Abstract
Background
MGMT methylation status can influence the therapeutic effect and prognosis of glioblastoma (GBM). There are conflicting results from studies evaluating the efficacy of bevacizumab (BV) when it is combined with temozolomide (TMZ) and radiotherapy (RT) in patients diagnosed with GBM with different MGMT methylation status.
Material/Methods
Data were extracted from publications in PubMed, Embase, and The Cochrane Library, with the last search performed March 23, 2016. Data on overall survival (OS), progression-free survival (PFS), and MGMT methylation status were obtained.
Results
Data from 3 clinical trials for a total of 1443 subjects were used for this meta-analysis. MGMT methylated and unmethylated patients showed improved PFS in the BV group (pooled HRs, 0.769, 95% CIs 0.604–0.978, P=0.032; 0.675, 95%CIs 0.466–0.979, P=0.038). For patients with either type of GBM, BV did not improve the OS based on the pooled HRs 1.132 (95% CIs 0.876–1.462; P=0.345) for methylated and 1.018 (95% CIs 0.879–1.179; P=0.345) for unmethylated.
Conclusions
Bevacizumab combined with temozolomide-radiotherapy correlated with improved PFS for treatment of patients with different MGMT methylation status of newly diagnosed GBM. There was insufficient evidence to determine the synergistic effects of combining BV with TMZ and RT on improving survival in patients with different MGMT methylation status.
MeSH Keywords: Chemoradiotherapy, Disease-Free Survival, Glioblastoma
Background
Glioma is the most common primary brain tumor in adults, with an unfavorable prognosis. Glioblastoma (GBM) is the most common and deadly histological type of glioma. Patients diagnosed with GBM have a median survival time of 12–18 months and few patients survive more than 5 years from initial diagnosis [1]. The classic treatment typically consists of maximal feasible resection, followed by radiotherapy combined with chemotherapy. One of the most commonly used chemotherapy drugs for the treatment of GBM is temozolomide (TMZ), a kind of alkylating agent that may sensitize the cells to radiation [2]. Patients are treated with temozolomide and radiation therapy show an improved median survival of 15 months [1,3]. Nevertheless, the prognosis for these patients remains poor and survival beyond 5 years remains quite low (about 9.8%) [3–5].
A variety of factors may influence the clinical benefit of temozolomide, including patient age, the extent of the required surgery, and the genetic composition of the individual [6,7]. The O6-methylguanine-DNA methyltransferase (MGMT) has been identified as a major mechanism by which cells can exhibit resistance to temozolomide [8]. Methylation of the promoter region of the MGMT gene may allow epigenetic silencing, resulting in low levels of MGMT in tumor cells. Temozolomide is more effective at causing cell death when tumors cells exhibit lower levels of MGMT [7]. Low levels of MGMT protein are associated with increased therapeutic effect of TMZ and improvement in OS in GBM patients [8,9]. Similarly, Martinez et al. [10] found that the methylation rate of MGMT in long-term surviving GBM patients was higher than the level measured in other GBM patients. To reduce MGMT levels and improve temozolomide activity, dose-dense (DD) schedules of temozolomide were designed. However, Gilbert et al. [8] found no improvement in median OS or median PFS efficacy for DD temozolomide for newly diagnosed GBM, regardless of the methylation status of MGMT. Given the low effectiveness of current therapies and poor treatment outcomes, new therapies are needed [11].
As a highly angiogenic tumor, there are large amounts of vascular endothelial growth factor (VEGF) present in GBM cells. The normal function of VEGF is to create new blood vessels, but an excessive level of VEGF may promote angiogenesis, increase vascular permeability, and stimulate tumor progression [4]. For this reason, VEGF has been considered as a potential therapeutic target of GBM [2]. In preclinical testing, bevacizumab (BV), a humanized recombinant monoclonal antibody against VEGF-A which interferes with the interaction of VEGF with its receptors [12], was found to inhibit angiogenesis and tumor growth and to enhance the anti-tumor activities of chemotherapy and radiotherapy (RT) [11]. In the clinical stage of testing, treatment using bevacizumab combined with irinotecan showed substantial anti-tumor effects and presented lower toxicity for recurrent GBM patients [13]. The follow-up phase II study confirmed clinical activity and safety [14,15]. There have been preliminary studies on the efficacy of bevacizumab in the treatment of newly diagnosed GBM [1,4,5,13,16–25]. In most of these studies, bevacizumab treatment was used as an auxiliary treatment combined with temozolomide and radiotherapy [4,5,16–18,22,25,26]. In several clinical trials, researchers evaluated the influence of MGMT methylation status on the treatment effect of bevacizumab [4,17,21], but the results are controversial.
