Abstract
Purpose. This study aimed to evaluate the clinical efficacy of using expression levels of CCL24 (eotaxin-2) mRNA on the ocular surface as a biomarker in patients with vernal keratoconjunctivitis (VKC) and atopic keratoconjunctivitis (AKC). Methods. Eighteen patients with VKC or AKC (VKC/AKC group) and 12 control subjects (control group) were enrolled in this study. The VKC/AKC clinical score was determined by objective findings in patients by using the 5-5-5 exacerbation grading scale. All subjects underwent modified impression cytology and specimens were obtained from the upper tarsal conjunctiva. Expression levels of CCL24 (eotaxin-2) mRNA on the ocular surface were determined using real-time reverse transcription polymerase chain reaction. Results. The VKC group was divided into two subgroups, depending on the clinical score: the active stage subgroup with 100 points or more of clinical scores and the stable stage subgroup with 100 points or less. CCL24 (eotaxin-2) mRNA expression levels in the active VKC/AKC stage subgroup were significantly higher than those in the stable VKC/AKC subgroup and the control group. Clinical scores correlated significantly with CCL24 (eotaxin-2) mRNA expression levels in the VKC group. Conclusions. CCL24 (eotaxin-2) mRNA expression levels on the ocular surface are a useful biomarker for clinical severity of VKC/AKC.
1. Introduction
Allergic conjunctival diseases (ACD) are conjunctival inflammatory disorders, characterized by an immediate hypersensitivity reaction associated with antigen-specific IgE antibodies [1]. Patients with ACD are usually categorized into the following types based on clinical criteria: seasonal allergic conjunctivitis (SAC), perennial allergic conjunctivitis (PAC), vernal keratoconjunctivitis (VKC), atopic keratoconjunctivitis (AKC), and giant papillary conjunctivitis (GPC) [2]. SAC and PAC are characterized by conjunctival hyperemia, conjunctival edema, and papillary hyperplasia of tarsal conjunctiva and are classified as mild ACD. In contrast, VKC is characterized by the development of proliferative lesions of the conjunctiva, including giant papillary proliferation of the tarsal conjunctiva and gelatinous cell infiltration of the limbal conjunctiva. VKC also complicates corneal disorders, such as shield ulcer and punctate corneal keratitis, and is classified as a severe ACD, because the visual prognosis may be poor. AKC usually develops in older patients with atopic dermatitis and also complicates severe ocular surface diseases, including giant papillary conjunctivitis, shield ulcer, and dry eye.
In shield ulcers of patients with VKC and AKC, depositions of major basic protein and eosinophil cationic protein (ECP), which comprise specific granules of eosinophils, have reportedly been observed histologically in the corneal ulcerative lesions [3, 4]. Furthermore, ECP levels in the tears of VKC and AKC patients are reportedly increased, in comparison with those in controls [5–7]. Therefore, the pathophysiological characteristics of these severe ACD, including VKC and AKC, include eosinophilic inflammation in the conjunctiva.
Eotaxin is a member of the CC chemokine family and is divided into three subfamilies, namely, CCL11/eotaxin-1, CCL24/eotaxin-2, and CCL26/eotaxin-3. Eotaxin-1, eotaxin-2, and eotaxin-3 interact with the CC chemokine receptor 3 (CCR3). Representative inflammatory cells expressing CCR3 on their cell surfaces are eosinophils, type-2 helper T cells (Th2), and basophils. IL-13, which is a Th2-derived cytokine, induces eotaxin in vitro through activation of the IL-4Rα receptor/STAT-6 pathway [8, 9]. Therefore, eotaxin-1, eotaxin-2, and eotaxin-3 are thought to be allergic inflammation-related chemokines.
In the eotaxin subfamily, it has been reported that increased eotaxin-2 levels in tears and expression of CCL24 (eotaxin-2) mRNA on the ocular surface are more common in VKC patients than those of eotaxin-1 and eotaxin-3 [10, 11]. However, the relationship between the expression levels of CCL24 (eotaxin-2) mRNA on the ocular surface and the severity of severe ACD, including VKC and AKC, has not been fully investigated.
In this study, we evaluated the clinical efficacy of using CCL24 (eotaxin-2) mRNA expression levels on the ocular surface in patients with severe ACD, including VKC and AKC, as a biomarker for severe ACD.
