Abstract
Herpes simplex virus 1 (HSV-1) is a nearly ubiquitous human pathogen, remaining dormant in its human host the majority of the time. The interaction between HSV-1 and the immune system represents a complicated balance of power that allows the virus to persist in the host for a lifetime. However, disruptions in the immune system can activate the virus with the potential to cause devastating infections in the central nervous system (CNS). We present a patient who suffered three consecutive yearly HSV-1 CNS episodes (encephalitis, seizure, and retinitis), each within days of his influenza vaccination. We highlight subtle immunologic defects in this patient that may have allowed unchecked viral replication and resultant disease manifestations, as well as the potential role of influenza vaccine in tipping this balance in favor of HSV-1.
Keywords: Herpes simplex, Acute retinal necrosis, Encephalitis, Influenza, Vaccination
Introduction
Between 52% and 84% of the human adult population is latently infected by HSV-1 [1]. The large double stranded DNA virus maintains lifelong infection by establishing a predominantly latent infection within the trigeminal ganglia that is punctuated by recurrent episodes of productive replication, or reactivation, that results in clinical disease and dissemination of virus either within the host or to new hosts. Rare manifestations of HSV-1 reactivation include Herpesvirus encephalitis (HSE) and acute retinal necrosis (ARN).
We present a case of HSV-1 HSE followed by post-HSE encephalitis or seizure and HSV-1 ARN in which HSV infection or reactivation was temporally associated with administration of influenza vaccination in three consecutive years.
Case presentation
A 47-year-old male with insulin-dependent diabetes mellitus, hypertension and dyslipidemia presented to an outside hospital in November of 2012 with acute fever, aphasia and dysnomia as well as a several day history of weakness, malaise and hiccups. He was tachycardic and laboratory evaluation revealed a leukocytosis (12,400/mm3) without bandemia as well as hyponatremia (129 meq/L), hypochloremia (93 meq/L) and hyperglycemia (270 mg/dL). He was admitted to the ICU for sepsis and dehydration. His lumbar puncture revealed clear fluid with elevated WBC (7), RBC (271) and glucose (188), normal protein and no organisms.
An MRI of his brain revealed edema of the left temporal lobe and pre-insular cortices without enhancement. His EEG demonstrated bilateral temporal lobe slowing without epileptiform changes, prompting treatment with intravenous acyclovir for presumed HSV encephalitis. His CSF PCR was positive for HSV-1 DNA at 100 copies/ml and negative for HSV-2.
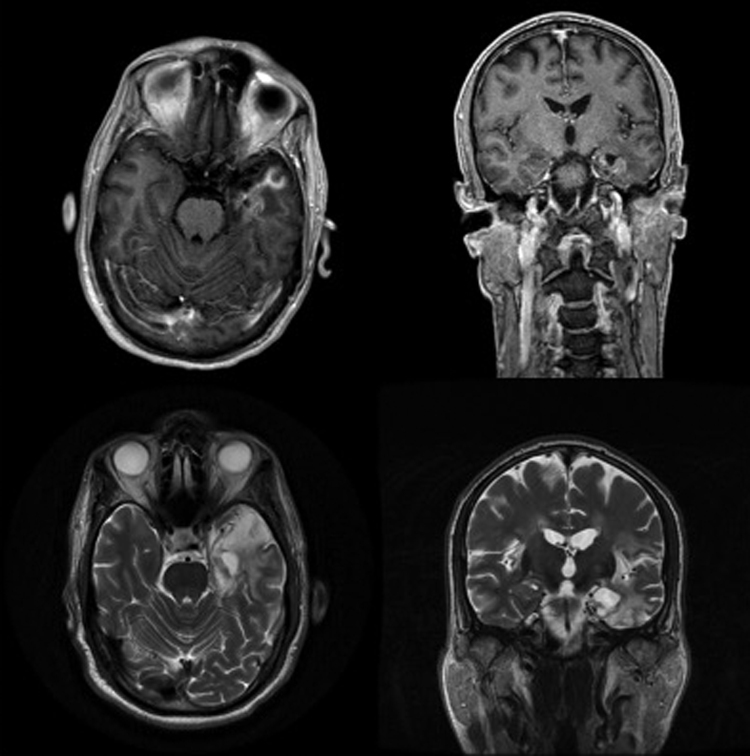
An MRI, repeated two weeks later, showed enhancement of the left temporal lobe and bilateral insular cortices (Fig. 1 top left and top right). His anti-herpetic therapy was transitioned to foscarnet 6 g IV BID and his condition improved. He was discharged to a rehabilitation facility after one month and experienced a good recovery.
