Abstract
Therapeutic strategies targeting the programmed cell death-ligand 1/programmed cell death-1 pathway have shown significant responses and good tolerability in solid malignancies. Although preclinical studies suggest that inhibiting programmed cell death-ligand 1/programmed cell death-1 interactions might also be highly effective in hematological malignancies, remarkably few clinical trials have been published. Determining patients who will benefit most from programmed cell death-ligand 1/programmed cell death-1-directed immunotherapy and whether programmed cell death-ligand 1/programmed cell death-1 are adequate prognostic markers becomes an increasingly important clinical question, especially as aberrant programmed cell death-ligand 1/programmed cell death-1 expression are key mediators of impaired anti-tumor immune responses in a range of B-cell lymphomas. Herein, we systematically review the published literature on the expression and prognostic value of programmed cell death-ligand 1/programmed cell death-1 in these patients and identify considerable differences in expression patterns, distribution and numbers of programmed cell death-ligand 1+/programmed cell death-1+cells, both between and within lymphoma subtypes, which is reflected in conflicting findings regarding the prognostic value of programmed cell death-ligand 1+/programmed cell death-1+ cells. This can be partly explained by differences in methodologies (techniques, protocols, cutoff values) and definitions of positivity. Moreover, lymphomagenesis, disease progression, and prognosis appear to be determined not only by the presence, numbers and distribution of specific subtypes of T cells, but also by other cells and additional immune checkpoints. Collectively, our findings indicate that programmed cell death-ligand 1/programmed cell death-1 interactions play an essential role in B-cell lymphoma biology and are of clinical importance, but that the overall outcome is determined by additional components. To categorize the exact prognostic value of programmed cell death-ligand 1/programmed cell death-1 expressing cells and cell types, efforts should be made to harmonize their assessment and interpretation, optimally within ongoing clinical immune checkpoint inhibitor trials, and to identify and validate novel high-throughput platforms.
Introduction
The immune checkpoint programmed cell death protein 1 (PD-1, CD279) and its ligand PD-L1 (B7-H1, CD274) have rapidly taken center stage in tumor immunology. This is because antibodies targeting this pathway have shown significant responses and good tolerability across a variety of solid malignancies, both in initial phase 1/2 studies and in recently published randomized trials or in combination with other substances.1–12 Although a plethora of preclinical studies suggest that inhibiting PD-L1/PD-1 interactions might also be highly effective in hematological malignancies,13,14 only few PD-L1/PD-1 antibody based clinical trials have been published to date. An initial phase I trial demonstrated a clinical benefit of the PD-1 antibody pidilizumab in several advanced hematological malignancies.15 Encouraging results were also observed in recently published phase II trials in relapsed follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL),16,17 as well as in relapsed/refractory Hodgkin lymphoma (HL) patients treated with nivolumab.18
Determining which patients benefit most from PD-L1/PD-1-directed immunotherapy is an important clinical question. Yet again, the solid oncology field appears to be one step ahead. Several retrospective and correlative studies examining the prognostic significance of tumor PD-L1 expression and PD-1 expression on tumor-infiltrating lymphocytes (TILs) have already been published, although the exact associations are somewhat controversial and appear to be dependent on tumor entity, treatment setting and the presence of other predictive factors or biomarkers.19–23
Similar studies have not been reported in hematological malignancies, even though most of these tumor types, and especially lymphomas, are increasingly understood to closely interact with their surrounding microenvironment.24 Importantly, we and others have shown that aberrant PD-L1 expression by lymphoma cells and increased expression of PD-1 on T cells are key mediators of impaired anti-tumor immune responses in a range of B-cell lymphomas, including DLBCL, FL and chronic lymphocytic leukemia (CLL),25–27 and that inhibiting their interaction restores immune function in preclinical models.28 However, PD-L1 is also expressed on other cell types and in peripheral tissues and is up-regulated during inflammation and in the tumor microenvironment.29–31 Similarly, PD-1 can be expressed on a variety of physiological immune cells, for example on CD4+ germinal center (GC) follicular helper T cells (TFH), which are required for GC development and high-affinity antibody production.32 As TFH cells also act as negative regulators of immune responses, their numbers and tissue distribution may shape the microenvironment in GC-type lymphomas.33 Indeed, across multiple solid cancer types, it was recently demonstrated that clinical responses were not only observed in patients with high tumor PD-L1 levels, but also when PD-L1 was expressed by tumor-infiltrating immune cells and when T helper type 1 (TH1) gene signatures and CTLA-4 expression were detected in baseline specimens.23
Herein, we aimed to collate and review data from the literature on the prognostic value of PD-L1 or PD-1 expression in patients with the most frequent types of B-cell lymphomas. We hypothesized that increased PD-L1/PD-1 expression confers an adverse prognosis, but that differences exist between lymphoma subtypes and between lymphoma and tumor infiltrating lymphocytes (TIL) expression. Such a systematic comparison has several clinical implications. First, it allows the identification of entity- and cell-type-specific expression patterns and their association with prognosis and survival. Second, it elucidates the clinical importance of this pathway in specific lymphomas, contributing to identifying patient groups that might benefit most from blocking PD-L1/PD-1 interactions. Ultimately, these findings provide direct translational guidance in the implementation and interpretation of assays and techniques assessing PD-L1 or PD-1 as biomarkers in future clinical trials of immune checkpoint inhibitors.
Methods and Materials
Full-text publications were included if they met prospectively defined criteria: i) investigated DLBCL, FL, CLL/small lymphocytic leukemia (SLL), Hodgkin lymphoma (HL) or primary mediastinal large B-cell lymphoma (PMBCL), ii) quantified PD-1/PD-L1 expression on tumor and/or microenvironmental components by immunohistochemistry (IHC) or flow cytometry, iii) described techniques and quantification methods, and iv) were written in English. Abstracts from conference proceedings were not reviewed, and less frequent B-cell lymphomas such as mantle cell, marginal zone and Burkitt lymphoma were not included. Suitable publications were retrieved from two independent MEDLINE database queries and information on study characteristics, methods/materials (examined tissues, techniques, quantification of PD-L1/PD-1 expression, antigens/antibodies, controls, statistical analyses), patients and treatment characteristics and findings on PD-L1/PD-1 expression and prognostic significance were extracted. The majority of retrieved results were excluded because studies examined T-cell or cutaneous lymphomas. An overview of key information on included studies can be found in Table 1. Expression patterns on lymphoma and lymphoma-associated immune and/or surrounding cells are summarized according to lymphoma type in Table 2 (DLBCL), Table 3 (FL), Table 4 (CLL/SLL) and Table 5 (HL). The prognostic value of PD-L1/PD-1 in all examined lymphoma types is depicted in Table 6.
Table 1.
Key information on included studies. Information on aim of study, patient/sample numbers, techniques and examined tissues and PD-L1/PD-1 scoring methods was extracted and is summarized according to B-NHL subtype.
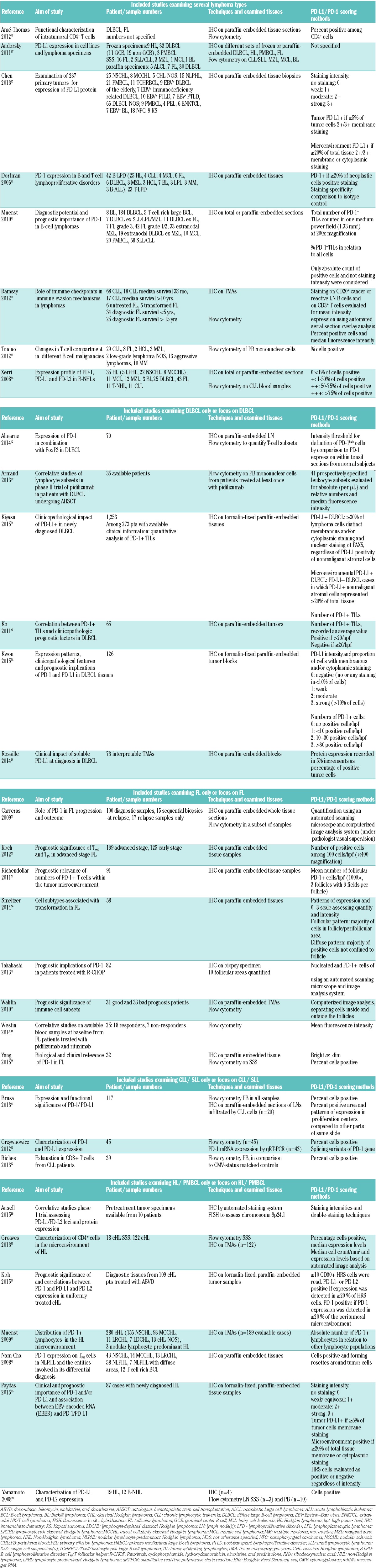
Table 2.
