Abstract
Background:
Tumour budding is a histological finding in epithelial cancers indicating an unfavourable phenotype. Previous studies have demonstrated that it is a negative prognostic indicator in colorectal cancer (CRC), and has been proposed as an additional factor to incorporate into staging protocols.
Methods:
A systematic review of papers until March 2016 published on Embase, Medline, PubMed, PubMed Central and Cochrane databases pertaining to tumour budding in CRC was performed. Study end points were the presence of lymph node metastases, recurrence (local and distal) and 5-year cancer-related death.
Results:
A total of 7821 patients from 34 papers were included, with a mean rate of tumour budding of 36.8±16.5%. Pooled analysis suggested that specimens exhibiting tumour budding were significantly associated with lymph node positivity (OR 4.94, 95% CI 3.96–6.17, P<0.00001), more likely to develop disease recurrence over the time period (OR 5.50, 95% CI 3.64–8.29, P<0.00001) and more likely to lead to cancer-related death at 5 years (OR 4.51, 95% CI 2.55–7.99, P<0.00001).
Conclusions:
Tumour budding in CRC is strongly predictive of lymph node metastases, recurrence and cancer-related death at 5 years, and its incorporation into the CRC staging algorithm will contribute to more effective risk stratification.
Keywords: colorectal cancer, tumour budding, prognosis, 5-year survival, lymph node positivity
The TNM staging system for colorectal cancer (CRC) stratifies patients into stages, which influences their treatment pathway; this is especially true for rectal cancer where treatment ranges from local polyp resection to neoadjuvant chemoradiotherapy, surgery and adjuvant chemotherapy. However, there are many flaws with this staging system, including the prognostic heterogeneity of patients with cancers at the same stage (Merkel et al, 2001; Bori et al, 2009), as well as under- and overtreatment of patient subsets (Merkel et al, 2001; Nagtegaal et al, 2007; Schiffmann et al, 2013; Lea et al, 2014; Li et al, 2014a), and no superior method of staging and prognostication is currently validated (Gao et al, 2012). The future of CRC care will rely on accurate staging and prognostic algorithms, potentially with the addition of demographic, biochemical, morphological, molecular and treatment-related parameters to improve accuracy. One such parameter for inclusion is tumour budding, a histological phenomenon in epithelial cancers when tumour cells or cell clusters migrate into the surrounding stroma by detaching from the invasive tumour front (Morodomi et al, 1989; Hase et al, 1993; Ueno et al, 2002b). It represents de-differentiation of epithelial cells into more invasive phenotypes in a process known as epithelial–mesenchymal transition (EMT; Kalluri and Weinberg, 2009).
Tumour budding in CRC has been shown significantly associated with lymphatic invasion (Morodomi et al, 1989; Okuyama et al, 2003a, 2003b; Park et al, 2005; Losi et al, 2006; Choi et al, 2007; Ogawa et al, 2009; Wang et al, 2009), and accordingly has a well-established association with lymph node metastases, and is a negative prognostic indicator in terms of recurrence and overall survival (Ueno et al, 2002a, 2004; Lugli et al, 2009; Wang et al, 2009; Kevans et al, 2011; Petrelli et al, 2015). An association with distant metastases has also been documented (Nakamura et al, 2005; Suzuki et al, 2009).
Despite this, tumour budding has not been incorporated into the TNM staging of CRC, partly due to the disagreements in its definition and identification, with ‘concerns over reproducibility of assessment, and the wide ranges of percentage of colorectal tumours reported to show budding in different studies' (Loughrey et al, 2014).
One other obstacle when trying to introduce budding in clinical practice is the fact that some methods for reporting are conceived for early stages (I–II) and some are for advanced disease (III); therefore, it is difficult to choose a method suitable for all stages.
The aim of the current study was to perform a systematic review and meta-analysis of the implications of tumour budding in operable CRC resection specimens on lymph node metastases, recurrence and cancer-related death.
Materials and methods
Search protocol
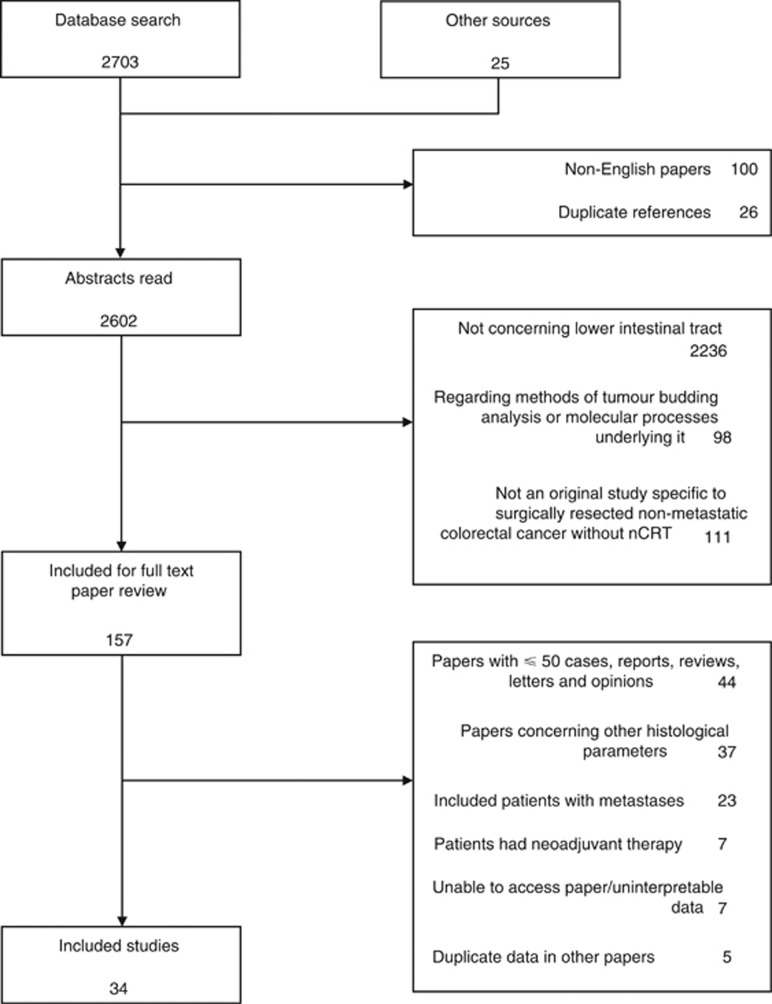
Original studies were searched for those that documented patients with surgically resected primary colorectal adenocarcinoma, where the specimens were assessed for the presence of tumour budding. The outcome measures chosen were lymph node metastases at resection, rates of recurrence and 5-year cancer-specific survival rates. Embase, Medline, PubMed, PubMed Central and Cochrane databases were searched using the following Boolean search term: budding AND (cancer OR carcinoma OR adenocarcinoma OR tumor OR tumour) for articles published up to March 2016. All search results were combined in a reference manager database (Endnote) and duplicates removed. Reference lists of included studies were also searched. The inclusion and exclusion criteria are shown in Figure 1.
Figure 1.
Study inclusion and exclusion criteria.
Data extraction
Two independent reviewers applied the inclusion criteria to retrieved study abstracts and selected full papers for data analysis. These same two reviewers extracted data from full text papers and applied exclusion criteria in order to identify the final included studies; discrepancies were verified by consensus. If multiple publications reported results in the same population, the most recent report or most comprehensive data were chosen. For each study, data on baseline characteristics (author institution, country, study period, total number of patients, sex, site of cancer, stage, histology, budding definition and methodology) were extracted. The number of patients exhibiting budding, those with co-existing lymph node metastases, merged rates of local or distant recurrence and 5-year cancer-related death rates were obtained where available. We described outcomes as odds ratios with 95% confidence intervals. Where these were not presented in a paper, we followed methods described by Parmar to extract them from Kaplan–Meier curves, or percentage survival. We contacted authors if data were not presented in a useable form.
