Abstract
Purpose of review
Current guidelines for cholesterol treatment emphasize the importance of engaging patients in a risk benefit discussion prior to initiating statin therapy.
Recent Findings
While current risk prediction algorithms are well defined, there is less data on how to communicate with patients about cardiovascular disease risk, benefits of treatment, and possible adverse effects.
Summary
We propose a four-part model for effective shared decision making: 1) assessing patient priorities, perceived risk, and prior experience with cardiovascular risk reduction, 2) arriving at a recommendation for therapy based on the patient’s risk of disease including other risk factors if needed, guideline recommendations, new clinical trial data, and patient preferences, 3) communicating this recommendation along with risks, benefits, and alternatives to therapy following best practices for discussing numeric risk, and 4) arriving at a shared decision with the patient with ongoing re-assessment as risk factors and patient priorities change.
Keywords: Shared decision making, risk communication, prevention
Introduction
“If it were not for the great variability among individuals medicine might as well be a science and not an art.”
-Osler
For many years, the importance of shared decision making (SDM) has been recognized. Multiple efforts have been undertaken to promote increased patient engagement in medical decision making. SDM involves: an understanding of patient preferences; an informed discussion highlighting therapeutic options including potential benefits and risks; and, a conversation between the patient and the provider to reach a decision about a therapeutic course. Fundamentally, the cornerstone of effective SDM is two-way communication between the patient and health-care provider. This presupposes three tenets: that providers have up to date information about the best medical evidence; provide unbiased recommendations; and, understand the patient’s goals and concerns.[1]
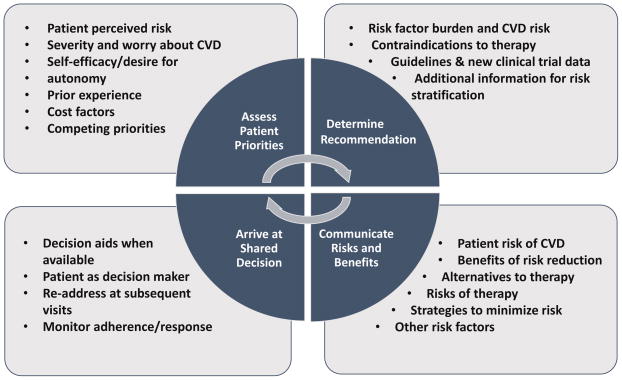
Current guidelines for cholesterol lowering highlight the importance of SDM in cardiovascular disease (CVD) prevention. The 2013 American College of Cardiology and American Heart Association (ACC/AHA) cholesterol guidelines recommend that clinicians and patients “engage in a discussion which considers the potential for ASCVD risk reduction benefits and for adverse effects, for drug-drug interactions, and patient preferences for treatment”.[2] But while the guidelines were clear on potential statin benefit groups, there was less clarity about how to conduct this comprehensive discussion. A subsequent report gave more detailed guidance on the clinician-patient risk discussion.[3*] In this review, we will expand on the clinician-patient risk discussion, focusing on four key components of SDM for CVD prevention: understanding patient priorities, formulating recommendations for CVD risk reduction, communicating risks and benefits, and arriving at a shared decision for risk reduction (Figure).
Figure. Shared Decision Making for Cardiovascular Disease Prevention.
The figure shows four components of the clinician patient discussion around cardiovascular disease prevention.
Understanding Patient Priorities
Successful SDM depends on clinicians having a clear understanding of patient goals and preferences. Before making a therapeutic recommendation, providers need to understand patient preferences and priorities. This includes knowledge of what patients believe is their personal risk of CVD, the perceived severity of this risk, the degree to which patients think they can affect this risk, and potential competing priorities.
Perceived CVD Risk
Evidence shows that patients are often unable to accurately estimate their personal risk of CVD. “Optimistic bias”, by which a person judges his or her risk as lower than predicted, is common.[4] In one Dutch cohort, nearly half of patients at high risk for CVD estimated their risk to be qualitatively “low.” On the other hand, nearly one fifth of adults with low risk thought their risk was high.[5] Recalibrating these misperceptions so that patients understand that they are in fact at higher risk than they previously suspected may help increase their motivation to engage in CVD risk reduction.
