Abstract
Disease bulk is an important prognostic factor in early stage Hodgkin lymphoma, but its definition is unclear in the computed tomography era. This retrospective analysis investigated the prognostic significance of bulky disease measured in transverse and coronal planes on computed tomography imaging. Early stage Hodgkin lymphoma patients (n=185) treated with chemotherapy with or without radiotherapy from 2000–2010 were included. The longest diameter of the largest lymph node mass was measured in transverse and coronal axes on pre-treatment imaging. The optimal cut off for disease bulk was maximal diameter greater than 7 cm measured in either the transverse or coronal plane. Thirty patients with maximal transverse diameter of 7 cm or under were found to have bulk in coronal axis. The 4-year overall survival was 96.5% (CI: 93.3%, 100%) and 4-year relapse-free survival was 86.8% (CI: 81.9%, 92.1%) for all patients. Relapse-free survival at four years for bulky patients was 80.5% (CI: 73%, 88.9%) compared to 94.4% (CI: 89.1%, 100%) for non-bulky; Cox HR 4.21 (CI: 1.43, 12.38) (P=0.004). In bulky patients, relapse-free survival was not impacted in patients treated with chemoradiotherapy; however, it was significantly lower in patients treated with chemotherapy alone. In an independent validation cohort of 38 patients treated with chemotherapy alone, patients with bulky disease had an inferior relapse-free survival [at 4 years, 71.1% (CI: 52.1%, 97%) vs. 94.1% (CI: 83.6%, 100%), Cox HR 5.27 (CI: 0.62, 45.16); P=0.09]. Presence of bulky disease on multidimensional computed tomography imaging is a significant prognostic factor in early stage Hodgkin lymphoma. Coronal reformations may be included for routine Hodgkin lymphoma staging evaluation. In future, our definition of disease bulk may be useful in identifying patients who are most appropriate for chemotherapy alone.
Introduction
The presence of bulky disease at presentation has long been considered a poor prognostic factor in early stage Hodgkin lymphoma (ESHL).1,2 Historically, bulk in the mediastinum was focused upon and defined using radiographic criteria from a standing posterior-anterior (PA) chest radiograph (CXR). In the 1989 Cotswolds revision of the Ann Arbor Staging system, bulk in the mediastinum was defined as “when the maximum width is equal or greater than one-third of the internal transverse diameter of the thorax at the level of T5/6” on a PA CXR and bulk at an alternate site was defined as any mass measuring 10 cm or more by any imaging study.3 Today, the presence of bulky disease in ESHL remains an unfavorable prognostic feature in all modern risk classification systems, but is variably defined as more than one-third of the mediastinal mass ratio (MMR), more than one-third of the mediastinal thoracic ratio (MTR), or any mass over 10 cm.4–7
Although definitions of disease bulk were originally developed in the CXR-era, computed tomography (CT) imaging is now the standard staging imaging modality in lymphoma.8 In the CT era, the definition of disease bulk remains elusive. Various retrospective studies have defined CT criteria for bulky disease associated with increased risk of relapse in ESHL, ranging from greater than 5 to 10 cm for the maximal mediastinal mass diameter in transverse plane.9–11 The recent “Lugano Classification” for initial staging in lymphoma retains the historical definition of bulk with a single nodal mass of 10 cm or more or mediastinal bulk more than one-third of the transthoracic diameter. However, with uncertainty remaining regarding an evidence-based definition of bulk in the CT-era, the authors also suggest elimination of the modifier “X” and propose recording the longest measurement by CT scan, presumably in the transverse plane.8 However, there remains a critical need for a bulky disease cut-off point in ESHL as increasingly patients are being treated with chemotherapy alone to avoid the late effects of radiotherapy, an approach that has generally excluded patients with disease bulk.12,13 For example, the recently published UK RAPID study included stage IA and IIA patients without mediastinal bulk defined as maximal mediastinal diameter of 33% or more of the internal thoracic diameter at T5–T6.12
Importantly, the transverse diameter of a lymph node mass may not reflect the longest dimension of an oblique or sagittal positioned mass in HL. On CT, lymph node masses can be measured in the transverse, coronal, and sagittal planes; however, measurements in axes other than the transverse are rarely reported in HL, and 3-dimensional or volumetric assessments of tumor bulk are difficult to routinely assess. In this study, we aimed to assess the prognostic significance of the longest diameter of the largest nodal mass measured in either the transverse and coronal planes using CT imaging.
Methods
Patients
For the training cohort of this retrospective study, we identified pediatric and adult patients with stage I-II classical HL treated at Memorial Sloan Kettering (MSK) Cancer Center between January 2000 and December 2010. Patients were included if they had pretreatment CT scan performed within 30 days of therapy initiation and received doxorubicin-containing chemotherapy with or without radiation at MSK.
