Abstract
Background
One third of patients with eosinophilic esophagitis (EoE) do not achieve histological remission with standard medical or dietary treatment. The outcome of these patients undergoing various rescue treatments is not known and whether these patients constitute a distinct subset remains unclear.
Objective
To analyze EoE treatment outcomes in a predominantly pediatric population, including after initial treatment failure (rescue treatment) for differences in outcomes and clinical presentation.
Methods
We identified 100 serial cases of confirmed EoE from our REDCap® database established at Massachusetts General Hospital starting from January 2007. Demographic data, clinical symptoms, treatment regimens, endoscopic findings, skin testing results, food triggers and clinical outcome of various rescue treatment strategies were presented. We defined clinical response as histological remission with peak eosinophil count of at least 6 biopsies less than 10 per high power field.
RESULTS
Ninety-seven EoE patients underwent initial treatments. Eighty-one elected dietary treatment (7 elemental diet, 54 multiple food elimination diet, and 20 milk-free diet and 16 elected medical treatment (15 swallowed fluticasone and 1 budesonide). Initial response rate to dietary and medical treatment was 67% (54/81) and 56% (9/16) respectively. Of the 34 who failed initial treatment, 24 of them elected various second treatment regimens (3 medical therapy, 2 milk-free diet, 14 multiple food elimination diet and 5 elemental diet) and 54% (13/24) achieved histological remission. Eight of the remaining 11 who failed second treatment underwent additional treatments and 2 ultimately achieved histological remission. The overall response rate by intention-to-treat analysis increased from 65% (63/97) with initial treatment to 78% (76/97) with rescue treatment, and further to 80% (78/97) with multiple rescue treatments. On a per-protocol basis, the overall response rate was 93% (78/84); however, patients who failed the first two rounds of therapy had only a 20% response rate. Patients who responded to initial treatment were found to have more symptoms and endoscopic abnormalities. In contrast, comparison of patients who failed both initial and rescue therapy to those who responded to rescue therapy did not identify any differentiating clinical features.
CONCLUSION
More than half of the patients who failed initial EoE treatment could still achieve histological remission with rescue treatments. Elemental diet is the most effective initial and rescue therapy in achieving histological remission. No clinical features could not identified to reliably predict response to rescue treatment.
Keywords: eosinophilic esophagitis, management, treatment, refractory
INTRODUCTION
Eosinophilic esophagitis (EoE) is an emerging disorder of the esophagus characterized by isolated esophageal eosinophilia in patients suffering from symptoms related to esophageal dysfunction. Topical steroids and dietary elimination have similar efficacy in achieving histologic remission (50–75%) and either is accepted as initial therapy in the treatment of EoE according to the most recently published guideline.1 Choice of initial therapy is driven by local expertise and patient preference. A third of EoE patients do not achieve histologic remission and/or symptom improvement after the initial therapy. The optimal treatment strategy for these patients as well as whether they represent a distinct subset of patients remains to be defined. In this report, we retrospectively analyzed a cohort of EoE patients who failed initial treatment and compared their clinical course and clinical features to patients who responded to initial treatment.
METHODS
Study Design
This was a retrospective cohort study of patients seen from January 2007 to June 2013 at the Massachusetts General Hospital Food Allergy Center (MGH FAC) with a diagnosis of EoE. We defined subgroups of this cohort based on response to the initial round of therapy and compared them with respect to subsequent clinical course as well as baseline or longitudinal clinical characteristics, including age at presentation, age at diagnosis, sex, atopic status, specific IgE testing, skin testing, patch testing, food triggers and number of food triggers. Participants were identified from the EoE biomarker database, an IRB-approved repository of disease-related information from patients who have EoE. Written informed consents were obtained from patients and all parents of adolescent patients. Assent forms were obtained from children older than 7 years old. The study was approved by the Partners Institutional Review Boards in Boston, MA.