Budding definition
There remains to be a consensus on the most accurate method of assessing tumour budding; currently there are variations in the cut-off for presence or absence of tumour budding – some groups use ⩾1 budding focus to define the presence of tumour budding, whereas other groups use >4 or >9 foci. For this reason, the defined term was catalogued from each included paper and displayed in the results. In papers where two methods of identifying tumour budding were evaluated, the results of the standard method were used for analysis. For the analysis, budding was determined to be either present or absent. Where budding was graded into groups (e.g., mild/moderate/severe), the results for the mild/moderate groups were combined and compared with the severe or highest grade group.
Quality analysis
This meta-analysis was conducted in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. We measured the quality of the studies on the basis of the Newcastle–Ottawa Scale that assesses the methodological quality of non-randomised cohort studies for meta-analysis. The studies were judged by two independent assessors using a nine-point scale comprising analysis on the selection of the study group, the comparability of cohorts and the ascertainment of outcome. Scores above 6 points were taken to denote studies of high methodological quality.
Statistical analysis
All analyses were conducted with the RevMan statistical package (Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Heterogeneity between studies for each of the outcome measures was tested with Cochran's Q test. The I2 statistic was calculated for an objective measure of heterogeneity. A fixed-effects meta-analysis was performed in all cases, and where there was appreciable heterogeneity (I2>50% or chi-squared P-values <0.10) a random-effects model was also used for meta-analysis. Corresponding funnel plots of Ln standard error as a function of effect size were used to examine the effect of publication bias visually, and were statistically tested using Eggers test. P-values >0.05 were indicative of no publication bias. For meta-analysis, Mantel–Haenszel odds ratios for lymph node metastasis, recurrence and 5-year cancer-related death were extracted and described with 95% confidence intervals. Sensitivity analysis was performed to identify if any methodological features were indicative of heterogeneity among studies. Studies were excluded if they had poor methodological quality (Newcastle–Ottawa scores <7), where the definitions of tumour budding were unclear or not predetermined in the Methods section, or where the rate of tumour budding in the study was outside of the mean rate of tumour budding among all studies±1 s.d. from the mean. Subgroup analysis was also carried out to compare whether tumour location (colon or rectum), T stage (some studies only included T1 and T2 tumours) and lymph node status affected results.
Results
Search results
The literature search revealed 2728 publications (Figure 2). Of the 143 included for full text review, 34 were ultimately included in the meta-analysis (Hase et al, 1993; Ueno et al, 2002a; Okuyama et al, 2003a, 2003b; Ueno et al, 2004; Prall et al, 2005; Wang et al, 2005; Yasuda et al, 2007; Kanazawa et al, 2008; Nakamura et al, 2008; Yamauchi et al, 2008; Lugli et al, 2009; Ogawa et al, 2009; Wang et al, 2009; Homma et al, 2010; Kajiwara et al, 2010; Komori et al, 2010; Tateishi et al, 2010; Akishima-Fukasawa et al, 2011; Reggiani Bonetti et al, 2011; Betge et al, 2012; Nakadoi et al, 2012; Sert Bektas et al, 2012; Zlobec et al, 2012; Wada et al, 2013; Huh et al, 2014; Lai et al, 2014; Nishida et al, 2014; Ryu et al, 2014; Gilardoni et al, 2015; Macias-Garcia et al, 2015; Miyachi et al, 2015; Tristante et al, 2015; Barresi et al, 2016). There were a total of 7821 patients included for analysis, with study groups ranging from 58 to 1114 patients. All studies were conducted in Europe or Asia. The main characteristics of eligible studies are characterised in Table 1. Most studies were retrospective and included patients with colon and rectal cancers from a mixture of stages. One study included patients with metastases at diagnosis (Kanazawa et al, 2008); these patients were excluded for analysis of disease recurrence and so the non-metastatic data are included here. There were 25 studies that correlated tumour budding status with lymph node metastases in the specimen, 12 that examined recurrence rates, and 9 that documented 5-year cancer-related death rates. The mean rate of tumour budding was 36.8±16.5%.
Figure 2.
Flow diagram of the systematic review and meta-analysis process.
Table 1. Overview of included studies.
|
AJCC stage |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Country | Study period | Site of cancer | No. of patients | I | II | III | IV | Newcastle–Ottawa Score |
| Hase | 1993 | Japan | 1970–1985 | CR | 663 | ✓ | ✓ | ✓ | 7 | |
| Ueno | 2002 | Japan | 1981–1994 | R | 437 | ✓ | 7 | |||
| Okuyama | 2003 | Japan | 1985–1997 | C | 196 | ✓ | ✓ | 8 | ||
| Okuyama | 2003 | Japan | 1985–1997 | R | 83 | ✓ | ✓ | 7 | ||
| Ueno | 2004 | Japan | 1960–1980 | R | 1114 | ✓ | ✓ | ✓ | 9 | |
| Prall | 2005 | Germany | 1994–1999 | CR | 182 | ✓ | ✓ | 8 | ||
| Wang | 2005 | China | 1969–2002 | CR | 159 | ✓ | ✓ | 8 | ||
| Yasuda | 2007 | Japan | NA | CR | 86 | ✓ | ✓ | 7 | ||
| Kanazawa | 2008 | Japan | 1996–2001 | CR | 133 | ✓ | ✓ | ✓ | 7 | |
| Nakamura | 2008 | Japan | 1986–1998 | C | 200 | ✓ | 7 | |||
| Yamauchi | 2008 | Japan | 1991–2001 | CR | 164 | ✓ | ✓ | 8 | ||
| Lugli | 2009 | Switzerland | 1987–1996 | CR | 273 | ✓ | ✓ | ✓ | 7 | |
| Ogawa | 2009 | Japan | 1995–2003 | CR | 83 | ✓ | ✓ | 8 | ||
| Wang | 2009 | Ireland | 1990–2004 | CR | 128 | ✓ | 9 | |||
| Homma | 2010 | Japan | 2000–2007 | R | 65 | ✓ | ✓ | 6 | ||
| Kajiwara | 2010 | Japan | 1985–2005 | CR | 244 | ✓ | ✓ | 8 | ||
| Komori | 2010 | Japan | 1990–2004 | CR | 111 | ✓ | ✓ | 8 | ||
| Tateishi | 2010 | Japan | 1992–2005 | CR | 322 | ✓ | ✓ | 8 | ||
| Akishima-Fukasawa | 2011 | Japan | 1989–2009 | CR | 111 | ✓ | ✓ | 7 | ||
| Reggiani Bonetti | 2011 | Italy | 1989–2004 | CR | 95 | ✓ | ✓ | 6 | ||
| Betge | 2012 | Austria | 1984–2005 | CR | 110 | ✓ | 8 | |||
| Nakadoi | 2012 | Japan | 1981–2008 | CR | 499 | ✓ | ✓ | 8 | ||
| Sert Bektas | 2012 | Turkey | 2003–2007 | CR | 73 | ✓ | ✓ | ✓ | 6 | |
| Wada | 2013 | Japan | 1995–2005 | CR | 120 | ✓ | ✓ | 8 | ||
| Zlobec | 2012 | Switzerland | 1987–1996 | CR | 127 | ✓ | ✓ | ✓ | 8 | |
| Nishida | 2014 | Japan | 2000–2011 | CR | 265 | ✓ | ✓ | 8 | ||
| Huh | 2014 | Korea | 2007–2009 | CR | 543 | ✓ | ✓ | 8 | ||
| Lai | 2014 | China | 1999–2007 | C | 135 | ✓ | 6 | |||
| Ryu | 2014 | Korea | 2003–2012 | CR | 179 | ✓ | ✓ | 8 | ||
| Gilardoni | 2015 | Italy | 2006–2009 | C | 196 | ✓ | ✓ | 8 | ||
| Macias-Garcia | 2015 | Spain | 2000–2011 | CR | 97 | ✓ | 8 | |||
| Tristante | 2015 | Spain | 2011–2014 | CR | 58 | ✓ | ✓ | 4 | ||
| Barresi | 2016 | Italy | NA | CR | 82 | ✓ | 7 | |||
| Miyachi | 2016 | Japan | 2001–2014 | CR | 653 | ✓ | 7 | |||
Abbreviations: C=colon only; CR=colon and rectum; NA=not available; R=rectum only.