The aim of our meta-analysis was to assess the effect of bevacizumab plus temozolomide-radiotherapy treatment for newly diagnosed glioblastoma with different MGMT methylation status based on data from clinic trails to determine if the inclusion of bevacizumab improves survival.
Material and Methods
Literature search strategy
We performed a comprehensive literature search to identify clinical trials that compared the outcomes of GBM patients with different MGMT methylation status that were treated with bevacizumab, temozolomide, and radiotherapy (BV/TMZ/RT) or with a combination of temozolomide and radiotherapy (TMZ/BV). The search was conducted using PubMed, Embase, and The Cochrane Library databases from inception through March 23, 2016. The following keywords and MeSH terms were used: ((O-6-methylguanine-DNA methyltransferase) OR (MGMT)) AND ((bevacizumab OR Avastin)) AND ((((radiotherapy OR radiotherapies OR (radiotherapy, targeted) OR (radiotherapies, targeted) OR (targeted radiotherapies) OR (targeted radiotherapy))) AND (temozolomide OR (8-carbamoyl-3-methylimidazo (5,1-d) -1,2,3,5-tetrazin-4 (3H) one) OR methazolastone OR (M and B 39831) OR (M and B-39831) OR temodar OR temodal OR (tmz-bioshuttle) OR (ccrg 81045) OR (CCRG-81045) OR (NSC 362856) OR (NSC-362856))) AND (glioma OR astrocytoma OR glioblastoma OR GBM OR oligodendroglioma OR ependymoma))). There were no language restrictions. Duplicate publications were considered only once. Any review articles, clearly irrelevant researches, or editorials were excluded. The remaining articles were carefully reviewed to determine whether they included useful information to the topic. Additionally, the reference sections were scanned manually for other relevant studies and review articles. Three reviewers independently performed the article search.
Study selection
The goal of this study was to investigate the treatment effect of bevacizumab combined with radiotherapy-temozolomide for newly diagnosed GBM with different MGMT methylation status. All prospective clinical trials that compared the outcomes of bevacizumab, temozolomide, and radiotherapy (BV/TMZ/RT, BV group) with those of temozolomide and radiotherapy (TMZ/RT, control group) for GBM patients with different MGMT methylation status were qualified for this meta-analysis. The selection criteria were: (1) clinical trials studies; (2) patients with previously untreated, histologically proven GBM with adequate organ and marrow function; (3) the status of MGMT methylation was determined; (4) patients were assigned to receive either TMZ/RT (control group) or BV/TMZ/RT (BV group); and (5) the studies reported information on OS and PFS. Studies were excluded if 1 of the following criteria existed: (1) no control group; (2) animal studies, pediatric studies, phase I studies, case reports, reviews, editorials/commentaries, meeting abstracts/summaries, letter to the editor or technical reports; (3) duplication of previous publications; or (4) OS and PFS data unavailable. All disagreements were resolved by consensus after discussion.
Data extraction
The 3 co-authors obtained the data from the selected studies. For each study, the following information was collected: the first author’s name, country, year of publication, number of treatment arms, study treatment groups, intervention details (including bevacizumab dose (mg/kg), TMZ dose (mg/kg), radiation therapy and total dose (Gy)), and outcomes (including median follow-up period, median PFS, median OS, hazard ratios (HRs), and the corresponding 95% confidence intervals (CIs)). Furthermore, through cautious review by the authors of the full text, controversy on inconsistent data from the eligible articles was settled by discussion.
Quality assessment
The quality assessment criteria of the Newcastle-Ottawa Scale (NOS) was used independently by 3 authors to evaluate the quality of the included studies [27]. Based on 3 major NOS components, a quality score was calculated and the maximum score was 9 points: (1) group selection; (2) comparability, and (3) assessment of outcome or exposure.
