were labeled 180° from the shield by uncaging with ultraviolet light (A). Labeled cells (black) move to the tailbud region marked by papc expression (red) in wild-type (B) and in single and double mutant embryos, including ppt;ntl (B), at bud stage. At five somites, the position of the labeled cells (red) relative to the ventral derived gta1-expressing cells (blue) was determined. In wild-type embryos (D), labeled cells form elongated arrays that overlap with gta1-expressing cells. In ppt (E), kny (F) and ntl (G) embryos, the labeled cells form shorter arrays that overlap with gta1 expression. By contrast, labeled cells in ppt;ntl (H) and kny;ntl (I) mutants do not form elongated arrays and remain in the posterior bud. (A) Lateral view. (B–I) Dorsal posterior views. Scale bars=100 μm.
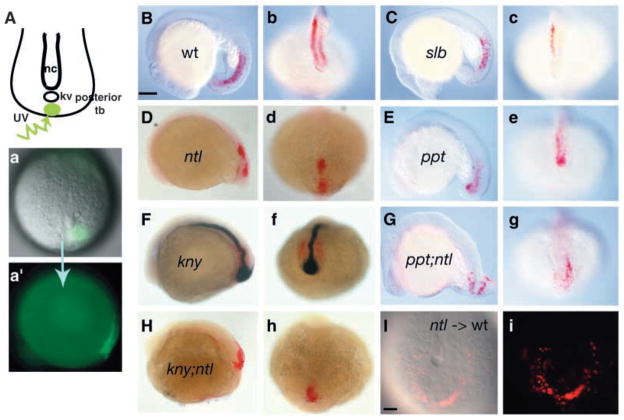
As tail extension continues, convergence and extension movements similar to those observed in the gastrula contribute to posterior body elongation along with movements that are unique to the developing tail (Kanki and Ho, 1997). Specifically, posterior tailbud cells move beneath the advancing anterior tailbud cells (subduction) and anteriorly but laterally around them (extension and laterad divergence, respectively) (Kanki and Ho, 1997). To examine these movements directly in ppt;ntl and kny;ntl mutants, we labeled uniformly sized small cell groups within the posterior tailbud before the tail extension phase as described previously (Topczewski et al., 2001) and assessed their position and extension at the 18-somite stage (Fig. 8A). In wild-type embryos (Fig. 8a,a′; n=32) the labeled cells were positioned beneath the ectoderm (Fig. 8B) and lateral to the midline (Fig. 8b) as expected for cells that have undergone subduction and anterior laterad movements. Similar position of labeled cells was observed in slb (Fig. 8C, c; n=6) mutants, and measurements revealed that AP extension of the labeled cell array was not significantly different from the wild type (98% of wild-type extension; P>0,42). In ppt (Fig. 8E,e; n=5) and kny single mutants (Fig. 8F,f; n=9) the labeled cells were found lateral to the midline in the deep mesendoderm, but the array extension was reduced compared to wild type (85% of wild-type extension for ppt; P<0.05; 82% of wild-type extension for kny). Whereas, in ntl embryos (Fig. 8D,d; n=11), labeled cells were positioned within the mesendoderm indicative of normal subduction, their position along the midline revealed that subsequent anterior laterad movements did not occur. Measurements indicated that extension of the labeled cell array was not reduced compared to the wild type (97% of wild-type length; n=6, P>0.38). These cell movement analyses, together with morphological observations (Fig. 2), support the notion that the functions of ntl and slb alone are not required for posterior body extension, but that ppt and kny are both essential for these movements. Furthermore, these studies reveal the requirement for ntl function in laterad divergence movements. In ppt;ntl (Fig. 8G,g) and kny;ntl (Fig. 8H,h), although some labeled cells were found within mesendoderm, others remained within the epiblast, indicating that subduction movements were impaired. Additionally, labeled cell arrays did not extend much (47% of wild-type length for ppt;ntl, n=5; 33% of wild-type length for kny;ntl, n=5), nor did they move laterally. Therefore, Ntl and non-canonical Wnt components synergistically regulate posterior tailbud extension cell movements. In addition, they co-operate to mediate normal subduction movements.
Fig. 8.
Ntl synergistically interacts with Ppt and Kny to regulate cell movements in the developing posterior body. At one somite, cells within the posterior tailbud were labeled by uncaging with ultraviolet light and, at 18 somites, the position of the labeled cells was determined (A). The labeled cells (red) are within the mesendoderm and lateral to the notochord (ntl in blue) in the wild-type embryos (B,b) and slb mutants (C,c). In ntl mutants (D,d), labeled cells are also within the mesendoderm (D) but are positioned medially (d). In ppt mutants (E,e), the cells occupy the mesendoderm (E) and are lateral to the notochord (e). In kny mutants, cells are within the mesendoderm (F) and lateral to the notochord (f). In ppt;ntl (G,g) and kny;ntl (H,h) mutants, labeled cells are present in both the mesendoderm and ectoderm and fail to move from the posterior bud laterally or to extend (g,h). (B–H) Lateral views. The ntl mutant cells transplanted into wild-type embryos undergo subduction and lateral divergence (I,i). (b–h,I,i) Dorsal posterior views. Scale bar=100 μm.
The failure of posterior tailbud cells in ntl mutants to undergo laterad divergence movements could simply reflect the absence of the midline tissues that normally extend from the anterior tailbud (Kanki and Ho, 1997). Alternatively, ntl mutant cells could be unable to respond to divergence signals or to execute such a movement. To distinguish between these possibilities, we carried out mosaic analyses. When ntl mutant cells were transplanted into the ventral region of wild-type gastrulae, they underwent normal subduction and, notably, also normal divergence (Fig. 8I,i; n=4). These results demonstrate that the divergence movement defect in ntl mutants is non-cell-autonomous and probably occurs because of the failure of the anterior tailbud tissue to extend posteriorly and form the posterior midline.
