Pain serves as a protective mechanism that leads to changes in movement.1,2 For more than 116 million Americans with chronic pain, however, the pain experience persists beyond a normal, protective phase and develops into a chronic, disabling disease.3 Altered movement may be appropriate in early protective phases, but, if maintained, this altered movement can contribute to poor recovery, continued disability, and decreased quality of life. The Institute of Medicine identifies chronic pain as a nervous system disease and a high-priority societal health concern.3 However, current management of this disease and its complications, such as movement impairments and subsequent reductions in function, is inadequate.3 The Institute of Medicine has called for the health care community to transform its understanding of pain as a key step for improving prevention, treatment, and assessment of pain.3
The Institute of Medicine specifically highlighted the need for “wider use of existing knowledge” as a main objective for transforming our understanding of pain.3 One way to meet this objective is to integrate existing knowledge into a comprehensive model that considers pain to be a dynamic, multifactorial experience that includes a movement component. Although researchers have extensively studied the sensory, psychological, and motor factors related to pain, they often have studied these factors separately or in limited combinations. Isolated investigation of these factors limits the advancement of our understanding of how pain impairs movement and how pain symptoms can cascade into a disabling disease process. Integrated study of sensory, psychological, and motor factors as primary drivers of the pain experience is needed to help establish the collective impact of pain processing on movement and recovery.
The purpose of this point of view is to introduce a conceptual model for studying pain with movement. First, we present a historical overview identifying existing bodies of knowledge that should be integrated in order to consider pain as a nervous system disease. Second, we propose a model for integrating pain with movement, which links nervous system processing with movement changes and recovery. This conceptual model will guide future studies that may transform our understanding of how pain is interrelated with movement and may potentially lead to the development of innovative evidence-based treatments that will optimize patient outcomes.
Historical Overview
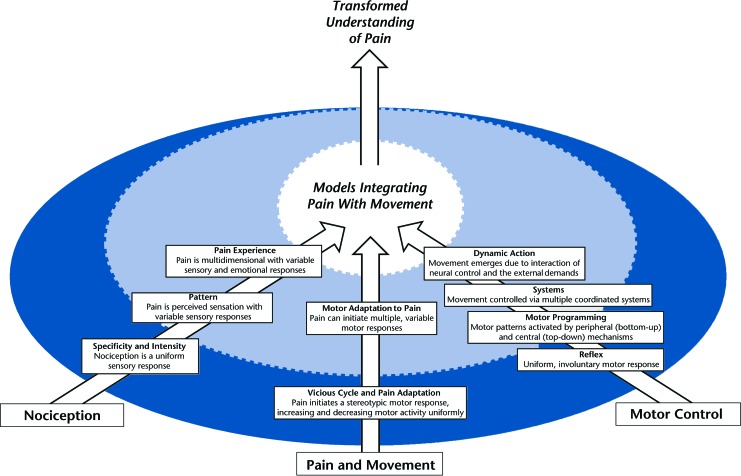
The individual study of nociception and motor control has resulted in mostly separate bodies of evidence describing the complexity and mechanisms of both pain and movement. Therefore, the study of pain and movement, although existent in the literature, is the exception and not the norm. Historically, these areas of study developed in similar directions, and they demonstrate common themes of advancement (Fig. 1). Early explanations of nociception and motor control portrayed the nervous system as acting in a uniform or predictable manner. As evidence of nervous system complexity grew, contemporary explanations described more dynamic processes of nociception and motor control. Researchers now recognize nervous system responses as variable within and across individuals. These areas of study have reached a critical point in which it is necessary to integrate their bodies of evidence in order to advance the understanding of pain as a nervous system disease. The advantage of integrating pain with movement is that researchers will be able to utilize established evidence to inform future study design, hypotheses, and interpretation of findings. As outlined below, these areas of study together drive the development of a model for integrating pain with movement.
Figure 1.
Historical overview of current knowledge. The bottom 3 arrows represent key areas of research requiring integration into conceptual models that combine pain with movement. The top arrow represents the result of integration: a transformed understanding of pain. The circle represents the pattern of historical development from characterizing uniform nervous system responses (dark blue) to dynamic nervous system responses (light blue).
Nociception
Early research defined pain as nociception, which required a peripheral stimulus generator.4,5 A critical transition in this area of study was the delineation between nociception and the pain experience. Nociception remains defined as a peripheral stimulus, but pain has been defined as a complex experience that includes sensory and emotional responses.6,7 The pain experience is separate from nociception and can exist without a peripheral stimulus.8 Individualized sensory responses, cognitive perceptions, and psychological functions may result in variable pain experiences. Although contemporary pain theories6,7 acknowledge this complex, multidimensional experience, most theories have minimized, simplified, or excluded the motor components of the pain experience.
