Abstract
Introduction. Patients with head and neck cancer suffer from various impairments due to the primary illness, as well as secondary consequences of the oncological treatment. This systematic review describes the effects of radiotherapy and/or chemotherapy on the functions of the upper aerodigestive tract in patients with head and neck cancer. Methods. A systematic literature search was performed by two independent reviewers using the electronic databases PubMed and Embase. All dates up to May 2016 were included. Results. Of the 947 abstracts, sixty articles met the inclusion criteria and described one or more aspects of the sequelae of radiotherapy and/or chemotherapy. Forty studies described swallowing-related problems, 24 described voice-related problems, seven described trismus, and 25 studies described general quality of life. Only 14 articles reported that speech pathologists conducted the interventions, of which only six articles described in detail what the interventions involved. Conclusion. In general, voice quality improved following intervention, whereas quality of life, dysphagia, and oral intake deteriorated during and after treatment. However, as a consequence of the diversity in treatment protocols and patient characteristics, the conclusions of most studies cannot be easily generalised. Further research on the effects of oncological interventions on the upper aerodigestive tract is needed.
1. Introduction
Head and neck oncological patients suffer from various functional, physical, and emotional impairments due to both the primary illness and the secondary consequences of the tumor treatment [1]. The oncological treatment of head and neck tumors depends on the location and the stage of the tumor, as well as the treatment preferences of the individual patient. Head and neck oncological treatment can include surgery, radiotherapy, chemotherapy, or combinations of these. The impact of head and neck oncological treatments on the anatomical structures, organ function, and the quality of life (QoL) should not be underestimated [2]. For instance, the implications of loss of function for people treated nonsurgically for head and neck cancer (HNC) and its detrimental effects on functioning and QoL are well documented [3].
In order to assist people with dysphagia to adjust to and live successfully with the sequelae of the primary condition, speech pathologists managing this caseload need to ensure posttreatment services are available [4] that address not only the physical but also the emotional and psychosocial needs. A qualitative study by Nund et al. [5] exploring dysphagia management by speech pathologists suggests that care givers generally feel ill-prepared for their role. Furthermore, this study suggests that clinicians should provide adequate and timely training and support to carers. Furthermore, Krisciunas et al. [6] concluded that within speech pathology there is no standardised therapy for HNC patients and scant evidence to support any particular protocol. As a result, institutions and individual speech pathologists need to develop their own protocols based on “standard” practices or anecdotal evidence.
Evidence-based practice (EBP) is hailed to be paramount in the practice of speech pathology [7]. The American Speech-Language-Hearing Association (ASHA) defines evidence-based practice as “…an approach in which current, high-quality research evidence is integrated with practitioner expertise and client preferences and values, into the process of making clinical decisions” [8]. Essentially, EBP involves moving the foundation for clinical decisions from clinical protocols centered solely on expert opinion to the integration of clinical expertise, the best current research evidence, and individual client values. To facilitate EBP in healthcare, clinical practice guidelines can be developed to summarise clinically relevant evidence [9].
Several reviews have been published about the outcomes after radiotherapy and/or chemotherapy in HNC patients (e.g., Frowen and Perry [10]; Jacobi et al. [11]; van der Molen et al. [12]; Paleri et al. [13]; Roe et al. [14]). Most of the reviews focused on selected functional domains in populations with HNC: health-related QoL [15], swallowing [13, 14, 16], and voice and speech [11]. Only the review by van der Molen et al. [12] covered a wider range of functional outcomes in patients with advanced HNC, including swallowing, mouth opening, nutrition, pain, and QoL. Further, the purpose of some studies was to provide evidence-based clinical guidelines (e.g., Paleri et al. [13]) and they did not perform systematic literature searches in line with the PRISMA guidelines [17]. As such, even though a number of reviews have been published over the last ten years, a comprehensive updated systematic review is needed that includes all functional domains affected by radiotherapy and/or chemotherapy in patients with head and neck carcinoma.
A systematic review was conducted to describe the effects of radiotherapy and/or chemotherapy on functions of the upper aerodigestive tract in patients with HNC and examined the evidence of interventions by speech pathologists.
2. Methods
A systematic literature search was performed by two independent reviewers. The electronic biomedical databases PubMed and Embase were used (search period from start of database until 5 May 2016). The searches were limited to English language publications. In PubMed the MeSH terms larynx or hypopharynx were combined with all MeSH terms related to head and neck neoplasms (Table 1). Next, the results were linked to all MeSH terms for chemotherapy or radiotherapy, after which the outcome was combined with all MeSH terms found for dysfunctions of the upper aerodigestive tract and limited with adults +19 years. The exact syntax of the literature search is presented in Table 1.
Table 1.
Search strategies per literature database.
| Database and search terms | Limits | Number of records | |
|---|---|---|---|
| Subject headings |
Embase: (larynx/OR pharynx/OR hypopharynx/) AND (neoplasm/OR larynx disorder/OR pharynx disorder/OR larynx cancer/OR larynx carcinoma/OR pharynx cancer/OR pharynx carcinoma/) AND (radiotherapy/OR chemotherapy/OR chemoradiotherapy/OR adjuvant therapy/OR drug therapy/) AND (speech sound disorder OR speech/or speech disorder/OR swallowing/OR dysphagia/OR dysphonia/OR voice disorder/OR aphonia/OR speech intelligibility/OR xerostomia/OR dysarthria/OR esophagus speech/OR larynx prosthesis/OR trismus/OR “quality of life”/) |
English | 201 |
|
| |||
| Subject headings | PubMed: (“Larynx”[Mesh] OR “Pharynx”[Mesh] OR “Hypopharynx”[Mesh]) AND (“Neoplasms”[Mesh] OR “Head and Neck Neoplasms”[Mesh] OR “Neoplasms, Second Primary”[Mesh] OR “Pharyngeal Neoplasms”[Mesh] OR “Oropharyngeal Neoplasms”[Mesh] OR “Tonsillar Neoplasms”[Mesh] OR “Nasopharyngeal Neoplasms”[Mesh] OR “Mouth Neoplasms”[Mesh] OR “Laryngeal Neoplasms”[Mesh] OR “Tongue Neoplasms”[Mesh] OR “Thyroid Neoplasms”[Mesh] OR “Salivary Gland Neoplasms”[Mesh] OR “Jaw Neoplasms”[Mesh] OR “Lip Neoplasms”[Mesh] OR “Thyroid Carcinoma, Anaplastic”[Mesh] OR “Neoplasms, Squamous Cell”[Mesh] OR “Neoplasms, Basal Cell”[Mesh] OR “Otorhinolaryngologic Neoplasms”[Mesh] OR “Hypopharyngeal Neoplasms”[Mesh] OR “Laryngeal Diseases”[Mesh] OR “Pharyngeal Diseases”[Mesh]) AND (“Radiotherapy”[Mesh] OR “Radiotherapy, Adjuvant”[Mesh] OR “Radiotherapy, High-Energy”[Mesh] OR “Radiotherapy, Image-Guided”[Mesh] OR “Radiotherapy, Intensity-Modulated”[Mesh] OR “Radiotherapy, Conformal”[Mesh] OR “Radiotherapy, Computer-Assisted”[Mesh] OR “Radiotherapy Planning, Computer-Assisted”[Mesh] OR “Radiotherapy Dosage”[Mesh] OR “Brachytherapy”[Mesh] OR “Radiosurgery”[Mesh] OR “Radiation Oncology”[Mesh] OR “Dose-Response Relationship, Radiation”[Mesh] OR “Consolidation Chemotherapy”[Mesh] OR “Induction Chemotherapy”[Mesh] OR “Maintenance Chemotherapy”[Mesh] OR “Chemotherapy, Adjuvant”[Mesh] OR “Chemotherapy, Cancer, Regional Perfusion”[Mesh] OR “Drug Therapy”[Mesh] OR “Drug Therapy, Combination”[Mesh] OR “Radiotherapy”[Mesh] OR “Radiation Dosage”[Mesh]) AND (“Articulation Disorders”[Mesh] OR “Speech”[Mesh] OR “Speech Sound Disorder”[Mesh] OR “Speech, Esophageal”[Mesh] OR “Speech, Alaryngeal”[Mesh] OR “Speech Intelligibility”[Mesh] OR “Speech Disorders”[Mesh] OR “Deglutition Disorders”[Mesh] OR “Deglutition”[Mesh] OR “Dysphonia” [Mesh] or “Voice Disorders” [Mesh] or “Hoarseness” [Mesh] or “Aphonia” [Mesh] OR “Xerostomia”[Mesh] OR “Dysarthria”[Mesh] OR “Larynx, Artificial”[Mesh] OR “Speech, Esophageal”[Mesh] OR “Trismus”[Mesh] OR “Quality of Life”[Mesh]) | Adult: 19+ years English |
304 |
|
| |||
| Free text | Embase: (larynx∗ or pharynx∗ OR hypopharyn∗ OR laryngo∗ OR larynge∗) AND (cancer OR cancers OR neoplasm∗ OR tumour∗ OR tumor OR tumors OR carcinoma∗) AND (radiation∗ OR radiotherap∗ OR chemotherap∗ OR adjuvant therap∗ OR radiochemotherap∗) AND (deglut∗ OR swallow∗ OR dysphag∗ OR speech∗ OR voic∗ OR hoarse∗ OR aphon∗ OR rough∗ OR articulat∗ OR dysphon∗ OR (quality AND life) OR xerostom∗ OR dysarthr∗ OR anarthr∗ OR trismus) | Publication date: last year | 397 |
|
| |||
| Free text | PubMed: As per Embase Free Text | Publication date: from 2015/05/05 to 2016/05/05 |
148 |
In Embase the thesaurus terms larynx or hypopharynx were linked to neoplasm and radiotherapy or chemotherapy. Next, the search outcome was combined with the following terms: dysphagia, speech, speech disorder, voice, dysphonia, xerostomia, quality of life, dysarthria, or trismus (see Table 1).
To identify the most recent publications, the search was complemented by free text words in PubMed and Embase (for the period after April 2015 until May 2016). Truncation symbols and wildcards were used to search for variant forms of words or word extensions: Laryn ∗, pharyn ∗ or hypopharyn ∗ were combined with cancer ∗, neoplasm ∗, tumour ∗ or carcinoma ∗. Furthermore, these free text words were combined with radiation ∗, radiotherap ∗, chemotherap ∗, adjuvant therap ∗ or radiochemotherap ∗ and, finally, combined with deglut ∗, swallow ∗, dysphag ∗, speech ∗, voic ∗, articulat ∗, dysphon ∗, quality of life ∗, xerostom ∗, dysarthr ∗ or anarthr ∗.
Only articles presenting both pre- and postintervention data of the upper aerodigestive tract functions of the participants were included. Review articles and studies with a population sample of less than 20 patients were excluded, as well as experiments on animals or articles not published in English. Furthermore, studies published before 1990, case reports, expert opinions, and articles describing combinations of therapy including surgical interventions were excluded.
Final decisions on inclusion were made based on the original articles by consensus between two expert reviewers in accordance with the PRISMA statement [17]. The reference lists of all the included articles were searched for additional literature. Next, the standard quality assessment QualSyst as described by Kmet et al. [18] was performed in order to evaluate the methodological strength and weaknesses of the included studies. All ratings were performed by two independent reviewers. After consensus, studies with poor methodology scores (<50%) were excluded. All included articles were classified according to the Australian National Health and Medical Research Council (NHMRC) Evidence Hierarchy [19]. Data were retrieved from all studies and tabulated; further details on selected speech pathology interventions were summarised separately.
3. Results
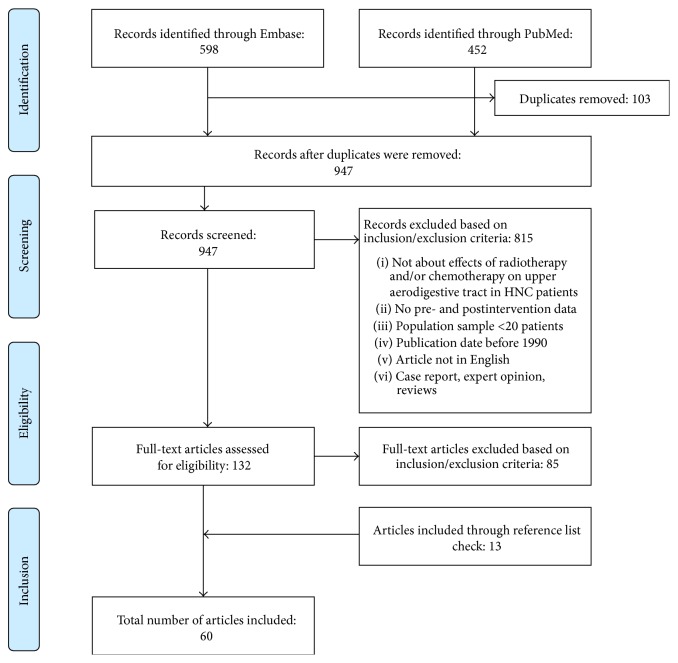
Using MeSH or thesaurus terms, 304 articles were located in PubMed and 201 in Embase. Free text word searches resulted in another 148 articles in PubMed and 397 in Embase. The combination of these searches, without overlap, yielded 947 articles. Figure 1 outlines the PRISMA reviewing process according to Moher et al. [20]. Sixty articles met all inclusion criteria.