Expression of PD-1 and PD-L1 on tumor infiltrating lymphocytes (TILs) and tumor cells in DLBCL.
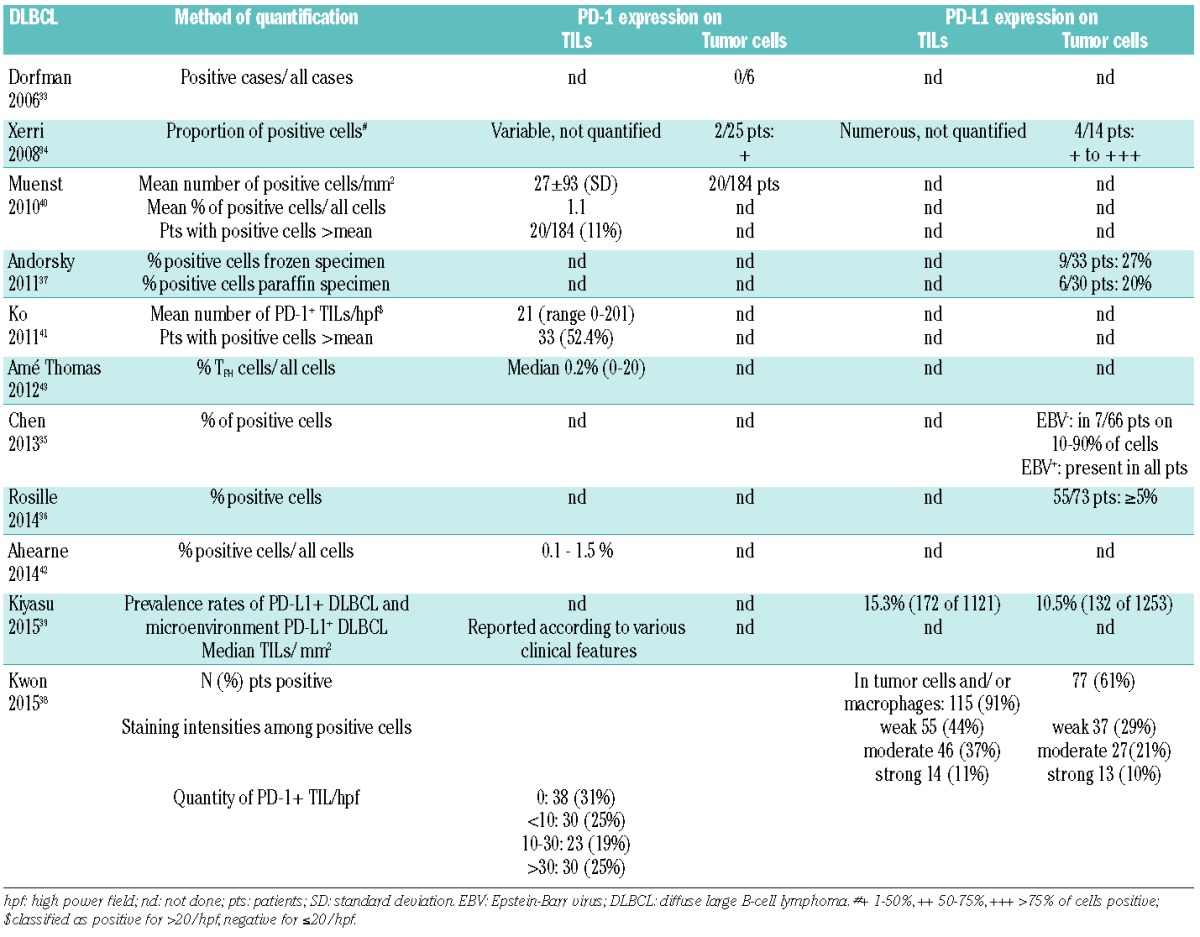
Table 3.
Expression of PD-1 and PD-L1 on tumor infiltrating lymphocytes (TILs) and tumor cells in FL.
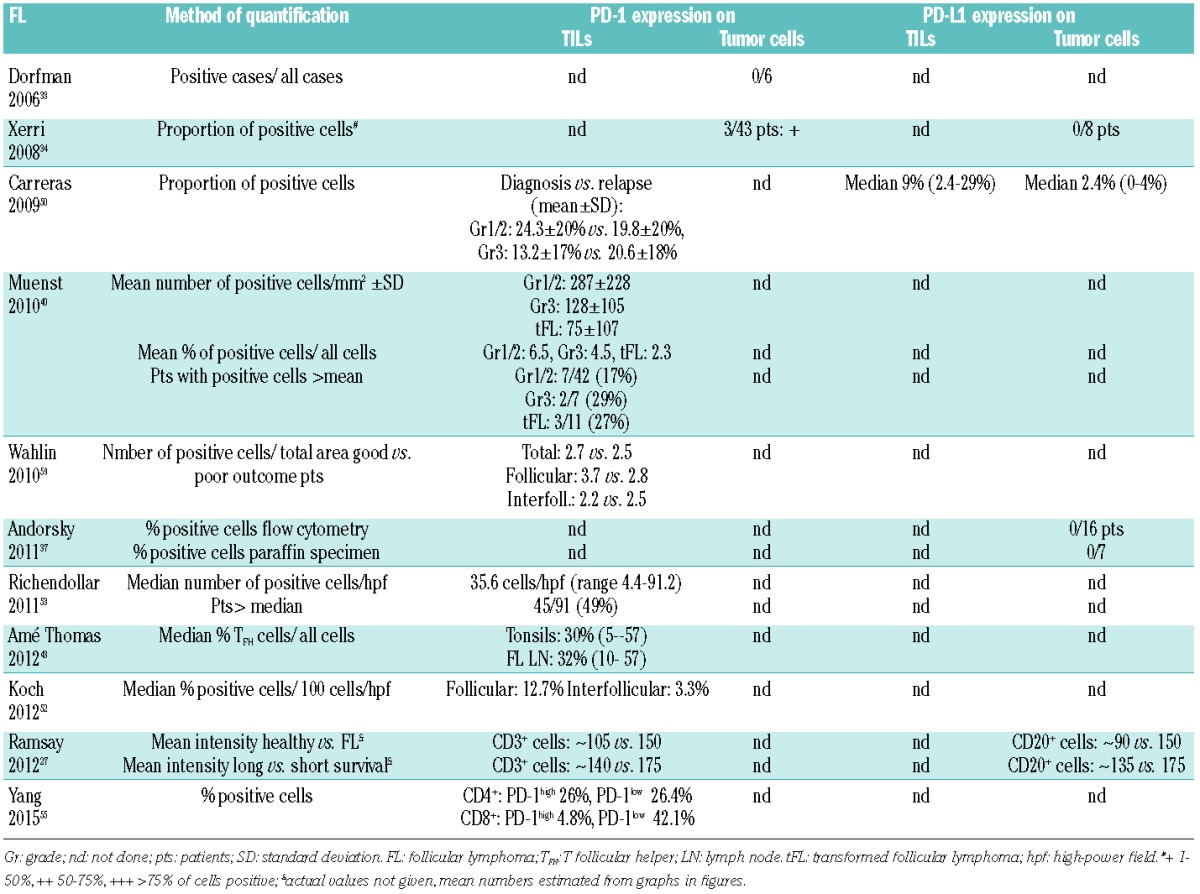
Table 4.
Expression of PD-1 and PD-L1 on tumor infiltrating lymphocytes (TILs) and tumor cells in CLL/SLL.
