Abstract
Accurate detection and characterization of cancers is key for providing timely intervention and effective treatments. Current imaging technologies are particularly limited when it comes to detecting very small tumors in vivo, i.e. very early cancers or metastases, differentiating viable tumor from surrounding dead tumor tissue, and evaluating tumor metabolism within tissue. Optoacoustic imaging offers potential solutions to these imaging problems because of its ability to image optical absorption properties of both intrinsic tissue chromophores and exogenous contrast agents without the involvement of ionizing radiation. Optoacoustic imaging uses pulsed laser to induce localized thermoelastic expansion that generates acoustic waves detectable by an ultrasound transducer. To date, Multispectral optoacoustic tomography (MSOT) has primarily been utilized in preclinical research; however, its use in translational and clinical research is expanding. This review focuses on the current and emerging applications of optoacoustic imaging for the molecular imaging of cancer using both exogenous and endogenous contrast agents and sheds light on potential future clinical applications.
Introduction
The accurate detection and localization of cancers in vivo is critical to medical decisions and improved treatments. Unfortunately, limitations of contrast (reporter) agents, resolution, and restrictions of depth reduce the ability of most imaging methodologies to detect and localize multiple contrast agents simultaneously, e.g., EGFR plus PD-L1 localization, restricting to ability to monitor tumors using imaging. Alternatively, optical imaging provides functional information and the ability to simultaneously detect multiple biomarkers as contrast agents; however, these approaches are restricted to superficial detection as light scattering degrades the spatial resolution at increased penetration depths. Multispectral optoacoustic tomography (MSOT) is emerging as an alternative modality that is not restricted by many of the limitations of the imaging used in the diagnosis and treatment of diseases (1, 2). MSOT was initially developed for research (1, 3, 4), but has been adapted for clinical uses (2).
Optoacoustic imaging is based upon a “light-in, sound-out” approach where absorption of near infrared light (NIR) within biological tissues generates ultrasonic waves with much less scattering, longer range of detection and higher accuracy compared to traditional optical imaging. The optoacoustic approach is unique with increased optical contrast and signal-to-noise ratios (1-17). Optoacoustic imaging retains the advantages of optical imaging including high specificity to identify functional and molecular processes in living organisms with high sensitivity. Most tissues are relatively transparent to NIR light in the range of 600 to 900 nm; therefore, use of NIR light excitation and ultrasound signals render photon scattering irrelevant to image formation, enabling high-resolution insights into the biological function of tumors and organs. Once the sound waves are generated, they obey the physical laws of sound transmission; the intensity of the sound increases with the number of molecules excited but is reduced by distance and the extent of ultrasound diffraction due to different densities of tissue. In MSOT, multiple spectral components of NIR light are varied automatically to excite specific molecules permitting accurate tomographic images to be constructed from the resulting ultrasonic signals. MSOT is also unique in its ability to detect multiple contrast agents simultaneously based upon differential spectral shape (Supplementary Fig. S1).
Imaging of tumors and cancer-related morphologic changes in tissues by MSOT is facilitated through exogenous contrast agents, including clinically approved optical dyes (e.g. indocyanine green), markers targeted to cell surface molecules, e.g., EGFR receptors (7, 8), the tumor microenvironment (e.g. pH)(1, 18), and endogenous absorbers (e.g., oxyhemoglobin)(2, 17, 19, 20). Use of MSOT in multiple tissue types and at varying depths, i.e at least 5 cm, can provide functional real time 3D information at high spatial resolution in vivo (2-4, 21) (Fig. 1, Supplementary Video S1). This ability will significantly impact clinical care in systemic diseases including cancer involving multiple organs. MSOT’s ability to identify tumors indicates great potential for clinical applicability for solid tumors (2), i.e. melanoma, head and neck, breast, pancreatic, prostate, colon, and potentially liver.
Figure 1.
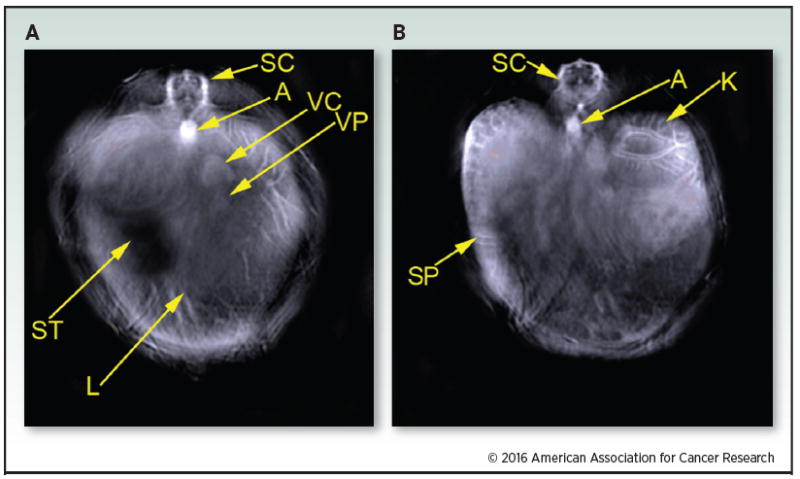
Single wavelength images were each captured using MSOT to form a background image. The representative images were taken at 900 nm. The image slices shown represents the region of the liver (A) and kidney (B). The following structures were identified:spinal cord (SC); aorta (A); vena cava (VC); vena porta (VP); liver (L); stomach (ST); kidney (K); spleen (SP).
This manuscript reviews current research as well as clinical applications of MSOT in oncology, including dynamic imaging of cancer biomarkers, nanoparticles, and real-time evaluation of cancer metabolism. Its aim is to highlight pertinent studies, especially of tumor associated molecules, with the potential for use in MSOT for the clinical management of cancer.
Endogenous Contrast Agents
Endogenously occurring molecules and exogenous materials investigated as contrast agents for optoacoustic imaging are in Tables 1 and 2. Of importance, to date there are relatively few endogenous contrast agents for MSOT.
Table 1.