Meanwhile, large numbers of adults hold pessimistic estimates of their CVD risks. In one study, nearly half of patients overestimated their 10-year risk of CVD by >20%.[6] Presenting these adults with better estimations of their own CVD risk might actually result in a decreased willingness to engage in CVD risk reduction. This may, in part, explain why interventions that utilize risk-communication and decision aids have not led to sustained changes in CVD risk factor profiles, despite improving patient accuracy of risk assessment.[7–9]
How might risk communication help motivate adults with “pessimistic bias”? Clinicians could focus on potential benefits of CVD risk reduction therapy. Focusing on this ‘positive-framing’ (benefits) over risk) is often preferred.[10] Framing risk reduction as a relative risk, rather than absolute risk, can also lead to increased motivation for behavior change.[11**]
Perceived Severity of CVD Risk
Equally important as understanding a patient’s perceived CVD risk is appreciating how much that risk translates into worry about CVD, which is largely driven by the perceived severity of a heart attack or stroke. While these two concepts are often correlated, they are not inextricably linked, and are often influenced by external factors. A patient who lost a parent at a young age to a heart attack may worry a great deal about developing heart disease despite being at low predicted risk. On the other hand, a person who has had several close friends and relatives experience uncomplicated myocardial infarctions may have less overall “worry”, even if at high perceived risk, due to lack of perceived severity of the event. This phenomenon has been demonstrated in the oncology literature: how much patients worry about breast cancer affects mammography rates more than their perceived risk.[12] Beyond affecting initiation of therapy, perceived risk and severity of CVD likely impact patients’ tolerance for side effects, adherence, and medication persistence.
Patient Autonomy and Perceived Self-Efficacy
Clinicians should also explore how empowered a patient feels that he/she can affect CVD risk. Certain risk factors, such as smoking and obesity, can be very difficult to manage, and might affect a patient’s perceived ability to affect other risk factors. A patient’s prior experience in risk factor modification should be explored. For example, for smoking, how many times has a patient tried to quit? Or for obesity, how many different “diets” has the patient undertaken?
Focusing on those risk factors patients feel empowered to modify may help improve willingness to engage in risk reduction strategies. Some patients may also hold a more deterministic view, noting that their risk is determined by factors beyond their control. This may be particularly relevant for patients with strong religious beliefs. In such patients, other benefits of CVD prevention efforts (i.e., greater quality of life), not just those related to risk-reduction, should be highlighted.
Part of the job of the clinician is to determine the degree to which a patient wants autonomy in the shared decision making process. Some patients may arrive without preconceived ideas about prevention therapy and desire in-depth communication. Other patients may bring strong beliefs, opinions, and/or prior experiences; while they may value the clinician’s input, they may have already arrived at a decision regarding certain therapies before a conversation begins. At the other end of the spectrum, some patients may bring a “whatever the doctor recommends” approach. Even in theses cases, it is important to explain why we, as a clinician, are recommending a certain approach so that the patient has a chance to evaluate whether the rationale fits with his or her belief system.
Competing Priorities
Patient preferences may preempt certain CVD prevention strategies. For example, patients who are averse to medications may be interested in lowering CVD risk but not if it requires taking a pill every day. In these patients, focusing on effective exercise and dietary modifications for CVD risk reduction will be more productive than a discussion of risks and benefits of statins. Conversely, other patients may be willing to take medication but not to alter their lifestyle. Knowing this in advance can help the provider frame the discussion towards therapies the patient is likely to accept.
An important, but often overlooked, competing priority is cost of therapy. This is particularly important in the context of novel therapies for lipid lowering such as the proprotein convertase subtilisin/kexin type 9 inhibitors or in patients who are uninsured, underinsured, or have copays or deductibles that make certain medications cost prohibitive. Most patients want to discuss costs with their providers, but research reveals that discussions about costs are rare.[13] One barrier to this may be a clinician’s lack of knowledge about out-of-pocket costs. Patients should know in advance that alternatives are often available within a therapeutic class that may be more cost-effective. Alternately, clinician referral to patient assistance programs can help offset the cost of medications. Patients should be encouraged to talk with their pharmacy or insurance company about the cost of medications and potential alternatives within the therapeutic class, and then to discuss the results of such conversations with the prescriber.