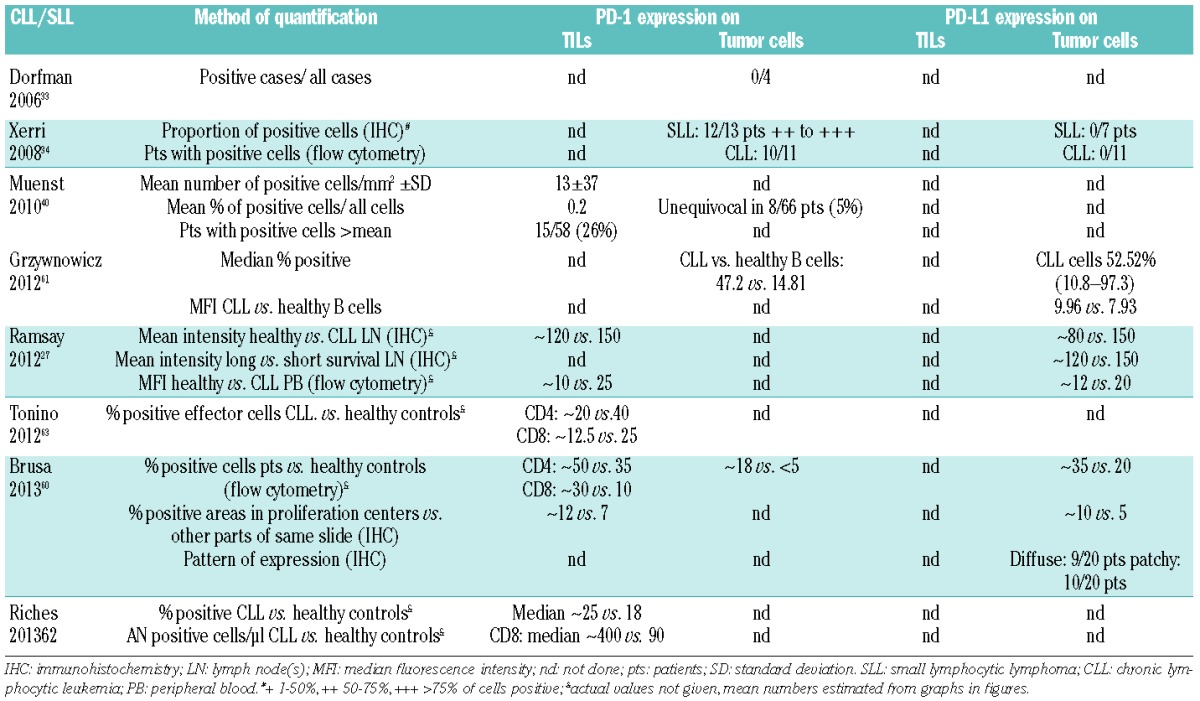
Table 5.
Expression of PD-1 and PD-L1 on tumor infiltrating lymphocytes (TILs) and tumor cells in HL/PMBCL.
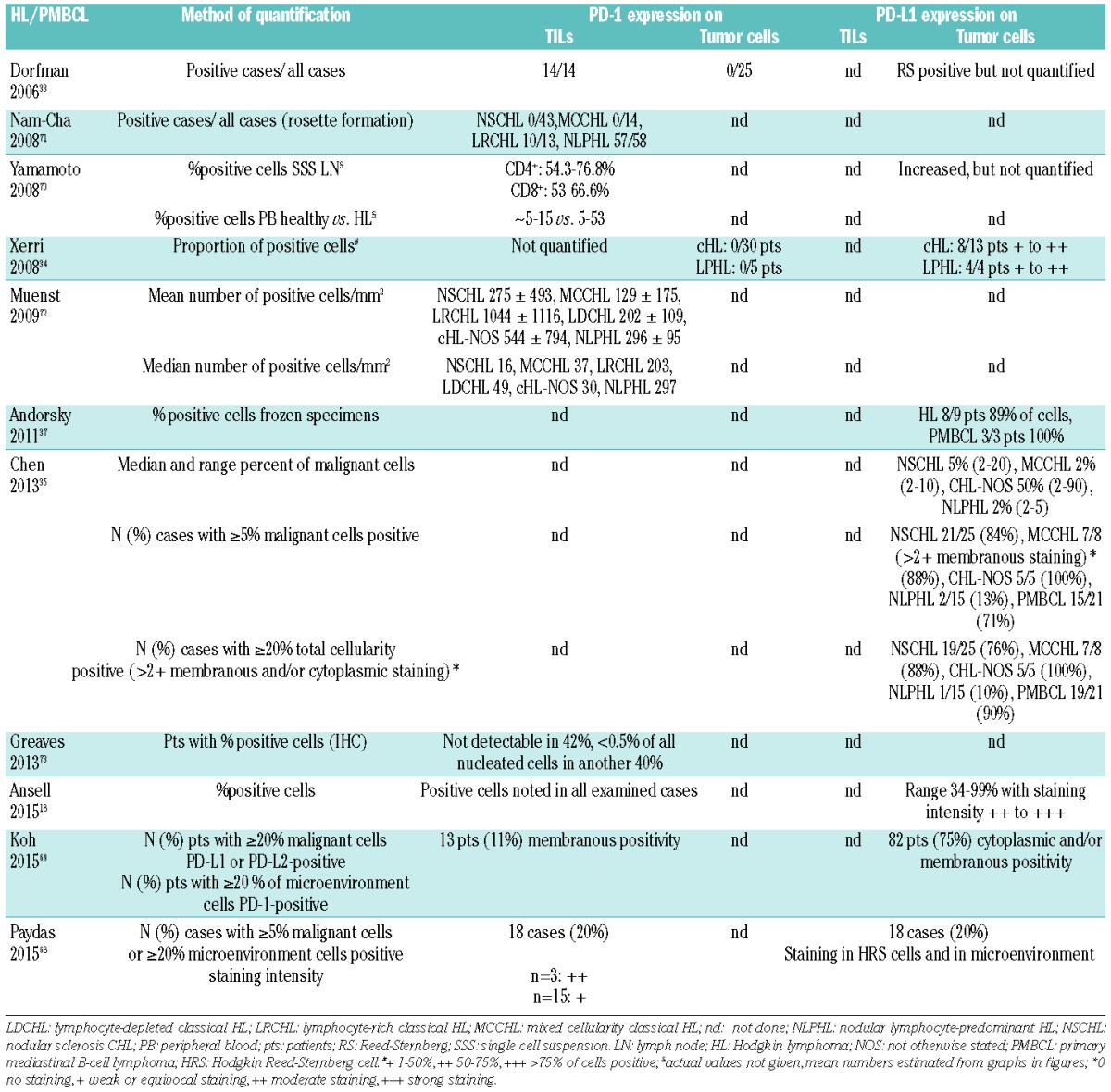
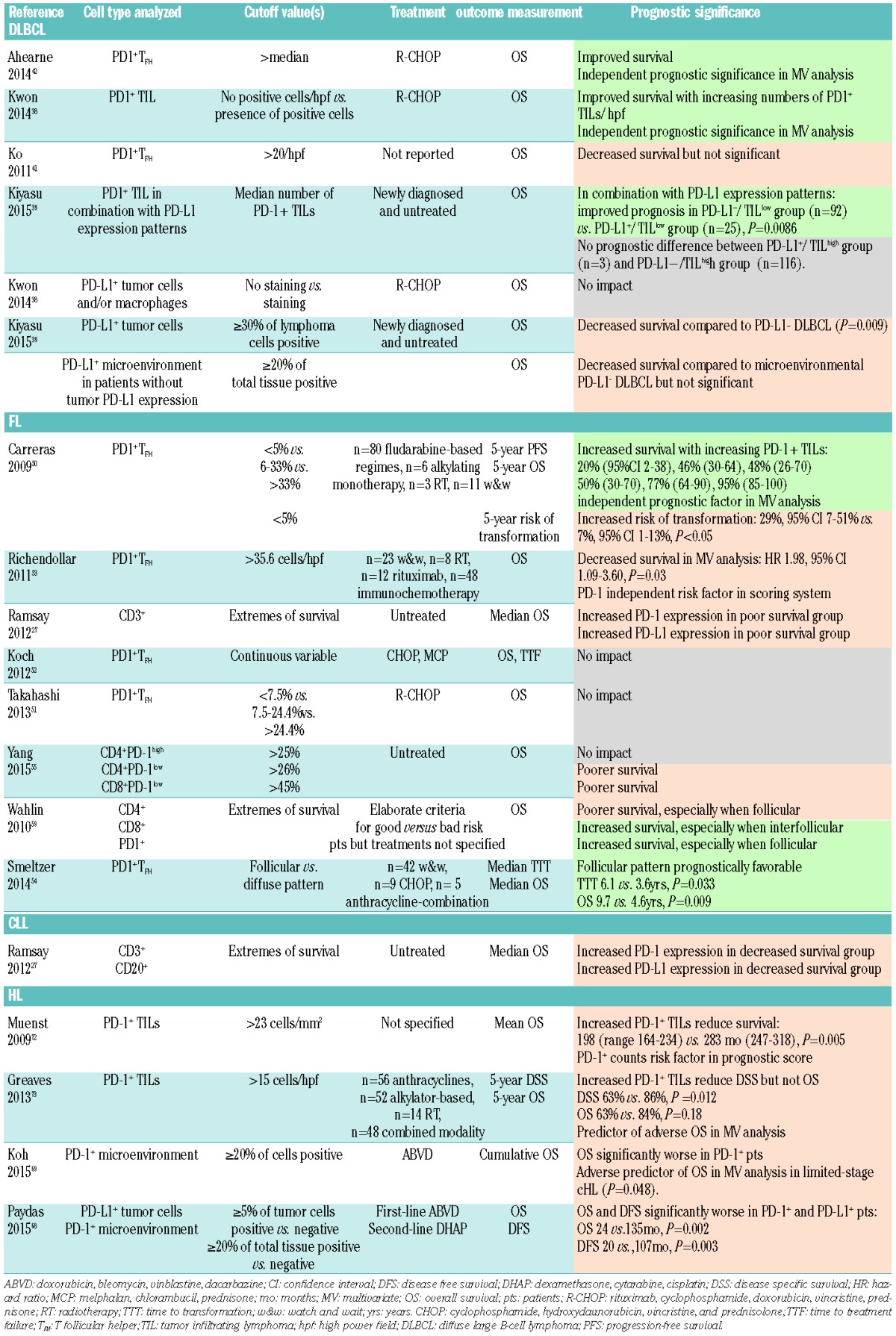
Table 6.