Discussion
Combined activities of non-canonical Wnt and Ntl regulate tail formation
The transcription factor No tail and the non-canonical Wnt signaling pathway components Slb (Wnt11), Ppt (Wnt5) and glypican Kny are all essential for convergence and extension movements during gastrulation (Heisenberg et al., 2000; Kilian et al., 2003; Topczewski et al., 2001). Here, we have analysed genetic interactions between these genes to ask whether they functionally interact during posterior body morphogenesis. The slb;ntl compound mutants display only additive defects, without further caudal deficiencies compared with ntl mutants. Accordingly, late expression of wnt11 in the posterior body (Fig. 2), but not anterior nor early gastrula expression, depends on ntl function (Makita et al., 1998). The absence of tail defects in slb single mutants suggests that additional partially overlapping signals must compensate for loss of posterior Wnt11 during tail formation, a notion supported by the stronger phenotype observed for slb;ppt double mutants (Fig. 1) (Kilian et al., 2003; Westfall et al., 2003). Similar to ntl mutants, ppt/wnt5 and kny single mutants exhibit shortened tails without loss of tail tissues (Hammerschmidt et al., 1996; Solnica-Krezel et al., 1996). In contrast to slb;ntl mutants, both ppt;ntl and kny;ntl double mutants display early tail elongation defects beyond those expected for the combined individual mutant phenotypes. Furthermore, ppt and kny expression persist in ntl mutants. Together, these data indicate that kny and ppt act in a parallel pathway that cooperates with ntl during posterior body development.
ppt;ntl and kny;ntl do not co-operate to specify posterior tissues
Genetic lesions and dominant negative interference have revealed roles for several genes in trunk and tail formation including ntl, other Tbx genes, papc, Fgf genes and caudal (Amaya et al., 1991; Chesley, 1935; Golling et al., 2002; Griffin et al., 1995; Halpern et al., 1993; Ho and Kane, 1990; Yamamoto et al., 1998). Because mutations in the murine brachyury gene or interference with Fgf signaling in frog and fish result in more severe posterior defects than those observed in zebrafish ntl mutants, redundant mechanisms have been proposed to operate downstream of Fgf during zebrafish trunk and tail development (Griffin et al., 1995). Therefore, it was possible that simultaneous inactivation of ntl and ppt or kny genes leads to synergistic loss of Fgf activity and the severe posterior defects in double mutants. However, we found no further reduction in expression of fgf3, fgf8 or the Fgf feedback antagonist sef compared with individual mutants. Based on these data, we propose that impaired Fgf activity does not account for the synergistic double mutant defects.
Previous studies in zebrafish have demonstrated overlapping function of Tbx genes during tail and trunk morphogenesis, with spt;ntl double mutants displaying more severe posterior defects than either individual mutant (Amacher et al., 2002; Griffin et al., 1998). Significant spt expression persists in ppt;ntl and kny;ntl double mutants, indicating that it is not synergistically regulated by Ntl and non-canonical Wnt components. In support of this, the ppt;ntl and kny;ntl compound mutants do not phenocopy the spt;ntl defects. Although spt;ntl double mutants exhibit severe trunk and tail mesoderm deficits, ppt;ntl and kny;ntl double mutants only have mesoderm deficiencies comparable to those observed in ntl single mutants (Amacher et al., 2002). Furthermore, expression of myoD in posterior somites is absent from spt;ntl and from double mutants for the Nodal cofactor oep and either ntl or spt (Amacher et al., 2002; Griffin et al., 1995; Griffin et al., 1998; Griffin and Kimelman, 2002; Schier et al., 1997) but not in ppt;ntl and kny;ntl double mutants.
The cad1 homeobox gene regulates posterior body specification in Drosophila and vertebrates (Golling et al., 2002; Joly et al., 1992; Macdonald et al., 1986; Macdonald and Struhl, 1986; Mlodzik et al., 1990; Subramanian et al., 1995). Mutational inactivation of and interference with Cad1 leads to posterior truncations in the zebrafish and Drosophila, and to anterior homeotic transformations in the mouse (Golling et al., 2002; Joly et al., 1992; Macdonald and Struhl, 1986; Subramanian et al., 1995). However, persistent expression of cad1 in the posterior tissues of double-mutant embryos indicates that their tail elongation defects must occur through a cad1-independent mechanism. Therefore, in contrast to Tbox and Nodal interactions and the cad1 gene, which promote posterior tissue specification and/or differentiation (Amacher et al., 2002; Griffin et al., 1998; Griffin and Kimelman, 2002; Schier et al., 1997), the ppt;ntl and kny;ntl mutant phenotypes probably arise by a distinct mechanism that involves regulation of morphogenetic processes.
How might bmp4 contribute to tail elongation?
