Abstract
The increasing demand for bone repair solutions calls for the development of efficacious bone scaffolds. Biphasic calcium phosphate (BCP) scaffolds with both macropores and micropores (MP) have improved healing compared to those with macropores and no micropores (NMP), but the role of micropores is unclear. Here, we evaluate capillarity induced by micropores as a mechanism that can affect bone growth in vivo. Three groups of cylindrical scaffolds were implanted in pig mandibles for three weeks: MP were implanted either dry (MP-Dry), or after submersion in phosphate buffered saline, which fills pores with fluid and therefore suppresses micropore-induced capillarity (MP-Wet); NMP were implanted dry. The amount and distribution of bone in the scaffolds were quantified using micro-computed tomography. MP-Dry had a more homogeneous bone distribution than MP-Wet, although the average bone volume fraction, , was not significantly different for these two groups (0.45±0.03 and 0.37±0.03, respectively). There was no significant difference in the radial bone distribution of NMP and MP-Wet, but the of NMP was significantly lower among the three groups (0.25±0.02). These results suggest that micropore-induced capillarity enhances bone regeneration by improving the homogeneity of bone distribution in BCP scaffolds. The explicit design and use of capillarity in bone scaffolds may lead to more effective treatments of large and complex bone defects.
Keywords: Bone regeneration, calcium phosphate scaffold, microporosity, capillary action, micro-computed tomography
1. Introduction
Two million people undergo bone repair surgery every year [1,2], and the demand is expected to increase with the aging global population [1,3,4]. The limited availability of the current gold-standard techniques, namely allografts and autografts, calls for alternative solutions [2,5–7]. Synthetic grafts made of metals, polymers and ceramics have been investigated [2,8–10]. However, the extensive research efforts to determine an optimal design for bone graft substitutes have not led to a well-defined solution [10,11], and the treatment of large, load-bearing bone defects remains a challenge [5,9,10,12–14].
Researchers studying bone repair and regeneration have investigated Biphasic Calcium Phosphates (BCP) as suitable materials for artificial bone scaffolds [3,15]. In bone scaffolds, large interconnected macropores (>300 μm) provide space for bone ingrowth, vascularization and innervation [9,10,13,16]. The chemical and structural similarity of BCP with bone mineral confers biocompatibility, bioactivity and osteoconductivity [9,15,17]. Loading scaffolds with exogenous cells and/or growth factors confers osteoinductivity [6,7,14,16,18,19]. However, harvesting cells and controlling their fate in vivo is challenging [6,7,14,16,18], and the use of growth factors has been linked to serious side effects ranging from ectopic bone formation to cancers, due to the difficulty in controlling release kinetics in vivo [5,7,16,20,21].
In recent years, attention has focused on the effects of incorporating controlled microporosity (< 20 μm) in macroporous BCP scaffolds [10,11,19]. Some studies have shown that the presence of micropores leads to the formation of bone in BCP scaffolds with macro- and micropores implanted in muscle, in the absence of exogenous biologics [22–24]. In bone defects, we and others have shown that BCP scaffolds with macro- and micropores (hereafter referred to as microporous, MP) show enhanced in vivo bone growth and overall improved healing compared to scaffolds with only macropores (hereafter referred to as non-microporous, NMP), regardless of prior loading with potent osteoinductive growth factors [25–28].
The role of micropores in enhancing scaffold performance is not well understood. Researchers have suggested that micropores provide additional surface area and a reservoir for the attachment of osteoinductive biomolecules and for the precipitation of biological apatite [29–31]. We previously demonstrated that micropores can serve as space for microscale bone growth. Indeed, cells trapped in micropores form bone in those micropores [25,26].
In a recent publication [32], we demonstrated that microporosity generates capillary forces that draw cells in the micropores of 2D BCP substrates in vitro when the substrate is put in contact with a cell suspension, and in the micropores of 3D MP scaffolds in vivo when the scaffold comes in contact with the physiological fluid in the defect at the time of implantation. That is to say that when the scaffolds are inserted in to the defect, micropore-induced capillary forces draw in cells and fluid into the scaffold macro- and micropores. That work raised the possibility of micropore-induced capillarity as a mechanism that enhances healing in MP scaffolds. Others have also investigated capillarity in vitro [33–35] in the context of a potential means to improve the efficacy of calcium phosphate bone scaffolds in vivo. This is the first work to study the mechanism in vivo, to our knowledge.
This study investigates the influence of micropore-induced capillarity on bone regeneration in BCP scaffolds implanted in porcine mandibular defects. Three groups were compared: MP scaffolds with either active (MP-Dry) or suppressed (MP-Wet) micropore-induced capillary forces, and NMP scaffolds that do not have micropore-induced capillarity because they do not have micropores. The amount and distribution of ingrown bone were quantitatively assessed using micro-computed tomography (micro-CT). The homogeneity of the bone distribution in the scaffold was considered an important measure of successful bone regeneration; several measures of homogeneity were considered including the depth of the bone growth from the scaffold-defect edge to the center of the scaffold and the local bone volume fraction at different radii.
2. Materials and Methods
2.1. Scaffold fabrication and characterization
BCP scaffolds were fabricated by directed deposition of a hydroxyapatite (HA) colloidal ink to generate a structure with periodic macropores, following the protocols described in our previous work, e.g. [27,36,32]. Briefly, HA powder of purity ≥ 97.0% (Riedel-de Haen, Seelze, Germany) was calcined at 1100 °C for 10 h, then ball-milled in 100% ethanol for 14 h, to decrease specific surface area and break up particle agglomerates. The HA powder was dispersed in deionized water and Darvan® 821A (R.T. Vanderbilt, Norwalk, CT). Methocel and 1-octanol were added to increase the viscosity of the slurry and to prevent foaming, and poly(ethylenimine) was added as a gelling agent. The pH of the slurry was adjusted during the process to optimize the rheology of the final HA ink. For MP scaffolds, poly(methyl methacrylate) (PMMA) microspheres (Matsumoto Microsphere M-100, Tomen America, New York, NY) with a nominal size of 5 μm (5.96 ± 2.00 μm with a range of 2–14 μm [36]) were added to the ink as sacrificial porogens in equal volume to the HA contained in the slurry; hence the MP scaffolds were nominally 50% microporous. HA ink was loaded in a syringe and a micro-robotic deposition system [37,38] was used to deposit scaffolds, 12 mm in diameter and 8 mm in height, with alternating layers of orthogonal rods. Deposited scaffolds were sintered at 1300 °C for two hours to burn out all of the chemical additives and porogens and to densify the powder. All MP and NMP scaffolds were machined to a diameter of 8 mm, and then autoclaved in individual sterilization pouches.
Scanning electron microscopy (SEM) was used to image rod microstructure in MP and NMP scaffolds. The samples were coated with gold–palladium and imaged using a Philips XL30 ESEM-FEG (FEI Company, Hillsboro, OR) microscope with a Secondary Electron detector at an acceleration voltage of 5 kV. The phases present in the final material were determined with X-Ray Diffraction (XRD) in [26]. The as-received powder was 100% HA, but the material underwent a phase transformation during sintering that led to a final composition of 87% HA and 13% β-TCP [26]. This combination of HA and β-TCP phases is referred to as biphasic calcium phosphate (BCP). Micropore diameter and interconnection size were measured in previous studies using the same material system [26,36].
2.2. In vivo surgeries
All animal experiments were done in accordance with the NIH Guide for the Care and Use of Laboratory Animals (8th ed.) and approved by the University of Wisconsin Institutional Animal Care and Use Committee.
2.2.1. Experimental groups
MP scaffolds were implanted in pig mandibular defects either in the as-sterilized condition (MP-Dry) or after they were submerged in sterile Phosphate Buffered Saline (PBS) (MP-Wet), which was used to fill both the macro- and micropores with fluid before implantation. Samples that were submerged in PBS (MP-Wet) were gently tapped on the PBS-filled container until air bubbles stopped coming out of the scaffold. All NMP scaffolds were implanted dry.
In [32], we showed that the micropores in MP scaffolds induce capillary forces that draw fluids such as PBS, cell suspensions, or fluid from the defect site into the scaffold macro- and micropores. Thus, the important difference between the MP-Wet and MP-Dry scaffolds was that capillary forces in the MP-Wet scaffolds filled the macro- and micropores with PBS prior to implantation, whereas the capillary forces in MP-Dry filled the macro- and micropores with physiological fluid containing endogenous cells and biomolecules from the mandibular defect at the time of implantation. In other words, MP-Wet scaffolds no longer had active micropore-induced capillary forces when they came in contact with the physiological fluid contained in the defect at the time of implantation; their pores had already been filled with PBS. In contrast, MP-Dry scaffolds had active micropore-induced capillary forces when implanted in the defect and were able to draw physiological fluid, with endogenous cells and biomolecules, and distribute it in the macro- and micropores at the time of implantation. In this paper, we will refer to MP-Wet scaffolds as scaffolds with “suppressed micropore-induced capillarity”, and to MP-Dry scaffolds as scaffolds with “active micropore-induced capillarity”. By definition, NMP scaffolds did not have micropore-induced capillary forces because they do not have micropores. The number of samples was n=7 for MP-Dry, n=5 for MP-Wet and n=8 for NMP. The number of samples for MP-Wet and MP-Dry differed from the initially planned n=8, because one MP-Dry and two MP-Wet samples were crushed and one MP-Wet sample was implanted in a pig that died due to post-surgery complications. The damage to the crushed samples was assessed during micro-CT imaging after samples were removed from the animal. Data from these samples could not be used in the analysis.