Prognostic significance of PD-L1 and PD-1 in different types of B-NHL and HL. Orange color signifies reduced survival, green color improved survival, gray color no association between PD-L1/PD-1 and survival.
Results
DLBCL
PD-L1/PD-1 expression on DLBCL cells
One of the first studies to characterize PD-L1/PD-1 expression in a series of 161 B-cell non-Hodgkin lymphoma (NHL) tissues contained only 25 DLBCL specimens, of which 4 out of 14 examined samples were PD-L1+ on 1–75% of tumor cells34 (Table 2). In a cohort comprising (Epstein-Barr virus) EBV+ and EBV− patients, the proportion of PD-L1+ malignant cells ranged from 10–90%.35 All EBV+ DLBCLs showed strong PD-L1 expression, in contrast to 11% of EBV− DLBCL patients. Another study found at least 5% of PD-L1+ tumor cells in 55 out of 73 interpretable tissue microarrays (TMAs), which did however not correlate with plasma PD-L1 levels.36 Slight differences were observed in frozen versus paraffin specimens, where heterogeneous PD-L1 tumor expression was observed in 27% of frozen and 20% of paraffin samples.37 A more recent study detected tumor PD-L1 expression in 61% of DLBCL TMAs, with variable intensities and proportions.38 Using a threshold of ≥30% of PD-L1+ malignant cells among all malignant cells, another recent study of a total of 1,253 DLBCL TMAs reported a tumor PD-L1+ prevalence rate of 11%.39 This was significantly associated with non-germinal center B-cell (GCB) type and EBV positivity, and with chromosome 9 gain but not structural abnormalities in chromosome 9p. PD-1 expression was initally not detected on DLBCL cells,33 but heterogenous expression in a small number of patients was subsequently described.34,40
PD-L1/PD-1 expression on DLBCL-associated immune cells
Initial studies described numerous PD-L1/PD-L2+ and variable, non-quantified amounts of PD-1+ reactive lymphocytes34 (Table 2). More recently, most DLBCL-infiltrating immune cells were characterized as PD-L1 expressing macrophages, with 30% of patients showing PD-L1 expression mainly in macrophages with little expression in tumor cells.38 Using a threshold of ≥20% PD-L1+ nonmalignant cells among the total tissue cellularity in PD-L1− patients, the study by Kiyasu et al. reported a microenvironment PD-L1+ prevalence rate of 15%.39 This was significantly associated with non-GCB type and EBV positivity, but not with gain of chromosome 9 nor structural abnormalities in chromosome 9p.
Increased PD-1+ TILs were detected in 11% of 184 DLBCL, but numbers and percentages were lower compared with FL and PMBCL.40 Similarly variable and low numbers of PD-1+ TILs were described in a Korean cohort.41 More than half of the included patients were classified PD-1+, with no differences between GCB subtypes. PD-1+ cases had significantly higher clinical stage (P=0.025) and higher International Prognostic Index (IPI) (P=0.026) than PD-1− patients. Subsequent studies classified PD1+CD4+ TILs in DLBCL as TFH cells, and noted reduced TFH numbers in DLBCL and reactive lymph nodes (LNs) compared to tonsils.42,43 CD4+ T-cell numbers correlated with both PD-1+ and FoxP3+ numbers.42 More recently, PD-1 was detected on TILs in all but two cases, and their quantity correlated positively with the level of PD-L1 expression in tumor cells (P=0.042) or in tumor cells/macrophages (P=0.03).38 In the study by Kiyasu et al., the number of PD-1+ TILs was significantly lower in PD-L1+ patients and in those with B symptoms (P=0.024), extranodal sites (P=0.042) and bulky disease (P=0.041), but higher in GCB-type DLBCL (P=0.034).39
Prognostic relevance of PD-1 expression in DLBCL
Distinct molecular subtypes determine biology and outcome in DLBCL,44,45 and molecular- and IHC-based algorithms have confirmed additional tumor-promoting roles of the microenvironment.46 However, findings regarding the prognostic relevance of TILs and tumor-associated macrophages (TAMs) are conflicting. Whereas infiltration with activated CD4+ cells generally correlates with better prognosis, the role of specific subtypes, such as FoxP3+ cells, has been largely contradictory.47–49 The same appears to be true for PD-1+ TILs in GC lymphomas (Table 6). While actual median values were not reported, the numbers of PD1+TFH (P=0.0007), FoxP3+ (P=0.0069), and total CD4+ cells (P=0.04) above the median were associated with improved overall survival (OS), and had independent prognostic significance in multivariate analyses.42 This was confirmed in more recent studies; although the quantity of PD-1+ TILs showed no significant association with clinicopathological variables, the presence of PD-1+ TILs (score 1–3) significantly prolonged OS (P=0.026) and progression-free survival (PFS) (P=0.005), and was an independent favorable prognostic factor in multivariate analyses.38 In contrast, in another study, patients with PD-1 expression >20/hpf had a trend to poorer OS (P=0.120).41 A similar trend was seen when groups were further refined to 1–10, 11–50, 51–100 and >100 PD-1+ cells/hpf, but numbers were too small to allow valid conclusions.
Prognostic relevance of PD-L1 expression in DLBCL
The prognostic relevance of cellular PD-L1 has only recently been explored (Table 6). Strong tumor and tumor/macrophage PD-L1 expression were significantly associated with B symptoms (P=0.005 tumor only, P=0.011 tumor and/or macrophages) and EBV infection (P=0.015 tumor only, P=0.020 tumor and/or macrophages), and tended to be higher in activated B-cell (ABC) than GCB DLBCL.38 This however did not correlate with survival, which is somewhat inconsistent with another report showing that increased plasma PD-L1 levels were associated with poorer prognosis in DLBCL patients.36 Inferior OS was also reported in patients with PD-L1+ DLBCL (P=0.0009), and the expression of PD-L1 maintained prognostic value for OS in multivariate analysis.39 Combining the median number of TILs with positive or negative PD-L1 expression patterns, the PD-L1+/TILlow group was significantly associated with poor prognosis compared to the PD-L1−/TILlow group, whereas no prognostic impact was observed in the other two groups (PD-L1+/TILhigh and PD-L1−/TILhigh).
FL
PD-L1/PD-1 expression on lymphoma cells
The majority of published studies reported virtually PD-L1 negative FL cells34,37,50 (Table 3). We found significantly increased PD-L1 on FL compared to healthy B cells, and on tumor cells from patients with <5-year (n=34) versus >15-year (n=25) survival.27 PD-1 was heterogeneously expressed on 1–50% of tumor cells in a minority of FL specimens,34 whereas others excluded PD-1 expression on B cells.33
PD-L1/PD-1 expression on FL-associated immune cells
PD-L1 expression was detected in some CD3+ cells in both reactive LN and FL samples50 (Table 3). A number of studies have characterized PD-1+ TFH cells, with similarly high proportions of TFH in tonsils and FL LNs (median 30% and 32%, respectively).43 At diagnosis (n=100), PD-1+ cells were mainly observed in follicular areas, but numbers were highly variable (mean 21.8%, range 0.12–73.6%) and similar to reactive tonsils.50 PD-1+ cells decreased with increasing histological grade (P=0.003), but correlated with the number of TRegs. PD-1+ cell numbers were also significantly lower in patients with poor performance status (P=0.014) and high serum lactate dehydrogenase (LDH, P=0.001). At relapse (n=32), the number of PD-1+ cells was similar to diagnosis for all grades. In transformed FL (n=10), PD-1+ numbers were significantly lower than either at diagnosis or relapse. Decreasing but numerous PD-1+ TILs with increasing grade (n=49) and transformation to DLBCL (n=11) were described by others.40 There might be an association between male gender and increased PD-1+ cells,51 but further confirmation is lacking.
