Abstract
Fusobacterium nucleatum has been associated with both periodontal disease and inflammatory bowel disease. This Gram-negative bacterium possesses a high inflammatory potential that may contribute to the disease process. We hypothesized that green and black tea polyphenols attenuate the inflammatory response of monocytes/macrophages mediated by F. nucleatum. We first showed that the tea extracts, EGCG and theaflavins reduce the NF-κB activation induced by F. nucleatum in monocytes. Since NF-κB is a key regulator of genes coding for inflammatory mediators, we tested the effects of tea polyphenols on secretion of IL-1β, IL-6, TNF-α, and CXCL8 by macrophages. A pre-treatment of macrophages with the tea extracts, EGCG, or theaflavins prior to a stimulation with F. nucleatum significantly inhibited the secretion of all four cytokines and reduced the secretion of MMP-3 and MMP-9, two tissue destructive enzymes. TREM-1 expressed by macrophages is a cell-surface receptor involved in the propagation of the inflammatory response to bacterial challenges. Interestingly, tea polyphenols inhibited the secretion/shedding of soluble TREM-1 induced by a stimulation of macrophages with F. nucleatum. The anti-inflammatory properties of tea polyphenols identified in the present study suggested that they may be promising agents for the prevention and/or treatment of periodontal disease and inflammatory bowel disease.
Periodontal disease is defined as a biofilm-associated inflammatory disorder affecting the supporting structures of the teeth, including the periodontal ligament and the alveolar bone. It is one of the most common chronic infections in adults and, if left untreated, may result in tooth loss and systemic complications such as cardiovascular disease, diabetes mellitus, rheumatoid arthritis, preterm birth, and respiratory infections1. Periodontal disease results from a dysbiosis in the gingival sulcus that causes a shift from symbiotic host-microbe interactions to a microflora dominated by strictly anaerobic Gram-negative bacterial species2. One of these, Fusobacterium nucleatum, is known to be present in higher numbers in diseased periodontal sites3. Due to its versatile adherence properties, F. nucleatum is a key bridging bacterium between early and late colonizers of the subgingival biofilm4. F. nucleatum is also a common resident of the human gastrointestinal tract. A number of recent reports have indicated that this bacterial species is present in higher numbers in the gastrointestinal tract of patients with inflammatory bowel disease (IBD) and that F. nucleatum may contribute to the pathogenesis of colorectal cancer5,6,7.
Inflammation is a complex pathophysiological phenomenon orchestrated by immune cells in response to infections and/or tissue damage. Although inflammation is an integral component of the host defense against pathogens, uncontrolled inflammation caused by a continuous bacterial challenge, as occurs during periodontitis, can lead to extensive secretion by mucosal and immune cells of pro-inflammatory mediators and matrix metalloproteinases (MMPs) that contribute to periodontal tissue destruction8. Monocytes and macrophages, which are present in higher numbers in active periodontal lesions than in inactive sites9, detect and respond to pathogens via toll-like receptors (TLR) and mediate both inflammation and resolution10. Following the recognition of pathogens by the TLR pathway, macrophages secrete a wide variety of cytokines through the nuclear factor-κB (NF-κB) pathway11. The Triggering Receptor Expressed on Myeloid Cells 1 (TREM-1) expressed by macrophages is a cell-surface receptor of the immunoglobulin superfamily that is also involved in the propagation of the inflammatory response to bacterial challenges12. Soluble TREM-1 (sTREM-1) can be released from the cell surface during the course of an infection and can also be used as a biomarker of systemic inflammation in systemic sepsis13, arthritis14 and IBD15. Recent studies have suggested that the sTREM-1 detected in gingival crevicular fluid, saliva, and serum is a biomarker of periodontal disease and a predictor of therapeutic outcome16,17,18,19.
Given the critical role played by the immune/inflammatory response in the pathogenesis of periodontitis that results in the local production of excessive levels of cytokines and tissue destructive enzymes, the modulation of the host response is seen as a valid adjunctive therapy to scaling and root planing in the treatment of periodontitis20,21. In this regard, plant polyphenols are bioactive molecules of interest due to their anti-inflammatory properties22. Tea, an aqueous aromatic infusion of the leaves of the Camellia sinensis plant, has a high polyphenol content23,24. While green tea (non-fermented) contains mainly catechins and their derivatives, the most important being epigallocatechin-3-gallate (EGCG), black tea (fermented) is characterized by the presence of theaflavins and their derivatives24,25. Many studies have shown that tea polyphenols may contribute to reducing the risk of cardiovascular disease and cancer and can exert a variety of other beneficial effects on human health24. In the present study, we investigated the effect of green and black tea polyphenols on F. nucleatum-mediated activation of the NF-κB signaling pathway and the secretion of pro-inflammatory mediators and MMPs by monocytes/macrophages.
Results
Cell viability assays
An MTT colorimetric assay was used to determine the non-cytotoxic concentrations of the green tea extract, the black tea extract, EGCG, and the theaflavins for use with U937-3xκB and U937 cells. This analysis was required to exclude the possibility that cytotoxicity related to the compounds tested might cause a decrease in NF-κB activation or cytokine and MMP secretion. For the U937-3xκB cell line, the non-cytotoxic concentrations were ≤125 μg/ml for the green tea extract, EGCG, and theaflavins, and ≤500 μg/ml for the black tea extract (Table 1). For the U937 macrophage-like cells, the non-cytotoxic concentrations were ≤62.5 μg/ml for the green tea extract and EGCG, ≤125 μg/ml for the theaflavins, and ≤500 μg/ml for the black tea extract (Table 1).
Table 1. Effect of the green tea extract, EGCG, black tea extract, and theaflavins on the viability of U937-3xκB and U937 macrophage-like cells.