Identification of contrast agents detectable using MSOT. Endogenous chromophores and exogenous contrast agents, i.e. organic dyes and nanoparticles, detectable using MSOT.
| Endogenous chromophores | Organic Dyes | Nanoparticles |
|---|---|---|
| Oxyhemoglobin | Indocyanine Green | Gold |
| Deoxyhemoglobin | Methylene Blue | Iron Oxide |
| Melanin | CF-750 | Silver |
| HiLite 750 | Tungsten | |
| IR-780 | Carbon Nanotubes | |
| IR800CW |
Table 2.
Several monoclonal antibodies to specific antigens associated with cancer have the potential to serve as targeted contrast agents for imaging of cancers by MSOT to provide tumor specific molecular information.
| Antigen category | Examples of antigens | mAbs raised against these targets | Tumors expressing antigen |
|---|---|---|---|
| Cell Growth and Differentiation | EGFR | Cetuximab, panitumumab, | Glioma, lung, breast, colon, and head and neck |
| ERBB2 | Trastuzumab and pertuzumab | Breast, colon, lung, ovarian and prostate | |
| ERBB3 | MM-121 | Breast, colon, lung, ovarian and prostate | |
| IGF1R | AVE1642, IMC-A12, MK-0646, R1507 and CP 751871 | Pancreas, Glioma, lung, breast, head and neck, prostate and thyroid | |
| TRAILR1 | TRA-8, Mapatumumab | Pancreas, colon, lung | |
| EPHA3 | KB004 and IIIA4 | Lung, kidney and colon melanoma, glioma and ALL | |
| MET | AMG 102 and METMAB | Breast, ovary and lung | |
| Targets of anti-angiogenic mAbs | VEGF | Bevacizumab | Vasculature |
| VEGFR | IM-2C6 and CDP791 | Epithelial Tumors | |
| Integrin ±V2 3 | Etaracizumab | Vasculature | |
| Integrin ±52 1 | Volociximab | Vasculature | |
| Glycoproteins expressed by solid tumors | EpCAM | IGN101 and adecatumumab | Breast, colon |
| CEA | Labetuzumab | Breast, colon | |
| Mucins (Muc 16) | Pemtumomab and oregovomab | Ovarian, breast, colon, lung | |
| TAG-72 | minretumomab | Breast, colon | |
| CAIX | cG250 | Renal cell carcinoma | |
| PSMA | J591 | Prostate | |
| Folate-binding protein | farletuzumab | Ovarian |
Hemoglobin (i.e., oxyhemoglobin and deoxyhemoglobin)
Changes in vasculature are often associated with oncologic, inflammatory, and immune disorders, but microvascular changes occur below the resolution of common clinical imaging modalities. MSOT identifies microvascularity and tissue oxygenation by hemoglobin absorption of multiple wavelengths of light to generate high optoacoustic contrast (17, 22). Because oxy- and deoxyhemoglobin each generate a unique optoacoustic signal, both oxy- and deoxyhemoglobin can be observed simultaneously without the addition of exogenous contrast agents using MSOT (Fig. 2). MSOT can distinguish between oxygenation states of hemoglobin, allowing visualization of differential blood saturation by oxygen within tissues including the capability to differentiate between ischemic areas of tumors and the surrounding tissue (1, 2). Of note, when an area becomes necrotic, it typically no longer contains hemoglobin; therefore, the necrotic area appears as “black” in MSOT reconstructions. MSOT can distinguish tumors from surrounding normal tissues by atypical vascularity and differences in tissue perfusion and oxygenation and can detect vascular changes as markers of responses to anti-neoplastic therapies.
Figure 2.
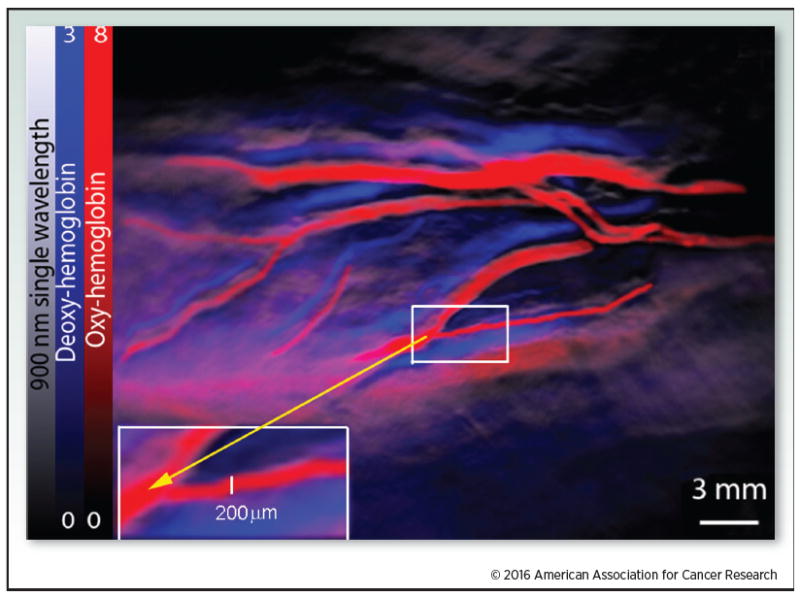
Oxy- and deoxy-hemoglobin were detected using MSOT. Oxy- and deoxyhemoglobin were visualized within a human wrist using a pre-clinical MSOT instrument. The red color bar represents oxyhemoglobin and the blue color bar represents deoxyhemoglobin. The gray scale image was provided using a 900 nm single wavelength.
Hemoglobin oxygenation has been used for tumor identification and characterization by detecting vascular abnormalities and oxygenation status (23). Currently, analytical methods, i.e. microbubble contrast identified using ultrasound, vascular spin labeling, and BOLD (Blood Oxygen Level Dependent), are used to identify thrombosis (24). However, recent studies have shown promising results for high resolution optoacoustic imaging of breast cancer based on tumor angiogenesis (1, 17, 25, 26); this has been utilized to monitor tumor responses to antiangiogenic agents, such as bevacizumab (23, 27). A recent advance is to use red blood cells that are homozygous for hemoglobin S; these cells deform under low oxygen causing thrombosis in areas of tumors that are ischemic (28); MSOT may be useful to image all forms of thrombotic therapy.
