Abstract
Given prostate cancer is driven, in part, by its responsiveness to androgens, treatments historically employ methods for their removal from circulation. Approaches as crude as castration, and more recently blockade of androgen synthesis or receptor binding, are still of limited use long term, since other steroids of adrenal origin or tumor origin can supersede that role as the ‘castration resistant’ tumor re-emerges. Broader inhibition of steroidogenesis using relatively nonselective P450 inhibitors such as ketoconazole is not an alternative since a general disruption of steroid biosynthesis is neither safe nor effective. The recent emergence of drugs more selectively targeting CYP17 have been more effective, and yet extension of life has been on the scale of months rather than years. It is now becoming clear this shortcoming arises from the adaptive capabilities of many tumors to initiate local steroid synthesis and/or become responsive to novel early pathway adrenal steroids that are synthesized when lyase activity is not selectively blocked, and ACTH rises in the face of declining cortisol feedback. Abiraterone has been described as a lyase selective inhibitor, yet its use still requires co-administration of prednisone to suppress such a rise of ACTH and fall in cortisol. So is creation of a selective lyase inhibitor even possible? Can C19 steroid production be achieved without a prominent decline in cortisol and corresponding rise in ACTH? Decades of scientific study of CYP17 in humans and nonhuman primates, as well as nature’s own experiments of gene mutations in humans, reveal ‘true’ or ‘isolated’ 17,20 lyase deficiency does quite selectively prevent C19 steroid biosynthesis whereas simple 17 hydroxylase deficiency also suppresses cortisol. We propose these known outcomes of natural mutations should be used to guide analysis of clinical trials and long term outcomes of CYP17 targeted drugs. In this review, we use that framework to re-evaluate the basic and clinical outcomes of many compounds being used or in development for treatment of castration resistant prostate cancer. Specifically, we include the nonselective drug ketoconazole, and then the CYP17 targeted drugs abiraterone, orteronel (TAK-700), galaterone (TOK-001), and VT464. Using this framework, we can fully discriminate the clinical outcomes for ketoconazole, a drug with broad specificity, yet clinically ineffective, from that of abiraterone, the first CYP17 targeted therapy that is limited by its need for prednisone co-therapy. We also can identify potential next generation CYP17 targeted drugs now emerging that show signs of being far more 17,20 lyase selective. We conclude that a future for improved therapy without substantial cortisol decline, thus avoiding prednisone co-administration, seems possible at long last.
Keywords: Prostate, Cancer, CYP17A1, Inhibitor, Lyase, Hydroxylase
1): The Problem
One area of modern medicine that continues to evade us is the treatment of prostate cancer. Like breast cancer, prostate cancer is commonly referred to as an ‘endocrine’ cancer due to the fact initial growth is driven by naturally occurring C19 bioactive steroids, including testosterone and its respective metabolites 1. Two recent approaches towards treatment of both of these steroid hormone-driven malignancies involve blockade of steroid binding to, or downregulation of androgen receptors (AR) 2 or estrogen receptors (ER) 3, respectively, or by inhibition of steroidogenic enzymes late in the steroidogenic pathway, particularly from the use of CYP19 (aromatase) inhibitors4. Steroid receptor blockade strategies, however, can fail due to treatment-induced removal of negative feedback regulation on hypothalamic gonadotropin releasing-hormone (GnRH) and pituitary gonadotropins, resulting in overdrive of endogenous steroid biosynthesis and so requiring accompanying GnRH analog therapy to ensure inhibition of pituitary gonadotropin release5. While steroid receptor antagonists may prove to be of future use, a more effective contemporary method is needed for controlling steroid biosynthesis in order to prevent induction of natural or drug-derived steroid ligands capable of driving tumor resurgence.
An alternative approach to this problem would be to inhibit the biosynthesis of dehydroepiandrostenedione (DHEA), which in humans and nonhuman primates is an obligatory intermediate in all C19 steroid biosynthesis from CYP17A1 6. There has indeed been a great deal of excitement in the oncology field over recent success in going beyond the initial use of general steroid biosynthesis inhibitors, such as ketoconazole 7, and testing of newer CYP17A1 inhibitors, such as abiraterone 8; 9; 10 to limit DHEA biosynthesis. Recent trials have suggested that the CYP17A1 inhibition strategy is indeed successful, with ~ 5 months of additional overall survival accruing to subjects receiving abiraterone who had already developed castration resistant prostate cancer 11; 12; 13; 14; 15. There is, nevertheless, a major problem that remains to be overcome; while tumor cells develop steroid producing ability of their own, as in the case of prostate cancer 10; 13; 14; 15, they are not the major biosynthetic source of steroid hormones in the body. That title is clearly claimed by the adrenal, which dwarfs steroidogenic output by testes, ovaries or other organs, including adipose and the brain. There is also the specific problem that the CYP17 enzyme which creates DHEA and all subsequent C19 steroid metabolites, is also the same enzyme necessary for cortisol biosynthesis. Complete and absolute inhibition of CYP17 in itself creates major problems that we will detail below. Not only is it possible for normally circulating C19 steroids to drive these malignancies, but suppression of normal circulating levels of DHEA and androstenedione (A4) can also lead tumors themselves to locally express CYP17 and other enzymes necessary to maintain local biosynthesis 16. In this review we will consider normal and alternate tumor biosynthesis of C19 steroids, and how the selective targeting in either case of 17,20 lyase activity inhibition, while sparing 17 hydroxylase activity, is the ‘holy grail’ necessary to overcome these problems.
Impact of CYP17 inhibition on the HPA axis
By far the greatest source of circulating C19 steroids is the adrenal cortex, producing mg quantities of DHEA production daily17. This function is normally undertaken by the zona reticularis (ZR) where, after adrenarche, CYP17 is co-expressed with abundant cytochrome b5 (CytB5) and lower levels of HSD3B2. This maximally enables 17,20 lyase oxidative cleavage of the 17-20 carbon-carbon bond within 17-hydroxypregnenolone to release DHEA 18; 19; 20; 21. Much of the DHEA so produced is then acted upon by SULT2A and the resulting sulfotransferase conjugation creates DHEAS, a unique circulating biomarker for adrenal androgen production. Constantly high circulating DHEA and DHEAS in males then provide the key steroidogenic precursors needed to form bioactive androgens in the adrenal ZR, the testicular Leydig cells, and also to be further converted in prostate tumor cells expressing the right enzymes 14; 15; 16. In addition, the adrenocortical zona fasciculata (ZF) continues to normally synthesize and releases considerable daily quantities of the glucocorticoid, cortisol 17. Such glucocorticoid steroidogenic production is critically dependent on 17-hydroxylation of pregnenolone into 17-hydroxypregnenolone, an enzymatic activity located within CYP17A1 immediately prior to its potential 17,20 lyase activity17.
An important point to recognize is that while DHEA from the ZR is also made under the predominant control of ACTH (Figure 1), it is only cortisol that provides effective negative feedback regulation of hypothalamic corticotropin releasing-hormone (CRH) and pituitary adrenocorticotropic hormone (ACTH) release 22. CRH is also enhanced by co-neuroendocrine release of vasopressin in its stimulation of pituitary ACTH release. Thus, it is the diminution of normal cortisol synthesis and release that leads to excess neuroendocrine drive to produce excessive amounts of steroid hormone intermediates earlier in the steroidogenic pathway, and in the case of excess ACTH, may even further promote alternate early steroidogenic pathway products to enter the circulation (Figure 1). In the ZR, where CYP17 is already abundant, excess CRH and ACTH may cause even higher CYP17 expression, and so continue to contribute to greater DHEA and DHEAS, even as 17-hydroxylase remains partly inhibited 14. It is already clear that biosynthetic inhibitors that cause generalized inhibition of CYP17A1 leading to additional blockade of cortisol biosynthesis and associated loss of feedback on the neuroendocrine axis, will result in increased ACTH release and as the remaining steroid pathway literally enters overdrive, there is now physiologically abnormal over-production of early steroidogenic pathway steroids in the ZF of the adrenal cortex, including pregnenolone (Figure 1). As this spills into the circulation, it can supplement continued conversion to DHEA (in the ZR) or to progesterone (in the ZF), and may even be converted to inappropriately high levels of aldosterone (in the ZG). These are indeed all observations that have been reported with the use of abiraterone, and such ACTH-induced aldosterone excess in particular is most likely responsible for abiraterone-associated increases in cardiovascular disorders 14; 15.
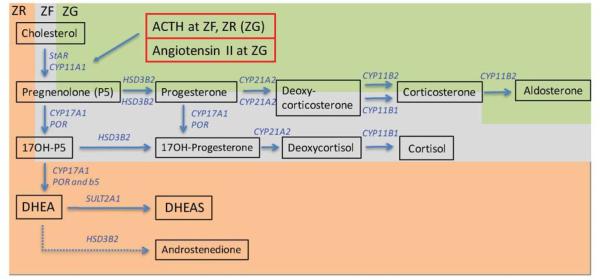
Figure 1. ACTH-dependent, specialized steroidogenic pathways of the adrenal cortex.