Formulating Recommendations for CVD Risk Reduction
After exploring their patients’ priorities for CVD prevention, clinicians need to determine their optimal recommendation (or recommendations) for therapy. This requires up-to-date knowledge of guidelines, novel trial data, known risks and benefits of therapies, and potential contraindications. Noting an inherent lag between clinical trial results and incorporation into guidelines, clinicians may choose to change practice in response to certain clinical trial data. For example, clinicians may be targeting lower systolic blood pressures in high-risk, non-diabetic adults without a history of stroke or heart failure based on the SPRINT trial prior to incorporation into blood pressure guidelines.[14] Many patients will fall into “grey areas”, in which guidelines are unclear or data are limited. Examples include high-risk younger adults, where data are lacking on the long-term risks and benefits of statin therapy, or adults with high blood pressure who may not have qualified for the SPRINT trial based on age or other comorbidities. Finally, it is important for clinicians to recognize that while current risk equations perform well for populations, the positive and negative predictive value of the risk equations can vary greatly. Thus, while CVD risk is a starting point for a discussion, it is important for clinicians to consider each patient individually. In certain cases, additional information may be needed to further risk-stratify the patient, such as a coronary artery calcium score or more detailed family history.
Communicating Risks and Benefits
Impact of Risk Presentation
After determining his or her recommendation (or recommendations) for CVD risk reduction, the clinician should discuss potential benefits and risks of those options with the patient. In CVD prevention, this starts with an accurate discussion about the patient’s risk of CVD, recognizing that there may be multiple ways to “accurately” communicate risk. The way that risk is presented can greatly influence a patient’s qualitative impression of it. Consider the following two scenarios:
Mr. Jones, you have a 10% chance of a heart attack in the next 10 years, meaning 10 in 100 people like you will have a heart attack or stroke. Over the long run, you have about a 50% chance of having a heart attack or stroke in your lifetime. This puts you in a high-risk category.
Mr. Jones, you have a 10% chance of heart attack in the next ten years. That means that 9 in 10 men like you would make it 10 years without any heart attack or stroke.
Neither of these statements is untrue, but both reflect different “agendas” on behalf of the provider. Fortunately, SDM does not require clinicians to enter into patient discussions with a “neutral” approach. Rather, patients expect as part of SDM that their provider will give them “sound counsel… based on their expertise and without bias”.[1] Clinicians frequently know up-front what his/her recommendation would be for the patient regarding preventive therapy and understand that how they frame risk is affected by their recommendation and might affect patient responses.
The literature is varied on the degree to which risk communication influences downstream behavior, but much is known on how risk communication formats affect patients in the short term. Although guidelines are based on predicted CVD risk in terms of a percent risk over a discrete time horizon, this may not be the optimal way to discuss risk with patients.[15*] It is well known that many people have difficulty comprehending basic probabilities. Yamagishi, for example, showed that people were more likely to rate risk as higher when presented as killing “1286 out of 10,000 people” rather than “24.14 out of 100 people”.[16] In a different study, barely half of adults could answer the basic question “how many times in 1000 flips of a coin would a coin come up heads?”[17]
Utilization of Alternate Risk Horizons
The current AHA/ACC lipid guidelines focus on 10-year risk of CVD to identify statin candidates among adults aged 40–75. While this framework is used throughout the literature, it may not be the best (or only) way to discuss risk with patients. Alternate presentations of risk may be more impactful.[18] Young people may respond better to long-term risk estimates such as 30-year or lifetime risk.[19] Notably, the AHA/ACC lipid guidelines recognize this and encourage providing lifetime risk estimation for adults ages 20–59, with particular emphasis for those under 40, to guide non-pharmacologic risk assessment. Others may be more motivated by the concept of “heart age,” which is commonly used in the U.K.’s cholesterol guidelines.[20] Finally, explaining how people compare with their peer group may increase the emotional response and perceived severity of risk among patients at low absolute, but high relative risk.[21] Along these lines, the AHA/ACC risk estimator provides the estimated risk for an individual who is similar to the patient (in age, sex, and race), but with optimal risk factors. In the table, we present some suggested guidelines for communication of CVD risk, including recommendations from Lipkus et al.[23]
Table.