Melanin
Melanin is another endogenous substance that acts as an optoacoustic agent over the wavelengths of NIR light pertinent to MSOT imaging. However, it must be noted that the strong optoacoustic signal obtained from high levels of melanin could inhibit the ability to detect other contrast agents. See section on melanomas.
Exogenous Contrast Agents
Many tumor types express unique substances which could be useful as cancer markers and/or could be developed as potentially clinically relevant targets for MSOT imaging to aid in diagnosis, staging, and characterization of common cancers, e.g., EGFR, PD-L1 and PD-1, folate receptors, thyroglobulin, and HER2 (Table 2). MSOT provides an excellent link between tumor imaging and delivery of selective therapy. Studies of some of these biomarkers as targets for MSOT are in early stages.
Organic dyes
Advantages of exogenous contrast materials for MSOT are that they provide a wider range of contrast agents and possess absorption spectra distinct from the endogenous signals of the tissues being imaged; therefore, their signals can be separated from the tissue background using spectral unmixing in similar fashion as in the case of other dynamic contrast enhanced methodologies such as fluorescent lifetime imaging (22). Organic dyes utilized in various clinical applications, including methylene blue (29) and indocyanine green (8, 30), can generate contrast for MSOT. NIR-reporter dyes can be created to detect cancers using tumor associated ligands, i.e. EGF, monoclonal antibodies, nanoparticles, or agents to evaluate tumor microenvironmental features, e.g., acidic pH or MMPs (see protolytic section, Fig. 3). A combination of endogenous and exogenous contrast agents improves the accuracy of sentinel lymph node biopsies and the characterization of patterns of lymphatic drainage of tumors (2, 31).
Figure 3.
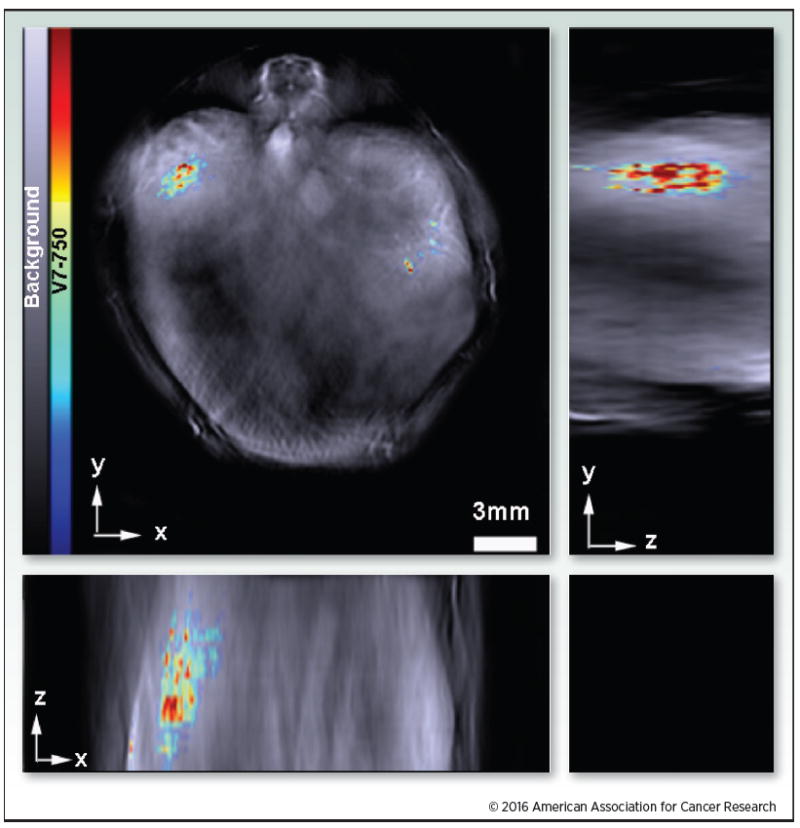
Accumulation of a pH responsive ligand-targeted probe within an orthotopic pancreatic tumor. The orthogonal image demonstrates 3D accumulation of V7-750 within the mouse in the xyz-plane. The rainbow color bar represents intensity of the V7-750 probe. Adapted from Kimbrough et al. (1).
Nanoparticles
Improving local delivery of drugs is essential in order to decrease chemotherapy morbidity and to improve survival. Utilization of nanoparticles for tumor imaging plus targeted drug delivery has been investigated using numerous nanoagents containing fluorescent dyes, gold, or silver for imaging and therapeutic drug delivery vehicles (32-35). To improve tumor delivery of nanoagents to tumors it is necessary to longitudinally track their biodistribution and accumulation, especially in the reticuloendothelial system, and to increase tumor accumulation and to decrease off-target uptake. Because MSOT detects distinct absorption spectra, it can monitor longitudinally the distribution of systemically administered nanoagents targeting tumors (Supplementary Video S2).
MSOT has identified tumor specific accumulation and biodistribution of targeted gold nanorods, mesoporous silica nanoparticles and liposomes in pancreatic tumors (9-11) and polymeric nanoparticles in breast tumors (35). Evaluation of tumor specificity, biodistribution and pharmacokinetics of tumor targeted nanoparticles, in the context of orthotopic and genetically modified organisms, represents an expanding, but inadequately developed use of MSOT; however, several light-absorbing materials, i.e. gold (9, 36-38), tungsten (39), iron-oxide nanoparticles (40), silver nanoparticles (41), carbon nanotubes (42, 43), or NIR-dye containing nanoparticles (10, 11, 44, 45), have been developed and have demonstrated utility for optoacoustic imaging and/or MSOT in animal models.
Targeted surface agents
Because exogenous agents can target cancer specific cell surface markers, some are used for both diagnostic and therapeutic targeting. Optical fluorescence imaging is a diagnostic approach in which a targeting agent is tagged with a fluorescent dye. Similarly, MSOT can be used by tagging the targeting agent with a contrast agent possessing absorption spectra within the NIR range (1, 7, 8). In practice, targeting agents may be used in combination with multiple organic dyes or nanoparticles and their spectra can be separated by spectral unmixing (8).