Bmps regulate cell fate, proliferation, survival and cell movements throughout development (reviewed in Ashique et al., 2002; Hammerschmidt and Mullins, 2002; Hogan, 1996a; Hogan, 1996b; Myers et al., 2002b). Recent studies in Xenopus revealed Notch-dependent and -independent functions for Bmp during tail outgrowth and patterning (Beck et al., 2001). Here, we show that bmp4 expression is lost at earlier developmental stages in ppt;ntl and kny;ntl double mutant embryos than in ntl individual mutants raising the possibility that the severe posterior defects are due to the earlier loss of bmp4 activity. According to the Xenopus model, Bmp4 signaling upstream of Notch promotes tailbud outgrowth. However, we did not observe enhanced reduction of notch6, notch1 or deltaC expression in double mutants compared with ntl single mutants (data not shown), although we cannot exclude the possibility that other notch and delta genes are involved. Further supporting the notion that Bmp4-dependent regulation of Notch signaling is probably not responsible for the synergistic tail phenotypes, single and double zebrafish mutants in Notch/Delta and their target genes exhibit segmentation and/or neurogenic phenotypes without loss of tail (Appel et al., 1999; Henry et al., 2002; Itoh et al., 2003; Oates and Ho, 2002; van Eeden et al., 1998). In Xenopus, Bmp signaling mediates tail somite formation by a mechanism that does not use Notch (Beck et al., 2001). Consistent with a role for Bmp signaling in tail mesoderm specification, ntl and compound mutants fail to form tail somites. However, this process does not appear to be synergistically affected in the compound mutants. In zebrafish, Bmp signaling was proposed to mediate posterior somite formation by ensuring proper tail progenitor movements during gastrulation (Myers et al., 2002a). Zebrafish mesodermal cells residing in the ventral no-convergence no-extension zone (NCEZ), where Bmp activity levels are the highest, do not undergo convergence and extension movements during gastrulation. Instead, they move vegetally to occupy the posterior tailbud (Myers et al., 2002a). In dorsalized zebrafish mutants with diminished Bmp activity and reduced NCEZ, the ventral cells fail to reach the tailbud, leading to tail truncations (Connors et al., 1999; Kishimoto et al., 1997; Miller-Bertoglio et al., 1997; Myers et al., 2002a). However, during early and late gastrulation, dorsal and ventral marker expression revealed overtly normal dorsoventral patterning in individual kny, ppt and ntl mutants (Rauch et al., 1997; Solnica-Krezel et al., 1996; Topczewski et al., 2001) and in kny;ntl and ppt;ntl compound mutants. Therefore, altered dorsoventral patterning in the gastrula does not underlie the tail elongation defects (data not shown). Accordingly, cell tracing experiments revealed normal movement of ventral mesodermal cells into the posterior tailbud in single and double mutants. Therefore, the posterior defects in double mutants are not due to a failure of contributing progenitors to move into the tailbud.
After the zebrafish tailbud forms, posterior Bmp signaling is promoted by the Chordin antagonist Tolloid/Minifin (Mfn) (Connors et al., 1999). Mutations that disrupt the mfn gene lead to mild dorsalization, loss of ventral tail tissues and reduction of somitic mesoderm. Despite these patterning defects and reduction of bmp4 and eve1 expression, extension of the tail is largely normal in mfn mutants (Connors et al., 1999). Furthermore, a dramatic downregulation of eve1 expression in ntl has been reported previously (Joly et al., 1993a) and we observed a comparable reduction of eve1 expression in kny;ntl and ppt;ntl double mutants (data not shown). Given normal extension of posterior body in ntl (Figs 2, 8) and mfn mutants, downregulation of eve1 in posterior tissues of kny;ntl and ppt;ntl double mutants during segmentation is unlikely to underlie their morphogenetic defects. However, as neither mfn nor ntl embryos exhibit complete loss of tailbud bmp4 expression as observed in kny;ntl and ppt;ntl compound mutants, the specific contribution of Bmp signaling to tail elongation will require further investigation.
Aberrant cell proliferation and death do not account for early posterior body shortening
Given that Bmp regulates cell proliferation and survival (Ashique et al., 2002), loss of bmp4 expression in ppt;ntl and kny;ntl mutants could lead to elevated cell death or reduced cell proliferation during tail elongation. In either case, double-mutant embryos would have fewer cells contributing to the posterior body. Our findings do not support this hypothesis. Rather, we found comparable levels of cell proliferation and death between compound and individual mutants before and during tail extension stages. Increased cell death occurred well after the double-mutant phenotype was manifest morphologically. At this time, cell death is visible in ntl and in compound mutants, and probably leads to the subsequent truncation and/or loss of posterior tissues. In support of this, Xenopus embryos with perturbed brachyury function and brachyury mouse mutants only exhibit apoptosis in posterior tissues after gastrulation defects are apparent (Chesley, 1935; Conlon and Smith, 1999; Yanagisawa et al., 1981). This increase in programmed cell death was proposed to be an indirect consequence of altered adhesion as reported for epithelial cells that occupy an inappropriate location (Conlon and Smith, 1999; Khwaja et al., 1997). Our cell labeling analysis supports this notion because ventral-derived posterior tailbud cells occupy the midline region, from which they are normally excluded (Kanki and Ho, 1997).
Convergence and extension genes interact with ntl to regulate tail-forming movements
Zebrafish tailbud morphogenesis entails regionalized cell movements (Kanki and Ho, 1997). Throughout the anterior and posterior tailbud, cells continue to undergo convergence and extension movements as observed during gastrulation until the tail eversion stage (Fig. 9) (Kanki and Ho, 1997). We link ntl with ppt and kny genes to the gastrulation-like convergence and extension movements within the tailbud. Between tailbud formation and the onset of tail extension, convergence and extension movements within the tailbud are normal in ntl mutants and impaired in single kny and ppt mutants, whereas double mutants have most severe tail convergence defects (Fig. 9). Tail convergence defects were apparent by broader gene expression domains before the onset of the tailbud extension stages. Therefore, ntl co-operates with ppt/wnt5 and kny/gpc4/6, possibly by regulating wnt11, to mediate the gastrulation-like convergence and extension movements throughout the tailbud.
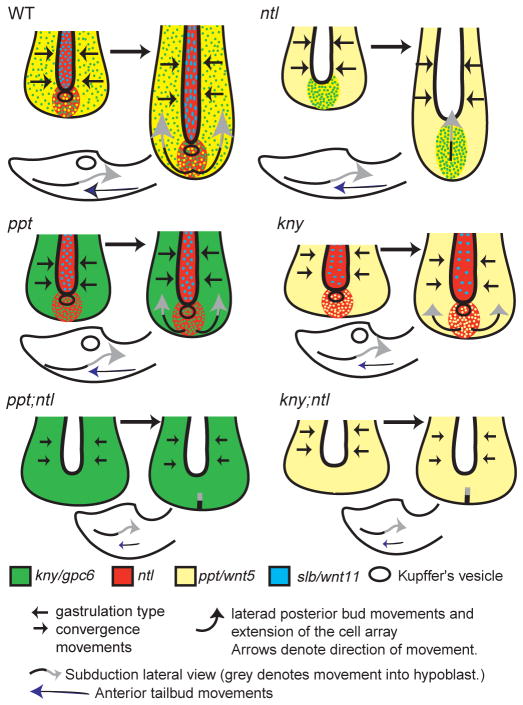
Fig. 9.