Fig. 1.
Herpes simplex encephalitis. MRI brain at one month (top left, top right) and one year (bottom left, bottom right) following the initial presentation. T1 with contrast axial (top left) and coronal (top right) images taken one month after the initial presentation showing enhancement in the left temporal lobe and subinsular cortex as well as signal abnormality in the right and left insular cortices. T2 without contrast axial (bottom left) and coronal (bottom right) images taken at one year when the patient presented with transient recrudescence of his symptoms.
His medical history was notable for cold sores as a child as well as an influenza vaccination ten days prior to this presentation (Fluvarix, 2012–2013 formula, GlaxoSmithKline, London, England). He tested negative for influenza A or B. His absolute lymphocyte counts were consistently low throughout his hospitalization, between 400 and 800/mm3 (normal range 1300–3600/mm3).
The patient presented eleven months later with aphasia and dysnomia three days after receiving an influenza vaccination (Agriflu, 2013–2014 formula, Novartis, Basel, Switzerland). He was afebrile with a normal total leukocyte count, however his lymphocyte count was again low at 1100/mm3. His symptoms resolved within hours without anti-viral treatment and a non-contrast MRI demonstrated only left temporal lobe encephalomalacia (Fig. 1 bottom left and bottom right), which was felt to be sequelae of his prior HSE. EEG demonstrated bilateral temporal slowing without epileptiform discharges. This episode was diagnosed as a temporal lobe seizure related to structural compromise and he was started on prophylactic levetiracetam (Keppra; LEV) at 500 mg BID.
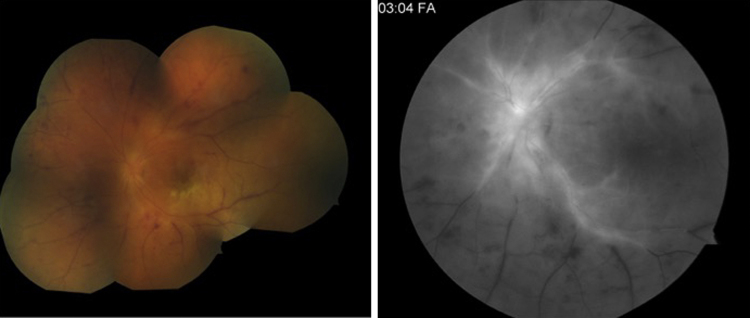
One year later, he developed eye pain, tearing and loss of vision in the left eye one day after receiving influenza vaccination (Fluzone, 2014–2015 formula, Sanofi Pasteur, Paris, France). On presentation two days later, his vision was limited to hand motion and he had a left afferent pupillary defect, conjunctival hyperemia, optic nerve and retinal edema and scattered retinal hemorrhages. He was referred to our retina service the following day, at which time his vision had deteriorated to light perception only. His fundus exam revealed mild vitritis, retinal whitening, vascular sheathing and peripheral choroidal effusions (Fig. 2 left). Fluorescein angiography demonstrated occlusive vasculitis (Fig. 2 right).
Fig. 2.
Acute retinal necrosis. Fundus photograph (left) showing retinal whitening, vascular occlusion, numerous retinal hemorrhages and disc edema. Flourescein angiogram (right) showing leaking and staining of the central retinal arterioles and venules and severe peripheral nonperfusion consistent with occlusive vasculitis.
An aqueous fluid sample was sent for PCR and the patient was empirically treated for viral retinitis (acute retinal necrosis) with intravitreal foscarnet (2.4 mg/0.1 ml), subtenon's triamcinolone (40 mg) and oral valacyclovir (1000 mg BID). The aqueous fluid PCR was positive for HSV-1 (150,000 copies/mL) and negative for HSV-2, VZV and CMV. Four days later, his visual acuity was still light perception although improvement was noted in his retinitis and vitritis. He was additionally treated with a 5-day course of oral prednisone at 60 mg daily.