In the zona glomerulosa (ZR, green shading), an absence of CYP17A1 leads to aldosterone as the major hormone released, while in the zona fasciculata (ZF, grey shading), expression of CYP17A1 without CytB5 leads to predominant 17-hydroxylase activity and cortisol as the major hormone released. In the zona reticularis (ZR, orange-pink shading), expression of CYP17A1 together with CytB5 now enhances 17,20 lyase activity. Coexpression of sulfotransferase, but diminished expression of 3beta-hydroxysteroid dehydrogenase, within the ZR leads to DHEA and DHEAS as the major products released post adrenarche, and androstenedione as a minor product (dashed arrow). Under physiological conditions, ACTH stimulated ZF and ZR steroidogenic function is regulated by cortisol negative feedback alone, while angiotensin II (and circulating K+) predominantly regulate ZG steroidogenic function.
Further complications caused by cortisol replacement
In order to counteract abiraterone-mediated progestogenic stimulation of prostate tumor survival, supplementation strategies employ prednisone or dexamethasone in conjunction with abiraterone to reverse elevations in circulating ACTH levels. While this co-treatment alleviates progestogenic excess 14; 15, it does not always alleviate aldosterone excess, and requires additional mineralocorticoid receptor antagonism in such situations 23. One immediate concern is that high levels of synthetic glucocorticoids, such as prednisone and dexamethasone, are also not therapies that can be tolerated for indefinite periods, and their inevitable forced withdrawal can lead to subsequent problems in simply regaining adrenal function in an already compromised patient. More recent data also suggest that prednisone itself may stimulate the mutant AR, further defeating the point of prednisone co-therapy 24. What is needed is a better way to starve prostate cancer of both C19 androgens and early steroid pathway intermediates, such as pregnenolone and progesterone, while preserving cortisol feedback, and thus avoiding a need for prednisone. But is that possible? The answer is “yes”, but only if we make inhibition selective for 17,20 lyase activity alone within CYP17A1.
Tumor survival strategies to escape CYP17 inhibition
As a result of CYP17A1 inhibition, prostate tumors will initially regress, but because of pituitary ACTH escape from cortisol negative feedback, increased ACTH stimulates adrenocortical steroid pathways causing excessive release of accumulating early pathway intermediates into the circulation. There is now an alternative option for malignant cells that survive through expression of an alternate mutated AR receptor 14; 25 that can bind and respond to these steroids, including progesterone and pregnenolone 10; 14. The continued excess of these progestogenic steroids appears to stimulate any newly expressed mutant AR 14, thus resulting in strong selection for malignant cells expressing the mutant AR and corresponding resurgent tumor growth. Such a situation potentially limits any further extension of cancer patient longevity using CYP17A1 inhibitors acting on 17 hydroxylase activity. It is thus not surprising that abiraterone only extends prostate cancer patient survival for ~ 5 months beyond the life expectancy provided by therapeutic or surgical castration 14, when prostate tumors become refractory to abiraterone therapy 14; 15.
In addition, abiraterone also inhibits HSD3B1. Some reports have indeed shown that HSD3B1 can be expressed in abiraterone-resistant tumors 26; 27; 28 and other reports show CRPC tumors can express HSD3B2 29. Since HSD3B isoforms are needed to convert DHEA to androstenedione, the assumption has been made this is a beneficial additional property, but other data regarding relative levels of CYP17 vs HSD3B activity suggest otherwise. Given human and nonhuman primate CYP17 is deficient in delta 4 lyase activity converting 17-hydroxy progesterone to androstenedione 6, then the major obligatory pathway for androstenedione production is via DHEA (Figure 1). This is the case not only in human adrenal ZR, but also in both gonads and the tumor. Previous mathematical modeling of relative enzyme activity confirms that progressive limitation of HSD3B activity relative to CYP17 raises DHEA production, and does not lower it 30.
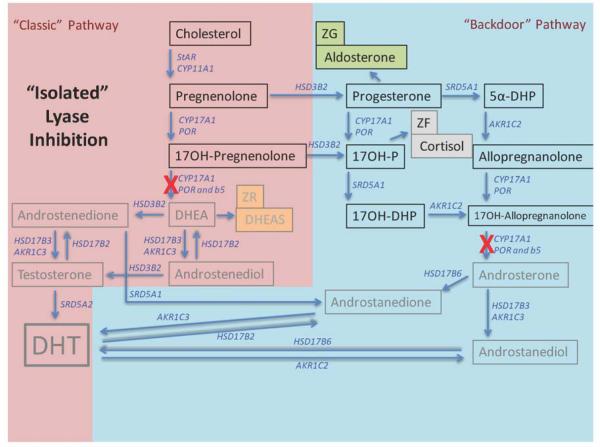
Beyond the major pathways indicated above, another consideration of relevance is the possible synthesis of C19 steroids by drug resistant tumors via alternative biosynthetic pathways. As shown in Figure 2, there are alternative pathways from progesterone or DHEA to 5 alpha DHT, including several other steroid metabolizing enzymes that are found in drug resistant CRPC tumors 16. The key point, nonetheless, is that CYP17A1 is still necessary for either of these pathways to receive DHEA substrate, or convert progesterone in particular to DHT independently of DHEA/androstenedione substrate. Clearly, based on Figure 2, if 17,20 lyase is selectively inhibited, not only is adrenal DHEA blocked without adrenal progesterone being elevated enough to enter the circulation, but also those tumors that initiate alternate steroid pathway biosynthesis will remain starved of C19 steroids in general, and DHT in particular, from other sources.
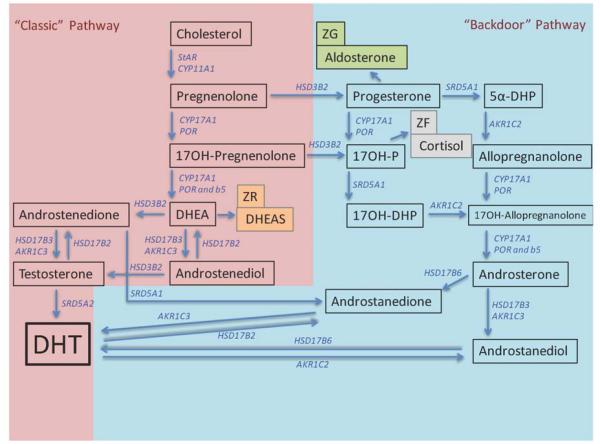
Figure 2. An alternative “backdoor” steroidogenic pathway to androgen production can operate in parallel to the “classical” steroidogenic pathway.
The “backdoor” pathway utilizes pregnenolone and progesterone to synthesize dihydrotestosterone, a highly potent androgen, without progressing through “classical” pathways of production to DHEA and androstenedione. Co-expression of CYP17A1 and CytB5, however, are required for both pathways to operate. Modified from 33.
2) The Search for a selective 17,20 Lyase inhibitor – is it even possible?
We now explore the evidence that selective lyase inhibition is indeed attainable. We then further explore how close the already established CYP inhibitors, and more recent compounds targeted to CYP17A1 in particular have progressed towards achieving that goal. Because the focus of this review is ultimately human therapy, we will focus only on the literature for species that show similar characteristics for CYP17A1 activity, namely humans and nonhuman primates, and we will also refer to data from the human-derived steroidogenic cell line, H295R, and its homologues. Before we begin, however, we first need to describe the normal role of CYP17A1 in steroid biosynthesis and ask what criteria can be effectively employed to distinguish between combined 17-hydroxylase and 17,20 lyase inhibition, and ‘true’, or ‘isolated’ 17,20 lyase deficiency. How much do circulating concentrations of steroids change in each instance? Can cortisol biosynthesis be preserved while completely inhibiting DHEA production? Fortunately the past 25 years have yielded an extensive understanding of the role of CYP17A1 in the normal adult male primate, and have allowed time to identify and characterize the outcomes of point mutations to the CYP17A1 gene itself, and indeed genes for accessory proteins (such as CytB5) that, when mutated selectively, impair 17,20 lyase activity while leaving 17 hydroxylase activity largely intact 31. In many clinical trials, there is little reference to this basic steroidogenic literature, and yet it is key to both an understanding of the problem and achieving future therapeutic success. The data are indeed already there to guide the way to achieving our goal of a CYP17A1 selective drug capable of “clean” androgen ablation without ACTH excess.