Synergistic interactions between Ntl and Ppt and Kny regulate specific cell movements underlying tail morphogenesis. During posterior body morphogenesis, convergence and extension movements as observed in the gastrula contribute to tail elongation until the tail everts. At the same time, laterad divergence movements of the posterior tailbud cells occur to avoid the midline, and they enter the mesendoderm by subducting beneath the advancing anterior tailbud cells at Kupffer’s vesicle in the wild type. In ntl mutants, convergence movements are relatively normal despite a lack of wnt11 in the notochord. During tail elongation, convergence and extension movements continue relatively normally. At this time, posterior tailbud cells fail to undergo laterad divergence and instead extend anteriorly. Movement of posterior tailbud cells into the mesendoderm occurs normally despite the lack of Kupffer’s vesicle, suggesting that the boundary between dorsal- and ventral-derived cells is maintained (if this distinction is required for normal subduction). In ppt mutants, gastrula-like convergence movements are impaired although initial positioning of cells within the posterior tailbud is normal. These cells undergo laterad divergence from the midline but it is reduced compared with the wild type, as is extension; movement into the mesendoderm is normal. In kny mutants, convergence and extension movements are impaired although ventral-derived posterior body precursor cells undergo normal epiboly movements (Topczewski et al., 2001) and contribute to the tailbud. In addition, tail-specific laterad divergence occurs and subduction movements position cells within the mesendoderm. In ppt;ntl and kny;ntl embryos, gastrulation-like convergence and extension movements are impaired, although posterior tailbud cells arrive at the bud on time. Like ppt;ntl double mutants, kny;ntl double mutant cells fail to undergo laterad divergence and do not move from the posterior bud. In addition, subduction movements into the mesendoderm are impaired but not completely blocked suggesting an additional role for Kny, Ppt and Ntl function.
Anterior mesendodermal tailbud cells ingress and move posteriorly within the dorsomedial posterior flow until exiting the flow and occupying more lateral regions. By contrast, posterior tailbud cells enter the mesendoderm beneath the dorsal derived anterior cells through a tailbud-specific subduction movement (Figs 1, 9) (Kanki and Ho, 1997). The posterior tailbud cell groups also diverge from the midline via laterad movement and extend anteroposteriorly (Fig. 9) (Kanki and Ho, 1997). In ntl single mutants, cells enter the mesendoderm but do not exhibit laterad movements even though the array extends normally (Fig. 9). Similarly, normal extension of dorsal mesoderm despite impaired convergence was observed in ntl gastrulae, but was attributed to epibolic movements (Glickman et al., 2003). Recent studies in Xenopus show that Xbra/ntl function promotes convergence and extension while inhibiting cell migration; therefore, it is possible that increased migration in the dorsal mesoderm of zebrafish ntl mutants contributes to its extension (Kwan and Kirschner, 2003). The cell behaviors driving extension of posterior tissues remain to be characterized.
In mosaic mouse embryos, brachyury mutant cells accumulate along the length of the primitive streak but are largely absent from the lateral paraxial mesoderm, a defect that has been associated with altered adhesion (Wilson and Beddington, 1997; Wilson et al., 1995). In the mouse, restoring Ntl activity in the streak is sufficient for cells to move into paraxial regions. Likewise, we found that ntl mutant cells in a wild-type host can undergo normal laterad divergence movements. Thus, the requirement for Ntl activity to promote lateral movement into paraxial mesoderm might be conserved between mouse and zebrafish (Wilson and Beddington, 1997). We hypothesize that the posterior tailbud cells upon subduction encounter the midline tissues extending from the anterior tailbud (Kanki and Ho, 1997), which serve as a barrier for their migration, resulting in the laterad divergence of their movement. It will be important to test whether a simple mechanical barrier or/and repulsive signals are involved. During tailbud extension stages, ntl and non-canonical Wnt signaling interact to regulate convergence and extension and tailbud unique movements. Cell labeling revealed a synergistic function between ntl and ppt, and between ntl and kny in facilitating posterior tailbud cell movements. In ppt;ntl and kny;ntl compound mutants, posterior tailbud cells fail to move from the posterior bud, fail to extend and exhibit impaired subduction movements (Fig. 9). We suggest that the tail extension defects in double-mutant embryos are due to the combined loss of ntl and parallel, partially overlapping non-canonical Wnt signaling inputs, which synergistically regulate posterior-specific cell movements but not posterior specification during tail morphogenesis. Moreover, genes required for cell movements in the gastrula interact to regulate cell movements within the developing posterior body, supporting the notion that tail formation is in part a continuation of mechanisms mediating gastrulation.
Acknowledgments
We thank L.S.-K. lab members for discussions and critical comments. We thank J. Clanton and W. Rogers for excellent fish care, C. Kimmel, C.-P. Heisenberg and M. Hammerschmidt for mutant lines, M. Westerfield, H. Takeda, P. Ingham, M. Ekker, Y. Grinblat, B. Thisse, C. Thisse, E. Weinberg and T. Jowett for probes, and R. Harland, S. Sokol and M. Tada for constructs. The Zeiss confocal microscope is supported by NIH grant 1S10RR015682. Work in E.M.G. laboratory is supported by grants from the government of Spain (FIS01/796 and MCYTBMC 2000-0540). Work in L.S.-K. lab is supported by NIH grant GM62283.