| Compounds | Cell viability (%) | |
|---|---|---|
| U937-3xκB | U937 macrophage-like cells | |
| Green tea extract (μg/ml) | ||
| 250 | 83.3 ± 6.7 | 58.4 ± 8.2 |
| 125 | 99.0 ± 7.3 | 89.2 ± 6.6 |
| 62.5 | 95.7 ± 1.8 | 113.4 ± 14.2 |
| EGCG (μg/ml) | ||
| 250 | 74.7 ± 3.5 | 17.5 ± 18.7 |
| 125 | 95.2 ± 2.5 | 46.6 ± 15.7 |
| 62.5 | 99.8 ± 5.2 | 87.5 ± 3.1 |
| Black tea extract (μg/ml) | ||
| 500 | 94.0 ± 2.6 | 98.7 ± 8.1 |
| 250 | 91.9 ± 3.6 | 101.7 ± 8.5 |
| 125 | 95.24 ± 1.3 | 107.2 ± 5.2 |
| Theaflavins (μg/ml) | ||
| 500 | 35.2 ± 6.0 | 41.0 ± 3.0 |
| 250 | 72.9 ± 2.7 | 67.6 ± 4.6 |
| 125 | 96.8 ± 8.2 | 108.8 ± 11.0 |
| None | 100 ± 0.8 | 100 ± 2.3 |
NF-κB activation
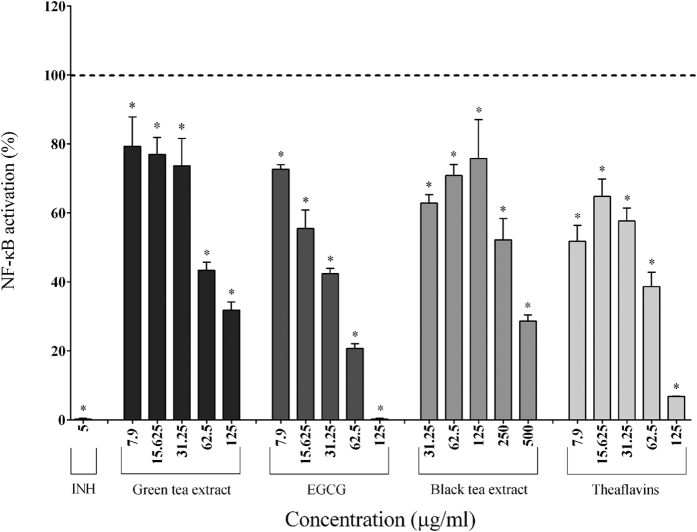
The ability of the tea polyphenols to prevent NF-κB activation was evaluated using two-fold serial dilutions beginning at their highest non-cytotoxic concentrations. In the absence of LPS stimulation, the tea polyphenols showed no inhibitory effects on the basal level of NF-κB activity in U937-3xκB cells (data not shown). In general, the tea polyphenols exhibited a comparable dose-dependent inhibitory effect on F. nucleatum-induced NF-κB activation in U937-3xκB cells (Fig. 1). More specifically, 62.5 μg/ml of the green tea extract, black tea extract, EGCG, and theaflavins reduced NF-κB activation induced by F. nucleatum (MOI of 100) by 56.65%, 29.14%, 79.28%, and 61.37%, respectively while 125 μg/ml of EGCG completely prevented NF-κB activation (Fig. 1). BAY-11-7082 (5 μg/ml), a commercial inhibitor was used as a positive control and totally prevented NF-κB activation.
Figure 1. Effect of the green tea extract, EGCG, black tea extract, and theaflavins on F. nucleatum-mediated activation of the NF-κB signaling pathway using the U937-3xκB cell model.
A value of 100% was assigned to the activation obtained with F. nucleatum at an MOI of 100 (-----) in the absence of tea polyphenols. The commercial inhibitor BAY-11-7082 (INH; 5 μg/ml) was used as a positive control. Results are expressed as the means ± SD of triplicate assays from three independent experiments. (*) Significant decrease (p < 0.001) compared to untreated F. nucleatum-stimulated cells.
Secretion of cytokines, MMPs, and sTREM-1
Since tea polyphenols reduced the activation of the NF-κB signaling pathway, we investigated their effects on inflammatory mediator secretion in a macrophage-like model (PMA-treated U937 cells) stimulated with F. nucleatum. Adherent macrophages were pre-treated for 2 h with the tea polyphenols and were then stimulated for 24 h with F. nucleatum (MOI of 100). The secretion of cytokines (IL-6, IL-1β, TNF-α, CXCL8) and MMPs (MMP-3, MMP-9) was then measured.
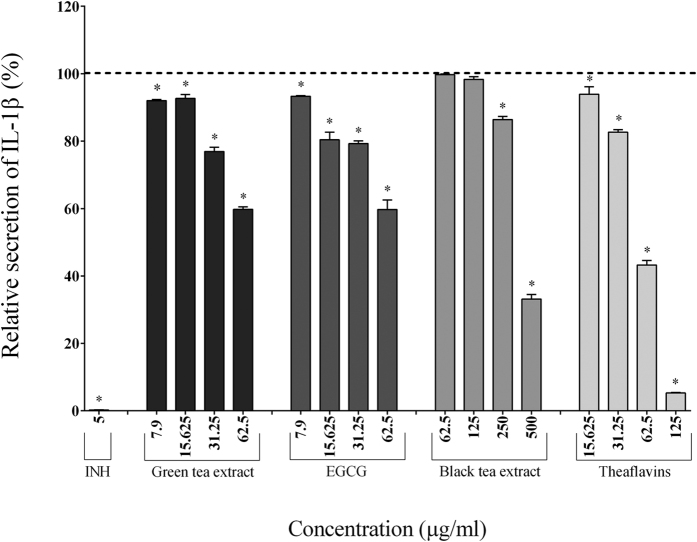
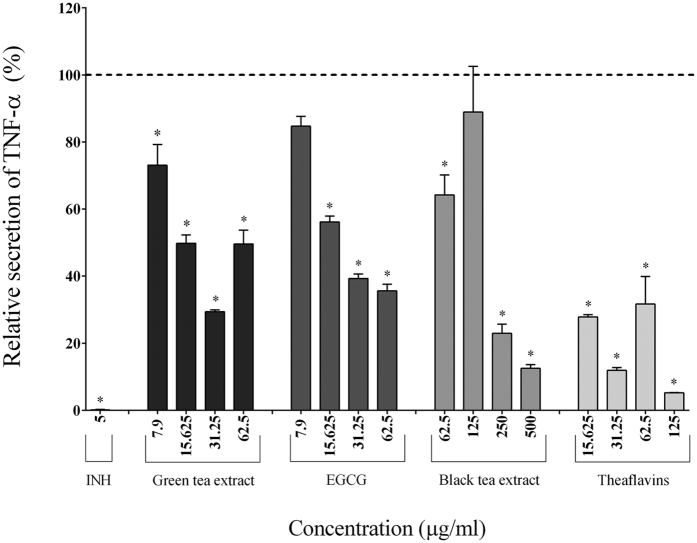
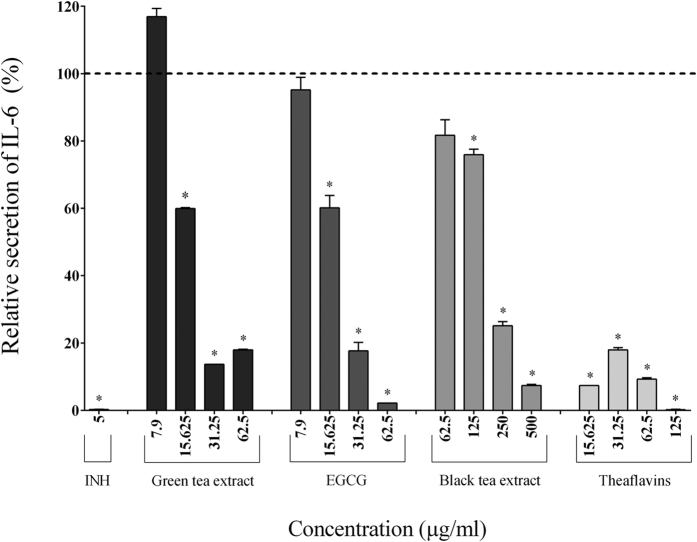
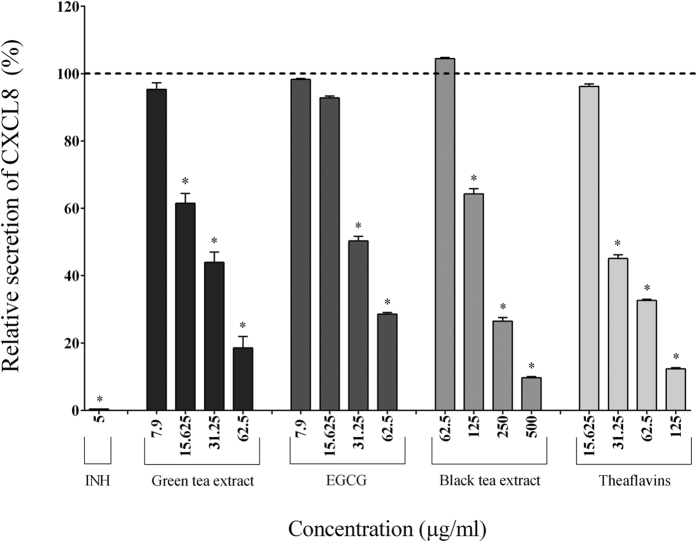
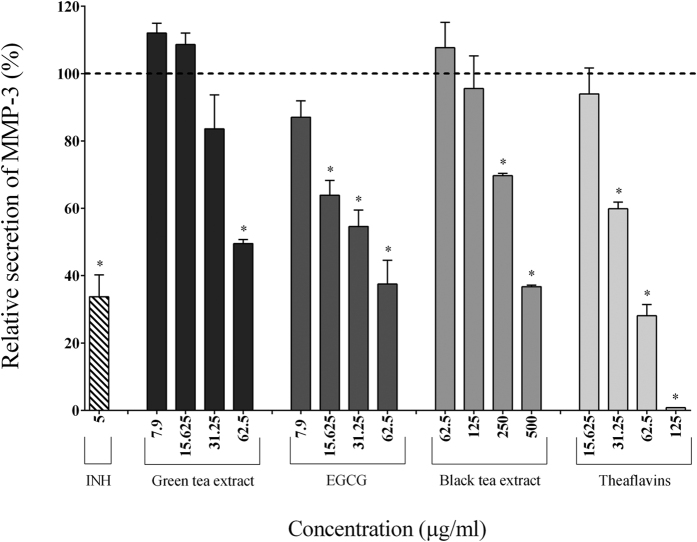
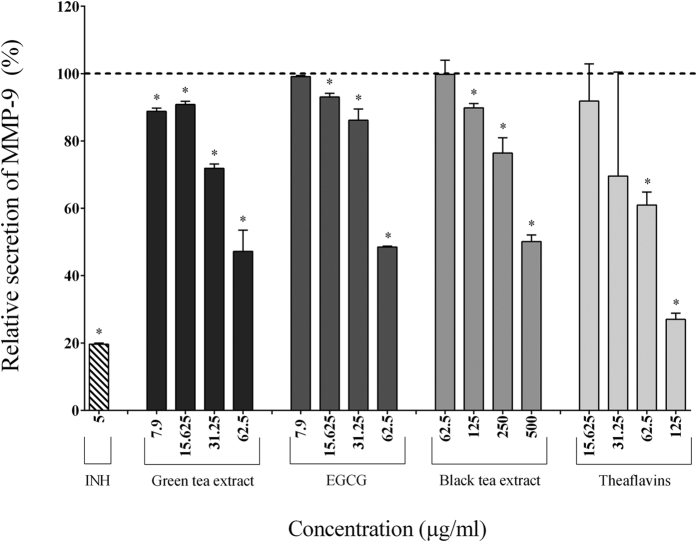
