Abstract
BACKGROUND
Negative urgency, defined as impulsive risk-taking during extreme negative emotional states, is the most important impulsivity-related trait for alcohol-related problems and alcohol dependence. However, how negative urgency imparts risk for alcohol-related problems is not yet well understood. Therefore, the goal of the current study was to examine how negative urgency relates to separable aspects of the emotional experience and alcohol-seeking behaviors.
METHODS
A total of 34 (19 women) community-dwelling, alcohol-using adults aged 21–32 (mean age=24.86, SD=3.40, 74.3% Caucasian) completed two counterbalanced intravenous alcohol self-administration sessions: one during a neutral mood condition and one during a negative mood condition.
RESULTS
Negative urgency was associated with 1) greater mood change following negative mood induction (F=4.38, df=15, p=.002, η2=0.87), but was unrelated to changes in craving or cortisol release in response to mood induction; 2) greater alcohol craving prior to and after an alcohol prime (F=3.27, p=.02, η2=0.86), but only in the negative and not the neutral mood condition; and 3) higher peak BrAC (F=2.13, df=42, p=.02, η2=0.48), continuing to increase intoxication level over a longer period (F=3.77, df=42, p<.001, η2=0.62), and more alcohol seeking (F=21.73, df=22, p<.001, η2=0.94) throughout the negative session. Negative urgency was associated with overall lower cortisol release.
CONCLUSIONS
These results highlight the importance of assessing behavioral indicators of negative urgency under mood condition, and suggest that negative urgency may amplify alcohol self-administration through increased negative emotional reactivity to mood events and increased alcohol craving after initial alcohol exposure, leading to maintenance of alcohol related behavior.
Keywords: Intravenous (IV) alcohol administration, negative urgency, mood induction, alcohol seeking, alcohol consumption
1.0 Introduction
Negative urgency, defined as impulsive risk-taking behavior during extreme negative emotional states, is the most important impulsivity-related trait for alcohol-related problems and alcohol dependence (Coskunpinar et al., 2013). There is an emerging consensus that negative urgency is a transdiagnostic endophenotype for a wide range of clinical disorders, most notably alcohol use problems (see a review by Cyders et al., in press and Verdejo-Garcia et al., 2010). Although evidence suggests negative urgency plays a predictive role in problematic alcohol-related behaviors (Coskunpinar et al., 2013; Settles et al., 2010), the mechanisms by which negative urgency imparts risk for alcohol-related problems is not yet well understood. Although, negative mood influences multiple types of alcohol-related behaviors (Cyders et al., in press), it remains unclear how negative urgency might relate to these separate aspects of mood, alcohol seeking, and consumption. This lack of understanding limits the development of effective treatment and prevention strategies that would mitigate negative urgency’s influence on clinical problems; therefore, the goal of the current study was to examine potential mood and alcohol-related mechanisms underlying negative urgency’s effects on alcohol seeking behaviors.
Research on negative urgency has begun to unravel potential neurocognitive underpinnings associated with its increased risk. Although some reports suggest that negative urgency is unrelated to the intensity or frequency of self-reported emotion (e.g., Cyders et al., 2009; Cyders and Coskunpinar, 2010), others suggest a relationship between negative urgency and physiologic correlates of emotional reactivity (e.g., Cyders et al., 2014), which then subsequently influence alcohol-seeking (e.g., Carney et al., 2000; Steptoe and Wardle, 1999). Proposed mechanisms include increased emotional reactivity (e.g., Albein-Urios et al., 2013; Cyders et al., 2014), increased attention to and salience of reward cues (e.g., Chester et al., in press; Cyders et al., 2014), increased reward circuitry activation (e.g., Wilbertz et al., 2014; Xue et al., 2010), and decreased ability to regulate emotional experiences (e.g., Hoptman et al., 2014; Muhlert and Lawrence, 2015). However, research has yet to directly examine these factors, perhaps in part because of limitations in the ability to measure alcohol related behaviors in experimental contexts.
The current study used the Computer-Assisted Alcohol Infusion System (CAIS; Plawecki et al., 2013; Zimmerman et al., 2008, 2009) to examine how negative urgency relates to alcohol and alcohol-related behaviors. Oral consumption results in a high variability of the time course of consequent breath alcohol concentrations (BrACs) across individuals (Ramchandani et al., 2009), which in turn confounds experimental interpretation. Therefore, the present study used intravenous alcohol self-administration because, compared to oral alcohol administration, it allows for safely administering more ecologically valid doses of alcohol, better controls and predicts BrAC so that subsequent infusion does not place one over a predetermined safety limit while ensuring consistency and accuracy in the application of infusion rate and dose, and ensures a consistent time course of brain alcohol exposure per alcohol reward across individuals (Gomez et al., 2012). CAIS directly controls the rate of IV alcohol administration, based on predictions of a physiologically based pharmacokinetic model with parameters tailored to the individual subjects (Plawecki et al., 2007; Ramchandani et al., 1999). We included two behavioral measures of IV alcohol seeking: self-administration of freely available alcohol for enjoyment (Free Access, FA) and self-administration requiring progressive work (PW) assessing motivation for access to successive alcohol rewards.
The goals of the current study were to examine how negative urgency relates to the effects of both negative mood induction and alcohol exposure on self-reported affect, cortisol, and craving. We also sought to determine how negative urgency is related to different alcohol-related behaviors (craving, ad lib consumption (FA), and work exerted to gain access to alcohol reward (PW) (Hobbs et al., 2005)).
We hypothesized that greater negative urgency would be related to: 1) greater mood change from negative mood induction (e.g., Albein-Urios et al., 2013; Cyders et al., 2014); 2) greater cortisol release to negative mood induction (increased physiologic reactivity in Cyders et al., 2014); 3) greater alcohol craving in response to an alcohol prime following negative mood induction (e.g., Chester et al., in press; Cyders et al., 2014); 4) greater cortisol release to the alcohol prime (e.g., increased physiologic reactivity in Cyders et al., 2014); 5) higher peak BrAC in the negative mood session (e.g., Coskunpinar et al., 2013; Settles et al., 2010); and 6) more work for alcohol rewards in the negative mood session (e.g., Carney et al., 2000; Steptoe and Wardle, 1999).
