Abstract
Background
Gamma knife radiosurgery is an effective and safe treatment modality in the management of pituitary adenomas. Internal carotid occlusion is a rare but possible complication of Gamma Knife Radiosurgery for lesions within the cavernous sinus.
Aim
To stress the importance of considering the Internal carotid artery as an organ at risk in cavernous sinus invading adenomas and reduce the dose delivered to this structure whenever possible.
Case description
We report two cases of asymptomatic occlusion of the intracavernous segment of the internal carotid artery seven years after treatment in acromegalic patients. After trans-sphenoidal surgery, residual tumour was treated with gamma knife radiosurgery. The maximal doses to the affected artery were higher than 40 Gy and the 90% isodose was close to the arterial wall.
Conclusion
Every effort should be done to minimize the radiation dose to the internal carotid artery. If not possible, “hot spots” exceeding the 90% isodose close to this vessel should be avoided.
Keywords: Pituitary adenoma, Gamma knife radiosurgery, Internal carotid artery occlusion, Growth hormone
1. Aim
To stress the importance of considering the Internal Carotid Artery as an organ at risk in cavernous sinus invading adenomas and reduce the dose higher than 90% isodose along the wall of this artery.
2. Introduction
Pituitary adenomas are benign tumours which usually grow causing compression of adjacent anatomical structures. However, some pituitary adenomas may infiltrate adjacent tissues. Six to ten percent of pituitary adenomas involve the cavernous sinus1, 2 and are considered to be invasive.3, 4 Dural wall invasion usually implies partial surgical removal of the tumour.5 Adjuvant treatment (i.e. conventional radiation therapy, radiosurgery or medication) is often necessary in these patients. Stereotactic radiosurgery and Radiotherapy are effective and safe radiation modalities to realize real salvage treatment for recurrent skull base tumours such as pituitary adenomas.6
In recent years, Gamma Knife Radiosurgery (GKRS) has emerged as an important treatment modality in the management of residual or recurrence of pituitary adenomas. Advances in computer guidance and imaging of the pituitary disease and surrounding normal structures have led to substantial improvements in GKRS; however, many unanswered questions regarding dose and complications still remain.
The major potential risks associated with the GKRS for pituitary adenomas are delayed optic neuropathy and new endocrine deficiencies. Vascular structures and cranial nerves within the cavernous sinus appear to be less susceptible to adverse radiation effects.7 Nowadays, only two cases of symptomatic internal carotid artery (ICA) occlusion were reported in patients following GKRS for a pituitary adenoma.8, 9 Other two cases of asymptomatic ICA stenosis after GKS for hormone-producing pituitary adenoma have been reported in the literature.10 We report two cases of asymptomatic ICA occlusion 7 years after GKS for a pituitary adenoma growth-hormone secreting with particular regards to the dose delivered to the ICA.
3. Materials and methods
3.1. Case report no. 1
A 30-year-old female patient presented at our institution with the diagnosis of acromegaly unresponsive to medical treatments. A contrast-enhanced magnetic resonance imaging (MRI) scan revealed the presence of a sellar lesion with an extension to the right cavernous sinus. In 2003, the patient underwent a trans-sphenoidal surgery with subtotal removal. In February 2004, the patient was treated with GKRS for persistent acromegaly activity of the residual intracavernous tumour. The treatment was performed with Gamma Plan 5.32 (Elekta instruments, Stockolm, Sweden). We prescribed 22 Gy at the 50% isodose. Treatment was performed with Leksell Gamma Knife C (Elekta instruments). The patient received postoperative pegvisomant treatment and was followed clinically and by MRI. GKRS achieved complete tumour growth control as confirmed by following MRI controls. Five years after GKRS, remission of acromegaly was achieved and medical treatments were interrupted. Seven years after GKRS, angio-MRI revealed occlusion of the cavernous segment of the right ICA (Fig. 1). The patient was asymptomatic and remained cured and asymptomatic 11 years after GKRS, no further treatments were started and the patient was annually followed with angio-MRI.
Fig. 1.