Quality of studies
The Newcastle–Ottawa scaling method assessed the methodological quality of the studies and scores are outlined in Table 1, with an overview of the scoring system method in Supplementary Material 1 and raw scores in Supplementary Material 2. The majority (81.6%) of studies had quality scores ⩾7, indicating good methodological quality of included studies, and four studies (15.6% Homma et al, 2010; Reggiani Bonetti et al, 2011; Sert Bektas et al, 2012; Lai et al, 2014) had acceptable scores of 6.
Definitions of tumour budding
There were considerable differences and an overt lack of standardisation of tumour budding between studies (Table 2). Twenty-eight studies used standard haematoxylin and eosin (H&E) staining, four studies used immunohistochemistry (IHC) and cytokeratin staining (Prall et al, 2005; Lugli et al, 2009; Ogawa et al, 2009; Zlobec et al, 2012), and two did not specify the technique (Gilardoni et al, 2015; Macias-Garcia et al, 2015), although one could assume standard H&E was used. A ‘budding focus' or its equivalent was defined as an isolated cancer cell or cluster of less than five cells in 24 studies. The remaining studies were either unclear about the definition of a budding focus (Yasuda et al, 2007; Kanazawa et al, 2008; Nakamura et al, 2008) or defined it as an isolated cancer cell or cluster of less than six cells (Okuyama et al, 2003a; Lugli et al, 2009; Reggiani Bonetti et al, 2011; Nakadoi et al, 2012; Zlobec et al, 2012), or a cluster of greater than four cancer cells (Okuyama et al, 2003b; Wang et al, 2005). Tumour budding was then defined according to the number of budding foci in the majority of studies; there was some heterogeneity with the classifications between studies. Ten studies identified tumour budding positivity if there were >4 budding foci present in the specimen. Other studies used >9 budding foci (eight studies), ⩾1 focus (five studies), >5 foci (three studies), >10, >16 or >24 foci (one study each). Three studies did not quantify the number of budding foci, but graded tumour budding as mild, moderate or severe, depending on subjective opinion (Hase et al, 1993) or the proportion of the tumour margin involved by budding (Kanazawa et al, 2008; Nakamura et al, 2008). For these studies, the severe group data were compared with mild and moderate (Kanazawa et al, 2008; Nakamura et al, 2008). One study was unclear as to the methodology used to determine tumour budding (Homma et al, 2010).
Table 2. Methods of defining tumour budding among included studies.
| Author | Year | Staining method | Definition of budding focus | Tumour budding method |
|---|---|---|---|---|
| Hase | 1993 | H&E | Cluster of <5 cancer cells | Graded into two groups: none/mild or moderate/severe at invasive front |
| Ueno | 2002 | H&E | Cluster of <5 cancer cells | >5 Budding foci |
| Okuyama | 2002 | H&E | Cluster of <6 cancer cells | ⩾1 Budding focus (Morodomi) |
| Okuyama | 2003 | H&E | Cluster of >4 cancer cells | ⩾1 Budding focus |
| Ueno | 2004 | H&E | Cluster of <5 cancer cells | >9 Budding foci |
| Prall | 2005 | Cytokeratin/IHC | Cluster of <5 cancer cells | >24 Budding foci |
| Wang | 2005 | H&E | Cluster of >4 cancer cells | ⩾1 Budding focus (Morodomi) |
| Yasuda | 2007 | H&E | Cluster of an unspecified number of cancer cells | ⩾1 Budding focus (Hase) |
| Kanazawa | 2008 | H&E | Cluster of an unspecified number of cancer cells | Mild: <1/3 invasive margin Moderate: 1/3–2/3 invasive margin Severe: >2/3 invasive margin |
| Nakamura | 2008 | H&E | Cluster of an unspecified number of cancer cells | As per Kanazawa above |
| Yamauchi | 2008 | H&E | Cluster of <5 cancer cells | >4 Budding foci (Ueno modified) |
| Lugli | 2009 | Cytokeratin/IHC | Cluster of <6 cancer cells | >16 Buds per hpf |
| Ogawa | 2009 | Cytokeratin/IHC | Cluster of <5 cancer cells | >9 Budding foci |
| Wang | 2009 | H&E | Cluster of <5 cancer cells | ⩾1 Budding focus |
| Homma | 2010 | H&E | Cluster of <5 cancer cells | Graded none, mild, mod, severe but did not delineate what comprised each |
| Kajiwara | 2010 | H&E | Cluster of <5 cancer cells | >9 Budding foci |
| Komori | 2010 | H&E | Cluster of <5 cancer cells | >4 Budding foci (Ueno modified) |
| Tateishi | 2010 | H&E | Cluster of <5 cancer cells | ⩾1 Budding focus (Ueno) |
| Akishima-Fukasawa | 2011 | H&E | Cluster of <5 cancer cells | >4 Budding foci (Ueno) |
| Reggiani Bonetti | 2011 | H&E | Cluster of <6 cancer cells | >4 Budding foci (Ueno) |
| Betge | 2012 | H&E | Cluster of <5 cancer cells | >9 Budding foci (Ueno modified) |
| Nakadoi | 2012 | H&E | Cluster of <6 cancer cells | >4 Budding foci (Ueno modified) |
| Sert Bektas | 2012 | H&E | Cluster of <5 cancer cells | >9 Budding foci (Ueno modified) |
| Wada | 2013 | H&E | Cluster of <5 cancer cells | >4 Budding foci (Ueno) |
| Zlobec | 2012 | Cytokeratin/IHC | Cluster of <6 cancer cells | >5 Budding foci |
| Nishida | 2014 | H&E | Cluster of <5 cancer cells | >4 Budding foci (Ueno) |
| Huh | 2014 | H&E | Cluster of <5 cancer cells | >5 Budding foci |
| Lai | 2014 | H&E | Cluster of <5 cancer cells | >9 Budding foci (Ueno modified) |
| Ryu | 2014 | H&E | Cluster of <5 cancer cells | >4 Budding foci (Ueno) |
| Gilardoni | 2014 | NS | Cluster of <5 cancer cells | >4 Budding foci (Ueno) |
| Macias-Garcia | 2014 | NS | Cluster of <5 cancer cells | >10 Budding foci (Ueno modified) |
| Tristante | 2014 | NS | NS | NS |
| Barresi | 2016 | H&E | Cluster of <5 cancer cells | >4 Budding foci (Ueno) |
| Miyachi | 2015 | H&E | Cluster of <5 cancer cells | >4 Budding foci (Ueno) |
Abbreviations: H&E=haematoxylin and eosin; IHC=Immunohistochemistry; NS, not significant.
Meta-analysis results
Lymph node metastasis
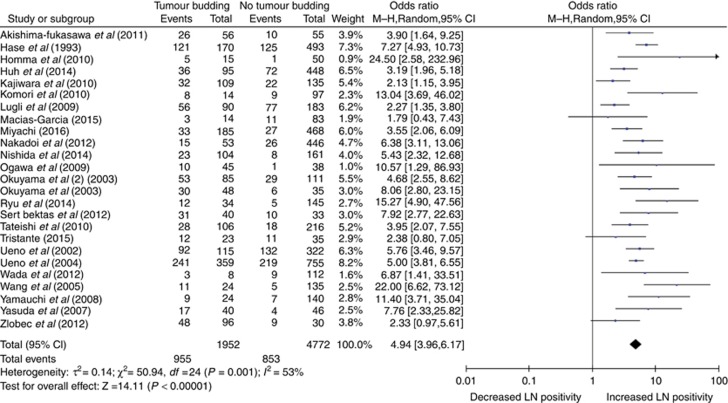
There were 25 included studies that compared the rate of tumour budding to regional lymph node metastases in the resected specimens, involving 6724 patients. The pooled random-effects analysis suggested that specimens exhibiting tumour budding were significantly associated with lymph node positivity (OR 4.94, 95% CI 3.96–6.17, P<0.00001; Figure 3). There was moderate heterogeneity between the studies (I2=53%) and thus a random-effects model is shown. When sensitivity analysis was performed, omission of those studies with Newcastle–Ottawa quality scores <7, those with an unclear definition of tumour budding or those which had overall rates of tumour budding outside of 1 s.d. of the mean tumour budding rate did not alter the overall effect of tumour budding on lymph node metastasis or affect heterogeneity (OR 4.90, CI 3.90–6.16, I2=55%, OR 5.13, CI 4.02–6.53, I2=52% and OR 3.98, CI 2.96–5.35, I2=54%, respectively). Subgroup analysis of studies by tumour location improved homogeneity; those that included rectal cancer only had an I2 of 0%, with a fixed-effects model OR remaining significant at 5.36, CI 4.25–6.75. Only one study in this group included colon cancer only. The remaining studies did not differentiate between colon and rectal cancers and had an OR for lymph node metastasis similar to the overall study group (OR 4.83, CI 3.61–6.45, I2=59%). Fourteen studies only examined lymph node metastases in T1 and T2 tumours, this group was also heterogeneous (I2=52%) with an OR of 5.67, CI 3.92–8.21. Conversely, the 11 studies that did not differentiate between T stage were also heterogeneous with I2=58% and OR 4.52, CI 3.39–6.02.