References
- Agathon A, Thisse C, Thisse B. The molecular nature of the zebrafish tail organizer. Nature, Lond. 2003;424:448–452. doi: 10.1038/nature01822. [DOI] [PubMed] [Google Scholar]
- Ajiro K, Yoda K, Utsumi K, Nishikawa Y. Alteration of cell cycle-dependent histone phosphorylations by okadaic acid. Induction of mitosis-specific H3 phosphorylation and chromatin condensation in mammalian interphase cells. J Biol Chem. 1996;271:13197–13201. doi: 10.1074/jbc.271.22.13197. [DOI] [PubMed] [Google Scholar]
- Amacher SL, Draper BW, Summers BR, Kimmel CB. The zebrafish T-box genes no tail and spadetail are required for development of trunk and tail mesoderm and medial floor plate. Development. 2002;129:3311–3323. doi: 10.1242/dev.129.14.3311. [DOI] [PubMed] [Google Scholar]
- Amaya E, Musci TJ, Kirschner MW. Expression of a dominant negative mutant of the FGF receptor disrupts mesoderm formation in Xenopus embryos. Cell. 1991;66:257–270. doi: 10.1016/0092-8674(91)90616-7. [DOI] [PubMed] [Google Scholar]
- Amaya E, Stein PA, Musci TJ, Kirschner MW. FGF signalling in the early specification of mesoderm in Xenopus. Development. 1993;118:477–487. doi: 10.1242/dev.118.2.477. [DOI] [PubMed] [Google Scholar]
- Appel B, Fritz A, Westerfield M, Grunwald DJ, Eisen JS, Riley BB. Delta-mediated specification of midline cell fates in zebrafish embryos. Curr Biol. 1999;9:247–256. doi: 10.1016/s0960-9822(99)80113-4. [DOI] [PubMed] [Google Scholar]
- Ashique AM, Fu K, Richman JM. Endogenous bone morphogenetic proteins regulate outgrowth and epithelial survival during avian lip fusion. Development. 2002;129:4647–4660. doi: 10.1242/dev.129.19.4647. [DOI] [PubMed] [Google Scholar]
- Beck CW, Slack JM. Analysis of the developing Xenopus tail bud reveals separate phases of gene expression during determination and outgrowth. Mech Dev. 1998;72:41–52. doi: 10.1016/s0925-4773(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Beck CW, Whitman M, Slack JM. The role of BMP signaling in outgrowth and patterning of the Xenopus tail bud. Dev Biol. 2001;238:303–314. doi: 10.1006/dbio.2001.0407. [DOI] [PubMed] [Google Scholar]
- Beddington RSP, Rashbass P, Wilson V. Brachyury – a gene affecting mouse gastrulation and early organogenesis. Development Suppl. 1992:157–165. [PubMed] [Google Scholar]
- Catala M, Teillet MA, Le Douarin N. Organization and development of the tail bud analyzed with the quail-chick chimaera system. Mech Dev. 1995;51:51–65. doi: 10.1016/0925-4773(95)00350-a. [DOI] [PubMed] [Google Scholar]
- Chadee DN, Taylor WR, Hurta RA, Allis CD, Wright JA, Davie JR. Increased phosphorylation of histone H1 in mouse fibroblasts transformed with oncogenes or constitutively active mitogen-activated protein kinase kinase. J Biol Chem. 1995;270:20098–20105. doi: 10.1074/jbc.270.34.20098. [DOI] [PubMed] [Google Scholar]
- Chesley P. Development of the short-tailed mutant in the house mouse. J Exp Zool. 1935;70:429–435. [Google Scholar]
- Cole LK, Ross LS. Apoptosis in the developing zebrafish embryo. Dev Biol. 2001;240:123–142. doi: 10.1006/dbio.2001.0432. [DOI] [PubMed] [Google Scholar]
- Conlon FL, Smith JC. Interference with brachyury function inhibits convergent extension, causes apoptosis, and reveals separate requirements in the FGF and activin signalling pathways. Dev Biol. 1999;213:85–100. doi: 10.1006/dbio.1999.9330. [DOI] [PubMed] [Google Scholar]
- Connors SA, Trout J, Ekker M, Mullins MC. The role of Tolloid/Mini fin in dorsoventral pattern formation of the zebrafish embryo. Development. 1999;126:3119–3130. doi: 10.1242/dev.126.14.3119. [DOI] [PubMed] [Google Scholar]
- Dorfman DM, Wilson DB, Bruns GA, Orkin SH. Human transcription factor GATA-2. Evidence for regulation of preproendothelin-1 gene expression in endothelial cells. J Biol Chem. 1992;267:1279–1285. [PubMed] [Google Scholar]
- Feldman B, Gates MA, Egan ES, Dougan ST, Rennebeck G, Sirotkin HI, Schier AF, Talbot WS. Zebrafish organizer development and germ-layer formation require Nodal-related signals. Nature. 1998;395:181–185. doi: 10.1038/26013. [DOI] [PubMed] [Google Scholar]
- Furthauer M, Lin W, Ang SL, Thisse B, Thisse C. Sef is a feedback-induced antagonist of Ras/MAPK-mediated FGF signalling. Nat Cell Biol. 2002;4:170–174. doi: 10.1038/ncb750. [DOI] [PubMed] [Google Scholar]
- Gaertner RA. Development of the posterior trunk and tail of the chick embryo. J Exp Zool. 1949;111:157–174. doi: 10.1002/jez.1401110202. [DOI] [PubMed] [Google Scholar]
- Gerhart J. The primacy of cell interactions in development. Trends Genet. 1989;5:233–237. doi: 10.1016/0168-9525(89)90093-0. [DOI] [PubMed] [Google Scholar]
- Glickman NS, Kimmel CB, Jones MA, Adams RJ. Shaping the zebrafish notochord. Development. 2003;130:873–887. doi: 10.1242/dev.00314. [DOI] [PubMed] [Google Scholar]
- Golling G, Amsterdam A, Sun Z, Antonelli M, Maldonado E, Chen W, Burgess S, Haldi M, Artzt K, Farrington S, et al. Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. Nat Genet. 2002;31:135–140. doi: 10.1038/ng896. [DOI] [PubMed] [Google Scholar]