An excellent example of a targeted molecule for MSOT is EGFR which has an external domain frequently targeted for therapeutic purposes (46, 47). This extracellular domain if overexpressed on cancer cells, can be targeted with nanoparticles or other optoacoustic contrast agents bound to ligands or antibodies to EGFR. Conjugation of organic dyes or gold nanoparticles to blocking (inhibitory) antibodies such as cetuximab could serve both for treatment and imaging (48).
Tumor microenvironment
Acidic pH and Tumor Imaging
Secondary to the production of lactic acid, most malignant tumors develop areas of low extracellular pH (generally pHe 6.4-6.8) compared to normal or uninvolved tissue, benign tumors, and most non-neoplastic diseases (pH 7.0-7.4). The acidic pH can be problematical for cancer therapy as some chemotherapeutic agents do not function below pHe 6.8. Acidic pH, pHe < 6.8, also facilitates the development of metastases and inhibits immune cell function. In general, detection of cancer based upon acidic pH using various methods of imaging has been suboptimal due to 1) the narrow targeting window of < Δ 1.0 pH between cancer and non-malignant tissue and 2) the high levels of pH specific agents, such as aliphatic amines or carboxylates, which have a pKa near physiological range used for MR imaging can buffer the tumor (49-51).
Because MSOT generally requires low concentrations of contrast agents, MSOT based contrast agents identifying pHe detect a variety of cancers. V7 is a peptide whose structure is modified at pHe ≤ 6.8 permitting V7 to integrate it into the membranes of cells (1,18). When this peptide is conjugated to a dye that absorbs NIR light, e.g., V7-750, it selectively images cells in acidic pHe areas (1, 18).
Protolytic evaluation
Several members of the family of matrix metalloproteinases (MMPs), the zinc-dependent endopeptidases degrading the extracellular matrix (ECM), are implicated in cancer progression. Specifically, gelatinases A and B (MMP-2 and -9) play a role in tumor invasion and angiogenesis which promote tumor aggressiveness. A MMP-2 cleavable probe has been created that is detectable using optoacoustic imaging (52). Utilizing a cleavable linker attached to an optoacoustic sensitive reporter and cell anchoring peptide represents a general approach that has been utilized to monitor the in vivo activity of other extracellular enzymes such as cathepsin D (53).
Clinical Cancer Imaging of Human Cancers
Imaging is important in the management of all cancer types including staging of primary tumors, intraoperative identification of surgical margins and determination of the effectiveness of neoadjuvant and definitive therapies. The goals of imaging vary with tumor type, location, and required depth for detection of tumor. For example, determining margins of a primary tumor of the ascending colon would seldom be important because very wide margins are utilized; however, imaging is likely to be important for therapeutic planning in patients with large cancers of the mouth or for application of novel therapies such as irreversible electroporation in patients with locally advanced pancreas cancer. The following describes imaging of cancers using MSOT.
Melanomas
Because most melanomas express melanin, MSOT can aid in the diagnosis and staging of melanomas (2), by identifying the depth of invasion and potential nodal metastases (2, 31). Also, melanomas can be identified due to their atypical patterns of vascularity. MSOT and optoacoustic imaging have been shown to detect melanin in sentinel lymph nodes in vivo and ex vivo with excellent concordance between in vivo imaging and ex vivo histology (2, 31). This offers the potential to identify non-invasively lymph nodes involved by tumor, potentially reducing the need for extensive surgical excisions and related complications. Identification by MSOT of micrometastatic disease permits more accurate sensitivity and specificity of sentinel lymph node biopsies, which will optimize surgical management as well as radiation and/or systemic therapies. Also, MSOT can be used to monitor potential metastatic sites for recurrence. While MSOT can detect melanoma based upon melanin, detection of amelanotic melanoma likely requires a tumor specific exogenous contrast agent. With practice, it should be possible to differentiate melanin with macrophages (diffusely scattered) vs melanin in micrometastasis.
Breast cancer imaging
Accurate preoperative imaging of the breast is important in staging and planning for optimal management of breast cancers. Because malignancies have increased vascular density in comparison to normal human breast tissue, MSOT based on oxyhemoglobin can distinguish between breast cancers and other abnormalities such as cysts as demonstrated using optoacoustic imaging (54). The diagnostic accuracy of MSOT may improve the detection of malignant masses especially in dense breasts, currently a problem for standard mammography (55). Alternatively, MSOT may be an important adjunct to mammography to characterize “suspicious” lesions. When axillary masses are identified in the absence of other clinical indications of breast cancer, MSOT can be used to identify metastatic disease, especially melanomas.
Head and neck lesions
Clinical versions of MSOT could detect metastatic nodal lesions of the head and neck, thyroid lesions, salivary gland tumors, and melanomas secondary to differences in neo-vascularity of benign versus malignant tumors, by identifying acidic areas and/or by targeting surface and other markers (e.g., melanin) of malignant cells; MSOT could image primary oral tumors including benign and malignant salivary tumors and squamous cell carcinomas (SCC), and MSOT could identify the extent of involvement of bone and vascular structures by SCCs. While novel fluorescent imaging approaches are currently in development in order to aid intraoperatively in identifying precise surgical margins of head and neck lesions (56), most of these same contrast agents could be used to identify head and neck lesions with greater depth using MSOT.
Prostate cancer
Patients with elevated levels of prostate specific antigen (PSA) undergo ultrasound (US) biopsies to identify cancers of the prostate (PrCa); however, the urologist is blind as to the presence of PrCa at biopsy sites (57). This is improved by magnetic resonance imaging (MRI) fused with US (MRI-US) to identify areas of the prostate that are suspicious for biologically aggressive and clinically relevant PrCa, however, MRI-US has limitations in visualization of biomarkers and functional properties of tumors (58). MSOT is likely to be a much more specific and sensitive approach to imaging small cryptic foci of PrCa which should be suitable with the current depth of detection based on vascular patterns or molecular markers and it could be combined with current ultrasound guidance of biopsies to improve detection.