Several studies have focused on localization patterns of TFH cells. While PD-1 expression generally correlated with T-cell content in both interfollicular and follicular zones, it was mainly expressed within52 or restricted to follicles.53 FoxP3+ cells were predominantly found interfollicularly, but a high follicular content of FoxP3+ and PD-1+ cells was associated with high interfollicular content of the same cell type.52 Regardless of region, PD-1 content decreased with stage, and the interfollicular PD-1 content decreased in patients with a high Follicular Lymphoma International Prognostic Index (FLIPI) score. More recent evidence suggests that PD-1+CD4+ cells consist of several sub-populations and include conventional TFH cells and PD-1+TIM-3+ exhausted T cells, which primarily reside in the interfollicular space.54 Functionally exhausted TIM-3+ cells were PD-1low in another study, while the majority of CD4+PD-1high T cells were conventional TFH cells.55 Others identified distinct functional T-cell populations displaying specific gene expression profiles on the basis of CD25, namely CD25+ follicular regulatory T cells and CD25-TFH.43 Changes in PD-1, PD-L1 and PD-L2 expression were analyzed with pidilizumab and rituximab treatment in relapsed FL patients.16 PD-L1 but not PD-1 or PD-L2 was significantly higher in blood T cells and monocytes of responders (n=18) than non-responders (n=7). Additional gene expression signature studies conducted in this trial suggested that T-effector cells had anti-tumor and TFH cells had pro-tumor effects, predicting tumor shrinkage and PFS. As this was not recapitulated in an external dataset of 191 patients largely treated with chemotherapy, the predictive power of the identified gene signature might only be relevant with PD-L1/PD-1 blockade.
Prognostic relevance of PD-1 expression in FL
Gene expression profiling studies demonstrated that the cellular microenvironment plays an essential role in lymphomagenesis and outcome in FL, with enrichment in T-cell and monocyte-restricted genes conferring a favorable prognosis, and with activated macrophages/dendritic genes conferring a poor prognosis.56 However, it appears that both survival and transformation into DLBCL are influenced by the presence and perifollicular versus follicular localization of specific T-cell subtypes, including FOXP3+ TRegs57,58 (Table 6). Several studies have assessed the prognostic relevance of PD-1+ T cells, but findings are contradictory; increased levels of PD-1+ TILs were associated with improved 5-year OS (P=0.004) and PFS in one study (P=0.038), but patients were treated independently of the number of PD-1+ cells, and there was no correlation to the type of therapy and therapeutic response.50 In contrast, increased levels were associated with reduced survival in another study.53 PD-1 was an independent risk factor (RF) in a scoring system predicting 10-year survival rates of 80%, 60%, and 15% in the low (0 RFs, n=14), intermediate (1/2 RFs, n=64) and high-risk group (3/4 RFs, n=13). Using an extremes of survival approach, we detected increased PD-1 expression on follicular T cells in poor outcome versus long-term surviving patients, as well as on CD3+ T cells from patients compared to healthy controls.27 Two studies found no impact on time to treatment failure or OS.51,52 The numbers of CD4+ cells were associated with poor outcome, and CD8+ and PD-1+ cells with improved outcome, independently of FLIPI.59 In another study, increased numbers of CD4+PD-1high TFH cells had no impact on survival (P=0.411), while that of exhausted CD4+PD-1low (P=0.007) and of CD8+PD-1+ (most likely also exhausted cytotoxic T cells) reduced survival (P=0.026).55
A potential prognostic role has also been attributed to patterns of PD-1+ TILs. The prognostic values of CD4+ and PD-1+ cells were accentuated when they were follicular, and that of CD8+ cells when they were interfollicular.59 Patients with PD-1+ in follicular patterns (i.e. TFH, n=38) also had prolonged time to transformation (TTT) and OS compared to patients with diffuse patterns (n=19), and transformation within one year occurred exclusively in patients with diffuse patterns.54 Multivariate analyses demonstrated that PD-1+ cells with diffuse patterns were associated with shorter TTT (HR 1.9, P=0.045) and inferior OS (HR 2.5, P=0.012), but that inferior outcome was also independently influenced by follicular dendritic cells (HR 3.0, P=0.004). In another study, transformation risk was significantly higher in patients (n=25) with less than 5% PD-1+ TILs compared to other patients.50
CLL/SLL
PD-L1/PD-1 expression on tumor cells
In initial IHC studies, neither PD-L1 nor PD-L2 were expressed on LN SLL/CLL cells34 (Table 4). Larger IHC studies later found significantly higher PD-L1 expression on CLL cells compared to control LN samples.27,60 Small vessels in CLL LNs also appear to express PD-L1 weakly, whereas this was confined to endothelial cells lining vessels in reactive LNs.60 Higher PD-L1 expression on CLL cells was also detected in blood in some27,60 but not all studies.61 PD-1 was strongly expressed on ≥50% of tumor cells in the majority of SLL LN specimens and on peripheral blood (PB) neoplastic cells from almost all CLL patients.34 Similar expression patterns were described in flow-cytometry-based studies.60,61 In contrast, the majority of examined SLL/CLL full tissue sections collected from three different institutions (n=58) were PD-1- in another study,40 similar to earlier findings of a lack of PD-1 expression on CLL cells.33
PD-L1/PD-1 expression on CLL/SLL-associated immune cells
PD-1+ TILs are generally exceptionally low in CLL/SLL compared to other lymphomas40 (Table 4). We found significantly increased PD-1 expression on T cells from CLL patients compared to reactive LNs, and on PB CLL T cells compared to age-matched healthy donor T cells (both P<0.01).27 While percentages and numbers of CD4+ and CD8+ T cells are significantly increased in CLL patients,60,62,63 marked differences exist in the composition of both CD4+ and CD8+ T-cell subsets. This includes decreased naïve and relatively increased effector cells, with differential PD-1 expression compared to age-matched controls, and in specific subpopulations such as BLIMP1HI CD4+ and CD8+ T cells and effector cells.60,62,63
Prognostic relevance of PD-L1/PD-1 expression in CLL
Studies assessing the prognostic value of PD-L1/PD-1 in CLL are lacking, and correlations between PD-L1/PD-1 and other conventional prognostic markers have not been identified.34,60,61 Using an extremes of survival approach and a limited number of patient samples, we found significantly increased expression of PD-L1 on CLL cells and of PD-1 on CD3+ T cells in poor prognosis patients (median survival 38 months, n=18) compared with good prognosis patients (median survival >10 years, n=17)27 (Table 6). This, however, was based on a relatively small sample size and requires confirmation in independent patient cohorts. Others described an association between stage, need of therapy and molecular markers and levels of CD4+ and CD8+ subsets, but the exact role of PD-1 has not been established.60
HL/PMBCL
PD-L1/PD-1 expression on HL and PMBCL cells
An underlying molecular mechanism leading to elevated PD-L1/PD-L2 transcription is present in most patients with HL and PMBCL, as frequent cytogenetic alterations involve chromosome 9p, the coding region for PD-L1/PD-L2.64–67 PD-L1 expression on malignant cells has been described by several studies for the majority of PMBCL patients and on Reed–Sternberg (RS) cells in patients with HL, mostly in conjunction with PD-L233–35,37,68–70 (Table 5). Expression seems to differ with histological subtype, with strong tumor PD-L1 expression in the majority of patients with nodular sclerosis classical HL (cHL), mixed cellularity cHL and cHL-not otherwise specified (NOS), but only in a small fraction of nodular lymphocyte-predominant HL patients.35 Although tumor infiltration varied widely in this cohort, tumor PD-L1 expression correlated with expression of PD-L1 on tumor-infiltrating macrophages. A more recent study reported PD-L1 positivity in only 20% of examined HL patients, with staining intensities and patterns not further specified.68 In contrast with findings in DLBCL, PD-L1 expression is not increased in EBV+ patients.35,68,70 RS cells and variants appear to lack PD-1 expression, suggesting a potentially mutually exclusive expression pattern with PD-L1.33,35
PD-L1/PD-1 on HL/PMBCL-associated immune cells