Figure 3.
The association of tumour budding with lymph node metastasis in resected colorectal cancer.
Local or distant recurrence
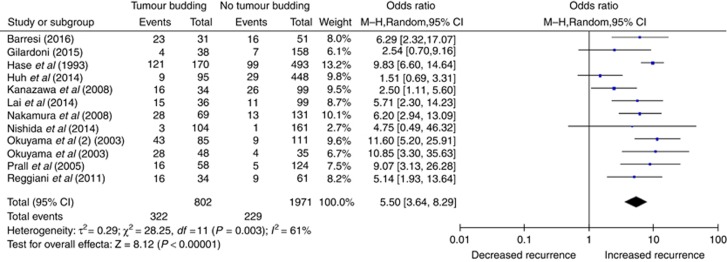
Twelve studies compared the rate of tumour budding with local or distant recurrence, involving 2773 patients. The pooled random-effects analysis suggested that specimens exhibiting tumour budding were significantly more likely to develop disease recurrence over the time period (OR 5.50, 95% CI 3.64–8.29, P<0.00001; Figure 4). There was moderate heterogeneity between the studies (I2=61%). Sensitivity analysis with omission of studies of poor methodological quality or those with unclear definitions of tumour budding did not change the effect of tumour budding on disease recurrence or affect heterogeneity results (OR 5.48, CI 3.35–8.97, I2=68% and OR 5.01, CI 2.88–8.73, I2=65%). Exclusion of studies with outlying rates of overall tumour budding did not improve heterogeneity with an I2 of 56%, and an OR of 4.54, CI 2.75–7.49. Subgroup analysis by tumour location could not be performed, as only one included study focussed on rectal cancer in relation to recurrence. In the group that examined recurrence in T1/T2 tumours only, three studies had a heterogeneous I2 of 50%, OR 2.87, CI 1.12–7.35. The remaining nine studies did not stratify by T stage and displayed improved homogeneity (I2=42%) and a fixed effects OR of 7.41, CI 5.77–9.50. In the four studies that only examined recurrence in node negative resections, the fixed-effects model gave an OR of 6.57, CI 4.18–10.32, I2=0%. The remaining seven studies included both node negative and node positive disease, and were heterogeneous (I2=75%), OR 4.91, CI 2.63–9.17.
Figure 4.
The association of tumour budding with local or distal recurrence in resected colorectal cancer.
Five-year cancer-related death rates
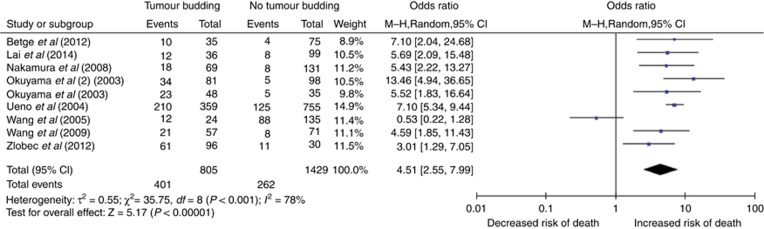
The rate of tumour budding with 5-year cancer-related death was documented in nine studies, involving 2234 patients. When analysed using a pooled random-effects model, the results demonstrate that CRCs with tumour budding were significantly more likely to lead to cancer-related death at 5 years (OR 4.51, 95% CI 2.55–7.99, P<0.00001; Figure 5). There was significant heterogeneity between the studies (I2=78%) and thus a random-effects model is shown. Sensitivity analysis excluding studies with Newcastle–Ottawa quality scores <7 and those with unclearly defined criteria for tumour budding did not change the overall effect of tumour budding on 5-year cancer-related death rates or on heterogeneity between studies (OR 4.39, CI 2.32–8.30, I2=80% and OR 3.58, CI 1.61–7.99, I2=85%). Including only those studies with rates of tumour budding within 1 s.d. of the overall mean rate improved I2 to 0%, with a fixed-effects model OR of 7.43, CI 5.84–9.45. Subgroup analysis by tumour location included two studies examining tumour budding with relation to 5-year cancer-related death in rectal cancer, OR 6.97, CI 5.29–9.19, I2=0%. Three studies included only colon cancers for analysis, OR 7.71, CI 4.46–13.33, I2=6%. The remaining four studies did not differentiate between colon and rectal cancers and were heterogeneous, I2=82%, OR 2.59, CI 0.85–7.92. Only one study examined 5-year cancer-related death in those with early (T1/T2) tumours, but when this was excluded, the remaining studies were homogenous (I2=0%) with the fixed-effects model giving a significant OR of 6.50, CI 5.19–8.14. Where node negative tumours only were included, the four studies were homogenous (I2=0%), with an OR of 5.43, CI 3.31–8.91, on the fixed-effects model. The remaining studies included node negative and positive disease and were heterogeneous (I2=89%), OR 3.86, CI 1.43–10.41.
Figure 5.
The association of tumour budding with 5-year cancer-related death in resected colorectal cancer.
Publication bias
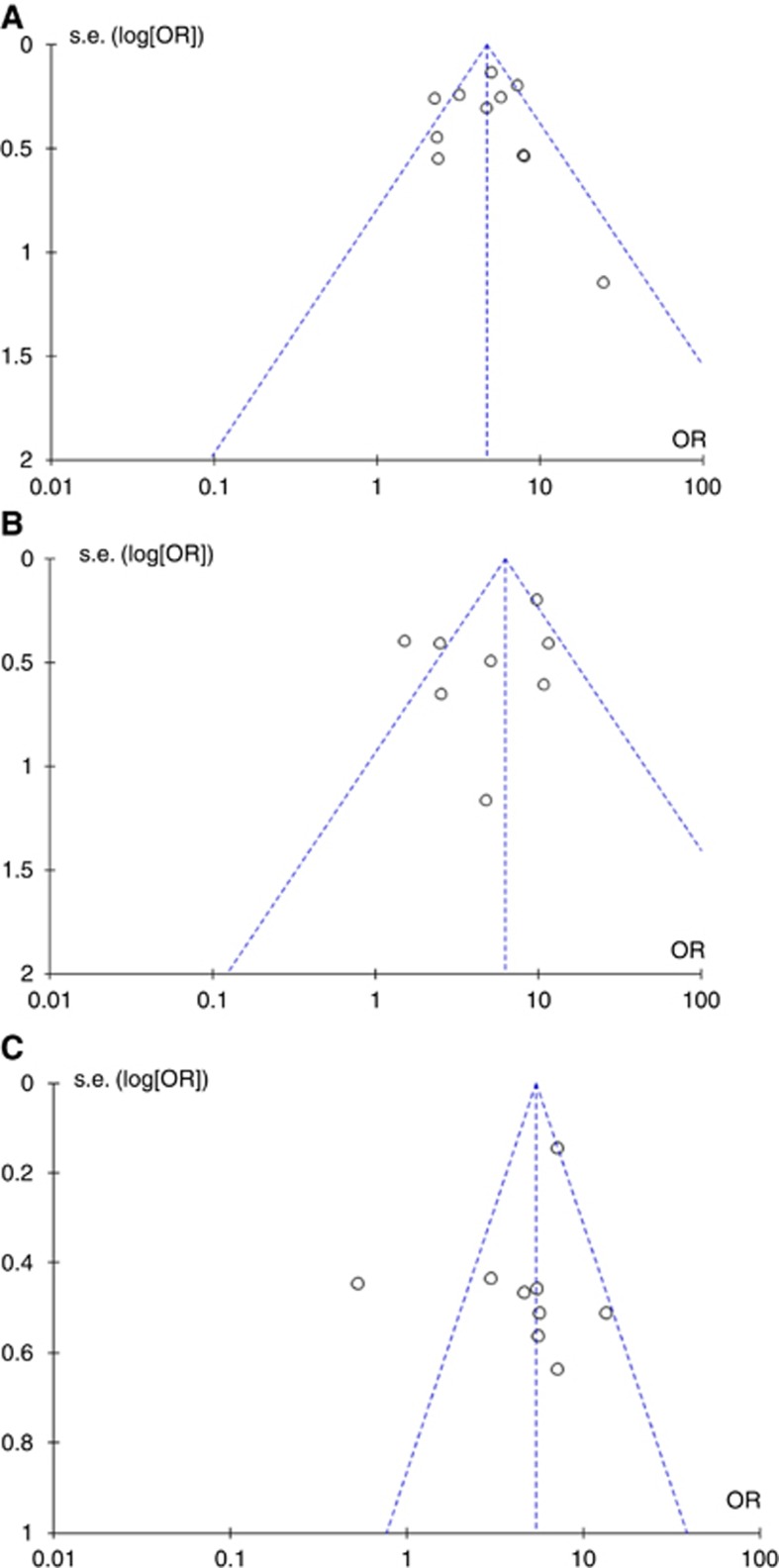
Funnel plots were created for each outcome measure. The shapes of the funnel plots and the statistical tests revealed no evidence of bias in any of the three groups (P-values for lymph node metastasis, recurrence and 5-year cancer-related death were 0.103, 0.402 and 0.363, respectively; Figure 6).