Ovarian cancer
Adnexal masses suspicious for malignancy are initially assessed with imaging such as transvaginal ultrasonography plus serum tumor markers such as CA-125. Both lack sensitivity and specificity (59) so diagnostic laparascopy is used, with biopsy to confirm the presence of malignancy. MSOT utilization prior to laparoscopy surgery could spare patients with benign masses the morbidity of more radical surgery, and would identify areas to biopsy to confirm metastatic cancer with a hand-held MSOT probe (60). By utilizing MSOT intraoperatively with minimally invasive surgery (MIS), the limitations of visual inspection and/or random biopsies during MIS could be reduced. MSOT would not be limited to the initial workup of an adnexal mass or staging for early ovarian cancer as it also could facilitate identification of small mesenteric metastases. Patients with advanced ovarian cancer undergoing interval cytoreductive surgeries after neoadjuvant chemotherapy (61) or patients with recurrent disease also may be candidates for secondary cytoreductive surgeries (62) using MSOT to guide therapy. Thus, when lower disease volume is encountered and complete resection translates to improved survival, MSOT would be of clinical interest.
Pre-operative Endoscopic and Laparoscopic Imaging for Resectable Tumors
Many cancers rely on complete resection for management. Pre-operative staging by MSOT could better identify patients who are surgical candidates or have borderline resectable disease that might benefit from neoadjuvant therapy to achieve resectability. Even apparently effective neoadjuvant therapy may be complicated by the inability to distinguish fibrous tissue and dead tumor from viable tumor (63). Another complication is the potential for small regional metastases (e.g. lymph nodes, mesenteric tumor deposits) that are not detected with standard state of the art imaging and negatively impact surgical outcomes. Inadequate detection of regional metastases is generally attributed to the low sensitivity of CT for small volume disease (64), leading to the addition of endoscopy and laparoscopy as part of the pre-operative staging for certain tumors.
Staging of malignancies such as lymphomas, pancreatic cancer, and neuroblastomas is important to their therapy. Because MSOT uses ultrasound transducers for signal detection, it can be adapted if endoscopic ultrasound is utilized. The application of MSOT to gastrointestinal cancers (esophageal, gastric, small bowel and colorectal cancers) may better characterize tumor depth of invasion and microscopic extent, resulting in more effective staging and treatment of these diseases. MSOT has a great potential for improving current endoscopic/laproscopic methods and could be coupled with fine needle aspiration with or without further injection of contrast agents.
Except for ultrasound, current laparoscopic optical imaging is limited by superficial visualization, so MSOT could provide molecular and functional detail beyond the information currently available. MSOT could provide more accurate delineation of tumor extent, lymphatic mapping, and/or detection of unknown regional metastases.
Clinical Sites Representing a Challenge to Imaging with MSOT
Transmission of ultrasound is optimal in tissues that have a high water content. Air is a much less efficient medium in transferring sound, so uses of current versions of MSOT to identify primary and metastatic lesions of the lung are not under development; however, MSOT may be used to identify pleural spread of tumors that cannot be detected by other imaging methods. Ultimately, bronchoscopic methods using transducers developed for endoscopic use may be developed to identify malignant lesions affecting the bronchi and adjacent tissues. Also, the air in the lung still may transfer the ultrasound images at somewhat less detail than in other tissues, particularly when intervening lung parenchyma is minimized by using navigational methods. While it is not currently known if MSOT can detect changes in bone, the density of the bone could present a challenge.
Intraoperative Imaging by MSOT
In rats, MSOT and optoacoustic imaging have been useful in detecting sentinel lymph nodes involved by cancer because MSOT is more accurate than currently used methods which utilize methylene blue dye plus radiotracers (44). The development of MSOT for sentinel lymph node detection may make the use of radiotracers obsolete, reducing exposure of patients and workers to radiation.
The use of MSOT intraoperatively to identify surgical margins may be a great advance for surgical management of cancers such as those of the oral cavity, anal area, lower rectum, and esophagus. Similarly, MSOT may permit accurate partial resections of tumors of the kidney, pancreas, larynx and other organs. Using biomarkers, i.e. HER2 or EGFR, MSOT may aid resection of minimal residual disease subsequent to therapy or prior surgical attempts at resection.
Discussion
Accurate detection and localization of cancers is key to providing more effective treatments. Current imaging technologies are limited for detecting small cancers or metastases, for differentiating viable tumor from surrounding nonviable tumor, and for evaluating tumor metabolism. With further development of contrast agents and hardware, MSOT offers solutions to these limitations. MSOT has primarily been utilized in research; however, its clinical uses are expanding, particularly in oncology.
MSOT has the potential for clinical usefulness across a broad spectrum of cancer management. It provides the ability to distinguish viable tumor from surrounding benign tissue by detecting differences in vascularity, perfusion, metabolism and molecular characteristics. Common cancer treatment changes, e.g. scarring, inflammation, may be difficult to distinguish from tumor using US, MRI, CT, PET, and SPECT; furthermore, residual or recurrent tumor could be identified with the relevant molecular information based upon endogenous and exogenous contrast agents below spatial resolution of these technologies. MSOT may separate benign from malignant tissues by detecting areas of ischemia via presence of deoxy-hemoglobin, low pH (by V7-750), metabolism (2-deoxyglucose), neovascularization and cell surface molecules of malignant tumors.
Combining MSOT with other approaches can improve the accuracy of cancer detection and diagnosis, staging, and aid in medical decision making. Intraoperative utilization of MSOT may improve detection of margins and metastases and assist in minimally invasive surgery. Visualization of vascularity of tumors may facilitate monitoring of successful treatment before anatomic tumor shrinkage and permit early detection of recurrence or metastases which may speed implementation of salvage therapies. As clinical use of MSOT increases, new applications of MSOT are likely.