For the independent validation cohort, adult patients with classical HL, stage I-II, with baseline CT scan performed within 30 days of therapy, and treated with chemotherapy alone at Dana Farber Cancer Institute (DFCI) or Mayo Clinic (Mayo) between January 2000 and December 2010 were included. These retrospective analyses were conducted on waivers of authorization approved by the institutional review boards at each institution.
Radiological assessment
Measurements for the training cohort were performed by a single board-certified radiologist (IB) who was blinded to patients’ clinical history, including treatment and outcome. All available pre-treatment imaging studies, including diagnostic computed tomography (dCT), FDG-PET and FDG-PET/CT were reviewed. CT scans were performed on GE Lightspeed helical scanners. MSK patients were scanned on dedicated PET/CT systems [Discovery STE, LS or 690 (GE Medical Systems) or Biograph 16 (Siemens Medical Systems)]. Images were reconstructed at 5- or 7.5-mm intervals in the Picture Archiving and Communication System (PACS) (Centricity; GE Medical Systems, Milwaukee, WI, USA). CT scans from outside institutions were digitized on PACS, reviewed and included only if of acceptable quality. Most measurements were obtained using dCT (134 of 185, 72%). Others were from low-dose CT performed in conjunction with FDG-PET imaging (51 of 185, 28%). Using calipers with measurements in centimeters, the longest diameter of the largest individual or conglomerate lymph node mass was measured in the transverse plane and coronal plane (see Figure 1). This measurement could lie obliquely or in any orientation, to ensure ascertainment of the maximal diameter. Coronal images were analyzed and re-formatted using the open-source software OsiriX.14 The quality of the coronal reconstruction was dependent on the slice thickness, ranging from 1.2 to 7.5 mm. The quality of coronal reconstructions was assessed using a subjective scale: 0-poor, 1-fair, 2-good.
Figure 1.
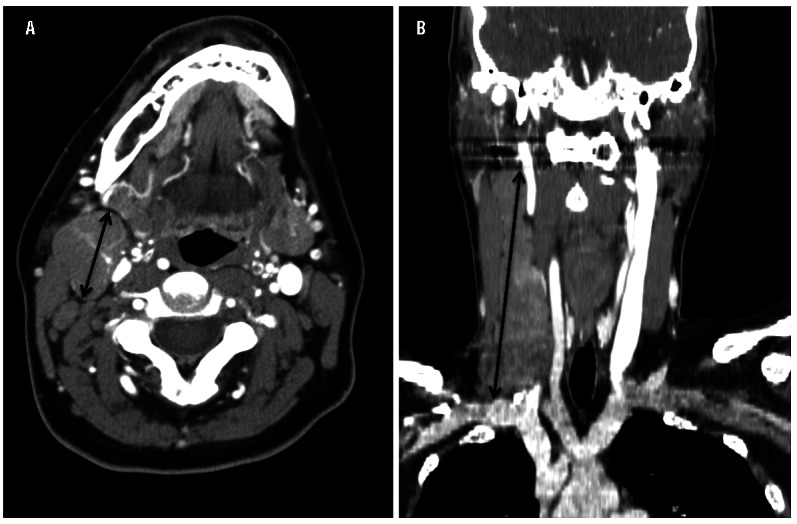
Representative images of the longest diameters measured using calipers of a right cervical mass in (A) transverse plane, 2.6 cm and (B) coronal plane, 12.1 cm.
For the validation cohorts, the measurements were performed in a similar manner as described for the training cohort. At DFCI, the measurements were performed by a single medical oncologist (AK) and at Mayo by a single board-certified radiologist (DW), both blinded to patient outcome. Coronal reconstructions were created using OsiriX and TeraRecon (TeraRecon Inc.) software at DFCI (n=25) and at Mayo (n=13), respectively. Measurements were made from dCT at baseline (23 of 38, 61%) and low-dose CT in conjunction with FDG-PET imaging (15 of 38, 39%).
Statistical analysis
Survival analyses were performed using the Kaplan-Meier method in SPSS 22 and R 3.2.3. Relapse-free survival (RFS) and overall survival (OS) were defined as the time from initiation of treatment until progression of disease or relapse (RFS) or until death from any cause (OS), respectively. Log rank tests were used to compare survival differences. P≤0.05 was considered statistically significant.
The Pearson correlation test assessed the degree of correlation between the maximal transverse and coronal diameters. With few deaths, only the association between disease bulk and RFS was assessed. To identify the optimal cut off for the transverse and coronal maximal diameters to predict outcome, we identified a range of potential cut-off points (in cm) based upon the distribution of the data (between the 10th and 90th percentiles), and examined their significance levels using log rank tests correlating with RFS. The cut-off point resulting in the maximal significance level was identified as the optimal cut off for the transverse and coronal dimensions, and this P-value was adjusted by the maximal χ2 method since multiple tests were performed.15 Further, we aimed to identify the most predictive “combined” criterion. By using concordance probability analysis, we identified the set of maximal diameter cut offs (i.e. transverse > X OR coronal > Y) that most strongly predicted RFS.16 The methods for determining bulky disease cut off, including maximal χ2 method and concordance probability analysis, are described in detail in the Online Supplementary Appendix.