Over the ensuing weeks severe retinal atrophy progressed with the development of several atrophic holes and the retina became totally detached. The patient underwent a pars plana vitrectomy, air-fluid exchange, endolaser and silicone oil fill. His vision improved to hand motion in the temporal field at one year, however his retina remains ischemic with a chronic inferotemporal detachment still under silicone oil tamponade. His exam also shows pallor of the optic nerve and severe vascular attenuation. He is being maintained on valacyclovir (500 mg daily) for life and has been advised to avoid future influenza vaccination.
Laboratory studies at this time again revealed decreased total lymphocytes 1200/mm3 as well as CD4T cells at 461/mm3 (normal 496–2186/mm3), despite overall normal total leukocyte counts. His immunglobulin subset levels were normal and demonstrated good reactivity to influenza, tetanus and Varicella zoster.
Discussion
Primary HSV infection
Upon initial oral mucocutaneous exposure, HSV-1 undergoes robust replication resulting in production of millions of progeny virions, which enter the distal axon terminals of peripheral neurons. The viral particles follow retrograde axonal transport to the nucleus where latent infection is established, usually in the trigeminal ganglion.
In the trigeminal nuclei, the viral genome circularizes and becomes coated with host cell protein complexes that inhibit viral gene transcription. Cellular stress, including apoptosis, hypoxia or loss of growth factor support, results in de-repression of viral transcription. This leads to reactivation marked by a highly ordered cascade of viral gene transcription culminating in viral DNA replication, followed by assembly of progeny virions.
The newly synthesized virions undergo anterograde axonal transport to the overlying skin and mucous membranes where they are released to infect neighboring cells or other hosts. Indeed, nearly 100% of infected, but asymptomatic, subjects intermittently shed HSV-1 DNA from either saliva or tears [2].
Host defense and the maintenance of viral latency
Under normal conditions, viral replication and morbidity to the host are limited by both pathogen and host factors, allowing the virus to persist for the host’s lifetime. Numerous host defense mechanisms are poised to limit HSV infection. Viral DNA, RNA and proteins are detected by the host via pathogen recognition receptors, such as the toll-like receptors (TLRs). Activation of these receptors results in release of pro-inflammatory cytokines, particularly type II interferons and IL-1β, which inhibit viral replication, promote destruction of infected cells and recruit phagocytes to clear infected cells.
Resident microglia and infiltrating CD8+ T cells provide additional defense in the form of direct cellular toxicity and anti-viral cytokines. Recurrent subclinical viral replication incites a local inflammatory response that triggers immune surveillance of infected trigeminal ganglia. This phenomenon, in conjunction with viral regulatory elements, is critical for repressing clinically apparent infection. Thus, clinical disease represents a tip in this balance in favor of viral proliferation.
CNS dissemination
Just as anterograde transport facilitates spread of virions from the trigeminal ganglia to the skin and mucous membranes, it also allows dissemination into the central nervous system (CNS), most commonly to the frontal and temporal cortices. Post-mortem histology and PCR performed on patients without clinically apparent infection demonstrates HSV-1 in the trigeminal ganglia of 60% of autopsy cases [3], a frequency that corroborates the prevalence estimates of 50–80% [1]. Both latent and replicative HSV-1 have been detected in the central nervous system of 30–55% of clinically asymptomatic autopsy cases [4], suggesting an alarmingly high rate of viral spread from the periphery to the CNS. Indeed, HSV-1 transcripts were detected in the CNS of all patients with HSV-1 positive trigeminal ganglia in a seven patient autopsy case series [5].
Despite the frequency of asymptomatic CNS infection, herpes encephalitis (HSE) is quite rare with prevalence estimates of 1/250-500,000. It is a potentially devastating infection characterized by necrotizing inflammation, predominantly in the temporal lobe. Even with antiviral therapy, the mortality rate is between 14% and 19%, and the rate of neurologic morbidity is 40–60% [6]. Notably, the viral load correlates with disease severity [7].
Post-HSE encephalitis occurs weeks to months after initial presentation in up to 25% of surviving patients and is characterized by progression or recrudescence of the original deficits. In contrast to the initial viral encephalitis, this syndrome is primarily immune-mediated, characterized by enhancement of previous brain lesions on MRI and CSF inflammation without detectable virus. Accordingly, post-HSE encephalitis responds to immunomodulatory therapy but not to extension of antiviral therapy [8]. This inflammatory response may be the result of persistent undetectable or abortive viral replication or a chronic immune response triggered by the initial infection.