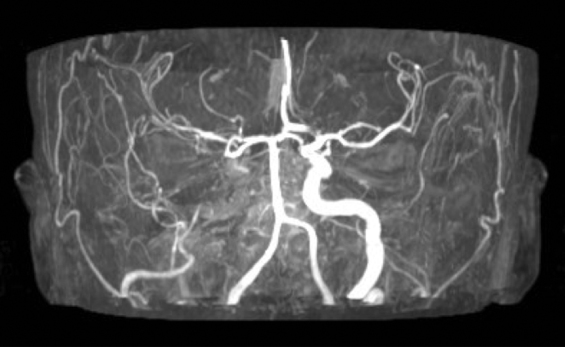
Angio-MRI showing complete right internal carotid artery occlusion in case 1.
3.2. Case report no. 2
A 35-year-old female patient presented at our institution with the endocrinological diagnosis of acromegaly unresponsive to medical treatments. A contrast-enhanced magnetic resonance imaging (MRI) scan revealed the presence of a sellar-suprasellar tumour with an extension to the left cavernous sinus. The patient underwent a trans-sphenoidal surgery with subtotal removal in 2006 and 2008. The pathological finding confirmed a mixed-cell adenoma, secreting growth hormone and prolactin. In April 2008 the patient was treated with GKRS for persistent acromegaly activity of the residual intracavernous lesion. We prescribed 23 Gy at the 50% isodose. Treatment was performed with Leksell Gamma Knife Perfexion (Elekta instruments). The patient received postoperative somatostatin analogues treatment. GKRS achieved complete tumour growth control, as confirmed by following MRI controls. In 2010 pegvisomant was started and a biochemical remission of acromegaly was achieved (normalization of serum IGF-1 concentrations). Seven years after GKRS, angio-MRI showed occlusion of the cavernous segment of the left ICA (Fig. 2). The patient was asymptomatic and was annually followed with angio-MRI.
Fig. 2.
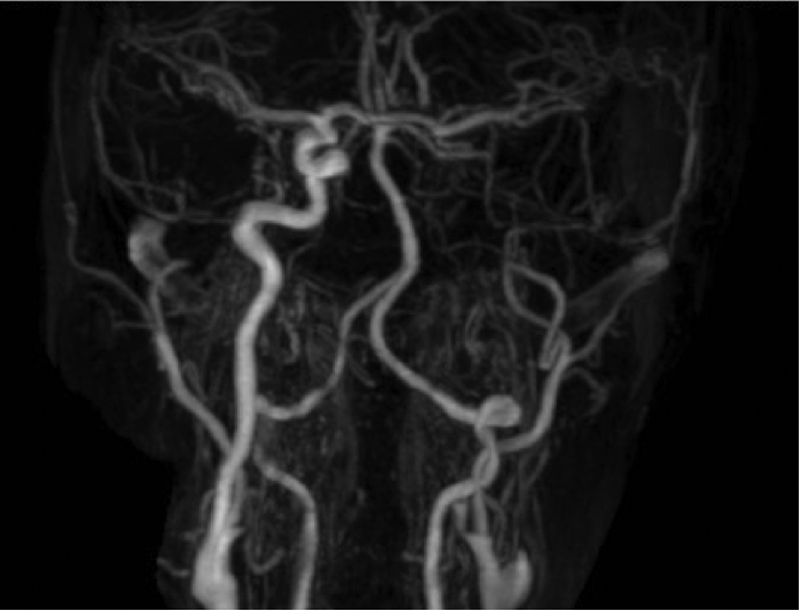
Angio-MRI showing complete left internal carotid artery occlusion in case 2.