Statistical analysis
The first outcome was OS, defined as the time from random assignment to a treatment group until death from any cause. PFS was the secondary outcome, defined as the time until either disease progression or death. The HRs of OS and PFS were used to assess the benefit of combined BV therapy in GBM patient populations with different MGMT methylation status. Two methods were used to measure the between-study heterogeneity: Cochran’s Q test and I2 statistic [28]. For Cochran’s Q test, a corresponding p-value below 0.05 was considered to show significant heterogeneity. For the I2 statistic, I2 >50% indicated significant heterogeneity. When heterogeneity was significant, pooled HRs were calculated using a random-effects model (I2 >50%) or a fixed-effects model (I2 ≤50%). The funnel plots and Egger’s tests were performed to statistically assess potential publication bias [29].
All statistical analyses were performed using STATA version 12 (Stata Corp LP, College Station, TX). All reported p-values were 2-sided and p<0.05 was considered to be significant.
Results
A total of 188 articles relevant to the searched keywords were initially identified; 5 of these articles were recognized as potentially relevant studies based on the titles and abstracts. Full texts were reviewed for a more detailed evaluation. Two papers were excluded because they were single-arm clinic trials that lacked a control group [22,30]. After evaluation, 3 studies met all the criteria for inclusion and none of the criteria for exclusion and were used for this meta-analysis [4,17,21]. The manual searching of reference lists produced no other publications eligible for inclusion. Figure 1 shows the study selection procedure.
Figure 1.
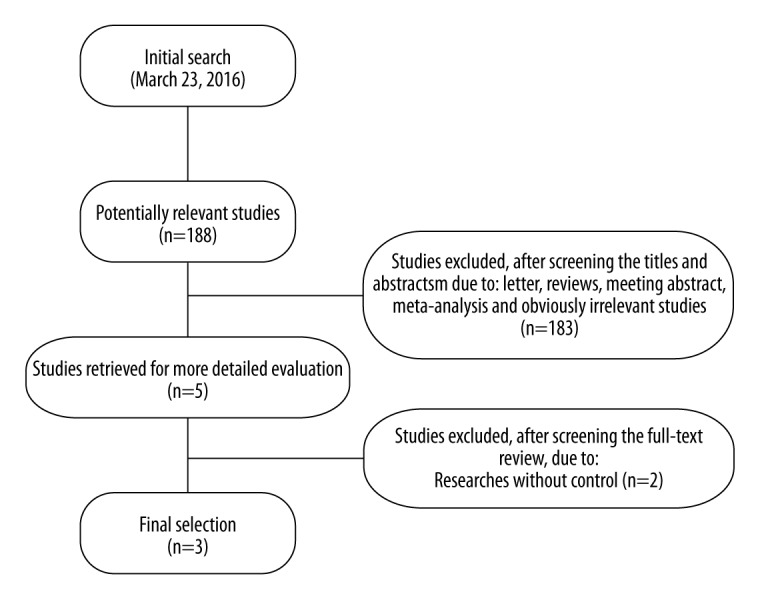
Flow chart of literature search and study selection.
A total of 1443 subjects were involved in this meta-analysis, of which 717 GBM patients received BV/TMZ/RT therapy and 726 GBM patients received TMZ/RT therapy. In the BV group, the numbers of MGMT methylated and unmethylated patients were 248 and 469, respectively. In the control group, the numbers of MGMT methylated and unmethylated patients were 248 and 478, respectively. The HRs of OS and its 95% CIs were extracted from the study [21], or calculated using other statistical information in the trials [4,17] by 3 independent authors using the method of Tierney et al. [31] and Hamling et al. [32]. Table 1 shows the general characteristics of the included studies. The NOS [27] quality assessment of the 3 selected studies is summarized in Table 2. All 3 studies were assessed and received an NOS quality score of 8 (Table 2).
Table 1.