What nature teaches us about the importance of independent control of 17-hydroxylase vs 17,20 lyase enzymatic activities within CYP17A1
With the cloning and expression of CYP17A1 cDNA, it was immediately apparent that the two previously reported 17-hydroxylase and 17,20 lyase enzymatic activities were inherent within one protein sequence 32. Since that time, the question has been: Is it truly possible to differentially regulate the two enzymatic activities within CYP17A1? We 6 have previously reviewed the evidence that there is both a clear preference of P450c17 CYP17A1 in humans for pregnenolone over progesterone, and further, that the relative delta 4 pathway 17,20 lyase insufficiency in humans, effectively disabling conversion of 17-hydroxyprogesterone into androstenedione, makes DHEA biosynthesis a prerequisite for androgenic and estrogenic C19 steroid biosynthesis. The studies of Miller and colleagues 33 confirm that the DHEA-route of biosynthesis of C19 steroids is preserved in many nonhuman primate species. Given that the 17-hydroxylase reaction converting pregnenolone to 17-hydroxypregnenolone occurs with higher substrate affinity than that of HSD3B conversion of pregnenolone to progesterone, then progesterone will only be synthesized in abundance when CYP17A1 expression is diminished or its activity is limited 6. While the absence of CYP17A1 activity is indeed the norm in both the adrenal ZG (Figure 1) and in ovarian follicle granulosa cells, it should be remembered that in the adrenal ZG, the additional abundance of CYP21A1 normally causes rapid conversion of any progesterone straight to deoxycorticosterone, and so limits release of progesterone into the circulation. In contrast, in the ovarian granulosa, the lack of CYP21A1 expression, except during mid-cycle luteinization 34, means that conversion of pregnenolone to progesterone is an end point, resulting in the release of this progestagen. It is therefore no surprise that under normal circumstances, while progesterone is made in both adrenal and ovary, circulating levels are relatively low and are only a reflection of the ovary when progesterone becomes an end product of granulosa cells of the dominant, pre-ovulatory follicle (late follicular phase of the menstrual cycle), and following ovulation when granulosa cells are luteinized (during the ovulatory LH surge) and form the majority of the corpus luteum (luteal phase). Luteinized granulosa cells within the post-ovulatory corpus luteum are mostly LH-driven and the fact they exhibit exaggerated HSD3B expression is what enables nM concentrations of progesterone in the circulation in support of potential fertilization, implantation and early pregnancy.
In the case of the adrenal ZF, however, expression of abundant 17-hydroxylase enables conversion of pregnenolone to 17-hydroxypregnenolone, and this is followed by the choice of further 17,20 lyase activity by CYP17A1 or further ‘dehydrogenase’ activity by HSD3B. In contrast to its 17-hydroxylase properties, 17,20 lyase activity within CYP17A1 is generally slower and the Km shows relatively lower affinity for 17-hydroxypregenolone, so HSD3B can compete effectively if HSD3B is sufficiently abundant and it becomes the predominant pathway. In the normal human ZF, once pregnenolone is converted to 17-hydroxypregnenolone, it is then converted to 17-hydroxyprogesterone. Once made, 17-hydroxyprogesterone cannot be effectively converted to androstenedione, since the human and nonhuman primate, CYP17A1 uniquely lacks effective delta 4 lyase activity 6 (Figure 1). Due to the additional presence of abundant CYP21A1 in the ZF, the fate of 17-hydroxyprogesterone in this zone of the adrenal cortex is also immediate conversion to deoxycortisol. Once again, while produced as an intermediate in the adrenal ZF itself, circulating levels of 17-hydroxyprogesterone are not normally a reflection of adrenal production, but of both ovarian pre-ovulatory theca and post-ovulatory luteinized theca cell function, particularly from the latter during the post-ovulatory luteal phase of the ovarian or menstrual cycle 35; 36. Only in conditions of CYP21A1 deficiency will circulating levels of 17-hydroxyprogesterone reflect adrenal activity 6; 37; 38 and so are used as diagnostic criteria for congenital adrenal hyperplasia 39; 40; 41; 42.
There are also normal circumstances during which 17,20 lyase activity can predominate and so lead to substantial production of DHEA and so feed into the C19 androgen pathway. This occurs in the adult adrenal ZR of both human and nonhuman primates where CytB5 is co-expressed with CYP17A1 at high levels, while competing HSD3B expression and activity is severely limited relative to CYP17A1. It is particularly noteworthy that the selective enhancement of 17,20 lyase activity is made possible relative to 17-hydroxylase activity by the presence of substantially elevated CytB5, and more so when it is recognized that electron transfer by CytB5 is also not required. Indeed activation of 17,20 lyase activity by CytB5 has been proposed to be purely allosteric 43; 44. Such an observation is not only critical in understanding normal physiologic regulation of DHEA synthesis in the normal adrenal goes beyond CYP17A1 expression alone, it also suggests selective inhibition of 17,20 lyase activity may indeed be possible.
Clearly, nature has revealed to us that the need to control DHEA production (for the sake of controlling C19 androgens) should not occur at the expense of diminishing an essential function, such as glucocorticoid production. When we also look further at hypothalamic-pituitary negative feedback regulation of adrenocortical steroidogenesis, exquisite feedback control exists for tightly maintaining cortisol in response to ACTH and there are further automated gain controls at the level of hypothalamo-pituitary 11BHSD expression (regulating intra-cellular inter-conversion between cortisol and inactive cortisone) 45, ACTH stimulating its own receptor level, and ACTH driven adrenal hyperplasia to fine tune the system 46; 47; 48. In contrast, while ACTH is at least one powerful stimulant of adrenal ZR DHEA synthesis and release, and this entire adrenal zone is geared to the additional production of sulfoconjugated DHEAS (Figure 1), neither DHEA nor its metabolites offer substantial negative feedback control on hypothalamo-pituitary regulation of adrenal cortex steroidogenesis 49.
Studies in subjects with CYP17 mutations
To summarize, while nature imposes tight control of 17-hydroxylase activity by simple control of expression relative to HSD3B and superimposed endocrine stimulation of pathway activity, the differential control of 17,20 lyase activity can be further achieved in humans and nonhuman primates through i) exploitation of competing reactions in the absence of significant delta 4 lyase activity and ii) further CYP17A1 allosteric interaction with CytB5 (through co-expression). The question now is can we exploit this pharmacologically by targeting the 17,20 lyase component of CYP17A1? Ironically, even before our more recent understanding of the dual nature of CYP17A1 enzymatic activity, nature had already run this experiment for us. With the discovery of natural CYP17A1 mutations 50; 51, many affected subjects have now been investigated for changes in steroid metabolism and associated signs and symptoms 42; 52; 53; 54. The findings of such studies certainly underscore the view that deficiency of 17-hydroxylase activity has the more substantial health consequences through disruption of cortisol biosynthesis (Figures 3 and 4) 42; 52; 53; 54. In contrast, a general finding in those proposed to have ‘true’, selective 17,20 lyase deficiency is they still have 17-hydroxylase activity sufficient for cortisol biosynthesis at basal levels (Figure 5), but have diminished capacity to raise cortisol synthesis through ACTH stimulation 31. The latter strongly suggests that such ‘true’ 17,20 lyase deficient subjects are indeed also showing some partial reduction in 17-hydroxylase activity.
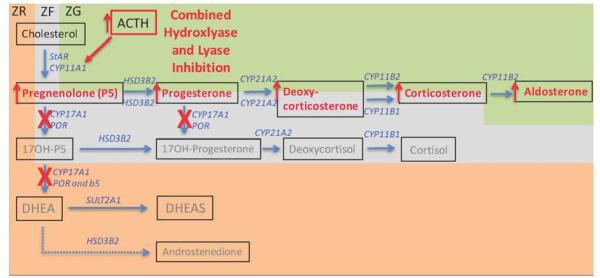
Figure 3. Altered steroid hormone production in the adrenal cortex induced following diminished CYP17A1 expression or selective 17-hydroxylase inhibition.
In the case of selective 17 hydroxylase inhibition, 17,20 lyase function is also correspondingly lost (red Xs).. While androgen biosynthesis is now prevented, cortisol is also lost (grey text). This releases negative feedback control of circulating ACTH, and the ensuing excess ACTH levels over-stimulate production and release of both aldosterone and early steroid pathway products into the circulation (red arrows). Other details are provided in the legend to Figure 1.
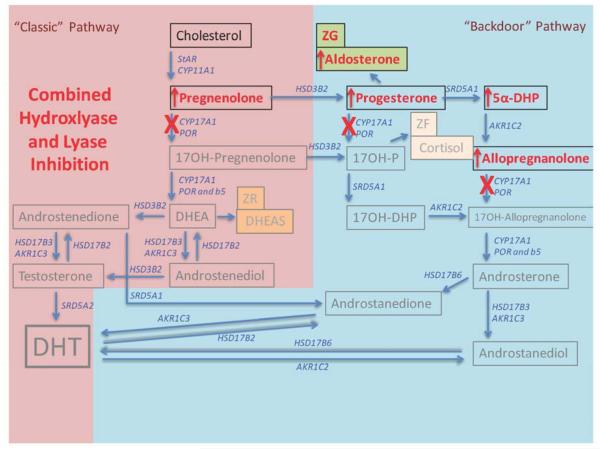
Figure 4. Altered steroid hormone production in both “classical” and “backdoor” pathways following diminished expression of CYP17A1 or selective 17-hydroxylase inhibition (red Xs).