In the absence of tea polyphenols, the stimulation of macrophages with F. nucleatum (MOI of 100) significantly increased the secretion of IL-1β (7.70-fold), TNF-α (84.57-fold), IL-6 (185.25-fold), CXCL8 (32.25-fold), MMP-3 (2.05-fold), and MMP-9 (2.04-fold) (Table 2). The secretion of pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) by macrophages stimulated with F. nucleatum was significantly and dose-dependently attenuated by the tea polyphenols compared to the control cells. The green tea extract, EGCG, and theaflavins (62.5 μg/ml) reduced the secretion of IL-1β by 40.49%, 40.27%, and 56.72%, respectively, TNF-α by 50.41%, 64.38%, and 68.30%, respectively, and IL-6 by 82.40%, 97.84%, and 85.39%, respectively (Figs 2, 3 and 4). At 62.5 μg/ml, the black tea extract had weak or no inhibitory effect on cytokine secretion. However, at 250 μg/ml, the black tea extract significantly reduced the secretion of IL-1β (13.57%), TNF-α (77.07%), and IL-6 (74.89%) (Figs 2, 3 and 4). The secretion of CXCL8 by F. nucleatum-stimulated macrophages was also significantly inhibited by the green tea extract (≥15.625 μg/ml), black tea extract (≥125 μg/ml), EGCG (≥31.25 μg/ml), and theaflavins (≥31.25 μg/ml) (Fig. 5). Lastly, the secretion of MMP-3 and MMP-9 by F. nucleatum-stimulated macrophages was also attenuated by the tea polyphenols. More specifically, at a concentration of 62.5 μg/ml, the green tea extract, EGCG, and theaflavins reduced the secretion of MMP-3 by 50.45%, 62.49%, and 71.91%, respectively, and the secretion of MMP-9 by 52.78%, 51.47%, and 39.02%, respectively (Figs 6 and 7). The black tea extract (250 μg/ml) attenuated the secretion of MMP-3 by 30.32% (Fig. 6) and MMP-9 by 23.59% (Fig. 7).
Table 2. Secretion of IL-1β, TNF-α, IL-6, CXCL8, MMP-3, MMP-9, and sTREM-1 by F. nucleatum-stimulated macrophages.
| Inflammatory mediators | Concentration (pg/ml) | Fold increase | |
|---|---|---|---|
| Control | F. nucleatum (MOI = 100) | ||
| IL-1β | 33.87 ± 1.67 | 260.9 ± 0.41 | 7.70 |
| TNF-α | 41.08 ± 29.05 | 3474 ± 26.25 | 84.57 |
| IL-6 | 3.180 ± 0.54 | 589.1 ± 22.27 | 185.25 |
| CXCL8 | 11359 ± 3142 | 366371 ± 1914 | 32.25 |
| MMP-3 | 19676 ± 68.69 | 40295 ± 3723 | 2.05 |
| MMP-9 | 118651 ± 1063 | 242000 ± 2465 | 2.04 |
| sTREM-1 | 685.5 ± 1.98 | 2317 ± 66.10 | 3.38 |
Figure 2. Effect of the green tea extract, EGCG, black tea extract, and theaflavins on the secretion of IL-1β by macrophages stimulated with F. nucleatum at an MOI of 100 (-----).
The commercial inhibitor BAY-11-7082 (INH; 5 μg/ml) was used as a positive control. Results are expressed as the means ± SD of triplicate assays from three independent experiments. (*) Significant decrease (p < 0.001) compared to untreated F. nucleatum-stimulated cells.
Figure 3. Effect of the green tea extract, EGCG, black tea extract, and theaflavins on the secretion of TNF-α by macrophages stimulated with F. nucleatum at an MOI of 100 (-----).
The commercial inhibitor BAY-11-7082 (INH; 5 μg/ml) was used as a positive control. Results are expressed as the means ± SD of triplicate assays from three independent experiments. (*) Significant decrease (p < 0.001) compared to untreated F. nucleatum-stimulated cells.