Characteristics of included trials.
| Author | Year | Country | Number | Mean age (years) | MGMT methylation status | Characteristics of treatment in each arm | Median OS (months) | Median PFS (months) | Median follow up | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | C | B | C | B | C | B | C | B | C | |||||
| Lai A | 2011 | USA | 41 | 43 | 57.4 | 59.4 | Methylated | BV+T–75+RT; BV+T–150 * | T–75+RT; T–150* | 24.7 | 26.7 | 17.5 | – | 24.2 |
| Gilbert MR | 2014 | USA | 90 | 85 | – | – | Methylated | BV+T–75+RT; BV+T–150 * | T–75+RT +P; T–150 +P * | 23.2 | – | 14.1 | – | 20.5 |
| Chinot OL | 2014 | French | 117 | 120 | 57 | 56 | Methylated | BV+T–75+RT; BV+T–150* | T–75+RT +P; T–150 +P * | – | – | – | – | 16.3 |
| Lai A | 2011 | USA | 29 | 28 | 57.4 | 59.4 | Unmethylated | BV+T–75+RT; BV+T–150 * | T–75+RT; T–150* | 15.9 | 18.2 | 10.5 | – | 24.2 |
| Gilbert MR | 2014 | USA | 215 | 214 | – | – | Unmethylated | BV+T–75+RT; BV+T–150 * | T–75+RT +P; T–150 +P * | 14.3 | – | 8.2 | – | 20.5 |
| Chinot OL | 2014 | French | 225 | 236 | 57 | 56 | Unmethylated | BV+T–75+RT; BV+T–150* | T–75+RT +P; T–150 +P * | – | – | – | – | 16.3 |
B – bevacizumab group; C – control; BV – bevacizumab 10 mg/kg biweekly; T-75 – temozolomide at 75 mg/m2 dailyl T-150 – temozolomide at 150 to 200 mg/m2/d daily first 5 days of every 28-day cycle; RT – totaling 60.0 Gy; P – placebo: * Post-RT phase.
Table 2.
Quality assessment criteria by the Newcastle-Ottawa Scale.
| Author | Year | Selection | Comparability | Outcome or exposure | Scores | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 1 | 2 | 3 | |||
| Lai A | 2011 | * | * | * | * | * | * | * | * | 8 |
| Gilbert MR | 2014 | * | * | * | * | * | * | * | * | 8 |
| Chinot OL | 2014 | * | * | * | * | * | * | * | * | 8 |
Survival analyses
For MGMT methylated and unmethylated patients, the addition of BV to TMZ-radiotherapy did not improve overall survival based on the pooled HRs of OS, which were 1.132 (95% CIs 0.876–1.462; P=0.345) and 1.018(95% CIs 0.879–1.179; P=0.345), respectively (Figure 2). The heterogeneity was not statistically significant as measured by χ2 or I2 testing (χ2=2.52, df=2, P=0.284; I2=20.5%; χ2=3.44, df=2, P=0.179; I2=41.8%). Of these 3 trials, 2 trials provided adequate data for the statistical analysis of PFS [4,21]. Both MGMT methylated and unmethylated patients obtained absolute benefit in terms of PFS in the BV group (pooled HRs, 0.769, 95% CIs 0.604–0.978, P=0.032; 0.675, 95%CIs 0.466–0.979, P=0.038; Figure 3). Because the heterogeneity was statistically significant in the MGMT unmethylated group (χ2=6.58, df=1, P=0.01; I2=84.8%), the pooled HRs were calculated using a random-effects model.
Figure 2.
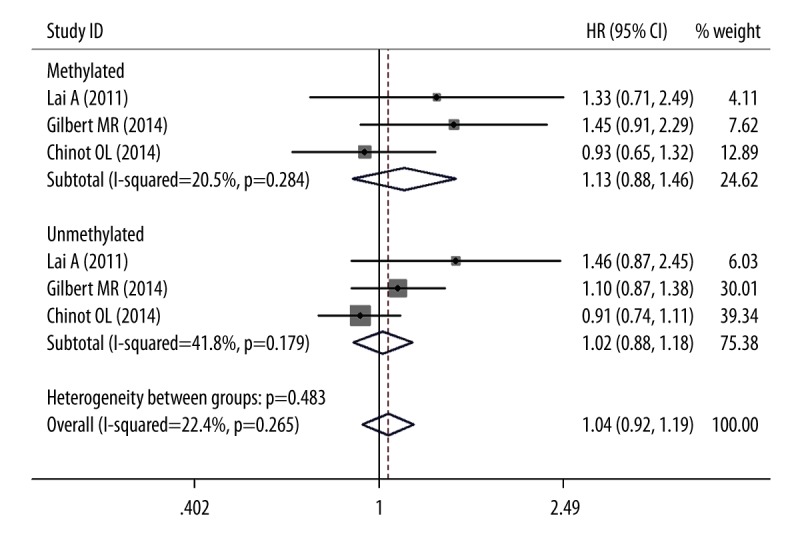
Forest plots for OS outcomes comparing BV/TMZ/RT with TMZ/RT.