While androgen biosynthesis is absent, cortisol is also absent (grey text), releasing negative feedback control on ACTH, and resulting in excessive ACTH drive also over-stimulating “backdoor” products of 5alpha-DHP and allopregnanolone, in addition to supraphysiologic over-stimulation of the adrenal ZG aldosterone pathway (red arrows; see also Figure 3).
Figure 5. Altered steroid hormone production in both “classical” and “backdoor” pathways following selectively diminished lyase activity within CYP17A1 (red Xs).
While androgen biosynthesis through both “classical” and “backdoor” pathways is now absent (grey text), sufficient cortisol biosynthesis remains to maintain negative feedback control on ACTH, thus avoiding the abnormal steroid hormone excesses of hydroxylase deficiency or blockade shown in Figures 3 and 4.
Structure-function studies on human and nonhuman primate CYP17A1 mutations, combined with our understanding of the importance of CytB5 co-expression, have started to unravel which amino acids in the primary CYP17A1 sequence are most important to 17,20 lyase activity. It is no surprise then that the amino acids most related to interaction with P450 oxido-reductase and CytB5 are indeed the ones most effective in reducing 17,20 lyase activity while maintaining the most hydroxylase activity 21; 55; 56. It is also no surprise that subjects with mutations in CytB5 are also found to result in truly selective, fully isolated 17,20 lyase deficient. The important difference is that at least one mutation of CytB5 results in complete loss of DHEA and its metabolites, while leaving cortisol at completely normal levels and normally responsive to ACTH challenge 57. The latter finding contrasts with multiple cases of CYP17A1 mutations classified as true lyase activity mutations wherein 17,20 lyase activity of the protein expressed in vitro is reduced to less than 10%, and an associated reduction of 17-hydroxlase activity to ~65 % of control is routinely observed 31; 57; 58. While circulating cortisol in such subjects would be near normal at rest, the adrenal capacity to respond with additional cortisol synthesis and release on ACTH challenge is usually limited. This leads us to the question: What happens to the levels of ACTH and circulating upstream steroids in individuals with each class of impaired CYP17A1 function?
Certainly if untreated, in the simplest case of severe 17-hydroxylase deficiency, ACTH is increased through lack of adrenal ZF cortisol negative feedback, and upstream steroids are elevated, especially including those that are not 17-hydroxylated. The result is that progesterone and corticosterone are particularly increased in the circulation (Figures 3 and 4)59. Of note, in addition to both cortisol and C19 steroids in general being equally depressed in these individuals (see for instance 60; 61), 17-hydroxyprogesterone is diminished in contrast to clear elevation of progesterone in these subjects (Figures 3 and 4).
In subjects with ‘true’ 17,20 lyase deficiency (wherein hydroxylation is largely still possible), near minimal levels of DHEA and associated C19 steroids are also seen in the circulation, but without a substantial loss of cortisol production (Figure 5), and further ACTH challenge may to some extent stimulate cortisol levels marginally higher 31; 62. While full steroid profiles are not yet published for subjects with “true” 17,20 lyase deficiency, reports to date suggest that they do show some increases in progesterone, but they also show more obvious increases in 17-hydroxyprogesterone, particularly when considered as a ratio to C19 steroid products 63. By comparison, in the subjects with “isolated” 17,20 lyase deficiency due to CytB5 mutations, there are no such pronounced increases in circulating levels of earlier steroid intermediates in the cortisol biosynthetic pathway, including 17-hydroxyprogesterone. Continued 17-hydroxylase activity is also indicated by normal low levels of corticosterone relative to cortisol, while the relative circulating concentrations of 17-hydroxyprogesterone are elevated when compared to the very low levels of androgens and estrogens 57; 63.
Thus far, the natural endocrine physiology of the adrenal and spontaneous mutation experiments of nature have given us much information about how selective/differential alteration of 17-hydroxylase and 17,20 lyase activity is possible, and what the consequences may be in the altered steroid profile in vivo of ‘true’ or ‘isolated’ forms of 17,20 lyase deficiency. The lessons of these in vivo observations are that 17-hydroxylase inhibition can suppress C19 steroid output of the adrenal ZF and ZR, but at a price associated with cortisol deficiency and ACTH excess (with Addison’s-like signs and symptoms) and so corresponding deleterious elevation of pregnenolone, progesterone, deoxycorticosterone and corticosterone in particular (Figures 3 and 4). In contrast, selective forms of 17,20 lyase inhibition do not drive such a strong increase in ACTH and not only avoids depression of cortisol, but also elevation of early steroid pathway intermediates (Figure 5). In testing pharmaceutical agents clinically in humans and nonhuman primates, in addition to loss of cortisol, substantial elevation of progesterone, but not 17-hydroxyprogesterone, provide a good set of indicators for 17-hydroxylase inhibition. In contrast, the more cortisol release is retained, and the less early steroid pathway intermediates are elevated (particularly those not 17-hydroxylated such as pregnenolone, progesterone, deoxycorticosterone and corticosterone), then the more selective the 17,20 lyase inhibition. While the ideal observation in this regard in normal subjects or nonhuman primates treated with a selective 17,20 lyase inhibitor would emulate that of subjects with naturally occurring CytB5 mutation, it is most likely the best that can be achieved with a drug targeted to the CYP17 binding/active site itself is the same as those homozygous for isolated 17,20 lyase deficiency, wherein lyase activity is <10% normal and hydroxylase remains at about 65% or more (see above).
3) Clinical Realities
Given these caveats, the remainder of this review will now focus on comparing what is known of putative adrenal CYP17 inhibitors in general, and of 17-hydroxylase vs 17,20 lyase selective inhibitors in particular in human subjects and nonhuman primates with normal CYP17A1 activity. In particular, we will further consider if the reported alterations in circulating steroids match that of human subjects with congenital 17-hydroxylase deficiency, or ‘isolated’ or ‘true’ 17,20 lyase deficiency, and which alternate steroid products arise in each case. We will then finish with a discussion of the likelihood that the development of a selective 17,20 lyase inhibitor can be achieved for clinical use that would be more beneficial in prostate cancer treatment than the drug currently most in use.
Reference data from use of general P450 inhibitors
Early compounds, such as ketoconazole, were developed as broad spectrum P450 inhibitors. It was therefore no surprise that while its use resulted in inhibition of 17-hydroxylase activity, general suppression of all adrenal steroids also occurred. Studies in H295R cells have clearly shown that ketoconazole not only inhibits CYP17A1, but also CYP11A1, CYP21A2, CYP11B1 and CYP19 64; 65. As such, while ketoconazole treatment has been used as a way of preventing C19 steroid production as an adjunct to anti-cancer treatments for breast and prostate cancer, it is not by any means a selective treatment that achieves our goal of doing so while maintaining sufficient cortisol output. Nor could it be considered safe for long-term use, as it induces Addison’s-like symptoms and hypotension. Likewise, while it may be that ketoconazole-induced generalized inhibition of adrenal P450’s , (including CYP11A1) prevents the production of large amounts of pregnenolone, progesterone, deoxycorticosterone and corticosterone that normally accompany more targeted 17-hydroxylase inhibition, it is also true that the required dose dependent decline in DHEA release parallels the decline in aldosterone output in monkey adrenal cell preparations 65, and so mineralocorticoid disruption will also occur. While the historic observations of ketoconazole’s effects may well be argued to have been pivotal in driving the use of steroidogenic inhibitors for prostate cancer therapy, its lack of specificity means ketoconazole is not an appropriate drug of choice for anti-prostate cancer therapy.
First generation CYP17 inhibitors without 17,20 lyase selectivity
The more recent development of CYP17A1 targeted inhibitors such as abiraterone has certainly led to the best current adjunct to chemotherapy for prostate cancer. The action of abiraterone is far more selective to CYP17A1 than ketoconazole, and clinical trials have demonstrated abiraterone to be successful in reducing C19 steroid products in general 11; 14; 15; 66; 67. Unfortunately, it also has significant off-target effects 15; 66; 68. Notable declines in circulating cortisol and corresponding simultaneous elevation of deoxycorticosterone and corticosterone 14; 66; 67 confirm the site of action is clearly more at the level of 17-hydroxylase 11; 15; 67. Substantial increases in pregnenolone and progesterone are also consistent with 17-hydroxylase inhibition, leading to elevated ACTH through lack of cortisol feedback 11; 23. Certainly in clinical use, the ACTH elevation can be reversed by co-treatment with glucocorticoids such as prednisone 11; 23; 69. Glucocorticoid replacement therapy can also lower the accumulation of pregnenolone and progesterone, and relieves hypertension with concomitant lowering of deoxycorticosterone 11; 67, but it also adds the further complications associated with long-term synthetic glucocorticoid therapy. While abiraterone does indeed represent a substantial advance in available therapy 11; 14; 15; 67, it does not yet present the profile expected of a truly selective 17,20 lyase inhibitor. Recent studies of tumor xenografts in mice have also shown that in the presence of abiraterone, further local CYP17A1 expression can be induced in the tumors and, more significantly, mutant AR expression has been reported 16; 25. As stated above, more recent data also now suggest that prednisone itself may stimulate the mutant AR, so defeating the point of prednisone co-therapy further 24.