Supplementary Material
Acknowledgments
Grant Support: This work was funded in part by the Cooperative Human Tissue Network (1CA183728) and the UAB Pancreatic (P50CA101955) and Breast (P50CA089019) SPOREs (to W.E. Grizzle).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Kimbrough CW, Khanal A, Zeiderman M, Khanal BR, Burton NC, McMasters KM, et al. Targeting acidity in pancreatic adenocarcinoma: multispectral optoacoustic tomography detects pH-low insertion peptide probes in vivo. Clinical cancer research. 2015;21:4576–85. doi: 10.1158/1078-0432.CCR-15-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoffels I, Morscher S, Helfrich I, Hillen U, Leyh J, Burton NC, et al. Noninvasive and nonradioactive determination of sentinel lymph node tumor status in melanoma using multispectral optoacoustic imaging. Science Translation Medicine. 2015;7:317. doi: 10.1126/scitranslmed.aad1278. [DOI] [PubMed] [Google Scholar]
- 3.Ntziachristos V, Razansky D. Molecular imaging by means of multispectral optoacoustic tomography (MSOT) Chemical reviews. 2010;110:2783–94. doi: 10.1021/cr9002566. [DOI] [PubMed] [Google Scholar]
- 4.Razansky D, Baeten J, Ntziachristos V. Sensitivity of molecular target detection by multispectral optoacoustic tomography (MSOT) Medical physics. 2009;36:939–45. doi: 10.1118/1.3077120. [DOI] [PubMed] [Google Scholar]
- 5.Kellnberger S, Deliolanis NC, Queirós D, Sergiadis G, Ntziachristos V. In vivo frequency domain optoacoustic tomography. Optics letters. 2012;37:3423–5. doi: 10.1364/OL.37.003423. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Allende PB, Glatz J, Koch M, Ntziachristos V. Enriching the interventional vision of cancer with fluorescence and optoacoustic imaging. Journal of Nuclear Medicine. 2013;54:664–7. doi: 10.2967/jnumed.111.099796. [DOI] [PubMed] [Google Scholar]
- 7.Hudson SV, Huang JS, Yin W, Albeituni S, Rush J, Khanal A, et al. Targeted noninvasive imaging of EGFR-expressing orthotopic pancreatic cancer using multispectral optoacoustic tomography. Cancer research. 2014;74:6271–9. doi: 10.1158/0008-5472.CAN-14-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimbrough CW, Hudson S, Khanal A, Egger ME, McNally LR. Orthotopic pancreatic tumors detected by optoacoustic tomography using Syndecan-1. Journal of surgical research. 2015;193:246–54. doi: 10.1016/j.jss.2014.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khanal A, Ullum C, Kimbrough CW, Garbett NC, Burlison JA, McNally MW, et al. Tumor targeted mesoporous silica-coated gold nanorods facilitate detection of pancreatic tumors using Multispectral optoacoustic tomography. Nano Research. 2015;8:3864–77. [Google Scholar]
- 10.Yin W, Kimbrough CW, Gomez-Gutierrez JG, Burns CT, Chuong P, Grizzle WE, et al. Tumor specific liposomes improve detection of pancreatic adenocarcinoma in vivo using optoacoustic tomography. J Nanobiotechnology. 2015;13:90. doi: 10.1186/s12951-015-0139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gurka MK, Pender D, Chuong P, Fouts B, Sobelov A, McNally M, et al. Identification of pancreatic tumors in vivo with ligand-targeted,pH responsive mesoporous silica nanoparticles by multispectral optoacoustic tomography. Journal of controlled release. 2016 doi: 10.1016/j.jconrel.2015.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuchin VV, Genina EA, Bashkatov AN, Simonenko GV, Odoevskaya OD, Altshuler GB. A pilot study of ICG laser therapy of acne vulgaris: photodynamic and photothermolysis treatment. Lasers in surgery and medicine. 2003;33:296–310. doi: 10.1002/lsm.10211. [DOI] [PubMed] [Google Scholar]
- 13.Li R, Wang P, Lan L, Lloyd FP, Goergen CJ, Chen S, et al. Assessing breast tumor margin by multispectral photoacoustic tomography. Biomedical optics express. 2015;6:1273–81. doi: 10.1364/BOE.6.001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deán-Ben XL, Razansky D. Adding fifth dimension to optoacoustic imaging: volumetric time-resolved spectrally enriched tomography. Light: Science & Applications. 2014;3:e137. [Google Scholar]
- 15.Ma R, Taruttis A, Ntziachristos V, Razansky D. Multispectral optoacoustic tomography (MSOT) scanner for whole-body small animal imaging. Optics express. 2009;17:21414–26. doi: 10.1364/OE.17.021414. [DOI] [PubMed] [Google Scholar]
- 16.Ntziachristos V, Ripoll J, Wang LV, Weissleder R. Looking and listening to light: the evolution of whole-body photonic imaging. Nature biotechnology. 2005;23:313–20. doi: 10.1038/nbt1074. [DOI] [PubMed] [Google Scholar]
- 17.Mason RP. Commentary on photoacoustic tomography. Journal of Nuclear Medicine. 2015;56:1815–6. doi: 10.2967/jnumed.115.165183. [DOI] [PubMed] [Google Scholar]
- 18.Reshetnyak YK. Imaging tumor acidity: pH-low insertion peptide probe for optoacoustic tomography. Clin Cancer Res. 2015;21:4502–4. doi: 10.1158/1078-0432.CCR-15-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stritzker J, Kirscher L, Scadeng M, Deliolanis NC, Morscher S, Symvoulidis P, et al. Vaccinia virus-mediated melanin production allows MR and optoacoustic deep tissue imaging and laser-induced thermotherapy of cancer. Proceedings of the National Academy of Sciences. 2013;110:3316–20. doi: 10.1073/pnas.1216916110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herzog E, Taruttis A, Beziere N, Lutich AA, Razansky D, Ntziachristos V. Optical imaging of cancer heterogeneity with multispectral optoacoustic tomography. Radiology. 2012;263:461–8. doi: 10.1148/radiol.11111646. [DOI] [PubMed] [Google Scholar]
- 21.Wang LV, Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science. 2012;335:1458–62. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tzoumas S, Deliolanis N, Morscher S, Ntziachristos V. Unmixing molecular agents from absorbing tissue in multispectral optoacoustic tomography. IEEE Trans Med Imaging. 2014;33:48–60. doi: 10.1109/TMI.2013.2279994. [DOI] [PubMed] [Google Scholar]