Figure 6.
Funnel plots for visual inspection of publication bias. Each point represents a standardised comparison of a separate study, comparing the outcome effect (OR) with the standard error of its logarithm (SE(log[OR])). (A) Lymph node metastases, (B) recurrence, (C) 5-year cancer-related death.
Discussion
The meta-analysis demonstrates that the histopathologic finding of tumour budding in resected CRC specimens is associated with concurrent lymph node metastases, disease recurrence and cancer-related death, despite variations in tumour budding definition between included studies. Tumour budding appears to indicate an aggressive phenotype, independent of staging according to the current TNM guidelines. Personalised patient care is an emerging concept in the future of cancer treatment (Piccart, 2013; Wazir and Mokbel, 2014; Jackson and Chester, 2015), and incorporation of tumour budding into the staging of CRC could assist in tailoring the management of currently contentious subgroups (Koelzer et al, 2016). Tumour budding may indicate early colorectal tumours requiring more rigorous treatment approaches, or its absence may aid decision-making in later stage cancers.
At present there is no proven benefit in treating early stage CRC patients with chemotherapy (Quasar Collaborative Group et al, 2007; Tournigand et al, 2012); however, we know there are subsets of stage I and II CRC who have poor prognostic outcomes. Although the current results suggest that tumour budding identifies high-risk early stage CRCs, it remains to be determined whether its presence indicates the need for further treatment in this subset. Clinical trials stratifying patients according to tumour budding status may be warranted.
Studies have also looked at tumour budding in other epithelial cancers, demonstrating a negative prognostic impact in oesophageal (Koike et al, 2008; Brown et al, 2010), breast (Salhia et al, 2015), pancreatic (Karamitopoulou et al, 2013) and lung (Masuda et al, 2012) carcinomas, but as yet, tumour budding remains a non-core component of colorectal adenocarcinoma pathologic staging (Koelzer et al, 2016) and is not formally part of staging of other cancers. Most studies advocate its identification using standard pathologic processing methods (H&E, light microscopy), with no extra staining or pathological processing required – thus this is potentially a cost-effective addition to current staging protocols. Cytokeratin staining appears to assist tumour budding detection and may be worthwhile investigating further (Suzuki et al, 2009; Puppa et al, 2012). Interestingly, although studies vary in their definition of how many cells constitute a tumour bud, and how many buds represent budding, and with considerable inter-observer variability in its reporting, most studies definitively demonstrate that the finding is a strong negative prognostic marker in CRC. A recent systematic review summarises methods of identifying tumour budding and their relationship to prognosticating CRC and surmises that despite differences in methodologies between studies, ‘most methods, if practised with care and some training, will yield relevant prognostic information as high-degree tumour budding places a patient at a significant risk for an adverse outcome' (van Wyk et al, 2015). However, it is clear that the definition needs standardisation, and for this, more prospective trials need to be designed to assess its reproducibility and inter-observer variation.
As the search herein was performed, other studies have also supported a negative prognostic role of tumour budding in CRC (Barresi et al, 2014; Graham et al, 2015). Petrelli et al (2015) recently undertook a systematic review of tumour budding and its relation to survival limited to stage II CRC. Their meta-analysis includes 10 studies and compares favourably with ours in that it also demonstrates a significantly increased OR for death at 5 years (OR 6.25, 95% CI 4.04–9.67, P<0.00001).
The results herein strongly demonstrate that tumour budding is a negative prognostic indicator in almost 7000 patients with CRC – these results remain robust even with sensitivity and subgroup analyses. Interestingly, when sensitivity analysis was performed only to include those studies with a percentage finding of tumour budding within 1 s.d. above and below the mean for all patients, the heterogeneity on the studies significantly decreased. This provides further evidence that standardisation of the definition and characterisation is imperative in the future of CRC staging.
Staging of CRC using the TNM system is notoriously inaccurate for prognosticating patient subsets, and thus many other prognostic markers have been studied. These include circulating and tumour markers such as cyclin D1 expression, CXCR4, VEGF, microRNA-21, survivin, CDKN2A hypermethylation, BRAF and K-ras mutation, as well as the findings of microsatellite instability, mucinous tumour phenotype and a high neutrophil–lymphocyte ratio (Guastadisegni et al, 2010; Chen et al, 2012; Safaee Ardekani et al, 2012; Verhulst et al, 2012; Huang et al, 2013; Xia et al, 2013; Xing et al, 2013; Lv et al, 2014; Wang et al, 2014; Li et al, 2014b, 2014c; Rui et al, 2015). Despite the fact that all are negative prognostic markers for overall survival in CRC, none have pooled hazard or odds ratios >2 on systematic review. Even more well-established prognostic indicators such as perineural invasion and high lymph node ratio are associated with relative risks for poorer overall survival of <2.5 (Ceelen et al, 2010; Knijn et al, 2015). The results of the current study demonstrate that tumour budding has an effect on survival that is markedly worse than any other previously studied pathological phenotype, with an OR of 4.5 for cancer-related death at 5 years in those exhibiting tumour budding. These findings support its inclusion into CRC pathologic staging as a marker of a more aggressive disease phenotype.
Early evidence suggests that tumours exhibiting budding do not respond to neoadjuvant chemotherapy (Bhangu et al, 2014; Rogers et al, 2014; Sannier et al, 2014). In this study, we excluded rectal cancers undergoing neoadjuvant chemoradiotherapy (nCRT). Although this subset of included rectal tumours are likely to be less advanced, we felt that nCRT inclusion may weaken the data, and a further meta-analysis of this subset is warranted when more results are available. It remains to be determined whether tumour budding should be seen as an indicator for a primary surgical approach in rectal cancer and foregoing nCRT as these patients are less likely to respond; more data are required to elucidate this point. However, those tumours exhibiting budding certainly are worth considering as a subset to target with adjuvant chemotherapy in CRC as a whole.