Subjects
Subjects were seen at the MGH FAC by one of two gastroenterologists and allergists. Patient records were selected for review if they met the diagnostic criteria of EoE as defined as having ≥ 15 eosinophils/HPF in at least one esophageal biopsy specimen, having no response to high dose proton-pump inhibitor (PPI) twice a day for at least 6 weeks, and exclusion of other causes of esophageal eosinophilia. Medications not known to affect esophageal eosinophilia, as well as asthma medications (including nasal and inhaled glucocorticoids), were permitted. A clinical history and family history of allergic disease (i.e., asthma, allergic rhinitis or atopic dermatitis) was recorded. Patient demographic and disease characteristics were evaluated. Duration of follow-up was defined as the number of months patients were followed at the MGH FAC since their first diagnosis of EoE.
Treatment selection
Patients elected to initiate either medical or dietary treatments. The risks and benefits of each therapeutic option were discussed. The initial therapy chosen for each patient was not randomly assigned but was negotiated among allergist, gastroenterologist, dietitian and patient based on multiple medical and socioeconomic factors including comprehensive medical history, physical examination, allergy testing, social environment and individual preferences.
Patients who failed to respond histologically (defined as peak eosinophil count/HPF <10) to initial treatment were offered the option to undergo rescue treatment. In our practice, we offered patients the following rescue treatment options: single or multiple food elimination, elemental diet, or topical swallowed corticosteroids (Fluticasone 110–220 mcg 2 puffs BID or viscous Budesonide 1–6 mg a day in single or divided dose). Choice and dosage of topical swallowed corticosteroids were determined by the treating physician. We offered patients salvage therapy if they failed both initial and rescue treatment. Similar to the initial therapy, choice of rescue and salvage therapies were individualized for each patient, depending on the many factors described above.
Endoscopy, Esophageal Biopsy Specimens, and Histologic Analysis
EGD with histological assessment was offered to patients who completed at least 8 weeks of treatment (regardless of regimens). EGD was performed using a Pentax EG-2990K Video Gastroscope (Pentax Medical Company, Montvale, NJ) and Radial-Jaw 4 grasp forceps (Boston Scientific, Natick, MA) were used to obtain biopsy specimens. At least 2 biopsy specimens were taken from the proximal, middle and distal esophagus for all endoscopies performed for diagnosis and treatment assessments. The peak eosinophil count/HPF was defined as the highest eosinophil count in either the proximal, mid or distal esophagus. The peak eosinophil count in biopsies was determined at 400× magnification (area 0.3 mm2) by MGH board-certified pathologists.
Remission Status
Due to the departure from symmetry, the median pre- and post-treatment peak eosinophil counts were calculated. Remission status following treatment and food reintroduction was dichotomized with remission being defined peak eosinophil count/HPF <10 and non-remission being defined as peak eosinophil count/HPF ≥ 10.
Diet Elimination and Reintroduction
For our retrospective analysis, we categorized dietary treatment into 3 groups: 1) milk-free diet (MFD), 2) multiple food elimination diet (MFED), and 3) elemental diet. Number and type of foods eliminated in MFED is highly individualized in our practice. The decision is based on multiple clinical and social factors such as history of IgE food allergies, allergy testing results, patient’s preference, social environment and input from our dietitian, allergist and gastroenterologist. Patients achieving histological remission with either MFED or elemental diet underwent systematic food reintroduction to identify potential food triggers. The reintroduction phase consisted of the addition of 1–2 food group(s) every 8–12 weeks. The order of food reintroduction was individualized based on allergy testing, physician and/or patient preference. An EGD with biopsy specimens was repeated 8–12 weeks after reintroduction. All patients remained on PPI BID throughout the elimination and subsequent challenge. Baked milk introduction was described previously by our group.2 Briefly, patients were instructed to ingest at least 3 to 4 servings per week of home-baked and/or store-bought baked milk products for at least 6 weeks. Examples include bread, muffins, cakes, and brownies. Ingestion of cheese, even if baked (e.g., pizza), was not regarded as baked milk in our clinical practice.