2.0 Materials and Methods
2.1 Participants
Participants were community-dwelling, alcohol-using men and women. Two samples were collected as part of a larger study of the effects of negative mood on alcohol self-administration across men and women (Cyders et al., in press): the FA sample, which was able to freely self-administer alcohol up to a maximal BrAC of 150 mg/dL (n=14), and the PW sample, which completed progressively longer work sets for alcohol rewards with a maximum BrAC of 120 mg/dL (n=20). Inclusion/exclusion criteria for both samples included: 21–35 years old, current alcohol users, good medical and mental health, able to understand and complete questionnaires in English, no past or present alcohol dependence, not currently pregnant or intending to become pregnant, or breastfeeding. The FA sample was recruited for social drinking (at least 4 standard drinks per week and at least two binge episodes per month- defined as 4 or more drinks at a time for women and 5 or more drinks at a time for men; NIH, 2014). The PW sample was recruited for heavier social drinking (consuming at least 7 drinks per week and at least one binge episode per week). The difference in recent drinking history reflected the reality that the PW paradigm requires more motivation (i.e., more effort to gain access to alcohol) than FA. It also reflects our experience that heavier drinkers performing the FA paradigm often rapidly reach the maximum allowable BrAC: a ceiling effect limiting interpretation. Given that a majority of study variables were obtained prior to completing the PW or FA paradigms specific tasks and that we employed a within-subject experimental design, the two samples were combined for all analyses with the exception of the PW-specific outcome variables.
2.2 Measures and Materials
2.2.1 The UPPS-P Impulsive Behavior Scale-Revised
(UPPS-P; Lynam et al., 2006) is a 59 item self-report scale, with responses ranging from 1 (agree strongly) to 4 (disagree strongly). The UPPS-P is designed to measure five sub-facets of trait impulsivity: sensation seeking, lack of planning, lack of perseverance, positive urgency, and negative urgency. The present study only used the negative urgency subscale, which had adequate reliability (α= 0.86). Items were coded so that higher mean scores represented higher levels of negative urgency.
2.2.2 The Affect Grid
(Russell et al., 1989) is a single-item, 2-dimensional scale designed to assess current mood along orthogonal axes of pleasure-displeasure and arousal-sleepiness. It has adequate correlations with other, longer measures of current mood states, such as the Mehrabian and Russell (1974) scale (r= 0.77), making it a more practical measure of current emotional ambience. In the present study, the pleasure-displeasure axis of the affect grid was used as a check for the effectiveness of the mood manipulation, with higher values indicating more pleasure.
2.2.3 The Alcohol Use Disorders Identification Test
(AUDIT; Saunders et al., 1993) is a ten-item scale that assesses hazardous alcohol consumption, abnormal alcohol consumption behavior, and alcohol related problems. Data obtained by the AUDIT allows for discriminating between hazardous and non-hazardous drinkers and responses show concurrent validity with other measures of alcohol use (Saunders et al., 1993). In the current study, the AUDIT was used to exclude participants with moderate to severe scores (AUDIT ≥ 16; Saunders et al., 1993).
2.2.4 Subjective Craving
Subjective experiences were assessed with 4-items taken from the Alcohol Urge Questionnaire (Bohn, 1994): “Having a drink now would make things seem just perfect”; “I want a drink so bad I can almost taste it”; “Nothing would be better than having a drink right now”; and “I crave a drink right now”. These items had acceptable internal reliability at each time point (α= 0.93 to 0.96).
2.2.5 Salivary Cortisol Collection
Saliva was collected using the Passive Drool method with the Saliva Collection Aid as described in the Salimetrics Saliva Collection Handbook (2013). Saliva samples were stored at −20°C and sent to the Salimetrics Lab for analysis. Greater cortisol levels indicate greater stress levels (De Kloet et al., 2006). Prior literature suggests that, in response to 20 min negative mood induction, 20 min neutral mood induction, 500kcalorie breakfast, and acute pain stressors, there is an average salivary cortisol increase of approximately 0.12μg/dL, 0.07 μg/dL, 0.08 μg/dL, and 0.10 μg/dL, respectively. To return to typical circadian cortisol levels, it takes roughly 15 min for both negative and neutral mood induction and 30 min for food consumption and acute pain (Cauter et al., 1992; Gadea et al., 2005; Zimmer et al., 2003). Based on a calorie content of 7 calories per gram of pure ethanol, and participants receiving an average of 2.7mL of ethanol in the priming session, we expected a negligible 0.003μg/dL increase in cortisol as a direct effect of the calories in the alcohol (Hamilton et al., 1991).
2.2.6 Life Events Narratives
(Abele, 1990) were used to induce either a negative or neutral mood. The negative life events narrative asks respondents to write about an event that made them particularly sad or upset in their lives. The neutral life events narrative asks respondents to write about their activities on a typical day. Both writing sessions required approximately twenty min each. Writing procedures are effective at inducing negative mood states (mean r= 0.52; e.g., Westermann et al., 1996). In order to increase the effect of the life events narrative, writing was paired with the musical mood induction (see below).
2.2.7 Musical Mood Induction Procedure
(MMIP; Västfjäll, 2002) was used to maintain the specified mood state. Initial song lists were taken from Västfjäll (2002). All songs were then rated by four trained raters, and songs that were not correctly categorized as negative or neutral were removed from the list. Listening to negative songs are associated with a more negative subjective mood rating compared to neutral songs (p<0.05; Västfjäll, 2002). Music was played continuously through over the ear headphones during the writing, priming, and alcohol self-administration sessions (songs and order of presentation are shown in Cyders et al., in press, Supplemental Table 1).
2.3 Procedure
All study documents and procedures were approved by the Institutional Review Board and Human Subjects Office. Participants were recruited through the use of advertisements posted in public areas (e.g. bars, liquor stores, etc.), on local college campuses, and on the Internet. Participants were provided informed consent and completed a screening session where they completed a series of questionnaires and interviews, including study measures listed above, to assess subject eligibility and to examine study hypotheses.
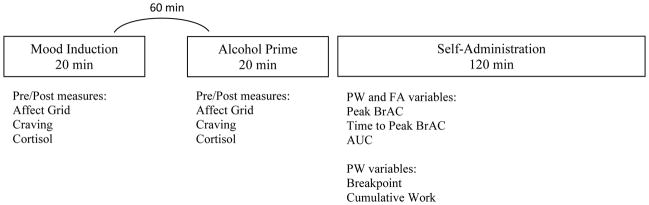
A timeline of study-relevant measurements and procedures during the two sessions is shown in Figure 1, including screening, writing the life narrative, alcohol prime, reading aloud the life narrative, and completion of the alcohol self-administration paradigm. Participants completed two counterbalanced IV alcohol self-administration sessions, scheduled approximately one week apart: one in which they engaged in a negative mood induction and one in which they engaged in a neutral mood induction.