- 23.Bohndiek SE, Sasportas LS, Machtaler S, Jokerst JV, Hori S, Gambhir SS. Photoacoustic tomography detects early vessel regression and normalization during ovarian tumor response to the antiangiogenic therapy trebananib. Journal of Nuclear Medicine. 2015;56:1942–7. doi: 10.2967/jnumed.115.160002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason RP, Zhao D, Liu L, Trawick ML, Pinney KG. A perspective on vascular disrupting agents that interact with tubulin: preclinical tumor imaging and biological assessment. Integr Biol (Camb) 2011;3:375–87. doi: 10.1039/c0ib00135j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buehler A, Kacprowicz M, Taruttis A, Ntziachristos V. Real-time handheld multispectral optoacoustic imaging. Optics letters. 2013;38:1404–6. doi: 10.1364/OL.38.001404. [DOI] [PubMed] [Google Scholar]
- 26.Mallidi S, Luke GP, Emelianov S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance. Trends in biotechnology. 2011;29:213–21. doi: 10.1016/j.tibtech.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruan Q, Xi L, Boye SL, Han S, Chen ZJ, Hauswirth WW, et al. Development of an anti-angiogenic therapeutic model combining scAAV2-delivered siRNAs and noninvasive photoacoustic imaging of tumor vasculature development. Cancer letters. 2013;332:120–9. doi: 10.1016/j.canlet.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choe SW, Terman DS, Rivers AE, Rivera J, Lottenberg R, Sorg BS. Drug-loaded sickle cells programmed ex vivo for delayed hemolysis target hypoxic tumor microvessels and augment tumor drug delivery. Journal of controlled release. 2013;171:184–92. doi: 10.1016/j.jconrel.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song KH, Stein EW, Margenthaler JA, Wang LV. Noninvasive photoacoustic identification of sentinel lymph nodes containing methylene blue in vivo in a rat model. Journal of biomedical optics. 2008;13:054033. doi: 10.1117/1.2976427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morscher S, Driessen WH, Claussen J, Burton NC. Semi-quantitative Multispectral Optoacoustic Tomography (MSOT) for volumetric PK imaging of gastric emptying. Photoacoustics. 2014;2:103–10. doi: 10.1016/j.pacs.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langhout GC, Grootendorst DJ, Nieweg OE, Wouters MW, van der Hage JA, Jose J. Detection of melanoma metastases in resected human lymph nodes by noninvasive multispectral photoacoustic imaging. Int. J Biomed Imaging. 2014;2014:163652. doi: 10.1155/2014/163652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chithrani DB. Nanoparticles for improved therapeutics and imaging in cancer therapy. Recent patents on nanotechnology. 2010;4:171–80. doi: 10.2174/187221010792483726. [DOI] [PubMed] [Google Scholar]
- 33.Ahmed N, Fessi H, Elaissari A. Theranostic applications of nanoparticles in cancer. Drug discovery today. 2012;17:928–34. doi: 10.1016/j.drudis.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Song W, Tang Z, Zhang D, Burton N, Driessen W, Chen X. Comprehensive studies of pharmacokinetics and biodistribution of indocyanine green and liposomal indocyanine green by multispectral optoacoustic tomography. RSC Advances. 2015;5:3807–13. [Google Scholar]
- 35.Balasundaram G, Ho CJH, Li K, Driessen W, Dinish US, Wong CL, et al. Molecular photoacoustic imaging of breast cancer using an actively targeted conjugated polymer. International journal of nanomedicine. 2015;10:387–97. doi: 10.2147/IJN.S73558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bao C, Beziere N, del Pino P, Pelaz B, Estrada G, Tian F, et al. Gold nanoprisms as optoacoustic signal nanoamplifiers for in vivo bioimaging of gastrointestinal cancers. Small. 2013;9:68–74. doi: 10.1002/smll.201201779. [DOI] [PubMed] [Google Scholar]
- 37.Conversano F, Soloperto G, Greco A, Ragusa A, Casciaro E, Chiriaco F, et al. Echographic detectability of optoacoustic signals from low-concentration PEG-coated gold nanorods. International journal of nanomedicine. 2012;7:4373–89. doi: 10.2147/IJN.S33908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vonnemann J, Beziere N, Bottcher C, Riese SB, Kuehne C, Dernedde J, et al. Polyglycerolsulfate functionalized gold nanorods as optoacoustic signal nanoamplifiers for in vivo bioimaging of rheumatoid arthritis. Theranostics. 2014;4:629–41. doi: 10.7150/thno.8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Z, Liu J, Wang R, Du Y, Ren J, Qu X. An efficient nano-based theranostic system for multi-modal imaging-guided photothermal sterilization in gastrointestinal tract. Biomaterials. 2015;56:206–18. doi: 10.1016/j.biomaterials.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 40.An Q, Liu J, Yu M, Wan J, Li D, Wang C, et al. Multifunctional magnetic Gd3+-based coordination polymer nanoparticles: combination of magnetic resonance and multispectral optoacoustic detections for tumor-targeted imaging in vivo. Small. 2015;11:5675–86. doi: 10.1002/smll.201501491. [DOI] [PubMed] [Google Scholar]
