Abstract
Donor lymphocyte infusions are used to treat relapse after allogeneic hematopoietic stem cell transplantation, but responses are inadequate. In addition to effector cells, infusions contain CD25+ regulatory T cells (Treg) that may suppress graft-versus-tumor responses. We undertook a phase I study of donor lymphocyte infusions depleted of CD25+ T cells in patients with hematologic malignancies who had relapsed after transplantation. Twenty-one subjects received CD25/Treg-depleted infusions following removal of CD25+ cells using antibody-conjugated magnetic beads. Sixteen subjects received prior cytoreductive therapy. Four were in complete remission at the time of infusion. Two dose levels were administered: 1×107 (n=6) and 3×107 CD3+ cells/kg (n=15). A median 2.3 log-depletion of CD4+CD25+FOXP3+ Treg was achieved. Seven subjects (33%) developed clinically significant graft-versus-host disease by 1 year, including one patient who died. At dose level 1, five subjects had progressive disease and one had stable disease. At dose level 2, nine subjects (60%) achieved or maintained responses (8 complete responses, 1 partial response), including seven with active disease at the time of infusion. A shorter period between relapse and infusion was associated with response at dose level 2 (P=0.016). The 1-year survival rate was 53% among patients treated with dose level 2. Four of eight subjects with acute myeloid leukemia remained in remission at 1 year. When compared to unmodified donor lymphocyte infusions in 14 contemporaneous patients meeting study eligibility, CD25/Treg depletion was associated with a better response rate and improved event-free survival. Circulating naïve and central memory CD4+ T cells increased after CD25/Treg-depleted infusion, but no immunophenotypic signature for response was noted. CD25/Treg-depleted donor infusion appears feasible and capable of inducing graft-versus-tumor responses without excessive graft-versus-host disease. (ClinicalTrials.gov NCT#00675831)
Introduction
Patients with a hematologic malignancy who relapse after allogeneic hematopoietic stem cell transplantation (HSCT) have a dismal prognosis. Re-establishing disease control through donor lymphocyte infusion (DLI) is one accepted approach, as this can invoke graft-versus-tumor effects mediated by recognition of minor histocompatibility antigens and tumor antigens on malignant cells.1 DLI has been successful in relapsed chronic myeloid leukemia with greater than 70% sustained response rates.2,3 Its efficacy in other diseases is limited by two factors. First, the potency and durability of the graft-versus-tumor effects vary, with response rates in diseases other than chronic myeloid leukemia being much lower.4–6 Initial responses to DLI can be as low as 15–29% in acute myeloid leukemia and 5–27% in acute lymphoid leukemia, with a median time of 10 months to leukemia recurrence.3,7,8 Second, DLI is often accompanied by toxicity from graft-versus-host disease (GvHD). The incidence of GvHD varies depending on the DLI cell dose, degree of HLA mismatch, and use of concurrent immunosuppression but ranges from 25% to 70% after DLI from fully HLA-matched related or unrelated donors.9–12 Strategies to enhance the efficacy of graft-versus-tumor effects after DLI have included ex vivo activation of effector cells with CD3/CD28-coated beads; incubation with interferon-γ, interleukin-2, and anti-CD3 to produce “cytokine-induced killer cells”; or incubation with interleukin-2 alone. These approaches have resulted in disappointing efficacy and/or unacceptable rates of GvHD.13–16
CD4+CD25+FoxP3+ regulatory T cells (Treg) account for ~5% to 10% of circulating CD4+ T cells in healthy individuals, can dominantly suppress auto-reactive T, B and natural killer effectors, and control innate and adaptive immune responses.17,18 In vitro Treg suppress proliferation of CD4+ and CD8+ T cells after polyclonal or antigenic stimulation.19 In vivo Treg can impede immune responses to solid and liquid tumors.20–23 They also play a role in alleviating GvHD as demonstrated by the inverse correlation between circulating Treg and the onset and severity of GvHD.24–28 Surprisingly, adoptive transfer or in vivo expansion of Treg improves GvHD without jeopardizing graft-versus-tumor effects.29–32
We hypothesized that, elimination of CD25+ cells, including CD25-expressing Treg, from DLI products by selective depletion of CD25+ cells might boost anti-tumor efficacy, with the risk that potency gained could be offset by worsened GvHD. The feasibility of large-scale CD25/Treg depletion from apheresis products has previously been demonstrated using bead-bound anti-CD25 antibodies and magnetic separation.33 We evaluated the feasibility and safety of administering CD25/Treg-depleted DLI to subjects with a hematologic malignancy who had relapsed after allogeneic HSCT, a population of patients for whom there are few effective therapeutic options.