Figure 1.
Timeline of study-relevant measures and procedures.
Note. PW= progressive work condition. FA= free access condition. BrAC= breath alcohol concentration. AUC= area under the alcohol exposure curve
At each session, participants did the following: 1) Participants first provided a BrAC and a urine sample for drug and pregnancy screen; any testing positive for marijuana were interviewed to ensure they were no longer under the effects of the drug (n=7 such participants completed the study). 2) Participants provided a saliva sample and mood rating, prior to and after completing the life event narrative, while listening to mood congruent music. The written narrative at this stage allowed for evaluation of mood effects on cortisol, affect, and craving without being confounded by the effects of alcohol.3) Participants were then given a standardized light breakfast (500 kcal), monitored by the hospital staff. Thirty minutes after breakfast, a member of the nursing staff inserted a 22 ga. indwelling venous catheter within the ante cubital fossa of the participant’s non-dominant arm. 4) Participants then provided a saliva sample, mood rating, and craving report prior to and after an alcohol prime. Prior to infusion, the subject’s age, height, weight, and gender were entered into the CAIS software, which transformed those measurements into the parameters of the physiologically-based pharmacokinetic model, tailoring the model’s estimation of future BrAC to the individual (Plawecki et al., 2007; Ramchandani et al., 1999). Participants were infused with a 6.0% (v/v) solution of ethanol in half-normal saline. The alcohol reward chosen for this study increased BrAC from its current value by 7.5 mg/dL in 2.5 min (a linear ascending limb slope of +3mg/dL/min), followed by a linear descent at −1.0 mg/dL/min until the next alcohol reward delivery began. A 20 min priming interval began with two prompted alcohol rewards (the priming exposure) yielding a BrAC of 15mg/dL BrAC after 5 min, consistent with ingesting one standard alcoholic drink. Participants were then informed by video-screen that no more drinks could be requested for the next 15 min; CAIS tracked the descending BrAC for 15 min resulting in a BrAC of approximately 5mg/dL at 20 min in all participants. Throughout the priming interval, participants continued to listen to mood congruent music. At the end of the priming interval, participants read their life narrative aloud so as to re-induce the mood condition (Westermann et al., 1996). 6) A 2-hour voluntary alcohol self-administration phase ensued, using either the FA or the PW paradigm. Participants were told they could self-administer either alcohol or water, as much or as little as they like, but that the session would still last 2 hours and they would be required to stay in the hospital until 5pm regardless of the intoxication level achieved. During both FA and PW sessions, the participant saw the message “the bar is temporarily closed” for the 2.5 min ascending limb of the rewards and whenever more alcohol would raise the BrAC above the safety limit (150mg/dL for FA or 120mg/dL for PW). To verify CAIS estimates, actual BrAC measurements were obtained intermittently throughout the experiment; always during reward delivery so the procedure would not interfere with the opportunity to work. Bathroom breaks occurred ad lib without disconnection from the CAIS apparatus, and the CAIS technician remained screened from the subject throughout the session. Participants were asked to complete an affect grid and craving scale near the end of the 2.5 min delivery period of either reward. Participants also completed the subjective questionnaire roughly every 30 min throughout the 2-hour session. At the end of each session, the IV catheter was removed and the subject was required to remain on the CRC until his/her BrAC was below 20mg/dL and the CRC nursing staff could no longer identify behavioral signs of intoxication, or until 5pm, whichever was later. Participants were provided lunch and dinner during their stay and were paid in cash upon discharge.
In the FA paradigm, a single button push initiated the chosen reward (“alcohol” or “water”). In the PW paradigm, participants performed the Constant Attention Task (CAT), an adaptive and cognitively effortful task which the subject could not perform successfully while paying attention to anything else. CAIS adjusts each CAT trial response window so that 50% of trials were successful, independent of fatigue, practice and intoxication. A predefined, progressive schedule of successful CAT trials, organized into work sets, was required to earn the selected reward (Plawecki, 2013). The previously chosen reward (“alcohol” or “water”) was delivered immediately upon completion of that set. The number of successful trials required to obtain a reward increased exponentially throughout the session and progress on the identical schedules for alcohol and water rewards were managed separately by CAIS.
2.4 Statistical Analyses
All analyses were conducted using SPSS 23.0. We combined data from the PW and FA samples (n=34) in order to increase power for all outcome variables in common and because the majority of assessments occurred prior to divergence in the methods between the two samples. For the three variables for which the samples were treated differently (peak BrAC, Time to Peak BrAC, and Area Under the Curve of BrAC), we controlled for group as a covariate in all analyses. Two controls were used in our study: 1) In each condition, the participants had the option to work for/infuse alcohol or saline, thereby controlling for any effects related to an alternative reinforce and 2) we included a neutral mood condition in order to control for any effects related to the task (writing, reading, infusion) itself. The data were then examined for missing data, skewness, kurtosis, and outliers. Second, we utilized a series of two repeated measures analyses of covariance (ANCOVA) (mood: negative or neutral) × 2 (time: pre/post alcohol prime) to examine how negative urgency relates to changes in self-reported mood, alcohol craving, cortisol release, and alcohol seeking across a range of variables, controlling for sex1 and age in all analyses (see Cyders et al., in press). Third, we conducted a series of repeated measures ANCOVA to examine how negative urgency relates to alcohol consumption and seeking behaviors across the neutral and negative mood sessions, controlling for group (PW vs. FA), age, and gender. Follow-up contrasts were conducted and significant interactions were probed (at one standard deviation above and below the mean of negative urgency) and graphed. Because of the small sample size, we examined effect sizes (partial η2) in addition to statistical tests to inform future work, using standard values to denote small (0.01), medium (0.06), and large (0.14) (Cohen, 1992).