Several early studies identified increased numbers of PD-1+ subsets, which frequently form rosettes around tumor cells, especially in lymphocyte-predominant Hodgkin lymphoma subtypes33,70–72 (Table 5). Elevated levels of PD-1+ TILs were also noted in blood T cells of HL patients (n=10) compared to healthy controls, and appeared to be higher in patients with active disease.70 In contrast, using both cHL-derived single-cell suspensions (n=18) and TMAs (n=122), our group found only little expression of PD-1 in TILs, with 40% of patients having less than 0.5% PD-1+ cells.73 In the phase I study on nivolumab, CD3+ TILs in available biopsy specimens largely expressed PD-1, albeit at similarly low levels.18 Recently published studies assessing diagnostic cHL TMAs reported PD-1 positivity on microenvironment cells in 11%69 and 20%68 of patients. Interestingly, there were no clear correlations between PD-L1 and PD-1 expression in either study.68,69 PD-1+ cell numbers were lower in both cHL patients with 9p24 gains and with higher amounts of FOXP3+ cells, but correlated with Granzyme-B and T-cell restricted intracellular antigen (TIA-1) expression in another study.72
Prognostic relevance of PD-1 expression in HL
Associations between microenvironment PD-1 expression and PD-1+ cell numbers and clinical variables or other known phenotypic parameters have not yet been identified.69,72 Regardless, an increased amount of PD-1+ TILs above the prognostic cutoff score (23 cells/mm2) was a stage-independent negative prognostic factor of OS (P=0.005)72 (Table 6). In a prognostic score incorporating numbers of PD-1, Granzyme-B, and FOXP3 expressing cells, different age- and stage-independent outcomes were found between risk groups (FOXP3−PD-1+GrB+ median survival 91 months vs. FOXP3+PD-1-Gr-B- not reached, P<0.0001). Similar associations were noted by our group: albeit expressed at low levels; patients with PD-1 expression in >15 cells/hpf had poorer 5-year disease specific survival, while OS was not affected.73 Multivariate analyses demonstrated that high PD-1 (P=0.007) and low FOXP3 expression (P=0.029) were predictors of adverse OS. Significantly reduced OS among PD-1+ patients was also reported in a recently published study, and multivariate analysis identified PD-1 expression as an independent prognostic marker for OS (P=0.019) along with high-risk IPS ≥3.69 This was, however, dependent on Ann Arbor clinical stage; in limited-stage cHL, PD-1-positive patients had a worse OS compared with PD-1-negative patients (P=0.048), whereas in advanced stage cHL PD-1-positive status was not associated with OS (P=0.13). Another study found median OS and disease-free survival (DFS) to be shorter in patients with PD-1 compared to those without PD-1 expression, as well as in patients with PD-L1 expression compared to those without, but none of these differences were statistically significant.68 Interestingly, co-expression of PD-1 and PD-L1 emerged as an independent risk factor for prognosis (OR 6.9, 95 % CI 1.9–24.3), and both OS and DFS were significantly reduced among patients with PD-1/PD-L1 coexpression compared to both PD-1 and PD-L1 negative patients.
Prognostic relevance of PD-L1 expression in HL
Koh et al. reported that patients with tumor PD-L1 expression were more likely to have a low level of lactate dehydrogenase (P=0.024) than PD-L1-negative patients, but neither PD-L1 nor PD-L2 expression were significantly associated with OS (P=0.477 and P=0.676)69 (Table 6).
Discussion
Preclinical studies suggest that PD-L1/PD-1 are key mediators of impaired anti-tumor immune responses in lymphomas.13,14 It is therefore reasonable to hypothesize that increased PD-L1/PD-1 expression confers an adverse prognosis, and that such patients might be prime candidates for therapeutic strategies targeting this axis. As prospective studies are currently lacking, we systematically reviewed published data on PD-L1/PD-1 expression and association with prognosis on B-cell lymphoma and lymphoma-associated cells.
We found that PD-L1 expression on DLBCL cells is very heterogeneous and present in only a small number of examined samples, while being affected by EBV status and potentially molecular subtype. On FL cells, PD-L1 is absent except in an extremes of survival approach. PD-L1 expression on CLL/SLL cells is increased on both LN and PB cells and in patients experiencing short-term survival. Malignant PMBCL and RS cells strongly express PD-L1 and PD-L2, especially in cHL subtypes, while being less affected by EBV serostatus. PD-1 expression was scarce on DLBCL and FL cells and absent on RS cells and variants, whereas highly conflicting findings exist in CLL.
PD-1+ TILs in DLBCL are predominantly TFH cells, and numbers are reduced compared to tonsils and other lymphomas. This appears unaffected by molecular subtype, but numbers increase with advanced disease. In FL, PD-1+ cells mainly reside in follicles. Their numbers are comparable to tonsils, but decrease with increasing histological grade, advanced stage and transformation. Several sub-populations of PD-1+CD4+ cells with distinct localization preferences and functions have been identified, including conventional TFH, exhausted and follicular regulatory T cells. Compared to other lymphomas, PD-1+ TILs numbers appears to be low in SLL/CLL LNs, but increased relative and absolute T cell numbers and functionally distinct subsets are present in blood. In HL, conflicting findings exist regarding the architectural structure of PD-1+ T-cell subsets and levels of PD-1+ TILs, potentially due to differences in examined histological subtypes and disease activity.
This heterogeneity within and across lymphoma entities is reflected by contradictory findings on the prognostic role of PD-1+ TILs, especially in DLBCL. On first sight, the same seems to be true for FL. However, both prognosis and transformation appear to be determined by follicular versus interfollicular localizations of exhausted versus functional or regulatory CD4+ and CD8+ cells. A more defined role exists in HL, where despite low and/or variable overall numbers, elevated numbers of PD-1+ TILs confer a poor prognosis. PD-L1 expression was generally found to be an adverse prognostic marker across examined lymphoma types.
Such heterogeneous findings can partly be explained by differences in the nature and composition of the examined cohorts (sample sizes, patient characteristics, treatment, etc.). Another explanation are differing methodologies, including the choice of reagents, analysis systems and definition of positivity and cutoff values. A validation study from a lymphoma consortium on the FL microenvironment reported considerable differences between manual scoring and automated microscopy systems and flow cytometry, which was also dependent on the investigating laboratory.74 Within semi-automated image analysis systems, a high concordance seems to exist.49 Among the included studies, expression was predominantly assessed by IHC. However, methods of quantifying positive cells and the definition of staining intensity and positivity varied widely. In selected studies, different counting methods were compared or verified with flow-cytometry results. Several studies have also accounted for intra- and inter-observer bias, showing good reproducibility especially in areas with fewer PD-1+ cells. Similar issues have been observed in solid malignancies, where the use of PD-L1 as a biomarker is confounded by detection antibodies, differing cutoffs and differences in tissue preparation and processing variability.75
It is also likely that biological behavior and prognosis are determined not only by overall PD-1+ TILs and tumor cells, but by functionally distinct subsets. PD-1+ numbers correlated with CD4+ T-cell and FoxP3+ numbers and GrB and TIA-1+ cells in several studies,42,52,72 and similar associations were found between distribution patterns of FoxP3+ and PD-1+ cells. Modulating effects might also be exerted by other microenvironment components such as TAMs,35 tumor-associated histiocytes,37 and small vessels.60 Studies in CLL, for example, suggest that monocyte-derived suppressor cells with high PD-L1 expression and/or skewed monocyte subpopulations are increased in patients and preclinical models and modulate T-cell responses.76,77 In multiple solid cancer types, clinical responses were observed in patients with high PD-L1 expression on tumor-infiltrating immune cells and in those with TH1 gene signatures and T-cell CTLA-4 expression at baseline.23 Immune dysfunction might also be mediated by other (potentially inducible) immune checkpoint receptor-ligand interactions, for example, by the binding of PD-1 to PD-L278 or by signaling via CD200, CD270 and CD276,27 or by additional tumor-associated and/or genetic determinants.22 Upregulation of TIM-3 was recently reported in preclinical models of lung adenocarcinoma, where tumors progressed following response to anti-PD-1 therapy.79 Optimally, the importance of these components should be assessed within one analysis and in conjunction with established clinic-pathological features.