Allergy testing
Skin prick testing (SPT) and atopy patch testing (APTs) were performed as previously described.3 Results of SPTs to foods based on their clinical history in addition to milk, wheat, soy, beef, corn, nuts and eggs were available in 83 patients. In some patients, APTs to milk, wheat, soy, beef, corn, nuts and eggs were obtained, unless the patient had a positive wheal-and-flare reaction (>3-mm wheal) on a SPT. Results of APT were available in 38 patients. Levels of specific IgE for milk, wheat, soy, beef, corn, nuts, seafood, and egg were available in 66 patients.
Statistical analysis
Descriptive data are expressed as the medians and interquartile ranges (IQR). Comparisons of demographic and disease-related variables between responders and non-responders were performed using the Mann Whitney U tests for continuous variables and Fisher’s exact test for discrete variables. The Wilcoxon paired signed rank test was used to determine statistical significance between pre- and post-treatment median peak eosinophil counts/HPF among each therapy. A p value of less than 0.05 was considered significant. Statistical analyses were performed using PASW Statistics 18.0 (SPSS Inc., Chicago, IL) and SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Demographics
One hundred and seventy nine patients were included in the EoE Biomarkers database at the MGH FAC. Twenty-five patients with PPI-responsive esophageal eosinophilia and 54 patients without specific pathology were excluded from further study. One hundred patients with confirmed EoE were included in the analysis. The median follow-up duration was 29.2 months (IQR = 11.8–56.9 months). Their demographics were summarized in table 1. The median age of the subjects was 13.0 (IQR = 8.0–18.0). Their symptoms, endoscopic findings and peak eosinophil count/HPF pre- and post- initial treatment were summarized in table 2. The most common presenting symptoms were heartburn (48%) and dysphagia (47%), followed by feeding dysfunction (40%), abdominal pain (38%) and vomiting (37%). Furrows were present in 56% of the diagnostic EGDs. Median peak eosinophil count/HPF pre-treatment was 20 (15–40). Milk, egg and wheat are among the most common food triggers identified (table 3).
Table 1.
Demographics of 100 EoE patients
| Gender (%) | |
| Male | 78.0 |
| Age | |
| median | 13.0 |
| interquartile range | 8.0–18.0 |
| Atopic past medical history (%) | |
| Bronchial asthma | 53.0 |
| Allergic rhinitis | 53.0 |
| IgE mediated Food allergy | 31.0 |
| Atopic dermatitis | 28.0 |
| Skin positivity for aeroallergens | 43.4 |
| Atopic family history (%) | |
| Bronchial asthma | 49.0 |
| Allergic rhinitis | 52.0 |
| Food allergy | 24.0 |
| Atopic dermatitis | 30.0 |
| Eosinophilic esophagitis | 6.0 |
Table 2.
Symptoms and endoscopic findings pre- and post- initial treatment. Comparisons of pre-treatment symptoms and endoscopic findings between responders (R) and non-responders (NR) were calculated using Fisher’s exact test while comparisons of median peak eosinophilic count/HPF were calculated using Mann Whitney U test.