Methods
This phase I, dose-escalation trial was approved by Dana-Farber/Harvard Cancer Center Institutional Review Board (ClinicalTrials.gov NCT#00675831). Its primary objectives were to determine the feasibility and safety of depleting CD25+ cells from leukapheresis products. The secondary objective was to assess response to the depleted DLI.
Patients’ characteristics
Eligible subjects were ≥18 years old, with relapsed hematologic malignancies (other than those with stable-phase chronic myeloid leukemia), ≥2 months after HLA –A, -B, -C and -DRB1-matched donor allogeneic HSCT with donor total leukocyte chimerism ≥20%, and disease involving ≤50% bone marrow cellularity and/or lymph nodes ≤5 cm. The subjects had been off systemic immune suppression for ≥2 weeks, without active GvHD, and had not received chemotherapy (except hydroxyurea) within 4 weeks or immunotherapy within 8 weeks of the DLI.
Donor leukocyte infusion
Original stem cell donors underwent one or two leukaphereses without growth-factor stimulation. If twice the CD3+ cell/kg target dose for infusion was not met, an unmanipulated DLI was administered. Planned dose levels were 1×107 and 3×107 CD3+ cells/kg; a lower dose of 1×106/kg was also envisaged if toxicities were encountered. CD25/Treg depletion was achieved using the CliniMACS CD25 Reagent System according to the manufacturer’s instructions (Miltenyi Biotec, Cambridge, MA, USA; IDE 13423). Release criteria included CD3+ cell dose, depletion of CD4+CD25high cells to ≤0.5%, and ≥70% viability. Standard methods were used for the immunophenotypic analyses of DLI products and recipients’ peripheral blood before and after CD25/Treg-depleted DLI (Online Supplementary Methods).
Study design and definitions
CD25/Treg-depleted DLI entailed a single infusion of fresh cells immediately following selection. Recipients not requiring chemotherapy within 8 weeks after DLI were evaluable for dose-limiting toxicities: grade III-IV acute GvHD, severe pancytopenia, or DLI-related CTCAE ≥ grade 3 toxicities unrelated to progressive disease or GvHD. Acute GvHD was assessed using consensus criteria, while chronic GvHD was graded “limited” or “extensive”.34,35 A complete response consisted of resolution of histological/radiological evidence of disease and chromosomal abnormalities (acute leukemia). A partial response was defined by ≥50% reduction, stable disease by <50% reduction or <50% increase, and progressive disease by ≥50% increase of lymph node or bone marrow disease burden.
Exploratory contemporaneous comparator cohort
In an exploratory analysis, a contemporaneous comparator cohort of patients from the Dana-Farber Harvard Cancer Center was identified. This cohort comprised 14 patients with relapsed hematologic malignancy (other than those with stable phase chronic myeloid leukemia) after HLA-matched HSCT, with ≥20% donor chimerism, who received unmanipulated DLI with 2–4×107 CD3+ cells/kg (dose level 2), and who were not on study secondary to the patients’ preference or logistical challenges.
Statistical analyses
Descriptive statistics were used for patient- and transplant-related characteristics. Fisher exact test, the χ2 test, or Wilcoxon-rank-sum tests were used for cohort comparisons. Overall survival and event-free survival were estimated using the Kaplan-Meier method. Differences in survival curves between cohorts were tested using the log-rank test. Cumulative incidences of non-relapse mortality and relapse/progression were constructed in a framework of competing risks and differences evaluated using the Gray test.36 Repeated measures analysis was performed for effect of time and response on immunophenotypic profiles. Immunophenotypic parameters were log- or square-root transformed to meet the normality assumption prior to modeling. Multiplicity was adjusted within each model. P-values were two-sided with a significance level of 0.05. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC, USA) and R version 2.15.12 (the CRAN project).