Tumour budding in CRC is strongly predictive of lymph node metastases, recurrence and cancer-related death at 5 years. Incorporation of this histological finding into the CRC staging algorithm is imminent, but will require standardisation of the pathological description of tumour budding.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Akishima-Fukasawa Y, Ishikawa Y, Akasaka Y, Uzuki M, Inomata N, Yokoo T, Ishii R, Shimokawa R, Mukai K, Kiguchi H, Suzuki K, Fujiwara M, Ogata K, Niino H, Sugiura H, Ichinose A, Kuroda Y, Kuroda D, Ishii T (2011) Histopathological predictors of regional lymph node metastasis at the invasive front in early colorectal cancer. Histopathology 59(3): 470–481. [DOI] [PubMed] [Google Scholar]
- Barresi V, Branca G, Ieni A, Reggiani Bonetti L, Baron L, Mondello S, Tuccari G (2014) Poorly differentiated clusters (PDCs) as a novel histological predictor of nodal metastases in pT1 colorectal cancer. Virchows Arch 464(6): 655–662. [DOI] [PubMed] [Google Scholar]
- Barresi V, Reggiani Bonetti L, Ieni A, Branca G, Tuccari G (2016) Histologic prognostic markers in stage IIA colorectal cancer: a comparative study. Scand J Gastroenterol 51(3): 314–320. [DOI] [PubMed] [Google Scholar]
- Betge J, Kornprat P, Pollheimer MJ, Lindtner RA, Schlemmer A, Rehak P, Vieth M, Langner C (2012) Tumor budding is an independent predictor of outcome in AJCC/UICC stage II colorectal cancer. Ann Surg Oncol 19(12): 3706–3712. [DOI] [PubMed] [Google Scholar]
- Bhangu A, Wood G, Brown G, Darzi A, Tekkis P, Goldin R (2014) The role of epithelial mesenchymal transition and resistance to neoadjuvant therapy in locally advanced rectal cancer. Colorectal Dis 16(4): O133–O143. [DOI] [PubMed] [Google Scholar]
- Bori R, Sejben I, Svebis M, Vajda K, Marko L, Pajkos G, Cserni G (2009) Heterogeneity of pT3 colorectal carcinomas according to the depth of invasion. Pathol Oncol Res 15(3): 527–532. [DOI] [PubMed] [Google Scholar]
- Brown M, Sillah K, Griffiths EA, Swindell R, West CM, Page RD, Welch IM, Pritchard SA (2010) Tumour budding and a low host inflammatory response are associated with a poor prognosis in oesophageal and gastro-oesophageal junction cancers. Histopathology 56(7): 893–899. [DOI] [PubMed] [Google Scholar]
- Ceelen W, Van Nieuwenhove Y, Pattyn P (2010) Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol 17(11): 2847–2855. [DOI] [PubMed] [Google Scholar]
- Chen JX, Tang XD, Xiang DB, Dong XL, Peng FY, Sun GY (2012) TNM stages and prognostic features of colorectal mucinous adenocarcinomas: a meta analysis. Asian Pac J Cancer Prev 13(7): 3427–3430. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Park KJ, Shin JS, Roh MS, Kwon HC, Lee HS (2007) Tumor budding as a prognostic marker in stage-III rectal carcinoma. Int J Colorectal Dis 22(8): 863–868. [DOI] [PubMed] [Google Scholar]
- Gao P, Zhou X, Wang ZN, Song YX, Tong LL, Xu YY, Yue ZY, Xu HM (2012) Which is a more accurate predictor in colorectal survival analysis? Nine data mining algorithms vs. the TNM staging system. PloS One 7(7): e42015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardoni E, Bernasconi DP, Poli S, Garancini M, Luperto M, Zucchini N, Bovo G, Totis M, Bugatti A, Gianotti L (2015) Surveillance for early stages of colon cancer: potentials for optimizing follow-up protocols. World J Surg Oncol 13: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham RP, Vierkant RA, Tillmans LS, Wang AH, Laird PW, Weisenberger DJ, Lynch CF, French AJ, Slager SL, Raissian Y, Garcia JJ, Kerr SE, Eun Lee H, Thibodeau SN, Cerhan JR, Limburg PJ, Smyrk TC (2015) Tumor budding in colorectal carcinoma: confirmation of prognostic significance and histologic cutoff in a population-based cohort. Am J Surg Pathol 39(10): 1340–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E (2010) Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer 46(15): 2788–2798. [DOI] [PubMed] [Google Scholar]
- Hase K, Shatney C, Johnson D, Trollope M, Vierra M (1993) Prognostic value of tumor ‘budding' in patients with colorectal cancer. Dis Colon Rectum 36(7): 627–635. [DOI] [PubMed] [Google Scholar]
- Homma Y, Hamano T, Otsuki Y, Shimizu S, Kobayashi H, Kobayashi Y (2010) Severe tumor budding is a risk factor for lateral lymph node metastasis in early rectal cancers. J Surg Oncol 102(3): 230–234. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Qi WX, He AN, Sun YJ, Shen Z, Yao Y (2013) The prognostic value of survivin expression in patients with colorectal carcinoma: a meta-analysis. Jpn J Clin Oncol 43(10): 988–995. [DOI] [PubMed] [Google Scholar]
- Huh JW, Kim HC, Kim SH, Park YA, Cho YB, Yun SH, Lee WY, Chun HK (2014) Mismatch repair system and p53 expression in patients with T1 and T2 colorectal cancer: predictive role of lymph node metastasis and survival. J Surg Oncol 109(8): 848–852. [DOI] [PubMed] [Google Scholar]
- Jackson SE, Chester JD (2015) Personalised cancer medicine. Int J Cancer 137(2): 262–266. [DOI] [PubMed] [Google Scholar]
- Kajiwara Y, Ueno H, Hashiguchi Y, Mochizuki H, Hase K (2010) Risk factors of nodal involvement in T2 colorectal cancer. Dis Colon Rectum 53(10): 1393–1399. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA (2009) The basics of epithelial-mesenchymal transition. J Clin Invest 119(6): 1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa H, Mitomi H, Nishiyama Y, Kishimoto I, Fukui N, Nakamura T, Watanabe M (2008) Tumour budding at invasive margins and outcome in colorectal cancer. Colorectal Dis 10(1): 41–47. [DOI] [PubMed] [Google Scholar]
- Karamitopoulou E, Zlobec I, Born D, Kondi-Pafiti A, Lykoudis P, Mellou A, Gennatas K, Gloor B, Lugli A (2013) Tumour budding is a strong and independent prognostic factor in pancreatic cancer. Eur J Cancer 49(5): 1032–1039. [DOI] [PubMed] [Google Scholar]
- Kevans D, Wang LM, Sheahan K, Hyland J, O'Donoghue D, Mulcahy H, O'Sullivan J (2011) Epithelial-mesenchymal transition (EMT) protein expression in a cohort of stage II colorectal cancer patients with characterized tumor budding and mismatch repair protein status. Int J Surg Pathol 19(6): 751–760. [DOI] [PubMed] [Google Scholar]
- Knijn N, Mogk SC, Teerenstra S, Simmer F, Nagtegaal ID (2015) Perineural invasion is a strong prognostic factor in colorectal cancer: a systematic review. Am J Surg Pathol 40(1): 103–112. [DOI] [PubMed] [Google Scholar]
- Koelzer VH, Zlobec I, Lugli A (2016) Tumor budding in colorectal cancer—ready for diagnostic practice? Hum Pathol 47(1): 4–19. [DOI] [PubMed] [Google Scholar]
- Koike M, Kodera Y, Itoh Y, Nakayama G, Fujiwara M, Hamajima N, Nakao A (2008) Multivariate analysis of the pathologic features of esophageal squamous cell cancer: tumor budding is a significant independent prognostic factor. Ann Surg Oncol 15(7): 1977–1982. [DOI] [PubMed] [Google Scholar]
- Komori K, Hirai T, Kanemitsu Y, Shimizu Y, Sano T, Ito S, Senda Y, Misawa K, Ito Y, Kato T (2010) Is ‘depth of submucosal invasion ⩾1000 microm' an important predictive factor for lymph node metastases in early invasive colorectal cancer (pT1)? Hepatogastroenterology 57(102-103): 1123–1127. [PubMed] [Google Scholar]