Acute retinal necrosis (ARN) is a rare and potentially devastating infection by HSV-1, HSV-2 or VZV characterized by rapidly progressive retinal necrosis, optic neuritis, retinal vasculitis and vitritis. The retinal necrosis is so severe that detachment ensues in 35–80% of patients [9]. It is caused by VZV about twice as frequently as HSV-1 in adults, and more commonly by HSV-2 in patients under 25 [9].
Brain-ocular transmission
There are numerous case reports of ARN following HSE [10]. In one series of 52 patients with ARN, 13% of ARN were preceded by HSE. Of those, five were caused by HSV-1 and two by VZV, opposite to the etiologic trend for ARN without HSE, though the significance of this is unclear since the total number of cases was small [9]. However, in a separate publication, the same authors detected concordance between HSV strains in CSF during HSE and ocular fluid during ARN in two patients [11]. Taken together, these data suggest that viral encephalitis may predispose patients to ARN and that HSV-1 may pose more of a risk for post-HSE ARN than VZV.
HSV may gain entry to the ocular environment via the nasociliary branch of the trigeminal nerve, which supplies the anterior segment of the eye, and probably transports the virions that cause keratitis or iritis. Alternatively, HSV may traverse the optic nerve after meningeal spread from the trigmeninal ganglion. Optic disc edema is, in fact, a common feature of ARN and there have been multiple cases of ARN that were preceded by optic neuritis [12]. Notably, intracerebral inoculation of HSV-1 results in retinitis in murine models [13].
Immune mechanisms for viral suppression
The ability of the host cell to sense and respond to HSV is highly complex with receptors poised to detect each intermediate (viral DNA, RNA and protein), and safeguarded with intrinsic regulatory components that limit the extent of inflammation and prevent tissue destruction and autoimmunity. One family of pathogen-sensing receptors is the toll-like receptors (TLRs). TLR3 recognizes double stranded RNA, TLR7 and TLR8 detect single stranded RNA and TLR9 binds viral DNA. Many of the signal cascades induced by TLR ligation converge to activate either interferon regulatory factor 3 (IRF3) or IRF7, both strong inducers of type I interferons (IFN). Polymorphisms in the TLR3-IFN pathway have been implicated in pediatric and adult herpetic encephalitis and may be quite common among patients with HSE [14]. Notably, these defects do not render the patients susceptible to mucocutaneous HSV-1 infections, or to other viral infections, but specifically to herpetic encephalitis.
While most TLRs are expressed in the CNS, there may be differences in the relative expression levels that render TLR3, in particular, more critical in the CNS. Teleologically, less redundancy in this system may serve to prevent immune-mediated CNS tissue destruction. TLR3 signals may also reduce immunopathology by providing anti-viral specificity to the immune response. Alterations in TLR3 signaling, therefore, may tip the immunologic balance from controlled viral occupancy toward rampant infection with uncontrolled CNS inflammation.
Additionally, specific immune modulating therapies may render patients susceptible to HSE or meningitis in the absence of mucocutaneous infection. The implicated medications include the TNFα inhibitors [15], as well as drugs that reduce immunologic surveillance by preventing leukocyte migration either out of the lymph nodes, in the case of the sphingosine-1-phosphate receptor antagonist Fingolimod [16], or into tissues, in the case of the α4 integrin antagonist Natalizumab [17]. These reports are rare among patients receiving these drugs, suggesting that patient-specific factors are also important, yet they highlight critical components of the CNS-specific immune response to herpesvirus infections and suggest, again, that there is a reduced immunologic redundancy in the CNS compared to the periphery.