Motor Control
Early motor control research focused on short-term and latent motor reflexes in response to peripheral stimuli.9–12 Advancements in this area of research suggested central and peripheral mechanisms controlled single- and multiple-joint motions, but still depicted these motions as simple, uniform motor responses.9,13,14 Contemporary theories now recognize that multiple systems contribute to complex, functional movement, and they also acknowledge that movement is often adaptable and variable.9,15–21 Although motor control studies often focus on motor changes due to adaptation (eg, error-based learning) and feedback (eg, visual and proprioceptive),22–24 the motor implications of pain are underrepresented in the literature.
Pain and Movement
A smaller area of study examines the relationship between pain and movement. We acknowledge this overlap of nociception and motor control research; however, current perspectives, as well as studies of pain and movement, are underdeveloped. Similar to nociception and motor control research, early theories on the relationship between pain and movement largely described reflexive and uniform motor responses to a painful stimulus.25,26 Voluntary movement during experimentally induced pain episodes resulted in decreased agonist muscle activity and increased antagonist muscle activity.27,28 More recent theories and research suggest a dynamic relationship—motor responses to pain are variable within and between individuals.1,2,29–31 Protective pain responses may vary based on the context, such as the pain location, state of chronicity, or type of activity.30,31 Although contemporary theories indeed recognize variable motor responses to pain, they often do not account for the potential influence of pain-related sensory or psychological factors.
Integrated Model
Although evidence suggests that sensory, psychological, and motor processing factors are related to pain, their potential interactions and the influence of these interactions on movement also must be considered. Researchers rarely consider interactions of all 3 factors, thus restricting our knowledge of their collective effect on movement and recovery.
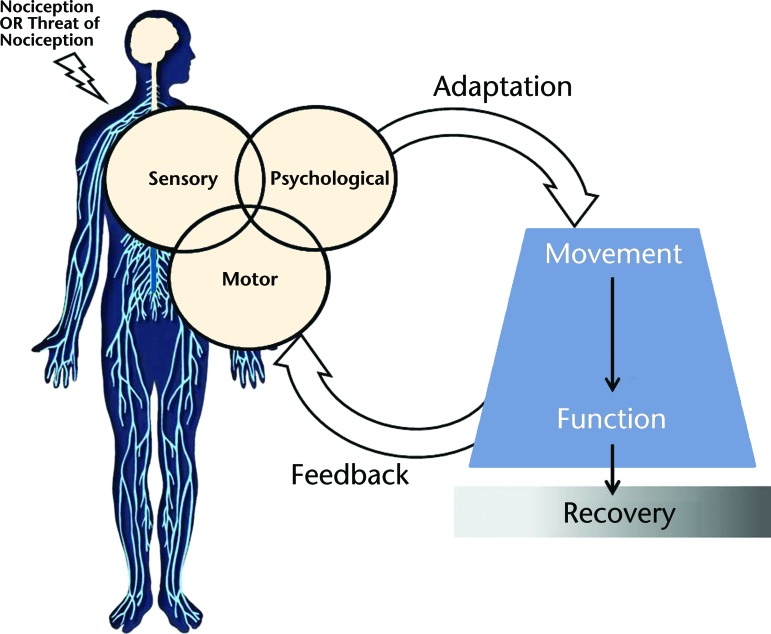
We propose a model for integrating pain with movement (Fig. 2) to advance our understanding of how pain—a nervous system disease—is interrelated with movement. Sensory, psychological, and motor factors are key components of brain and spinal cord processing of pain. Pain processing results in adaptation and feedback, the drivers of movement changes. Concurrent examination of sensory, psychological, and motor factors may provide insight into variability of movement within and between people experiencing pain. Movement changes that persist beyond their original protective stage may lead to changes in function and further affect recovery. Integration of pain-related processing factors with movement and recovery demonstrates alignment with the Institute of Medicine's definition of pain as a nervous system disease.3 Integration of this existing knowledge leads to a conceptual model that better characterizes the multidimensional pain experience.
Figure 2.
Model for integrating pain with movement. Yellow indicates nervous system processing. Blue indicates the results of adaptation and feedback. Gray shading indicates recovery spectrum.