Figure 1.
PRISMA flowchart.
Table 2 shows the outcomes of the QualSyst critical appraisal tool by Kmet et al. [18]. As all studies had sufficient methodological quality, no further studies were excluded; the overall methodological quality ranged from adequate to good with 0 studies ranked as poor, 3 studies as adequate, 3 studies as good, and 54 studies as strong. Based on the NHMRC Evidence Hierarchy [19], 6 studies were classified as level II evidence and 54 studies as level III evidence.
Table 2.
Methodological quality based on QualSyst critical appraisal tool by Kmet et al. 2004 [18] and NHMRC 1999 [19] evidence level of included articles.
| Reference | Kmet score (%) | Methodological quality1 | NHMRC level of evidence2 |
|---|---|---|---|
| Aaltonen et al. 2014 [21] | 25/28 (89%) | Strong | II |
| Ackerstaff et al. 2009 [22] | 22/28 (79%) | Good | II |
| Agarwal et al. 2009 [23] | 19/24 (79%) | Good | III-2 |
| Agarwal et al. 2011 [45] | 17/20 (85%) | Strong | III-3 |
| Akst et al. 2004 [46] | 17/20 (85%) | Strong | III-3 |
| Al-Mamgani et al. 2012 [24] | 19/20 (95%) | Strong | III-3 |
| Al-Mamgani et al. 2012 [47] | 21/22 (95%) | Strong | III-3 |
| Al-Mamgani et al. 2013 [25] | 21/22 (95%) | Strong | III-3 |
| Al-Mamgani et al. 2015 [26] | 21/22 (95%) | Strong | III-3 |
| Bansal et al. 2004 [27] | 14/24 (58%) | Adequate | III-3 |
| Bibby et al. 2008 [28] | 18/22 (82%) | Strong | III-2 |
| Bottomley et al. 2014 [78] | 24/28 (86%) | Strong | II |
| Buchbinder et al. 1993 [48] | 14/26 (54%) | Adequate | III-1 |
| Caudell et al. 2010 [49] | 21/22 (95%) | Strong | III-3 |
| Christianen et al. 2015 [50] | 21/22 (95%) | Strong | III-3 |
| Cohen et al. 2006 [51] | 19/20 (95%) | Strong | III-3 |
| Dornfeld et al. 2007 [29] | 17/22 (77%) | Strong | III-3 |
| Dijkstra et al. 2007 [52] | 19/22 (86%) | Strong | III-3 |
| Feng et al. 2007 [53] | 19/22 (86%) | Strong | III-3 |
| Feng et al. 2010 [54] | 20/20 (100%) | Strong | III-3 |
| Frowen et al. 2010 [16] | 22/22 (100%) | Strong | III-2 |
| Haderlein et al. 2014 [55] | 17/20 (85%) | Strong | III-3 |
| Hutcheson et al. 2014 [56] | 18/20 (90%) | Strong | III-3 |
| Jacobi et al. 2016 [30] | 17/18 (94%) | Strong | III-3 |
| Karlsson et al. 2015 [31] | 26/28 (93%) | Strong | II |
| Karlsson et al. 2016 [32] | 18/20 (90%) | Strong | III-3 |
| Kazi et al. 2008 [33] | 17/20 (85%) | Strong | III-2 |
| Kerr et al. 2015 [34] | 19/20 (95%) | Strong | III-2 |
| Kotz et al. 2012 [57] | 24/28 (86%) | Strong | II |
| Kraaijenga et al. 2014 [35] | 19/20 (95%) | Strong | III-3 |
| Kumar et al. 2014 [58] | 19/20 (95%) | Strong | III-2 |
| Lazarus et al. 2014 [36] | 19/20 (95%) | Strong | III-3 |
| List et al. 1999 [59] | 15/18 (83%) | Strong | III-3 |
| McLaughlin et al. 2010 [60] | 19/20 (95%) | Strong | III-3 |
| Mittal et al. 2001 [37] | 16/20 (80%) | Strong | III-3 |
| Murry et al. 1998 [61] | 11/20 (55%) | Adequate | III-3 |
| Niedzielska et al. 2010 [38] | 17/20 (85%) | Strong | III-2 |
| Nourissat et al. 2010 [62] | 23/26 (88%) | Strong | III-3 |
| Ottoson et al. 2014 [63] | 19/22 (86%) | Strong | III-3 |
| Pauli et al. 2013 [64] | 19/22 (86%) | Strong | III-3 |
| Pauloski et al. 2006 [65] | 18/20 (90%) | Strong | III-3 |
| Rademaker et al. 2003 [66] | 17/20 (85%) | Strong | III-3 |
| Remmelts et al. 2013 [39] | 18/20 (90%) | Strong | III-3 |
| Salama et al. 2008 [67] | 17/20 (85%) | Strong | III-3 |
| Sanguineti et al. 2014 [40] | 19/20 (95%) | Strong | III-3 |
| Scrimger et al. 2007 [68] | 18/20 (90%) | Strong | III-3 |
| Spector et al. 1999 [41] | 17/22 (77%) | Good | III-3 |
| Starmer et al. 2014 [69] | 18/20 (90%) | Strong | III-3 |
| Stenson et al. 2010 [70] | 16/20 (80%) | Strong | III-3 |
| Strigari et al. 2010 [71] | 17/20 (85%) | Strong | III-3 |
| Tuomi et al. 2015 [42] | 18/20 (90%) | Strong | III-2 |
| Urdaniz et al. 2005 [77] | 18/20 (90%) | Strong | III-2 |
| Vainshtein et al. 2015 [72] | 20/24 (83%) | Strong | III-3 |
| van der Molen et al. 2011 [73] | 24/26 (92%) | Strong | II |
| van der Molen et al. 2012 [44] | 16/20 (80%) | Strong | III-3 |
| van der Molen et al. 2013 [74] | 19/20 (95%) | Strong | III-3 |
| Verdonck-de Leeuw et al. 1999 [43] | 18/20 (90%) | Strong | III-2 |
| Verdonck-de Leeuw et al. 2014 [79] | 16/20 (80%) | Strong | III-2 |
| Vlacich et al. 2014 [75] | 18/20 (90%) | Strong | III-3 |
| Wilson et al. 2011 [76] | 18/20 (90%) | Strong | III-3 |
1Methodological quality: strong > 80%; good 60–79%; adequate 50–59%; poor < 50%.
2NHMRC evidence hierarchy designates the following hierarchy: level I (evidence obtained from a systematic review of all relevant RCTs), level II (evidence obtained from at least one properly designed RCT), level III-1 (evidence obtained from well-designed pseudo-RCTs [alternate allocation or some other method]), level III-2 (evidence obtained from comparative studies with concurrent controls and allocation not randomised [cohort studies], case control studies, or interrupted time series with a control group), level III-3 (evidence obtained from comparative studies with historical control, two or more single-arm studies, or interrupted time series without a parallel control group), and level IV (evidence obtained from case series, either posttest or pretest and posttest).
All 60 studies focused on different functions of the upper aerodigestive tract following radiotherapy and/or chemotherapy for HNC. The following constructs were evaluated across the different studies: communication (voice and speech), functions of the digestive tract (oral intake, weight loss, dysphagia, trismus, xerostomia, and tube dependency), QoL, and overall survival rates.
Table 3 provides a summary of the 60 retrieved observational and intervention studies that met the inclusion criteria. The first column presents the reference of the author(s). The second column represents the number of subjects, the third column represents the etiology of the head and neck malignancies, and the 4th column displays the staging of the malignancies. The 5th column shows whether voice and/or speech, digestive tract, and QoL were studied. The 6th and 7th columns show the evaluation techniques and the treatment, respectively. The 8th column presents the follow-up and the last column describes the author's key findings.
Table 3.
Overview of included observational and intervention studies (N = 60) that met eligibility criteria.
| Reference | Subjects | Carcinoma | Staging | Topic | Evaluation technique | Treatment(s) | Follow-up | Key findings/author's conclusions |
|---|---|---|---|---|---|---|---|---|
| Aaltonen et al. 2014 [21] | N = 56 | Glottic = 56 (100%) | T1a = 56 (100%) | V | Videolaryngostroboscopy Expert rating (GRBAS) Patient self-rating (VAS) hoarseness and impact on everyday life |
Group 1: laser surgery (n = 31) Group 2: RT (n = 25) |
6, 24 months | Similar overall voice quality for both groups. Laser surgery yielded more breathiness compared to RT |
|
| ||||||||
| Ackerstaff et al. 2009 [22] |
N = 207 | Oral cavity = 40 (19%) Oropharyngeal = 129 (62%) Hypopharyngeal = 38 (19%) |
T3 = 65 (31%) T4 = 142 (69%) |
V D QoL |
EORTC QLQ-C30 EORTC QLQ-H&N35 Trial-specific questionnaires |
Group 1: intra-arterial cisplatin 4 weekly (n = 104) + RT Group 2: intravenous cisplatin 3 weekly (n = 103) + RT |
7 weeks; 3 months; 1, 2, 5 years | Both groups showed improved oral intake and voice quality, at 1-year follow-up often better compared to baseline |
|
| ||||||||
| Agarwal et al. 2009 [23] |
N = 50 | Glottic = 50 (100%) | T1 = 33 (66%) T2 = 17 (34%) |
V | Voice analysis Acoustic parameters: frequency, intensity, perturbation Patient-reported improvement in voice quality |
RT | 3–6 months | A trend for improvement in voice quality following RT was found |
|
| ||||||||
| Agarwal et al. 2011 [45] |
N = 47 | Oropharyngeal Hypopharyngeal Laryngeal (No details provided) |
T1 T2 T3 T4 (No details provided) |
D | Videofluoroscopy PSS-HN |
CRT | 2, 6, 12 months | Significant impairment of swallowing was found: most frequently residue and aspiration |
|
| ||||||||
| Akst et al. 2004 [46] |
N = 196 | Oral cavity = 12 (6%) Base of tongue = 41 (21%) Tonsil = 41 (21%) Other oropharyngeal = 15 (8%) Hypopharyngeal = 34 (17%) Laryngeal = 50 (26%) Unknown = 3 (1%) |
T1 = 15 (8%) T2 = 42 (21%) T3 = 65 (33%) T4 = 70 (36%) Unknown = 4 (2%) |
D | Presence of feeding tube Presence of tracheotomy Level of oral diet |
CRT | 3, 6, 12, 24 months | A majority of patients did not need a tracheotomy but need a feeding tube during treatment. At 1-year follow-up most patients had a (nearly) normal oral intake. Patients with tumor stage IV and age ≥ 60 had prolonged feeding tube use and slower recovery |
|
| ||||||||
| Al-Mamgani et al. 2012 [24] |
N = 170 | Supraglottic = 121 (71%) Glottic = 49 (29%) |
T3 = 170 (100%) | V QoL |
EORTC QLQ-C30 EORTC QLQ-H&N35 VHI |
Group 1: CRT (n = 48) Group 2: RT (n = 122) |
2, 4, 6 weeks; 3, 6, 12 months | Adding chemotherapy to RT did not diminish QoL or voice handicap |
|
| ||||||||
| Al-Mamgani et al. 2012 [47] |
N = 176 | Hypopharyngeal = 176 (100%) | T1 = 18 (10%) T2 = 55 (31%) T3 = 56 (32%) T4a = 35 (20%) T4b = 12 (7%) |
D QoL |
Tube dependency EORTC QLQ-C30 EORTC QLQ-H&N35 |
Group 1: CRT (n = 102) Group 2: RT (n = 74) |
2, 4, 6 weeks; 3, 6 months; 1, 2 years | CRT significantly improved functional outcome. Acute toxicity increased but late radiation side effects did not increase |
|
| ||||||||
| Al-Mamgani et al. 2013 [25] |
N = 1050 | Glottic = 1050 (100%) | T1a = 551 (52%) T1b = 168 (16%) T2a = 209 (20%) T2b = 122 (12%) |
V QoL |
EORTC QLQ-C30 EORTC QLQ-H&N35 VHI |
RT | 4, 6 weeks; 3, 6, 12, 18, 24, 36, 48 months | Excellent outcome with good QoL and VHI scores |
|
| ||||||||
| Al-Mamgani et al. 2015 [26] |
N = 30 | Glottic = 30 (100%) | T1a = 30 (100%) | V | Laryngoscopy VHI |
Single vocal cord RT | 4, 6, 12 weeks; 6, 12, 18 months | Single vocal cord RT showed better voice quality compared to whole larynx RT |
|
| ||||||||
| Bansal et al. 2004 [27] |
N = 45 | Base of tongue = 20 (44%) Tonsil = 10 (22%) Unknown = 15 (33%) |
Stage III = 17 (38%) Stage IV = 23 (51%) Not reported = 5 (11%) |
V QoL |
Acute and late morbidity scoring of skin, oropharyngeal mucosa, salivary glands, larynx, and oesophagus (LENT/SOMA) EORTC QLQ-C30 |
RT | 1, 4 months | During RT a decline in all QoL domains was found. QoL improved after 1 month but did not reach pre-RT levels |