Results
In total, there were 185 patients who met inclusion criteria for this study. Of the 185 images examined, 158 (85%) were characterized by the interpreting radiologist as good quality for coronal measurement assessment and 27 fair quality (15%). Patients’ characteristics are shown in Table 1. The median age of patients was 41 years (range 9 to 85) with slight female predominance (57%). Of the histological subtypes of classical HL, the predominant was the nodular sclerosing subtype (78%). Overall, 95% of patients presented with stage II disease by Ann Arbor classification. An effusion (pericardial or pleural) or extranodal disease (lung, chest wall, or thyroid involvement) was present in a minority of patients. Using the German Hodgkin Study Group system, 71% of patients met criteria for unfavorable risk disease.5
Table 1.
Patient’s characteristics of early stage Hodgkin lymphoma, n=185.
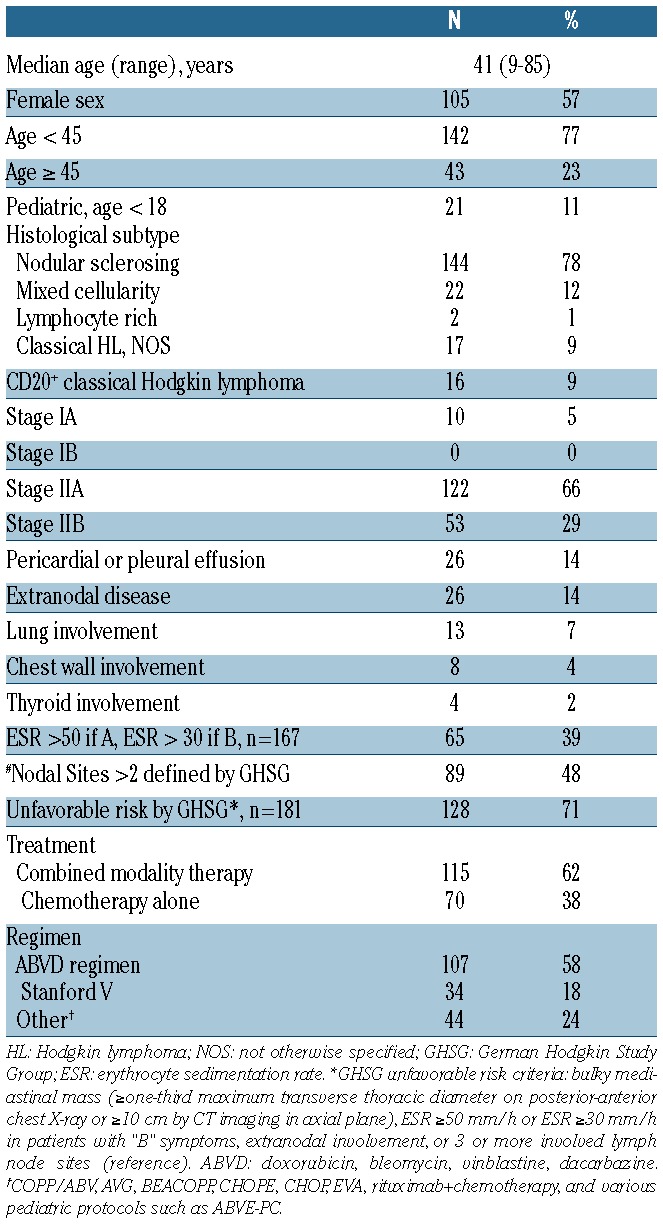
All patients were treated with standard or novel doxorubicin-based chemotherapy regimens (Table 1). The vast majority of patients were treated with ABVD or Stanford V, and others were treated with various doxorubicin-containing regimens, including clinical trial protocols. A proportion of CD20+ HL patients were treated with rituximab-containing regimens (6 of 16). One hundred and fifteen patients (62%) were treated with combined modality therapy (CMT) and 70 patients (38%) with chemotherapy alone.
Outcomes
Of the 185 patients, 23 patients relapsed and 6 patients died after initial therapy: 4 deaths due to progressive HL after multiple lines of salvage treatment, one death from secondary myelodysplastic syndromes (MDS), and one death of unknown cause. The median follow up for all patients was five years. The 4-year OS was 96.5% (CI: 93.2%, 100%) and the 4-year RFS was 86.8% (CI: 81.8%, 92.1%).