Figure 4. Effect of the green tea extract, EGCG, black tea extract, and theaflavins on the secretion of IL-6 by macrophages stimulated with F. nucleatum at an MOI of 100 (-----).
The commercial inhibitor BAY-11-7082 (INH; 5 μg/ml) was used as a positive control. Results are expressed as the means ± SD of triplicate assays from three independent experiments. (*) Significant decrease (p < 0.001) compared to untreated F. nucleatum-stimulated cells.
Figure 5. Effect of the green tea extract, EGCG, black tea extract, and theaflavins on the secretion of CXCL8 by macrophages stimulated with F. nucleatum at an MOI of 100 (-----).
The commercial inhibitor BAY-11-7082 (INH; 5 μg/ml) was used as a positive control. Results are expressed as the means ± SD of triplicate assays from three independent experiments. (*) Significant decrease (p < 0.001) compared to untreated F. nucleatum-stimulated cells.
Figure 6. Effect of the green tea extract, EGCG, black tea extract, and theaflavins on the secretion of MMP-3 by macrophages stimulated with F. nucleatum at an MOI of 100 (-----).
The commercial inhibitor BAY-11-7082 (INH; 5 μg/ml) was used as a positive control. Results are expressed as the means ± SD of triplicate assays from three independent experiments. (*) Significant decrease (p < 0.001) compared to untreated F. nucleatum-stimulated cells.
Figure 7. Effect of the green tea extract, EGCG, black tea extract, and theaflavins on the secretion of MMP-9 by macrophages stimulated with F. nucleatum at an MOI of 100 (-----).
The commercial inhibitor BAY-11-7082 (5 μg/ml) was used as a positive control. Results are expressed as the means ± SD of triplicate assays from three independent experiments. (*) Significant decrease (p < 0.001) compared to untreated F. nucleatum-stimulated cells.
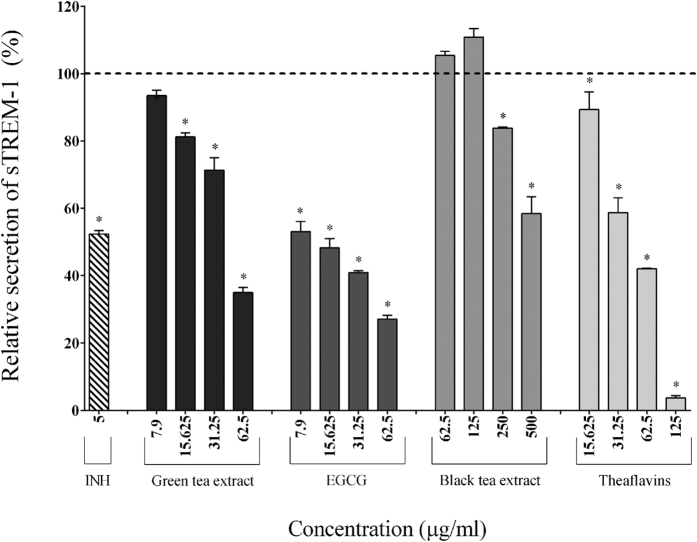
We also investigated the ability of tea polyphenols to inhibit the secretion/shedding of sTREM-1 induced by the stimulation of macrophages with F. nucleatum. F. nucleatum significantly increased the secretion/shedding of sTREM-1 (3.38-fold) (Table 2), while the tea polyphenols caused a significant and dose-dependent inhibition (Fig. 8). More specifically, 62.5 μg/ml of the green tea extract, EGCG, and theaflavins decreased secretion/shedding by 65%, 72.5%, and 57.93%, respectively. The black tea extract (250 μg/ml) reduced the secretion/shedding of sTREM-1 by 16.18%.
Figure 8. Effect of the green tea extract, EGCG, black tea extract, and theaflavins on the secretion of sTREM-1 by macrophages stimulated with F. nucleatum at an MOI of 100 (-----).
The commercial inhibitor BAY-11-7082 (5 μg/ml) was used as a positive control. Results are expressed as the means ± SD of triplicate assays from three independent experiments. (*) Significant decrease (p < 0.001) compared to untreated F. nucleatum-stimulated cells.
Discussion
F. nucleatum is a Gram-negative strictly anaerobic bacterium that is considered a normal resident of the oral cavity. Most research on F. nucleatum has focused on its contribution to the pathogenesis of gingivitis and periodontitis through the establishment of a pathogenic subgingival biofilm4 and the induction of a host inflammatory response26. Recent evidence has shown that F. nucleatum, by virtue of its adhesive, invasive, and pro-inflammatory properties, may also be involved in IBD and, consequently, in colorectal cancer5,6,7. This bacterial species colonizes the mucus layer and has been associated with a robust local inflammatory response in human colorectal carcinomas27,28. Periodontal disease and IBD such as ulcerative colitis and Crohn’s disease share some similarities in terms of their pathogenic process. First, diseased sites contain high numbers of macrophages29,30. Second, pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, are thought to play a critical role in the tissue damage observed with these diseases31. Given their anti-inflammatory properties, tea polyphenols show great potential as agents for the prevention and treatment of periodontal disease and IBD. To this end, we investigated the effect of green and black tea polyphenols on the activation of the NF-κB signaling pathway and the secretion of pro-inflammatory mediators by monocytes/macrophages challenged with F. nucleatum.
NF-κB is one of the main pathways used by host cells to respond to microbial challenges. Moreover, NF-κB is often referred to as a central mediator of the human immune response because it plays an essential role in several aspects of human health, including the development of innate and adaptive immunity. As such, plant-derived phytochemicals are promising lead compounds for the development of potent and safe inhibitors for cancer and inflammatory disorders driven by NF-κB32.
Since tea polyphenols, including EGCG and theaflavins, have been reported to possess immunomodulatory properties33, we evaluated the effect of tea polyphenols on F. nucleatum-induced NF-κB activation. We previously showed that F. nucleatum can strongly activate the NF-κB signaling pathway in the U937-3xκB cell line34, as did Kostic et al., who suggested that F. nucleatum induces an NF-κB-driven pro-inflammatory response that may promote colorectal cancer35. We found that 125 μg/ml of EGCG and theaflavins almost completely prevented NF-κB activation. The green tea extract and, to a lesser extent, the black tea extract also attenuated NF-κB activation by F. nucleatum. Given that both extracts have a comparable concentration in total polyphenols, it is likely that the differences between the two extracts in regard to inhibition of NF-κB are related to the composition and proportions of individual catechins and theaflavins.
Our results were in agreement with several other studies showing that both green tea catechins and black tea theaflavins inhibit the activation of NF-κB in cultured cell lines. In lipopolysaccharide-activated macrophages and epidermal cells treated with the tumor promoter 12-O-tetradecanoylphorbol-13-acetate (TPA), black tea theaflavins and EGCG inhibited the phosphorylation of IκB, preventing NF-κB from translocating to the nucleus and binding to DNA36,37. Moreover, in intestinal epithelial cells, EGCG is the most potent inhibitor of the IκB kinase activity of intestinal epithelial cells of any green tea catechin38. Similarly, the oral administration of EGCG markedly attenuates the severity of acetic acid-induced colitis in rats and reduces TNF-α and IFN-γ levels in plasma as well as NF-κB expression in the colon39.