2.2.2. Surgical Procedures
Eleven pigs, aged 4-6 months and weighing 75-91 kg, were used in this study. Pigs were fasted for approximately 12 h prior to surgery and sedated with telazol (6-8 mg/kg IM), ketamine (1-6 mg/kg IM) and glycopyrrolate (0.005-0.01 mg/kg IM). Buprenorphine (0.01-0.02 mg/kg IM) was administered prior to induction with propofol (4-10 mg/kg). Pigs were maintained with isoflurane (0-4%) gas in oxygen administered through an endotracheal tube. A dose of Procaine Penicillin-G (6,600-20,000 IU/kg IM) was administered along with fentanyl patch(es) at a dose of 3-5 μg/kg/h. Vital signs were monitored for the duration of the surgery. Retromandibular and submandibular incisions were made through skin and subcutaneous tissues, down to the inferior border of the mandible, avoiding the facial artery and vein. The periosteum was carefully cut and reflected to expose the mandibular cortex. Cylindrical defects were created with a 8 mm cannulated drill bit and a variable speed surgical drill (Stryker EHD, MI) under continuous irrigation with saline. Three bicortical defects were drilled in the ramus of each hemi-mandible, for six defects per pig, and scaffolds were press-fit in the defects. Visible bone debris generated from creating the defect were cleared from the defect manually. No blood or marrow was aspirated from the defect. Because the thickness of the ramus differed between pigs, the depth of each defect was assessed and the scaffolds were gently cut to the appropriate height as needed, prior to being implanted in the defect. This procedure ensured that the scaffold was flush with both sides of the bicortical defect and minimized irritation that might otherwise be caused if the scaffold protruded. A second dose of perioperative antibiotics (Procaine Penicillin-G) was then administered. The periosteum was closed over the bone and sutured with interrupted and continuous running 3-0 absorbable suture. The deep and subcutaneous tissues and the skin, were closed with a continuous 3-0 absorbable suture. Pigs were returned to a clean, dry pen for recovery and were maintained on a soft, nutritionally complete diet for 7-10 days. Note that 24 defects in the eleven pigs were used for this study, while the remaining 42 were used as part of another study using scaffolds that were the same size and composition, but had different rod configurations. The two studies were conducted simultaneously to ensure that scaffolds from each treatment group were spread across several pigs for both studies.
2.2.3. Scaffold retrieval
The pigs were euthanized three weeks after surgery. The mandibles were then excised and placed in PBS-soaked gauze on ice for less than 48 hours. The soft tissue was removed and the bone calluses that formed over the defects were carefully removed using a Dremel rotary drill (Robert Bosch Tool Corp, Racine, WI) with a high-speed saw blade in order to uncover the implanted scaffolds. A trephine bur (10 mm ID) was used to core out the scaffolds from the mandible. Cored samples consisted of a scaffold with ingrown bone, surrounded by a ring of native bone. Cored samples were 10 mm in diameter and approximately 10 mm in height. They were rinsed and stored individually in 15 ml of 10% neutral buffered formalin. The formalin solution was refreshed after 48 hours. After two weeks in formalin, samples were transferred to 15 ml of 70% ethanol in deionized water until they were processed for histology.
2.3. Histological evaluation
Histology was used to qualitatively examine bone growth in the macropores and cells and bone in micropores. It was not used to conduct any quantitative analysis. Two to five scaffolds from each treatment group were embedded in PMMA following the method described in [39]. Briefly, scaffolds were dehydrated with increasing concentrations of ethanol from 70% to 100% and then infiltrated under vacuum with methyl methacrylate (Sigma-Aldrich, St. Louis, MO). After polymerization, samples were cut with a diamond wire saw (Escil, Chassieu, France) into sections approximately 800 μm thick. Sections from the central region of each scaffold were mounted on microscope slides with cured Eukitt medium (Electron Microscopy Science, Hatfield, PA) and were polished to a thickness of 400 μm. Slides were stained for 4 min with a solution of Stevenel’s blue at 60°C. The Stevenel’s blue staining solution was prepared by mixing a 13 mg/ml aqueous solution of methylene blue and a 20 mg/ml aqueous solution of potassium permanganate (both from Sigma-Aldrich, St. Louis, MO) in a boiling water bath. The solution was then filtered at room temperature on a 0.22 µm filter membrane (Merck Millipore, Darmstadt, Germany). The slides were rinsed in DI water at 60°C and counterstained for 1 min with Van Gieson’s picro-fuchsin. The Van Gieson’s picro-fuchsin was prepared by mixing 15 ml of a 1% acid fuchsin aqueous solution with 100 ml of saturated aqueous picric acid (both from Sigma-Aldrich, St. Louis, MO). The mixture was kept in the dark at room temperature. The histology slides were imaged with 5x, 10x, 20x and 50x N PLAN objectives mounted on a DM LM microscope (Leica Microsystems, Wetzlar, Germany). ImageJ (NIH, Bethesda, MD) was used to measure the diameter of 42 red blood cells in a blood vessel and of 38 aggregated cells in micropores, in four different regions of MP-Dry samples.
2.4. Micro-computed tomography imaging and image processing
2.4.1. Imaging procedure and parameters
Micro-CT was used to image each of the implanted scaffolds that were retrieved from the mandibles, and then these data were used to quantify the bone growth in each scaffold. Cored samples were imaged with a MicroXCT-400 scanner (Xradia, Inc., CA). All scans were performed through a 1 mm-thick Al filter, using a 0.5x magnifying lens, at 80 keV, 8 W, a binning parameter of 1 and an exposure time in single mode of 2 s. The scanning rotation step was 0.25° and the resulting voxel size was 15 μm for all samples. After reconstruction with XMReconstructor software (Xradia, Inc., CA), the 3D z-stack of micro-CT images for each scaffold, where z is the coordinate along the long axis of the sample, consisted of 400-500 images, each with 1024x1024 pixels. The number of images in the stack depended on the height of the individual sample.
2.4.2. Segmentation algorithm
The micro-CT images were processed using a custom segmentation algorithm adapted from [40] in order to extract quantitative information. Briefly, for each micro-CT image in the 3D z-stacks, the algorithm yielded a 2D (x,y)-matrix with pixels labeled as “scaffold”, “bone” or “background”. Each scaffold was first digitally isolated from the surrounding native bone and its orientation was adjusted so that the micro-CT images displayed vertical and/or horizontal scaffold rods. This step was essential so that the periodicity of the scaffold could be used in subsequent steps. In each image, the rod orientation was determined with a correlation operation. Pixels corresponding to rods were temporarily removed, and bone pixels were identified from background based on a threshold calculated to minimize within-group variance. Errors in labeling were fine-tuned using the scaffold structure and material gradient detection.
The accuracy of the segmentation algorithm was evaluated by comparing the results of the automated segmentation to those of manual segmentation, pixel by pixel, for a representative subset of images from different samples [40]. Manual segmentation is considered to be the gold standard. It is a time-consuming process consisting of manually identifying and labeling pixels on each micro-CT image.
2.5. Analysis of bone growth using segmented micro-CT data
The amount and distribution of ingrown bone in the scaffolds were quantified from the segmented micro-CT data and compared across groups. In the following, the term “volume” refers to a number of pixels in the 3D z-stack of segmented images. A macropore volume corresponds to the sum of the volumes of bone and background within the macropore.
2.5.1. Average bone volume fraction
The average bone volume fraction in the sample, was calculated and compared across groups. is given by where BV and MV are the volumes of bone and macropores in the scaffold, respectively. As is the average bone volume fraction over the entire sample, it does not account for the spatial variations in bone within the scaffold. The values for are reported as the average for each group ± the Standard Error (SE) of the mean.
2.5.2. Distribution of bone
The bone distribution was quantified for the three treatment groups and the data were represented in three ways. First, a 2D heatmap of bone volume fraction was generated from the 3D z-stack of segmented images corresponding to each sample. The heatmaps quantitatively illustrate the volume fraction of bone in the z-direction, for all (x, y). For every location (x,y) in the image stack, the volumes of bone and macropores along z, BV(x,y) and MV(x,y) respectively, were calculated. A 2D (x,y)-matrix of bone volume fraction BVF(x,y) was then determined, where BVF(x,y) was given by BVF(x,y) = BV(x,y) / MV(x,y) at each (x,y). The BVF(x,y) matrix was mapped to RGB values in order to generate a heatmap of bone volume fraction. Therefore, the heatmap does not represent the bone distribution in one micro-CT slice of the scaffold; rather, the heatmap shows the bone volume fraction along the sample height projected in a 2D representation.