Disease bulk
Training cohort
In most cases the longest diameter in the transaxial and coronal plane was measured from the same lymph node mass; however, in 13 cases the longest diameters were from different sites. In univariate analyses, both transverse maximal diameter and coronal maximal diameter were significantly associated with RFS (Online Supplementary Table S1). Using log rank tests to compare RFS, the optimal cut-off point for transverse maximal diameter was 7 cm (P=0.01, after adjustment by the maximal χ2 method P=0.02). The optimal cut-off point for coronal max diameter was 10.5 cm (P=0.005, after adjustment P=0.007). Using the concordance probability technique to determine optimal combined criterion, the criteria of transverse max diameter more than 7.0 cm OR coronal max diameter more than 7.0 cm was the best predictor for progression (Online Supplementary Appendix). Figure 2 demonstrates that the transverse and coronal maximal diameters are highly correlated (correlation coefficient R=0.856; P<0.001).
Figure 2.
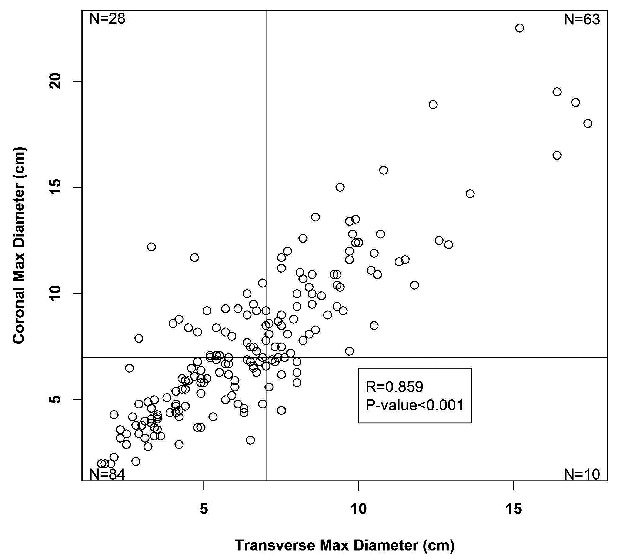
Correlation between maximal transverse and coronal measurements with lines for the 7 cm maximal transverse and coronal cut-offs for disease bulk.
Using a cut-off point of more than 7cm in maximal transverse dimension or maximal coronal dimension (>7cm in MTD or MCD), approximately half of the patients are defined as bulky (101 of 187, 54%). Seventy-three patients had disease bulk more than 7.0 cm by transverse criteria and 10 of these patients had disease bulk in the transverse plane alone without disease bulk in the coronal plane. Ninety-one patients had disease bulk more than 7.0 cm by coronal criteria with 28 patients identified as having disease bulk more than 7.0 cm on coronal imaging that was not observed in the transaxial plane. Of the 101 patients with bulky disease (>7cm in MTD or MCD), 83 patients had evidence of mediastinal disease bulk, 17 with other sites of supradiaphragmatic bulk, and one with infradiaphragmatic disease bulk (Table 2).
Table 2.
Relapse-free survival (RFS) at four years for bulky disease subgroups defined as more than 7cm in transverse or coronal plane. The corresponding hazard ratios (HR) are reported for the bulky disease subgroups when compared to the complementary non-bulky disease subgroup.
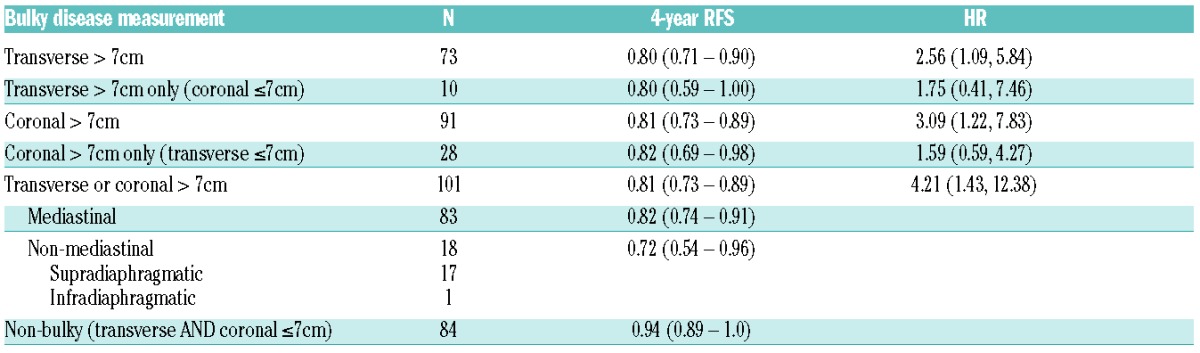
Univariate analyses for RFS demonstrated that disease bulk defined as more than 7cm was associated with increased risk for relapse, with similar findings for transverse versus coronal plane measurements and for mediastinal versus non-mediastinal disease (Table 2). The presence of bulky disease, defined as more than 7cm in maximal transverse dimension or maximal coronal dimension (>7cm in MTD or MCD), significantly correlated with RFS. At four years, relapse-free survival for bulky patients was 80.5% (CI: 73%, 88.9%) compared to 94.4% (CI: 89.1%, 100%) for non-bulky; Cox HR 4.21 (CI: 1.43, 12.38) (P=0.004) (Figure 3A). There was no significant difference in OS between patients with bulky versus non-bulky disease [Cox HR 4.63 (CI 0.54, 40.04); P=0.126]. In an outcomes analysis of bulky patients (Figure 3B) there is no apparent difference between RFS for patients uniquely identified in this study with coronal bulk alone (coronal > 7cm and transverse ≤7cm) versus traditional definition of bulk using transverse measurement (transverse > 7cm) [Cox HR 0.91 (CI: 0.33, 2.51); P=0.846].