Since NF-κB is a key regulator of over 500 genes, including those coding for the expression of pro-inflammatory mediators40, inhibitors of this transcription factor hold great promise for the prevention and treatment of chronic inflammatory disorders41. In this context, we investigated the effect of tea polyphenols on inflammatory mediator secretion using a macrophage model stimulated with F. nucleatum. Macrophages play a central role in the coordinated resolution of inflammation and the return to tissue homeostasis and are actively involved in all phases of inflammation. They are positioned directly beneath the surface epithelium in the gingiva and the bowel and are the first immune cell population to encounter microbial challenges29,30. Another important feature of monocytes/macrophages is their ability to secrete cytokines and chemokines. In some cases, this inflammatory response in infected periodontal pockets or in the gastrointestinal tract is continuous, resulting in a chronic inflammatory state. Consequently, the mechanisms underlying the destructive processes associated with periodontitis or IBD are not only related to the direct tissue damage caused by bacteria but also to indirect damage mediated by the uncontrolled host inflammatory response. Most, if not all, of the chronic inflammatory states involved in these pathological conditions are characterized by an overproduction of cytokines (TNF-α, IL-6), chemokines (CXCL8), and MMPs42. Elevated levels of these mediators act to amplify the inflammatory process by attracting additional inflammatory cells to the site, thus contributing to tissue destruction and the clinical symptoms observed42. In the present study, we showed that green and black tea extracts as well as their major polyphenols, EGCG and theaflavins, significantly and dose-dependently reduce the secretion of three major pro-inflammatory cytokines (IL-1β, TNF-α, and IL-6) by F. nucleatum-stimulated macrophages.
IL-1β and IL-6 are signature innate cytokines in periodontal disease and have been associated with inflammatory cell migration and osteoclastogenesis43,44. In IBD, IL-1β is involved in the increased recruitment of neutrophils and the activation of innate lymphoid cells (ILCs)45, while IL-6 activates T cells and macrophages, recruits immune cells, and activates acute-phase proteins. Interestingly, the blockade of IL-1β or IL-6 signaling with monoclonal antibodies is effective in suppressing chronic intestinal inflammation in mouse models. All these findings suggest that these two cytokines may be potential therapeutic targets for the treatment of IBD45.
TNF-α is a multi-effect cytokine with many functions, ranging from cell migration to tissue destruction26,45. Its role and contribution to tissue destruction and bone loss has been clearly documented in periodontal disease46. In IBD, TNF-α is a central pro-inflammatory cytokine and causes barrier alterations and promotes the cell death of intestinal epithelial cells and Paneth cells47,48. TNF-α also promotes tissue destruction by increasing the production of MMPs by myofibroblasts49. Interestingly, the administration of an anti-TNF-α monoclonal antibody (infliximab) significantly reduces the clinical symptoms and radiographic and clinical progression of bone loss in both rheumatoid arthritis (human model) and periodontal disease (primate model)50,51. Moreover, TNF-α-specific antibodies (infliximab and certolizumab) may alleviate IBD by simultaneously suppressing several pro-inflammatory pathways45.
F. nucleatum-stimulated macrophages secreted higher levels of the chemokine CXCL8 than of any of the other cytokines investigated. Chemokines are chemotactic cytokines that play a very important role in the migration of phagocytic cells to sites of infection52,53. In addition, CXCL8-mediated chemotactic and activation effects on neutrophils in the inflamed gingiva may contribute to periodontal tissue destruction54. The fact that tea polyphenols inhibited the secretion of CXCL8 by macrophages suggested that they have the potential to reduce the influx of inflammatory cells to diseased sites and the amplification of bacteria-induced inflammatory processes. In the case of periodontal disease and IBD, the significant inhibition of the secretion of all the cytokines tested may have a major indirect impact given that anti-inflammatory modalities can indirectly exert antimicrobial effects. Indeed, periodontal or intestinal dysbiosis is crucially dependent on an inflammatory environment since, for example, inflammatory tissue breakdown products are used as nutrients8,55.
The activation of TREM-1 expressed on neutrophils and monocytes amplifies the expression of various pro-inflammatory cytokines, chemokines, and cell surface receptors56. Controlling this amplified inflammatory response may have a significant impact on the severity of periodontal disease and IBD57,58. Moreover, bacterial challenges induce the release of the soluble form of TREM-1 (sTREM-1) in humans and in mouse models59,60. Patients with moderate or severe ulcerative colitis and Crohn’s disease have been reported to have increased levels of sTREM-161. sTREM-1 has also been detected in gingival crevicular fluid and is present in higher concentrations in diseased periodontal sites16. However, the exact role of sTREM-1 in the inflammatory cascade remains unclear. Some studies have shown that the upregulation of sTREM-1 production is mediated by bacterial challenges62 and concluded that it may be a specific marker of infections in various pathologies13,62. When sTREM-1 is detected in serum it may have been released by circulating leukocytes during the course of a systemic infection or may have been in focal infections, eventually entering the blood stream63. Interestingly, we found that tea polyphenols significantly reduce sTREM-1 levels. To the best of our knowledge, no studies have investigated the potential of F. nucleatum to induce the secretion/shedding of sTREM-1 or the potential of polyphenols to reduce this trend. Bostanci et al. recently showed that P. gingivalis induces TREM-1 expression in monocytes concomitantly with an increased release of sTREM-164. They also reported that doxycycline reduces the expression of TREM-1 and the secretion of sTREM-165.
Macrophages produce several MMPs, which are a group of genetically distinct but structurally-related enzymes involved in the degradation of extracellular matrix and basement membrane proteins during normal tissue turnover and tissue destructive processes66. MMP-3 and MMP-9 have been strongly associated with the progression of periodontitis66. In IBD, particularly Crohn’s disease, MMPs such as collagenases and stromelysins, which can degrade the extracellular matrix, cause ulceration and result in tissue destruction67,68. High levels of extracellular MMPs, which can be upregulated by pro-inflammatory cytokines, have been detected in areas of tissue injury and in ulceration foci in IBD patients68. MMP inhibitors efficiently prevent tissue destruction in other inflammatory processes such rheumatoid arthritis, aphthous disease of oral mucosa, and periodontal disease69,70. Interestingly, we showed that tea polyphenols, including EGCG and theaflavins, reduce MMP-3 and MMP-9 secretion by F. nucleatum-stimulated macrophages. These findings were in agreement with those of a study by Oka et al., who showed that MMP-2 and MMP-9 activities are lower in theaflavin-treated rat osteoclast precursor cells than in control osteoclasts71 and of a study by Yun et al., who reported that EGCG has an inhibitory effect on the gene expression of MMP-9 in osteoblasts and on the formation of osteoclasts72.
Conclusion
In conclusion, we investigated the anti-inflammatory potential of green and black tea extracts, EGCG, and theaflavins using in vitro models related to periodontal disease and IBD. All tea compounds tested exhibited comparable effects, including the capacity to reduce the activation of NF-κB, the secretion of pro-inflammatory mediators and MMPs, and the secretion/shedding of sTREM-1. Given that pathological inflammation involves a loss of tolerance and/or of regulatory processes, the anti-inflammatory properties of tea polyphenols suggested that they may represent promising preventive or therapeutic agents.