Regardless of the expression and functions of PD-L1/PD-1 expressing cell subsets, blocking PD-L1/PD-1 interactions is safe and effective in patients with relapsed/refractory FL, DLBCL and HL.16–18 This indicates that PD-L1/PD-1 expression on tumor cells or TILs cannot be used in isolation to predict outcome of treatment for individual patients. This is further supported by observations that the numbers of PD-L1+ Tregs, CD4+ and CD8+ central memory cells, and PD-L1+ monocytes increased during treatment.17 PD-1/PD-L1/PD-L2 expression changes could also be noted in responding versus non-responding patients.16 Altogether, this work highlights that PD-L1/PD-1 expression on tumor cells and the microenvironment is only one aspect, albeit an essential one, determining the biology of lymphomas, and that the inclusion of additional components will be required to form prognostic models.
Therefore, attempts should be made to harmonize quantification methods and reporting of PD-L1/PD-1, optimally in the context of clinical studies on immune checkpoint inhibitors. Clinical study strategies should also include the identification of additional potential biomarkers using high-throughput technologies such as whole-exome sequencing, gene expression signatures/patterns, epigenetic modifications, protein microarrays and flow and mass cytometry. To address the challenges of assay comparability, performance standardization, interpretation of test results and safe translation into patient care, the US Food and Drug Administration (FDA), the American Association for Cancer Research (AACR) and the American Society of Clinical Oncology (ASCO) recently convened a workshop entitled “Complexities in Personalized Medicine: Harmonizing Companion Diagnostics Across a Class of Targeted Therapies”. As a collaboration between several companies, a blueprint proposal was developed with the goal to agree on and deliver a package of information/data upon which analytic comparison of various diagnostic assays may be conducted in non-small cell lung cancer treated with PD1/PD-L1 inhibitors.80 It is anticipated that the proposed study will build the preclinical evidence for PD-L1/PD-1 diagnostic characterization and lead to post-approval studies that will help inform personalized treatment decisions, and ultimately be applied to other tumor entities as well.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/10/1144
References
- 1.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–2028. [DOI] [PubMed] [Google Scholar]
- 2.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–2532. [DOI] [PubMed] [Google Scholar]
- 3.Robert C, Long GV, Brady B, et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N Engl J Med. 2015;372(4):320–330. [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti–Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non–Small-Cell Lung Cancer. J Clin Oncol. 2015;33(18):2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. [DOI] [PubMed] [Google Scholar]
- 8.Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–1117. [DOI] [PubMed] [Google Scholar]
- 9.Topalian SL, Sznol M, McDermott DF, et al. Survival, Durable Tumor Remission, and Long-Term Safety in Patients With Advanced Melanoma Receiving Nivolumab. J Clin Oncol. 2014;32(10):1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamid O, Robert C, Daud A, et al. Safety and Tumor Responses with Lambrolizumab (Anti–PD-1) in Melanoma. N Engl J Med. 2013;369(2):134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer JR, Tykodi SS, Chow LQM, et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N Engl J Med. 2012;366(26):2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med. 2012;366(26):2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greaves P, Gribben JG. The role of B7 family molecules in hematologic malignancy. Blood. 2013;121(5):734–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi L, Chen S, Yang L, Li Y. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J Hematol Oncol. 2013;6(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I Safety and Pharmacokinetic Study of CT-011, a Humanized Antibody Interacting with PD-1, in Patients with Advanced Hematologic Malignancies. Clin Cancer Res. 2008;14(10):3044–3051. [DOI] [PubMed] [Google Scholar]
- 16.Westin JR, Chu F, Zhang M, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15(1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armand P, Nagler A, Weller EA, et al. Disabling Immune Tolerance by Programmed Death-1 Blockade With Pidilizumab After Autologous Hematopoietic Stem-Cell Transplantation for Diffuse Large B-Cell Lymphoma: Results of an International Phase II Trial. J Clin Oncol. 2013;31(33):4199–4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 Blockade with Nivolumab in Relapsed or Refractory Hodgkin’s Lymphoma. N Engl J Med. 2015;372(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang A, Wang HY, Liu Y, et al. The prognostic value of PD-L1 expression for non-small cell lung cancer patients: a meta-analysis. Eur J Surg Oncol. 2015;41(4):450–456. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Kang S, Shen J, et al. Prognostic significance of programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) Expression in epithelial-originated cancer: a meta-analysis. Medicine (Baltimore). 2015;94(6):e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu P, Wu D, Li L, Chai Y, Huang J. PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PLoS One. 2015;10(6):e0131403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sui X, Ma J, Han W, et al. The anticancer immune response of anti-PD-1/PD-L1 and the genetic determinants of response to anti-PD-1/PD-L1 antibodies in cancer patients. Oncotarget. 2015;6(23):19393–19404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burger JA, Gribben JG. The microenvironment in chronic lymphocytic leukemia (CLL) and other B cell malignancies: Insight into disease biology and new targeted therapies. Semin Cancer Biol. 2014;24:71–81. [DOI] [PubMed] [Google Scholar]
- 25.Gribben JG, Riches JC. Immunotherapeutic strategies including transplantation: eradication of disease. Hematology Am Soc hematol Educ Program. 2013;2013(1):151–157. [DOI] [PubMed] [Google Scholar]
- 26.Kiaii S, Clear AJ, Ramsay AG, et al. Follicular Lymphoma Cells Induce Changes in T-Cell Gene Expression and Function: Potential Impact on Survival and Risk of Transformation. J Clin Oncol. 2013;31(21): 2654–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramsay AG, Clear AJ, Fatah R, Gribben JG. Multiple inhibitory ligands induce impaired T-cell immunologic synapse function in chronic lymphocytic leukemia that can be blocked with lenalidomide: establishing a reversible immune evasion mechanism in human cancer. Blood. 2012;120(7):1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McClanahan F, Hanna B, Miller S, et al. PD-L1 Checkpoint Blockade Prevents Immune Dysfunction and Leukemia Development in a Mouse Model of Chronic Lymphocytic Leukemia. Blood. 2015;126(2):203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fife BT, Pauken KE. The role of the PD-1 pathway in autoimmunity and peripheral tolerance. Ann N Y Acad Sci. 2011;1217:45–59. [DOI] [PubMed] [Google Scholar]
- 30.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nature Rev Immunol. 2008;8(6):467–477. [DOI] [PubMed] [Google Scholar]
- 31.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crotty S. Follicular helper CD4 T cells (TFH). Annu Rev Immunol. 2011;29:621–663. [DOI] [PubMed] [Google Scholar]
- 33.Dorfman DM, Brown JA, Shahsafaei A, Freeman GJ. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2006;30(7):802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xerri L, Chetaille B, Seriari N, et al. Programmed death 1 is a marker of angioimmunoblastic T-cell lymphoma and B-cell small lymphocytic lymphoma/chronic lymphocytic leukemia. Hum Pathol. 2008;39(7): 1050–1058. [DOI] [PubMed] [Google Scholar]
- 35.Chen BJ, Chapuy B, Ouyang J, et al. PD-L1 expression is characteristic of a subset of aggressive B-cell lymphomas and virus-associated malignancies. Clin Cancer Res. 2013;19(13):3462–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossille D, Gressier M, Damotte D, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014;28(12):2367–2375. [DOI] [PubMed] [Google Scholar]
- 37.Andorsky DJ, Yamada RE, Said J, Pinkus GS, Betting DJ, Timmerman JM. Programmed Death Ligand 1 Is Expressed by Non–Hodgkin Lymphomas and Inhibits the Activity of Tumor-Associated T Cells. Clin Cancer Res. 2011;17(13):4232–4244. [DOI] [PubMed] [Google Scholar]
- 38.Kwon D, Kim S, Kim PJ, et al. Clinicopathological analysis of programmed cell death-1 and programmed cell death-ligand 1 expression in the tumor microenvironments of diffuse large B-cell lymphomas. Histopathology. 2016;68(7):1079–1089. [DOI] [PubMed] [Google Scholar]