Best Practices for Communication of Cardiovascular Disease Risk Estimates
| Recommendation | Rationale |
|---|---|
| Assess patient estimated risk at baseline | Patients may have optimistic or pessimistic biases which may require different communication strategies |
| Use round numbers and avoid decimals | Simple numbers are more easy for patients to understand |
| Maintain a consistent denominator when presenting proportions | Larger numerators lead to higher perceived risk, regardless of the denominator: patients presented with a 1 in 10 chance may consider that lower than if it were presented as 10 out of 100 |
| Avoid using excessively small percentages | Small numbers close to zero may be perceived as no risk. This is particularly important when considering the European lipid guidelines that focus on SCORE estimation for 10-year risk of CVD death[22] |
| Provide context for risk estimates when available, i.e. “high-risk is >7.5%” | Putting risk into context may help improve comprehension beyond just providing numeric estimates |
| Consider alternatives to 10-year risk | Lifetime or 30 year risk may be more impactful in young adults, heart age may be more motivating for behavior change |
| Limit the number of statistics/graphics when discussing risk | Providing too many estimates can lead to “information overload” |
| Utilize decision aids and visual graphics, but explain them to the patient | Graphics can improve comprehension but may require explanation |
| Avoid using number needed to treat (NNT) | NNT leads to poor comprehension of benefit |
| Note differences in responses to relative risk and absolute risk | Relative risk reductions are perceived as greater than when presented as absolute risk reductions. Absolute risk reductions allow for direct comparisons of risks and benefits. |
Risks, Benefits, and Alternatives to Therapy
When discussing preventive therapy, benefits can be particularly hard to quantify given that the “benefits” of risk reduction are not directly experienced by the patient. There are several ways to present the potential benefit of preventive therapy: absolute risk reduction, relative risk reduction, or number needed to treat (NNT). In general, presenting relative risks is more motivating than absolute risk. For example, when discussing potential statin benefit, stating that there is a 30% reduction in risk of heart disease is perceived as more impactful than “statins can reduce your risk from 8% to 6%.” NNT can be a difficult concept to explain and may actually lessen understanding of potential benefit.[11**]
Absolute risk reduction in ASCVD may be preferred to relative risk reduction when comparing different therapies or if comparing potential risks and benefits harm as it maintains a stable denominator. (EDITOR’S NOTE: THIS LAST SENTENCE REQUIRES CLARIFICATION OR CORRECTION – MEANING IS NOT CLEAR.)For example, in a trial involving intermediate risk individuals without CVD, daily rosuvastatin conferred an absolute risk reduction of cardiovascular death, myocardial infarction, or stroke of 1.1%, but also increased the risk of cataract surgery by (0.7%; p=0.02). Presented as relative risks, daily rosuvastatin led to a 24% reduction in CV death, MI, or stroke and a 23% increase in the risk of cataract surgery.[24] Without knowing what the baseline risk was in the trial, these relative risk estimates are difficult to compare. Finally, in discussing risks and benefits, clinicians should go beyond numbers and consider the seriousness and reversibility of outcomes. Another important concept is the multiplicative effect of risk reduction over time. Clinical trials often show the benefit of preventive therapies in relatively short windows. Long-term studies have shown that over time, the absolute risk reduction of therapies increases.[25]
Graphics can help improve understanding of risks and benefits but can also quickly lead to information overload. Unfortunately, data are limited on how to optimize the presentation of CVD risk, and focus groups have shown differing results in terms of patient preference.[20] Some patients prefer icon arrays (a.k.a. crowd charts/Cates plots Figure 1), whereas others prefer bar graphs or other displays of risk. Graphics that focus only on the numerator, while ignoring the denominator (such as bar charts), may lead people to make choices that are more risk-averse. Recognizing that patient preferences vary, clinicians should have different formats available to communicate risk with patients, and ask them which they prefer, rather than implementing a “one-size-fits-all” approach.
Part of a benefit discussion also includes a discussion of potential risks. This is particularly challenging in the context of CVD prevention as the risks may be unclear. For example, with statin therapy, myalgias are commonly reported in observational studies, but in pooled analysis of randomized clinical trials they were no more common in the intervention arms compared with placebo arm. In another example, the risk of bleeding with low-dose aspirin remains difficult to accurately quantify in routine clinical care. Acknowledging grey areas where risk information is unknown can help preserve trust between patients and providers.
When applicable, providers should use this discussion to inform patients about potential medication interactions or adverse reactions. Asking what patients already know about potential risks or side-effects can also allow this discussion to be tailored to correct any pre-existing misinformation. During the risk discussion, patients should be assessed for contraindications and relative contraindications to therapy (e.g. postural hypotension as contraindication to lower systolic blood pressure goals, prior severe bleeding or thrombocytopenia as relative contraindications to aspirin therapy). In addition, patients can be educated on ways to minimize side effects, e.g. reducing alcohol intake to decrease the risk of stain myopathy.