One additional drug with abiraterone like properties is orteronel (TAK-700). Extensive studies have been published on this compound and that of Yamaoka et al 70 gives a detailed study of this compound’s effects in both H295R and nonhuman primate adrenocortical cells, as well as in vivo effects in nonhuman primates. While the compound has previously been shown to inhibit 17,20 lyase activity using human P450c17 preparations and 17-hydroxypregenolone as substrate, comparative effects on 17-hydroxylase vs 17,20 lyase activity were not reported. More recently, using a monkey adrenal microsomal preparation, the ED50 for effects of TAK-700 on 17-hydroxylase vs 17,20 lyase were reported to be similar at 38 vs 27 nM, respectively. This difference in ED50 was comparable to that observed for abiraterone run in parallel. In a human P450c17 preparation, the affinity for TAK-700 was less, but at the same time the inhibitory discrimination of lyase vs hydroxylase activity was reportedly improved at 140 vs 760 nM respectively. In monkey adrenal cells, dose dependent suppression of DHEA and androstenedione was slightly better than for cortisol, but cortisol levels were still lowered up to 80% when DHEA and androstenedione output were at basal. While the ability to show some degree of separation for the dose dependent inhibition of C19 steroid products compared to cortisol was better for TAK-700 than was observed for ketoconazole, the decline in cortisol output in response to TAK-700 was still associated with corresponding dose dependent increases in corticosterone and progesterone, clearly indicating 17-hydroxylase inhibition was significant in addition to any observed 17,20 lyase inhibition. This likely explains TAK-700’s clinical use in conjunction with prednisone, and its tendency to produce signs of mineralocorticoid excess 14, while data for changes in pregnenolone remain unreported 70.
In vivo studies, reported by Yamaoka et al 2012 70, also confirmed that when TAK-700 inhibited testosterone down to minimum levels it was associated with substantial loss (90%) of circulating DHEA, but cortisol levels were also reduced by more than 60%. While further circulating levels of corticosterone, progesterone, pregnenolone, and indeed ACTH were not reported, it is also relevant that in the H295 cell model, side by side comparison of the effects of TAK-700 and abiraterone showed similar effects to inhibit DHEA output more effectively than cortisol, suggesting while there is some lyase specificity in comparison to ketoconazole, both TAK-700 and abiraterone dose dependently increased aldosterone ahead of progesterone over the same dose range. This confirms the inhibitory effects of these drugs on 17,20 lyase vs 17-hydroxylase activity is similar and is consistent with the observation in vivo that suppression of testosterone and/or circulating DHEA is accompanied by an approximate 60% loss of cortisol and likely a substantial rise in progesterone and pregnenolone. As such, TAK-700 appears to have about the same lack of 17,20 lyase specificity in vivo as abiraterone, and it is likely clinically that the same undesirable co-dosing with a synthetic glucocorticoid will be needed to prevent the anticipated rise in ACTH 14.
Next generation selective CYP17 lyase inhibitors
There are already enough data emerging to show that the dose dependent inhibition of C19 steroids such as DHEA/androstenedione, and the further metabolites such as testosterone can be achieved with some degree of separation from the decline in cortisol. Detailed data on TAK-700 and abiraterone therapy gives some hope that a more selective 17,20 lyase inhibitor is both possible and could clinically be more successful 71. If we go back to our natural mutation experiments and look at the in vivo effects of selective lyase deficiency due to mutation of CYP17A1 itself, we can see that 17-hydroxylase activity levels can be retained at 65% even with near complete 17,20 lyase inhibition, and cortisol levels in vivo can be largely maintained at rest even if they do not substantially increase further on ACTH challenge. The ideal drug, therefore, is not likely to exceed these values. In vivo, for those with tumors expressing mutant AR, the pivotal goal of any therapy is to starve the tumor of C19 androgen, but without a dramatic rise in ACTH that would otherwise drive increases in upstream early ZF alternate pathway steroids such as pregnenolone and progesterone, or require the use of prednisone to suppress them (because of their combined mutant AR stimulating effects) 16. Certainly data from those with naturally occurring CYP17A1 mutations resulting in ‘true’ 17,20 lyase inhibition suggest this is possible 31; 50. Given the availability of nonhuman primate models, primate adrenocortical cell models, and indeed H295 cell models, the tools are in place to ‘work up’ such compounds to a considerable degree before clinical trials begin. In addition, the existence of ketoconazole as a negative control and abiraterone or TAK-700 as reference compounds for partial 17,20 lyase activity should make such assays reliable. The goal then is to exceed the specificity of abiraterone or TAK-700 as indicated by equal ablation of circulating C19 steroids, but with minimal loss of cortisol or rise in ACTH, and so minimal corresponding elevation of pregnenolone or progesterone or indeed corticosterone or aldosterone. Based on the realization that these are the markers that identify a truly selective lyase inhibitors necessary to more effectively treat castration resistant prostate cancer with the least adverse side effects, at least two promising drugs are in development for clinical use that fulfil those criterion 72; 73.
Galaterone (TOK-001) has been proposed to act as both a CYP17A1 inhibitor and AR antagonist 14; 74. Nonetheless, very little information is publically available regarding its effects on steroid profiles in vitro or in vivo beyond putative ED50 values 75; 76; 77. The compound has been described as a 17,20 lyase inhibitor and the assay used to determine ED50 values was human P450c17 expressed in E. Coli using 17-hydroxypregnenolone as substrate. Complete data on relative hydroxylase vs lyase specificity have only been presented in preliminary form, but studies in steroidogenic cells also suggest Galaterone is indeed capable of suppression of testosterone synthesis without substantial suppression of cortisol 14. While a crystal structure has been reported for P450c17 crystalized with abiraterone and galaterone, there is no apparently obvious difference in the orientation of the substrate in each case 78 and it has also been further proposed that galaterone’s main action may be at the level of the AR itself 74; 76. Clinical trial data do indicate prednisone co-administration is not required, and mineralocorticoid excess is not observed 74, but complete clinical trial data of any effects on circulating ACTH or cortisol in humans have not been reported. It is reasonable to conclude that Galaterone shows 17,20 lyase selectivity exceeding that of abiraterone, but just how much more selective it is remains unclear.
VT464 is another recently developed compound proposed to act as a selective lyase inhibitor, and more complete data is available in the public domain to support this claim. A review of preliminary data released suggest the IC50 for Human CYP17 lyase activity is ten times lower than for hydroxylase 15 and in nonhuman primates VT464 was able to suppress circulating testosterone as effectively as abiraterone, but with minimally depressed cortisol (remaining at 82% control compared to only 9% with aberaterone), and without associated increases in pregnenolone, progesterone and mineralocorticoids otherwise observed with abiraterone 15. Like Galaterone, VT464 is also in use in clinical trials without co-administration of prednisone. Together with the clear lack of suppression of circulating cortisol in nonhuman primates, these data argue that VT464 may indeed be a selective 17,20 lyase inhibitor.
4) Commentary on uses of selective 17,20 lyase inhibitors
If continued monitoring of these same parameters along with tracking of circulating ACTH in human trials confirms these new drugs are the long sought after 17,20 lyase inhibitors, then we may for the first time be able to control both circulating C19 androgen precursors (and so androgens) that initially drive tumor growth, and alternatively derived steroids in drug resistant tumors without diminishing circulating cortisol and thus elevating circulating ACTH (with all its deleterious side effects). The immediate relevance is first and foremost improving the treatment of castration resistant prostate cancer, and hopefully the second would be extending life beyond the ~5 months achieved so far with abiraterone in clinical trials (see above). Given the availability now of drugs showing greater 17,20 lyase selectivity, we should know the answer to this question quite soon.
There are also other diseases of androgen, but not cortisol, excess which may also benefit from treatment with such new compounds. One prevalent example is polycystic ovary syndrome (PCOS), a condition afflicting ~15% of women. Ovarian androgen excess is present in the majority of women with PCOS and comprises one of the key diagnostic criteria, namely hyperandrogenism, intermittent or absent menstrual cycles and polycystic ovaries (at least two out of these three required for diagnosis)79. The excess androgen results in hirsutism, acne and disrupted ovarian, hypothalamic and adipose function80. Oral contraceptives effectively lower androgens and block the effect of androgens via suppression of ovarian androgen production and by increasing sex hormone-binding globulin. To especially block the effects of androgen in skin pilosebaceous units or hair follicles, androgen action is diminished either through the use of competitive androgen receptor antagonists such as spironolactone, cyproterone acetate and flutamide, or by using finasteride to inhibit 5α-reductase and preventing the conversion of testosterone to its more potent form, 5α-dihydrotestosterone. The selection of antiandrogen therapy is guided by presenting symptoms 81.