The second way to represent the bone distribution was to quantify the extent of the radial bone growth from the scaffold-defect edge toward the center by identifying a bone growth front on the heatmap corresponding to each sample. To find the bone growth front, the coordinate system was first converted from Cartesian (x,y,z) to polar coordinates (r,θ,z). Here, r = 0 corresponded to the center of the scaffold, R was the scaffold radius and θ = 0 corresponded to the +x-axis. After this step, the BVF(x,y) values from the heatmaps were referred to as BVF(r,θ). A custom ImageJ (NIH, Bethesda, MD) routine was used to assess BVF(r,θ) for r incrementally from 0 to R and θ from 0 to 360° on each individual heatmap. For each θ, the value of r at which BVF(r,θ) became larger than 0.2 was detected; the collection of these r values for all θ constituted a “contour” defined as the bone growth front for each sample. Individual sample contours were averaged within each group to give an average contour. The 0.2 threshold was chosen to detect the bone growth front for all groups, while avoiding the detection of local heterogeneities. This choice was guided by qualitatively estimating the contour of the bone growth front using the heatmaps. The radial depth of the bone growth front was expressed as a percent of the radial distance from the scaffold-defect edge where is the normalized radius, for all θ. The radial depth of the bone growth front for each group is reported as the average ± SE of the mean. Note that the bone growth front only marks the limit from the center of the scaffold where BVF(r,θ) is higher than the defined threshold; it does not give the absolute values of BVF(r,θ) within or beyond the contour. By implication, there can be bone in the center of the scaffold beyond the contour limit, but the fraction is below the 0.2 threshold for BVF(r,θ). Note that the bone growth front is generated from the heatmap and therefore is not from a single micro-CT slice, but rather from the collapsed or projected data previously described.
Finally, the bone volume fraction as a function of normalized radius, was calculated to assess the homogeneity of the bone distribution. This representation of the data collapses all of the 3D data onto a single curve. The radial bone volume fraction was determined in increments of 0.1R. is given by: where are the volumes of bone and macropore at respectively. The data for each group were fit to an exponential curve according to Equation (1) using MATLAB (MathWorks, Natick, MA), in order to compare the characteristics of the curves for the different groups.
| (1) |
BVF(0) was the volume fraction of bone at the center of the defect, k was the growth rate of the exponential.
at all was also compared to the average bone volume fraction, , as a measure of the homogeneity of the bone distribution. This was done by calculating the root-mean-square deviation (RMSD) across all of from 1. The RMSD was averaged across samples within each group. Because bone grows radially from the scaffold-defect edge to the center in a cylindrical bone defect, is higher than at the scaffold-defect edge and lower in the center. The larger the RMSD, the larger the discrepancy between and and hence the less homogeneous the bone distribution.
2.6. Statistical Methods
One-way ANOVA followed by a post-hoc Tukey’s HSD test were used to detect statistically significant differences between treatment groups, with a significance level of p < 0.05 unless otherwise stated. The statistical comparisons were made using OriginPro 2016 (OriginLab, Northampton, MA). One-Way ANOVA accounts for unequal sample numbers [41]. All error bars correspond to the SE of the mean to indicate the uncertainty about the value of the mean for each measurement [42].
3. Results
3.1. Scaffold characterization
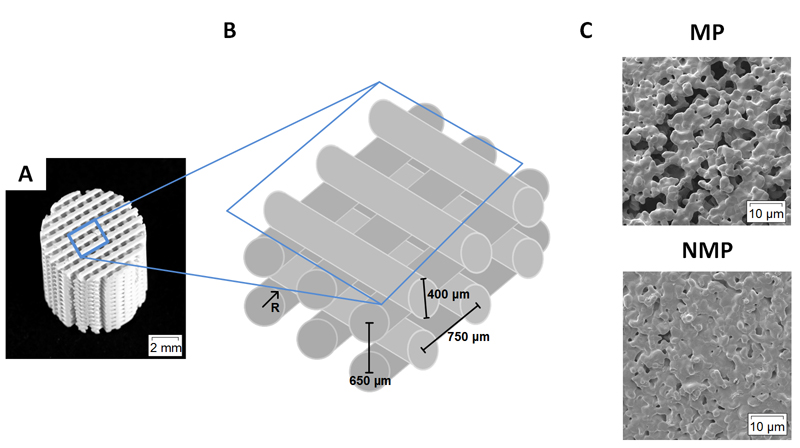
Implanted scaffolds were 8 mm in diameter and 8 mm in height (Fig. 1A). Rods were nominally 400 μm in diameter with in-plane and out-of-plane center-to-center spacing of 750 μm and 650 μm, respectively [36]. The space between scaffold rods constitutes the macropore space (Fig. 1B). MP scaffolds had microporous rods as a result of the use of a sacrificial porogen in the ink [25–27,36,32]. The porogen gave a micropore size distribution of 5.3 ± 4.1 μm [26,36] with an average interconnection size of 2.2 μm [26]. NMP rods were considered solid with micropores (< 1 μm) caused by incomplete sintering (Fig. 1C). The micropores in NMP were not interconnected and do not generate capillarity. The scaffold material for both MP and NMP scaffolds had a final composition of 87% HA and 13% β-TCP, as determined by XRD [26].
Figure 1. Scaffold macro- and microstructure.
A. Photograph of a BCP scaffold used in this study. Scaffolds were 8 mm diameter and consisted of alternating layers of orthogonal rods. B. Schematic showing a lattice of scaffold rods. So-called macropores make the space between the rods. C. Scanning electron micrographs of rod microstructure. Rods were either microporous (MP) or solid and non-microporous (NMP).
3.2. Histological observation of bone and cells in macropores and micropores
This section qualitatively describes the bone and cells in the macropores of scaffolds from all groups and in the micropores of MP scaffolds. The quantitative analysis based on micro-CT data is presented in section 3.3.
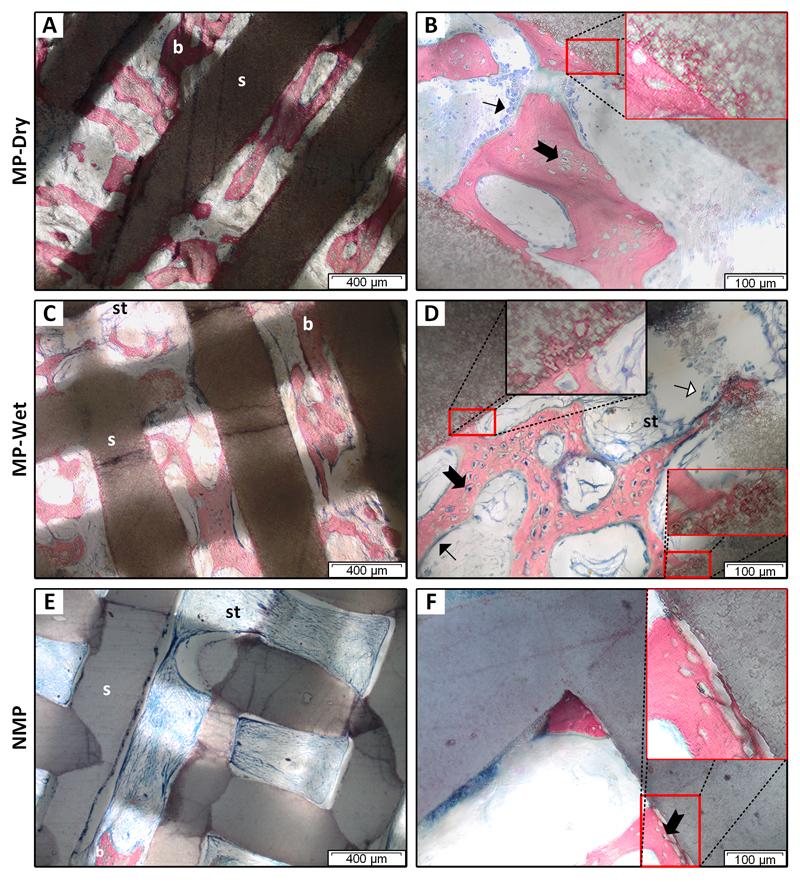
Mineralized bone was observed in the macropores of all treatment groups (Fig. 2). Most of the bone was found in the center of macropores away from the scaffold rods, although in places it was apposed to the rods. In MP scaffolds, apposed bone in the macropores appears anchored into the rods through the presence of bone tissue in micropores (Fig. 2 B, D). In contrast, apposed bone is not anchored in NMP rods (Fig. 2F). NMP had less bone in the macropores and large amounts of fibrous soft tissue (Fig. 2E) compared to MP-Dry and MP-Wet. Soft tissue in all NMP scaffolds (Fig. 2E) and most MP-Wet ones (Fig. 2D) contracted away from the scaffold rod surfaces. This was likely an artifact of histologic sample preparation. Notably, it was rarely observed in MP-Dry.
Figure 2. Tissue and cells in the scaffold macro- and micropores.