3.0 Results
3.1 Sample Characteristics and Preliminary Analyses
A total of 34 (19 women) community-dwelling alcohol-using adults aged 21–32 (mean age=24.86, SD=3.40) completed the study. The sample was mostly Caucasian (74.3%), with 17.1% African American, 5.7% Asian, and 2.9% Latino. The overall sample’s mean AUDIT score was 10.1 (SD=3.35); not surprisingly, the only notable difference between the two samples was in AUDIT score, with the PW sample having significantly higher AUDIT scores (AUDIT=11.14, SD=3.34) than the FA sample (AUDIT=8.62, SD=2.79; t(32)= 3.22, p= 0.03). The PW and FA groups did not differ on age (t=−0.27, p=.79), sex (t=−1.21, p=.23) or negative urgency (t=.86, p=.39), suggesting no bias across the groups on our independent variables and moderator variables that might be confounding our results. Not surprisingly, the groups did differ on peak BrAC (t=−6.64, p<.001), time to peak BrAC (t=−6.81, p<.001), and AUC (t=−4.92, p<.001). The sample overall reported a mean negative urgency of 2.00 (SD=0.67). All cortisol measurements were within the expected range based on these increases and the daily circadian rhythm observed by Aardal and Holm (1995), suggesting that there were no ceiling or floor effects (i.e., too high or low cortisol concentrations, respectively) within the cortisol data. The average peak BrAC (M= 100.5 mg/dl, SD= 38.72) occurred 84.5 min into the 140-min study protocol. Affect valence ratings and craving data collected shortly before the peak BrAC were used in the analyses.
3.2 Mood Induction
3.2.1 Effects on self-reported affect
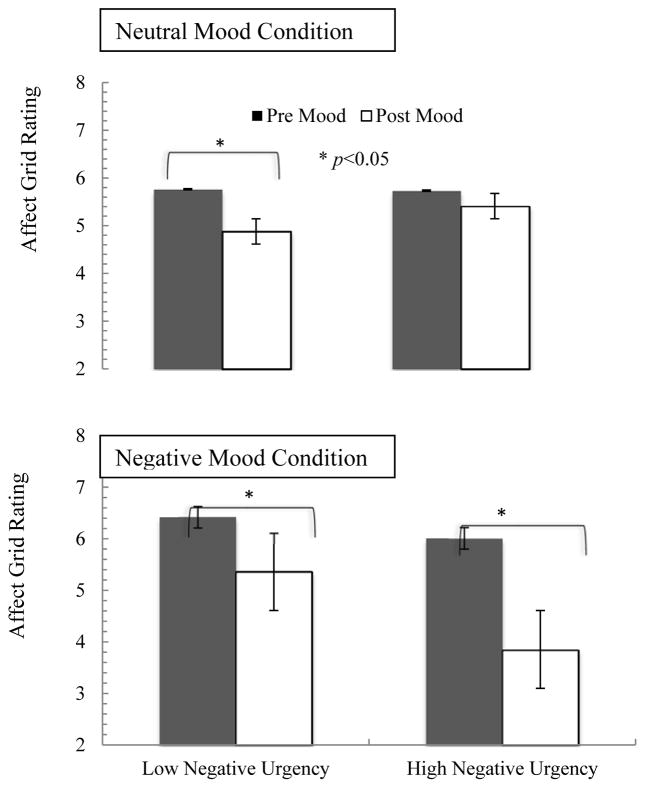
The 2 (mood: negative, neutral) × 2 (time: pre/post mood induction) repeated measures ANCOVA suggested a significant mood by time by negative urgency three-way interaction (F=4.38, df=15, p=.002, η2=0.87).2 Follow-up contrasts indicate that there was a trend toward a significant negative urgency by time interaction in the negative mood condition (F=2.03, p=.08, η2=0.75), but not in the neutral mood condition (F=1.21, p=.36, η2=0.64). In the negative mood condition, those higher in negative urgency had overall greater mood change following mood induction at both time points, but this difference was larger post mood induction (Figure 2).
Figure 2.
Self-reported mood changes in response to mood inductions by negative urgency
Note. Higher affect grid rating indicates more positive mood
3.2.2 Effects on alcohol craving
The 2 (mood: negative, neutral) × 2 (time: pre/post mood induction) repeated measures ANCOVA indicated that mood induction had no significant effects on alcohol craving, and was unrelated to negative urgency (all p’s>.40).
3.2.3 Effects on cortisol release
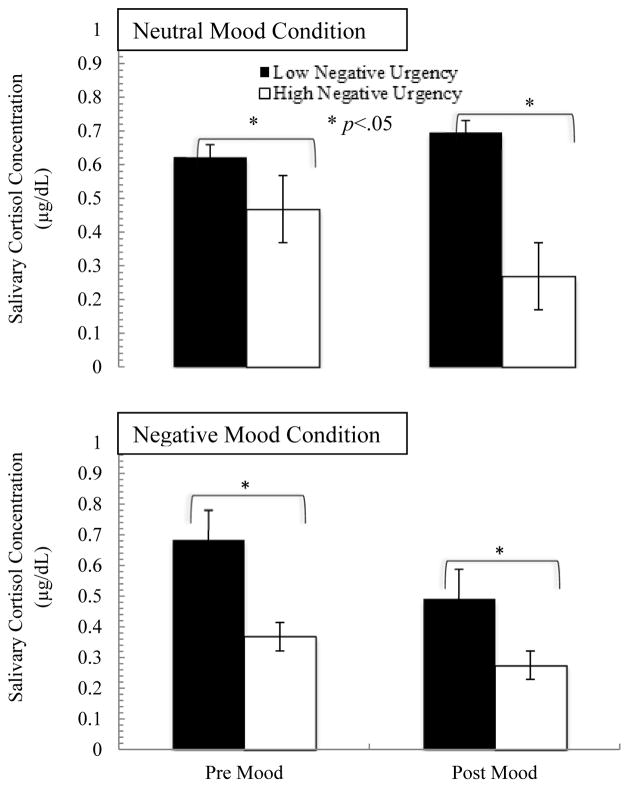
The 2 (mood: negative, neutral) × 2 (time: pre/post mood induction) repeated measures ANCOVA indicated a significant mood by time interaction (F=5.30, p=.04, η2=0.29), and a trend toward a mood by time by negative urgency interaction (F=1.96, p=.10, η2=0.74). Probing of the significant mood by time interaction indicated that there were decreases in cortisol in both conditions in response to the mood condition (Figure 3); higher negative urgency was associated with lower cortisol release in all conditions.
Figure 3.
Cortisol Release across conditions and negative urgency level
3.3 Alcohol Prime
3.3.1 Effects on self-reported affect
The 2 (mood: negative, neutral) × 2 (time: pre/post alcohol prime) repeated measures ANCOVA indicated no significant relationship between negative urgency and self-reported affect change in response to the alcohol prime (all p’s>.40).