2. Subjects and Methods
This study was approved by the institutional review board of the Nihon University School of Medicine and adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all study subjects.
2.1. Subjects
This study included 18 consecutive patients with VKC or AKC treated at the Department of Ophthalmology, Nihon University Itabashi Hospital, Tokyo, Japan, from August 2012 to January 2015 (AKC/VKC group), and 12 healthy volunteers who did not have any personal or family history of atopic disease, were not affected by ocular surface diseases, or have a history of wearing contact lenses as controls (control group). Demographic data of the subjects are shown in Table 1. Objective diagnoses were made in VKC and AKC patients by means of slit-lamp clinical examination and serum examination for antigen-specific IgE antibodies, according to the Japanese guidelines for ACD [2]. Patients with ocular surface disease other than ACD, including lagophthalmos, blepharospasm, conjunctival chalasis, dry eye, infectious conjunctivitis, infectious keratitis, Stevens-Johnson syndrome, and ocular pemphigoid, were excluded. Nontreated patients or patients treated with antiallergic ophthalmic solutions alone, such as mast cell stabilizers, histamine H1 receptor antagonists, corticosteroids, and immunosuppressive agents, were included in the study. Patients who used oral medicines or injections for treating allergic diseases and those who received immunotherapy were excluded from the study.
Table 1.
Subjects and their demographic data.
| Control | AKC/VKC | P value | |
|---|---|---|---|
| Number of subjects | 12 | 18 | |
| Age (years) (mean ± SD) |
25.5 ± 2.07 | 21.9 ± 13.1 | 0.272 |
| Gender (male : female) |
10 : 2 | 14 : 4 | 0.709 |
| Positive ratio of CCL24 (eotaxin-2) mRNA expression | |||
| Positive | 8 | 17 | 0.046 |
| Negative | 4 | 1 |
AKC: atopic keratoconjunctivitis; VKC: vernal keratoconjunctivitis.
Negative: below the lowest level of detection limit of real-time reversed transcription polymerase chain reaction.
2.2. Clinical Score Grading
Clinical scores of objective findings in the AKC/VKC group were determined using the 5-5-5 exacerbation grading scale for ACD [12]. The AKC/VKC group was divided into two subgroups depending on the clinical score: the active stage subgroup with clinical scores of 100 points or more (n = 6) and the stable stage subgroup with 100 points or less (n = 12).
2.3. Modified Impression Cytology
Modified impression cytology was performed after instillation of topical oxybuprocaine 0.4% (Benoxil, Santen, Osaka, Japan). Schirmer's test strips (Schirmer Tear Production Measuring Strips, Showa Yakuhin Kako, Tokyo, Japan) were applied to the upper tarsal conjunctiva, pressed gently using a glass rod, and then removed. The membrane was preserved in RNAlater RNA Stabilization Reagent (Qiagen, Hilden, Germany) until analysis.
2.4. Real-Time Reverse Transcription Polymerase Chain Reaction
Total RNA was harvested from each Schirmer tested paper using an RNeasy® Mini Kit (QIAGEN, Hilden, Germany) following the manufacturer's instructions. cDNA was then synthesized using a High-Capacity cDNA Reverse Transcription Kit (Life Technologies Japan, Tokyo, Japan), according to the manufacturer's instructions.
To detect expression of CCL24 (eotaxin-2) mRNA, real-time reverse transcription polymerase chain reaction (real-time RT-PCR) was performed using a commercial PCR master mix (TaqMan Universal PCR Master Mix; Life Technologies, Tokyo, Japan) and predesigned primers (Life Technologies) for CCL24 (Eotaxin-2; Hs00171082_m1). Samples were analyzed using the Step One Plus™ real-time PCR system (Life Technologies) and comparative threshold (Ct) values were obtained. Target Ct values were normalized to those of GAPDH (Hs99999905_m1) in the same sample. Data were analyzed using the ΔΔCt method.
2.5. Statistical Analysis
Differences between AKC/VKC and control groups were identified using Welch's t-test or the chi-square test. The results for CCL24 (eotaxin-2) mRNA expression on the ocular surface were evaluated using the nonparametric Steel-Dwass test. Spearman's rank correlation coefficient was used to evaluate whether CCL24 (eotaxin-2) mRNA expression correlated with the clinical score. A P value of <0.05 was regarded as statistically significant.