Results
Feasibility
Between May, 2008 and October, 2011, 24 subjects were enrolled and evaluable for feasibility. Two subjects received unmanipulated DLI because of an insufficient CD3+ cell collection from the donor after two leukaphereses. One subject developed GvHD shortly after enrollment, so DLI was not pursued. Of the 21 CD25/Treg-depleted products administered, 20 were collected in a single leukapheresis. The median CD3+ recovery after CD25 depletion was 73.3% (range, 46.1 – 107.7%) (Table 1). The median log-depletion of CD4+CD25high cells was 2.46 (range, 0.83 – 3.37), while that of CD4+CD25+FoxP3+ Treg was 2.28 (range, 0.73–3.33) (Figure 1). A similar log-depletion (median 2.32) of Treg, based on CD4+CD25+CD127low surface expression, was achieved. At dose level 2, the median number of residual Treg infused was 4.24 × 103/kg (range, 0.64 × 103/kg to 95.66 × 103/kg).
Table 1.
Efficiency of processing and CD25/Treg depletion.
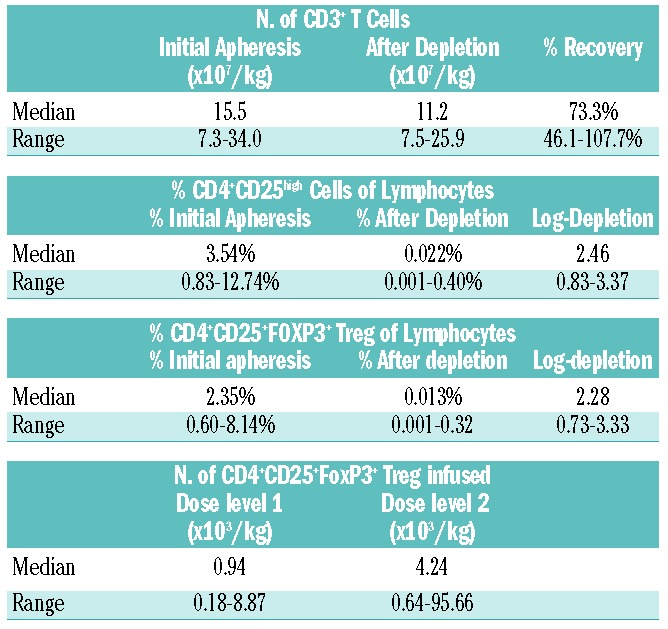
Figure 1.
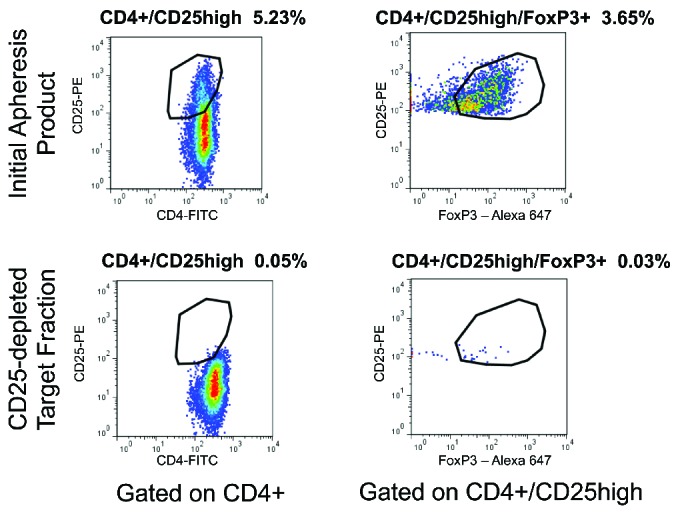
Flow cytometric analysis of CD4+CD25+FoxP3+ cells confirms Treg depletion. The initial apheresis product (top plots) and target fraction after CD25 depletion using the CliniMACS system (bottom plots) were subjected to extracellular staining for CD4 and CD25 expression and intracellular staining for FoxP3 protein. As shown for a representative DLI product, gating on CD45+CD4+ cells demonstrated reduction in cells expressing high levels of CD25 (left plots). Gating on CD4+CD25high cells demonstrated reduction in cells expressing FoxP3 (right plots) after the depletion process.