3.3.2 Effects on alcohol craving
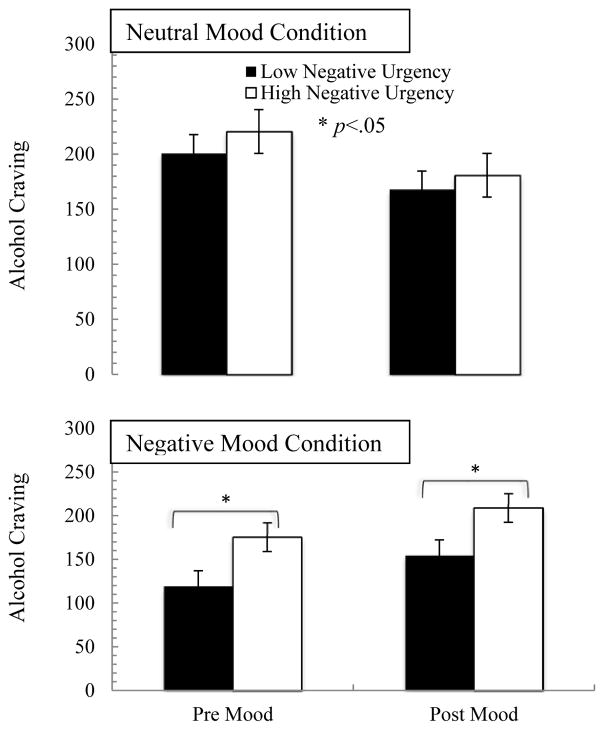
The 2 (mood: negative, neutral) × 2 (time: pre/post alcohol prime) repeated measures ANCOVA indicated a significant mood by time by negative urgency interaction (F=3.27, p=.02, η2=0.86). Follow up contrasts indicated that there was no significant time by negative urgency interaction in the neutral mood condition (F=0.87, p=.62, η2=0.61), but there was a trend in the negative mood condition (F=2.33, p=.07, η2=0.81). In the neutral mood condition, negative urgency was unassociated with alcohol craving changes. However, in the negative mood condition, higher negative urgency was associated with greater alcohol craving both prior to and following the mood induction (Figure 4).
Figure 4.
Alcohol craving in response to alcohol prime by mood, time and negative urgency
3.3.3 Effects on cortisol release
The 2 (mood; negative, neutral) × 2 (time: pre/post alcohol prime) repeated measures ANCOVA indicated no significant relationship between negative urgency and cortisol release in response to the alcohol prime (all p’s>.30).
3.4 Alcohol Seeking
3.4.1 Effects on peak BrAC and consumption patterns
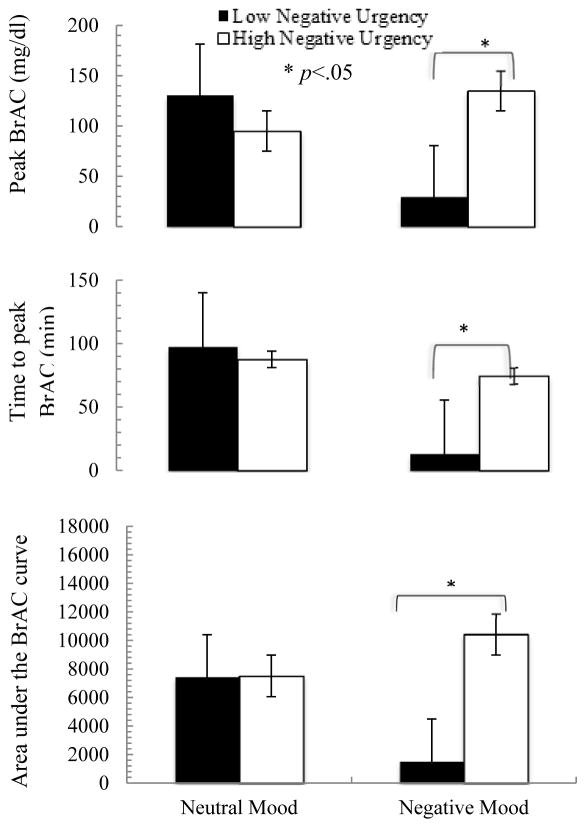
Three variables were analyzed to demonstrate alcohol consumption patterns: peak BrAC (highest BrAC achieved during the alcohol self-administration session), time to peak BrAC (experimental time at which the peak BrAC was achieved), and area under the alcohol exposure curve (AUC).
3.4.1.1 Peak BrAC
For peak BrAC, there were significant effects of mood (F=14.38, df=42, p<.001, η2=0.26) and a mood by negative urgency interaction (F=2.13, df=42, p=.02, η2=0.48).3 In the neutral mood condition, there was no relationship between negative urgency and peak BrAC; however, in the negative mood condition, participants higher in negative urgency reached a greater peak BrAC than those lower in negative urgency (Figure 5). This effect is, in part, related to increased peak BrAC in negative as compared to neutral mood conditions for those higher in negative urgency, as well as decreased peak BrAC in negative as compared to neutral mood conditions for those lower in negative urgency.
Figure 5.
Alcohol consumption behavior changes by mood and negative urgency
3.4.1.2 Time to peak BrAC
For time to peak BrAC, there was a significant effect of mood (F=18.37, df=42, p=<.001, η2=0.30) and a mood by negative urgency interaction (F=3.77, df=42, p<.001, η2=0.62).4 There was no relationship between negative urgency and time to peak BrAC in the neutral mood condition; however, in the negative mood condition, participants higher in negative urgency took a longer time to reach peak BrAC than those lower in negative urgency (Figure 5). This effect was driven mostly by a reduction in time to peak BrAC in the negative as compared to the neutral condition for those low in negative urgency; those high in negative urgency showed similar time to peak BrAC across mood conditions.
3.4.1.3 AUC
For AUC, there were significant effects of mood (F=12.01, df=42, p=.001, η2=0.22) and a mood by negative urgency interaction (F=2.73, df=42, p=.004, η2=0.54).5 There was no relationship between negative urgency and AUC in the neutral mood condition; in the negative mood condition, participants higher in negative urgency had a greater AUC than those lower in negative urgency (Figure 5). This effect was driven by both an increase in AUC in the negative as compared to the neutral condition for those higher in negative urgency, as well as a reduction in AUC in the negative as compared to the neutral condition for those lower in negative urgency.