- Lai YH, Wu LC, Li PS, Wu WH, Yang SB, Xia P, He XX, Xiao LB (2014) Tumour budding is a reproducible index for risk stratification of patients with stage II colon cancer. Colorectal Dis 16(4): 259–264. [DOI] [PubMed] [Google Scholar]
- Lea D, Haland S, Hagland HR, Soreide K (2014) Accuracy of TNM staging in colorectal cancer: a review of current culprits, the modern role of morphology and stepping-stones for improvements in the molecular era. Scand J Gastroenterol 49(10): 1153–1163. [DOI] [PubMed] [Google Scholar]
- Li J, Guo BC, Sun LR, Wang JW, Fu XH, Zhang SZ, Poston G, Ding KF (2014. a) TNM staging of colorectal cancer should be reconsidered by T stage weighting. World J Gastroenterol 20(17): 5104–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MX, Liu XM, Zhang XF, Zhang JF, Wang WL, Zhu Y, Dong J, Cheng JW, Liu ZW, Ma L, Lv Y (2014. b) Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review and meta-analysis. Int J Cancer 134(10): 2403–2413. [DOI] [PubMed] [Google Scholar]
- Li Y, Wei J, Xu C, Zhao Z, You T (2014. c) Prognostic significance of cyclin D1 expression in colorectal cancer: a meta-analysis of observational studies. PloS One 9(4): e94508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losi L, Ponti G, Gregorio CD, Marino M, Rossi G, Pedroni M, Benatti P, Roncucci L, de Leon MP (2006) Prognostic significance of histological features and biological parameters in stage I (pT1 and pT2) colorectal adenocarcinoma. Pathol Res Pract 202(9): 663–670. [DOI] [PubMed] [Google Scholar]
- Loughrey MB, Quirke P, Shepherd NA (2014) Standards and datasets for reporting cancers Dataset for colorectal cancer histopathology reports. Version 3. The Royal College of Pathologists: London, July 2014. [Google Scholar]
- Lugli A, Karamitopoulou E, Panayiotides I, Karakitsos P, Rallis G, Peros G, Iezzi G, Spagnoli G, Bihl M, Terracciano L, Zlobec I (2009) CD8+ lymphocytes/ tumour-budding index: an independent prognostic factor representing a 'pro-/anti-tumour' approach to tumour host interaction in colorectal cancer. Br J Cancer 101(8): 1382–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv S, Yang Y, Kwon S, Han M, Zhao F, Kang H, Dai C, Wang R (2014) The association of CXCR4 expression with prognosis and clinicopathological indicators in colorectal carcinoma patients: a meta-analysis. Histopathology 64(5): 701–712. [DOI] [PubMed] [Google Scholar]
- Macias-Garcia F, Celeiro-Munoz C, Lesquereux-Martinez L, Gude-Sampedro F, Uribarri-Gonzalez L, Abdulkader I, Alvarez-Castro A, Dominguez-Munoz JE (2015) A clinical model for predicting lymph node metastasis in submucosal invasive (T1) colorectal cancer. Int J Colorectal Dis 30(6): 761–768. [DOI] [PubMed] [Google Scholar]
- Masuda R, Kijima H, Imamura N, Aruga N, Nakamura Y, Masuda D, Takeichi H, Kato N, Nakagawa T, Tanaka M, Inokuchi S, Iwazaki M (2012) Tumor budding is a significant indicator of a poor prognosis in lung squamous cell carcinoma patients. Mol Med Rep 6(5): 937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel S, Mansmann U, Papadopoulos T, Wittekind C, Hohenberger W, Hermanek P (2001) The prognostic inhomogeneity of colorectal carcinomas stage III: a proposal for subdivision of stage III. Cancer 92(11): 2754–2759. [PubMed] [Google Scholar]
- Miyachi H, Kudo SE, Ichimasa K, Hisayuki T, Oikawa H, Matsudaira S, Kouyama Y, Kimura YJ, Misawa M, Mori Y, Ogata N, Kudo T, Kodama K, Hayashi T, Wakamura K, Katagiri A, Baba T, Hidaka E, Ishida F, Kohashi K, Hamatani S (2015) Management of T1 colorectal cancers after endoscopic treatment based on the risk stratification of lymph node metastasis. J Gastroenterol Hepatol 31(6): 1126–1132. [DOI] [PubMed] [Google Scholar]
- Morodomi T, Isomoto H, Shirouzu K, Kakegawa K, Irie K, Morimatsu M (1989) An index for estimating the probability of lymph node metastasis in rectal cancers. Lymph node metastasis and the histopathology of actively invasive regions of cancer. Cancer 63(3): 539–543. [DOI] [PubMed] [Google Scholar]
- Nagtegaal ID, Gosens MJ, Marijnen CA, Rutten HJ, van de Velde CJ, van Krieken JH (2007) Combinations of tumor and treatment parameters are more discriminative for prognosis than the present TNM system in rectal cancer. J Clin Oncol 25(13): 1647–1650. [DOI] [PubMed] [Google Scholar]
- Nakadoi K, Tanaka S, Kanao H, Terasaki M, Takata S, Oka S, Yoshida S, Arihiro K, Chayama K (2012) Management of T1 colorectal carcinoma with special reference to criteria for curative endoscopic resection. J Gastroenterol Hepatol 27(6): 1057–1062. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Mitomi H, Kanazawa H, Ohkura Y, Watanabe M (2008) Tumor budding as an index to identify high-risk patients with stage II colon cancer. Dis Colon Rectum 51(5): 568–572. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Mitomi H, Kikuchi S, Ohtani Y, Sato K (2005) Evaluation of the usefulness of tumor budding on the prediction of metastasis to the lung and liver after curative excision of colorectal cancer. Hepatogastroenterology 52(65): 1432–1435. [PubMed] [Google Scholar]
- Nishida T, Egashira Y, Akutagawa H, Fujii M, Uchiyama K, Shibayama Y, Hirose Y (2014) Predictors of lymph node metastasis in T1 colorectal carcinoma: an immunophenotypic analysis of 265 patients. Dis Colon Rectum 57(8): 905–915. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Yoshida T, Tsuruta T, Tokuyama W, Adachi S, Kikuchi M, Mikami T, Saigenji K, Okayasu I (2009) Tumor budding is predictive of lymphatic involvement and lymph node metastases in submucosal invasive colorectal adenocarcinomas and in non-polypoid compared with polypoid growths. Scand J Gastroenterol 44(5): 605–614. [DOI] [PubMed] [Google Scholar]
- Okuyama T, Nakamura T, Yamaguchi M (2003. a) Budding is useful to select high-risk patients in stage II well-differentiated or moderately differentiated colon adenocarcinoma. Dis Colon Rectum 46(10): 1400–1406. [DOI] [PubMed] [Google Scholar]
- Okuyama T, Oya M, Ishikawa H (2003. b) Budding as a useful prognostic marker in pT3 well- or moderately-differentiated rectal adenocarcinoma. J Surg Oncol 83(1): 42–47. [DOI] [PubMed] [Google Scholar]
- Park SY, Choe G, Lee HS, Jung SY, Park JG, Kim WH (2005) Tumor budding as an indicator of isolated tumor cells in lymph nodes from patients with node-negative colorectal cancer. Dis Colon Rectum 48(2): 292–302. [DOI] [PubMed] [Google Scholar]
- Petrelli F, Pezzica E, Cabiddu M, Coinu A, Borgonovo K, Ghilardi M, Lonati V, Corti D, Barni S (2015) Tumour budding and survival in stage II colorectal cancer: a systematic review and pooled analysis. J Gastrointest Cancer 46(3): 212–218. [DOI] [PubMed] [Google Scholar]
- Piccart M (2013) Personalised cancer management: closer, but not here yet. Ann Oncol 24(8): 1951–1955. [DOI] [PubMed] [Google Scholar]
- Prall F, Nizze H, Barten M (2005) Tumour budding as prognostic factor in stage I/II colorectal carcinoma. Histopathology 47(1): 17–24. [DOI] [PubMed] [Google Scholar]
- Puppa G, Senore C, Sheahan K, Vieth M, Lugli A, Zlobec I, Pecori S, Wang LM, Langner C, Mitomi H, Nakamura T, Watanabe M, Ueno H, Chasle J, Conley SA, Herlin P, Lauwers GY, Risio M (2012) Diagnostic reproducibility of tumour budding in colorectal cancer: a multicentre, multinational study using virtual microscopy. Histopathology 61(4): 562–575. [DOI] [PubMed] [Google Scholar]