It is important in discussions about CVD prevention to consider CVD risk reduction as a whole. At a given time, there may be multiple opportunities for CVD risk reduction, such as in a patient with dyslipidemia, uncontrolled hypertension, and a sedentary lifestyle. Addressing all at once in the first visit can be confusing or overwhelming and a patient may have greater motivation to address particular issues at that time. For example, the patient may be reluctant to begin cholesterol lowering medication, but willing to work on blood pressure control or increased exercise. Focusing on potential benefits across the different areas of CVD prevention can also help highlight the multifactorial benefits of lifestyle modification, which are a cornerstone of risk reduction efforts.[26]
Arriving at Shared Decisions for CVD Prevention
The final piece of SDM involves coming to a decision regarding the choice for or against a recommended therapy. A Cochrane review of SDM showed that decision aids can help improve knowledge, make patients feel more informed, participate more deeply in SDM, and better understand risks and benefits.[27] The Mayo Clinic website (shareddecisions.mayoclinic.org) offers a number of well-designed decision aids that can be useful to help anchor conversations about CVD risk and prevention therapies. The availability of much of the information needed to calculate CVD risk electronically makes it possible to design point-of-care decision aids that integrate real-time patient level information into risk estimators and may reduce the burden on providers.
Comprehensive conversation about CVD risk and risk reduction strategies can be time-consuming. However, these types of discussions occur regularly in doctor’s offices regarding a number of other potential interventions, such as the risks and benefits of routine cancer screening (mammography, colonoscopy) and other preventive therapies (e.g. vaccination). Cardiologists routinely see patients in clinic for “perioperative risk assessment,” where they assess a patient’s risk of complications (e.g. STS score), formulate a recommendation for both therapy and perioperative management (e.g. hold anticoagulation, continue beta blocker), discuss potential risks with the patient (based on risk estimation and other characteristics), and arrive at a shared decision regarding how to proceed based on patient priorities (including risk tolerance, perceived benefits of surgery). This paradigm follows what is outlined above for CVD risk reduction.
Given time constraints and competing priorities, it may not be possible to cover all potential areas for CVD risk reduction (lipids, smoking, blood pressure, diet, exercise, diabetes control, etc.), nor all components specified above (patient priorities, CVD risk communication, discussion of therapeutic risks/benefits, and arriving at a decision) in one visit. Moreover, patients may want time to read, digest, and better understand the information, or time to discuss the decision with family and/or friends. Meanwhile, the patient and clinician may gather additional information (e.g., prior medical records, more detailed family history, atherosclerosis imaging). However, SDM is a process, not a single conversation. Fortunately, comprehensive CVD risk reduction for primary prevention can take place over multiple visits, which may help improve patient comprehension and sustainability of changes.
Next, not all of this conversation needs to take place between the physician or visit provider and the patient. Other clinicians, including trained nurse clinicians and pharmacists can be well (if not better) equipped to engage in these conversations. Integrating other members of the health care team into these discussions can help ease the time burden on individual visits.
Many patients are reluctant to initiate medication treatment if they think it is “lifelong”. While consistent adherence to preventive interventions over the long-term is critical to achieving the best outcomes, patients should be reminded that no decision is necessarily a lifelong one. Providers should acknowledge clinical evidence is constantly changing, and if new information becomes available that changes the risk/benefit equation or new therapeutic options become available, then prior decisions should be readdressed. Similarly, patient preferences, perceived risk, and desire to engage in therapy may change over time, which may also prompt re-assessment of risk. Patient reluctance for lifelong therapy may provide a strong incentive to engage in lifestyle modification with the ultimate goal of decreasing the need for pharmacologic therapy, particularly as it relates to blood pressure lowering.
Successful CVD prevention requires ongoing monitoring and re-evaluation. At every visit, patients should be asked about adherence to medications and lifestyle intervention and potential barriers. This includes perceived side effects, changes in insurance or financial constraints affecting the affordability of medications, and changes in diet and exercise patterns. Regular measurement of lipid panels can help assess adherence and provide positive reinforcement to patients who are not otherwise “seeing” any noticeable benefit of statin therapy. Ongoing conversations about CVD prevention also allow clinicians to understand changes in patient priorities and to re-assess their approach to CVD prevention in the context of new guidelines and trial evidence.