| Overall | 63 Responder (R) | 34 Non-responders (NR) | R versus NR P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Δ | Pre | Post | Δ | Pre | |
| Symptoms | % | % | % | % | % | % | |||
| Heartburn | 48 | 10 | 49 | 10 | −40 | 18 | 3 | −15 | ** |
| Dysphagia | 47 | 14 | 48 | 10 | −38 | 18 | 6 | −12 | ** |
| Feeding dysfunction | 40 | 6 | 37 | 5 | −32 | 12 | 0 | −12 | * |
| Abdominal Pain | 38 | 11 | 38 | 6 | −32 | 12 | 6 | −6 | * |
| Vomiting | 37 | 8 | 38 | 5 | −33 | 3 | 3 | 0 | ** |
| Weight loss | 21 | 9 | 19 | 10 | −10 | 12 | 6 | −6 | NS |
| Cough | 16 | 3 | 16 | 3 | −13 | 3 | 3 | 0 | NS |
| Impaction | 11 | 0 | 11 | 0 | −11 | 6 | 0 | −6 | NS |
|
| |||||||||
| Endoscopic findings | |||||||||
| Furrows | 56 | 35 | 56 | 22 | −33 | 18 | 21 | 3 | ** |
| Edema | 40 | 18 | 37 | 13 | −24 | 12 | 6 | −6 | * |
| White plaques | 30 | 18 | 22 | 3 | −19 | 21 | 12 | −9 | NS |
| rings | 28 | 16 | 29 | 13 | −16 | 6 | 3 | −3 | * |
| Normal | 11 | 36 | 14 | 43 | 29 | 0 | 9 | 9 | * |
| Stricture | 3 | 0 | 3 | 0 | −3 | 0 | 0 | 0 | NS |
| Small caliber | 1 | 0 | 2 | 0 | −2 | 0 | 0 | 0 | NS |
|
| |||||||||
| Median peak eso/HPF | |||||||||
| Prox | 20 | 0 | 15 | 0 | 28 | 18 | ** | ||
| Med | 20 | 0 | 20 | 0 | 30 | 20 | ** | ||
| distal | 25 | 2 | 25 | 0 | 30 | 25 | NS | ||
p < 0.05,
p < 0.005,
NS = p >0.05.
Table 3.
Food triggers of EoE identified by histology and/or symptoms. Histological remission and recurrence is defined as peak eos <10/hpf and ≥ 10/hpf respectively.
| Food Triggers | Confirmed* | Probable** | Total |
|---|---|---|---|
| Milk | 29 | 11 | 40 |
| Wheat | 7 | 5 | 12 |
| Egg | 7 | 3 | 10 |
| Beef | 4 | 6 | 10 |
| Corn | 5 | 4 | 9 |
| Soy | 3 | 6 | 9 |
| Seafood | 0 | 5 | 5 |
| Nuts | 1 | 3 | 4 |
| Rice | 1 | 0 | 1 |
| Beans | 1 | 0 | 1 |
A confirmed food trigger is a food that causes recurrence of disease after its reintroduction, or its single avoidance leads to histologic remission.
A probable trigger is a food that causes worsening of symptoms during reintroduction (but no histological assessment), or it is part of the 2–3 food elimination diet that led to remission (but no further reintroduction was conducted). For example, if a patient achieved histological remission with milk and wheat elimination diet, he/she may not want to pursue further reintroduction and simply avoid both milk and wheat.
Clinical outcome of initial treatment
Of the 100 confirmed EoE patients identified, ninety-seven underwent initial treatments. Eighty-one elected dietary treatment and 16 elected medical treatment (figure 1). The rates of achieving histologic remission with initial dietary and pharmacological therapy were 67% (54/81) and 56% (9/16) respectively. Among those who elected dietary therapy, response rate to elemental diet, MFED and MFD are 100% (7/7), 63% (34/54) and 65% (13/20) respectively. Three elemental diet and 19 MFED responders successfully completed the reintroduction phase (at the time of the manuscript submission) and milk was identified as the only trigger in 6 patients. Together with the 5 MFD responders, a total of 11 milk-mediated EoE patients elected heated/baked milk challenge, 72% (n=8/11) tolerated baked milk (figure 1).
Figure 1.