Figure 3.
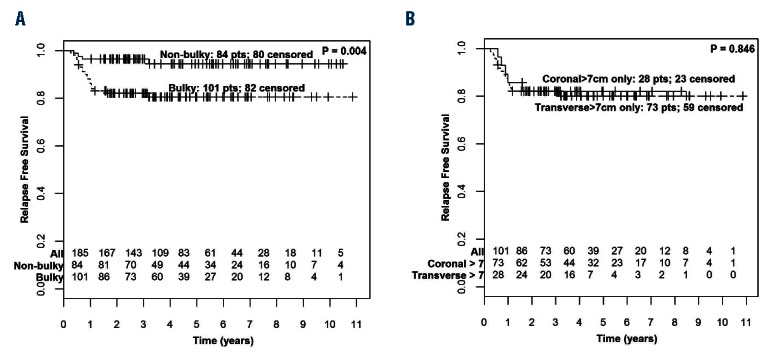
Relapse-free survival (RFS) by presence of bulky disease. (A) RFS for non-bulky versus bulky disease (transverse or coronal max diameter > 7cm). (B) RFS for coronal bulk alone (coronal max measurement > 7cm, transverse max, measurement ≤ 7cm) compared to traditional definition of bulk (transverse max, measurement >7cm).
Analysis of bulk stratified by therapy (CMT vs. chemotherapy alone) demonstrated that patients with bulky disease treated with chemotherapy alone had a particularly unfavorable prognosis with 4-year RFS of 55.2% (CI: 40.2%, 75.7%) (Figure 4). The definition of bulk as more than 7 cm in MTD or MCD was a powerful prognostic factor in patients treated with chemotherapy alone. However, for patients who received CMT, this definition of disease bulk was not prognostic; rather the traditional definition of bulky disease (>10cm in transverse plane) delineated a group with increased risk of relapse (P=0.001) (Online Supplementary Figure S1).
Figure 4.
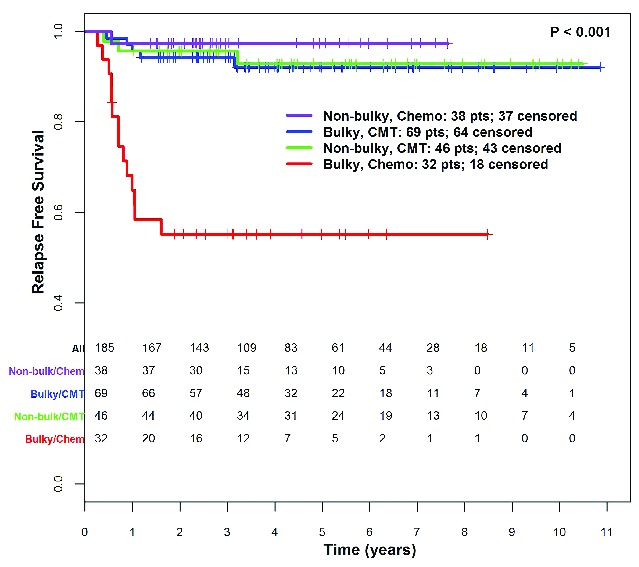
Relapse-free survival by presence of bulky disease (transverse or coronal max, diameter > 7cm) and treatment [chemotherapy alone (Chemo) vs. combined modality therapy (CMT)].
Validation cohort
The novel MSK definition of disease bulk (>7cm in MTD or MCD) was prognostically relevant among patients who received chemotherapy alone, and, therefore, this prognostic factor was examined in an independent validation set of 38 patients with stage II disease treated with chemotherapy alone (36 with 4–6 cycles of ABVD, 1 with 4 cycles of BCVPP, and 1 with 3 cycles of MOPP and 3 cycles of ABVD). The median follow up was four years for this cohort. Nineteen patients (50%) had disease bulk and 5 patients (13%) were identified to have disease bulk on coronal measurement, but not by transverse measurement. Among the 38 patients, there were 6 relapse events and 2 deaths, one undifferentiated sarcoma eight years post treatment in a 53-year old and one bleomycin-related respiratory failure in a 67-year old; both deaths occurred in patients with non-bulky disease. Disease bulk was associated with a trend toward inferior RFS: in bulky patients, 4-year RFS was 71.1% (CI: 52.1%, 97%) versus 4-year RFS of 94.1% (CI: 83.6%, 100%) in non-bulky patients [Cox HR 5.27 (CI: 0.62, 45.16); P=0.09] (Figure 5).