3. Results
3.1. Expression Levels of Eotaxin-2 mRNA on Ocular Surface
For CCL24 (eotaxin-2) mRNA expression on the ocular surface, 6 of 6 patients in the active stage subgroup of AKC/VKC group were CCL24- (eotaxin-2-) positive, with median (range) levels of 133 (27.6–232). Eleven of 12 patients in the stable stage subgroup of AKC/VKC group were CCL24- (eotaxin-2-) positive, with median (range) levels of 5.99 (0.140–33.2); the remaining patient had levels below the lower limit of detection. Eight of 12 patients in the control group were CCL24- (eotaxin-2-) positive, with median (range) levels of 0.98 (0.08–20.2), while 4 patients had levels below the lower limit of detection.
The expression levels of CCL24 (eotaxin-2) mRNA on the ocular surface were significantly higher in the active stage than in the stable stage AKC/VKC subgroup and the control group (both P < 0.01; Steel-Dwass test; Figure 1).
Figure 1.
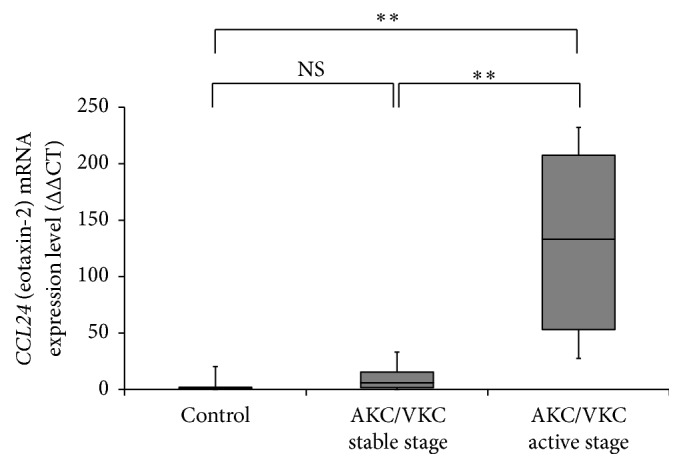
The levels of CCL24 (eotaxin-2) mRNA expression on the ocular surface in AKC/VKC and control group. The levels of CCL24 (eotaxin-2) mRNA expression on the ocular surface in the active stage subgroup of the AKC/VKC group were significantly higher than those in the stable stage subgroup of AKC/VKC group and the control group (P < 0.01, P < 0.01, respectively, Steel-Dwass test). ∗ p < 0.05; NS: not significant.
3.2. Relationship between Clinical Severity and Expression Levels of CCL24 (Eotaxin-2) mRNA
The median value (range) of the clinical scores in the active and stable stage subgroups of AKC/VKC group were 13 (2–33) and 128 (343–112), respectively. In patients with AKC/VKC, clinical scores were significantly correlated with the levels of (CCL24) eotaxin-2 mRNA expression on the ocular surface (ρ = 0.795, P < 0.01, Spearman's rank correlation coefficient; Figure 2).
Figure 2.

Comparison of clinical scores and levels of CCL24 (eotaxin-2) mRNA expression on the ocular surface. In the patients with AKC/VKC, clinical scores were significantly correlated with the levels of CCL24 (eotaxin-2) mRNA expression on the ocular surface (ρ = 0.795, P < 0.01, Spearman's rank correlation coefficient).
3.3. Case Presentation
3.3.1. Patient 1
A 10-year-old boy was diagnosed as having VKC and had been under treatment for VKC by a local doctor for 6 years. His clinical observation of VKC repeated exacerbation and remission, and his severity of VKC was different in right and left active giant papillae and exfoliative epithelial keratopathy was present in his right eye; his clinical score was 212, and his CCL24 (eotaxin-2) mRNA expression levels on the ocular surface were 203.2. In his left eye, papillary lesions and hyperemia at the upper palpebral conjunctiva were observed, and the clinical score was 2, while the CCL24 (eotaxin-2) mRNA expression level on the ocular surface was 17.2 (Figure 3).
Figure 3.