Subjects receiving CD25/Treg-depleted donor lymphocyte infusions
The median age of the 21 subjects evaluable for safety and efficacy was 42 years (range, 19–71 years) (Table 2). The majority (n=15, 71%) had relapsed acute myeloid leukemia, acute lymphoblastic leukemia, or myelodysplastic syndrome. Thirteen subjects (62%) received cells from matched siblings, eight from matched unrelated donors. All had received peripheral blood stem cell grafts; one was CD34-selected. Eleven subjects (52%) received myeloablative conditioning; ten received reduced-intensity conditioning. Two subjects (9.5%) had prior grade I acute GvHD and four subjects (19.0%) had prior chronic GvHD after HSCT but did not have symptoms at the time of the DLI. The median time from HSCT to relapse was 6.5 months (range, 3 – 30 months), while the median time from relapse to DLI was 2.8 months (range, 1 – 15 months).
Table 2.
Demographics of the study subjects.
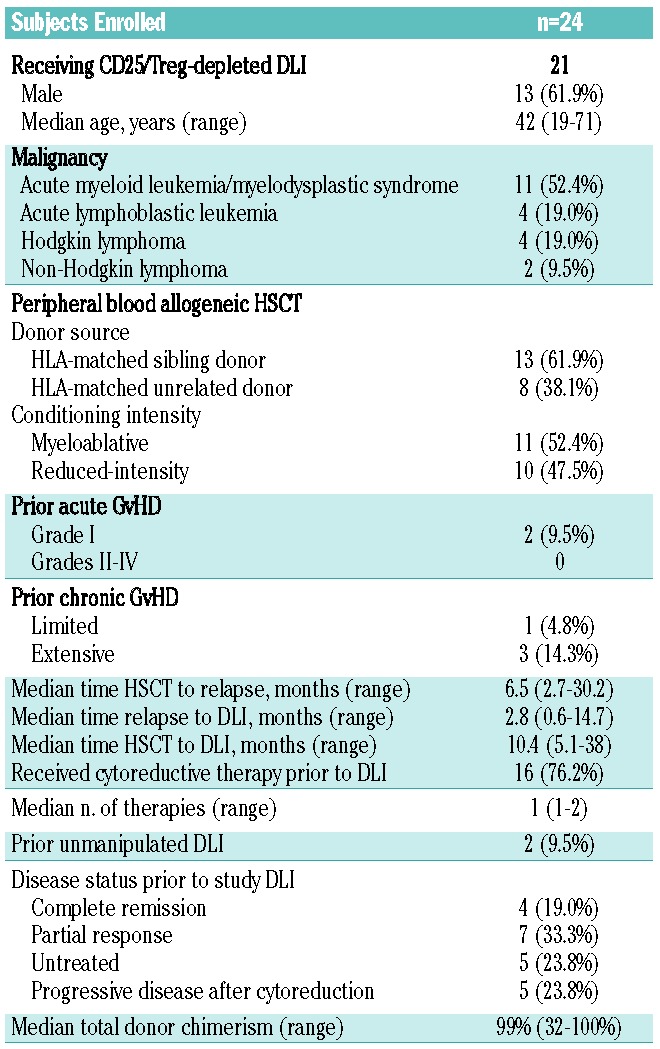
Sixteen (76%) subjects received cytoreductive therapy after discontinuation of immune suppression at the discretion of the treating clinician, all more than 4 weeks prior to DLI. The median number of therapies after relapse in these 16 subjects was one (range, 1 – 2). Two subjects, one with Hodgkin lymphoma and one with acute myeloid leukemia, had received prior unmanipulated DLI without achieving a sustained response. Four (19%) subjects were in complete response and seven (33%) in partial response prior to DLI. Five (24%) had morphological disease without receipt of cytoreductive therapy since relapse, and five (24%) had progressive/persistent disease despite therapy. The median total leukocyte donor chimerism was 99%, (range, 32% –100%) (Online Supplementary Table S1). The median follow-up among survivors was 44 months (range, 26 – 59 months).