Another area where selective lyase inhibition may also be of value is in premature adrenarche. The past decade of study suggests that in such subjects premature elevation of adrenal androgens is coincident with premature expression of Cytb5 and reduction of HSD3B2 82 within the ZR. Recent studies also suggest that bioactive androgens may be derived from adrenal androstenedione itself or 11BOH-androstenedione, but both are still dependent on 17,20 lyase activity. It is therefore highly likely that the use of a selective lyase inhibitor would moderate premature symptoms and avoid the need for subtype specific HSD17B inhibitors which are proposed for use but don’t yet exist. One reason this approach may be appealing is while the increased expression of HSD17B5 is observed in ZR development 82; 83, it is also widely expressed in other tissues including kidney, bladder as well as prostate and testis 84. Thus while a specific HSD17B5 inhibitor may be attractive as a treatment, it may also have undesired side effects. It is more likely the best approach is a combined use of CYP17A1 lyase inhibitor alone or in combination with a selective HSD17B isoform inhibitors at more modest doses, so avoiding unwanted side effects.
In closing, it is important to recognize that only by considering decades of biomolecular study of CYP17A1 expression and function in the normal and abnormal adrenal that we can fully understand the true action of CYP17A1 inhibitors. As we now approach the third generation of inhibitors, namely those truly selective for 17,20 lyase activity, we may finally be turning the corner in our battle to treat castration resistant prostate cancer (and potentially other hyperandrogenic responses) with a scalpel-like approach instead of a hammer because we considered both basic science and clinical outcomes together.
Highlights.
Using CYP17 inhibitors to target prostate cancer has had only limited success.
Advances are limited by a failure to properly allow for effects on adrenal steroids.
Naturally occurring CYP17 mutation studies provides much needed reference data.
Based on such analysis, at least two new lyase specific drugs are identified.
Use of lyase selective inhibitors avoids the need for prednisone cotreatment.
Acknowledgements & Disclosure
DHA was funded by a research grant from Viamet and IMB and DHA were/are consultants for Viamet/Innocrin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anik A, Abaci A. Endocrine cancer syndromes: an update. Minerva Pediatr. 2014;66:533–47. [PubMed] [Google Scholar]
- 2.Tian X, He Y, Zhou J. Progress in antiandrogen design targeting hormone binding pocket to circumvent mutation based resistance. Front Pharmacol. 2015;6:57. doi: 10.3389/fphar.2015.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonnell DP, Wardell SE, Norris JD. Oral Selective Estrogen Receptor Downregulators (SERDs), a Breakthrough Endocrine Therapy for Breast Cancer. J Med Chem. 2015;58:4883–7. doi: 10.1021/acs.jmedchem.5b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chumsri S. Clinical utilities of aromatase inhibitors in breast cancer. Int J Womens Health. 2015;7:493–9. doi: 10.2147/IJWH.S69907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Lauro L, Pizzuti L, Barba M, Sergi D, Sperduti I, Mottolese M, Amoreo CA, Belli F, Vici P, Speirs V, Santini D, De Maria R, Maugeri-Sacca M. Role of gonadotropin-releasing hormone analogues in metastatic male breast cancer: results from a pooled analysis. J Hematol Oncol. 2015;8:53. doi: 10.1186/s13045-015-0147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conley AJ, Bird IM. The role of cytochrome P450 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the delta 5 and delta 4 pathways of steroidogenesis in mammals. Biol Reprod. 1997;56:789–99. doi: 10.1095/biolreprod56.4.789. [DOI] [PubMed] [Google Scholar]
- 7.Leibowitz-Amit R, Seah JA, Atenafu EG, Templeton AJ, Vera-Badillo FE, Alimohamed N, Knox JJ, Tannock IF, Sridhar SS, Joshua AM. Abiraterone acetate in metastatic castration-resistant prostate cancer: a retrospective review of the Princess Margaret experience of (I) low dose abiraterone and (II) prior ketoconazole. Eur J Cancer. 2014;50:2399–407. doi: 10.1016/j.ejca.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Gupta E, Guthrie T, Tan W. Changing paradigms in management of metastatic Castration Resistant Prostate Cancer (mCRPC) BMC Urol. 2014;14:55. doi: 10.1186/1471-2490-14-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Bishop AC, Alyamani M, Garcia JA, Dreicer R, Bunch D, Liu J, Upadhyay SK, Auchus RJ, Sharifi N. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature. 2015;523:347–51. doi: 10.1038/nature14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mostaghel EA. Beyond T and DHT - novel steroid derivatives capable of wild type androgen receptor activation. Int J Biol Sci. 2014;10:602–13. doi: 10.7150/ijbs.8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attard G, Reid AH, Auchus RJ, Hughes BA, Cassidy AM, Thompson E, Oommen NB, Folkerd E, Dowsett M, Arlt W, de Bono JS. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab. 2012;97:507–16. doi: 10.1210/jc.2011-2189. [DOI] [PubMed] [Google Scholar]
- 12.Grist E, Attard G. The development of abiraterone acetate for castration-resistant prostate cancer. Urol Oncol. 2015;33:289–94. doi: 10.1016/j.urolonc.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Labrie F. Combined blockade of testicular and locally made androgens in prostate cancer: a highly significant medical progress based upon intracrinology. J Steroid Biochem Mol Biol. 2015;145:144–56. doi: 10.1016/j.jsbmb.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Stein MN, Patel N, Bershadskiy A, Sokoloff A, Singer EA. Androgen synthesis inhibitors in the treatment of castration-resistant prostate cancer. Asian J Androl. 2014;16:387–400. doi: 10.4103/1008-682X.129133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin L, Hu Q. CYP17 inhibitors--abiraterone, C17,20-lyase inhibitors and multi-targeting agents. Nat Rev Urol. 2014;11:32–42. doi: 10.1038/nrurol.2013.274. [DOI] [PubMed] [Google Scholar]
- 16.Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, Marck B, Matsumoto AM, Simon NI, Wang H, Chen S, Balk SP. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–13. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing Y, Lerario AM, Rainey W, Hammer GD. Development of adrenal cortex zonation. Endocrinol Metab Clin North Am. 2015;44:243–74. doi: 10.1016/j.ecl.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273:3158–65. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- 19.Conley AJ, Pattison JC, Bird IM. Variations in adrenal androgen production among (nonhuman) primates. Semin Reprod Med. 2004;22:311–26. doi: 10.1055/s-2004-861548. [DOI] [PubMed] [Google Scholar]
- 20.Lee-Robichaud P, Akhtar ME, Akhtar M. Control of androgen biosynthesis in the human through the interaction of Arg347 and Arg358 of CYP17 with cytochrome b5. Biochem J. 1998;332:293–6. doi: 10.1042/bj3320293. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pattison JC, Abbott DH, Saltzman W, Conley AJ, Bird IM. Plasticity of the zona reticularis in the adult marmoset adrenal cortex: voyages of discovery in the New World. J Endocrinol. 2009;203:313–26. doi: 10.1677/JOE-08-0554. [DOI] [PubMed] [Google Scholar]