|
| ||||||||
| Bibby et al. 2008 [28] |
N = 30 | Glottic = 30 (100%) | T1 = 21 (70%) T2 = 9 (30%) |
V QoL |
Voice analysis Patient self-rating voice quality VR-QoL |
RT | 3, 6, 12 months | After RT expert-rated perceptual auditory outcomes, patient self-rated VAS and all subscales of VR-QoL showed significant improvement |
|
| ||||||||
| Bottomley et al. 2014 [78] |
N = 450 | Laryngeal Hypopharyngeal (No details provided) |
T2 T3 T4 (No details provided) |
QoL | EORTC QLQ-C30 EORTC QLQ-H&N35 |
Group 1: sequential CRT (n = 224) Group 2: alternating CRT (n = 226) |
6, 12, 18, 24, 36, 48 months | The HRQoL scores of the majority of patients returned to baseline after therapy. No group differences were found |
|
| ||||||||
| Buchbinder et al. 1993 [48] |
N = 21 | No details provided | No details provided | D | MIO | Group 1: RT + unassisted exercise Group 2: RT + stacked tongue depressors combined + unassisted exercise Group 3: RT + TheraBite® system combined + unassisted exercise |
2, 4, 6, 8, 10 weeks | The highest increase in MIO was reached in group 3 |
|
| ||||||||
| Caudell et al. 2010 [49] |
N = 83 | Nasal cavity = 3 (4%) Nasopharyngeal = 7 (8%) Oral cavity = 1 (1%) Oropharyngeal = 44 (53%) Hypopharyngeal = 6 (7%) Laryngeal = 17 (21%) Unknown = 5 (6%) |
Tx-2 = 28 (34%) T3-T4 = 55 (66%) |
D | Videofluoroscopy PEG dependency |
Group 1: CRT (n = 70) Group 2: RT (n = 13) |
12 months | Mean dose to the larynx greater than 41 Gy and a receiving volume greater than 24% showed association with increased PEG dependency and aspiration |
|
| ||||||||
| Christianen et al. 2015 [50] |
N = 238 | Nasopharyngeal = 8 (3%) Oral cavity = 11 (5%) Oropharyngeal = 71 (30%) Hypopharyngeal = 12 (5%) Laryngeal = 136 (57%) |
T1-T2 = 161 (68%) T3-T4 = 77 (32%) |
D | Grade of swallowing dysfunction according to the RTOG/EORTC Late Radiation Morbidity Scoring Criteria | Group 1: conventional RT (n = 33) Group 2: accelerated RT (n = 155) Group 3: CRT (n = 50) |
6, 12, 18, 24 months | Patterns of swallowing dysfunction may be caused by radiobiological mechanisms of radiation induced damage and recovery. No group differences were found |
|
| ||||||||
| Cohen et al. 2006 [51] |
N = 53 | Oral cavity = 14 (26%) Oropharyngeal = 11 (21%) Hypopharyngeal = 3 (6%) Laryngeal = 9 (17%) Supraglottic = 13 (24%) Unknown = 3 (6%) |
T0 = 3 (6%) T1 = 3 (6%) T2 = 17 (32%) T3 = 30 (56%) |
D QoL |
PSS-HN Head and Neck RT Questionnaire (selected questions) FACT-H&N |
CRT | 3, 6, 12, 18, 24, 36, 48, 60 months | Most patients returned to pretreatment function (QoL and performance) by 12 months |
|
| ||||||||
| Dornfeld et al. 2007 [29] |
N = 27 | Oral cavity = 1 (4%) Oropharyngeal = 16 (59%) Hypopharyngeal = 1 (4%) Laryngeal = 6 (22%) Unknown = 3 (11%) |
Tx = 2 (7%) T1 = 6 (22%) T2 = 7 (26%) T3 = 5 (19%) T4 = 7 (26%) |
V D QoL |
Weight Type of diet Type of speech Presence of PEG tube HNCI |
CRT | 1 year | Speech, diet, and QoL outcomes showed an inverse relationship with the delivered radiation dose to the larynx |
|
| ||||||||
| Dijkstra et al. 2007 [52] |
N = 29 | Parotid = 4 (14%) Maxilla = 4 (14%) Gingiva = 2 (7%) Floor of mouth = 3 (10%) Trigonum retromolare = 4 (14%) Oropharyngeal = 8 (27%) Other localization = 4 (14%) |
No details provided | D | MIO | RT | 12–48 weeks | Increase in mouth opening was significantly less in the group of patients with trismus related to head and neck cancer and is difficult to treat with exercise therapy |
|
| ||||||||
| Feng et al. 2007 [53] |
N = 36 | Base of tongue = 19 (53%) Tonsil = 12 (33%) Nasopharyngeal = 5 (14%) |
T1 = 2 (5%) T2 = 11 (31%) T3 = 9 (25%) T4 = 14 (39%) |
D QoL |
Videofluoroscopy Esophagogram HNQoL UWQoL EORTC Late Radiation Morbidity Scale |
CRT | 3 months | Statistically significant dose-volume effect relationships for dysphagia and aspiration were found. Reducing the doses to the swallowing structures may improve swallowing |
|
| ||||||||
| Feng et al. 2010 [54] |
N = 73 | Base of tongue = 38 (52%) Tonsil = 35 (48%) |
T1 = 9 (12%) T2 = 29 (40%) T3 = 17 (23%) T4 = 18 (25%) |
D | Videofluoroscopy UWQoL (swallowing question) HNQoL (eating domain) Observer rated dysphagia |
CRT | 3, 6, 12, 18, 24 months | Long-term measures of swallowing were slightly worse than pretherapy measures |
|
| ||||||||
| Frowen et al. 2010 [16] |
N = 81 | Base of tongue = 19 (24%) Soft palate = 2 (2%) Tonsil = 26 (32%) Supraglottic = 8 (10%) Hypopharyngeal = 8 (10%) Laryngeal = 18 (22%) |
T1 = 11 (14%) T2 = 27 (33%) T3 = 31 (38%) T4 = 12 (15%) |
D | Videofluoroscopy | Group 1: CRT (n = 23) Group 2: CT (n = 58) |
3, 6 months | Swallowing in both groups was best at baseline; a decline at 3 months and an improvement at 6 months after therapy were shown. Baseline levels were not reached. Predictors for swallowing outcome were intoxications, tumor size, RT technique, and baseline level of swallowing. Patients who received conformal RT had a very low risk of penetration and aspiration of liquids by 6 months after treatment |
|
| ||||||||
| Haderlein et al. 2014 [55] |
N = 45 | Oropharyngeal = 3 (7%) Hypopharyngeal = 18 (40%) Laryngeal = 24 (53%) |
T2 = 15 (33%) T3 = 17 (38%) T4 = 13 (29%) |
D QoL |
PEG dependency EORTC QLQ-C30 |
CRT | 3–6- month intervals | Almost 50% of patients had deterioration of swallowing function after CRT |
|
| ||||||||
| Hutcheson et al. 2014 [56] |
N = 47 | Nasopharyngeal = 1 (2%) Oral cavity = 1 (2%) Oropharyngeal = 41 (88%) Hypopharyngeal = 2 (4%) Supraglottic = 2 (4%) |
T1 = 16 (34%) T2 = 14 (30%) T3 = 12 (25%) T4 = 5 (11%) |
D | Videofluoroscopy PSS-HN MDADI |
Group 1: RT (n = 23) Group 2: CRT (n = 23) Group 3: surgery (n = 1) |
6, 12, 24 months | Two years after therapy, mild deterioration of swallowing without chronic aspiration was found |
|
| ||||||||
| Jacobi et al. 2016 [30] |
N = 34 | Nasopharyngeal = 6 (18%) Oral cavity/oropharyngeal = 15 (44%) Hypopharyngeal = 13 (38%) |
T1 = 6 (18%) T2 = 13 (38%) T3 = 11 (32%) T4 = 4 (12%) |
V | Speech analysis | CRT | 10 weeks; 1 year | Received dose to tongue and velopharynx were most relevant for speech and voice quality |
|
| ||||||||
| Karlsson et al. 2015 [31] |
N = 74 | Laryngeal = 74 (100%) | T0 = 1 (1%) T1 = 44 (60%) T2 = 22 (30%) T3 = 6 (8%) T4 = 1 (1%) |
V QoL |
EORTC QLQ-C30 EORTC QLQ-H&N35 S-SECEL |
Group 1: CRT + voice rehabilitation (n = 37) Group 2: CRT only (n = 37) |
1, 6 months | Patients treated with voice rehabilitation experienced benefits of therapy on communication and HRQoL |
|
| ||||||||
| Karlsson et al. 2016 [32] |
N = 40 | Laryngeal = 40 (100%) | Tis = 2 (5%) T1 = 20 (50%) T2 = 13 (33%) T3 = 5 (12%) |
V QoL |
EORTC QLQ-C30 EORTC QLQ-H&N35 S-SECEL Perceptual and acoustic voice analysis |
RT (1 subject received concomitant chemotherapy) | 1, 6, 12 months | One year after treatment most outcomes showed no significant improvements compared to baseline measurements |
|
| ||||||||
| Kazi et al. 2008 [33] |
N = 21 | Hypopharyngeal = 8 (38%) Laryngeal = 10 (48%) Supraglottic = 3 (14%) |
Stage III Stage IV No details provided |
V | Voice analysis Electroglottography |
CRT | 1, 6, 12 months | Patients treated with CRT had a better voice quality compared to patients after total laryngectomy |
|
| ||||||||
| Kerr et al. 2015 [34] |
N = 200 | Tongue base = 77 (38%) Tonsil/soft palate = 123 (62%) |
T0-T1 = 42 (21%) T2 = 72 (36%) T3 = 48 (24%) T4 = 38 (19%) |
V D |
KPS ECOG toxicity and response criteria scale PSS-HN RBHOMS VHI-10 Edmonton Self-Assessment Scale (Self-rated Xerostomia) |
Group 1: 3DCRT (n = 83) Group 2: IMRT (n = 117) |
3, 6, 12, 24 months | IMRT showed better functional outcomes compared to 3DCRT, both 3–6 and 12–24 months after treatment |
|
| ||||||||
| Kotz et al. 2012 [57] |
N = 26 | Nasopharyngeal = 1 (4%) Tongue base = 11 (42%) Tonsil = 11 (42%) Oropharyngeal = 1 (4%) Glottic = 1 (4%) Unknown = 1 (4%) |
T2 = 1 (4%) T3 = 5 (19%) T4 = 20 (77%) |
D | PSS-HN (eating in public and normalcy of diet) FOIS |
Group 1: CRT + prophylactic swallowing therapy (n = 13) Group 2: CRT (n = 13) |
3, 6, 9, 12 months | Prophylactic swallowing therapy improves swallowing at 3 and 6 months; later there were no group differences |
|
| ||||||||
| Kraaijenga et al. 2014 [35] |
N = 22 | Nasopharyngeal = 4 (18%) Oral cavity/ oropharyngeal = 10 (46%) Hypopharyngeal/ laryngeal = 8 (36%) |
T1 = 5 (23%) T2 = 9 (41%) T3 = 7 (32%) T4 = 1 (4%) |
V D QoL |
Videofluoroscopy Acoustic analysis Presence of feeding tube FOIS Pain (VAS) Trismus QoL aspects (based on EORTC QLQ-C30 and EORTC QLQ-H&N35) SWAL-QoL VHI |
CRT | 2, 6 years | Functional swallowing and voice problems at 6 years after treatment were minimal, possibly due to preventive swallowing rehabilitation programs |
|
| ||||||||
| Kumar et al. 2014 [58] |
N = 46 | Tonsil = 19 (41%) Base of tongue = 22 (47%) Pharyngeal wall = 3 (7%) Unknown = 2 (5%) |
T0 = 2 (4%) T1 = 15 (33%) T2 = 14 (30%) T3 = 12 (26%) T4 = 3 (7%) |
D | Videofluoroscopy | CRT | From <6 to >18 months | Aspiration and penetration were associated with dose and volume delivered to the floor of mouth muscles |
|
| ||||||||
| Lazarus et al. 2014 [36] |
N = 29 | Nasopharyngeal = 3 (10%) Oropharyngeal = 18 (63%) Pharyngeal = 1 (3%) Hypopharyngeal = 1 (3%) Laryngeal = 5 (18%) Unknown primary = 1 (3%) |
Stage I = 2 (7%) Stage II = 1 (4%) Stage III = 5 (17%) Stage IVa = 21 (72%) |
D V QoL |
Tongue strength, jaw ROM, and tongue ROM Saliva weight Eating Assessment Tool MDADI Speech Handicap Index EORTC QLQ-H&N35 PSS-HN (normalcy of diet, eating in public, and understandability of speech) KPS |
CRT | 3, 6 months |
Patients performed worse in oral outcomes, performance status, and QoL after treatment |
|
| ||||||||
| List et al. 1999 [59] |
N = 64 | Nasopharyngeal = 1 (2%) Oral cavity = 6 (9%) Oropharyngeal = 34 (53%) Hypopharyngeal = 10 (16%) Laryngeal = 9 (14%) Other = 4 (6%) |
Stage III = 4 (6%) Stage IV = 60 (94%) |
D QoL |
KPS PSS-HN McMaster University Head and Neck RT Questionnaire (selected questions) FACT-H&N |
CRT | 1, 3, 6, 9, 12 months | Decline of QoL and functional aspects resolved 1 year after treatment; however, oral intake stayed restricted |
|
| ||||||||
| McLaughlin et al. 2010 [60] |
N = 91 | Nasopharyngeal = 9 (10%) Oral cavity = 19 (21%) Oropharyngeal = 32 (35%) Hypopharyngeal = 7 (8%) Laryngeal = 12 (13%) Unknown = 4 (4%) Other = 8 (9%) |