For arterious venous malformation the rate of vessel obliteration depends on the marginal dose administered to the nidus. In order to analyze the dosimetry delivered to the ICA, we retrospectively created a volume of the intracavernous portion of the ICA. In both cases the ICA received a maximal dose higher than 35 Gy and the 90% isodose was close to the arterial wall (Fig. 3). In case no. 1 the maximal dose received by the ICA was 44.3 Gy (1 mm3) while the median dose delivered to the artery was 26.4 Gy (delivered to 164.4 mm3, i.e. 67% of the ICA volume). 50% of the vessel received 34.5 Gy while 3% of the ICA (6.3 mm3) received the 90% isodose. In case no. 2 the maximal dose received by the ICA was 44.7 Gy (1 mm3) while the median dose delivered to the artery was 29 Gy (delivered to 206.2 mm3, i.e. 62% of the ICA volume). 50% of the vessel received 30 Gy while 9% of the ICA (29.6 mm3) received the 90% isodose. The total energy (integral dose) absorbed by the ICA was 6.5 mJ and 9.7 mJ in case no. 1 and no. 2, respectively. These parameters show that in both cases the wall of the ICA received a very high dose. All dosimetric parameters are reported in Table 1.
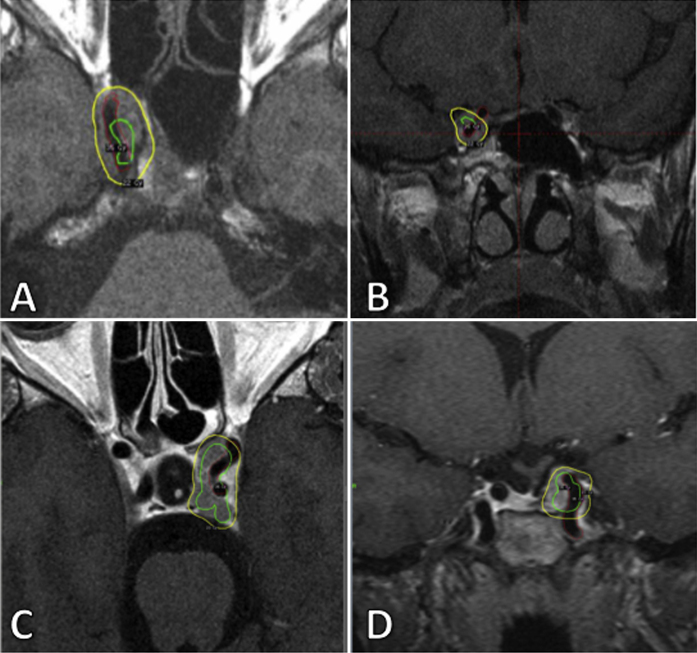
Fig. 3.
Axial (A, C) and coronal (B, D) images showing the ICA (in red) the prescription isodose (yellow line) and the isodose of 35 Gy. Figures A, B refer to case 1 and figures C and D refer to case 2.
Table 1.
Dosimetric parameters to the ICA in cases 1 and 2.
| Case report no.1 | |
| Marginal dose to the tumour | 22 Gy at 50% isodose |
| Internal carotid artery volume | 247.1 mm3 |
| Minimum dose | 1.3 Gy to 247 mm3 |
| Maximum dose | 44.3 Gy to 1 mm3 |
| Mean dose | 26.4 ± 10.7 Gy to 164.4 mm3 |
| Integral dose | 6.5 mJ |
| D50% | 30 Gy |
| V50% | 73% (180.1 mm3) |
| V90% | 3% (6.3 mm3) |
| V12 to the skull | 6.93 cm3 |
| Case report no. 2 | |
| Marginal dose to the tumour | 23 Gy at 50% isodose |
| Internal carotid artery volume | 334.4 mm3 |
| Minimum dose | 2.8 Gy to 334 mm3 |
| Maximum Dose | 44.7 Gy to 1 mm3 |
| Mean dose | 29.0 Gy ± 11.4 Gy to 206.2 mm3 |
| Integral dose | 9.7 mJ |
| D50% | 34.5 Gy |
| V50% | 74% (246.3 mm3) |
| V90% | 9% (29.6 mm3) |
| V12 to the skull | 7.20 cm3 |
4. Discussion
Stereotactic radiosurgery safely provides a high tumour control rate for patients with recurrent or residual pituitary adenomas. The incidence of treatment-induced endocrinopathy, visual dysfunction, and/or vascular complications appears to be low.