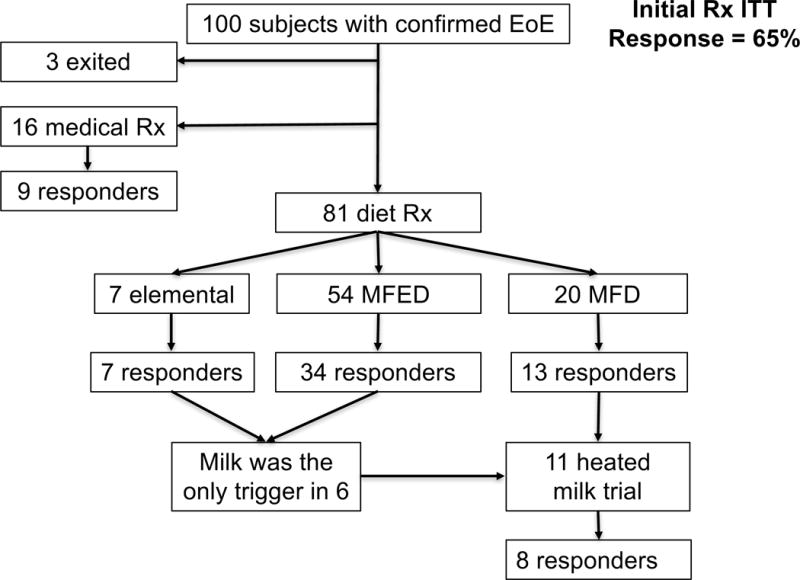
Clinical outcomes after initial treatment for EoE. MFED = multiple food elimination diet; MFD = milk-free diet
Clinical outcome of rescue treatment
Twenty four of the 34 patients (71%) who failed initial therapy elected to undergo rescue therapy. The choice of rescue therapy and their outcomes were summarized in figure 2A. The choice of rescue therapy was individualized based on clinical history, as well as physicians’ and patient’s preference. Among the 7 patients who failed initial medical therapy, 2 lost follow-up and 5 elected dietary therapy as rescue treatment: 0/2 responded to MFD, 1/2 to MFED, and 1/1 to elemental diet. Of the 7 that failed initial MFD, 3 were lost to follow-up, 2 failed MFED and 2 tried topical steroids and 1 responded. Ten of the twenty who failed initial MFED underwent a second round of MFED with 60% response rate. Overall, 54% of patients responded to individualized rescue therapy (13/24). Results are summarized in figure E1.
Figure 2.
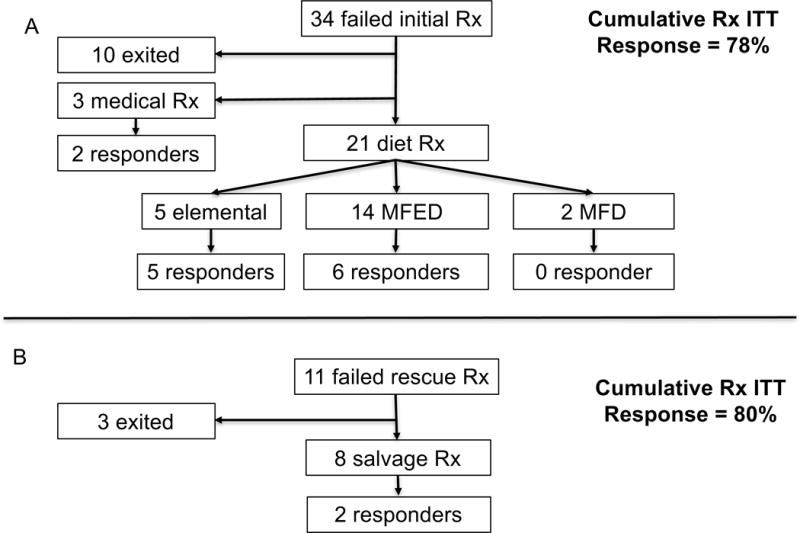
A, Clinical outcomes of the 34 patients who failed initial treatment of EoE; B, Outcomes of 11 patients who failed rescues treatment. MFED = multiple food elimination diet; MFD = milk-free diet
Clinical outcome of those who failed rescue treatment
Eleven patients failed both initial and rescue treatments (Figure 2B). Their subsequent clinical course and clinical features were summarized in table 4. Five patients failed to respond to both topical steroids and extensive elimination diets. Xolair (n = 2) and oral viscous Asacol 1000 mg BID (n = 1) failed to achieve histologic remission in these refractory patients. Interestingly, one patient who failed both 8 food elimination diet (wheat, milk, soy, egg, nuts, seafood, beef and corn) and subsequently 4-food elimination diet (wheat, milk, soy and egg) responded to a 2-food elimination diet (wheat and milk). His treating physician speculated that a 2-food elimination diet was easier to follow, resulting in improved adherence. Another patient who failed swallowed fluticasone and 10-food elimination diet eventually responded to budesonide. EoE treatment was put on hold in 1 patient because of other more serious medical problems, 2 lost follow-up, and 1 is undergoing treatment at the time of manuscript submission.