Stage II = 1 (1%) Stage III = 21 (23%) Stage IV = 69 (76%) |
D | Weight loss Aspiration Overall nutritional status Duration G-tube placement Treatment-related complications |
CRT | 6, 12 months | Patients treated with CRT could be managed without nutritional support via G-tube. Dysphagia at baseline and advanced tumor stage are associated with increased risk of longer G-tube dependency |
|
| ||||||||
| Mittal et al. 2001 [37] |
N = 39 | Nasopharyngeal = 4 (10%) Oropharyngeal = 17 (44%) Hypopharyngeal = 7 (18%) Laryngeal = 5 (13%) Unknown = 6 (15%) |
Stage III = 5 (13%) Stage IV = 34 (87%) |
D V |
Videofluoroscopy Saliva production PSS-HN FACT-H&N Fisher-Logemann Test of Articulation Competence |
Group 1: CRT with TDC (n = 18) Group 2: CRT without TDC (n = 21) |
3 months | Patients treated with TDC had better oral intake, swallowing function, and articulation |
|
| ||||||||
| Murry et al. 1998 [61] | N = 37 | Oropharyngeal = 19 (52%) Hypopharyngeal = 6 (16%) Laryngeal = 12 (32%) |
T3 T4 (No details provided) |
D | HNRQ Questionnaire on swallowing |
CRT | 6 months | During treatment QoL and swallowing function decreased acutely and significantly. Six months after therapy QoL exceeded pretreatment level. Recovery was site-specific: oropharyngeal tumor patients had poorest outcome, whereas hypopharyngeal tumor patients showed most rapid recovery. Physical recovery followed psychosocial recovery. Organ preservation treatment may improve swallowing after treatment |
|
| ||||||||
| Niedzielska et al. 2010 [38] |
N = 45 | Laryngeal = 45 (100%) | T1 = 24 (53%) T2 = 21 (47%) |
V | Videolaryngostroboscopy Acoustic analysis |
RT | 1–3 years | All irradiated patients showed reduced vibration of the vocal cords. Except for some of the acoustic parameters, most data were comparable to a healthy control group (N = 24) |
|
| ||||||||
| Nourissat et al. 2010 [62] |
N = 535 | Oral cavity = 63 (12%) Oropharyngeal = 17 (3%) Hypopharyngeal = 8 (1%) Supraglottic = 100 (19%) Glottic = 347 (65%) |
T1 = 329 (61%) T2 = 206 (39%) |
D QoL |
Weight KPS EORTC QLQ-C30 Structured general questionnaire |
RT | Direct posttherapy | The occurrence of adverse effects of RT appeared to be one of the main reasons for weight loss. Correlations were found between genetic factors associated with the adverse effects of cancer treatments |
|
| ||||||||
| Ottoson et al. 2014 [63] |
N = 101 | Oral cavity = 20 (20%) Oropharyngeal = 62 (61%) Hypopharyngeal = 8 (8%) Laryngeal = 11 (11%) |
T1 = 11 (10%) T2 = 16 (16%) T3 = 28 (28%) T4 = 46 (46%) |
D | Videofluoroscopy BMI |
RT | 5 years | Dysphagia with aspiration was related to unintentional weight loss and a lower BMI |
|
| ||||||||
| Pauli et al. 2013 [64] |
N = 75 | Sinus, nose = 6 (8%) Salivary gland = 10 (13%) Gingiva, buccal = 6 (8%) Tongue, floor of mouth = 15 (20%) Tonsil = 24 (32%) Base of tongue, oropharyngeal = 11 (15%) Other = 3 (4%) |
T0 = 5 (7%) T1 = 13 (17%) T2 = 29 (39%) T3 = 9 (12%) T4 = 18 (24%) Unknown = 1 (1%) |
D QoL |
MIO Patient-reported outcome Gothenburg Trismus Questionnaire EORTC QLQ-C30 EORTC QLQ-H&N35 HADS |
Group 1: surgery (n = 8) Group 2: surgery + RT (n = 15) Group 3: surgery + CRT (n = 6) Group 4: RT (n = 16) Group 5: CRT (n = 29) Group 6: no treatment (n = 1) |
3, 6, 12 months | Trismus was a major side effect of the treatment of head and neck cancer and deteriorates HRQoL |
|
| ||||||||
| Pauloski et al. 2006 [65] |
N = 170 | Nasopharyngeal = 8 (5%) Oral cavity = 15 (9%) Oropharyngeal = 80 (47%) Hypopharyngeal = 14 (8%) Laryngeal = 42 (25%) Unknown = 11 (6%) |
Stage IV = 122 (72%) Other = 48 (28%) |
D | Videofluoroscopy Oral intake |
Group 1: CRT (n = 147) Group 2: RT (n = 22) Unknown (n = 1) |
1, 3, 6, 12 months | In both groups limitations in oral intake and diet after cancer treatment were significantly related to reduced laryngeal elevation and reduced cricopharyngeal opening due to treatment |
|
| ||||||||
| Rademaker et al. 2003 [66] |
N = 255 | Nasopharyngeal = 13 (5%) Oral cavity = 25 (10%) Oropharyngeal = 118 (46%) Hypopharyngeal = 22 (9%) Laryngeal = 59 (23%) Unknown = 18 (7%) |
Stage II = 16 (6%) Stage III = 48 (19%) Stage IV = 187 (73%) Unknown = 4 (2%) |
D | Percentage of oral intake Food consistencies |
CRT | 1, 3, 6, 12 months | Eating ability decreased during treatment and improved 12 months after treatment to near pretreatment levels |
|
| ||||||||
| Remmelts et al. 2013 [39] |
N = 248 | Glottic = 248 (100%) | Tis = 26 (10%) T1a = 103 (42%) T1b = 42 (17%) T2 = 77 (31%) |
V | VHI (physical subscale) 5-item questionnaire |
Group 1: RT (n = 159) Group 2: laser surgery (n = 89) |
12 months | VHI scores were comparable for both groups. Regarding laryngeal preservation surgery is the treatment of first choice |
|
| ||||||||
| Salama et al. 2008 [67] |
N = 95 | Nasopharyngeal = 4 (4%) Oral cavity = 8 (9%) Oropharyngeal = 49 (52%) Hypopharyngeal = 5 (5%) Laryngeal = 22 (23%) Other = 7 (7%) |
Tx = 7 (7%) T1 = 15 (16%) T2 = 19 (20%) T3 = 16 (17%) T4 = 38 (40%) |
D | Videofluoroscopy SPS |
CRT | 1-2 months | Improvement of swallowing ability compared to baseline was associated with advanced tumor stage |
|
| ||||||||
| Sanguineti et al. 2014 [40] |
N = 124 | Base of tongue = 54 (43%) Soft palate = 2 (2%) Tonsil = 59 (48%) Pharyngeal wall = 1 (1%) Unknown = 8 (6%) |
T0 = 8 (6%) T1 = 37 (30%) T2 = 49 (40%) T3 = 14 (11%) T4 = 16 (13%) |
V | CTCAE FACT-HN (items HN4 and HN10) |
Group 1: CRT (n = 108) Group 2: RT (n = 16) |
3, 6, 12, 18, 24, 36, 48, 60 months | Mild voice changes were common and strictly correlated to mean dose to larynx and should be kept under 50 Gy |
|
| ||||||||
| Scrimger et al. 2007 [68] |
N = 47 | Nasopharyngeal = 10 (21%) Oral cavity = 20 (43%) Oropharyngeal = 9 (19%) Hypopharyngeal/ laryngeal = 6 (13%) Unknown primary = 2 (4%) |
T0 = 2 (4%) T1 = 7 (15%) T2 = 20 (42%) T3 = 12 (26%) T4 = 6 (13%) |
D QoL |
Mouth saliva flow UW-QoL RTOG late-toxicity scale XQoL |
Group 1: RT (n = 5) Group 2: CRT (n = 12) Group 3: surgery + RT (n = 30) |
3, 6, 12 months | Nonsurgery resulted in better QoL questionnaire scores compared to surgery. Patients with good saliva production did not exhibit better QoL after RT than patients with less saliva production |
|
| ||||||||
| Spector et al. 1999 [41] |
N = 659 | Glottic = 659 (100%) | T1 = 659 (100%) | V | Voice preservation | Group 1: low-dose RT (n = 90) Group 2: high-dose RT (n = 104) Group 3: conservation surgery (n = 404) Group 4: endoscopic resection (n = 61) |
5 years | Groups 2–4 had similar unaided laryngeal voice preservation rates; however group 1 had significant lower unaided laryngeal voice preservation |
|
| ||||||||
| Starmer et al. 2014 [69] |
N = 71 | Oropharyngeal = 71 (100%) | T1 = 24 (34%) T2 = 19 (27%) T3 = 13 (18%) T4 = 12 (17%) Unknown = 3 (4%) |
D | Videofluoroscopy FOIS |
Group 1: CRT (n = 65) Group 2: RT (n = 6) |
1–18 months | Patients undergoing nonsurgical treatment for oropharyngeal tumors were at risk for posttreatment dysphagia |
|
| ||||||||
| Stenson et al. 2010 [70] | N = 111 | Buccal = 4 (3%) Alveolus/gingivae = 7 (6%) Floor of mouth = 32 (29%) Tongue = 50 (45%) Palate/oral cavity NOS = 4 (4%) Trigonum retromolare = 13 (12%) Unknown = 1 (1%) |
T1 = 9 (8%) T2 = 15 (14%) T3 = 20 (18%) T4 = 67 (60%) |
D | Videofluoroscopy SPS |
Group 1: CRT (n = 84) Group 2: surgery + CRT (n = 27) |
2, 4, 6, 8, 10, 12, 16, 20, 24, 30, 36 months | Ninety-two percent of all patients were able to maintain weight via oral route. Both groups showed comparable overall survival. Ninety-two percent of all patients had a sufficient oral intake |
|
| ||||||||
| Strigari et al. 2010 [71] |
N = 63 | Nasopharyngeal = 44 (70%) Floor of mouth/oral cavity = 2 (3%) Oropharyngeal = 11 (17%) Hypopharyngeal = 4 (7%) Unknown primary = 2 (3%) |
T1-T2 = 17 (23%) T3-T4 = 46 (73%) |
D | Saliva flow Xerostomia related questionnaires RTOG late-toxicity scale |
RT | 3, 6, 12, 18, 24 months | The mean score on the xerostomia related questionnaire increased (worsened) after RT and decreased (improved) over time in all patients |
|
| ||||||||
| Tuomi et al. 2015 [42] |
N = 67 | Supraglottic = 13 (19%) Glottic = 54 (81%) |
Tis = 2 (3%) T1 = 41 (61%) T2 = 17 (25%) T3 = 6 (9%) T4 = 1 (2%) |
V D QoL |
Acoustic analysis EORTC QLQ-C30 EORTC QLQ-H&N35 S-SECEL |
RT | 1 month | Patients treated for supraglottic tumors experienced more problems in eating and swallowing prior to therapy compared to glottic tumors and demonstrated significant HRQoL reduction after treatment. In contrast, glottic tumors presented with inferior voice quality |
|
| ||||||||
| Urdaniz et al. 2005 [77] |
N = 60 | Paranasal sinuses = 3 (5%) Nasopharyngeal = 3 (5%) Oral cavity = 2 (3%) Oropharyngeal = 25 (42%) Hypopharyngeal = 9 (15%) Laryngeal = 18 (30%) |
T2 = 2 (3%) T3 = 20 (33%) T4 = 38 (64%) |
QoL | EORTC QLQ-C30 EORTC QLQ-H&N35 |
Group 1: hyperfractionated concomitant boost RT + cisplatin (n = 30) Group 2: hyperfractionated conventional RT + cisplatin (n = 30) |
1 month | QoL in both groups was relatively good. QoL improved in the follow-up period |
|
| ||||||||
| Vainshtein et al. 2015 [72] |
N = 40 | Base of tongue = 18 (45%) Tonsil = 22 (55%) |
T1 = 8 (20%) T2 = 20 (50%) T3 = 8 (20%) T4 = 4 (10%) |
D QoL |
HNQoL UWQoL XQoL |
CRT | 1, 3, 6, 12, 18, 24 months | At 6.5 years after therapy patients showed a stable or improved HRQoL in most domains comparable with baseline and 2 years after therapy |
|
| ||||||||
| van der Molen et al. 2011 [73] |
N = 49 | Nasopharyngeal = 7 (14%) Oral cavity/ oropharyngeal = 24 (49%) Hypopharyngeal/ laryngeal = 18 (37%) |
T1 = 8 (16%) T2 = 15 (31%) T3 = 19 (39%) T4 = 7 (14%) |
D | Videofluoroscopy MIO BMI FOIS VAS pain |
Group 1: standard rehabilitation (n = 28) Group 2: experimental rehabilitation (n = 27) |
10 weeks | (Preventive) rehabilitation in head and neck cancer patients was feasible and improved functional outcomes after therapy |
|
| ||||||||
| van der Molen et al. 2012 [44] |
N = 55 | Nonlaryngeal = 36 (65%) Laryngeal = 19 (35%) |
T1 = 8 (15%) T2 = 15 (27%) T3 = 21 (38%) T4 = 11 (20%) |
V QoL |
Acoustic analysis Study-specific QoL questionnaire |
CRT | 10 weeks; 1 year | CRT effects 10 weeks after therapy were worse than 1 year after therapy, and both were worse than baseline |
|
| ||||||||
| van der Molen et al. 2013 [74] |
N = 55 | Nasopharyngeal = 7 (13%) Oral cavity/ oropharyngeal = 29 (53%) Hypopharyngeal/ laryngeal = 19 (34%) |