- Gont LK, Steinbeisser H, Blumberg B, De Robertis EM. Tail formation as a continuation of gastrulation: the multiple cell populations of the Xenopus tailbud derive from the late blastopore lip. Development. 1993;119:991–1004. doi: 10.1242/dev.119.4.991. [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Kimelman D. One-eyed pinhead and Spadetail are essential for heart and somite formation. Nat Cell Biol. 2002;4:821–825. doi: 10.1038/ncb862. [DOI] [PubMed] [Google Scholar]
- Griffin K, Patient R, Holder N. Analysis of FGF function in normal and no tail zebrafish embryos reveals separate mechanisms for formation of the trunk and the tail. Development. 1995;121:2983–2994. doi: 10.1242/dev.121.9.2983. [DOI] [PubMed] [Google Scholar]
- Griffin KJ, Amacher SL, Kimmel CB, Kimelman D. Molecular identification of spadetail: regulation of zebrafish trunk and tail mesoderm formation by T-box genes. Development. 1998;125:3379–3388. doi: 10.1242/dev.125.17.3379. [DOI] [PubMed] [Google Scholar]
- Griffith CM, Wiley MJ, Sanders EJ. The vertebrate tailbud: three germ layers from one tissue. Anat Embryol. 1992;185:101–113. doi: 10.1007/BF00185911. [DOI] [PubMed] [Google Scholar]
- Halpern ME, Ho RK, Walker C, Kimmel CB. Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell. 1993;75:99–111. [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Mullins MC. Dorsoventral patterning in the zebrafish: bone morphogenetic proteins and beyond. Results Probl Cell Differ. 2002;40:72–95. doi: 10.1007/978-3-540-46041-1_5. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Nusslein-Volhard C. The expression of a zebrafish gene homologous to Drosophila snail suggests a conserved function in invertebrate and vertebrate gastrulation. Development. 1993;119:1107–1118. doi: 10.1242/dev.119.4.1107. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, Brand M, van Eden FJM, Furutani-Seiki M, Kelsh RN, Odenthal J, Warga RM, et al. Mutations affecting morphogenesis during gastrulation and tail formation in the zebrafish, Danio rerio. Development. 1996;123:143–151. doi: 10.1242/dev.123.1.143. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Nusslein-Volhard C. The function of silberblick in the positioning of the eye anlage in the zebrafish embryo. Dev Biol. 1997;184:85–94. doi: 10.1006/dbio.1997.8511. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JCF, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- Henry CA, Urban MK, Dill KK, Merlie JP, Page MF, Kimmel CB, Amacher SL. Two linked Hairy/Enhancer of split-related zebrafish genes, her1 and her7, function together to refine alternating somite boundaries. Development. 2002;129:3693–3704. doi: 10.1242/dev.129.15.3693. [DOI] [PubMed] [Google Scholar]
- Ho RK, Kane DA. Cell-autonomous action of zebrafish spt-1 mutation in specific mesodermal precursors. Nature. 1990;348:728–730. doi: 10.1038/348728a0. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996a;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996b;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo. Plainview, NY: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- Isaacs HV, Tannahill D, Slack JM. Expression of a novel FGF in the Xenopus embryo. A new candidate inducing factor for mesoderm formation and anteroposterior specification. Development. 1992;114:711–720. doi: 10.1242/dev.114.3.711. [DOI] [PubMed] [Google Scholar]
- Isaacs HV, Pownall ME, Slack JM. eFGF regulates Xbra expression during Xenopus gastrulation. EMBO J. 1994;13:4469–4481. doi: 10.1002/j.1460-2075.1994.tb06769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Joly JS, Maury M, Joly C, Duprey P, Boulekbache H, Condamine H. Expression of a zebrafish caudal homeobox gene correlates with the establishment of posterior cell lineages at gastrulation. Differentiation. 1992;50:75–87. doi: 10.1111/j.1432-0436.1992.tb00488.x. [DOI] [PubMed] [Google Scholar]
- Joly JS, Joly C, Schulte-Merker S, Boulekbache H, Condamine H. The ventral and posterior expression of the zebrafish homeobox gene eve1 is perturbed in dorsalized and mutant embryos. Development. 1993a;119:1261–1275. doi: 10.1242/dev.119.4.1261. [DOI] [PubMed] [Google Scholar]
- Joly JS, Maury M, Joly C, Boulekbache H, Condamine H. Ventral and posterior expression of the homeobox gene eve1 in zebrafish (Brachydanio rerio) is repressed in dorsalized embryos. CR Seances Soc Biol Filiales. 1993b;187:356–363. [PubMed] [Google Scholar]
- Kanki JP, Ho RK. The development of the posterior body in zebrafish. Development. 1997;124:881–893. doi: 10.1242/dev.124.4.881. [DOI] [PubMed] [Google Scholar]
- Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer P, Mathieu M, Mason I, Dickson C. Secretion and mitogenic activity of zebrafish FGF3 reveal intermediate properties relative to mouse and Xenopus homologues. Oncogene. 1996;12:1503–1511. [PubMed] [Google Scholar]
- Kilian B, Mansukoski H, Barbosa FC, Ulrich F, Tada M, Heisenberg CP. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech Dev. 2003;120:467–476. doi: 10.1016/s0925-4773(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Kimelman D, Kirschner M. Synergistic induction of mesoderm by FGF and TGF-β and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987;51:869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Law RD. Cell lineage of zebrafish blastomeres. III Clonal analyses of the blastula and gastrula stages. Dev Biol. 1985;108:94–101. doi: 10.1016/0012-1606(85)90012-0. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Lee KH, Zon L, Hammerschmidt M, Schulte-Merker S. The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development. 1997;124:4457–4466. doi: 10.1242/dev.124.22.4457. [DOI] [PubMed] [Google Scholar]