Figure 3.
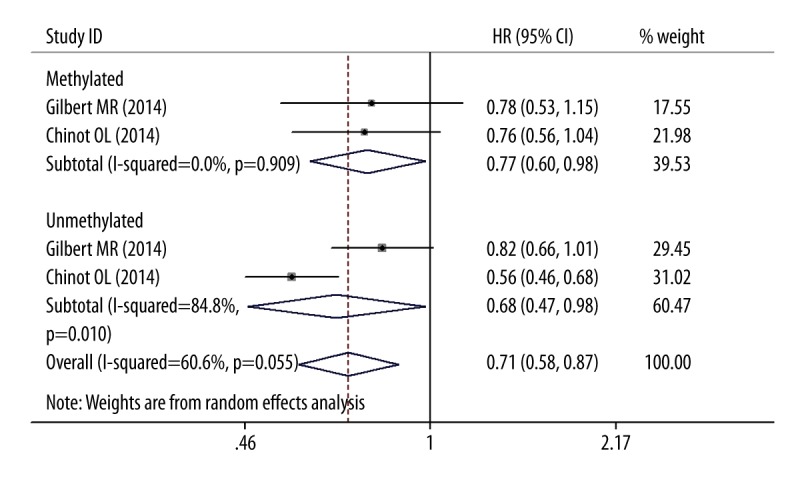
Forest plots for PFS outcomes comparing BV/TMZ/RT with TMZ/RT.
Assessment of publication bias
The publication bias of the included studies was evaluated by funnel plot analyses and Egger’s linear regression tests. The symmetry of the funnel plot and Egger’s test indicated that there was no publication bias (Egger’s test: t=2.34, P=0.08) (Figure 4).
Figure 4.
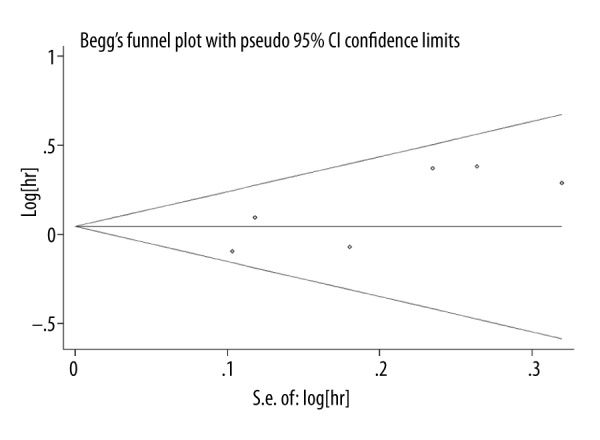
Funnel plot for publication bias.
Discussion
The current meta-analysis assessed the benefit of using bevacizumab combined with RT and TMZ in newly diagnosed GBM patients with methylated and unmethylated MGMT. The benefit of adjuvant BV in patients with newly diagnosed GBM and different MGMT methylation status was not clearly documented in initial studies [4,17,21]. Although bevacizumab showed substantial anti-tumor effect and lower toxicity for recurrent GBM patients [13–15] and the US Food and Drug Administration approved the use of bevacizumab for patients with recurrent GBM [4], our meta-analysis did not find evidence for synergistic effects of combining BV with TMZ and RT on improving survival in glioblastoma with different MGMT methylation status.