T1 = 8 (15%) T2 = 15 (27%) T3 = 21 (38%) T4 = 11 (20%) |
D | Videofluoroscopy MIO Study-specific structured questionnaire |
CRT | 10 weeks; 1 year | A correlation between doses and structures was found for dysphagia and trismus |
|
| ||||||||
| Verdonck-de Leeuw et al. 1999 [43] |
N = 60 | Glottic = 60 (100%) | T1 = 60 (100%) | V | Videolaryngostroboscopy Voice quality rating Self-rating of vocal performance and quality |
RT | 0.5–10 years | Voice and its characteristics improved after treatment but did not reach pretreatment levels in half of the patients |
|
| ||||||||
| Verdonck-de Leeuw et al. 2014 [79] |
N = 164 | Oral/oropharyngeal = 95 (58%) Hypopharyngeal/ laryngeal = 69 (42%) |
No details provided | QoL | EORTC QLQ-C30 EORTC QLQ-H&N35 |
CRT | 6 weeks; 6, 12, 18, 24 months | Significant difference in HRQoL between survivors and nonsurvivors in favor of survivors was found |
|
| ||||||||
| Vlacich et al. 2014 [75] |
N = 141 | Sinus/nasal cavity = 2 (1%) Nasopharyngeal = 12 (9%) Oral cavity = 5 (4%) Oropharyngeal = 82 (58%) Hypopharyngeal = 6 (4%) Laryngeal = 30 (21%) Unknown = 4 (3%) |
Stage III = 42 (30%) Stage IVa = 81 (57%) Stage IVb = 18 (13%) |
D | PEG requirement | CRT | 12 months | IMRT dose to the inferior constrictor correlated with persistent dysphagia requiring prolonged PEG use |
|
| ||||||||
| Wilson et al. 2011 [76] |
N = 167 | Nasopharyngeal = 5 (3%) Oropharyngeal = 66 (39%) Hypopharyngeal = 21 (13%) Laryngeal = 63 (38%) Unknown primary = 12 (7%) |
T1 = 37 (22%) T2 = 37 (22%) T3 = 37 (22%) T4 = 44 (27%) Unknown = 12 (7%) |
D QoL |
MDADI UWQoL |
Group 1: CRT (n = 104) Group 2: RT (n = 63) |
3, 6, 12 months | HRQoL deteriorated significantly after treatment. Little improvement may be expected 3 to 12 months after treatment |
3DCRT: 3D conformal radiotherapy; BMI: body mass index; CRT: chemoradiotherapy; CTCAE: common terminology criteria for adverse events; D: digestive tract; ECOG: Eastern Cooperative Oncology Group; EORTC QLQ-C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire; EORTC QLQ-H&N35: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Module Head and Neck Cancer; FACT-HN: functional assessment of cancer therapy-head and neck; FOIS: functional oral intake scale; GRBAS: grade, roughness, breathiness, asthenia, strain scale; HADS: hospital anxiety and depression scale; HNCI: head and neck cancer inventory; HNQoL: head and neck quality of life; HNRQ: head and neck radiotherapy questionnaire; HRQoL: health-related quality of life; IMRT: intensity-modulated radiation therapy; KPS: Karnofsky performance status scale; LENT/SOMA: late effects normal tissue-subjective, objective, management, analytic scales; MDADI: MD Anderson dysphagia inventory; MIO: maximum incisal opening; NOS: not otherwise specified; PEG: percutaneous endoscopic gastrostomy; PSS-HN: performance status scale for head and neck cancer patients; QoL: quality of life; RBHOMS: Royal Brisbane Hospital outcome measure for swallowing; ROM: range of motion; RT: radiotherapy; RTOG: Radiation Therapy Oncology Group; S-SECEL: Swedish version of the self-evaluation of communication experiences after laryngeal cancer; SPS: swallowing performance status scale; TDC: tissue/dose compensation; UWQoL: University of Washington Quality of Life Questionnaire; V: voice and/or speech; VAS: visual analog scale; VHI: voice handicap index; VHI-10: voice handicap index-10; VR-QoL: voice-related quality of life; XQoL: xerostomia questionnaire.
3.1. Voice and/or Speech Function
Twenty-four studies evaluated voice and/or speech function [21–43] with a follow-up time ranging from 1-month follow-up [42] to ten-year follow-up [43]. Most studies included patients with laryngeal tumors only; however 11 studies [22, 27, 29, 30, 33–37, 44] also included nonlaryngeal tumors. Seventeen studies [21, 23, 25, 26, 28, 30–32, 34–36, 38–43] included patients with low-grade tumors (i.e., T1 and T2) and 15 studies included patient with advanced tumors [22, 24, 27, 29–37, 40, 42, 44].
Nine studies [23, 28, 30, 32, 33, 35, 38, 42, 44] used acoustic analysis to evaluate voice quality, six studies [24–26, 34, 35, 39] used the Voice Handicap Index, and three studies [21, 38, 43] used videolaryngostroboscopy. In several studies, either descriptions of how voice quality was evaluated were missing or nonvalidated tools were used (e.g., patients self-reporting or trial-specific questionnaires). Only four studies [23, 31, 40, 44] reported whether the patient received any voice therapy.
All the studies reported good to excellent outcomes for voice quality at long-term follow-up. Some studies specifically reported pre- to posttreatment improvements of voice or speech quality following radiotherapy and/or chemotherapy [22, 23, 28]. However, other studies [36, 40, 42, 44] reported a deterioration after therapy at long-term follow-up. Al-Mamgani et al. [26] found a better voice outcome in case of single vocal cord irradiation compared with irradiation of the whole larynx. Mittal et al. [37] concluded that radiation with tissue/dose compensation (TDC) improved articulatory outcome compared to radiation without TDC.
3.2. Functions of the Digestive Tract
Forty studies [16, 22, 29, 34–37, 42, 45–76] describe the effects of radiotherapy and/or chemotherapy on the functions of the digestive tract and used a variety of outcome measures. Of these 40 studies, 16 studies [16, 35, 37, 45, 49, 53, 54, 56, 58, 63, 65, 67, 69, 70, 73, 74] used videofluoroscopy to measure physiological changes in swallowing function. Eight studies [29, 35, 46, 47, 49, 55, 60, 75] used feeding tube dependency as a (dichotomous) outcome, whereas seven studies [35, 46, 57, 65, 66, 69, 73] described the level of oral intake in more detail. Only four studies [35, 36, 56, 76] used a condition specific validated measure for swallowing disorders (e.g., MDADI).
With regard to nutritional status, five studies [29, 60, 62, 63, 73] used the body mass index as an outcome or reported specifically on weight gain or loss. Seven studies [35, 36, 48, 52, 64, 73, 74] used the presence of trismus as an outcome by reporting on the maximum distance of mouth opening. Saliva flow (as a measure of xerostomia) was used in four studies [36, 37, 68, 71].
Follow-up times in these studies range from immediately after therapy [62] to 6 years after therapy [35], describing both low stage tumors and more advanced tumors. Thirty-two studies used the TNM-classification system, stage was described in six other studies, and the remaining two studies did not report on tumor stage or grade. However, it was unclear whether the clinical TNM-score or the pathological TNM-score was used to describe the severity of the disease. Eight studies [16, 35, 46, 48, 52, 56, 57, 69, 73, 74] described whether the patients received functional treatment (by a speech pathologist); the remainder of the articles did not mention whether the patient received any additional treatment.
Nine studies reported impaired swallowing function following radiotherapy and/or chemotherapy [36, 45, 50, 54–56, 69, 75, 76].
Five studies [16, 51, 59, 61, 66] showed that swallowing was least affected at baseline, worst immediately following posttreatment (0–3 months after treatment), and improved by 6–12 months after treatment and later. However, swallowing usually did not return to pretreatment functioning level. In four studies [49, 53, 58, 74], a relation between dose-volume, dysphagia, and aspiration was found. Caudell et al. [49] showed that a mean radiation dosage >41 Gy with >24% volume of the larynx being radiated was associated with increased percutaneous endoscopic gastrostomy (PEG) dependency and aspiration. Akst et al. [46] correlated advanced tumor stage and age >60 years with a deterioration of swallowing.
Ackerstaff et al. [22] demonstrated improved oral intake postradiotherapy and/or chemotherapy. Stenson et al. [70] stated that weight remained unchanged after treatment (via oral route), whereas Nourissat et al. [62] described a mean weight loss of 2.2 kg posttreatment.
3.3. QoL
Twenty-five studies [22, 24, 25, 27–29, 31, 32, 35, 36, 42, 44, 47, 51, 53, 55, 59, 62, 64, 68, 72, 76–79] described the short- and long-term effects of treatment for HNC on patients' general QoL. The European Organization for Research and Treatment of Cancer (EORTC) C30-questionnaire was used in fifteen studies [22, 24, 25, 27, 31, 32, 35, 42, 47, 55, 62, 64, 77–79] and the more HNC specific EORTC H&N35 was used in thirteen studies [22, 24, 25, 31, 32, 35, 36, 42, 47, 64, 77–79]. Other questionnaires that were used included the University of Washington QoL Questionnaire (UWQoL) [53, 68, 72, 76], the Head and Neck QoL or HNQoL [53, 72], and the Xerostomia related QoL or XQoL [68, 72]. Follow-up time for QoL was up to six years after treatment [35], including patients with tumors that were early staged and patients with advanced tumors.
Although three studies [28, 72, 77] demonstrated improvements in QoL, four studies [22, 36, 42, 54] reported a decrease in general QoL as a result of radiotherapy and/or chemotherapy. Bansal et al. [27] found a significant decline in physical, social, and emotional functioning as well as in global health scores following a course of radiotherapy. However, the patients' functional scores improved one month after treatment but did not reach pretreatment levels. The health-related QoL (HRQoL) scores of the majority of patients in the Bottomley et al. [78] study returned to baseline at 48-month follow-up. These findings support the findings of Ackerstaff et al. [22], Cohen et al. [51], Karlsson et al. [32], List et al. [59], and Wilson et al. [76], who suggested that HRQoL deteriorates significantly immediately after treatment, with variable degrees of improvement 3–72 months after treatment.
3.4. Reported (Efficacy of) Speech Pathology Interventions
We assessed the speech pathology interventions against the following criteria: (a) whether a detailed description of the intervention was provided; (b) whether the authors provided a description of treatment duration and intensity; and (c) what the speech pathology intervention outcomes were. The reported efficacy of 14 speech pathology intervention studies aimed at addressing problems in dysphagia, speech, voice, and trismus is summarised in Table 4.
Table 4.