Figure 5.
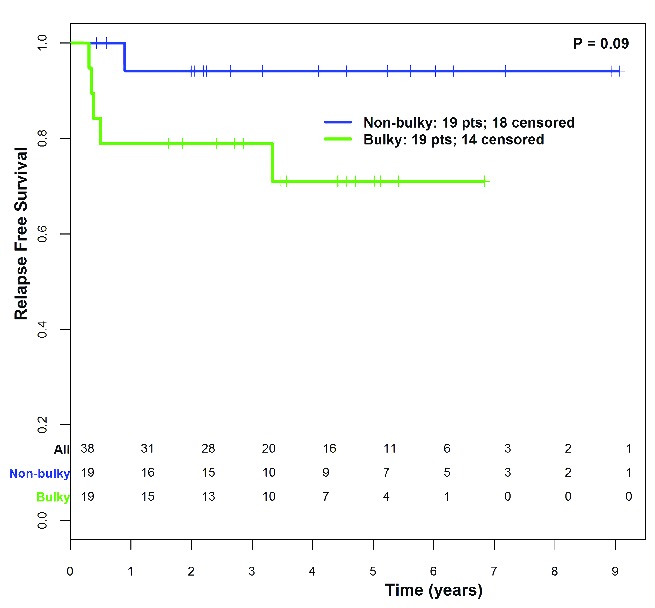
Relapse-free survival by presence of bulky disease (transverse or coronal max, diameter > 7cm) in validation cohort patients (n=38) treated with chemotherapy alone.
Discussion
The importance of re-examining the definition of bulk is based upon improved multidimensional CT data quality and evolving treatment strategies, including reduction and elimination of radiotherapy. Previous definitions of disease bulk were formulated in the era when combined modality therapy was the standard of care for all ESHL patients.17 Our proposed definition of disease bulk (>7cm in transverse or coronal plane) has heightened prognostic relevance for patients treated with chemotherapy alone. To our knowledge, this is the first study to analyze the prognostic significance of bulky disease defined in both transverse and coronal planes in the era of CT imaging. This study demonstrates that, when measured in the coronal plane, bulky disease is significantly associated with inferior outcome.
The early stage risk classification systems (including GHSG, EORTC, NCIC, and NCCN) use MMR and MTR to define mediastinal bulky disease; however, in modern clinical practice and in clinical trials, bulky disease is often defined by the maximal transverse diameter of the largest lymph node mass more than 10 cm, likely because this measurement is a quick and reproducible method of assessing disease bulk on CT imaging. Other studies have suggested alternate definitions of mediastinal bulky disease, including lymph node masses greater than 5 cm, 6 cm, or 7.5 cm.9,10,17,18 Herein the optimal criterion for bulky disease of more than 7 cm in MTD or MCD falls within the range of previously reported CT bulk definitions. Our data show that bulky disease in any location, in the mediastinum or an alternate site, is of prognostic value.
Coronal and sagittal reformations in CT studies provide additional diagnostic and clinical information in various clinical settings.19,20 Evaluation of coronal images in HL is important for disease bulk positioned obliquely in the mediastinum or for conglomerate lymph node mass extending along the cranio-caudal axis, such as involvement of contiguous cervical, supraclavicular, and infraclavicular lymph nodes. We found additional patients (approx. 30%) with bulky disease were identified using CT coronal reformations that would not have otherwise been identified using transaxial imaging alone, identifying additional patients at increased risk of relapse. Importantly, patients with coronal-only bulk had similar outcomes when compared to patients with bulky disease defined traditionally using transverse measurements. These data have the potential to add to the “Lugano classification” system by clarifying the importance of measuring disease bulk in the coronal axis in addition to the transverse axis.
OsiriX software was used to create coronal reformats from transaxial CT images in this study; however, coronal and sagittal reformations are a routine component of modern multi-planar CT protocols. Obtaining these measurements on modern pre-treatment staging CT scans can easily be performed in routine practice.