Table 4.
Clinical summary of 11 patients who failed initial and rescue treatment.
| Age | Sex | Atopic Hx | Family Hx | #EGD | Treatment that failed to achieve histologic remission (peak eso < 10/hpf) | Salvage treatment(s) | |
|---|---|---|---|---|---|---|---|
| Initial Rx | Second treatment | ||||||
| Non-responder to rescue treatment | |||||||
| 10 | M | BA, AR, FA, AD, AS | BA, FA, AD | 16 | Swallowed fluticasone | Milk-free diet | No histological remission to (1) 8 food EED or (2) Xolair |
| 13 | M | BA,AR,FA | BA,AR,FA | 18 | 8 food EED | Elemental | No histological remission to (1) 4 month of Asacol viscous solution 1000mg PO BID, (2) 3 month of swallowed fluticasone BID, and (3) 6 month of Xolair |
| 9 | M | BA, EoE, AD | 11 | Milk-free diet | Budesonide | No histological remission to (1) wheat and soy-free diet, (2) 8 food EED (treating M.D. suspected non-compliance, (3) 3 food EED (wheat/soy/milk-free) elimination diet, (4) 6 food EED, (5) second attempt of 8 food EED with rice elimination, and (6) milk-free elimination diet. | |
| 9 | M | BA, AR, AS | BA | 9 | 8 food EED | Elemental | No histological remission to budesonide and fluticasone. Although he did not achieve histologic remission, he had histological improvement with elemental diet, budesonide and fluticasone, with peak eosinophil count dropped from > 80 to 15, 23 and 24 eos/hpf respectively. He developed Cushingoid faces with budesonide and therefore was put on fluticasone long-term. |
| 6 | F | BA, AR | BA | 14 | 8 food EED | Elemental + fruit + chicken + turkey + broccoli and carrot | No histological remission to budesonide for 6 months. |
| Responder to rescue treatment | |||||||
| 21 | M | AR, AS | BA, AR | 4 | Swallowed fluticasone | 10 food SGED | Histological remission with Budesonide |
| 13 | M | BA, AR | BA, AR | 3 | 8 food EED | 4 food EED (wheat, milk, soy, egg-free) | Histological remission with wheat and milk-free diet. Reason for previous failure to multiple-food elimination diet was thought to be non-compliance. |
| Undetermined response to rescue treatment | |||||||
| 25 | F | AR | AD | 5 | 8 food EED | Milk and soy free diet | She is being treated with swallowed fluticasone, histology pending |
| 15 | F | BA, AR, FA, AD, AS | AR, FA | 4 | Milk-free | 8 food EED | EoE treatment was put on hold because of other more serious medical problems |
| 15 | M | AR, AD | 3 | Swallowed fluticasone | Milk-free diet | Loss of follow-up | |
| 18 | M | BA, AR, AS | BA, AR, FA | 3 | 8 food EED | 8 food EED | Loss of follow-up |
BA= bronchial asthma; AR = allergic rhinitis; FA = IgE mediated food allergy strongly suggested by clinical history and allergy testing, AD = atopic dermatitis; AS = aeroallergen sensitivity as documented by positive skin testing and/or elevated specific IgE to aeroallergens; EED = empirical elimination diet; EoE = eosinophilic esoiphagitis; SGED = Skin test and specific IgE guided elimination diet. Histological remission is defined as peak eosinophil count < 10/hfp in at least 6 esophageal biopsies
Clinical features of the responders and non-responders
We compared the clinical features of responders and non-responders to initial treatment. Responders to initial treatment had significantly more symptoms (heartburn, dysphasia, feeding dysfunction, abdominal pain and vomiting) and endoscopic findings (furrows, edema and rings) [table 2]. Non-responders had a higher peak eosinophil count/HPF in the proximal and mid esophagus when compared to responders (28 vs 20, p<0.005; 30 vs 20, p<0.005 respectively). Next, we investigated if there were any factors that were associated with response to rescue therapy. There were no statistical differences between symptoms, endoscopic findings, peak eosinophil counts/hpf, age, gender, ethnicity, concurrent atopic diseases, family history, skin testing results, specific food triggers or number of food triggers between the responders and non-responders to rescue treatments.