Overview of speech pathology interventions aimed at addressing problems in dysphagia, speech, voice, and trismus (n = 14).
| Reference | Topic | General description of intervention and treatment intensity/duration | Description of specific exercises | Conclusions specific to therapy |
|---|---|---|---|---|
| Agarwal et al. 2009 [23] |
Voice | All patients received counseling and voice therapy by a trained speech pathologist No specific data provided on treatment frequency/intensity |
No description of exercises provided | Forty-seven of 50 patients showed compliance to the therapy. No specific conclusions of influence of provided therapy on primary outcomes described |
|
| ||||
| Akst et al. 2004 [46] |
Swallowing | Swallowing evaluation and intervention when clinically indicated | No description of exercises provided | No specific conclusions of influence of provided therapy on primary outcomes described |
|
| ||||
| Buchbinder et al. 1993 [48] |
Trismus | Six to 10 exercise sessions per day for a 10-week period | Group 1: unassisted exercises: reach maximum MIO and closing, jaw motion to left, right, and protrusively Group 2: unassisted exercises: reach maximum MIO and closing, jaw motion to left, right, and protrusively. Stacked tongue depressors, to mechanically increase MIO (5 × 30 seconds per session) Group 3: unassisted exercises: reach maximum MIO and closing, jaw motion to left, right, and protrusively. Combined with the TheraBite System (5 × 30 seconds per session) |
The first four weeks no differences between groups were found. After week 4 minimal improvements in groups 1 and 2 were found and group 3 still improved. The highest increment in MIO was reached in group 3 |
|
| ||||
| Dijkstra et al. 2007 [52] |
Trismus | Physical therapy for trismus, median of 4 sessions | Physical therapy consisting of (i) Active range of motion (ii) Hold and relax (iii) Manual stretching (iv) Joint distraction Following therapeutic tools are used in described cohort: (i) Rubber plugs (ii) Tong blades (iii) Dynamic bite opener (iv) TheraBite System |
MIO increases significantly after physical therapy. History of HNC decreases the effect of physical therapy, compared to other trismus patients |
|
| ||||
| Frowen et al. 2010 [16] |
Swallowing | All patients were seen by a speech pathologist as an aspect of regular care | No description of exercises provided | No specific conclusions of influence of provided therapy on primary outcomes described |
|
| ||||
| Hutcheson et al. 2014 [56] |
Swallowing | All patients received prophylactic swallowing therapy to avoid nothing by mouth periods during treatment No specific data provided on treatment frequency/intensity |
Targeted swallowing exercises | No specific conclusions of influence of provided therapy on primary outcomes described |
|
| ||||
| Karlsson et al. 2015 [31] |
Voice | Group 1: voice therapy group received 10 × 30-minute sessions over 10 weeks Group 2: vocal hygiene group: 1 session for vocal hygiene advice |
Group 1: voice therapy consisting of relaxation, respiration, posture, and phonation exercises Group 2: vocal hygiene advice |
Patients treated with voice therapy experienced greater improvements compared to patients that only received vocal hygiene advice. Group 1 showed a significant better functional communication and HRQoL |
|
| ||||
| Kotz et al. 2012 [57] |
Swallowing | Group 1: weekly treatment by speech pathologist and daily 3 × 10 home sessions of exercises. Group 2: swallowing assessment and treatment if necessary after treatment | Group 1: prophylactic swallowing therapy consisting of effortful swallow, tongue base retraction exercises, super supraglottic swallow, and the Mendelssohn maneuver Group 2: control group only receive symptomatic dysphagia treatment |
Prophylactic swallowing therapy improves swallowing at 3 and 6 months; later there were no group differences found |
|
| ||||
| Kraaijenga et al. 2014 [35] |
Swallowing and voice | Daily practice from the start of the treatment until 1 year after treatment | Two combined groups: TheraBite System and standard logopedic swallowing exercises (the same cohort as van der Molen et al. 2011 [73]) | Minimal voice and swallowing difficulties were found 60 months after treatment in patients treated with prophylactic swallowing exercises |
|
| ||||
| Sanguineti et al. 2014 [40] |
Voice | 75.8% of the patients received speech therapy. No therapy was provided to 30 patients No specific data provided on treatment frequency/intensity |
No description of exercises provided | No specific conclusions of influence of provided therapy on primary outcomes described |
|
| ||||
| Starmer et al. 2014 [69] |
Swallowing | Patients received prophylactic swallowing and trismus exercises | No description of exercises provided | No specific conclusions of influence of provided therapy on primary outcomes described |
|
| ||||
| van der Molen et al. 2011 [73] |
Swallowing | Patients received instructions in advance of their oncological treatment. Three times daily exercises | Group 1: range-of-motion exercises and three strengthening exercises, that is, the effortful swallow, the Masako maneuver, and the super supraglottic swallow. Stretch holding for 10–30 seconds at a point of mild discomfort Group 2: stretch of the mouth using the TheraBite System and a strengthening exercise: swallowing with the tongue elevated to the palate while maintaining mouth opening at 50% of its maximum. Stretch holding for 10–30 seconds at a point of mild discomfort |
Similar outcomes in both groups were found. Preventive rehabilitation can improve early posttreatment functional outcomes |
|
| ||||
| van der Molen et al. 2012 [44] |
Voice | No specific speech or voice therapy | N/A | N/A |
|
| ||||
| van der Molen et al. 2013 [74] |
Swallowing and trismus | Study was aimed at describing dose-effect relationships in two treatment groups described in earlier study. References to other published study where treatment regime is described | Group 1: standard exercises Group 2: experimental exercises |
Any possible difference between the two included treatment groups is not described, nor possible influence of the respective treatments |
HNC: head and neck cancer; HRQoL: health-related quality of life; MIO: maximum incisal opening; ROM: range of motion.
Of the 60 articles included in this review, 14 studies [16, 23, 29, 31, 35, 40, 44, 46, 48, 56, 57, 69, 73, 74] reported whether there was any treatment for the sequelae of radiotherapy and/or chemotherapy. Of these intervention studies, five focused on voice-related problems [23, 31, 35, 40, 44], two focused on trismus [48, 52], seven focused on swallowing disorders [16, 35, 46, 56, 57, 69, 73], and one study reported on both swallowing disorders and trismus [74].
The three studies that investigated the treatment of trismus [48, 52, 74] presented the most detailed information on what the interventions involved. The study by Dijkstra et al. [52] described a wide variety of trismus-specific therapies, suggesting that most patients received a combination of therapies. The patients in the van der Molen et al. [44] and Kraaijenga et al. [35] studies did not receive any speech therapy. The remainder of the studies reported that patients received speech therapy; however, most of these studies did not provide specific data on treatment duration or intensity. None of the voice-related studies provided information on the specific exercises prescribed to patients except Karlsson et al. [31].
Of the eight studies on swallowing disorders, only Kotz et al. [57] and van der Molen et al. [73] described the prescribed exercises in detail. The aim of the latter study was to compare the effectiveness of experimental rehabilitation to standard rehabilitation in 49 advanced HNC patients. The authors concluded that preventive rehabilitation is feasible and effective in reducing the extent and/or severity of various functional short-term effects of chemoradiotherapy [74]. This finding is supported by the 6-year follow-up study by Kraaijenga et al. [35]. Kotz et al. [57] described a temporary improvement. These are the only studies that provided detailed information about the speech pathology intervention and reported on the effectiveness of the intervention.
4. Discussion
In total, 60 studies met the inclusion criteria. The studies described the effects of radiotherapy and/or chemotherapy on the functions of the upper aerodigestive tract in patients with HNC. The articles yielded by this systematic review vary in their findings regarding tumor characteristics and treatment modalities. As a result of this variability, no statistical pooling was possible. We also set out to investigate the involvement of speech pathologists in treating patients with HNC.
When considering treatment outcomes, voice quality worsened at the start of radiotherapy and/or chemotherapy but eventually improved after therapy finished. Dysphagia can be a major side effect of HNC and its treatment. The high incidence of dysphagia in this study population can cause serious secondary consequences, such as malnutrition, dehydration, an increased risk of aspiration, and, at worst, death [80]. As dysphagia is a common sequela to oncological treatment, early detection and treatment are needed to avoid or minimise serious secondary complications [81].
The general description of the study population in Table 3 shows that there was great variability in both the location of the tumor and the grading/staging, making comparisons of these studies difficult. As the follow-up times varied in each study, the outcomes may be noncomparable. Thus, this review shows that there is a need for more standardised approaches to research in this field.
Additionally, a large range of outcome measures were used, some of which are not validated. This calls into question the reliability of results reported in some of the studies. The use of validated and standardised assessments in future research would provide more robust findings.
When considering the functional outcomes of radiotherapy and/or chemotherapy, one of the most important factors is whether the patient had received voice or swallowing therapy. Interestingly, only 14 of the 60 included studies reported whether the patients received any speech therapy. Thus, in 46 articles functional results, such as voice quality, are presented with no specification of whether the patient received therapy. As some of these studies have a follow-up of >2 years, it is fair to assume that patients sought help for voice or swallowing problems. Therefore, the involvement of speech therapy may be underreported, suggesting that the presented outcomes in these studies are biased and raise questions about their reliability.
When information was provided about treatment, only six articles [31, 48, 52, 57, 73, 74] described in detail the treatment intensity as the actual treatment. Furthermore, these studies are the only five that include conclusions about the efficacy of speech therapy in this specific population. In the context of EBP, this finding demonstrates the need for more research into the efficacy of speech pathology interventions for patients with HNC receiving radiotherapy and/or chemotherapy.
To enable the objective reporting of the effectiveness of radiation and/or chemotherapy, baseline measurements of different aspects of voice quality and swallowing are required. To manage expectations, healthcare professionals and patients need to be made aware that some aspects of both voice and swallowing commonly do not recover to the level prior to the oncological intervention [16]. Regarding effectiveness of voice treatments, the following multidimensional assessment is recommended [82]: a videolaryngostroboscopy recording of the laryngeal structures and the vocal fold vibration; an acoustic and a perceptual analysis of voice; a voice-related questionnaire on QoL (e.g., the Voice Handicap Index) [83]; and a functional health status questionnaire. Such a protocol would be in line with the recommendations for functional assessment of voice pathology as described by the Committee on Phoniatrics of the European Laryngological Society [84].
When describing aspects of swallowing function, both fiber optic endoscopic evaluation of swallowing and videofluoroscopy are considered to be the gold standard in dysphagia assessment [85]. In addition to these tools, questionnaires on HRQoL and functional health status are recommended and should be integrated in the overall swallowing assessment protocol. Repeated measurements of outcome measures should be performed in order to monitor any side effects of the oncological intervention, to detect spontaneous recovery, and to measure the effects of the speech pathology interventions. Apart from baseline measurements, posttreatment and follow-up measurements should be used to monitor functional and QoL outcomes.
Additional research is needed to develop clinical practice guidelines to support evidence-based practice in the area of dysphagia, speech, voice, and trismus following radiotherapy and/or chemotherapy in patients with head and neck carcinoma. These practice guidelines should bring together the best available current evidence within a specific clinical area, formulating evidence-based recommendations for clinicians and present choices between different interventions that have an impact on health and use of resources [86]. This systematic review summarised the effects of radiotherapy and/or chemotherapy on the function of the upper aerodigestive tract in patients with head and neck cancer. However, because of the marked variation in treatment protocols and patient characteristics, outcome data from the included studies cannot be easily generalised. Recommendations for future studies advocate the use of a multidimensional assessment protocol, using well-validated measures and standardised pretreatment, posttreatment, and follow-up measurements, thus allowing for future meta-analysis of homogeneous outcomes.
5. Conclusion
The studies included in this systematic review described a wide variety of outcomes in patients with HNC following radiotherapy and/or chemotherapy. The findings about the long-term functional implications of radiotherapy and/or chemotherapy in patients with HNC are inconclusive as a result of the wide range of outcome measures used and the possible influence of underreported speech therapy.
Future researchers need to consider targeting more homogeneous groups using standardised treatment protocols to improve the treatment outcomes, thereby decreasing the side effects of the oncological treatments. Findings of these studies need to inform the decision-making process in the treatment of HNC so complications can be better predicted with due consideration of the possible negative side effects to the upper aerodigestive tract. Although the main objective of most studies was to determine curing rates, the importance of the functional implications of the side effects of oncology treatments should not be overlooked, particularly their impact on QoL. Finally, more research is needed to gain a full understanding of the complexity and variety in the effects of radiotherapy and/or chemotherapy on the functions of the upper aerodigestive tract following HNC.
Competing Interests
All authors declare no competing interests or financial disclosures.