Initial State
Nociception with tissue damage, or the threat of potential tissue damage, initiates the pain experience.8 A person's initial state may influence his or her pain experience—which includes, but is not limited to, the person's age, sex, gender role, genotype features, socioeconomic status, or education level. Although these factors may be important, they often are unmodifiable during a particular pain episode and, therefore, are not the primary focus of this model. Furthermore, in this model, the movement patterns that occur within the initial state (ie, movement that occurs when the individual is pain-free) are used as a comparison for individual recovery. Thus, the initial state factors may be more important when describing between-subject differences, but are not as essential to this model's framework when describing within-subject changes over time.
Nervous System Processing
Sensory, psychological, and motor factors interact with each other to influence the initial state. Not only are these important factors to consider in any nervous system disease, but each one demonstrates an established, unidimensional association with pain. For example, central sensitization,32 fear and catastrophizing,33,34 and altered neuromuscular activation31,35 are all associated with higher pain levels. Although these processes occur simultaneously, no single factor has shown a strong enough association to fully explain the variability of the pain experience. However, levels of sensitivity (sensory component), negative psychological functioning (psychological component), and motor excitability (motor component) together could have an interactive influence on adaptation and feedback, which then drive movement changes. Individual differences in multiple sensory, psychological, or motor factors and their interactions may explain individual variability in movement during an episode of pain.
Adaptation and Feedback
In this model, changes in motor activity or movement occur after nervous system processing. This is the first opportunity for individual adaptation in response to pain or to the threat of pain. Adaptation, as represented in this model, refers to a change in motor activity or movement patterns that were previously consistent with the initial state. Early changes in motor activity and movement are most likely responses meant to protect the individual from further injury. These changes can occur in a muscle, in a single joint, or across multiple joints, and they also may differ in magnitude (how much change occurs) and duration (how long the changes last). Once changes occur, feedback is sent back to the nervous system. This feedback can include numerous sensory signals, body awareness, or environmental features. Based on continued nervous system processing and further adaptation, initial movement changes may be facilitated, inhibited, or further altered.
Changes in movement are an important aspect of the pain experience, but these changes cannot be predicted by considering sensory, psychological, or motor factors in isolation. Furthermore, specific movement changes that result from interactive nervous system processing, adaptation, and feedback are still unknown. Immediate modulation of functional activities occurs following experimentally induced pain, providing evidence of short-term, protective responses to pain.31,36 Movement changes that are initially protective may persist due to continued pain, the type of injury, or functional necessity. Prolonged movement changes compared with movement in the initial state may suggest a long-term behavioral alteration that could directly perpetuate the pain experience. Although longitudinal studies have not been conducted to confirm this hypothesis, a continuous relationship among nervous system processing, feedback, and adaptation not only may contribute to individual differences in function and behavior but also may ultimately affect individual recovery.
Recovery
In this model, recovery is defined as return to movement patterns and function consistent with the initial state. Although short-term changes in movement often are necessary in a protective capacity, researchers now believe the persistence of altered movement is a key characteristic of chronic pain.1,2,37,38 We propose a spectrum of recovery that includes complete recovery, delayed recovery, or chronic behavioral changes. When all components of the model lead to continuation of typically short-term changes in movement, delayed recovery is more likely. If nervous system processing, adaptation, and feedback relationships promote persistence of these movement changes, chronic behavioral changes that prevent recovery may result. Thus, this model suggests that, even when a person is still experiencing pain, he or she has the ability to recover once movement consistent with the initial state is achieved.
Significance of Integrating Pain With Movement for Physical Therapists
The American Physical Therapy Association Vision Statement describes physical therapists as health care professionals who are “transforming society by optimizing movement to improve the human experience.”39 Understanding the interplay among pain-related nervous system processing, movement impairments, and recovery is necessary for physical therapists to reach their full potential as movement experts. The model for integrating pain with movement encourages physical therapists to view pain with movement as a complex, dynamic relationship. Physical therapists must learn to assess and treat pain with movement using rehabilitation approaches based on individual or subgroup differences in nervous system processing. Physical therapists' use of this integrated model will potentially accelerate its translation from theory to application in clinical practice. By embracing this integrated perspective, physical therapists will be better equipped to treat pain conditions, optimize recovery, and prevent pain-related disability.
General Significance of Integrating Pain With Movement
The model for integrating pain with movement provides a conceptual framework for future efforts to transform our understanding of pain.3 The model contains elements that have not been fully considered previously in the study of pain and movement. This model highlights the iterative nature of the pain experience, providing an explanation for the variability in movement, function, and recovery—pain affects movement, while movement further affects pain. In addition, the model incorporates recovery into the overall pain experience and aligns predictors of recovery with the current definition of pain as a nervous system disease.3 Finally, the framework of the model is designed to support interdisciplinary collaboration and promote translation of research findings into clinically useful pain management and rehabilitative strategies.