Materials and Methods
Tea polyphenols
The commercial green and black tea extracts (Hangzhou Gosun Biotechnologies, China) had polyphenol contents of 98.4% and 92%, respectively. Stock solutions were freshly prepared by dissolving the tea powder (20 mg of green tea extract or 10 mg of black tea extract) in 1 ml of sterile warm distilled water and filtering the solution through a 0.22-μm pore size membrane filter. EGCG (Sigma-Aldrich, Canada), the predominant catechin in the green tea extract (47.9%), was also dissolved in sterile distilled water at a concentration of 10 mg/ml and was sterilized by filtration. The theaflavin preparation was purchased from DeHe Biotechnology (China). According to the product specifications, the preparation is a mixture of theaflavin, theaflavin-3-gallate, theaflavin-3′-gallate, and theaflavin-3,3′-digallate, with more than 80% purity. A stock solution was prepared by dissolving 20 mg of powder in 1 ml of 95% ethanol.
Bacteria and growth conditions
F. nucleatum ATCC 25586 was grown anaerobically (80% N2, 10% CO2, 10% H2) for 24 h at 37 °C in Todd-Hewitt broth (THB; Becton, Dickinson and Company, USA) supplemented with 0.001% hemin and 0.0001% vitamin K.
Activation of the NF-κB transcription factor
The human monoblastic leukemia cell line U937 3xκB-LUC, a subclone of the U937 cell line stably transfected with a luciferase gene coupled to a promoter of three NF-κB- binding sites, was kindly provided by R. Blomhoff (University of Oslo, Norway)73. The cells were routinely cultivated in Roswell Park Memorial Institute 1640 medium (RPMI-1640; Life Technologies Inc., Canada) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 μg/ml of penicillin G/streptomycin, and 75 μg/ml of hygromycin B at 37 °C in a 5% CO2 atmosphere. To investigate the effect of tea polyphenols on F. nucleatum-induced NF-κB activation, U937 3xκB-LUC cells (106 cells/ml) were pre-incubated with the compounds (non-cytotoxic concentrations; in RPMI containing 1% FBS) for 30 min in the wells of a black wall, black bottom 96-well microplate (Greiner Bio-One North America Inc., USA). They were then stimulated for 6 h with F. nucleatum at a multiplicity of infection (MOI) of 100. The bacterial suspension was prepared from an overnight culture of F. nucleatum. Wells with no F. nucleatum or no compounds were used as controls. An assay using the commercial inhibitor BAY-11-7082 (5 μg/ml; EMD Millipore, Canada) was used as a positive control for the inhibition of the NF-κB signaling pathway. NF-κB activation was determined by measuring luciferase activity following the addition of Bright-Glo reagent (Promega Corporation, USA) in accordance with the manufacturer’s protocol. Luminescence was monitored using a Synergy 2 microplate reader (BioTek Instruments, USA).
Cytokine and MMP secretion by macrophages
U937 human monocytes (CRL-1593.2; American Type Culture Collection, USA) were cultivated in RPMI-1640 supplemented with 10% heat-inactivated FBS and 100 μg/ml of penicillin G/streptomycin at 37 °C in a 5% CO2 atmosphere. The monocytes (2.5 × 105 cells/ml) were incubated in RPMI-10% FBS containing 100 ng/ml of phorbol myristic acid (PMA; Sigma-Aldrich, Canada) for 48 h to induce differentiation into adherent macrophage-like cells74. The adherent macrophage-like cells were harvested by gentle scraping followed by centrifugation at 1,200 × g for 5 min. The cells were washed, suspended in RPMI-1% FBS at a concentration of 1 × 106 cells/ml, seeded in the wells of a 12-well microplate (1 × 106 cells/well), and incubated overnight at 37 °C in a 5% CO2 atmosphere. The macrophage-like cells were then pre-treated for 2 h with either green tea extract, black tea extract, EGCG, or theaflavins (non-cytotoxic concentrations; in RPMI containing 1% FBS) prior to being stimulated with F. nucleatum at an MOI of 100. An assay using a commercial inhibitor (BAY-11-7082; 5 μg/ml) was used as a positive inhibitory control. After a 24-h incubation at 37 °C in a 5% CO2 atmosphere, the culture medium supernatants were collected and were stored at −20 °C until used. Cells incubated in culture medium with or without tea polyphenols and stimulated or not with bacteria were used as controls. Enzyme-linked immunosorbent assay (ELISA) kits (eBioscience Inc., USA; R&D Systems, USA) were used to determine IL-1β, IL-6, CXCL8, TNF-α, MMP-3, MMP-8, MMP-9, and sTREM-1 concentrations according to the manufacturers’ protocols.
Effects of tea polyphenols on cell viability
Prior to evaluating the anti-inflammatory properties of tea polyphenols, we determined their cytotoxic effect on the two cell lines to exclude the possibility that toxicity related to the compounds might cause a decrease in NF-κB activation or in cytokine and MMP secretion. The cells were treated for 24 h with either green tea extract, black tea extract, EGCG, or theaflavins at concentrations of 500, 250, 125, 62.5, 31.25, 15.625, and 7.9 μg/ml. A colorimetric MTT cell viability assay (Roche Diagnostics, Germany) using 3-[4,5-diethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide as the substrate was performed according to the manufacturer’s protocol. Untreated control cells were assigned a value of 100%.
Statistical analysis
Unless indicated otherwise, all experiments were performed in triplicate in three independent experiments. The data are expressed as means ± standard deviations (SD). Statistical analyses were performed using a one-way analysis of variance with a post hoc Bonferroni multiple comparison test (GraphPad Software Inc., USA). All results were considered statistically significant at p < 0.001.
Additional Information
How to cite this article: Lagha, A. B. and Grenier, D. Tea polyphenols inhibit the activation of NF-κB and the secretion of cytokines and matrix metalloproteinases by macrophages stimulated with Fusobacterium nucleatum. Sci. Rep. 6, 34520; doi: 10.1038/srep34520 (2016).
Acknowledgments
This study was funded by the Laboratoire de Contrôle microbiologique of Université Laval.
Footnotes
Author Contributions A.B.L. conducted the experiments, A.B.L. and D.G. analyzed the results, D.G. contributed reagents/materials. The manuscript was written by A.B.L. and D.G.