References
- 1.Kreeft A. M., Van Der Molen L., Hilgers F. J., Balm A. J. Speech and swallowing after surgical treatment of advanced oral and oropharyngeal carcinoma: a systematic review of the literature. European Archives of Oto-Rhino-Laryngology. 2009;266(11):1687–1698. doi: 10.1007/s00405-009-1089-2. [DOI] [PubMed] [Google Scholar]
- 2.Rogers S. N., Ahad S. A., Murphy A. P. A structured review and theme analysis of papers published on ‘quality of life’ in head and neck cancer: 2000–2005. Oral Oncology. 2007;43(9):843–868. doi: 10.1016/j.oraloncology.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Takes R. P., Strojan P., Silver C. E., et al. Current trends in initial management of hypopharyngeal cancer: the declining use of open surgery. Head and Neck. 2012;34(2):270–281. doi: 10.1002/hed.21613. [DOI] [PubMed] [Google Scholar]
- 4.Nund R. L., Ward E. C., Scarinci N. A., Cartmill B., Kuipers P., Porceddu S. V. Survivors' experiences of dysphagia-related services following head and neck cancer: implications for clinical practice. International Journal of Language & Communication Disorders. 2014;49(3):354–363. doi: 10.1111/1460-6984.12071. [DOI] [PubMed] [Google Scholar]
- 5.Nund R. L., Ward E. C., Scarinci N. A., Cartmill B., Kuipers P., Porceddu S. V. Carers' experiences of dysphagia in people treated for head and neck cancer: a qualitative study. Dysphagia. 2014;29(4):450–458. doi: 10.1007/s00455-014-9527-8. [DOI] [PubMed] [Google Scholar]
- 6.Krisciunas G. P., Sokoloff W., Stepas K., Langmore S. E. Survey of usual practice: dysphagia therapy in head and neck cancer patients. Dysphagia. 2012;27(4):538–549. doi: 10.1007/s00455-012-9404-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Speech Pathology Australia. Evidence-Based Practice in Speech Pathology. 2010. http://www.speechpathologyaustralia.org.au/library/position_statements/EBP_in_SP.pdf. [Google Scholar]
- 8.American Speech-Language-Hearing Association (ASHA) Evidence-Based Practice in Communication Disorders: Position Statement. 2005. [Google Scholar]
- 9.Miao M., Power E., O'Halloran R. Factors affecting speech pathologists' implementation of stroke management guidelines: a thematic analysis. Disability and Rehabilitation. 2015;37(8):674–685. doi: 10.3109/09638288.2014.932444. [DOI] [PubMed] [Google Scholar]
- 10.Frowen J. J., Perry A. R. Swallowing outcomes after radiotherapy for head and neck cancer: a systematic review. Head and Neck. 2006;28(10):932–944. doi: 10.1002/hed.20438. [DOI] [PubMed] [Google Scholar]
- 11.Jacobi I., Van Der Molen L., Huiskens H., Van Rossum M. A., Hilgers F. J. M. Voice and speech outcomes of chemoradiation for advanced head and neck cancer: a systematic review. European Archives of Oto-Rhino-Laryngology. 2010;267(10):1495–1505. doi: 10.1007/s00405-010-1316-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Der Molen L., Van Rossum M. A., Burkhead L. M., Smeele L. E., Hilgers F. J. M. Functional outcomes and rehabilitation strategies in patients treated with chemoradiotherapy for advanced head and neck cancer: a systematic review. European Archives of Oto-Rhino-Laryngology. 2009;266(6):889–900. doi: 10.1007/s00405-008-0817-3. [DOI] [PubMed] [Google Scholar]
- 13.Paleri V., Roe J. W. G., Strojan P., et al. Strategies to reduce long-term postchemoradiation dysphagia in patients with head and neck cancer: an evidence-based review. Head and Neck. 2014;36(3):431–433. doi: 10.1002/hed.23251. [DOI] [PubMed] [Google Scholar]
- 14.Roe J. W. G., Carding P. N., Dwivedi R. C., et al. Swallowing outcomes following Intensity Modulated Radiation Therapy (IMRT) for head & neck cancer—a systematic review. Oral Oncology. 2010;46(10):727–733. doi: 10.1016/j.oraloncology.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 15.Klein J., Livergant J., Ringash J. Health related quality of life in head and neck cancer treated with radiation therapy with or without chemotherapy: a systematic review. Oral Oncology. 2014;50(4):254–262. doi: 10.1016/j.oraloncology.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Frowen J., Cotton S., Corry J., Perry A. Impact of demographics, tumor characteristics, and treatment factors on swallowing after (chemo)radiotherapy for head and neck cancer. Head and Neck. 2010;32(4):513–528. doi: 10.1002/hed.21218. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A., Altman D. G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine. 2009;6(7) doi: 10.1371/journal.pmed.1000100.e1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kmet L. M., Lee R. C., Cook L. S. Standard Quality Assessment Criteria for Evaluating Primary Research Papers from a Variety of Fields. 13. HTA Initiative; 2004. [Google Scholar]
- 19.NHMRC. A Guide to the Development, Implementation and Evaluation of Clinical Practice Guidelines. 1999. [Google Scholar]
- 20.Moher D., Liberati A., Tetzlaff J., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. 2009;6(7) doi: 10.1371/journal.pmed.1000097.e1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aaltonen L.-M., Rautiainen N., Sellman J., et al. Voice quality after treatment of early vocal cord cancer: a randomized trial comparing laser surgery with radiation therapy. International Journal of Radiation Oncology Biology Physics. 2014;90(2):255–260. doi: 10.1016/j.ijrobp.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 22.Ackerstaff A. H., Balm A. J. M., Rasch C. R. N., et al. First-year quality of life assessment of an intraarterial (RADPLAT) versus intravenous chemoradiation phase III trial. Head and Neck. 2009;31(1):77–84. doi: 10.1002/hed.20937. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal J. P., Baccher G. K., Waghmare C. M., et al. Factors affecting the quality of voice in the early glottic cancer treated with radiotherapy. Radiotherapy and Oncology. 2009;90(2):177–182. doi: 10.1016/j.radonc.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Al-Mamgani A., Tans L., Van Rooij P., Levendag P. C. A single-institutional experience of 15 years of treating T3 laryngeal cancer with primary radiotherapy, with or without chemotherapy. International Journal of Radiation Oncology Biology Physics. 2012;83(3):1000–1006. doi: 10.1016/j.ijrobp.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 25.Al-Mamgani A., van Rooij P. H., Woutersen D. P., et al. Radiotherapy for T1-2N0 glottic cancer: a multivariate analysis of predictive factors for the long-term outcome in 1050 patients and a prospective assessment of quality of life and voice handicap index in a subset of 233 patients. Clinical Otolaryngology. 2013;38(4):306–312. doi: 10.1111/coa.12139. [DOI] [PubMed] [Google Scholar]
- 26.Al-Mamgani A., Kwa S. L. S., Tans L., et al. Single vocal cord irradiation: image guided intensity modulated hypofractionated radiation therapy for T1a glottic cancer: early clinical results. International Journal of Radiation Oncology Biology Physics. 2015;93(2):337–343. doi: 10.1016/j.ijrobp.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Bansal M., Mohanti B. K., Shah N., Chaudhry R., Bahadur S., Shukla N. K. Radiation related morbidities and their impact on quality of life in head and neck cancer patients receiving radical radiotherapy. Quality of Life Research. 2004;13(2):481–488. doi: 10.1023/B:QURE.0000018491.80646.bc. [DOI] [PubMed] [Google Scholar]
- 28.Bibby J. R. L., Cotton S. M., Perry A., Corry J. F. Voice outcomes after radiotherapy treatment for early glottic cancer: assessment using multidimensional tools. Head and Neck. 2008;30(5):600–610. doi: 10.1002/hed.20750. [DOI] [PubMed] [Google Scholar]
- 29.Dornfeld K., Simmons J. R., Karnell L., et al. Radiation doses to structures within and adjacent to the larynx are correlated with long-term diet- and speech-related quality of life. International Journal of Radiation Oncology Biology Physics. 2007;68(3):750–757. doi: 10.1016/j.ijrobp.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 30.Jacobi I., Navran A., van der Molen L., Heemsbergen W. D., Hilgers F. J. M., van den Brekel M. W. M. Radiation dose to the tongue and velopharynx predicts acoustic-articulatory changes after chemo-IMRT treatment for advanced head and neck cancer. European Archives of Oto-Rhino-Laryngology. 2016;273(2):487–494. doi: 10.1007/s00405-015-3526-8. [DOI] [PubMed] [Google Scholar]
- 31.Karlsson T., Johansson M., Andréll P., Finizia C. Effects of voice rehabilitation on health-related quality of life, communication and voice in laryngeal cancer patients treated with radiotherapy: a randomised controlled trial. Acta Oncologica. 2015;54(7):1017–1024. doi: 10.3109/0284186x.2014.995773. [DOI] [PubMed] [Google Scholar]
- 32.Karlsson T., Bergström L., Ward E., Finizia C. A prospective longitudinal study of voice characteristics and health-related quality of life outcomes following laryngeal cancer treatment with radiotherapy. Acta Oncologica. 2016;55(6):693–699. doi: 10.3109/0284186x.2016.1150604. [DOI] [PubMed] [Google Scholar]
- 33.Kazi R., Venkitaraman R., Johnson C., et al. Electroglottographic comparison of voice outcomes in patients with advanced laryngopharyngeal cancer treated by chemoradiotherapy or total laryngectomy. International Journal of Radiation Oncology, Biology, Physics. 2008;70(2):344–352. doi: 10.1016/j.ijrobp.2007.06.040. [DOI] [PubMed] [Google Scholar]
- 34.Kerr P., Myers C. L., Butler J., Alessa M., Lambert P., Cooke A. L. Prospective functional outcomes in sequential population based cohorts of stage III/ IV oropharyngeal carcinoma patients treated with 3D conformal vs. intensity modulated radiotherapy. Journal of Otolaryngology—Head and Neck Surgery. 2015;44, article 17 doi: 10.1186/s40463-015-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kraaijenga S. A. C., van der Molen L., Jacobi I., Hamming-Vrieze O., Hilgers F. J. M., van den Brekel M. W. M. Prospective clinical study on long-term swallowing function and voice quality in advanced head and neck cancer patients treated with concurrent chemoradiotherapy and preventive swallowing exercises. European Archives of Oto-Rhino-Laryngology. 2014;272(11):3521–3531. doi: 10.1007/s00405-014-3379-6. [DOI] [PubMed] [Google Scholar]
- 36.Lazarus C. L., Husaini H., Hu K., et al. Functional outcomes and quality of life after chemoradiotherapy: baseline and 3 and 6 months post-treatment. Dysphagia. 2014;29(3):365–375. doi: 10.1007/s00455-014-9519-8. [DOI] [PubMed] [Google Scholar]
- 37.Mittal B. B., Kepka A., Mahadevan A., et al. Tissue/dose compensation to reduce toxicity from combined radiation and chemotherapy for advanced head and neck cancers. International Journal of Cancer. 2001;96:61–70. doi: 10.1002/ijc.10360. [DOI] [PubMed] [Google Scholar]
- 38.Niedzielska G., Niedzielski A., Toman D. Voice after radiotherapy of the larynx carcinoma. Radiotherapy and Oncology. 2010;97(2):276–280. doi: 10.1016/j.radonc.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 39.Remmelts A. J., Hoebers F. J. P., Klop W. M. C., Balm A. J. M., Hamming-Vrieze O., Van Den Brekel M. W. M. Evaluation of lasersurgery and radiotherapy as treatment modalities in early stage laryngeal carcinoma: tumour outcome and quality of voice. European Archives of Oto-Rhino-Laryngology. 2013;270(7):2079–2087. doi: 10.1007/s00405-013-2460-x. [DOI] [PubMed] [Google Scholar]
- 40.Sanguineti G., Ricchetti F., McNutt T., Wu B., Fiorino C. Dosimetric predictors of dysphonia after intensity-modulated radiotherapy for oropharyngeal carcinoma. Clinical Oncology. 2014;26(1):32–38. doi: 10.1016/j.clon.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Spector J. G., Sessions D. G., Chao K. S., et al. Stage I (t1 N0 M0) squamous cell carcinoma of the laryngeal glottis: therapeutic results and voice preservation. Head & Neck. 1999;21(8):707–717. doi: 10.1002/(sici)1097-0347(199912)21:8<707::aid-hed5>3.3.co;2-u. [DOI] [PubMed] [Google Scholar]
- 42.Tuomi L., Karlsson T., Johansson M., Finizia C. Health-related quality of life and voice following radiotherapy for laryngeal cancer-a comparison between glottic and supraglottic tumours. Acta Oncologica. 2015;54(1):73–79. doi: 10.3109/0284186X.2014.925576. [DOI] [PubMed] [Google Scholar]
- 43.Verdonck-de Leeuw I. M., Keus R. B., Hilgers F. J. M., et al. Consequences of voice impairment in daily life for patients following radiotherapy for early glottic cancer: voice quality, vocal function, and vocal performance. International Journal of Radiation Oncology, Biology, Physics. 1999;44(5):1071–1078. doi: 10.1016/s0360-3016(99)00110-8. [DOI] [PubMed] [Google Scholar]