The standard of care for patients with ESHL and bulky mediastinal disease is CMT.21–23 In the modern treatment of HL, there is interest in limiting or eliminating the use of radiotherapy in select patients to decrease long-term radiotherapy-related toxicities. Many clinical trials have aimed to eliminate radiotherapy in select ESHL patients, such as the United Kingdom RAPID, NCIC HD6, MSK, EORTC/LYSA/FIL H10, and SWOG ESHL trials, and have been limited to patients with non-bulky disease.12,24–26 In the current retrospective analysis, we analyzed risk factors for relapse in patients treated with chemotherapy alone versus CMT, and identified a novel definition of disease bulk (>7cm in MTD or MCD) that is prognostically most relevant for patients treated with chemotherapy alone. This novel definition was evaluated in an independent cohort of patients treated with chemotherapy alone. Unfortunately, in this validation cohort, the sample size was small and there were few relapse events, such that the RFS difference in this validation cohort was not statistically significant but showed a trend towards this (P=0.09). However, findings in the validation cohort were consistent with the training set and demonstrate a clinically meaningful difference with most relapse events (5 of 6) occurring among patients with disease bulk. These data provide preliminary evidence that suggest a potential for caution against eliminating radiotherapy in patients with disease more than 7 cm in MTD or MCD as our retrospective analysis suggests that chemotherapy alone is associated with poor outcomes in bulky patients [in the training set: 4-year RFS of 55.2% (CI: 40.2%, 75.7%); in the validation set: 4-year RFS 71.1% (CI: 52.1%, 97%)]. A corollary of these data is that patients without disease bulk per our definition appear to have excellent relapse-free survival when treated with chemotherapy alone [in the training set: 4-year RFS of 97.4% (CI: 92.4%, 100%); in the validation set: 4-year RFS 94% (CI: 83.6%, 100%)]. Full course chemotherapy, as was commonly administered for patients included in this retrospective analysis, is likely not necessary for these non-bulky patients who have an excellent prognosis and would be appropriate candidates for the RAPID or CALGB/Alliance 50604 risk-adapted treatment paradigms with abbreviated chemotherapy regimens (i.e. 3–4 cycles of ABVD) for PET-negative patients.12,13 Finally, the training data also suggest that patients with very bulky disease with masses more than 10 cm in the transverse plane have a high risk of relapse even when treated with standard CMT, suggesting a role for more intensive chemotherapy such as escalated BEACOPP or incorporation of novel agents, such as brentuximab vedotin or nivolumab, in this patient population.
A limitation of this study is that interim and end-of-treatment PET scans were not routinely available. Preliminary data from the British Columbia Cancer Agency reports excellent outcomes for bulky HL patients who achieve a negative PET scan after full-course ABVD without additional consolidative RT, and there is an ongoing CALGB/Alliance 50801 study exploring interim PET-based risk-adapted therapy in HL patients with disease bulk.27 These studies will further clarify whether achievement of an interim or end-of-treatment PET may overcome the negative prognostic value of disease bulk at initial presentation. In addition, the current study was inadequately powered to assess association with OS due to few deaths. Furthermore, due to a limited number of patients and events, subset analyses were underpowered and predominantly univariate analyses were performed. We hope future studies in larger data sets can provide external validation.
In conclusion, our study demonstrates that measurement of bulky disease on coronal reformations in addition to standard transaxial measurements identifies additional patients with disease bulk, and our data suggest that these patients are potentially at increased risk of relapse when treated with chemotherapy alone versus combined modality therapy. Routine review of transverse and coronal reformats on staging CT examinations in ESHL is feasible and has clinical impact.
Acknowledgments
The authors would like to thank collaborators at Dana Farber Cancer Institute and Mayo Clinic for their important contributions toward validating this work.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/10/1237
Funding
This research was supported by the Lymphoma Research Foundation and NIH.
References
- 1.Mauch P, Goodman R, Hellman S. The significance of mediastinal involvement in early stage Hodgkin’s disease. Cancer. 1978;42(3):1039–1045. [DOI] [PubMed] [Google Scholar]
- 2.Schomberg PJ, Evans RG, O’Connell MJ, et al. Prognostic significance of mediastinal mass in adult Hodgkin’s disease. Cancer. 1984;53(2):324–328. [DOI] [PubMed] [Google Scholar]
- 3.Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J Clin Oncol. 1989;7(11):1630–1636. [DOI] [PubMed] [Google Scholar]
- 4.Eghbali H, Raemaekers J, Carde P, Group EL. The EORTC strategy in the treatment of Hodgkin’s lymphoma. Eur J Haematol. 2005;(Suppl):135–40. [DOI] [PubMed] [Google Scholar]