References
- Pizzo G., Guiglia R., Lo Russo L. & Campisi G. Dentistry and internal medicine: from the focal infection theory to the periodontal medicine concept. Eur J Intern Med 21, 496–502, 10.1016/j.ejim.2010.07.011 (2010). [DOI] [PubMed] [Google Scholar]
- Berezow A. B. & Darveau R. P. Microbial shift and periodontitis. Periodontol 2000 55, 36–47, 10.1111/j.1600-0757.2010.00350.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ximenez-Fyvie L. A., Haffajee A. D. & Socransky S. S. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J Clin Periodontol 27, 648–657 (2000). [DOI] [PubMed] [Google Scholar]
- Socransky S. S. & Haffajee A. D. Dental biofilms: difficult therapeutic targets. Periodontol 2000 28, 12–55 (2002). [DOI] [PubMed] [Google Scholar]
- Bashir A., Miskeen A. Y., Hazari Y. M., Asrafuzzaman S. & Fazili K. M. Fusobacterium nucleatum, inflammation, and immunity: the fire within human gut. Tumour Biol 37, 2805–2810, 10.1007/s13277-015-4724-0 (2016). [DOI] [PubMed] [Google Scholar]
- Allen-Vercoe E., Strauss J. & Chadee K. Fusobacterium nucleatum: an emerging gut pathogen? Gut Microbes 2, 294–298, 10.4161/gmic.2.5.18603 (2011). [DOI] [PubMed] [Google Scholar]
- Underwood M. A. Intestinal dysbiosis: novel mechanisms by which gut microbes trigger and prevent disease. Prev Med 65, 133–137, 10.1016/j.ypmed.2014.05.010 (2014). [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends Immunol 35, 3–11, 10.1016/j.it.2013.09.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappa U., Reinking-Zappa M., Graf H. & Espeland M. Cell populations and episodic periodontal attachment loss in humans. J Clin Periodontol 18, 508–515 (1991). [DOI] [PubMed] [Google Scholar]
- Anderson K. V. Toll signaling pathways in the innate immune response. Curr Opin Immunol 12, 13–19 (2000). [DOI] [PubMed] [Google Scholar]
- Uehara A. & Takada H. Functional TLRs and NODs in human gingival fibroblasts. J Dent Res 86, 249–254 (2007). [DOI] [PubMed] [Google Scholar]
- Gibot S. & Massin F. Soluble form of the triggering receptor expressed on myeloid cells 1: an anti-inflammatory mediator? Intensive Care Med 32, 185–187, 10.1007/s00134-005-0018-0 (2006). [DOI] [PubMed] [Google Scholar]
- Gibot S. et al. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med 350, 451–458, 10.1056/NEJMoa031544 (2004). [DOI] [PubMed] [Google Scholar]
- Collins C. E. et al. Elevated synovial expression of triggering receptor expressed on myeloid cells 1 in patients with septic arthritis or rheumatoid arthritis. Ann Rheum Dis 68, 1768–1774, 10.1136/ard.2008.089557 (2009). [DOI] [PubMed] [Google Scholar]
- Park J. J. et al. Correlation of serum-soluble triggering receptor expressed on myeloid cells-1 with clinical disease activity in inflammatory bowel disease. Dig Dis Sci 54, 1525–1531, 10.1007/s10620-008-0514-5 (2009). [DOI] [PubMed] [Google Scholar]
- Bisson C. et al. Increased gingival crevicular fluid levels of soluble triggering receptor expressed on myeloid cells (sTREM) -1 in severe periodontitis. J Clin Periodontol 39, 1141–1148, 10.1111/jcpe.12008 (2012). [DOI] [PubMed] [Google Scholar]
- Bostanci N., Ozturk V. O., Emingil G. & Belibasakis G. N. Elevated oral and systemic levels of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) in periodontitis. J Dent Res 92, 161–165, 10.1177/0022034512470691 (2013). [DOI] [PubMed] [Google Scholar]
- Belibasakis G. N., Ozturk V. O., Emingil G. & Bostanci N. Soluble triggering receptor expressed on myeloid cells 1 (sTREM-1) in gingival crevicular fluid: association with clinical and microbiologic parameters. J Periodontol 85, 204–210, 10.1902/jop.2013.130144 (2014). [DOI] [PubMed] [Google Scholar]
- Bostanci N. et al. Porphyromonas gingivalis regulates TREM-1 in human polymorphonuclear neutrophils via its gingipains. PLoS One 8, e75784, 10.1371/journal.pone.0075784 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannobile W. V. Host-response therapeutics for periodontal diseases. J Periodontol 79, 1592–1600, 10.1902/jop.2008.080174 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preshaw P. M. Host response modulation in periodontics. Periodontol 2000 48, 92–110, 10.1111/j.1600-0757.2008.00252.x (2008). [DOI] [PubMed] [Google Scholar]
- El Gharras H. Polyphenols: food sources, properties and applications a review. Int J Food Sci Tech 44, 2512–2518, 10.1111/j.1365-2621.2009.02077.x (2009). [DOI] [Google Scholar]
- Graham H. N. Green tea composition, consumption, and polyphenol chemistry. Prev Med 21, 334–350 (1992). [DOI] [PubMed] [Google Scholar]
- Higdon J. V. & Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr 43, 89–143, 10.1080/10408690390826464 (2003). [DOI] [PubMed] [Google Scholar]
- Chen H., Shurlknight K., Leung T. & Sang S. Structural identification of theaflavin trigallate and tetragallate from black tea using liquid chromatography/electrospray ionization tandem mass spectrometry. J Agric Food Chem 60, 10850–10857, 10.1021/jf303749z (2012). [DOI] [PubMed] [Google Scholar]
- Cekici A., Kantarci A., Hasturk H. & Van Dyke T. E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000 64, 57–80, 10.1111/prd.12002 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy A. N. et al. Fusobacterium is associated with colorectal adenomas. PLoS One 8, e53653, 10.1371/journal.pone.0053653 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellarin M. et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 22, 299–306, 10.1101/gr.126516.111 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasturk H., Kantarci A. & Van Dyke T. E. Oral inflammatory diseases and systemic inflammation: role of the macrophage. Front Immunol 3, 118, 10.3389/fimmu.2012.00118 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl A. A., Erben U., Kredel L. I. & Siegmund B. Diversity of intestinal macrophages in inflammatory bowel diseases. Front Immunol 6, 613, 10.3389/fimmu.2015.00613 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldoveanu A. C., Diculescu M. & Braticevici C. F. Cytokines in inflammatory bowel disease. Rom J Intern Med 53, 118–127 (2015). [DOI] [PubMed] [Google Scholar]
- Golan-Goldhirsh A. & Gopas J. Plant derived inhibitors of NF-κB. Phytochem Rev 13, 107–121, 10.1007/s11101-013-9293-5 (2013). [DOI] [Google Scholar]
- Clarke J. O. & Mullin G. E. A review of complementary and alternative approaches to immunomodulation. Nutr Clin Pract 23, 49–62 (2008). [DOI] [PubMed] [Google Scholar]
- Ben Lagha A., Dudonne S., Desjardins Y. & Grenier D. Wild blueberry (Vaccinium angustifolium Ait.) polyphenols target Fusobacterium nucleatum and the host inflammatory response: Potential innovative molecules for treating periodontal diseases. J Agric Food Chem 63, 6999–7008, 10.1021/acs.jafc.5b01525 (2015). [DOI] [PubMed] [Google Scholar]
- Kostic A. D. et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14, 207–215, 10.1016/j.chom.2013.07.007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M. H., Lin-Shiau S. Y., Ho C. T., Lin J. H. & Lin J. K. Suppression of lipopolysaccharide-induced nuclear factor-kappaB activity by theaflavin-3,3′-digallate from black tea and other polyphenols through down-regulation of IkappaB kinase activity in macrophages. Biochem Pharmacol 59, 357–367 (2000). [DOI] [PubMed] [Google Scholar]