- 44.van der Molen L., Van Rossum M. A., Jacobi I., et al. Pre- and posttreatment voice and speech outcomes in patients with advanced head and neck cancer treated with chemoradiotherapy: expert listeners' and patient's perception. Journal of Voice. 2012;26(5):664.e25–664.e33. doi: 10.1016/j.jvoice.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 45.Agarwal J., Palwe V., Dutta D., et al. Objective assessment of swallowing function after definitive concurrent (Chemo)radiotherapy in patients with head and neck cancer. Dysphagia. 2011;26(4):399–406. doi: 10.1007/s00455-011-9326-4. [DOI] [PubMed] [Google Scholar]
- 46.Akst L. M., Chan J., Elson P., Saxton J., Strome M., Adelstein D. Functional outcomes following chemoradiotherapy for head and neck cancer. Otolaryngology—Head and Neck Surgery. 2004;131(6):950–957. doi: 10.1016/j.otohns.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 47.Al-Mamgani A., Mehilal R., Van Rooij P. H., Tans L., Sewnaik A., Levendag P. C. Toxicity, quality of life, and functional outcomes of 176 hypopharyngeal cancer patients treated by (Chemo)radiation: the impact of treatment modality and radiation technique. The Laryngoscope. 2012;122(8):1789–1795. doi: 10.1002/lary.23387. [DOI] [PubMed] [Google Scholar]
- 48.Buchbinder D., Currivan R. B., Kaplan A. J., Urken M. L. Mobilization regimens for the prevention of jaw hypomobility in the radiated patient: a comparison of three techniques. Journal of Oral and Maxillofacial Surgery. 1993;51(8):863–867. doi: 10.1016/s0278-2391(10)80104-1. [DOI] [PubMed] [Google Scholar]
- 49.Caudell J. J., Schaner P. E., Desmond R. A., Meredith R. F., Spencer S. A., Bonner J. A. Dosimetric factors associated with long-term dysphagia after definitive radiotherapy for squamous cell carcinoma of the head and neck. International Journal of Radiation Oncology, Biology, Physics. 2010;76(2):403–409. doi: 10.1016/j.ijrobp.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 50.Christianen M. E. M. C., Verdonck-De Leeuw I. M., Doornaert P., et al. Patterns of long-term swallowing dysfunction after definitive radiotherapy or chemoradiation. Radiotherapy and Oncology. 2015;117(1):139–144. doi: 10.1016/j.radonc.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 51.Cohen E. E. W., Haraf D. J., List M. A., et al. High survival and organ function rates after primary chemoradiotherapy for intermediate-stage squamous cell carcinoma of the head and neck treated in a multicenter phase II trial. Journal of Clinical Oncology. 2006;24(21):3438–3444. doi: 10.1200/JCO.2006.05.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dijkstra P. U., Sterken M. W., Pater R., Spijkervet F. K. L., Roodenburg J. L. N. Exercise therapy for trismus in head and neck cancer. Oral Oncology. 2007;43(4):389–394. doi: 10.1016/j.oraloncology.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Feng F. Y., Kim H. M., Lyden T. H., et al. Intensity-modulated radiotherapy of head and neck cancer aiming to reduce dysphagia: early dose-effect relationships for the swallowing structures. International Journal of Radiation Oncology Biology Physics. 2007;68(5):1289–1298. doi: 10.1016/j.ijrobp.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 54.Feng F. Y., Kim H. M., Lyden T. H., et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. Journal of Clinical Oncology. 2010;28(16):2732–2738. doi: 10.1200/jco.2009.24.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haderlein M., Semrau S., Ott O., Speer S., Bohr C., Fietkau R. Dose-dependent deterioration of swallowing function after induction chemotherapy and definitive chemoradiotherapy for laryngopharyngeal cancer. Strahlentherapie und Onkologie. 2014;190(2):192–198. doi: 10.1007/s00066-013-0493-0. [DOI] [PubMed] [Google Scholar]
- 56.Hutcheson K. A., Lewin J. S., Holsinger F. C., et al. Long-term functional and survival outcomes after induction chemotherapy and risk-based definitive therapy for locally advanced squamous cell carcinoma of the head and neck. Head and Neck. 2014;36(4):474–480. doi: 10.1002/hed.23330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kotz T., Federman A. D., Kao J., et al. Prophylactic swallowing exercises in patients with head and neck cancer undergoing chemoradiation: a randomized trial. Archives of Otolaryngology—Head and Neck Surgery. 2012;138(4):376–382. doi: 10.1001/archoto.2012.187. [DOI] [PubMed] [Google Scholar]
- 58.Kumar R., Madanikia S., Starmer H., et al. Radiation dose to the floor of mouth muscles predicts swallowing complications following chemoradiation in oropharyngeal squamous cell carcinoma. Oral Oncology. 2014;50(1):65–70. doi: 10.1016/j.oraloncology.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 59.List M. A., Siston A., Haraf D., et al. Quality of life and performance in advanced head and neck cancer patients on concomitant chemoradiotherapy: a prospective examination. Journal of Clinical Oncology. 1999;17(3):1020–1028. doi: 10.1200/JCO.1999.17.3.1020. [DOI] [PubMed] [Google Scholar]
- 60.McLaughlin B. T., Gokhale A. S., Shuai Y., et al. Management of patients treated with chemoradiotherapy for head and neck cancer without prophylactic feeding tubes: the University of Pittsburgh experience. The Laryngoscope. 2010;120(1):71–75. doi: 10.1002/lary.20697. [DOI] [PubMed] [Google Scholar]
- 61.Murry T., Madasu R., Martin A., Robbins K. T. Acute and chronic changes in swallowing and quality of life following intraarterial chemoradiation for organ preservation in patients with advanced head and neck cancer. Head and Neck. 1998;20(1):31–37. doi: 10.1002/(sici)1097-0347(199801)20:1<31::aid-hed6>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 62.Nourissat A., Bairati I., Samson E., et al. Predictors of weight loss during radiotherapy in patients with stage I or II head and neck cancer. Cancer. 2010;116(9):2275–2283. doi: 10.1002/cncr.25041. [DOI] [PubMed] [Google Scholar]
- 63.Ottosson S., Lindblom U., Wahlberg P., et al. Weight loss and body mass index in relation to aspiration in patients treated for head and neck cancer: a long-term follow-up. Supportive Care in Cancer. 2014;22(9):2361–2369. doi: 10.1007/s00520-014-2211-6. [DOI] [PubMed] [Google Scholar]
- 64.Pauli N., Johnson J., Finizia C., Andréll P. The incidence of trismus and long-term impact on health-related quality of life in patients with head and neck cancer. Acta Oncologica. 2013;52(6):1137–1145. doi: 10.3109/0284186X.2012.744466. [DOI] [PubMed] [Google Scholar]
- 65.Pauloski B. R., Rademaker A. W., Logemann J. A., et al. Relationship between swallow motility disorders on videofluorography and oral intake in patients treated for head and neck cancer with radiotherapy with or without chemotherapy. Head and Neck. 2006;28(12):1069–1076. doi: 10.1002/hed.20459. [DOI] [PubMed] [Google Scholar]
- 66.Rademaker A. W., Vonesh E. F., Logemann J. A., et al. Eating ability in head and neck cancer patients after treatment with chemoradiation: a 12-month follow-up study accounting for dropout. Head and Neck. 2003;25(12):1034–1041. doi: 10.1002/hed.10317. [DOI] [PubMed] [Google Scholar]
- 67.Salama J. K., Stenson K. M., List M. A., et al. Characteristics associated with swallowing changes after concurrent chemotherapy and radiotherapy in patients with head and neck cancer. Archives of Otolaryngology—Head and Neck Surgery. 2008;134(10):1060–1065. doi: 10.1001/archotol.134.10.1060. [DOI] [PubMed] [Google Scholar]
- 68.Scrimger R., Kanji A., Parliament M., et al. Correlation between saliva production and quality of life measurements in head and neck cancer patients treated with intensity-modulated radiotherapy. American Journal of Clinical Oncology. 2007;30(3):271–277. doi: 10.1097/01.coc.0000258081.70643.3d. [DOI] [PubMed] [Google Scholar]
- 69.Starmer H. M., Tippett D., Webster K., et al. Swallowing outcomes in patients with oropharyngeal cancer undergoing organ-preservation treatment. Head and Neck. 2014;36(10):1392–1397. doi: 10.1002/hed.23465. [DOI] [PubMed] [Google Scholar]
- 70.Stenson K. M., Kunnavakkam R., Cohen E. E. W., et al. Chemoradiation for patients with advanced oral cavity cancer. The Laryngoscope. 2010;120(1):93–99. doi: 10.1002/lary.20716. [DOI] [PubMed] [Google Scholar]
- 71.Strigari L., Benassi M., Arcangeli G., Bruzzaniti V., Giovinazzo G., Marucci L. A novel dose constraint to reduce xerostomia in head-and-neck cancer patients treated with intensity-modulated radiotherapy. International Journal of Radiation Oncology Biology Physics. 2010;77(1):269–276. doi: 10.1016/j.ijrobp.2009.07.1734. [DOI] [PubMed] [Google Scholar]
- 72.Vainshtein J. M., Moon D. H., Feng F. Y., Chepeha D. B., Eisbruch A., Stenmark M. H. Long-term quality of life after swallowing and salivary-sparing chemo-intensity modulated radiation therapy in survivors of human papillomavirus-related oropharyngeal cancer. International Journal of Radiation Oncology Biology Physics. 2015;91(5):925–933. doi: 10.1016/j.ijrobp.2014.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van der Molen L., van Rossum M. A., Burkhead L. M., Smeele L. E., Rasch C. R. N., Hilgers F. J. M. A randomized preventive rehabilitation trial in advanced head and neck cancer patients treated with chemoradiotherapy: feasibility, compliance, and short-term effects. Dysphagia. 2011;26(2):155–170. doi: 10.1007/s00455-010-9288-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Molen L., Heemsbergen W. D., De Jong R., et al. Dysphagia and trismus after concomitant chemo-Intensity-Modulated Radiation Therapy (chemo-IMRT) in advanced head and neck cancer; dose–effect relationships for swallowing and mastication structures. Radiotherapy and Oncology. 2013;106(3):364–369. doi: 10.1016/j.radonc.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 75.Vlacich G., Spratt D. E., Diaz R., et al. Dose to the inferior pharyngeal constrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiotherapy and Oncology. 2014;110(3):435–440. doi: 10.1016/j.radonc.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 76.Wilson J. A., Carding P. N., Patterson J. M. Dysphagia after nonsurgical head and neck cancer treatment: patients' perspectives. Otolaryngology—Head and Neck Surgery. 2011;145(5):767–771. doi: 10.1177/0194599811414506. [DOI] [PubMed] [Google Scholar]
- 77.Urdaniz J. I. A., De La Vega F. A., Burgaleta A. M., et al. Quality of life in patients with locally advanced head and neck cancer treated with chemoradiotherapy. Comparison of two protocols using the EORTC questionnaires (QLQ-C30, H and N35) Clinical and Translational Oncology. 2005;7(9):398–403. doi: 10.1007/bf02716585. [DOI] [PubMed] [Google Scholar]
- 78.Bottomley A., Tridello G., Coens C., et al. An international phase 3 trial in head and neck cancer: quality of life and symptom results: EORTC 24954 on behalf of the EORTC head and neck and the EORTC radiation oncology group. Cancer. 2014;120(3):390–398. doi: 10.1002/cncr.28392. [DOI] [PubMed] [Google Scholar]
- 79.Verdonck-De Leeuw I. M., Buffart L. M., Heymans M. W., et al. The course of health-related quality of life in head and neck cancer patients treated with chemoradiation: A Prospective Cohort Study. Radiotherapy and Oncology. 2014;110(3):422–428. doi: 10.1016/j.radonc.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 80.Langendijk J. A., Doornaert P., Verdonck-de Leeuw I. M., Leemans C. R., Aaronson N. K., Slotman B. J. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. Journal of Clinical Oncology. 2008;26(22):3770–3776. doi: 10.1200/jco.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 81.Jensen K., Bonde Jensen A., Grau C. The relationship between observer-based toxicity scoring and patient assessed symptom severity after treatment for head and neck cancer. A correlative cross sectional study of the DAHANCA toxicity scoring system and the EORTC quality of life questionnaires. Radiotherapy and Oncology. 2006;78(3):298–305. doi: 10.1016/j.radonc.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 82.Speyer R. Effects of voice therapy: a systematic review. Journal of Voice. 2008;22(5):565–580. doi: 10.1016/j.jvoice.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 83.Jacobson B. H., Johnson A., Grywalski C., et al. The Voice Handicap Index (VHI) American Journal of Speech-Language Pathology. 1997;6(3):66–69. [Google Scholar]
- 84.Dejonckere P. H., Bradley P., Clemente P., et al. A basic protocol for functional assessment of voice pathology, especially for investigating the efficacy of (phonosurgical) treatments and evaluating new assessment techniques. European Archives of Oto-Rhino-Laryngology. 2001;258(2):77–82. doi: 10.1007/s004050000299. [DOI] [PubMed] [Google Scholar]
- 85.Speyer R., Baijens L., Heijnen M., Zwijnenberg I. Effects of therapy in oropharyngeal dysphagia by speech and language therapists: a systematic review. Dysphagia. 2010;25(1):40–65. doi: 10.1007/s00455-009-9239-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.World Health Organization. WHO Handbook for Guideline Development. 2012. http://apps.who.int/iris/bitstream/10665/75146/1/9789241548441_eng.pdf. [Google Scholar]