- 5.Engert A, Schiller P, Josting A, et al. Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2003;21(19):3601–3608. [DOI] [PubMed] [Google Scholar]
- 6.Meyer RM, Gospodarowicz MK, Connors JM, et al. Randomized comparison of ABVD chemotherapy with a strategy that includes radiation therapy in patients with limited-stage Hodgkin’s lymphoma: National Cancer Institute of Canada Clinical Trials Group and the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23(21):4634–4642. [DOI] [PubMed] [Google Scholar]
- 7.Hoppe RT, Advani RH, Ai WZ, et al. Hodgkin lymphoma, version 2.2012 featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10(5):589–597. [DOI] [PubMed] [Google Scholar]
- 8.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendenhall NP, Cantor AB, Barre DM, Lynch JW, Jr, Million RR. The role of prognostic factors in treatment selection for early-stage Hodgkin’s disease. Am J Clin Oncol. 1994;17(3):189–195. [DOI] [PubMed] [Google Scholar]
- 10.North LB, Fuller LM, Hagemeister FB, Rodgers RW, Butler JJ, Shullenberger CC. Importance of initial mediastinal adenopathy in Hodgkin disease. Am J Roentgenology. 1982;138(2):229–235. [DOI] [PubMed] [Google Scholar]
- 11.Bradley AJ, Carrington BM, Lawrance JA, Ryder WD, Radford JA. Assessment and significance of mediastinal bulk in Hodgkin’s disease: comparison between computed tomography and chest radiography. J Clin Oncol. 1999;17(8):2493–2498. [DOI] [PubMed] [Google Scholar]
- 12.Radford J, Illidge T, Counsell N, et al. Results of a Trial of PET-Directed Therapy for Early-Stage Hodgkin’s Lymphoma. N Engl J Med. 2015;372(17):1598–1607. [DOI] [PubMed] [Google Scholar]
- 13.Straus D, Pitcher BN, Kostakoglu L, et al. Initial Results of US Intergroup Trial of response-adapted chemotherapy or chemotherapy/radiation therapy based on PET for non-bulky Stage I and II Hodgkin lymphoma (HL) (CALGB/Alliance 50604). ASH Annual Meeting Abstracts. Blood. 2015;126(23):578. [Google Scholar]
- 14.Rosset A, Spadola L, Pysher L, Ratib O. Informatics in radiology (infoRAD): navigating the fifth dimension: innovative interface for multidimensional multimodality image navigation. Radiographics. 2006; 26(1):299–308. [DOI] [PubMed] [Google Scholar]
- 15.Mazumdar M, Glassman JR. Categorizing a prognostic variable: review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med. 2000;19(1):113–132. [DOI] [PubMed] [Google Scholar]
- 16.Gonen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92(4):965–970. [Google Scholar]
- 17.Klimm B, Goergen H, Fuchs M, et al. Impact of risk factors on outcomes in early-stage Hodgkin’s lymphoma: an analysis of international staging definitions. Ann Oncol. 2013;24(12):3070–6. [DOI] [PubMed] [Google Scholar]
- 18.Picardi M, De Renzo A, Pane F, et al. Randomized comparison of consolidation radiation versus observation in bulky Hodgkin’s lymphoma with post-chemotherapy negative positron emission tomography scans. Leuk Lymphoma. 2007;48(9):1721–1727. [DOI] [PubMed] [Google Scholar]
- 19.Sandrasegaran K, Rydberg J, Tann M, Hawes DR, Kopecky KK, Maglinte DD. Benefits of routine use of coronal and sagittal reformations in multi-slice CT examination of the abdomen and pelvis. Clin Radiol. 2007;62(4):340–347. [DOI] [PubMed] [Google Scholar]
- 20.Wei SC, Ulmer S, Lev MH, Pomerantz SR, Gonzalez RG, Henson JW. Value of coronal reformations in the CT evaluation of acute head trauma. Am J Neuroradiol. 2010; 31(2):334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behar RA, Horning SJ, Hoppe RT. Hodgkin’s disease with bulky mediastinal involvement: effective management with combined modality therapy. Int J Radiat Oncol Biol Phys. 1993;25(5):771–776. [DOI] [PubMed] [Google Scholar]
- 22.Hughes-Davies L, Tarbell NJ, Coleman CN, et al. Stage IA-IIB Hodgkin’s disease: management and outcome of extensive thoracic involvement. Int J Radiat Oncol Biol Phys. 1997;39(2):361–369. [DOI] [PubMed] [Google Scholar]
- 23.Leopold KA, Canellos GP, Rosenthal D, Shulman LN, Weinstein H, Mauch P. Stage IA-IIB Hodgkin’s disease: staging and treatment of patients with large mediastinal adenopathy. J Clin Oncol. 1989;7(8):1059–1065. [DOI] [PubMed] [Google Scholar]
- 24.Raemaekers JM, Andre MP, Federico M, et al. Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: Clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2014;32(12):1188–1194. [DOI] [PubMed] [Google Scholar]
- 25.Meyer RM, Gospodarowicz MK, Connors JM, et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin’s lymphoma. N Engl J Med. 2012;366(5):399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Straus DJ, Portlock CS, Qin J, et al. Results of a prospective randomized clinical trial of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) followed by radiation therapy (RT) versus ABVD alone for stages I, II, and IIIA nonbulky Hodgkin disease. Blood. 2004;104(12):3483–3489. [DOI] [PubMed] [Google Scholar]
- 27.Savage KJ, Connors JM, Villa DR, et al. Advanced Stage Classical Hodgkin Lymphoma Patients with a Negative PET-Scan Following Treatment with ABVD Have Excellent Outcomes without the Need for Consolidative Radiotherapy Regardless of Disease Bulk at Presentation Blood (ASH Annual Meeting Abstracts). 2015;126. [Google Scholar]