Histology slices were taken at the center of the scaffolds. Samples were stained with Stevenel’s blue and counterstained with picro-fuchsin. Bone was pink/red; soft tissue, osteoid, cell cytoplasm were light blue; and cell nuclei were dark blue. Mineralized bone (b) was observed in macropores between scaffold rods (s) for all three groups. Fibrous soft tissue (st) was prevalent in the center of NMP scaffolds (E). Osteoblast-like cells (➙) lined mineralized bone in the macropores (B, D). Osteocytes (➞) were in lacunae (B, D, F). Osteoclast-like cells (⇾) were found on rod and bone surfaces in some areas (D). In the macropores of MP scaffolds, mineralized bone is anchored in rods (B, D, insets). In NMP, bone is not anchored (F, inset). Soft tissue in MP-Wet and NMP is contracted and frequently not in contact with the rods (C- E).
Bone in macropores had lacunae containing osteocytes (Fig. 2 B, D, F). Some lacunae appeared empty, suggesting that the polishing for histology removed the osteocytes from those lacunae. Bone was lined with cells morphologically similar to osteoblasts (Fig. 2 B, D), characterized by their cuboidal shape [43]. Cells morphologically similar to osteoclasts, characterized by their larger size, multiple nuclei and ruffled membrane were found on rod and bone surfaces, likely resorbing the calcium phosphate material [43–45] (Fig. 3 A, B, D, E). Cells were abundant in the soft tissue in the macropores of MP-Dry (Fig. 2 B). Blood vessels containing red blood cells (RBCs) were also observed in the macropores of MP-Dry (Fig. 3A). RBCs have a characteristic discoid shape [46] and stain uniformly due to their lack of nucleus [47]. In Fig. 3A, the average diameter of the RBCs in the blood vessel was 3.4 ± 0.8 µm. The presence of blood vessels indicates that extensive vasculature developed to sustain nutrient and waste transport in the growing bone. The soft tissue contraction in the macropores of MP-Wet and NMP scaffolds made it difficult to observe cells and blood vessels in the macropores (Fig. 2 C-F).
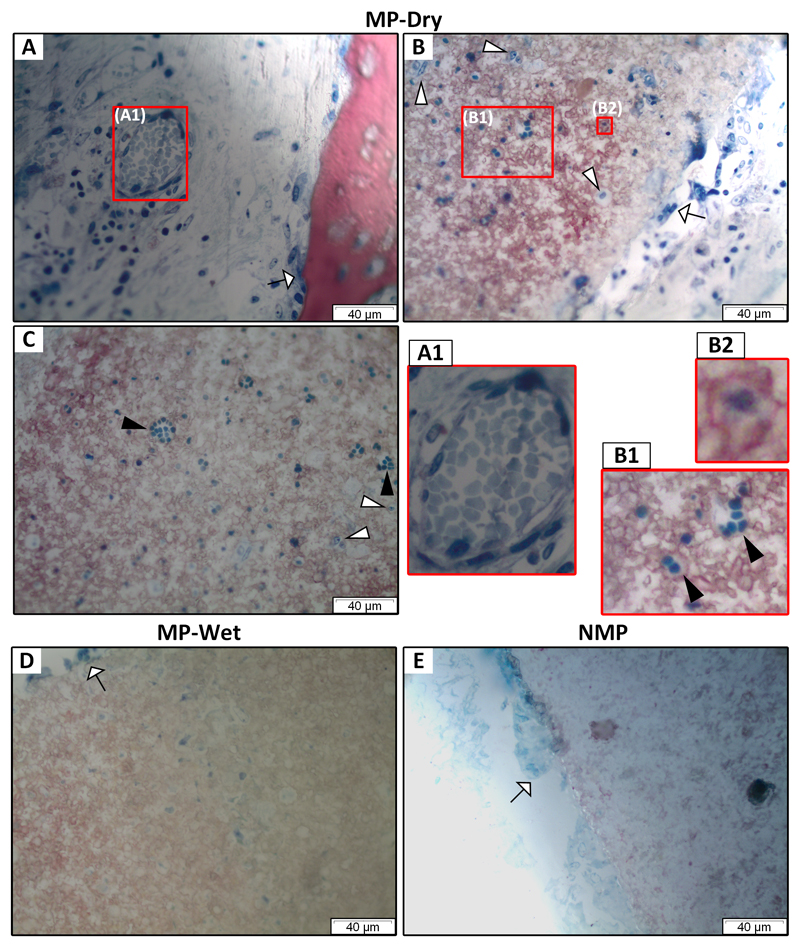
Figure 3. Blood vessel in macropore and scaffold microstructure after implantation.
(A-C) for MP-dry, (D) for MP-Wet and (E) for NMP. A blood vessel in a macropore (A1) confirms that vasculature develops in implanted scaffolds. Red blood cells inside the blood vessel appear aggregated and without nuclei. Blue-stained cells, which are not identified, are visible within the micropores of rods (A-D). In contrast, there are no cells in the rods of NMP which does not have micropores (E). In MP-Dry, some cells appear in aggregates and are uniformly dark blue (►) with no cell nucleus (B, C). They conform to the shape of surrounding cells and the pore walls (B1). Other cells in micropores are predominantly isolated, show an apparent nucleus, and stain lighter blue (▻). In some micropores, cells are surrounded by bone (B2). Osteoclast-like cells (⇾), characterized by their larger size and multiple nuclei, are on bone (A) and rod surfaces (B, D, E).
Cells were in the micropores of both MP-Dry and MP-Wet scaffolds, though the density was higher in MP-Dry (Fig. 3 B-D). It was not possible to identify the cells in the micropores. In MP-Dry only, some cells in the micropores appeared in aggregates (Fig. 3 B1, C). They were 3.0 ± 0.5 µm in diameter, had a uniform dark blue color, with no apparent nucleus, and concavities on their surface. This morphology is similar to that of RBCs seen in blood vessels in the macropores (Fig. 3A). These aggregated cells were not observed in the micropores of MP-Wet (Fig. 3D). To note, no cells were observed in the rods of NMP as these samples have no micropores other than those (< 1 µm) caused by sintering (Fig. 3E).
Bone was observed in the micropores of MP scaffolds. Fig. 3B2 shows a cell in a micropore surrounded by bone; the cell likely synthesized bone after having migrated or having been drawn by capillarity through the microporous network. Bone filled some micropores that either do not contain cells or in which cells are buried in the bone and are not visible in the histologic section (Fig. 2 B, D).
3.3. Quantification of bone regeneration using segmented micro-CT data
3.3.1. The custom algorithm accurately segments micro-CT images
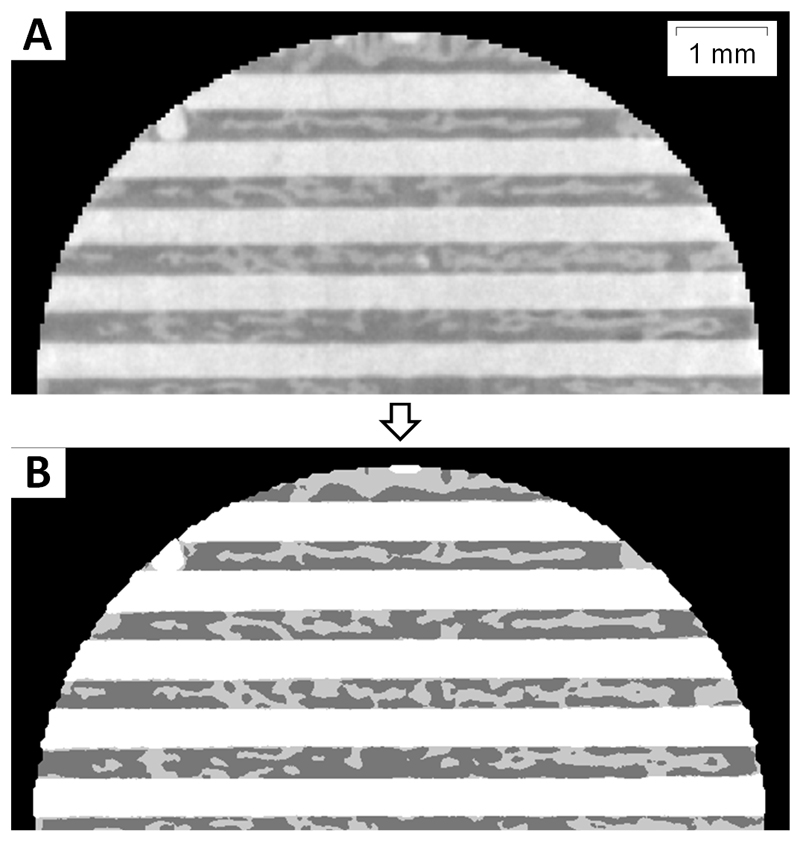
The segmentation algorithm processed 500 images per hour. The segmentation results were more than 92% accurate compared to manual segmentation, which is considered the gold standard [40]. A representative image demonstrating the results of the segmentation algorithm is shown in Figure 4.