- 39.Kiyasu J, Miyoshi H, Hirata A, et al. Expression of programmed cell death ligand 1 is associated with poor overall survival in patients with diffuse large B-cell lymphoma. Blood. 2015;126(19):2193–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muenst S, Hoeller S, Willi N, Dirnhofera S, Tzankov A. Diagnostic and prognostic utility of PD-1 in B cell lymphomas. Dis Markers. 2010;29(1):47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ko YS, Oh YH, Park CK, et al. Prognostic Implication of Programmed Death-1-Positive Tumor-infiltrating Lymphocytes in Diffuse Large B-Cell Lymphoma. Korean J Pathol. 2011;45(6):573–581. [Google Scholar]
- 42.Ahearne MJ, Bhuller K, Hew R, Ibrahim H, Naresh K, Wagner SD. Expression of PD-1 (CD279) and FoxP3 in diffuse large B-cell lymphoma. Virchows Arch. 2014;465(3): 351–358. [DOI] [PubMed] [Google Scholar]
- 43.Ame-Thomas P, Le Priol J, Yssel H, et al. Characterization of intratumoral follicular helper T cells in follicular lymphoma: role in the survival of malignant B cells. Leukemia. 2012;26(5):1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(25):1937–1947. [DOI] [PubMed] [Google Scholar]
- 45.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. [DOI] [PubMed] [Google Scholar]
- 46.Scott DW. Cell-of-Origin in Diffuse Large B-Cell Lymphoma: Are the Assays Ready for the Clinic? Am Soc Clin oncol Educ Book. 2015;35:e458–466. [DOI] [PubMed] [Google Scholar]
- 47.Hasselblom S, Sigurdadottir M, Hansson U, Nilsson-Ehle H, Ridell B, Andersson PO. The number of tumour-infiltrating TIA-1+ cytotoxic T cells but not FOXP3+ regulatory T cells predicts outcome in diffuse large B-cell lymphoma. Br J Haematol. 2007;137(4):364–373. [DOI] [PubMed] [Google Scholar]
- 48.Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica. 2008;93(2):193–200. [DOI] [PubMed] [Google Scholar]
- 49.Coutinho R, Clear AJ, Mazzola E, et al. Revisiting the immune microenvironment of diffuse large B-cell lymphoma using a tissue microarray and immunohistochemistry: robust semi-automated analysis reveals CD3 and FoxP3 as potential predictors of response to R-CHOP. Haematologica. 2015;100(3):363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carreras J, Lopez-Guillermo A, Roncador G, et al. High numbers of tumor-infiltrating programmed cell death 1-positive regulatory lymphocytes are associated with improved overall survival in follicular lymphoma. J Clin Oncol. 2009;27(9):1470–1476. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi H, Tomita N, Sakata S, et al. Prognostic significance of programmed cell death-1-positive cells in follicular lymphoma patients may alter in the rituximab era. Eur J Haematol. 2013;90(4):286–290. [DOI] [PubMed] [Google Scholar]
- 52.Koch K, Hoster E, Unterhalt M, et al. The composition of the microenvironment in follicular lymphoma is associated with the stage of the disease. Hum Pathol. 2012;43(12):2274–2281. [DOI] [PubMed] [Google Scholar]
- 53.Richendollar BG, Pohlman B, Elson P, Hsi ED. Follicular programmed death 1-positive lymphocytes in the tumor microenvironment are an independent prognostic factor in follicular lymphoma. Hum Pathol. 2011;42(4):552–557. [DOI] [PubMed] [Google Scholar]
- 54.Smeltzer JP, Jones JM, Ziesmer SC, et al. Pattern of CD14+ follicular dendritic cells and PD1+ T cells independently predicts time to transformation in follicular lymphoma. Clin Cancer Res. 2014;20(11):2862–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang ZZ, Grote DM, Ziesmer SC, Xiu B, Novak AJ, Ansell SM. PD-1 expression defines two distinct T-cell sub-populations in follicular lymphoma that differentially impact patient survival. Blood Cancer J. 2015;5:e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351(21): 2159–2169. [DOI] [PubMed] [Google Scholar]
- 57.Lee AM, Clear AJ, Calaminici M, et al. Number of CD4+ Cells and Location of Forkhead Box Protein P3–Positive Cells in Diagnostic Follicular Lymphoma Tissue Microarrays Correlates With Outcome. J Clin Oncol. 2006;24(31):5052–5059. [DOI] [PubMed] [Google Scholar]
- 58.Farinha P, Al-Tourah A, Gill K, Klasa R, Connors JM, Gascoyne RD. The architectural pattern of FOXP3-positive T cells in follicular lymphoma is an independent predictor of survival and histologic transformation. Blood. 2010;115(2):289–295. [DOI] [PubMed] [Google Scholar]
- 59.Wahlin BE, Aggarwal M, Montes-Moreno S, et al. A unifying microenvironment model in follicular lymphoma: outcome is predicted by programmed death-1–positive, regulatory, cytotoxic, and helper T cells and macrophages. Clin Cancer Res. 2010;16(2): 637–650. [DOI] [PubMed] [Google Scholar]
- 60.Brusa D, Serra S, Coscia M, et al. The PD-1/PD-L1 axis contributes to T-cell dysfunction in chronic lymphocytic leukemia. Haematologica. 2013;98(6):953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grzywnowicz M, Zaleska J, Mertens D, et al. Programmed death-1 and its ligand are novel immunotolerant molecules expressed on leukemic B cells in chronic lymphocytic leukemia. PLoS One. 2012;7(4):e35178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Riches JC, Davies JK, McClanahan F, et al. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood. 2013;121(9): 1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tonino SH, van de Berg PJ, Yong SL, et al. Expansion of effector T cells associated with decreased PD-1 expression in patients with indolent B cell lymphomas and chronic lymphocytic leukemia. Leuk Lymphoma. 2012;53(9):1785–1794. [DOI] [PubMed] [Google Scholar]
- 64.Twa DD, Chan FC, Ben-Neriah S, et al. Genomic rearrangements involving programmed death ligands are recurrent in primary mediastinal large B-cell lymphoma. Blood. 2014;123(13):2062–2065. [DOI] [PubMed] [Google Scholar]
- 65.Green MR, Monti S, Rodig SJ, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198(6):851–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joos S, Otano-Joos M, Ziegler S, et al. Primary mediastinal (thymic) B-cell lymphoma is characterized by gains of chromosomal material including 9p and amplification of the REL gene. Blood. 1996;87(4): 1571–1578. [PubMed] [Google Scholar]
- 68.Paydas S, Bağır E, Seydaoglu G, Ercolak V, Ergin M. Programmed death-1 (PD-1), programmed death-ligand 1 (PD-L1), and EBV-encoded RNA (EBER) expression in Hodgkin lymphoma. Ann Hematol. 2015;94(9):1545–1552. [DOI] [PubMed] [Google Scholar]
- 69.Koh YW, Jeon YK, Yoon DH, Suh C, Huh J. Programmed death 1 expression in the peritumoral microenvironment is associated with a poorer prognosis in classical Hodgkin lymphoma. Tumour Biol. 2016;37(6):7507–7514. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto R, Nishikori M, Kitawaki T, et al. PD-1/PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111(6):3220–3224. [DOI] [PubMed] [Google Scholar]
- 71.Nam-Cha SH, Roncador G, Sanchez-Verde L, et al. PD-1, a follicular T-cell marker useful for recognizing nodular lymphocyte-predominant Hodgkin lymphoma. Am J Surg Pathol. 2008;32(8):1252–1257. [DOI] [PubMed] [Google Scholar]
- 72.Muenst S, Hoeller S, Dirnhofer S, Tzankov A. Increased programmed death-1+ tumor-infiltrating lymphocytes in classical Hodgkin lymphoma substantiate reduced overall survival. Hum Pathol. 2009;40(12):1715–1722. [DOI] [PubMed] [Google Scholar]
- 73.Greaves P, Clear A, Owen A, et al. Defining characteristics of classical Hodgkin lymphoma microenvironment T-helper cells. Blood. 2013;122(16):2856–2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sander B, de Jong D, Rosenwald A, et al. The reliability of immunohistochemical analysis of the tumor microenvironment in follicular lymphoma: a validation study from the Lunenburg Lymphoma Biomarker Consortium. Haematologica. 2014;99(4): 715–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. [DOI] [PubMed] [Google Scholar]
- 76.Jitschin R, Braun M, Buettner M, et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood. 2014;124(5):750–760. [DOI] [PubMed] [Google Scholar]
- 77.Hanna BS, McClanahan F, Yazdanparast H, et al. Depletion of CLL-associated patrolling monocytes and macrophages controls disease development and repairs immune dysfunction in vivo. Leukemia. 2016;30(3):570–579. [DOI] [PubMed] [Google Scholar]
- 78.Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. [DOI] [PubMed] [Google Scholar]
- 79.Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nature communications. 2016;7:10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.A Blueprint Proposal for Companion Diagnostic Comparability. Available at: http://www.aacr.org/AdvocacyPolicy/GovernmentAffairs/Pages/industry-working-group-blueprint-proposal.aspx#.VyEupkfVt5A [Last accessed: April 27th 2016.]