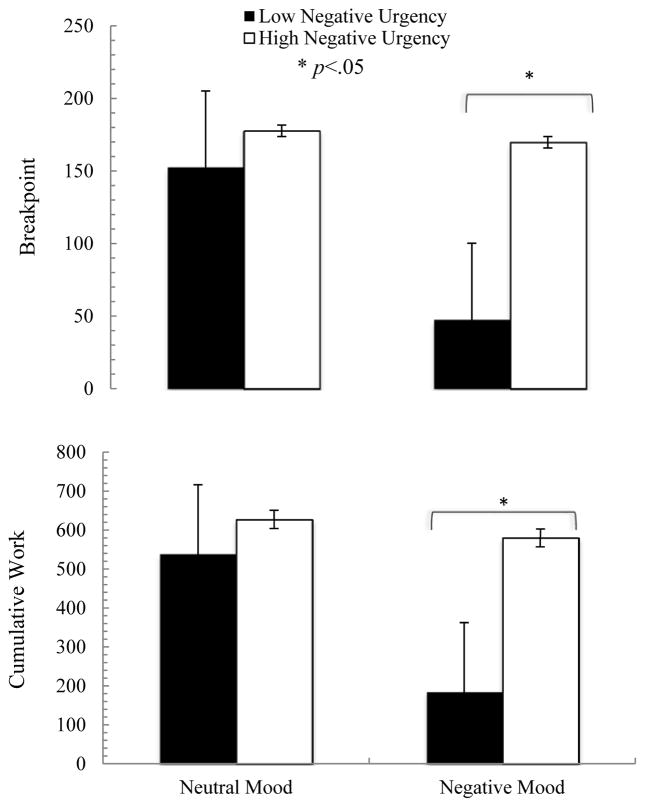
3.4.2 Effects on motivation to work for alcohol
Motivation to work for alcohol was assessed in the PW sample only, as the FA sample did not have to work to receive alcohol. Two variables were analyzed to demonstrate motivation for alcohol rewards: breakpoint (number of total CAT trials in the last earned alcohol reward) and cumulative work (total number of completed CAT trials for alcohol rewards across the entire infusion session).
3.4.2.1 Breakpoint
For breakpoint, there were significant effects of mood by negative urgency interaction (F=14.48, df=22, p<.001, η2=0.91).6 There was no relationship between breakpoint and negative urgency in the neutral mood condition; however, in the negative mood condition, participants higher in negative urgency exhibited a higher breakpoint than those at a lower level of negative urgency (Figure 6). This effect was mostly driven by a reduction in breakpoint for those low in negative urgency between the neutral and the negative mood conditions.
Figure 6.
Progressive work alcohol seeking behavior changes by mood and negative urgency
3.4.2.2 Cumulative Work
Similarly, for cumulative work, there were significant effects of mood (F=5.80, df=22, p=.03, η2=0.21) and mood by negative urgency interaction (F=21.73, df=22, p<.001, η2=0.94).7 There was no relationship between cumulative work and negative urgency in the neutral mood condition; however, in the negative mood condition, participants higher in negative urgency exhibited a higher cumulative work than those at a lower level of negative urgency (Figure 6). This effect was mostly driven by a reduction in cumulative work for those lower in negative urgency between the neutral and the negative mood conditions.
4.0 Discussion
Overall, this initial study found that negative urgency is differentially related to separable aspects of the emotional experience and alcohol-related behaviors. 1) Negative urgency was related to greater mood change following negative mood induction, but was unrelated to craving or cortisol release changes in response to mood induction. 2) Negative urgency was related to greater alcohol craving prior to and after an alcohol prime, but only in the negative and not the neutral mood condition. 3) Negative urgency was related to peak BrAC, increasing intoxication level over time, and more alcohol seeking throughout the negative session.
Importantly, negative urgency was related to emotion, craving, alcohol consumption, and alcohol seeking primarily only during negative, but not neutral, mood conditions. This segregation suggests that in measuring behavioral indicators of negative urgency, the mood context of the measurement should be considered. Although negative urgency has been shown to be a general risk factor for problematic alcohol consumption (e.g., Coskunpinar et al., 2013), very little work has suggested how negative urgency affects these outcomes in the moment of the experience of negative emotional experiences. Importantly, this pattern was driven both by increases in negative mood behavior related to high negative urgency and decreases in negative mood behavior related to low negative urgency. Negative mood seems to decrease alcohol seeking for those with low negative urgency, but increase, or at least maintain, it for those with high negative urgency. Consequently, negative mood becomes a risk factor only for those at higher levels of negative urgency.
Our data suggest that negative urgency might affect alcohol consumption and seeking in negative emotional states by leading individuals to experience 1) more negative emotional reactivity and 2) increased alcohol craving to the first alcohol exposure. These two factors appear to increase or at least maintain alcohol seeking, leading those with high negative urgency to show more consumption and alcohol seeking persistence during negative mood contexts (compared to those with lower negative urgency).
These results are the first empirical data supporting a role for negative urgency in alcohol-seeking and consuming behaviors in an IV alcohol self-administration paradigm. This finding is important because IV alcohol design controls for pharmacokinetic differences, thus ensuring similar brain exposure to alcohol at each dose across individuals with different levels of tolerance. Additionally, IV alcohol effects are assessed in the absence of alcohol cues and expectancies that might affect the experience of alcohol consumption. Recent work has related negative urgency with increased responses to alcohol cues (e.g., Cyders et al., 2014). The current study suggests that negative urgency not only appears to be important in responding to alcohol cues (e.g., Cyders et al., 2014), but also in seeking pharmacological effects of alcohol, sans any effects of alcohol cue or contexts.
In the current study, negative urgency was unrelated to cortisol release in response to mood induction or alcohol prime (although there was a trend for negative urgency to relate overall to lower cortisol release across all conditions). However, it’s important to note that our mood manipulation decreased, rather than increased, cortisol release. Additionally, our participants showed no effect of alcohol prime on cortisol release. Methodological factors likely limited our cortisol results: 1) The timing of the cortisol measure might have been too long after completion of the writing narrative to capture cortisol increase (Gadea et al., 2005) or 2) The timing of study sessions in the morning, when cortisol levels typically drop dramatically, might have reduced ability to show sharp, reliable increases in cortisol. Future work might consider evaluating cortisol via real-time blood measures that might be sensitive to these changes, and should include more powerful mood manipulations, such as reading sequentially more depressing statements for 20 min or talking to another person about a negative event (Engert et al., 2014; Gadea et al., 2005).