Figure 4. Representative image showing results from the automated segmentation algorithm used to quantify bone ingrowth in scaffold macropores.
A. Original 2D micro-CT image from a 3D z-stack of images corresponding to a MP-Dry scaffold. In this image, we see horizontal rods from one scaffold layer. Mineralized bone is in the macropore space between the scaffold rods. B. Label matrix resulting from the segmentation of A. Scaffold pixels are white, bone pixels are light gray and soft tissue or background pixels are dark gray.
3.3.2. Microporosity increases the average bone volume fraction
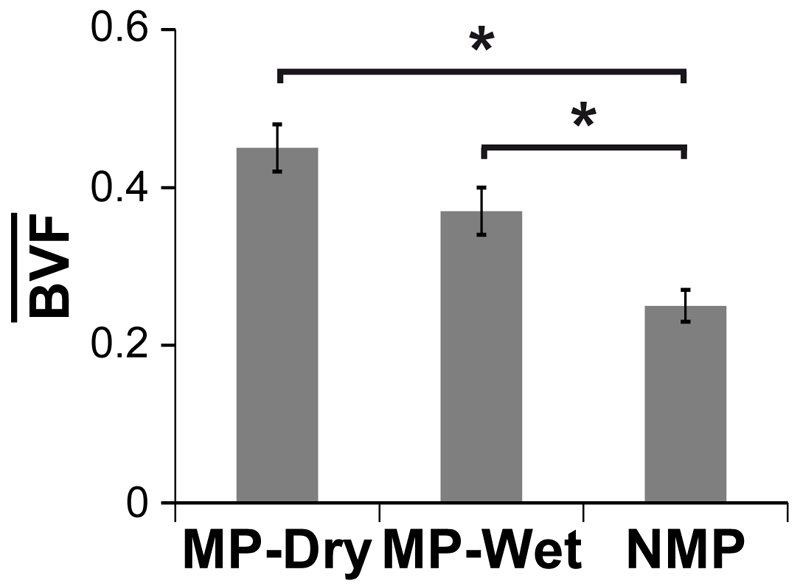
The average bone volume fraction, is one measure to compare the extent of bone regeneration across treatment groups. Both types of MP scaffolds, MP-Wet and MP-Dry, had a significantly higher than NMP (p < 0.05). The for MP-Dry, MP-Wet, and NMP were 0.45±0.03, 0.37±0.03 and 0.25±0.02, respectively, reported as the average ± SE of the mean. There was no significant difference in between MP-Dry and MP-Wet (p = 0.15), indicating that the suppression of capillary forces by filling the pores with PBS prior to implantation had no effect on the average bone volume fraction (Fig. 5).
Figure 5. Average bone volume fraction in the scaffolds,
Both types of MP scaffolds had a significantly higher than NMP (p < 0.05). There was no difference in between MP-Dry and MP-Wet (p = 0.10).
3.3.3. Micropore-induced capillarity enhances the bone distribution
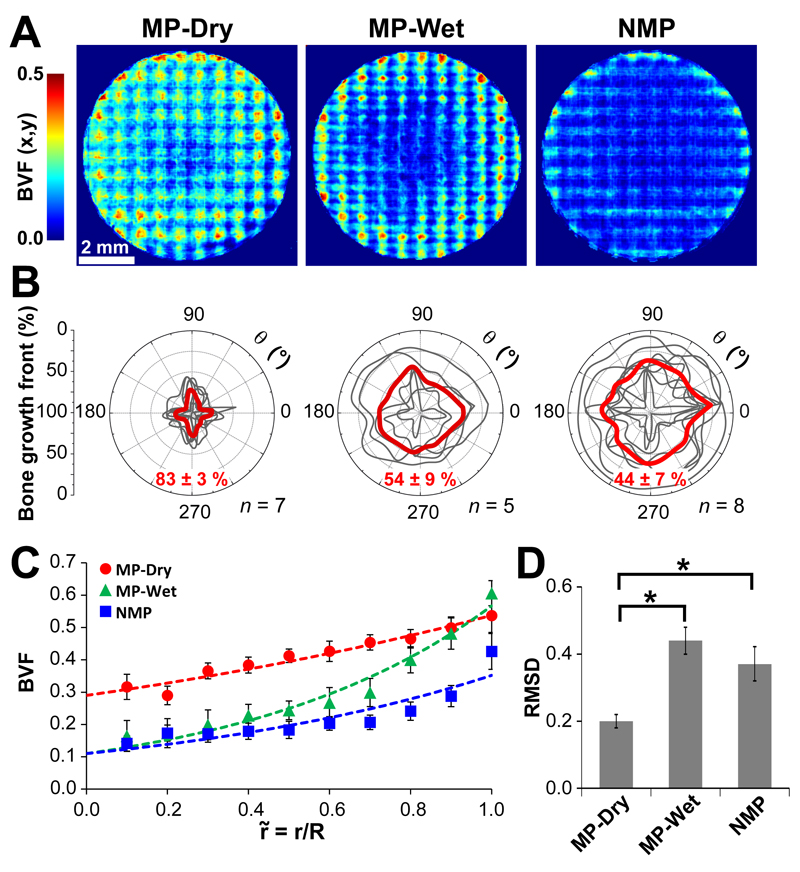
is an important measure of bone regeneration, however it is not a measure of bone distribution. The bone distribution in the scaffolds is an important parameter for the assessment of the overall efficacy of the scaffold. The distribution was evaluated for each group by examining the micro-CT data in three different ways: by using heatmaps to illustrate the bone volume fraction in the z-direction for all (x,y) (Fig.6A); by quantitatively determining a bone growth front as a function of angle θ (Fig. 6B); and by collapsing all of the data onto a single curve of (Fig. 6C).
Figure 6. Quantitative evaluation of the bone distribution.
A. Average heatmaps of bone volume fraction. The BVF(x,y) values for MP-Dry are qualitatively more homogeneous throughout the heatmap compared to MP-Wet and NMP. Both MP-Wet and NMP have a distinct central region with less bone, while the central region is less distinct for MP-Dry. B. Bone growth front. The bone growth front for each individual sample is shown in gray and the average for each group is in red. The growth front in MP-Dry extends the furthest to the center and shows less variability compared to MP-Wet and NMP. The front is significantly closer to the center in MP-Dry than in MP-Wet and NMP (p < 0.01). There is no difference in the depth of the growth front between MP-Wet and NMP. C. Radial bone volume fraction, For all groups, is higher at the scaffold-defect edge than in the center of the scaffold. The dashed lines correspond to the exponential fits given by Equation (1) and Table 1. MP-Wet and NMP have a larger discrepancy in between the periphery and the center as compared to MP-Dry. D. Average root-mean-square deviation, RMSD, of from 1 across all . RMSD is significantly less (p > 0.05) for MP-Dry than for MP-Wet and NMP. There is no difference between MP-Wet and NMP. Therefore, is closer to for MP-Dry compared to MP-Wet and NMP.
The average heatmaps (Fig. 6A) collapse the 3D data into a 2D image to show the spatial distribution of BVF(x,y), the bone volume fraction along z. In all treatment groups, BVF(x,y) is higher at the scaffold-defect edge than in the center of the scaffold. However, the heatmap of MP-Dry shows higher BVF(x,y) in the center of the scaffold compared to the two other groups. MP-Wet and NMP each show a large area near the center that contains little bone, and have dense bone at the periphery. While the heatmaps provide a quantitative representation of the bone volume fraction in z, they represent the data semi-quantitatively in the x,y-plane and do not fully illustrate the variation in individual samples or across groups. The heatmaps also show the periodic structure of the scaffolds relative to the ingrown bone.
The contour of the bone growth front for each individual sample and the average for each group are shown in Fig. 6B. The depth of the bone growth front and the shape of the resulting contour illustrate the variation in the radial bone growth both within each group and across groups. The average radial depth of the bone growth front is 83 ± 3% for MP-Dry, 54 ± 9% for MP-Wet, and 44 ± 7% for NMP, reported as the average ± SE of the mean. Thus the bone growth front extends significantly further to the center in MP-Dry than in MP-Wet and NMP (p < 0.01). However, the front is not significantly different in MP-Wet versus NMP. The shape and depth of the growth front have less variability for the MP-Dry samples. In contrast, the growth front for NMP has a larger range in depth and shape. The contour for MP-Wet samples is qualitatively more similar to that from NMP than MP-Dry in terms of depth and shape. Note that the contour defines the region of the scaffold where BVF(r,θ) is larger than the 0.2 threshold; therefore, there could be bone in the center of the scaffold beyond the contour limit, but with a BVF(r,θ) smaller than 0.2.