- 41.Homan KA, Souza M, Truby R, Luke GP, Green C, Vreeland E, et al. Silver nanoplate contrast agents for in vivo molecular photoacoustic imaging. ACS nano. 2012;6:641–50. doi: 10.1021/nn204100n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De La Zerda A, Zavaleta C, Keren S, Vaithilingam S, Bodapati S, Liu Z, et al. Carbon nanotubes as photoacoustic molecular imaging agents in living mice. Nature nanotechnology. 2008;3:557–62. doi: 10.1038/nnano.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pramanik M, Song KH, Swierczewska M, Green D, Sitharaman B, Wang LV. In vivo carbon nanotube-enhanced non-invasive photoacoustic mapping of the sentinel lymph node. Physics in medicine and biology. 2009;54:3291. doi: 10.1088/0031-9155/54/11/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim C, Song KH, Gao F, Wang LV. Sentinel lymph nodes and lymphatic vessels: noninvasive dual-modality in vivo mapping by using indocyanine green in rats--volumetric spectroscopic photoacoustic imaging and planar fluorescence imaging. Radiology. 2010;255:442–50. doi: 10.1148/radiol.10090281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beziere N, Lozano N, Nunes A, Salichs J, Queiros D, Kostarelos K, et al. Dynamic imaging of PEGylated indocyanine green (ICG) liposomes within the tumor microenvironment using multi-spectral optoacoustic tomography (MSOT) Biomaterials. 2015;37:415–24. doi: 10.1016/j.biomaterials.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 46.Bonner JA, Buchsbaum DJ, Russo SM, Fiveash JB, Trummell HQ, Curiel DT, et al. Anti-EGFR-mediated radiosensitization as a result of augmented EGFR expression. International journal of radiation oncology, biology physics. 2004;59:2–10. doi: 10.1016/j.ijrobp.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 47.Buchsbaum DJ, Bonner JA, Grizzle WE, Stackhouse MA, Carpenter M, Hicklin DJ, et al. Treatment of pancreatic cancer xenografts with Erbitux (IMC-C225) anti-EGFR antibody, gemcitabine, and radiation. International Journal of Radiation Oncology Biology Physics. 2002;54:1180–93. doi: 10.1016/s0360-3016(02)03788-4. [DOI] [PubMed] [Google Scholar]
- 48.Markman B, Javier Ramos F, Capdevila J, Tabernero J. EGFR and KRAS in colorectal cancer. Advances in clinical chemistry. 2010;51:71–119. doi: 10.1016/s0065-2423(10)51004-7. [DOI] [PubMed] [Google Scholar]
- 49.Chen LQ, Randtke EA, Jones KM, Moon BF, Howison CM, Pagel MD. Evaluations of tumor acidosis within in vivo tumor models using parametric maps generated with AcidoCEST MRI. Molecular Imaging and Biology. 2015:1–9. doi: 10.1007/s11307-014-0816-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gillies RJ, Raghunand N, Garcia-Martin ML, Gatenby RA. pH imaging. A review of pH measurement methods and applications in cancers. IEEE Eng Med Biol Mag. 2004;23:57–64. doi: 10.1109/memb.2004.1360409. [DOI] [PubMed] [Google Scholar]
- 51.Gillies RJ, Raghunand N, Karczmar GS, Bhujwalla ZM. MRI of the tumor microenvironment. Journal of magnetic resonance imaging. 2002;16:430–50. doi: 10.1002/jmri.10181. [DOI] [PubMed] [Google Scholar]
- 52.Levi J, Kothapalli SR, Ma TJ, Hartman K, Khuri-Yakub BT, Gambhir SS. Design, synthesis, and imaging of an activatable photoacoustic probe. Journal of the American Chemical Society. 2010;132:11264–9. doi: 10.1021/ja104000a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tung CH, Mahmood U, Bredow S, Weissleder R. In vivo imaging of proteolytic enzyme activity using a novel molecular reporter. Cancer research. 2000;60:4953–8. [PubMed] [Google Scholar]
- 54.Heijblom M, Piras D, Brinkhuis M, Van Hespen J, Van den Engh F, Van der Schaaf M, et al. Photoacoustic image patterns of breast carcinoma and comparisons with magnetic resonance imaging and vascular stained histopathology. Scientific reports. 2015;5:11778. doi: 10.1038/srep11778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jackson VP, Hendrick RE, Feig SA, Kopans DB. Imaging of the radiographically dense breast. Radiology. 1993;188:297–301. doi: 10.1148/radiology.188.2.8327668. [DOI] [PubMed] [Google Scholar]
- 56.Rosenthal EL, Warram JM, de Boer E, Chung TK, Korb ML, Brandwein-Gensler M, et al. Safety and tumor specificity of cetuximab-IRDye800 for surgical navigation in head and neck cancer. Clin Cancer Res. 2015;21:3658–66. doi: 10.1158/1078-0432.CCR-14-3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boesen L, Noergaard N, Chabanova E, Logager V, Balslev I, Mikines K, Thomsen HS. Early experience with multiparametric magnetic resonance imaging-targeted biopsies under visual transrectal ultrasound guidance in patients suspicious for prostate cancer undergoing repeated biopsy. Scand J Urol. 2015;49:25–34. doi: 10.3109/21681805.2014.925497. [DOI] [PubMed] [Google Scholar]
- 58.Cash H, Günzel K, Maxeiner A, Stephan C, Fischer T, Durmus T, et al. Prostate cancer detection on transrectal ultrasonography-guided random biopsy despite negative real-time magnetic resonance imaging/ultrasonography fusion-guided targeted biopsy: reasons for targeted biopsy failure. BJU Int. 2015 Sep 19; doi: 10.1111/bju.13327. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 59.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Management of adnexal masses. Obstet Gynecol. 2007;110:201–14. doi: 10.1097/01.AOG.0000263913.92942.40. [DOI] [PubMed] [Google Scholar]
- 60.Cibula D, Oonk MH, Abu-Rustum NR. Sentinel lymph node biopsy in the management of gynecologic cancer. Curr Opin Obstet Gynecol. 2015;27:66–72. doi: 10.1097/GCO.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 61.Seward SM, Winer I. Primary debulking surgery and neoadjuvant chemotherapy in the treatment of advanced epithelial ovarian carcinoma. Cancer Metastasis Rev. 2015;34:5–10. doi: 10.1007/s10555-014-9536-y. [DOI] [PubMed] [Google Scholar]
- 62.Frederick PJ, Ramirez PT, McQuinn L, Milam MR, Weber DM, Coleman RL, et al. Preoperative factors predicting survival after secondary cytoreduction for recurrent ovarian cancer. Int J Gynecol Cancer. 2011;21:831–6. doi: 10.1097/IGC.0b013e31821743f9. [DOI] [PubMed] [Google Scholar]
- 63.Tzeng CW, Fleming JB, Lee JE, Xiao L, Pisters PW, Vauthey JN, et al. Defined clinical classifications are associated with outcome of patients with anatomically resectable pancreatic adenocarcinoma treated with neoadjuvant therapy. Annals of surgical oncology. 2012;19:2045–53. doi: 10.1245/s10434-011-2211-4. [DOI] [PubMed] [Google Scholar]
- 64.Pietryga JA, Morgan DE. Imaging preoperatively for pancreatic adenocarcinoma. J Gastrointest Oncol. 2015;6:343–57. doi: 10.3978/j.issn.2078-6891.2015.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