To our knowledge, only two cases of symptomatic internal carotid artery occlusion were reported in patients following GKRS for pituitary adenomas. Lim et al. reported the first case.8 A patient treated with GKRS for a residual pituitary adenoma suffered a cerebral infarction with internal carotid artery occlusion 4 years after radiosurgery. The patient had no history or signs of heart disease or metabolic disorder which could predispose to cerebrovascular events. So it is likely that cranial irradiation led to accelerated atherosclerotic changes. The irradiated dose of the cavernous segment of the carotid artery was below 20 Gy. The patient presented with hemiparesis and facial palsy that recovered completely with conservative management. Hidemichi Ito et al. reported another case.9 A patient treated with GKRS for a recurrent pituitary adenoma suffered severe stenosis of cavernous internal carotid artery 5 years after radiosurgery. The patient had no history of heart disease or metabolic disorder that could predispose to cerebrovascular complications. The irradiated dose of the cavernous segment of the carotid artery was 20–22 Gy. The patient presented with amaurosis. In this case, percutaneous transluminal angioplasty was performed successfully with anatomical and functional improvement.
Other two cases of asymptomatic ICA stenosis after GKRS for pituitary adenoma have been reported in the literature by Bruce E. Pollock et al.10 He reported the results of stereotactic radiosurgery in 43 patients with hormone-producing pituitary adenomas, operated between 1990 and 1999. In two patients, asymptomatic stenosis of the ICA developed. The radiation dose directed to the tumour margin and the maximum radiation dose were 25 and 50 Gy, respectively, for one patient and 30 and 60 Gy for the other; one patient also had received previous fractionated radiation therapy (45 Gy).
There have been occasional reports of stenosis or occlusion of major cerebral arteries occurring several years after stereotactic radiosurgery for other diseases,11, 12 such as arteriovenous malformations and meningiomas. Nevertheless, very little information is available on the actual irradiation dose to which the affected artery itself had been exposed. Barami et al.13 reported a risk of ICA occlusion or stenosis when the margin dose was 13–18 Gy and the calculated radiation dose to the arteries was 25–36 Gy. Hin et al. recommended restricting the dose to the cavernous portion of the carotid artery to less than 30 Gy with the notion that this might help avoid post-radiosurgery stenosis14 even if reaching this goal is not so easy in patient with cavernous sinus invading lesions. As suggested by Abeloos L. et al.15 dose heterogeneity inside the target volume can produce hot spots of dose (i.e. 90% isodose) inside the internal carotid artery that can lead to a vascular occlusion, especially in arteries surrounded by the tumour that could affect their wall. In our case we found the same dose heterogeneity as shown in Fig. 3. Therefore, the authors recommend shifting the hot spot during the dosimetry planning in order to reduce the incidence of such vascular injury. We personally think that high dose delivered to the artery can cause their occlusion inducing an endothelial destruction followed by spindle-shaped cell proliferation in the subendothelial region and in the connective tissue stroma as described in arterio-venous malformations,16 especially if the artery is totally surrounded by the tumour and the connective tissue stroma may be invaded. Due to the rarity of the phenomenon, it is difficult to explain why carotid occlusion sometimes happens in a symptomatic rather than an asymptomatic way. Our case reports suggest that patients who undergo GKRS for tumours involving cavernous sinus should be monitored for arterial stenosis in the long-term follow-up. We recommend to perform MRI control annually and to add an angio-MRI sequence if changes indicate carotid restriction or occlusion.