The bone volume fraction as a function of the normalized radius, was compared between groups (Fig. 6C) in order to further assess the homogeneity of the bone distribution. For all groups, is larger at the defect periphery than in the center, consistent with bone growth from the scaffold-defect edge. The parameters of the exponential fits and the corresponding R-squared values are given in Table 1. BVF(0) for MP-Dry is three times higher than for MP-Wet and NMP, indicating that the bone volume fraction in the center of the scaffolds, i.e. at low values of , is higher in MP-Dry. The exponential coefficient, k, for MP-Dry is nearly three times smaller than for MP-Wet and twice smaller than for NMP. This indicates that bone distribution is more homogeneous in MP-Dry, compared to the two other groups. The large gradient in the near the edge of the defect and the more pronounced slope of the curve for large for MP-Wet and NMP shows a high discrepancy between the values at the center and those at the edge for those scaffolds. This indicates that bone is more concentrated at the periphery of the scaffold than in the center in MP-Wet and NMP as compared to MP-Dry.
Table 1.
Parameters of the exponential fits for the for the data shown in Fig. 6C.
| BVF(0) | k | R2 | |
|---|---|---|---|
| MP-Dry | 0.29 | 0.62 | 0.95 |
| MP-Wet | 0.11 | 1.65 | 0.96 |
| NMP | 0.11 | 1.17 | 0.81 |
was compared to the average (Fig. 5) using the RMSD (Fig. 6D). The RMSD was significantly less for MP-Dry (0.20 ± 0.02) than for MP-Wet (0.44 ± 0.04) and NMP (0.37 ± 0.05) (p < 0.05). There was no significant difference between the RMSD values for MP-Wet and NMP (p = 0.41). The result indicates that the radial bone volume fraction was overall closer to the average for the scaffold, in MP-Dry compared to MP-Wet and NMP.
Together, the different metrics considered above, i.e. BVF(x,y), the bone growth front, and with RMSD, all collectively show that the ingrown bone is more homogeneously distributed in MP-Dry scaffolds than in MP-Wet and NMP. They also show that MP-Wet and NMP have a similar distribution of bone relative to MP-Dry.
4. Discussion
This study investigated the effects of micropore-induced capillarity on bone regeneration in BCP scaffolds that were implanted in porcine mandibular defects for three weeks. To our knowledge, this is the first work that investigated the role of capillarity specifically for enhancing bone growth in vivo. The results demonstrated that microporosity increases the average bone volume fraction in the scaffold, but that micropore-induced capillarity alone enhances bone distribution.
In the histological evaluation, bone in macropores appeared healthy and active with osteocytes in lacunae, osteoblast-like cells lining bone and osteoclast-like cells on bone and BCP rods. In MP scaffolds, bone was also found surrounding cells trapped in micropores (Fig. 3B2). Some bone in the macropores extended into the micropores (Fig. 2B, D). These results confirmed our previous findings that scaffold microstructure provides space for microscale bone growth, leading to an interpenetrating scaffold-bone composite at multiple length scales [26].
The presence of cells in the micropores can be attributed to two mechanisms based on the results from this study. The presence of cells in micropores of MP-Wet scaffolds suggests that cells have the capacity to migrate through the microporous network. The presence of more cells, and cells that may be RBCs, in the micropores of MP-Dry suggests that in addition to migration, capillary forces also draw cells into micropores. This is supported by our previous work [32]. Others have shown that cells penetrate micropores in vitro under the influence of capillary forces in scaffolds dipped vertically or horizontally in cell suspensions [33–35]. In [32], we showed that micropore-induced capillarity operates not only in vitro to draw cells into the micropores of 2D substrates, but also in vivo right at the time of implantation when the dry microporous scaffold comes in contact with physiological fluid containing endogenous cells and biomolecules in the defect. In the present study, we showed that this micropore-induced capillarity mechanism significantly enhances bone distribution in scaffolds in vivo (Fig. 6). Indeed, the presence of more cells in the micropores of MP-Dry suggests that micropore-induced capillary forces draw cells and biomolecules from the physiological fluid in the defect and distribute them not only in the micropores, but also in the macropores throughout the scaffold. This initial seeding of the scaffold macropores and micropores with native cells and biomolecules may be the reason why bone growth is more homogeneous in MP-Dry than in MP-Wet. In MP-Wet, the micropore-induced capillarity was used prior to implantation to fill the macro- and micropores with PBS. Therefore this initial seeding of cells and biomolecules does not occur at the time of implantation; the pores are already filled with PBS. However, cells and biomolecules can migrate and diffuse, respectively, into the scaffold macro- and micropores during the three-week implantation time.
Aggregates of cells lacking nuclei were present in the micropores of MP-Dry. These cells stained homogeneously and had a different morphology compared to other nucleated cells in micropores. The size and morphology were consistent with RBCs found in blood vessels in macropores (Fig. 3A) and with published characteristics of RBCs: absence of a nucleus and dimensions of 2 to 8μm [46]. When stained, RBCs can shrink in size and appear darker than other cells [46]. The presence of these cells leads us to hypothesize that RBCs are also drawn through the microporous network by capillarity and that the capillary forces are large enough to draw several cells simultaneously into a single micropore. This is significant because RBCs do not have the capacity to actively migrate since they lack a nucleus. They could not travel through the microporous network unless driven by an active mechanism such as capillarity. These results suggest that capillarity increases not only the amount, but also the variety of cells in the micropores. We emphasize that whether the presence of cells in scaffold micropores affects bone growth in the macropores is not known and needs further investigation.
The similarity in between MP-Dry and MP-Wet indicates that the average bone volume fraction is not significantly affected by the suppression of capillary forces (Fig. 5), even though the bone distribution is. The difference between MP-Dry and MP-Wet is that, at the time of implantation, MP-Dry have the capacity to generate capillary forces and draw fluid and cells into the micropores. In contrast, the capillary forces have been eliminated in MP-Wet by submerging scaffolds in PBS before implantation. One possibility for the similarity in for these two groups is that the PBS did not saturate the entire pore space in MP-Wet. Others have shown that air can be trapped in porous calcium phosphate scaffolds [48]. If regions of MP-Wet scaffolds with empty, dry micropores came in contact with the pool of blood at the time of implantation, they might generate capillary forces in the MP-Wet scaffold. This would decrease the difference in between MP-Wet and MP-Dry. Another explanation is that capillary forces may affect the bone distribution more than they affect the average bone volume fraction. In this case, despite the absence of capillary forces at the time of implantation, microporosity would still constitute an advantage that is responsible for the higher in MP-Wet compared to NMP. Nutrients and osteoinductive biomolecules, including bone morphogenetic proteins, present in the native bone environment [49,50], may be able to diffuse in and out of PBS-filled macro- and micropores under the influence of concentration gradients, and the micropores would play a role of reservoirs for these molecules. These molecules may contribute to the higher in MP-Wet compared to NMP. Biological apatite may also precipitate in the micropores [19], though it was not possible to observe this in this study.
The MP-Dry treatment had a more homogeneous distribution of bone by multiple measures compared to MP-Wet and NMP. The less homogeneous bone distribution in MP-Wet and NMP compared to MP-Dry is manifested by a dense ring of bone near the scaffold-defect edge and a large region with little bone in the center of the scaffold (Fig. 6). The bone growth front extends further towards the center and has less variability in shape and radial depth in MP-Dry compared to MP-Wet and NMP. The lesser variability of the bone growth front within the MP-Dry group indicates that capillary forces may improve the consistency of bone growth into the scaffolds, which is manifested through the enhancement of bone distribution. In MP-Wet and NMP, the higher variability of the bone growth front indicates that, in the absence of a driving force that homogenizes bone distribution, in this case capillarity, the amount and distribution of growth are less certain.
The results of this study support our hypothesis that micropore-induced capillarity is responsible for the enhancement in bone distribution that we have observed in microporous BCP scaffolds compared to non-microporous ones. To our knowledge, we are the first to show that capillary forces enhance the homogeneity of bone distribution in scaffolds in vivo. A homogeneous bone distribution is considered an important condition for bone implant success, especially in large defects. It reduces the occurrence of large empty spaces that compromise the mechanical properties of the implanted scaffold [51]. In addition, effective vascularization is a prerequisite for sustainable bone growth in the scaffold [9,14] to ensure nutrient and waste transport. A homogeneous bone distribution therefore implies that extensive vasculature has developed throughout the scaffold. Effective vascularization throughout the scaffold decreases the risk for tissue necrosis at the center of the defect [52].
The higher average bone volume fraction in both types of MP scaffolds compared to NMP confirms and strengthens previous results obtained in smaller defects with the same scaffold system as was used in this work. In [27], we implanted MP and NMP scaffolds with and without bone morphogenetic protein-2 (BMP-2), a potent osteoinductive molecule, in 5 mm defects. Scaffolds containing BMP-2 in [27] would be classified as “wet” in the present study because the BMP-2 was incorporated via a BMP-2-saturated, liquid-gelatin suspension. With the increase in the defect size to 8 mm here combined with the lack of BMP-2 compared to [27], we expected to have smaller for both MP and NMP samples. However, this was not the case. here was 0.42 ± 0.02 for all the MP scaffolds, MP-Dry and MP-Wet combined, and 0.25 ± 0.02 for NMP. In [27], was 0.39 ± 0.02 for all MP and 0.34 ± 0.02 for all NMP, irrespective of whether the scaffolds contained BMP-2 or not. Hence, the increase in the defect size and the absence of BMP-2 did not affect the in MP samples in this study, whereas they resulted in a decrease in for NMP. These results highlight the advantage conferred by microporosity in BCP scaffolds for the treatment of large defects. It is not possible to compare the distribution of bone between this study and [27] as the distribution was not evaluated in the same way.