Summary
Researchers have studied pain-related sensory, psychological, and motor factors extensively, but often separately. Isolated investigation of these factors limits our understanding of how pain is interrelated with movement. The model for integrating pain with movement encourages an integrated research approach that emphasizes a dynamic relationship among nervous system pain processing, movement adaptations, and recovery. Future studies that utilize this integrated model will be better positioned to answer meaningful questions by using existing knowledge to transform our understanding of pain diseases.3 An integrated research approach is critical for developing appropriate risk assessment tools and comprehensive, long-term rehabilitative strategies for pain management, recovery of movement and function, and prevention of pain-related disability.
Footnotes
All authors provided concept/idea/project design and writing. Dr George and Dr Fox provided consultation (including review of manuscript before submission).
The authors thank Dr Mark Bishop for his contributions during development of this model. They thank Mr Daniel Ortiz and Mr Eric Spector for their assistance with graphical design.
Funding for Dr Butera's PhD training is being provided by the Brooks-PHHP Research Collaboration. Dr George is supported by Brooks Rehabilitation. The manuscript was written while Dr Fox received support from the National Institutes of Health Rehabilitation Research Career Development Program (NIH K12 HD055929).
References
- 1. Hodges PW, Smeets RJ. Interaction between pain, movement, and physical activity: short-term benefits, long-term consequences, and targets for treatment. Clin J Pain. 2015;31:97–107. [DOI] [PubMed] [Google Scholar]
- 2. Hodges PW, Tucker K. Moving differently in pain: a new theory to explain the adaptation to pain. Pain. 2011;152(3 suppl):S90–S98. [DOI] [PubMed] [Google Scholar]
- 3. Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 4. Wall PD. The gate control theory of pain mechanisms: a re-examination and re-statement. Brain. 1978;101:1–18. [DOI] [PubMed] [Google Scholar]
- 5. Perl ER. Ideas about pain: a historical view. Nat Rev Neurosci. 2007;8:71–80. [DOI] [PubMed] [Google Scholar]
- 6. Melzack R, Casey KL. Sensory, motivational, and central control determinants of pain: a new conceptual model. In: Kenshalo DR, ed. The Skin Senses. Springfield, IL: CC Thomas; 1968:423–443. [Google Scholar]
- 7. Melzack R. From the gate to the neuromatrix. Pain. 1999;(suppl 6):S121–S126. [DOI] [PubMed] [Google Scholar]
- 8. Merskey H, Bogduk N. Classification of Chronic Pain. Seattle, WA: International Association for the Study of Pain Press; 1994. [Google Scholar]
- 9. Shumway-Cook A, Woollacott MH. Motor Control: Translating Research Into Clinical Practice. Baltimore, MD: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 10. Sherrington CS. The Integrative Action of the Nervous System. New Haven, CT: Yale University Press; 1906. [Google Scholar]
- 11. Schaltenbrand G. The development of human motility and motor disturbances. Arch Neurol Psychiatry. 1928;20:720. [Google Scholar]
- 12. Weisz S. Studies in equilibrium reaction. J Nerv Ment Dis. 1938;88:150–162. [Google Scholar]
- 13. Keele SW. Movement control in skilled motor performance. Psychol Bull. 1968;70:387. [Google Scholar]
- 14. Forssberg H, Grillner S, Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Res. 1975;85:103–107. [DOI] [PubMed] [Google Scholar]
- 15. Bernstein N. The Control and Regulation of Movements. London, United Kingdom: Pergamon Press; 1967. [Google Scholar]
- 16. Latash ML, Scholz JP, Schöner G. Motor control strategies revealed in the structure of motor variability. Exerc Sport Sci Rev. 2002;30:26–31. [DOI] [PubMed] [Google Scholar]
- 17. Scholz JP, Schöner G. The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res. 1999;126:289–306. [DOI] [PubMed] [Google Scholar]
- 18. Stergiou N, Harbourne RT, Cavanaugh JT. Optimal movement variability: a new theoretical perspective for neurologic physical therapy. J Neurol Phys Ther. 2006;30:120–129. [DOI] [PubMed] [Google Scholar]