5. Conclusions
These two cases represent the only documented ICA occlusion cases in a population of more than 400 pituitary adenomas treated with GKRS at our institution. The good tumour growth control achieved after GKRS and the natural history of cavernous sinus invading pituitary adenomas may suggest that these cases of ICA occlusions have to be considered as irradiation injury secondary to GKRS. At the time of planning, every effort should be made to minimize the radiation dose to the ICA avoiding hot spots exceeding the 90% isodose close to this vessel.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Ahmadi J., North C.M., Segall H.D., Zee C.S., Weiss M.H. Cavernous sinus invasion by pituitary adenomas. Am J Neuroradiol. 1985 doi: 10.2214/ajr.146.2.257. [DOI] [PubMed] [Google Scholar]
- 2.Falbusch R., Buchfelder M. Transsphenoidal surgery of parasellar pituitary adenomas. Acta Neurochir. 1988 doi: 10.1007/BF01401978. [DOI] [PubMed] [Google Scholar]
- 3.Martins A.N., Hayes G.J., Kempe L.G. Invasive pituitary adenomas. J Neurosurg. 1965 doi: 10.3171/jns.1965.22.3.0268. [DOI] [PubMed] [Google Scholar]
- 4.Lundberg P.O., Drettner B., Hemmingsson A., Stenkvist B., Wide L. The invasive pituitary adenoma. Arch Neurol. 1977 doi: 10.1001/archneur.1977.00500240030005. [DOI] [PubMed] [Google Scholar]
- 5.Sekhar L.N., Burgess J., Akin O. Anatomical study of the cavernous sinus emphasizing operative approaches and related vascular and neural reconstruction. Neurosurgery. 1987 doi: 10.1227/00006123-198712000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Krengli M., Apicella G., Deantonio L., Paolini M., Masini L. Stereotactic radiation therapy for skull base recurrences: is a salvage approach still possible? Rep Pract Oncol Radiother. 2015;20(6):430–439. doi: 10.1016/j.rpor.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson I.M.D., Noren G. Gamma knife radiosurgery for pituitary tumours. Clin Endocrinol Metab. 1999 doi: 10.1053/beem.1999.0033. [DOI] [PubMed] [Google Scholar]
- 8.Lim Y.J., Leem W., Park J.T., Kim T.S., Rhee B.A., Kim G.K. Cerebral infarction with ICA occlusion after gamma knife radiosurgery for pituitary adenoma: a case report. Stereotact Funct Neurosurg. 1999 doi: 10.1159/000056449. [DOI] [PubMed] [Google Scholar]
- 9.Ito H., Onodera H., Sase T. Percutaneous transluminal angioplasty in a patient with internal carotid artery stenosis following gamma knife radiosurgery for recurrent pituitary adenoma. Surg Neurol Int. 2015 doi: 10.4103/2152-7806.157795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollock B.E., Nippoldt T.B., Stafford S.L., Foote R.L., Abboud C.F. Results of stereotactic radiosurgery in patients with hormone-producing pituitary adenomas: factors associated with endocrine normalization. J Neurosurg. 2002 doi: 10.3171/jns.2002.97.3.0525. [DOI] [PubMed] [Google Scholar]
- 11.Lunsford L.D., Kondziolka D., Flickinger J.C. Stereotactic radiosurgery for arteriovenous malformations of the brain. J Neurosurg. 1991 doi: 10.3171/jns.1991.75.4.0512. [DOI] [PubMed] [Google Scholar]
- 12.Marks M.P., Delapaz R.L., Fabrikant J.I. Intracranial vascular malformations: imaging of charged-particle radiosurgery – Part II: Complications. Radiology. 1988 doi: 10.1148/radiology.168.2.3293113. [DOI] [PubMed] [Google Scholar]
- 13.Barami K., Grow A., Brem S., Dagnew E., Sloan A.E. Vascular complications after radiosurgery for menigiomas. Neurosurg Focus. 2007 doi: 10.3171/foc.2007.22.3.10. [DOI] [PubMed] [Google Scholar]
- 14.Shin M., Kurita H., Sasaki T., Tago M., Morita A., Ueki K. Stereotactic radiosurgery for pituitary adenoma invading the cavernous sinus. J Neurosurg. 2000 doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 15.Abeloos L., Levivier M., Devriendt D., Massager N. Internal carotid occlusion following gamma knife radiosurgery for cavernous sinus meningioma. Stereotact Funct Neurosurg. 2007 doi: 10.1159/000107365. [DOI] [PubMed] [Google Scholar]
- 16.Szeifert G.T., Levivier M., Lorenzoni J., Nyáry I., Major O., Kemeny A.A. Morphological observations in brain arteriovenous malformations after gamma knife radiosurgery. Prog Neurol Surg. 2013;27:119–129. doi: 10.1159/000341772. [DOI] [PubMed] [Google Scholar]