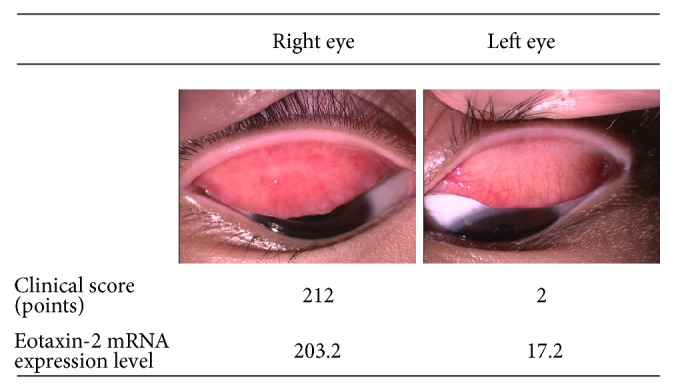
Comparison of the CCL24 (eotaxin-2) mRNA expression in bilateral eyes of a vernal keratoconjunctivitis (VKC) patient. A 10-year-old boy had VKC, showing laterality in the severity of the objective findings. The levels of CCL24 (eotaxin-2) mRNA expression on the ocular surface were low (17.2) in the left eye with mild VKC and high (203.2) in the right eye with severe VKC.
3.3.2. Patient 2
A 10-year-old girl was diagnosed as having VKC and had been under treatment for VKC by a doctor for 1 year. She experienced pain in her right eye and also had recurrence of corneal plaque and was referred to our hospital. At her first examination, she demonstrated shield ulcer in her right eye, giant papillae, and palpebral conjunctiva with a velvety appearance. Topical 0.1% tacrolimus ophthalmic suspension (Talymus® Ophthalmic Suspension 0.1%, Senju Pharmaceutical, Co., Ltd., Osaka, Japan) and 2% sodium cromoglicate ophthalmic solution (Intal® Ophthalmic Solution 2%, Sanofi, Tokyo, Japan) were administered. At commencement of treatment with tacrolimus ophthalmic suspension, her clinical score was 213 and the level of CCL24 (eotaxin-2) mRNA expression on the ocular surface was 349.6. During the first 10 weeks of treatment, the shield ulcer was relieved and the giant papillae disappeared. At this point, the clinical score was 3 points and the CCL24 (eotaxin-2) mRNA expression level on the ocular surface was 9.6 (Figure 4).
Figure 4.
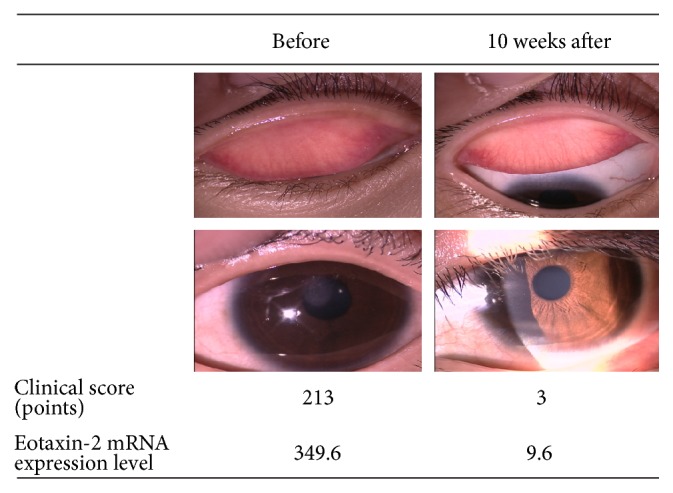
Comparison of CCL24 (eotaxin-2) mRNA expression levels before and after treatment. A 10-year-old girl with vernal keratoconjunctivitis (VKC) was treated with tacrolimus ophthalmic suspension and disodium cromoglicate ophthalmic solution. With this treatment, her clinical score and levels of CCL24 (eotaxin-2) mRNA expression decreased, as compared to those before treatment.
4. Discussion
In this study, we assessed the usefulness of CCL24 (eotaxin-2) mRNA expression levels on the ocular surface as a biomarker of the severity of ACD. We found that these levels correlated well with the clinical score reflecting objective findings in patients with AKC and VKC. As a method for sampling the ocular surface to test the expression levels of CCL24 (eotaxin-2) mRNA, we used a modified impression cytology method. This method entailed a membrane biopsy technique, as used for impression cytology, but used filter paper instead of nitrocellulose membrane for the biopsy. In the conventional impression cytology method, the specimen obtained using a nitrocellulose membrane is examined histologically. However, the major concern of this method is the ocular sensation of a foreign body and the ocular pain experienced after the examination; repeated examinations for follow-up of the biomarker may cause discomfort for the patients. Changing to the filter paper for impression cytology sampling and using a quantitative method (real-time RT-PCR) allowed assessment of the biomarker on the ocular surface. The specimens obtained by modified impression cytology most likely included conjunctival epithelial and invading inflammatory cells, in addition to tears and mucin. It was therefore considered useful for investigating inflammation-associated factors expressed by conjunctival epithelial cells and inflammatory cells as potential biomarkers of allergic inflammation at the ocular surface.