Conclusion
While the importance of shared decision making and patient engagement in making treatment decisions is widely recognized, there are only limited data to guide how such discussions should be conducted. Before making treatment recommendations, clinicians should assess a patient’s baseline understanding of their risk of CVD and their willingness to engage in prevention therapies. Recommendations for CVD prevention should consider a patient’s potential benefit which may require the gathering of more information.. In communicating a recommendation, clinicians need to inform patients of their risk of CVD. Best practices for discussing numeric risk should be followed to help ensure comprehension, and decision aids can be useful. Ultimately the patient is the decision maker, and clinicians should routinely re-engage in a discussion about CVD prevention to ensure adherence and address changes in risk and patient preferences. Future research should focus on determining how differential framing of CVD risk and therapeutic benefits affects patient behavior and adherence to prevention strategies.
Key Points.
Shared decision making should start with an assessment of patient priorities and their own perceived risk of heart disease
Patients’ comprehension of numeric estimates of risks and benefits is impacted by how that risk is presented to
Risk reduction in CVD prevention should cover multiple risk factors and occur on an ongoing basis to encourage adherence and adapt to patient changes
Acknowledgments
AM Navar receives research support from Regeneron and Sanofi pharmaceuticals and consulting fees from Sanofi. She also receives research support from the NIH (T32HL069749).
SS Martin is listed as a coinventor on a pending patent filed by Johns Hopkins University for a method of low-density lipoprotein cholesterol estimation. He has grant support from the PJ Schafer Cardiovascular Research Fund, American Heart Association, Aetna Foundation, and Google. He has received honoraria from the American College of Cardiology for dyslipidemia-related educational activities. He has served as a consultant to Pressed Juicery, Abbott Nutrition, Quest Diagnostics, and the Pew Research Center.
N Stone has no conflicts of interest to declare.
We would like to think Dr. Allan Sniderman for his assistance in reviewing this manuscript.
Financial Support and Sponsorship:
AM Navar is supported through research funding from the National Institutes of Health T32HL069749.
Footnotes
Conflicts of Interest:
AM Navar receives research support from Regeneron and Sanofi pharmaceuticals and consulting fees from Sanofi.
SS Martin is listed as a coinventor on a pending patent filed by Johns Hopkins University for a method of low-density lipoprotein cholesterol estimation. He has grant support from the PJ Schafer Cardiovascular Research Fund, American Heart Association, Aetna Foundation, and Google. He has received honoraria from the American College of Cardiology for dyslipidemia-related educational activities. He has served as a consultant to Pressed Juicery, Abbott Nutrition, Quest Diagnostics, and the Pew Research Center.
N Stone has no conflicts of interest to declare.
References
- 1.Alston C, Paget L, Halvorson G, et al. Communicating with Patients on Health Care Evidence. 2012 http://nam.edu/wp-content/uploads/2015/06/VSRT-Evidence.pdf.
- 2.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63:2889–2934. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3*.Martin SS, Sperling LS, Blaha MJ, et al. Clinician-patient risk discussion for atherosclerotic cardiovascular disease prevention: importance to implementation of the 2013 ACC/AHA Guidelines. Journal of the American College of Cardiology. 2015;65:1361–1368. doi: 10.1016/j.jacc.2015.01.043. Review of the role of shared decision making in implementation of the lipid guideines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samsa GP, Cohen SJ, Goldstein LB, et al. Knowledge of risk among patients at increased risk for stroke. Stroke; a journal of cerebral circulation. 1997;28:916–921. doi: 10.1161/01.str.28.5.916. [DOI] [PubMed] [Google Scholar]
- 5.van der Weijden T, van Steenkiste B, Stoffers HE, et al. Primary prevention of cardiovascular diseases in general practice: mismatch between cardiovascular risk and patients’ risk perceptions. Medical decision making: an international journal of the Society for Medical Decision Making. 2007;27:754–761. doi: 10.1177/0272989X07305323. [DOI] [PubMed] [Google Scholar]
- 6.Frijling BD, Lobo CM, Keus IM, et al. Perceptions of cardiovascular risk among patients with hypertension or diabetes. Patient education and counseling. 2004;52:47–53. doi: 10.1016/s0738-3991(02)00248-3. [DOI] [PubMed] [Google Scholar]