Although the current study has limitations, including a relatively small, homogeneous sample, these findings do suggest viability of this line of research. The effect sizes for many of the study findings suggest robustness of these negative urgency effects and they can be used in future research planning. Future work should also examine these effects across a range of alcohol use disorder severity, as the current sample was primarily comprised of moderate to heavy social drinkers, all of whom had no current or past history of an alcohol use disorder. Finally, the current work, and its potentially compromised cortisol manipulation, does not allow for conclusions concerning the relationship between negative urgency and physiological HPA axis activation.
5.0 Conclusions
The current data are an important first step in determining the mechanisms by which negative urgency imparts its high risk for problematic alcohol consumption. In combination with recent work, it appears that greater negative urgency is related to 1) experiencing more negative emotional reactivity in the moment (e.g., Albein-Urios et al., 2013; Cyders et al., 2014) and 2) being more reactive to alcohol cues (e.g., Chester et al., in press; Cyders et al., 2014) and to the initial experience of alcohol (current study), all of which likely increase or at least maintain negative mood related alcohol seeking and consumption. In simpler terms, those high in negative urgency are not demotivated to seek alcohol in the context of a negative mood, as those at lower levels seem to be.
Acknowledgments
We want to express our gratitude to the Indiana (CTSI) Clinical Research Center (UL1TR001108) and Nicholas Grahame, Sage Bates, and Eva Agriyou for their continued effort in making this project possible.
7.0 Research Support
This work was supported by the National Institute of Alcohol Abuse and Alcoholism [grant number K01AA020102 (MAC)], the Indiana Alcohol Research Center [grant number P60AA07611], and an Indiana University – Purdue University, Indianapolis University Fellowship (JDV).
Footnotes
See Cyders et al., in press, for a full discussion of sex effects in the current data.
There was also a significant time by sex interaction (F=5.06, df=15, p=.04, η2=0.25) on self-reported mood change.
There was also a mood by age interaction (F=17.71, df=42, p<.001, η2=0.30).
There were also mood by sex (F=10.47, df=42, p=.002, η2=0.20) and mood by age (F=12.68, df=42, p=.001, η2=0.23) interactions.
There was also a mood by age interaction (F=14.47, df=42, p<.001, η2=0.26).
There were also mood by age (F=5.82, df=22, p=.03, η2=0.21) and mood by sex (F=5.32, df=22, p=.03, η2=0.20) interactions.
There were also mood by age (F=23.42, df=22, p<.001, η2=0.52), and mood by sex (F=6.56, df=22, p=.02, η2=0.23) interactions.
References
- Aardal E, Holm AC. Cortisol in saliva-reference ranges and relation to cortisol in serum. Clin Chem Lab Med. 1995;33:927–932. doi: 10.1515/cclm.1995.33.12.927. [DOI] [PubMed] [Google Scholar]
- Abele A. Recall of positive and negative life events. Studies of mood-inducing effect and production of texts. Z Exp Angew Psychol. 1990;37:181–207. [PubMed] [Google Scholar]
- Albein-Urios N, Martinez-Gonzalez JM, Lozano Ó, Moreno-López L, Soriano-Mas C, Verdejo-Garcia A. Negative urgency, disinhibition and reduced temporal pole gray matter characterize the comorbidity of cocaine dependence and personality disorders. Drug Alcohol Depend. 2013;132:231–237. doi: 10.1016/j.drugalcdep.2013.02.008. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Carney MA, Armeli S, Tennen H, Affleck G, O’Neil TP. Positive and negative daily events, perceived stress, and alcohol use: a diary study. J Consult Clin Psychol. 2000;68:788–798. [PubMed] [Google Scholar]
- Chester DS, Lynam DR, Milich R, DeWall CN. How do negative emotions impair self-control? A Neural model of negative urgency. NeuroImage. doi: 10.1016/j.neuroimage.2016.02.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra K, Segal Z, Buis T, Kennedy S, Levitan R. Investigating associations between cortisol and cognitive reactivity to sad mood provocation and the prediction of relapse in remitted major depression. Asian J Psychiatr. 2008;1:33–36. doi: 10.1016/j.ajp.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Coskunpinar A, Dir AL, Cyders MA. Multidimensionality in impulsivity and alcohol use: a meta-analysis using the UPPS model of impulsivity. Alcohol Clin Exp Res. 2013;37:1441–1450. doi: 10.1111/acer.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Coskunpinar A. Is urgency emotionality? Separating urgent behaviors from effects of emotional experiences. Pers Individ Dif. 2010;48:839–844. doi: 10.1016/j.paid.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Coskunpinar A, VanderVeen JD. American Psychiatric Association. Urgency- A common transdiagnostic endophenotype for maladaptive risk-taking. In: Zeigler-Hill V, editor. The Dark Side of Personality. Arlington (VA): American Psychiatric Publishing; in press. [Google Scholar]
- Cyders MA, Dzemidzic M, Eiler WJ, Coskunpinar A, Karyadi K, Kareken DA. Negative urgency and ventromedial prefrontal cortex responses to alcohol cues: FMRI evidence of emotion-based impulsivity. Alcohol Clin Exp Res. 2014;38:409–417. doi: 10.1111/acer.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, Flory K, Rainer S, Smith GT. The role of personality dispositions to risky behavior in predicting first-year college drinking. Addiction. 2009;104:193–202. doi: 10.1111/j.1360-0443.2008.02434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyders MA, VanderVeen JD, Plawecki M, Millward JB, Hayes J, Kareken DA, O’Connor S. Gender specific effects of mood on alcohol seeking behaviors: Preliminary findings using intravenous alcohol self-administration. Alcohol Clin Exp Res. :40. doi: 10.1111/acer.12955. in press. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kloet C, Vermetten E, Geuze E, Kavelaars A, Heijnen C, Westenberg H. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40:550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Engert V, Smallwood J, Singer T. Mind your thoughts: Associations between self-generated thoughts and stress-induced and baseline levels of cortisol and alpha-amylase. Biol Psychol. 2014;103:283–291. doi: 10.1016/j.biopsycho.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Gadea M, Gómez C, González-Bono E, Espert R, Salvador A. Increased cortisol and decreased right ear advantage (REA) in dichotic listening following a negative mood induction. Psychoneuroendocrino. 2005;30:129–138. doi: 10.1016/j.psyneuen.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Gomez R, Behar KL, Watzl J, Weinzimer SA, Gulanski B, Sanacora G, … Petrakis IL. Intravenous ethanol infusion decreases human cortical γ-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biol Psychiat. 2012;71:239–246. doi: 10.1016/j.biopsych.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton EMN, Whitney EN, Sizer FS. Nutrition: Concepts and controversies. 5. Wadsworth; California: 1991. [Google Scholar]