Micropore-induced capillarity in BCP scaffolds clearly enhances the efficacy of bone regeneration by improving the homogeneity of bone distribution throughout the scaffold. This mechanism is especially relevant for the treatment of large, critical size defects that can be on the order of centimeters [53]. We have shown that capillary forces in BCP scaffolds can be tailored through the control of scaffold microporosity [32]. Thus, scaffolds with specifically designed microstructures can help to direct the infiltration of cells and fluid relevant to bone regeneration in a way that maximizes bone ingrowth. Ultimately, this can be exploited to guide bone growth in large and complex defects.
5. Conclusions
This study is the first to investigate the effects of micropore-induced capillarity on bone regeneration in vivo through the quantification of the distribution of ingrown bone in implanted BCP scaffolds. We showed that bone distribution was more homogeneous in MP scaffolds with active micropore-induced capillarity (MP-Dry) than in MP scaffolds with suppressed micropore-induced capillarity (MP-Wet), although no significant difference was observed in the average bone volume fractions, for these two groups. The radial bone distribution was not significantly different when comparing MP-Wet and NMP, although NMP had a lower than both MP-Wet and MP-Dry. Cells and bone were found in scaffold micropores, primarily in MP-Dry, but occasionally also in MP-Wet. It is not known whether cells in micropores influence bone growth in macropores. The results of this study clearly show that capillarity enhances bone distribution in scaffold macropores. The result of enhanced bone distribution is especially relevant for the treatment of large defects where the ingrown bone must reach the center of the defect to ensure implant success. In future studies, designed microporosity and macroporosity in structural scaffolds can be used to direct or drive the blood and marrow components deep into the scaffold through the use of capillarity to better address regeneration in large, complex bone defects.
Acknowledgements
This work was funded by a grant from the AO Foundation (S-11-17W) and in part by a National Science Foundation grant (CMMI 09-00184). LER acknowledges the Région Rhône-Alpes for International Cooperation and Mobility (CMIRA) and the GIS Matériaux (Grenoble, France) for travel fellowships. AJWJ acknowledges the support of the Nanosciences Foundation program for Chairs of Excellence (Grenoble, France). CP acknowledges the European Commission (FP7) for a European Research Council grant (GA259370). The funding sources were not involved in the study design, collection and processing of data, report preparation or decision to publish. We also acknowledge staff of the Imaging Technology Group at the Beckman Institute for Advanced Science and Technology, part of the University of Illinois at Urbana-Champaign. In particular we acknowledge L. Yin, S. J. Robinson, C. L. Wallace and T. Ross for training in the use of micro-CT instruments, scanning electron microscopy, and image processing software, respectively.
Contributor Information
Laurence E. Rustom, Email: rustom2@illinois.edu.
Thomas Boudou, Email: thomas.boudou@grenoble-inp.fr.
Siyu Lou, Email: lousiyushanghai@gmail.com.
Isabelle Pignot-Paintrand, Email: isabelle.paintrand@grenoble-inp.fr.
Brett W. Nemke, Email: brett.nemke@wisc.edu.
Yan Lu, Email: yan.lu@wisc.edu.
Mark D. Markel, Email: mark.markel@wisc.edu.
Catherine Picart, Email: catherine.picart@grenoble-inp.fr.
References
- [1].Kinaci A, Neuhaus V, Ring DC. Trends in bone graft use in the United States. Orthopedics. 2014;37:e783–8. doi: 10.3928/01477447-20140825-54. [DOI] [PubMed] [Google Scholar]
- [2].Campana V, Milano G, Pagano E, Barba M, Cicione C, Salonna G, Lattanzi W, Logroscino G. Bone substitutes in orthopaedic surgery: from basic science to clinical practice. J Mater Sci Mater Med. 2014;25:2445–61. doi: 10.1007/s10856-014-5240-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Habraken W, Habibovic P, Epple M, Bohner M. Calcium phosphates in biomedical applications: materials for the future? Mater Today. 2015 [Google Scholar]
- [4].UCBPI Office. Census Bureau Releases Comprehensive Analysis of Fast-Growing 90-and-Older Population - Aging Population - Newsroom - U.S. Census Bureau. [accessed March 14, 2016]; (n.d.). https://www.census.gov/newsroom/releases/archives/aging_population/cb11-194.html.
- [5].Bigham-Sadegh A, Oryan A. Basic concepts regarding fracture healing and the current options and future directions in managing bone fractures. Int Wound J. 2015;12:238–47. doi: 10.1111/iwj.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Faour O, Dimitriou R, Cousins CA, Giannoudis PV. The use of bone graft substitutes in large cancellous voids: any specific needs? Injury. 2011;42(Suppl 2):S87–90. doi: 10.1016/j.injury.2011.06.020. [DOI] [PubMed] [Google Scholar]
- [7].Griffin KS, Davis KM, McKinley TO, Anglen JO, Chu T-MG, Boerckel JD, Kacena MA. Evolution of Bone Grafting: Bone Grafts and Tissue Engineering Strategies for Vascularized Bone Regeneration. Clin Rev Bone Miner Metab. 2015;13:232–244. [Google Scholar]
- [8].Hollister SJ. Scaffold design and manufacturing: from concept to clinic. Adv Mater. 2009;21:3330–42. doi: 10.1002/adma.200802977. [DOI] [PubMed] [Google Scholar]
- [9].Brydone AS, Meek D, Maclaine S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc Inst Mech Eng H. 2010;224:1329–43. doi: 10.1243/09544119JEIM770. [DOI] [PubMed] [Google Scholar]
- [10].Bohner M, Loosli Y, Baroud G, Lacroix D. Commentary: Deciphering the link between architecture and biological response of a bone graft substitute. Acta Biomater. 2011;7:478–84. doi: 10.1016/j.actbio.2010.08.008. [DOI] [PubMed] [Google Scholar]
- [11].Lapczyna H, Galea L, Wüst S, Bohner M, Jerban S, Sweedy A, Doebelin N, van Garderen N, Hofmann S, Baroud G, Müller R, et al. Effect of grain size and microporosity on the in vivo behaviour of β-tricalcium phosphate scaffolds. Eur Cells Mater. 2014;28:299–319. doi: 10.22203/ecm.v028a21. [DOI] [PubMed] [Google Scholar]
- [12].Hollister SJ, Murphy WL. Scaffold translation: barriers between concept and clinic. Tissue Eng Part B Rev. 2011;17:459–74. doi: 10.1089/ten.teb.2011.0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Loh QL, Choong C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng Part B Rev. 2013;19:485–502. doi: 10.1089/ten.teb.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu JX, Buza JA, Leucht P. Clinical Aspects of Fracture Healing: An Overview. Clin Rev Bone Miner Metab. 2015;13:208–221. [Google Scholar]
- [15].Moore WR, Graves SE, Bain GI. Synthetic bone graft substitutes. ANZ J Surg. 2001;71:354–361. [PubMed] [Google Scholar]
- [16].Fernandez-Yague MA, Abbah SA, McNamara L, Zeugolis DI, Pandit A, Biggs MJ. Biomimetic Approaches in Bone Tissue Engineering: Integrating Biological and Physicomechanical Strategies. Adv Drug Deliv Rev. 2014;84:1–29. doi: 10.1016/j.addr.2014.09.005. [DOI] [PubMed] [Google Scholar]
- [17].Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–91. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- [18].El-Ghannam A. Bone reconstruction: from bioceramics to tissue engineering. Expert Rev Med Devices. 2005;2:87–101. doi: 10.1586/17434440.2.1.87. [DOI] [PubMed] [Google Scholar]
- [19].LeGeros RZ. Calcium phosphate-based osteoinductive materials. Chem Rev. 2008;108:4742–53. doi: 10.1021/cr800427g. [DOI] [PubMed] [Google Scholar]
- [20].Schmidt-Bleek K, Willie BM, Schwabe P, Seemann P, Duda GN. BMPs in bone regeneration: Less is more effective, a paradigm-shift. Cytokine Growth Factor Rev. 2015;27:141–148. doi: 10.1016/j.cytogfr.2015.11.006. [DOI] [PubMed] [Google Scholar]