- Krauss S, Johansen T, Korzh V, Moens U, Ericson JU, Fjose A. Zebrafish pax[zf-a]: a paired box-containing gene expressed in the neural tube. EMBO J. 1991;10:3609–3619. doi: 10.1002/j.1460-2075.1991.tb04927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KM, Kirschner MW. Xbra functions as a switch between cell migration and convergent extension in the Xenopus gastrula. Development. 2003;130:1961–1972. doi: 10.1242/dev.00412. [DOI] [PubMed] [Google Scholar]
- Macdonald PM, Struhl G. A molecular gradient in early Drosophila embryos and its role in specifying the body pattern. Nature. 1986;324:537–545. doi: 10.1038/324537a0. [DOI] [PubMed] [Google Scholar]
- Macdonald PM, Ingham P, Struhl G. Isolation, structure, and expression of even-skipped: a second pair-rule gene of Drosophila containing a homeobox. Cell. 1986;47:721–734. doi: 10.1016/0092-8674(86)90515-5. [DOI] [PubMed] [Google Scholar]
- Mahadevan LC, Willis AC, Barratt MJ. Rapid histone H3 phosphorylation in response to growth factors, phorbol esters, okadaic acid, and protein synthesis inhibitors. Cell. 1991;65:775–783. doi: 10.1016/0092-8674(91)90385-c. [DOI] [PubMed] [Google Scholar]
- Makita R, Mizuno T, Kuroiwa A, Koshida S, Takeda H. Zebrafish wnt11: pattern and regulation of the expression by the yolk cell and no tail activity. Mech Dev. 1998;71:165–176. doi: 10.1016/s0925-4773(98)00013-6. [DOI] [PubMed] [Google Scholar]
- Miller-Bertoglio VE, Fisher S, Sanchez A, Mullins MC, Halpern ME. Differential regulation of chordin expression domains in mutant zebrafish. Dev Biol. 1997;192:537–550. doi: 10.1006/dbio.1997.8788. [DOI] [PubMed] [Google Scholar]
- Mlodzik M, Gibson G, Gehring WJ. Effects of ectopic expression of caudal during Drosophila development. Development. 1990;109:271–277. doi: 10.1242/dev.109.2.271. [DOI] [PubMed] [Google Scholar]
- Myers DC, Sepich DS, Solnica-Krezel L. Bmp activity gradient regulates convergence and extension during zebrafish gastrulation. Dev Biol. 2002a;243:81–98. doi: 10.1006/dbio.2001.0523. [DOI] [PubMed] [Google Scholar]
- Myers DC, Sepich DS, Solnica-Krezel L. Convergence and extension in vertebrate gastrulae: cell movements according to or in search of identity? Trends Genet. 2002b;18:447–455. doi: 10.1016/s0168-9525(02)02725-7. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Trout J, Connors SA, Andermann P, Weinberg E, Mullins MC. Dorsal and intermediate neuronal cell types of the spinal cord are established by a BMP signaling pathway. Development. 2000;127:1209–1220. doi: 10.1242/dev.127.6.1209. [DOI] [PubMed] [Google Scholar]
- Oates AC, Ho RK. Hairy/E(spl)-related (Her) genes are central components of the segmentation oscillator and display redundancy with the Delta/Notch signaling pathway in the formation of anterior segmental boundaries in the zebrafish. Development. 2002;129:2929–2946. doi: 10.1242/dev.129.12.2929. [DOI] [PubMed] [Google Scholar]
- Odenthal J, Haffter P, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, et al. Mutations affecting the formation of the notochord in the zebrafish, Danio rerio. Development. 1996;123:103–115. doi: 10.1242/dev.123.1.103. [DOI] [PubMed] [Google Scholar]
- Rauch GJ, Hammerschmidt M, Blader P, Schauerte HE, Strahle U, Ingham PW, McMahon AP, Haffter P. Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harbor Symp Quant Biol. 1997b;62:227–234. [PubMed] [Google Scholar]
- Reifers F, Bohli H, Walsh E, Crossley PH, Stainier DYR, Brand M. Fgf8 is mutated in zebrafish acerebellar (ace) mutants and is required for maintenance of midbrain-hindbrain boundary development and somitogenesis. Development. 1998;125:2381–2395. doi: 10.1242/dev.125.13.2381. [DOI] [PubMed] [Google Scholar]
- Schier AF, Shen MM. Nodal signalling in vertebrate development. Nature. 2000;403:385–389. doi: 10.1038/35000126. [DOI] [PubMed] [Google Scholar]
- Schier AF, Nuehauss SCF, Helde KA, Talbot WS, Driever W. The one-eyed pinhead gene functions in mesoderm and endoderm formation in zebrafish and interacts with no tail. Development. 1997;124:327–342. doi: 10.1242/dev.124.2.327. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC. Tail (end) bud contributions to the posterior region of the chick embryo. J Exp Zool. 1977;201:227–246. [Google Scholar]
- Schoenwolf GC. Histological and ultrastructural studies of secondary neurulation in mouse embryos. Am J Anat. 1984;169:361–376. doi: 10.1002/aja.1001690402. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Smith JL. Gastrulation and early mesodermal patterning in vertebrates. Methods Mol Biol. 2000;135:113–125. doi: 10.1385/1-59259-685-1:113. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Smith JC. Mesoderm formation in response to Brachyury requires FGF signalling. Curr Biol. 1995;5:62–67. doi: 10.1016/s0960-9822(95)00017-0. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Ho RK, Herrmann BG, Nusslein-Volhard C. The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development. 1992;116:1021–1032. doi: 10.1242/dev.116.4.1021. [DOI] [PubMed] [Google Scholar]