DISCUSSION
We demonstrated that rescue therapy is highly effective at inducing disease remission in patients who failed initial standard EoE treatment and that there were no clinical factors that could reliably predict responsiveness to rescue or salvage treatment.
We were able to identify 100 patients with confirmed EoE from the entire 179-patient database. The male/female ratio, prevalence of concurrent atopic diatheses are similar to those seen in previous studies.4,5
Milk, egg and wheat were the three most common food triggers identified, as proven with provocative challenge or single elimination followed by histologic assessment. This is similar to previous published study.3 Our response rate to achieve histologic remission with initial pharmacologic and dietary therapy was similar to those published in literature.1,6–11
We performed an extensive chart review of all 100 patients with confirmed EoE. We analyzed their treatment outcomes and compared their clinical characteristics. We found that more than half of the patients who failed initial EoE treatment (either pharmacological or dietary) could still achieve histological remission (defined as < 10 eos/HPF) with individualized rescue therapy. In our experience, response rate increased from 65% with initial treatment to 80% with rescue treatment on an as treated basis. For both initial and rescue therapy, elemental diet is superior to restricted diet in achieving histologic remission.
Patients who responded to initial treatment were found to have more symptoms and endoscopic abnormalities. In contrast, comparison of patients who failed both initial and rescue therapy to those who responded to rescue therapy did not identify any differentiating clinical features.
Esophageal fibrosis and stricture are long-term sequelae of untreated EoE.12 Given one third of the patients with EoE will fail initial therapy of either topical steroids or MFED, optimal strategy for rescue therapy to achieve disease remission is clinically relevant and important. As evidenced by our data, the implementation of an individualized rescue treatment program can salvage at least 50% more patients who failed the initial therapy. We showed that rescue treatment with MFED was highly effective in patients who failed initial MFED. Only a fraction of these patients reported non-adherence to initial MFED, thus self-report of diet adherence should not preclude motivated patients from trying a second attempt of MFED. Further prospective studies are warranted to elucidate the most effective rescue treatment strategy.
To conclude, more than half of EoE patients who failed initial therapy can still achieve histological remission with implementation of individualized rescue treatment. It is worthwhile to repeat MFED in selected patients who failed initial MFED. Elemental diet is superior to MFED in achieving histologic remission as an initial and rescue therapy. Medical therapy is effective in rescuing patients who failed initial dietary therapy.
Supplementary Material
What is already known about this topic?
Approximately one third of the EoE patients do not achieve histologic remission with topical steroids or dietary treatment.
What does this article add to our knowledge?
More than half of the patients who failed initial EoE treatment (either pharmacological or dietary) could still achieve histological remission with individualized rescue treatment.