In this study, we elucidated a significant correlation between clinical observations and CCL24 expression on the ocular surface of AKC/VKC patients with or without treatment with ophthalmic solutions. These results suggest that CCL24 expression on the ocular surface is suitable as a biomarker of severe allergic conjunctival diseases. In previous reports, it has been demonstrated that eotaxin-1 plays a critical role in eosinophilic infiltration in the conjunctiva and cornea of patients with ACD [10, 11, 13, 14]. However, the concentration of eotaxin-2 in tears has been reported to be higher than those of eotaxin-1 in ACD patients [10]. Therefore, in this study, we investigated the usefulness of CCL24 (eotaxin-2) mRNA expressed on the ocular surface as a biomarker for patients with AKC/VKC. We found that expression levels of CCL24 (eotaxin-2) mRNA on the ocular surface were significantly increased and correlated with the clinical score in the active stage of VKC.
Eotaxin-1 is reportedly produced by corneal keratinocytes [13, 14], conjunctival fibroblasts [11], CD68-positive cells in the conjunctiva, and eosinophils in the conjunctiva [15]. However, the cellular source of eotaxin-2 in tears was not fully understood. Previously, we have reported that the tear levels of eotaxin-2 correlated significantly with those of eosinophil cationic protein and that epithelial cells in conjunctival smears of patients with VKC expressed eotaxin-2, based on immunohistochemistry [10]. Leonardi and colleagues [11] reported that tear levels of eotaxin-1 and eotaxin-2 significantly correlated with the percentage of eosinophils in tears. Therefore, conjunctival epithelial cells and eosinophils are thought to be candidate eotaxin-2-producing cells on the ocular surface. Furthermore, expression levels of CCL24 (eotaxin-2) mRNA in modified impression cytology may be a good biomarker for evaluating allergic inflammation in the conjunctivas of patients with AKC/VKC.
In these case reports, we showed differences between the right and left eye in the severity of VKC based on the quantitative analysis of CCL24 (eotaxin-2) mRNA levels. Furthermore, in the 10-year-old girl with VKC, we were able to show the therapeutic effect of treatment by tacrolimus instillation by the reduction of both clinical scores and expression levels of CCL24 (eotaxin-2) mRNA. Therefore, in AKC/VKC patients undergoing treatment, monitoring of the expression levels of CCL24 (eotaxin-2) mRNA may provide a useful index of exacerbation and therapeutic response.
The limitation of this study included the small sample size, and a lack of patients with mild ACD, such as SAC and PAC. Further investigation is necessary for verifying the usefulness of CCL24 (eotaxin-2) mRNA levels as a biomarker of ACD in a large sample that includes patients with SAC and PAC. Another limitation of this study was that AKC/VKC patients not receiving treatment and those only receiving treatment with ophthalmic solutions were enrolled. The CCL24 mRNA expression on the ocular surface may be affected by treatment with ophthalmic solutions. However, the efficacy of the therapeutic agent is one of the items measured by a biomarker. Therefore, further investigation on the therapeutic effect of antiallergic treatment in a large cohort, including untreated patients with allergic conjunctival diseases, using eotaxin-2 expression as a biomarker, will be necessary in the future.
5. Conclusion
Expression levels of CCL24 (eotaxin-2) mRNA on the ocular surface are a useful biomarker of the clinical severity of AKC/VKC.