- Sepich DS, Myers DC, Short R, Topczewski J, Marlow F, Solnica-Krezel L. Role of the zebrafish trilobite locus in gastrulation movements of convergence and extension. Genesis. 2000;27:159–173. doi: 10.1002/1526-968x(200008)27:4<159::aid-gene50>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Slack JM, Darlington BG, Heath JK, Godsave SF. Mesoderm induction in early Xenopus embryos by heparin-binding growth factors. Nature. 1987;326:197–200. doi: 10.1038/326197a0. [DOI] [PubMed] [Google Scholar]
- Smith JC, Price BM, Green JB, Weigel D, Herrmann BG. Expression of a Xenopus homolog of Brachyury (T) is an immediate-early response to mesoderm induction. Cell. 1991;67:79–87. doi: 10.1016/0092-8674(91)90573-h. [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L, Schier AF, Driever W. Efficient recovery of ENU-induced mutations from the zebrafish germline. Genetics. 1994;136:1401–1420. doi: 10.1093/genetics/136.4.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnica-Krezel L, Stemple DL, Mountcastle-Shah E, Rangini Z, Neuhauss SCF, Malicki J, Schier A, Stainier DYR, Zwartkruis F, Abdelilah S, et al. Mutations affecting cell fates and cellular rearrangements during gastrulation in zebrafish. Development. 1996;123:117–128. doi: 10.1242/dev.123.1.67. [DOI] [PubMed] [Google Scholar]
- Subramanian V, Meyer BI, Gruss P. Disruption of the murine homeobox gene Cdx1 affects axial skeletal identities by altering the mesodermal expression domains of Hox genes. Cell. 1995;83:641–653. doi: 10.1016/0092-8674(95)90104-3. [DOI] [PubMed] [Google Scholar]
- Talbot WS, Trevarrow W, Halpern ME, Melby AE, Farr G, Postlethwait JH, Jowett T, Kimmel CB, Kimelman D. A homeobox gene essential for zebrafish notochord development. Nature. 1995;378:150–157. doi: 10.1038/378150a0. [DOI] [PubMed] [Google Scholar]
- Tam PPL. The histogenetic capacity of tissues in the caudal end of the embryonic axis of the mouse. J Embryol Exp Morphol. 1984;82:253–266. [PubMed] [Google Scholar]
- Tam PP. A study of the pattern of prospective somites in the presomitic mesoderm of mouse embryos. J Embryol Exp Morphol. 1986;92:269–285. [PubMed] [Google Scholar]
- Thisse C, Thisse B. High resolution whole-mount in situ hybridization. In: Westerfield M, editor. Zebrafish Science Monitor. Vol. 5. Eugene, OR: The Institute of Neuroscience, University of Oregon; 1998. pp. 8–9. [Google Scholar]
- Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, Hammerschmidt M, Postlethwait J, Solnica-Krezel L. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1:251–264. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Tsang M, Friesel R, Kudoh T, Dawid IB. Identification of Sef, a novel modulator of FGF signalling. Nat Cell Biol. 2002;4:165–169. doi: 10.1038/ncb749. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Slack JMW. The Xenopus tail forming region. Development. 1995;121:249–262. [Google Scholar]
- van Eeden FJ, Holley SA, Haffter P, Nusslein-Volhard C. Zebrafish segmentation and pair-rule patterning. Dev Genet. 1998;23:65–76. doi: 10.1002/(SICI)1520-6408(1998)23:1<65::AID-DVG7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Warga RM, Nusslein-Volhard C. spadetail-dependent cell compaction of the dorsal zebrafish blastula. Dev Biol. 1998;203:116–121. doi: 10.1006/dbio.1998.9022. [DOI] [PubMed] [Google Scholar]
- Weinberg ES, Allende ML, Kelly CS, Abdelhamid A, Murakami T, Andermann P, Doerre OG, Grunwald DJ, Riggleman B. Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development. 1996;122:271–280. doi: 10.1242/dev.122.1.271. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book. Eugene, OR: University Oregon Press; 1995. [Google Scholar]
- Westfall TA, Brimeyer R, Twedt J, Gladon J, Olberding A, Furutani-Seiki M, Slusarski DC. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/β-catenin activity. J Cell Biol. 2003;162:889–898. doi: 10.1083/jcb.200303107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson V, Beddington R. Expression of T protein in the primitive streak is necessary and sufficient for posterior mesoderm movement and somite differentiation. Dev Biol. 1997;192:45–58. doi: 10.1006/dbio.1997.8701. [DOI] [PubMed] [Google Scholar]
- Wilson V, Manson L, Skarnes WC, Beddington RS. The T gene is necessary for normal mesodermal morphogenetic cell movements during gastrulation. Development. 1995;121:877–886. doi: 10.1242/dev.121.3.877. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Amacher SL, Kim SH, Geissert D, Kimmel CB, De Robertis EM. Zebrafish paraxial protocadherin is a downstream target of spadetail involved in morphogenesis of gastrula mesoderm. Development. 1998;125:3389–3397. doi: 10.1242/dev.125.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa KO, Fujimoto H, Urushihara H. Effects of the brachyury (T) mutation on morphogenetic movement in the mouse embryo. Dev Biol. 1981;87:242–248. doi: 10.1016/0012-1606(81)90147-0. [DOI] [PubMed] [Google Scholar]
- Yang X, Castilla LH, Xu X, Li C, Gotay J, Weinstein M, Liu PP, Deng CX. Angiogenesis defects and mesenchymal apoptosis in mice lacking SMAD5. Development. 1999;126:1571–1580. doi: 10.1242/dev.126.8.1571. [DOI] [PubMed] [Google Scholar]