- Quasar Collaborative Group, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ (2007) Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 370(9604): 2020–2029. [DOI] [PubMed] [Google Scholar]
- Reggiani Bonetti L, Di Gregorio C, De Gaetani C, Pezzi A, Barresi G, Barresi V, Roncucci L, Ponz de Leon M (2011) Lymph node micrometastasis and survival of patients with Stage I (Dukes' A) colorectal carcinoma. Scand J Gastroenterol 46(7-8): 881–886. [DOI] [PubMed] [Google Scholar]
- Rogers AC, Gibbons D, Hanly AM, Hyland JM, O'Connell PR, Winter DC, Sheahan K (2014) Prognostic significance of tumor budding in rectal cancer biopsies before neoadjuvant therapy. Mod Pathol 27(1): 156–162. [DOI] [PubMed] [Google Scholar]
- Rui Y, Wang C, Zhou Z, Zhong X, Yu Y (2015) K-Ras mutation and prognosis of colorectal cancer: a meta-analysis. Hepatogastroenterology 62(137): 19–24. [PubMed] [Google Scholar]
- Ryu HS, Kim WH, Ahn S, Kim DW, Kang SB, Park HJ, Park YS, Lee CH, Lee HS (2014) Combined morphologic and molecular classification for predicting lymph node metastasis in early-stage colorectal adenocarcinoma. Ann Surg Oncol 21(6): 1809–1816. [DOI] [PubMed] [Google Scholar]
- Safaee Ardekani G, Jafarnejad SM, Tan L, Saeedi A, Li G (2012) The prognostic value of BRAF mutation in colorectal cancer and melanoma: a systematic review and meta-analysis. PLoS One 7(10): e47054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhia B, Trippel M, Pfaltz K, Cihoric N, Grogg A, Ladrach C, Zlobec I, Tapia C (2015) High tumor budding stratifies breast cancer with metastatic properties. Breast Cancer Res Treat 150(2): 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sannier A, Lefevre JH, Panis Y, Cazals-Hatem D, Bedossa P, Guedj N (2014) Pathological prognostic factors in locally advanced rectal carcinoma after neoadjuvant radiochemotherapy: analysis of 113 cases. Histopathology 65(5): 623–630. [DOI] [PubMed] [Google Scholar]
- Schiffmann L, Eiken AK, Gock M, Klar E (2013) Is the lymph node ratio superior to the Union for International Cancer Control (UICC) TNM system in prognosis of colon cancer? World J Surg Oncol 11: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sert Bektas S, Inan Mamak G, Ciris IM, Bozkurt KK, Kapucuoglu N (2012) Tumor budding in colorectal carcinomas. Turk Patoloji Derg 28(1): 61–66. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Togashi K, Nokubi M, Koinuma K, Miyakura Y, Horie H, Lefor AT, Yasuda Y (2009) Evaluation of venous invasion by Elastica van Gieson stain and tumor budding predicts local and distant metastases in patients with T1 stage colorectal cancer. Am J Surg Pathol 33(11): 1601–1607. [DOI] [PubMed] [Google Scholar]
- Tateishi Y, Nakanishi Y, Taniguchi H, Shimoda T, Umemura S (2010) Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod Pathol 23(8): 1068–1072. [DOI] [PubMed] [Google Scholar]
- Tournigand C, Andre T, Bonnetain F, Chibaudel B, Lledo G, Hickish T, Tabernero J, Boni C, Bachet JB, Teixeira L, de Gramont A (2012) Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter sInternational Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 30(27): 3353–3360. [DOI] [PubMed] [Google Scholar]
- Tristante E, Martinez CM, Jimenez S, Mora L, Carballo F, Martinez-Lacaci I, de Torre-Minguela C (2015) Association of a characteristic membrane pattern of annexin A2 with high invasiveness and nodal status in colon adenocarcinoma. Transl Res 166(2): 196–206. [DOI] [PubMed] [Google Scholar]
- Ueno H, Mochizuki H, Shinto E, Hashiguchi Y, Hase K, Talbot IC (2002. a) Histologic indices in biopsy specimens for estimating the probability of extended local spread in patients with rectal carcinoma. Cancer 94(11): 2882–2891. [DOI] [PubMed] [Google Scholar]
- Ueno H, Murphy J, Jass JR, Mochizuki H, Talbot IC (2002. b) Tumour 'budding' as an index to estimate the potential of aggressiveness in rectal cancer. Histopathology 40(2): 127–132. [DOI] [PubMed] [Google Scholar]
- Ueno H, Price AB, Wilkinson KH, Jass JR, Mochizuki H, Talbot IC (2004) A new prognostic staging system for rectal cancer. Ann Surg 240(5): 832–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wyk HC, Park J, Roxburgh C, Horgan P, Foulis A, McMillan DC (2015) The role of tumour budding in predicting survival in patients with primary operable colorectal cancer: a systematic review. Cancer Treat Rev 41(2): 151–159. [DOI] [PubMed] [Google Scholar]
- Verhulst J, Ferdinande L, Demetter P, Ceelen W (2012) Mucinous subtype as prognostic factor in colorectal cancer: a systematic review and meta-analysis. J Clin Pathol 65(5): 381–388. [DOI] [PubMed] [Google Scholar]
- Wada H, Shiozawa M, Sugano N, Morinaga S, Rino Y, Masuda M, Akaike M, Miyagi Y (2013) Lymphatic invasion identified with D2-40 immunostaining as a risk factor of nodal metastasis in T1 colorectal cancer. Int J Clin Oncol 18(6): 1025–1031. [DOI] [PubMed] [Google Scholar]
- Wang HS, Liang WY, Lin TC, Chen WS, Jiang JK, Yang SH, Chang SC, Lin JK (2005) Curative resection of T1 colorectal carcinoma: risk of lymph node metastasis and long-term prognosis. Dis Colon Rectum 48(6): 1182–1192. [DOI] [PubMed] [Google Scholar]
- Wang LM, Kevans D, Mulcahy H, O'Sullivan J, Fennelly D, Hyland J, O'Donoghue D, Sheahan K (2009) Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am J Surg Pathol 33(1): 134–141. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yao X, Ge J, Hu F, Zhao Y (2014) Can vascular endothelial growth factor and microvessel density be used as prognostic biomarkers for colorectal cancer? A systematic review and meta-analysis. ScientificWorldJournal 2014: 102736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wazir U, Mokbel K (2014) Emerging gene-based prognostic tools in early breast cancer: first steps to personalised medicine. World J Clin Oncol 5(5): 795–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Yang B, Zhai X, Liu X, Shen K, Wu Z, Cai J (2013) Prognostic role of microRNA-21 in colorectal cancer: a meta-analysis. PloS One 8(11): e80426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing X, Cai W, Shi H, Wang Y, Li M, Jiao J, Chen M (2013) The prognostic value of CDKN2A hypermethylation in colorectal cancer: a meta-analysis. Br J Cancer 108(12): 2542–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi H, Togashi K, Kawamura YJ, Horie H, Sasaki J, Tsujinaka S, Yasuda Y, Konishi F (2008) Pathological predictors for lymph node metastasis in T1 colorectal cancer. Surg Today 38(10): 905–910. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Inomata M, Shiromizu A, Shiraishi N, Higashi H, Kitano S (2007) Risk factors for occult lymph node metastasis of colorectal cancer invading the submucosa and indications for endoscopic mucosal resection. Dis Colon Rectum 50(9): 1370–1376. [DOI] [PubMed] [Google Scholar]
- Zlobec I, Bihl MP, Foerster A, Rufle A, Lugli A (2012) The impact of CpG island methylator phenotype and microsatellite instability on tumour budding in colorectal cancer. Histopathology 61(5): 777–787. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.