How does this study impact current management guidelines?
Patients who fail initial therapy should be offered rescue treatment. Patients who fail initial multiple food elimination diet should be offered a second trial of multiple food elimination diet, as evidenced by the high rescue response rate.
Acknowledgments
Funding Sources: Demarest Lloyd Jr Foundation
Abbreviations
- EoE
eosinophilic esophagitis
- sIgE
specific IgE
- MFD
milk-free diet
- MFED
multiple food elimination diet
- IQR
interquartile range
- APT
atopy patch test
- SPT
skin prick test
- PPI
proton pump inhibitor
- EGD
esophagogastroduodenoscopy
- HPF
high power field’
Contributor Information
John Leung, Email: jleung3@tuftsmedicalcenter.org.
Raman Mehrzad, Email: raman_m1@hotmail.com.
Navneet Virk Hundal, Email: NVIRKHUNDAL@mgh.harvard.edu.
Alexandra Alejos, Email: AALEJOS@mgh.harvard.edu.
Paul E Hesterberg, Email: phesterberg@mgh.harvard.edu.
Aubrey J Katz, Email: ajkatz@mgh.harvard.edu.
Qian Yuan, Email: qyuan@mgh.harvard.edu.
References
- 1.Dellon ES, Gonsalves N, Hirano I, et al. ACG Clinical Guideline: Evidenced Based Approach to the Diagnosis and Management of Esophageal Eosinophilia and Eosinophilic Esophagitis (EoE) Am J Gastroenterol. 2013;108:679–692. doi: 10.1038/ajg.2013.71. [DOI] [PubMed] [Google Scholar]
- 2.Leung J, Hundal NV, Katz AJ, et al. Tolerance of baked milk in patients with cow’s milk–mediated eosinophilic esophagitis. J Allergy Clin Immunol. 2013;132:1215–1216.e1. doi: 10.1016/j.jaci.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spergel JM, Brown-Whitehorn TF, Cianferoni A, et al. Identification of causative foods in children with eosinophilic esophagitis treated with an elimination diet. J Allergy Clin Immunol. 2012;130:461–467.e5. doi: 10.1016/j.jaci.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: a 10-year experience in 381 children. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2005;3:1198–1206. doi: 10.1016/s1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 5.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351:940–941. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 6.Kagalwalla AF, Amsden K, Shah A, et al. Cow’s Milk Elimination: A Novel Dietary Approach to Treat Eosinophilic Esophagitis. J Pediatr Gastroenterol Nutr. 2012 doi: 10.1097/MPG.0b013e318268da40. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22820121 [Accessed September 28, 2012] [DOI] [PubMed]
- 7.Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2006;4:1097–1102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Gonsalves N, Yang G-Y, Doerfler B, et al. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterology. 2012;142:1451–1459.e1. doi: 10.1053/j.gastro.2012.03.001. quiz e14–15. [DOI] [PubMed] [Google Scholar]
- 9.Kelly KJ, Lazenby AJ, Rowe PC, et al. Eosinophilic esophagitis attributed to gastroesophageal reflux: Improvement with an amino acid-based formula. Gastroenterology. 1995;109:1503–1512. doi: 10.1016/0016-5085(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 10.Markowitz JE, Spergel JM, Ruchelli E, et al. Elemental diet is an effective treatment for eosinophilic esophagitis in children and adolescents. Am J Gastroenterol. 2003;98:777–782. doi: 10.1111/j.1572-0241.2003.07390.x. [DOI] [PubMed] [Google Scholar]
- 11.Henderson CJ, Abonia JP, King EC, et al. Comparative dietary therapy effectiveness in remission of pediatric eosinophilic esophagitis. J Allergy Clin Immunol. 2012;129:1570–1578. doi: 10.1016/j.jaci.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145:1230–1236.e1–2. doi: 10.1053/j.gastro.2013.08.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