- 22.Veldhuis JD, Sharma A, Roelfsema F. Age-dependent and gender-dependent regulation of hypothalamic-adrenocorticotropic-adrenal axis. Endocrinol Metab Clin North Am. 2013;42:201–25. doi: 10.1016/j.ecl.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pia A, Vignani F, Attard G, Tucci M, Bironzo P, Scagliotti G, Arlt W, Terzolo M, Berruti A. Strategies for managing ACTH dependent mineralocorticoid excess induced by abiraterone. Cancer Treat Rev. 2013;39:966–73. doi: 10.1016/j.ctrv.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Richards J, Lim AC, Hay CW, Taylor AE, Wingate A, Nowakowska K, Pezaro C, Carreira S, Goodall J, Arlt W, McEwan IJ, de Bono JS, Attard G. Interactions of abiraterone, eplerenone, and prednisolone with wild-type and mutant androgen receptor: a rationale for increasing abiraterone exposure or combining with MDV3100. Cancer Res. 2012;72:2176–82. doi: 10.1158/0008-5472.CAN-11-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, Nelson PS, Montgomery RB. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants. Clin Cancer Res. 2011;17:5913–25. doi: 10.1158/1078-0432.CCR-11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang KH, Li R, Kuri B, Lotan Y, Roehrborn CG, Liu J, Vessella R, Nelson PS, Kapur P, Guo X, Mirzaei H, Auchus RJ, Sharifi N. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell. 2013;154:1074–84. doi: 10.1016/j.cell.2013.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofland J, van Weerden WM, Dits NF, Steenbergen J, van Leenders GJ, Jenster G, Schroder FH, de Jong FH. Evidence of limited contributions for intratumoral steroidogenesis in prostate cancer. Cancer Res. 2010;70:1256–64. doi: 10.1158/0008-5472.CAN-09-2092. [DOI] [PubMed] [Google Scholar]
- 28.Wu G, Huang S, Nastiuk KL, Li J, Gu J, Wu M, Zhang Q, Lin H, Wu D. Variant allele of HSD3B1 increases progression to castration-resistant prostate cancer. Prostate. 2015;75:777–82. doi: 10.1002/pros.22967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arai S, Shibata Y, Nakamura Y, Kashiwagi B, Uei T, Tomaru Y, Miyashiro Y, Honma S, Hashimoto K, Sekine Y, Ito K, Sasano H, Suzuki K. Development of prostate cancer in a patient with primary hypogonadism: intratumoural steroidogenesis in prostate cancer tissues. Andrology. 2013;1:169–74. doi: 10.1111/j.2047-2927.2012.00026.x. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen PT, Lee RS, Conley AJ, Sneyd J, Soboleva TK. Variation in 3beta-hydroxysteroid dehydrogenase activity and in pregnenolone supply rate can paradoxically alter androstenedione synthesis. J Steroid Biochem Mol Biol. 2012;128:12–20. doi: 10.1016/j.jsbmb.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Miller WL. The syndrome of 17,20 lyase deficiency. J Clin Endocrinol Metab. 2012;97:59–67. doi: 10.1210/jc.2011-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshimoto FK, Auchus RJ. The diverse chemistry of cytochrome P450 17A1 (P450c17, CYP17A1) J Steroid Biochem Mol Biol. 2015;151:52–65. doi: 10.1016/j.jsbmb.2014.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller WL. Steroidogenic enzymes. Endocr Dev. 2008;13:1–18. doi: 10.1159/000134751. [DOI] [PubMed] [Google Scholar]
- 34.Amin M, Simerman A, Cho M, Singh P, Briton-Jones C, Hill D, Grogan T, Elashoff D, Clarke NJ, Chazenbalk GD, Dumesic DA. 21-Hydroxylase-derived steroids in follicles of nonobese women undergoing ovarian stimulation for in vitro fertilization (IVF) positively correlate with lipid content of luteinized granulosa cells (LGCs) as a source of cholesterol for steroid synthesis. J Clin Endocrinol Metab. 2014;99:1299–306. doi: 10.1210/jc.2013-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fanelli F, Gambineri A, Belluomo I, Repaci A, Di Lallo VD, Di Dalmazi G, Mezzullo M, Prontera O, Cuomo G, Zanotti L, Paccapelo A, Morselli-Labate AM, Pagotto U, Pasquali R. Androgen profiling by liquid chromatography-tandem mass spectrometry (LC-MS/MS) in healthy normal-weight ovulatory and anovulatory late adolescent and young women. J Clin Endocrinol Metab. 2013;98:3058–67. doi: 10.1210/jc.2013-1381. [DOI] [PubMed] [Google Scholar]
- 36.Sanders SL, Stouffer RL. Localization of steroidogenic enzymes in macaque luteal tissue during the menstrual cycle and simulated early pregnancy: immunohistochemical evidence supporting the two-cell model for estrogen production in the primate corpus luteum. Biol Reprod. 1997;56:1077–87. doi: 10.1095/biolreprod56.5.1077. [DOI] [PubMed] [Google Scholar]
- 37.Malikova J, Fluck CE. Novel insight into etiology, diagnosis and management of primary adrenal insufficiency. Horm Res Paediatr. 2014;82:145–57. doi: 10.1159/000363107. [DOI] [PubMed] [Google Scholar]
- 38.New M. 21-Hydroxylase Deficiency. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, McLachlan R, New M, Rebar R, Singer F, Vinik A, Weickert MO, editors. Classical & Nonclassical Congenital Adrenal Hyperplasia. MDText.com, Inc.; South Dartmouth (MA): 2006. 2000. Endotext [Internet] [Google Scholar]
- 39.Clayton PE, Miller WL, Oberfield SE, Ritzen EM, Sippell WG, Speiser PW, Group ELCW. Consensus statement on 21-hydroxylase deficiency from the European Society for Paediatric Endocrinology and the Lawson Wilkins Pediatric Endocrine Society. Horm Res. 2002;58:188–95. doi: 10.1159/000065490. [DOI] [PubMed] [Google Scholar]
- 40.Honour JW. 17-Hydroxyprogesterone in children, adolescents and adults. Ann Clin Biochem. 2014;51:424–40. doi: 10.1177/0004563214529748. [DOI] [PubMed] [Google Scholar]
- 41.Sahmay S, Tuten A, Gurleyen H, Oncul M, Benian A, Tamer Erel C. Diagnosis of late-onset congenital adrenal hyperplasia in clinical practice: current evaluation. Minerva Endocrinol. 2014;39:215–22. [PubMed] [Google Scholar]
- 42.Witchel SF, Miller WL. Prenatal treatment of congenital adrenal hyperplasia-not standard of care. J Genet Couns. 2012;21:615–24. doi: 10.1007/s10897-012-9508-8. [DOI] [PubMed] [Google Scholar]
- 43.Peng HM, Liu J, Forsberg SE, Tran HT, Anderson SM, Auchus RJ. Catalytically relevant electrostatic interactions of cytochrome P450c17 (CYP17A1) and cytochrome b5. J Biol Chem. 2014;289:33838–49. doi: 10.1074/jbc.M114.608919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshimoto FK, Zhou Y, Peng HM, Stidd D, Yoshimoto JA, Sharma KK, Matthew S, Auchus RJ. Minor activities and transition state properties of the human steroid hydroxylases cytochromes P450c17 and P450c21, from reactions observed with deuterium-labeled substrates. Biochemistry. 2012;51:7064–77. doi: 10.1021/bi300895w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agarwal AK, Monder C, Eckstein B, White PC. Cloning and expression of rat cDNA encoding corticosteroid 11 beta-dehydrogenase. J Biol Chem. 1989;264:18939–43. [PubMed] [Google Scholar]
- 46.Hofland J, Delhanty PJ, Steenbergen J, Hofland LJ, van Koetsveld PM, van Nederveen FH, de Herder WW, Feelders RA, de Jong FH. Melanocortin 2 receptor-associated protein (MRAP) and MRAP2 in human adrenocortical tissues: regulation of expression and association with ACTH responsiveness. J Clin Endocrinol Metab. 2012;97:E747–54. doi: 10.1210/jc.2011-2328. [DOI] [PubMed] [Google Scholar]
- 47.Spiga F, Lightman SL. Dynamics of adrenal glucocorticoid steroidogenesis in health and disease. Mol Cell Endocrinol. 2015;408:227–34. doi: 10.1016/j.mce.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Webb TR, Clark AJ. Minireview: the melanocortin 2 receptor accessory proteins. Mol Endocrinol. 2010;24:475–84. doi: 10.1210/me.2009-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lisansky J, Peake GT, Strassman RJ, Qualls C, Meikle AW, Risch SC, Fava GA, Zownir-Brazis M, Hochla P, Britton D. Augmented pituitary corticotropin response to a threshold dosage of human corticotropin-releasing hormone in depressives pretreated with metyrapone. Arch Gen Psychiatry. 1989;46:641–9. doi: 10.1001/archpsyc.1989.01810070067011. [DOI] [PubMed] [Google Scholar]
- 50.Miller WL, Geller DH, Auchus RJ. The molecular basis of isolated 17,20 lyase deficiency. Endocr Res. 1998;24:817–25. doi: 10.3109/07435809809032692. [DOI] [PubMed] [Google Scholar]
- 51.Schulze JJ, Lorentzon M, Ohlsson C, Lundmark J, Roh HK, Rane A, Ekstrom L. Genetic aspects of epitestosterone formation and androgen disposition: influence of polymorphisms in CYP17 and UGT2B enzymes. Pharmacogenet Genomics. 2008;18:477–85. doi: 10.1097/FPC.0b013e3282fad38a. [DOI] [PubMed] [Google Scholar]
- 52.Auchus RJ. The genetics, pathophysiology, and management of human deficiencies of P450c17. Endocrinol Metab Clin North Am. 2001;30:101–19. doi: 10.1016/s0889-8529(08)70021-5. vii. [DOI] [PubMed] [Google Scholar]
- 53.Auchus RJ. The classic and nonclassic concenital adrenal hyperplasias. Endocr Pract. 2015;21:383–9. doi: 10.4158/EP14474.RA. [DOI] [PubMed] [Google Scholar]
- 54.Sharp L, Cardy AH, Cotton SC, Little J. CYP17 gene polymorphisms: prevalence and associations with hormone levels and related factors. a HuGE review. Am J Epidemiol. 2004;160:729–40. doi: 10.1093/aje/kwh287. [DOI] [PubMed] [Google Scholar]