Because of the disparate molecular composition of tumor cells, glioblastoma is a heterogeneous disease with different prognoses [33]. Based on specific molecular alterations, specific treatment strategies can be designed which should improve treatment success rates [34]. In clinical practice, researchers found that MGMT promoter methylation status strongly influences the therapeutic effect of chemoradiotherapy for GBM [34]. In the EORTC-NCIC trial, patients with a methylated MGMT promoter showed improved PFS and OS after treatment with temozolomide and radiotherapy [9]. Patients with an unmethylated MGMT promoter showed no improvement in median survival [3]. To reduce the resistance of TMZ due to a lack of MGMT promoter methylation, different dosing regimens of TMZ were introduced into the treatment of glioblastoma. Weiler et al. [35] performed a 7 days on/7 days off regimen in newly diagnosed glioblastoma patients and reported a more favorable outcome in patients with methylated MGMT promoters. In contrast, Gilbert et al. [8] did not find significant improvement in OS or PFS using a DD temozolomide regimen (days 1 through 21 of a 28-day cycle) in newly diagnosed GBM, irrespective of MGM methylation. Given the limited improvement of OS in GBM and because VEGF plays a key in glioma biology, especially for GBM [1], bevacizumab is a reasonable drug to use for treatment of patients with newly diagnosed GBM. Bevacizumab is currently used for recurrent GBM treatment in over 60 countries [13–15,33]. However, in 2 large clinical studies (AVAglio and RTOG 0825) [4,21], the OS of patients with GBM was not improved by treatment with BV combined TMZ and radiotherapy. Because there is no benefit of adjuvant BV in GBM patients, and given the poor survival rate, alternative treatment strategies are needed. Raizer et al. performed a phase II study to investigate the effect of combining erlotinib and bevacizumab in MGMT unmethylated GBM patients [25]. Although the combination treatment is tolerable, it did not increase survival. In our meta-analysis, BV combined with TMZ and radiotherapy also did not increase survival in MGMT methylated and unmethylated GBM patients.
In preclinical models, bevacizumab magnified the anti-tumor effects of chemotherapy and radiotherapy [11]. Our analysis found that PFS was prolonged in GBM patients with different MGMT methylation status compared to the control groups, but there was no effect on OS. This may be due to the following reasons. First of all, as a growing tumor, prevention of new blood vessel formation by BV may cause augmentative hypoxia and block nutrient absorption. However, increased hypoxia may stimulate tumor progression in a variety of ways, like advancing angiogenesis, infestation of tumor cell, and resistance to apoptosis [36]. Secondly, preclinical studies established that use of antiangiogenic agents could lead to normalization of abnormal tumor vessels, which may mitigate the resistance of tumor cells to radiation therapy and chemotherapy [37]. The use of BV may relieve the vasogenic edema and improve PFS based on the vessel normalization, but may not prolong the time of vessel normalization sufficiently to provide a more sustained benefit. Growth of the tumor was observed in nearly half of the patients with GBM from the time of surgery until the time of chemoradiation [38]. This suggests that shortening the interval between surgery and subsequent therapy may be essential [39]. However, all clinical trials included in our meta-analysis used bevacizumab in combination with standard temozolomide and radiotherapy, and alternate treatment may provide different results.
There are several limitations of our current meta-analysis. First, the statistical power was limited because the analysis was based on summary data rather than utilization of the individual patient data. Second, this analysis was limited to the articles that were identified and indexed by the selected databases, so some relevant studies may have been missed. Third, the methylation status of MGMT was not used as a stratification factor for randomization of the 3 reports used for this analysis. This may have biased our conclusions. Finally, relevant confounding factors that are recognized to affect the OS and PFS at the patient level (e.g., age, performance status, and operation type [40]) were not assessed in this meta-analysis.
Conclusions
Bevacizumab combined with temozolomide and radiotherapy improved PFS for treatment of patients with different MGMT methylation status of newly diagnosed GBM. However, no improvement of overall survival was observed for patients with methylated or unmethylated MGMT. There is insufficient evidence to determine the synergistic effects of combining BV with TMZ and RT on improving survival in patients with different MGMT methylation status. Finally, further large-scale, randomized, controlled trials are needed to assess the effects of bevacizumab combined with temozolomide and radiotherapy or other targeted glioblastoma therapies in patients with different MGMT methylation status.