- [21].Tannoury CA, An HS. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014;14:552–9. doi: 10.1016/j.spinee.2013.08.060. [DOI] [PubMed] [Google Scholar]
- [22].Habibovic P, Yuan H, van den Doel M, Sees TM, van Blitterswijk CA, de Groot K. Relevance of osteoinductive biomaterials in critical-sized orthotopic defect. J Orthop Res. 2006;24:867–76. doi: 10.1002/jor.20115. [DOI] [PubMed] [Google Scholar]
- [23].Habibovic P, Sees TM, Van Blitterswijk CA, De Groot K. Influence of physico-chemical properties, macro- And microstructure on osteoinductive potential of calcium-phosphate ceramics. Key Eng Mater. 2006;309-311:1307–10. [Google Scholar]
- [24].Le Nihouannen D, Daculsi G, Saffarzadeh A, Gauthier O, Delplace S, Pilet P, Layrolle P. Ectopic bone formation by microporous calcium phosphate ceramic particles in sheep muscles. Bone. 2005;36:1086–93. doi: 10.1016/j.bone.2005.02.017. [DOI] [PubMed] [Google Scholar]
- [25].Lan Levengood SK, Polak SJ, Poellmann MJ, Hoelzle DJ, Maki AJ, Clark SG, Wheeler MB, Wagoner Johnson AJ. The effect of BMP-2 on micro- and macroscale osteointegration of biphasic calcium phosphate scaffolds with multiscale porosity. Acta Biomater. 2010;6:3283–91. doi: 10.1016/j.actbio.2010.02.026. [DOI] [PubMed] [Google Scholar]
- [26].Lan Levengood SK, Polak SJ, Wheeler MB, Maki AJ, Clark SG, Jamison RD, Wagoner Johnson AJ. Multiscale osteointegration as a new paradigm for the design of calcium phosphate scaffolds for bone regeneration. Biomaterials. 2010;31:3552–63. doi: 10.1016/j.biomaterials.2010.01.052. [DOI] [PubMed] [Google Scholar]
- [27].Polak SJ, Levengood SKL, Wheeler MB, Maki AJ, Clark SG, Wagoner Johnson AJ. Analysis of the roles of microporosity and BMP-2 on multiple measures of bone regeneration and healing in calcium phosphate scaffolds. Acta Biomater. 2011;7:1760–71. doi: 10.1016/j.actbio.2010.12.030. [DOI] [PubMed] [Google Scholar]
- [28].Annaz B, Hing KA, Kayser M, Buckland T, Di Silvio L. Porosity variation in hydroxyapatite and osteoblast morphology: a scanning electron microscopy study. J Microsc. 2004;215:100–10. doi: 10.1111/j.0022-2720.2004.01354.x. [DOI] [PubMed] [Google Scholar]
- [29].Li X, van Blitterswijk CA, Feng Q, Cui F, Watari F. The effect of calcium phosphate microstructure on bone-related cells in vitro. Biomaterials. 2008;29:3306–16. doi: 10.1016/j.biomaterials.2008.04.039. [DOI] [PubMed] [Google Scholar]
- [30].Habibovic P, Yuan H, van der Valk CM, Meijer G, van Blitterswijk CA, de Groot K. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials. 2005;26:3565–75. doi: 10.1016/j.biomaterials.2004.09.056. [DOI] [PubMed] [Google Scholar]
- [31].Habibovic P, Sees TM, van den Doel MA, van Blitterswijk CA, de Groot K. Osteoinduction by biomaterials--physicochemical and structural influences. J Biomed Mater Res A. 2006;77:747–62. doi: 10.1002/jbm.a.30712. [DOI] [PubMed] [Google Scholar]
- [32].Polak SJ, Rustom LE, Genin GM, Talcott M, Wagoner Johnson AJ. A mechanism for effective cell-seeding in rigid, microporous substrates. Acta Biomater. 2013;9:7977–86. doi: 10.1016/j.actbio.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bai H, Wang D, Delattre B, Gao W, De Coninck J, Li S, Tomsia AP. Biomimetic gradient scaffold from ice-templating for self-seeding of cells with capillary effect. Acta Biomater. 2015;20:113–9. doi: 10.1016/j.actbio.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Oh DS, Hyuk Kim Y, Ganbat D, Han M-H, Lim P, Back J-H, Lee HY, Tawfeek H. Bone marrow absorption and retention properties of engineered scaffolds with micro-channels and nano-pores for tissue engineering: A proof of concept. Ceram Int. 2013;39:8401–8410. [Google Scholar]
- [35].Oh DS, Kim YJ, Hong M-H, Han M-H, Kim K. Effect of capillary action on bone regeneration in micro-channeled ceramic scaffolds. Ceram Int. 2014;40:9583–9589. [Google Scholar]
- [36].Cordell JM, Vogl ML, Wagoner Johnson AJ. The influence of micropore size on the mechanical properties of bulk hydroxyapatite and hydroxyapatite scaffolds. J Mech Behav Biomed Mater. 2009;2:560–70. doi: 10.1016/j.jmbbm.2009.01.009. [DOI] [PubMed] [Google Scholar]
- [37].Hoelzle DJ, Alleyne AG, Wagoner Johnson AJ. Micro-robotic deposition guidelines by a design of experiments approach to maximize fabrication reliability for the bone scaffold application. Acta Biomater. 2008;4:897–912. doi: 10.1016/j.actbio.2008.02.018. [DOI] [PubMed] [Google Scholar]
- [38].Hoelzle DJ, Svientek SR, Alleyne AG, Wagoner Johnson AJ. Design and manufacture of combinatorial calcium phosphate bone scaffolds. J Biomech Eng. 2011;133:101001. doi: 10.1115/1.4005173. [DOI] [PubMed] [Google Scholar]
- [39].Sterchi DL, Eurell JAC. An evaluation of methylmethacrylate mixtures for hard tissue embedding. J Histotechnol. 1995;18:45–50. [Google Scholar]
- [40].Polak SJ, Candido S, Levengood SKL, Wagoner Johnson AJ. Automated segmentation of micro-CT images of bone formation in calcium phosphate scaffolds. Comput Med Imaging Graph. 2012;36:54–65. doi: 10.1016/j.compmedimag.2011.07.004. [DOI] [PubMed] [Google Scholar]
- [41].Analysis of Variance -ANOVA. [accessed August 10, 2016]; (n.d.). https://medicine.tcd.ie/neuropsychiatric-genetics/assets/pdf/2009_3_ANOVA.pdf.
- [42].Altman DG, Bland JM. Standard deviations and standard errors. BMJ. 2005;331:903. doi: 10.1136/bmj.331.7521.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Simmons DJ, Menton DN, Russell JE, Smith R, Walker WV. Bone cell populations and histomorphometric correlates to function. Anat Rec. 1988;222:228–236. doi: 10.1002/ar.1092220303. [DOI] [PubMed] [Google Scholar]
- [44].Jordan HE. Further evidence concerning the function of osteoclasts. Anat Rec. 1921;20:280–295. [Google Scholar]
- [45].Yamada S, Heymann D, Bouler JM, Daculsi G. Osteoclastic resorption of biphasic calcium phosphate ceramic in vitro. J Biomed Mater Res. 1997;37:346–52. doi: 10.1002/(sici)1097-4636(19971205)37:3<346::aid-jbm5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- [46].Ponder E, Yeager JF, Charipper HA. Studies in comparative Haematology-I. Camelidae. Q J Exp Physiol. 1928;19:115–126. [Google Scholar]
- [47].Thompson EL. Time and rate of loss of nuclei by the red blood cells of human embryos. Anat Rec. 1951;111:317–325. doi: 10.1002/ar.1091110304. [DOI] [PubMed] [Google Scholar]
- [48].Stähli C, Bohner M, Bashoor-Zadeh M, Doebelin N, Baroud G. Aqueous impregnation of porous beta-tricalcium phosphate scaffolds. Acta Biomater. 2010;6:2760–72. doi: 10.1016/j.actbio.2010.01.018. [DOI] [PubMed] [Google Scholar]
- [49].Ji W, Wang H, van den Beucken JJJP, Yang F, Walboomers XF, Leeuwenburgh S, Jansen JA. Local delivery of small and large biomolecules in craniomaxillofacial bone. Adv Drug Deliv Rev. 2012;64:1152–64. doi: 10.1016/j.addr.2012.03.003. [DOI] [PubMed] [Google Scholar]
- [50].Mercado-Pagán ÁE, Stahl AM, Shanjani Y, Yang Y. Vascularization in bone tissue engineering constructs. Ann Biomed Eng. 2015;43:718–29. doi: 10.1007/s10439-015-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Woodard JR, Hilldore AJ, Lan SK, Park CJ, Morgan AW, Eurell JAC, Clark SG, Wheeler MB, Jamison RD, Wagoner Johnson AJ. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials. 2007;28:45–54. doi: 10.1016/j.biomaterials.2006.08.021. [DOI] [PubMed] [Google Scholar]
- [52].Nguyen LH, Annabi N, Nikkhah M, Bae H, Binan L, Park S, Kang Y, Yang Y, Khademhosseini A. Vascularized bone tissue engineering: approaches for potential improvement. Tissue Eng Part B Rev. 2012;18:363–82. doi: 10.1089/ten.teb.2012.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gugala Z, Gogolewski S. Regeneration of Segmental Diaphyseal Defects in Sheep Tibiae Using Resorbable Polymeric Membranes: A Preliminary Study. J Orthop Trauma. 1999;13:187–195. doi: 10.1097/00005131-199903000-00006. [DOI] [PubMed] [Google Scholar]