- 7.Koelewijn-van Loon MS, van der Weijden T, Ronda G, et al. Improving lifestyle and risk perception through patient involvement in nurse-led cardiovascular risk management: a cluster-randomized controlled trial in primary care. Preventive medicine. 2010;50:35–44. doi: 10.1016/j.ypmed.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Edwards A, Thomas R, Williams R, et al. Presenting risk information to people with diabetes: evaluating effects and preferences for different formats by a web-based randomised controlled trial. Patient education and counseling. 2006;63:336–349. doi: 10.1016/j.pec.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 9.Krones T, Keller H, Sonnichsen A, et al. Absolute cardiovascular disease risk and shared decision making in primary care: a randomized controlled trial. Annals of family medicine. 2008;6:218–227. doi: 10.1370/afm.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodyear-Smith F, Arroll B, Chan L, et al. Patients prefer pictures to numbers to express cardiovascular benefit from treatment. Annals of family medicine. 2008;6:213–217. doi: 10.1370/afm.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Annals of internal medicine. 2014;161:270–280. doi: 10.7326/M14-0295. Systematic review of risk communication strategies. [DOI] [PubMed] [Google Scholar]
- 12.McCaul KD, Branstetter AD, Schroeder DM, Glasgow RE. What is the relationship between breast cancer risk and mammography screening? A meta-analytic review. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 1996;15:423–429. doi: 10.1037//0278-6133.15.6.423. [DOI] [PubMed] [Google Scholar]
- 13.Alexander GC, Casalino LP, Meltzer DO. Patient-physician communication about out-of-pocket costs. Jama. 2003;290:953–958. doi: 10.1001/jama.290.7.953. [DOI] [PubMed] [Google Scholar]
- 14.Group SR, Wright JT, Jr, Williamson JD, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. The New England journal of medicine. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. Latest guidelines on risk assessment including how to assess other risk factors and importance of communication with the patient. [DOI] [PubMed] [Google Scholar]
- 16.Yamagishi K. When a 12.86% mortality is more dangerous than 24.14%: implications for risk communication. App Cog Psych. 1999;11:12. [Google Scholar]
- 17.Schwartz LM, Woloshin S, Black WC, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Annals of internal medicine. 1997;127:966–972. doi: 10.7326/0003-4819-127-11-199712010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Soureti A, Hurling R, Murray P, et al. Evaluation of a cardiovascular disease risk assessment tool for the promotion of healthier lifestyles. European journal of cardiovascular prevention and rehabilitation: official journal of the European Society of Cardiology, Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. 2010;17:519–523. doi: 10.1097/HJR.0b013e328337ccd3. [DOI] [PubMed] [Google Scholar]
- 19.Frileux S, Munoz Sastre MT, Mullet E, Sorum PC. The impact of the preventive medical message on intention to change behavior. Patient education and counseling. 2004;52:79–88. doi: 10.1016/s0738-3991(03)00003-x. [DOI] [PubMed] [Google Scholar]
- 20.Goldman RE, Parker DR, Eaton CB, et al. Patients’ perceptions of cholesterol, cardiovascular disease risk, and risk communication strategies. Annals of family medicine. 2006;4:205–212. doi: 10.1370/afm.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fair AK, Murray PG, Thomas A, Cobain MR. Using hypothetical data to assess the effect of numerical format and context on the perception of coronary heart disease risk. American journal of health promotion: AJHP. 2008;22:291–296. doi: 10.4278/061030140R2.1. [DOI] [PubMed] [Google Scholar]
- 22.Task Force for the management of dyslipidaemias of the European Society of C, the European Atherosclerosis S. Catapano AL, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Atherosclerosis. 2011;217(Suppl 1):S1–44. doi: 10.1016/j.atherosclerosis.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: suggested best practices and future recommendations. Medical decision making: an international journal of the Society for Medical Decision Making. 2007;27:696–713. doi: 10.1177/0272989X07307271. [DOI] [PubMed] [Google Scholar]
- 24.Yusuf S, Bosch J, Dagenais G, et al. Cholesterol Lowering in Intermediate-Risk Persons without Cardiovascular Disease. The New England journal of medicine. 2016 doi: 10.1056/NEJMoa1600176. [DOI] [PubMed] [Google Scholar]
- 25.Ford I, Murray H, McCowan C, Packard CJ. Long-Term Safety and Efficacy of Lowering Low-Density Lipoprotein Cholesterol With Statin Therapy: 20-Year Follow-Up of West of Scotland Coronary Prevention Study. Circulation. 2016;133:1073–1080. doi: 10.1161/CIRCULATIONAHA.115.019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;63:2960–2984. doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Stacey D, Legare F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. The Cochrane database of systematic reviews. 2014;1:CD001431. doi: 10.1002/14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]