- 55.Fluck CE, Pandey AV. Clinical and biochemical consequences of p450 oxidoreductase deficiency. Endocr Dev. 2011;20:63–79. doi: 10.1159/000321221. [DOI] [PubMed] [Google Scholar]
- 56.Fluck CE, Pandey AV, Huang N, Agrawal V, Miller WL. P450 oxidoreductase deficiency - a new form of congenital adrenal hyperplasia. Endocr Dev. 2008;13:67–81. doi: 10.1159/000134826. [DOI] [PubMed] [Google Scholar]
- 57.Idkowiak J, Randell T, Dhir V, Patel P, Shackleton CH, Taylor NF, Krone N, Arlt W. A missense mutation in the human cytochrome b5 gene causes 46,XY disorder of sex development due to true isolated 17,20 lyase deficiency. J Clin Endocrinol Metab. 2012;97:E465–75. doi: 10.1210/jc.2011-2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marsh CA, Auchus RJ. Fertility in patients with genetic deficiencies of cytochrome P450c17 (CYP17A1): combined 17-hydroxylase/17,20-lyase deficiency and isolated 17,20-lyase deficiency. Fertil Steril. 2014;101:317–22. doi: 10.1016/j.fertnstert.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 59.Qiao J, Chen X, Zuo CL, Gu YY, Liu BL, Liang J, Lu YL, Tang JF, Wu YX, Chen MD, Chen JL, Wu WL, Song HD. Identification of steroid biosynthetic defects in genotype-proven heterozygous individuals for 17alpha-hydroxylase/17,20-lyase deficiency. Clin Endocrinol (Oxf) 2010;72:312–9. doi: 10.1111/j.1365-2265.2009.03607.x. [DOI] [PubMed] [Google Scholar]
- 60.Belgini DR, Mello MP, Baptista MT, Oliveira DM, Denardi FC, Garmes HM, Grassiotto Oda R, Benetti Pinto CL, Marques-de-Faria AP, Maciel-Guerra AT, Guerra-Junior G. Six new cases confirm the clinical molecular profile of complete combined 17alpha-hydroxylase/ 17,20-lyase deficiency in Brazil. Arq Bras Endocrinol Metabol. 2010;54:711–6. doi: 10.1590/s0004-27302010000800008. [DOI] [PubMed] [Google Scholar]
- 61.Neres MS, Auchus RJ, Shackleton CH, Kater CE. Distinctive profile of the 17-hydroxylase and 17,20-lyase activities revealed by urinary steroid metabolomes of patients with CYP17 deficiency. Arq Bras Endocrinol Metabol. 2010;54:826–32. doi: 10.1590/s0004-27302010000900009. [DOI] [PubMed] [Google Scholar]
- 62.Van Den Akker EL, Koper JW, Boehmer AL, Themmen AP, Verhoef-Post M, Timmerman MA, Otten BJ, Drop SL, De Jong FH. Differential inhibition of 17alpha-hydroxylase and 17,20-lyase activities by three novel missense CYP17 mutations identified in patients with P450c17 deficiency. J Clin Endocrinol Metab. 2002;87:5714–21. doi: 10.1210/jc.2001-011880. [DOI] [PubMed] [Google Scholar]
- 63.Hicks RA, Yee JK, Mao CS, Graham S, Kharrazi M, Lorey F, Lee WP. Precursor-to-product ratios reflect biochemical phenotype in congenital adrenal hyperplasia. Metabolomics. 2014;10:123–131. doi: 10.1007/s11306-013-0558-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gal M, Orly J. Selective inhibition of steroidogenic enzymes by ketoconazole in rat ovary cells. Clin Med Insights Reprod Health. 2014;8:15–22. doi: 10.4137/CMRH.S14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van der Pas R, Hofland LJ, Hofland J, Taylor AE, Arlt W, Steenbergen J, van Koetsveld PM, de Herder WW, de Jong FH, Feelders RA. Fluconazole inhibits human adrenocortical steroidogenesis in vitro. J Endocrinol. 2012;215:403–12. doi: 10.1530/JOE-12-0310. [DOI] [PubMed] [Google Scholar]
- 66.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–71. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 67.Thoma C. Pharmacology: How abiraterone works. Nat Rev Urol. 2015;12:363–363. doi: 10.1038/nrurol.2015.131. [DOI] [PubMed] [Google Scholar]
- 68.Conteduca V, Caffo O, Derosa L, Veccia A, Petracci E, Chiuri VE, Santoni M, Santini D, Fratino L, Maines F, Testoni S, De Giorgi U. Metabolic syndrome in castration-resistant prostate cancer patients treated with abiraterone. Prostate. 2015;75:1329–38. doi: 10.1002/pros.23014. [DOI] [PubMed] [Google Scholar]
- 69.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Jr., Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Flechon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI, Investigators, C.-A.-. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamaoka M, Hara T, Hitaka T, Kaku T, Takeuchi T, Takahashi J, Asahi S, Miki H, Tasaka A, Kusaka M. Orteronel (TAK-700), a novel non-steroidal 17,20-lyase inhibitor: effects on steroid synthesis in human and monkey adrenal cells and serum steroid levels in cynomolgus monkeys. J Steroid Biochem Mol Biol. 2012;129:115–28. doi: 10.1016/j.jsbmb.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 71.Rafferty SW, Eisner JR, Moore WR, Schotzinger RJ, Hoekstra WJ. Highly-selective 4-(1,2,3-triazole)-based P450c17a 17,20-lyase inhibitors. Bioorg Med Chem Lett. 2014;24:2444–7. doi: 10.1016/j.bmcl.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 72.Bambury RM, Rathkopf DE. Novel and next-generation androgen receptor-directed therapies for prostate cancer: Beyond abiraterone and enzalutamide. Urol Oncol. 2015 doi: 10.1016/j.urolonc.2015.05.025. [DOI] [PubMed] [Google Scholar]
- 73.Yin L, Hu Q, Hartmann RW. Recent progress in pharmaceutical therapies for castration-resistant prostate cancer. Int J Mol Sci. 2013;14:13958–78. doi: 10.3390/ijms140713958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Njar VC, Brodie AM. Discovery and development of Galeterone (TOK-001 or VN/124-1) for the treatment of all stages of prostate cancer. J Med Chem. 2015;58:2077–87. doi: 10.1021/jm501239f. [DOI] [PubMed] [Google Scholar]
- 75.Gomez L, Kovac JR, Lamb DJ. CYP17A1 inhibitors in castration-resistant prostate cancer. Steroids. 2015;95:80–7. doi: 10.1016/j.steroids.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bruno RD, Vasaitis TS, Gediya LK, Purushottamachar P, Godbole AM, Ates-Alagoz Z, Brodie AM, Njar VC. Synthesis and biological evaluations of putative metabolically stable analogs of VN/124-1 (TOK-001): head to head anti-tumor efficacy evaluation of VN/124-1 (TOK-001) and abiraterone in LAPC-4 human prostate cancer xenograft model. Steroids. 2011;76:1268–79. doi: 10.1016/j.steroids.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Handratta VD, Vasaitis TS, Njar VC, Gediya LK, Kataria R, Chopra P, Newman D, Jr., Farquhar R, Guo Z, Qiu Y, Brodie AM. Novel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft model. J Med Chem. 2005;48:2972–84. doi: 10.1021/jm040202w. [DOI] [PubMed] [Google Scholar]
- 78.DeVore NM, Scott EE. Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001. Nature. 2012;482:116–9. doi: 10.1038/nature10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fauser BC, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, Lobo R, Carmina E, Chang J, Yildiz BO, Laven JS, Boivin J, Petraglia F, Wijeyeratne CN, Norman RJ, Dunaif A, Franks S, Wild RA, Dumesic D, Barnhart K. Consensus on women's health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97:28–38. doi: 10.1016/j.fertnstert.2011.09.024. e25. [DOI] [PubMed] [Google Scholar]
- 80.Dumesic DA, Oberfield SE, Stener-Victorin E, Marshall JC, Laven JS, Legro RS. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr Rev. 2015;36:487–525. doi: 10.1210/er.2015-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and Pcos Society Disease State Clinical Review: Guide to the Best Practices in the Evaluation and Treatment of Polycystic Ovary Syndrome - Part 1. Endocr Pract. 2015;21:1291–300. doi: 10.4158/EP15748.DSC. [DOI] [PubMed] [Google Scholar]
- 82.Rege J, Rainey WE. The steroid metabolome of adrenarche. J Endocrinol. 2012;214:133–43. doi: 10.1530/JOE-12-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hui XG, Akahira J, Suzuki T, Nio M, Nakamura Y, Suzuki H, Rainey WE, Sasano H. Development of the human adrenal zona reticularis: morphometric and immunohistochemical studies from birth to adolescence. J Endocrinol. 2009;203:241–52. doi: 10.1677/JOE-09-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Azzarello J, Fung KM, Lin HK. Tissue distribution of human AKR1C3 and rat homolog in the adult genitourinary system. J Histochem Cytochem. 2008;56:853–61. doi: 10.1369/jhc.2008.951384. [DOI] [PMC free article] [PubMed] [Google Scholar]