- 19. VanSant AF. Rising from a supine position to erect stance: description of adult movement and a developmental hypothesis. Phys Ther. 1988;68:185–192. [DOI] [PubMed] [Google Scholar]
- 20. Kugler P, Turvey M. Information, Natural Law, and the Self-Assembly of Rhythmic Movement. Hillsdale, NJ: Lawrence Erlbaum Associates Inc; 1987. [Google Scholar]
- 21. Perry SB. Clinical implications of a dynamic systems theory. J Neurologic Phys Ther. 1998;22:4–10. [Google Scholar]
- 22. Adams JA. Historical review and appraisal of research on the learning, retention, and transfer of human motor-skills. Psychol Bull. 1987;101:41–74. [Google Scholar]
- 23. Guadagnoli MA, Kohl RM. Knowledge of results for motor learning: relationship between error estimation and knowledge of results frequency. J Mot Behav. 2001;33:217–224. [DOI] [PubMed] [Google Scholar]
- 24. Diedrichsen J, White O, Newman D, Lally N. Use-dependent and error-based learning of motor behaviors. J Neurosci. 2010;30:5159–5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roland MO. A critical review of the evidence for a pain-spasm-pain cycle in spinal disorders. Clin Biomech (Bristol, Avon). 1986;1:102–109. [DOI] [PubMed] [Google Scholar]
- 26. Lund JP, Donga R, Widmer CG, Stohler CS. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991;69:683–694. [DOI] [PubMed] [Google Scholar]
- 27. Svensson P, Arendt-Nielsen L, Houe L. Sensory-motor interactions of human experimental unilateral jaw muscle pain: a quantitative analysis. Pain. 1996;64:241–249. [DOI] [PubMed] [Google Scholar]
- 28. Graven-Nielsen T, Svensson P, Arendt-Nielsen L. Effects of experimental muscle pain on muscle activity and co-ordination during static and dynamic motor function. Electroencephalogr Clin Neurophysiol. 1997;105:156–164. [DOI] [PubMed] [Google Scholar]
- 29. Hodges PW, Moseley GL, Gabrielsson A, Gandevia SC. Experimental muscle pain changes feedforward postural responses of the trunk muscles. Exp Brain Res. 2003;151:262–271. [DOI] [PubMed] [Google Scholar]
- 30. van Dieën JH, Selen LP, Cholewicki J. Trunk muscle activation in low-back pain patients, an analysis of the literature. J Electromyogr Kinesiol. 2003;13:333–351. [DOI] [PubMed] [Google Scholar]
- 31. van den Hoorn W, Hug F, Hodges PW, et al. Effects of noxious stimulation to the back or calf muscles on gait stability. J Biomech. 2015;48:4109–4115. [DOI] [PubMed] [Google Scholar]
- 32. Correa JB, Costa LO, de Oliveira NT, et al. Central sensitization and changes in conditioned pain modulation in people with chronic nonspecific low back pain: a case-control study. Exp Brain Res. 2015;233:2391–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. George SZ, Calley D, Valencia C, Beneciuk JM. Clinical investigation of pain-related fear and pain catastrophizing for patients with low back pain. Clin J Pain. 2011;27:108–115. [DOI] [PubMed] [Google Scholar]
- 34. Picavet HS, Vlaeyen JW, Schouten JS. Pain catastrophizing and kinesiophobia: predictors of chronic low back pain. Am J Epidemiol. 2002;156:1028–1034. [DOI] [PubMed] [Google Scholar]
- 35. Arendt-Nielsen L, Graven-Nielsen T, Svarrer H, Svensson P. The influence of low back pain on muscle activity and coordination during gait: a clinical and experimental study. Pain. 1996;64:231–240. [DOI] [PubMed] [Google Scholar]
- 36. Hodges PW, Tsao H, Sims K. Gain of postural responses increases in response to real and anticipated pain. Exp Brain Res. 2015;233:2745–2752. [DOI] [PubMed] [Google Scholar]
- 37. Madeleine P, Mathiassen SE, Arendt-Nielsen L. Changes in the degree of motor variability associated with experimental and chronic neck-shoulder pain during a standardised repetitive arm movement. Exp Brain Res. 2008;185:689–698. [DOI] [PubMed] [Google Scholar]
- 38. Moseley GL, Hodges PW. Reduced variability of postural strategy prevents normalization of motor changes induced by back pain: a risk factor for chronic trouble? Behav Neurosci. 2006;120:474–476. [DOI] [PubMed] [Google Scholar]
- 39. American Physical Therapy Association. Vision Statement for the Physical Therapy Profession and Guiding Principles to Achieve the Vision. Available at: http://www.apta.org/Vision/ Accessed June 17, 2016.