Footnotes
Source of support: Medical Science and Technology Development Project of Shandong Province, China (grant no. 2015WS0395) and Natural Science Foundation of Shandong Province, China (grant no. ZR2015HL018)
Conflict of interest statement
The authors declare that they have no competing interests.
References
- 1.Vredenburgh JJ, Desjardins A, Kirkpatrick JP, et al. Addition of bevacizumab to standard radiation therapy and daily temozolomide is associated with minimal toxicity in newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2012;82(1):58–66. doi: 10.1016/j.ijrobp.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 2.Narayana A, Gruber D, Kunnakkat S, et al. A clinical trial of bevacizumab, temozolomide, and radiation for newly diagnosed glioblastoma. J Neurosurg. 2012;116:341–45. doi: 10.3171/2011.9.JNS11656. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. New Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai A, Filka E, McGibbon B, et al. Phase II pilot study of bevacizumab in combination with temozolomide and regional radiation therapy for up-front treatment of patients with newly diagnosed glioblastoma multiforme: interim analysis of safety and tolerability. Int J Radiat Oncol Biol Phys. 2008;71:1372–80. doi: 10.1016/j.ijrobp.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 6.Wick W, Platten M, Weller M. New (alternative) temozolomide regimens for the treatment of glioma. Neuro Oncol. 2009;11:69–79. doi: 10.1215/15228517-2008-078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baur M, Preusser M, Piribauer M, et al. Frequent MGMT (0(6)-methylguanine-DNA methyltransferase) hypermethylation in long-term survivors of glioblastoma: A single institution experience. Radiol Oncol. 2010;44:113–20. doi: 10.2478/v10019-010-0023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: A randomized phase III clinical trial. J Clin Oncol. 2013;31:4085–91. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. New Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 10.Martinez R, Schackert G, Yaya-Tur R, et al. Frequent hypermethylation of the DNA repair gene MGMT in long-term survivors of glioblastoma multiforme. J Neurooncol. 2007;83:91–93. doi: 10.1007/s11060-006-9292-0. [DOI] [PubMed] [Google Scholar]
- 11.Field KM, Jordan JT, Wen PY, et al. Bevacizumab and glioblastoma: Scientific review, newly reported updates, and ongoing controversies. Cancer. 2015;121:997–1007. doi: 10.1002/cncr.28935. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Gulotta B, Thomas A, et al. Large-volume low lesion predicts poor survival in bevacizumab-treated glioblastoma patients. Neuro Oncol. 2016;18(5):735–43. doi: 10.1093/neuonc/nov268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vredenburgh JJ, Desjardins A, Herndon JE, II, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–29. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 14.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–45. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 16.Chinot OL, de La Motte Rouge T, Moore N, et al. AVAglio: Phase 3 trial of bevacizumab plus temozolomide and radiotherapy in newly diagnosed glioblastoma multiforme. Adv Ther. 2011;28:334–40. doi: 10.1007/s12325-011-0007-3. [DOI] [PubMed] [Google Scholar]
- 17.Lai A, Tran A, Nghiemphu PL, et al. Phase II study of bevacizumab plus temozolomide during and after radiation therapy for patients with newly diagnosed glioblastoma multiforme. J Clin Oncol. 2011;29:142–48. doi: 10.1200/JCO.2010.30.2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vredenburgh JJ, Desjardins A, Reardon DA, et al. The addition of bevacizumab to standard radiation therapy and temozolomide followed by bevacizumab, temozolomide, and irinotecan for newly diagnosed glioblastoma. Clin Cancer Res. 2011;17:4119–24. doi: 10.1158/1078-0432.CCR-11-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagane M, Nishikawa R, Narita Y, et al. Phase II study of single-agent bevacizumab in Japanese patients with recurrent malignant glioma. Jpn J Clin Oncol. 