- Nomura M., Ma W., Chen N., Bode A. M. & Dong Z. Inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced NF-kappaB activation by tea polyphenols, (-)-epigallocatechin gallate and theaflavins. Carcinogenesis 21, 1885–1890 (2000). [DOI] [PubMed] [Google Scholar]
- Yang F. et al. The green tea polyphenol (-)-epigallocatechin-3-gallate blocks nuclear factor-kappa B activation by inhibiting I kappa B kinase activity in the intestinal epithelial cell line IEC-6. Mol Pharmacol 60, 528–533 (2001). [PubMed] [Google Scholar]
- Ran Z. H., Chen C. & Xiao S. D. Epigallocatechin-3-gallate ameliorates rats colitis induced by acetic acid. Biomed Pharmacother 62, 189–196, 10.1016/j.biopha.2008.02.002 (2008). [DOI] [PubMed] [Google Scholar]
- Pahl H. L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 18, 6853–6866, 10.1038/sj.onc.1203239 (1999). [DOI] [PubMed] [Google Scholar]
- Souza J. A., Rossa C. Jr., Garlet G. P., Nogueira A. V. & Cirelli J. A. Modulation of host cell signaling pathways as a therapeutic approach in periodontal disease. J Appl Oral Sci 20, 128–138 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P. C. et al. Inflammatory disease processes and interactions with nutrition. Br J Nutr 101 Suppl 1, S1–45, 10.1017/S0007114509377867 (2009). [DOI] [PubMed] [Google Scholar]
- Fonseca J. E., Santos M. J., Canhao H. & Choy E. Interleukin-6 as a key player in systemic inflammation and joint destruction. Autoimmun Rev 8, 538–542, 10.1016/j.autrev.2009.01.012 (2009). [DOI] [PubMed] [Google Scholar]
- Graves D. T., Fine D., Teng Y. T., Van Dyke T. E. & Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol 35, 89–105, 10.1111/j.1600-051X.2007.01172.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath M. F. Cytokines in inflammatory bowel disease. Nat Rev Immunol 14, 329–342, 10.1038/nri3661 (2014). [DOI] [PubMed] [Google Scholar]
- Liu Y. C., Lerner U. H. & Teng Y. T. Cytokine responses against periodontal infection: protective and destructive roles. Periodontol 2000 52, 163–206, 10.1111/j.1600-0757.2009.00321.x (2010). [DOI] [PubMed] [Google Scholar]
- Su L. et al. TNFR2 activates MLCK-dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology 145, 407–415, 10.1053/j.gastro.2013.04.011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther C. et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature 477, 335–339, 10.1038/nature10400 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sabatino A. et al. Functional modulation of Crohn’s disease myofibroblasts by anti-tumor necrosis factor antibodies. Gastroenterology 133, 137–149, 10.1053/j.gastro.2007.04.069 (2007). [DOI] [PubMed] [Google Scholar]
- Assuma R., Oates T., Cochran D., Amar S. & Graves D. T. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol 160, 403–409 (1998). [PubMed] [Google Scholar]
- Breedveld F. C. et al. Infliximab in active early rheumatoid arthritis. Ann Rheum Dis 63, 149–155 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther S. A. & Cyster J. G. Chemokines as regulators of T cell differentiation. Nat Immunol 2, 102–107, 10.1038/84205 (2001). [DOI] [PubMed] [Google Scholar]
- Gerard C. & Rollins B. J. Chemokines and disease. Nat Immunol 2, 108–115, 10.1038/84209 (2001). [DOI] [PubMed] [Google Scholar]
- Scott D. A. & Krauss J. Neutrophils in periodontal inflammation. Front Oral Biol 15, 56–83, 10.1159/000329672 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamboli C. P., Neut C., Desreumaux P. & Colombel J. F. Dysbiosis in inflammatory bowel disease. Gut 53, 1–4 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk M., Bouchon A., Seibold F. & Mueller C. TREM-1-expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J Clin Invest 117, 3097–3106, 10.1172/JCI30602 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchon A., Dietrich J. & Colonna M. Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J Immunol 164, 4991–4995 (2000). [DOI] [PubMed] [Google Scholar]
- Colonna M. TREMs in the immune system and beyond. Nat Rev Immunol 3, 445–453, 10.1038/nri1106 (2003). [DOI] [PubMed] [Google Scholar]
- Gibot S. et al. Surface and soluble triggering receptor expressed on myeloid cells-1: expression patterns in murine sepsis. Crit Care Med 33, 1787–1793 (2005). [DOI] [PubMed] [Google Scholar]
- Gibot S. Clinical review: role of triggering receptor expressed on myeloid cells-1 during sepsis. Crit Care 9, 485–489, 10.1186/cc3732 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzivras M. et al. Role of soluble triggering receptor expressed on myeloid cells in inflammatory bowel disease. World J Gastroenterol 12, 3416–3419 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibot S. et al. A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J Exp Med 200, 1419–1426, 10.1084/jem.20040708 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleharski J. R. et al. A role for triggering receptor expressed on myeloid cells-1 in host defense during the early-induced and adaptive phases of the immune response. J Immunol 170, 3812–3818 (2003). [DOI] [PubMed] [Google Scholar]
- Bostanci N., Thurnheer T. & Belibasakis G. N. Involvement of the TREM-1/DAP12 pathway in the innate immune responses to Porphyromonas gingivalis. Mol Immunol 49, 387–394, 10.1016/j.molimm.2011.09.012 (2011). [DOI] [PubMed] [Google Scholar]
- Bostanci N. & Belibasakis G. N. Doxycycline inhibits TREM-1 induction by Porphyromonas gingivalis. FEMS Immunol Med Microbiol 66, 37–44, 10.1111/j.1574-695X.2012.00982.x (2012). [DOI] [PubMed] [Google Scholar]
- Sorsa T. et al. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann Med 38, 306–321, 10.1080/07853890600800103 (2006). [DOI] [PubMed] [Google Scholar]
- Shanahan F. Crohn’s disease. Lancet 359, 62–69, 10.1016/S0140-6736(02)07284-7 (2002). [DOI] [PubMed] [Google Scholar]
- von Lampe B., Barthel B., Coupland S. E., Riecken E. O. & Rosewicz S. Differential expression of matrix metalloproteinases and their tissue inhibitors in colon mucosa of patients with inflammatory bowel disease. Gut 47, 63–73 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L., Drummond A. H. & Galloway W. A. Metalloproteinase inhibitors as therapeutics. Clin Exp Rheumatol 11 Suppl 8, S91–S94 (1993). [PubMed] [Google Scholar]
- Ryan M. E., Ramamurthy S. & Golub L. M. Matrix metalloproteinases and their inhibition in periodontal treatment. Curr Opin Periodontol 3, 85–96 (1996). [PubMed] [Google Scholar]
- Oka Y. et al. Tea polyphenols inhibit rat osteoclast formation and differentiation. J Pharmacol Sci 118, 55–64 (2012). [DOI] [PubMed] [Google Scholar]
- Yun J. H. et al. Inhibitory effects of green tea polyphenol (-)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J Periodontal Res 39, 300–307, 10.1111/j.1600-0765.2004.00743.x (2004). [DOI] [PubMed] [Google Scholar]
- Carlsen H., Moskaug J. O., Fromm S. H. & Blomhoff R. In vivo imaging of NF-kappa B activity. J Immunol 168, 1441–1446 (2002). [DOI] [PubMed] [Google Scholar]
- Rovera G., Santoli D. & Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci USA 76, 2779–2783 (1979). [DOI] [PMC free article] [PubMed] [Google Scholar]