Acknowledgments
The authors wish to thank Mrs. Akiko Tomioka (Department of Visual Sciences, Division of Ophthalmology, Nihon University School of Medicine) for her expert technical assistance.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Leonardi A. Allergy and allergic mediators in tears. Experimental Eye Research. 2013;117:106–117. doi: 10.1016/j.exer.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Takamura E., Uchio E., Ebihara N., et al. Japanese guideline for allergic conjunctival diseases. Allergology International. 2011;60(2):191–203. doi: 10.2332/allergolint.11-RAI-0335. [DOI] [PubMed] [Google Scholar]
- 3.Trocme S. D., Kephart G. M., Bourne W. M., Buckley R. J., Gleich G. J. Eosinophil granule major basic protein deposition in corneal ulcers associated with vernal keratoconjunctivitis. American Journal of Ophthalmology. 1993;115(5):640–643. doi: 10.1016/s0002-9394(14)71463-1. [DOI] [PubMed] [Google Scholar]
- 4.Messmer E. M., May C. A., Stefani F. H., Welge-Luessen U., Kampik A. Toxic eosinophil granule protein deposition in corneal ulcerations and scars associated with atopic keratoconjunctivitis. American Journal of Ophthalmology. 2002;134(6):816–821. doi: 10.1016/S0002-9394(02)01726-9. [DOI] [PubMed] [Google Scholar]
- 5.Leonardi A., Borghesan F., Faggian D., Secchi A., Plebani M. Eosinophil cationic protein in tears of normal subjects and patients affected by vernal keratoconjunctivitis. Allergy. 1995;50(7):610–613. doi: 10.1111/j.1398-9995.1995.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 6.Shoji J., Kitazawa M., Inada N., et al. Efficacy of tear eosinophil cationic protein level measurement using filter paper for diagnosing allergic conjunctival disorders. Japanese Journal of Ophthalmology. 2003;47(1):64–68. doi: 10.1016/s0021-5155(02)00630-5. [DOI] [PubMed] [Google Scholar]
- 7.Montan P. G., van Hage-Hamsten M. Eosinophil cationic protein in tears in allergic conjunctivitis. British Journal of Ophthalmology. 1996;80(6):556–560. doi: 10.1136/bjo.80.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsukura S., Stellato C., Georas S. N., et al. Interleukin-13 upregulates eotaxin expression in airway epithelial cells by a STAT6-dependent mechanism. American Journal of Respiratory Cell and Molecular Biology. 2001;24(6):755–761. doi: 10.1165/ajrcmb.24.6.4351. [DOI] [PubMed] [Google Scholar]
- 9.Zimmermann N., Hershey G. K., Foster P. S., Rothenberg M. E. Chemokines in asthma: cooperative interaction between chemokines and IL-13. The Journal of Allergy and Clinical Immunology. 2003;111(2):227–242. doi: 10.1067/mai.2003.139. [DOI] [PubMed] [Google Scholar]
- 10.Shoji J., Inada N., Sawa M. Evaluation of eotaxin-1, -2, and -3 protein production and messenger RNA expression in patients with vernal keratoconjunctivitis. Japanese Journal of Ophthalmology. 2009;53(2):92–99. doi: 10.1007/s10384-008-0628-5. [DOI] [PubMed] [Google Scholar]
- 11.Leonardi A., Jose P. J., Zhan H., Calder V. L. Tear and mucus eotaxin-1 and eotaxin-2 in allergic keratoconjunctivitis. Ophthalmology. 2003;110(3):487–492. doi: 10.1016/S0161-6420(02)01767-0. [DOI] [PubMed] [Google Scholar]
- 12.Shoji J., Inada N., Sawa M. Evaluation of novel scoring system named 5-5-5 exacerbation grading scale for allergic conjunctivitis disease. Allergology International. 2009;58(4):591–597. doi: 10.2332/allergolint.09-oa-0100. [DOI] [PubMed] [Google Scholar]
- 13.Fukagawa K., Nakajima T., Saito H., et al. IL-4 induces eotaxin production in corneal keratocytes but not in epithelial cells. International Archives of Allergy and Immunology. 2000;121(2):144–150. doi: 10.1159/000024310. [DOI] [PubMed] [Google Scholar]
- 14.Kumagai N., Fukuda K., Ishimura Y., Nishida T. Synergistic induction of eotaxin expression in human keratinocytes by TNF-α and IL-4 or IL-13. Investigative Ophthalmology & Visual Science. 2000;41(6):1448–1453. [PubMed] [Google Scholar]
- 15.Abu El-Asrar A. M., Struyf S., Al-Kharashi S. A., Missotten L., Van Damme J., Geboes K. Chemokines in the limbal form of vernal keratoconjunctivitis. British Journal of Ophthalmology. 2000;84(12):1360–1366. doi: 10.1136/bjo.84.12.1360. [DOI] [PMC free article] [PubMed] [Google Scholar]