Influenza vaccination as a trigger for HSV reactivation
There are very rare reports of neurologic complications, including encephalitis, following influenza vaccination, totaling 13 out of 38 million doses over 10 years in one Japanese study [18] and, not surprisingly, the underlying pathology is unknown. Proposed immunologic mechanisms include molecular mimicry, whereby immunity to a vaccine peptide triggers a demyelinating immune response against a similar host protein; and autoinflammation, for which vaccination triggers robust inflammation that results in generalized tissue damage within the CNS. In support of the latter theory, a recent murine study showed that intranasal infection with a non-neurotropic influenza virus resulted in reduced levels of neurotrophin in the brain and activation of brain microglial cells [19]. The reduction in neurotrophic factors could trigger robust HSV replication, overwhelming anti-viral immune surveillance. Alternatively, the local immune response at the site of vaccination could distract normal immunologic surveillance, such that the number of T cells normally present near infected neurons was effectively decreased, resulting in loss of immunologic control.
In our patient, there was a reproducible temporal relationship between sequential influenza vaccinations and HSV reactivation. He first presented with encephalitis just days after vaccination. The following year, he presented with similar, albeit milder symptoms. While HSV was not detected in his CSF at the second presentation, it may have represented transient viral reactivation. His final vaccination preceded HSV infection at a second neurologic site, the retina.
Given the widespread safe and efficacious use of influenza vaccinations even in persons with significant immune compromise, there were likely multiple host and viral factors that contributed to our patient’s disease. Our patient did not have a recent history of orolabial herpetic infection, nor did he suffer from frequent colds or other viral illnesses to suggest a generalized immunologic deficiency. In fact, his immune studies demonstrated normal humoral immunity. He does, however, appear to have 25–75% fewer circulating CD4T cells than normal during these episodes. This could translate to a relative paucity in immune surveillance within the CNS. Indeed, impaired cellular immunity has been previously reported in a subset of ARN patients [20]. A subtle immunologic deficiency in our patient may have lowered the threshold for clinically significant HSV reactivation in the CNS. Subsequently, vaccination may have drawn the T cells normally involved in CNS immune surveillance toward the vaccine response, allowing viral reactivation to proceed unchecked. Additionally, vaccine-induced inflammatory cytokines may have triggered viral reactivation directly, or indirectly via neurotoxicity or loss of neurotrophic support.
The current influenza vaccines are extraordinarily safe and efficacious, even in patients with severe immune deficiencies and pre-existing CNS disease, with the reported neurologic sequelae existing at the level of case reports. This case divulges, not the perils of influenza vaccination, but the complexity of the HSV-1-host interface, and begs for greater understanding, and therapeutic manipulation, of the forces that govern it.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Funding
This work was supported by an unrestricted grant from the Research to Prevent Blindness Foundation.
References
- 1.Pebody R.G., Andrews N., Brown D., Gopal R., De Melker H., Francois G. The seroepidemiology of herpes simplex virus type 1 and 2 in Europe. Sex Transm Infect. 2004;80(3):185–191. doi: 10.1136/sti.2003.005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufman H.E., Azcuy A.M., Varnell E.D., Sloop G.D., Thompson H.W., Hill J.M. HSV-1 DNA in tears and saliva of normal adults. Invest Ophthalmol Vis Sci. 2005;46(1):241–247. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Motani H., Sakurada K., Ikegaya H., Akutsu T., Hayakawa M., Sato Y. Detection of herpes simplex virus type 1 DNA in bilateral human trigeminal ganglia and optic nerves by polymerase chain reaction. J Med Virol. 2006;78(12):1584–1587. doi: 10.1002/jmv.20742. [DOI] [PubMed] [Google Scholar]