- Hobbs M, Remington B, Glautier S. Dissociation of wanting and liking for alcohol in humans: a test of the incentive-sensitisation theory. Psychopharmacology. 2005;178:493–499. doi: 10.1007/s00213-004-2026-0. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Antonius D, Mauro CJ, Parker EM, Javitt DC. Cortical thinning, functional connectivity, and mood-related impulsivity in schizophrenia: relationship to aggressive attitudes and behavior. Am J Psychiat. 2014;171:939–948. doi: 10.1176/appi.ajp.2014.13111553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner C, Huffziger S, Liebsch K. Rumination, distraction and mindful self-focus: Effects on mood, dysfunctional attitudes and cortisol stress response. Psychol Med. 2009;39:219–228. doi: 10.1017/S0033291708003553. [DOI] [PubMed] [Google Scholar]
- Lynam D, Smith G, Whiteside S, Cyders M. The UPPS-P: Assessing five personality pathways to impulsive behavior. Purdue University; Indiana: 2006. [Google Scholar]
- Mehrabian A, Russell JA. An approach to environmental psychology. the MIT Press; 1974. [Google Scholar]
- Muhlert N, Lawrence AD. Brain structure correlates of emotion-based rash impulsivity. NeuroImage. 2015;115:138–146. doi: 10.1016/j.neuroimage.2015.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki MH, DeCarlo RA, Ramchandani VA, O’Connor S. Improved transformation of morphometric measurements for a priori parameter estimation in a physiologically-based pharmacokinetic model of ethanol. Biomed Signal Process Control. 2007;2:97–110. doi: 10.1016/j.bspc.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plawecki MH, Wetherill L, Vitvitskiy V, Kosobud A, Zimmermann US, Edenberg HJ, O’Connor S. Voluntary intravenous self-administration of alcohol detects an interaction between GABAergic manipulation and GABRG1 polymorphism genotype: A pilot study. Alcohol Clin Exp Res. 2013;37:E152–E160. doi: 10.1111/j.1530-0277.2012.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani VA, Bolane J, Li TK, O’Connor S. A physiologically-based pharmacokinetic (PBPK) model for alcohol facilitates rapid BrAC clamping. Alcohol Clin Exp Res. 1999;23:617–623. [PubMed] [Google Scholar]
- Ramchandani VA, Plawecki M, Li TK, O’Connor S. Intravenous ethanol infusions can mimic the time course of breath alcohol concentrations following oral alcohol administration in health volunteers. Alcohol Clin Exp Res. 2009;33:938–944. doi: 10.1111/j.1530-0277.2009.00906.x. [DOI] [PubMed] [Google Scholar]
- Russell JA, Weiss A, Mendelsohn GA. Affect grid: A single-item scale of pleasure and arousal. J Pers Soc Psychol. 1989;57:493. [Google Scholar]
- Salimetrics LLC, SalivaBio LLC, editors. [accessed on [19 October 2015]];Saliva Collection and Handling Advice. 2013 from http://www.salimetrics.com/collection-supplies.
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption--II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Settles RF, Cyders M, Smith GT. Longitudinal validation of the acquired preparedness model of drinking risk. Psychol Addict Behav. 2010;24:198–208. doi: 10.1037/a0017631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Wardle J. Mood and drinking: A naturalistic diary study of alcohol, coffee and tea. Psychopharmacology. 1999;141:315–321. doi: 10.1007/s002130050839. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism. [accessed on [19 October 2015]];Moderate and binge drinking. 2014 from http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- Van Cauter E, Shapiro ET, Tillil H, Polonsky KS. Circadian modulation of glucose and insulin responses to meals: relationship to cortisol rhythm. Am J Physiol Endocrinol Metab. 1992;262:E467–E475. doi: 10.1152/ajpendo.1992.262.4.E467. [DOI] [PubMed] [Google Scholar]
- Västfjäll D. Emotion induction through music: A review of the musical mood induction procedure. Music Sci. 2002;5:173–211. [Google Scholar]
- Verdejo-García A, del Mar Sánchez-Fernández M, Alonso-Maroto LM, Fernández-Calderón F, Perales JC, Lozano Ó, Pérez-García M. Impulsivity and executive functions in polysubstance-using rave attenders. Psychopharmacology. 2010;210:377–392. doi: 10.1007/s00213-010-1833-8. [DOI] [PubMed] [Google Scholar]
- Westermann R, Spies K, Stahl G, Hesse FW. Relative effectiveness and validity of mood induction procedures: a meta-analysis. Eur J Soc Psychol. 1996;26:557–580. [Google Scholar]
- Wilbertz T, Deserno L, Horstmann A, Neumann J, Villringer A, Heinze HJ, … Schlagenhauf F. Response inhibition and its relation to multidimensional impulsivity. Neuroimage. 2014;103:241–248. doi: 10.1016/j.neuroimage.2014.09.021. [DOI] [PubMed] [Google Scholar]
- Xue G, Lu Z, Levin IP, Bechara A. The impact of prior risk experiences on subsequent risky decision-making: the role of the insula. Neuroimage. 2010;50:709–716. doi: 10.1016/j.neuroimage.2009.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C, Basler HD, Vedder H, Lautenbacher S. Sex differences in cortisol response to noxious stress. Clin J Pain. 2003;19:233–239. doi: 10.1097/00002508-200307000-00006. [DOI] [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Laucht M, Vitvitskiy V, Plawecki MH, Mann KF, O’Connor S. Offspring of parents with an alcohol use disorder prefer higher levels of brain alcohol exposure in experiments involving computer-assisted self-infusion of ethanol (CASE) Psychopharmacology. 2009;202:689–697. doi: 10.1007/s00213-008-1349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, Mick I, Vitvitskyi V, Plawecki MH, Mann KF, O’Connor S. Development and pilot validation of computer-assisted self-infusion of ethanol (CASE): A new method to study alcohol self-administration in humans. Alcohol Clin Exp Res. 2008;32:1321–1328. doi: 10.1111/j.1530-0277.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]