2012;42:887–95. doi: 10.1093/jjco/hys121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lou E, Peters KB, Sumrall AL, et al. Phase II trial of upfront bevacizumab and temozolomide for unresectable or multifocal glioblastoma. Cancer Med. 2013;2:185–95. doi: 10.1002/cam4.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–22. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 22.Omuro A, Beal K, Gutin P, et al. Phase II study of bevacizumab, temozolomide, and hypofractionated stereotactic radiotherapy for newly diagnosed glioblastoma. Clin Cancer Res. 2014;20:5023–31. doi: 10.1158/1078-0432.CCR-14-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carlson JA, Reddy K, Gaspar LE, et al. Hypofractionated-intensity modulated radiotherapy (hypo-IMRT) and temozolomide (TMZ) with or without bevacizumab (BEV) for newly diagnosed glioblastoma multiforme (GBM): A comparison of two prospective phase II trials. J Neurooncol. 2015;123:251–57. doi: 10.1007/s11060-015-1791-4. [DOI] [PubMed] [Google Scholar]
- 24.Ney DE, Carlson JA, Damek DM, et al. Phase II trial of hypofractionated intensity-modulated radiation therapy combined with temozolomide and bevacizumab for patients with newly diagnosed glioblastoma. J Neurooncol. 2015;122:135–43. doi: 10.1007/s11060-014-1691-z. [DOI] [PubMed] [Google Scholar]
- 25.Raizer JJ, Giglio P, Hu J, et al. A phase II study of bevacizumab and erlotinib after radiation and temozolomide in MGMT unmethylated GBM patients. J Neurooncol. 2016;126(1):185–92. doi: 10.1007/s11060-015-1958-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Linde ME, Verhoeff JJ, Richel DJ, et al. Bevacizumab in combination with radiotherapy and temozolomide for patients with newly diagnosed glioblastoma multiforme. Oncologist. 2015;20:107–8. doi: 10.1634/theoncologist.2014-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niyazi M, Flieger M, Ganswindt U, et al. Validation of the prognostic Heidelberg re-irradiation score in an independent mono-institutional patient cohort. Radiat Oncol. 2014;9:128. doi: 10.1186/1748-717X-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27:954–70. doi: 10.1002/sim.3013. [DOI] [PubMed] [Google Scholar]
- 33.Sandmann T, Bourgon R, Garcia J, et al. Patients with proneural glioblastoma may derive overall survival benefit from the addition of bevacizumab to first-line radiotherapy and temozolomide: Retrospective analysis of the AVAglio trial. J Clin Oncol. 2015;33:2735–44. doi: 10.1200/JCO.2015.61.5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weller M, Wick W, Hegi ME, et al. Should biomarkers be used to design personalized medicine for the treatment of glioblastoma? Fut Oncol. 2010;6:1407–14. doi: 10.2217/fon.10.113. [DOI] [PubMed] [Google Scholar]
- 35.Weiler M, Hartmann C, Wiewrodt D, et al. Chemoradiotherapy of newly diagnosed glioblastoma with intensified temozolomide. Int J Radiat Oncol Biol Phys. 2010;77:670–76. doi: 10.1016/j.ijrobp.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 36.Lu-Emerson C, Duda DG, Emblem KE, et al. Lessons from anti-vascular endothelial growth factor and anti-vascular endothelial growth factor receptor trials in patients with glioblastoma. J Clin Oncol. 2015;33:1197–213. doi: 10.1200/JCO.2014.55.9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carmeliet P, Jain RK. Principles and mechanisms of vessel normalization for cancer and other angiogenic diseases. Nat Rev Drug Discov. 2011;10:417–27. doi: 10.1038/nrd3455. [DOI] [PubMed] [Google Scholar]
- 38.Pirzkall A, McGue C, Saraswathy S, et al. Tumor regrowth between surgery and initiation of adjuvant therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2009;11:842–52. doi: 10.1215/15228517-2009-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun MZ, Oh T, Ivan ME, et al. Survival impact of time to initiation of chemoradiotherapy after resection of newly diagnosed glioblastoma. J Neurosurg. 2015;122:1144–50. doi: 10.3171/2014.9.JNS14193. [DOI] [PubMed] [Google Scholar]
- 40.Field KM, Drummond KJ, Yilmaz M, et al. Clinical trial participation and outcome for patients with glioblastoma: Multivariate analysis from a comprehensive dataset. J Clin Neurosci. 2013;20:783–89. doi: 10.1016/j.jocn.2012.09.013. [DOI] [PubMed] [Google Scholar]