- 4.Fraser N.W., Lawrence W.C., Wroblewska Z., Gilden D.H., Koprowski H. Herpes simplex type 1 DNA in human brain tissue. Proc Natl Acad Sci U S A. 1981;78(10):6461–6465. doi: 10.1073/pnas.78.10.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steiner I., Mador N., Reibstein I., Spivack J.G., Fraser N.W. Herpes simplex virus type 1 gene expression and reactivation of latent infection in the central nervous system. Neuropathol Appl Neurobiol. 1994;20(3):253–260. doi: 10.1111/j.1365-2990.1994.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 6.Raschilas F., Wolff M., Delatour F., Chaffaut C., De Broucker T., Chevret S. Outcome of and prognostic factors for herpes simplex encephalitis in adult patients: results of a multicenter study. Clin Infect Dis. 2002;35(3):254–260. doi: 10.1086/341405. [DOI] [PubMed] [Google Scholar]
- 7.Whitley R.J. Herpes simplex encephalitis: adolescents and adults. Antiviral Res. 2006;71(2–3):141–148. doi: 10.1016/j.antiviral.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Gnann J.W., Skoldenberg B., Hart J: Gnann J.W. (Clin Infect Dis 2015; 61 683–691). herpes simplex encephalitis: lack of clinical benefit of long-term valacyclovir therapy. Clin Infect Dis. 2016;62(4):530. doi: 10.1093/cid/civ1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vandercam T., Hintzen R.Q., de Boer J.H., Van der Lelij A. Herpetic encephalitis is a risk factor for acute retinal necrosis. Neurology. 2008;71(16):1268–1274. doi: 10.1212/01.wnl.0000327615.99124.99. [DOI] [PubMed] [Google Scholar]
- 10.Ganatra J.B., Chandler D., Santos C., Kuppermann B., Margolis T.P. Viral causes of the acute retinal necrosis syndrome. Am J Ophthalmol. 2000;129(2):166–172. doi: 10.1016/s0002-9394(99)00316-5. [DOI] [PubMed] [Google Scholar]
- 11.Maertzdorf J., Van der Lelij A., Baarsma G.S., Osterhaus A.D., Verjans G.M. Herpes simplex virus type 1 (HSV-1)–induced retinitis following herpes simplex encephalitis: indications for brain-to-eye transmission of HSV-1. Ann Neurol. 2000;48(6):936–939. [PubMed] [Google Scholar]
- 12.Bert R.J., Samawareerwa R., Melhem E.R. CNS MR and CT findings associated with a clinical presentation of herpetic acute retinal necrosis and herpetic retrobulbar optic neuritis: five HIV-infected and one non-infected patients. AJNR Am J Neuroradiol. 2004;25(10):1722–1729. [PMC free article] [PubMed] [Google Scholar]
- 13.Norgren R.B., Jr., Lehman M.N. Retrograde transneuronal transport of herpes simplex virus in the retina after injection in the superior colliculus, hypothalamus and optic chiasm. Brain Res. 1989;479(2):374–378. doi: 10.1016/0006-8993(89)91644-2. [DOI] [PubMed] [Google Scholar]
- 14.Mork N., Kofod-Olsen E., Sorensen K.B., Bach E., Orntoft T.F., Ostergaard L. Mutations in the TLR3 signaling pathway and beyond in adult patients with herpes simplex encephalitis. Genes Immun. 2015;16(8):552–566. doi: 10.1038/gene.2015.46. [DOI] [PubMed] [Google Scholar]
- 15.Bradford R.D., Pettit A.C., Wright P.W., Mulligan M.J., Moreland L.W., McLain D.A. Herpes simplex encephalitis during treatment with tumor necrosis factor-alpha inhibitors. Clin Infect Dis. 2009;49(6):924–927. doi: 10.1086/605498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfender N., Jelcic I., Linnebank M., Schwarz U., Martin R. Reactivation of herpesvirus under fingolimod: a case of severe herpes simplex encephalitis. Neurology. 2015;84(23):2377–2378. doi: 10.1212/WNL.0000000000001659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fine A.J., Sorbello A., Kortepeter C., Scarazzini L. Central nervous system herpes simplex and varicella zoster virus infections in natalizumab-treated patients. Clin Infect Dis. 2013;57(6):849–852. doi: 10.1093/cid/cit376. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama T., Onoda K. Vaccine adverse events reported in post-marketing study of the Kitasato Institute from 1994 to 2004. Vaccine. 2007;25(3):570–576. doi: 10.1016/j.vaccine.2006.05.130. [DOI] [PubMed] [Google Scholar]
- 19.Sadasivan S., Zanin M., O'Brien K., Schultz-Cherry S., Smeyne R.J. Induction of microglia activation after infection with the non-neurotropic A/CA/04/2009 H1N1 influenza virus. PLoS One. 2015;10(4):e0124047. doi: 10.1371/journal.pone.0124047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rochat C., Polla B.S., Herbort C.P. Immunological profiles in patients with acute retinal necrosis. Graefes Arch Clin Exp Ophthalmol. 1996;234(9):547–552. doi: 10.1007/BF00448798. [DOI] [PubMed] [Google Scholar]