Safety and toxicities
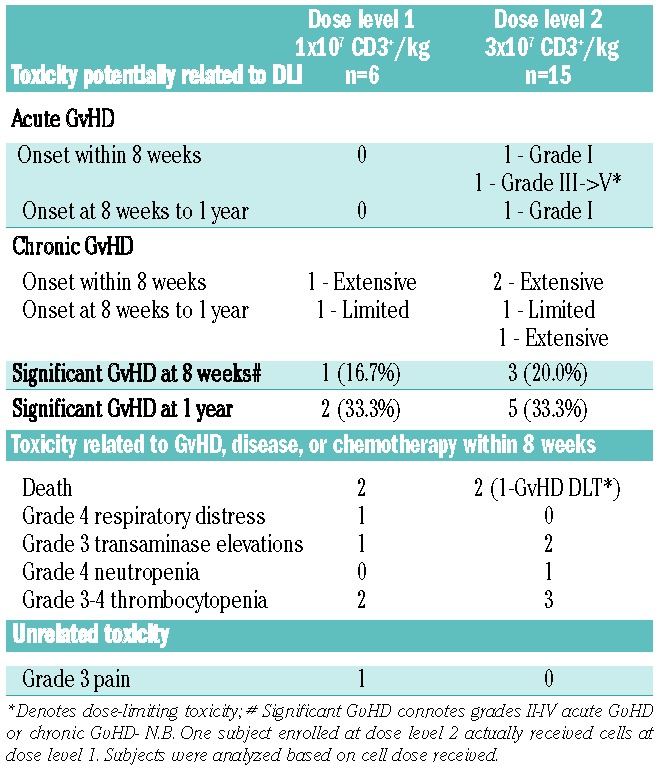
No significant infusion reactions occurred. Five subjects were enrolled (6 treated) at dose level 1 (1×107 CD3+ cells/kg recipient). Given that no significant adverse events occurred at this dose, escalation to dose level 2 (3×107 CD3+ cells/kg) was allowed for the subsequent 15 subjects. Three subjects with acute myleloid leukemia and two with acute lymphoblastic leukemia required cytotoxic therapy for disease progression within 8 weeks of DLI (n=2, dose level 1; n=3, dose level 2). The maximum-tolerated dose was not reached. One severe adverse event was observed in the first 8 weeks: namely, grade III acute GvHD (stage 2 gastrointestinal) 25 days after DLI at dose level 2, eventually leading to death (Table 3). There were two cases of grade I acute GvHD at dose level 2: one in the first 8 weeks, and one between 8 weeks and 1 year. Three subjects developed extensive chronic GvHD within 8 weeks following DLI: one at dose level 1 and two at dose level 2. After 8 weeks, three additional cases of chronic GvHD developed, yielding observed rates of significant GvHD of 19% (n=4) at 8 weeks and of 33% (n=7) at 1 year. All other adverse events observed, namely respiratory distress, transaminase elevations, cytopenias, or death were deemed related to GvHD, disease progression, subsequent cytoreductive therapy, or unrelated rather than secondary to CD25/Treg-depleted DLI.
Table 3.
Toxicity after CD25/Treg-depleted DLI.
Efficacy
Twenty-one subjects were evaluated for response at 8 weeks. Only those with evidence of relapse/disease progression received additional therapy following DLI. At dose level 1, four (67%) of six subjects were alive 8 weeks after DLI, but all had persistent or progressive disease (Table 4). At 1 year, two (33%) of those six subjects remained alive. At dose level 2, 13 (87%) of 15 subjects were alive 8 weeks after DLI. One succumbed to acute GvHD and one to disease progression. Eight subjects at dose level 2 had a complete response (53%), for an overall response (complete responses + partial responses) rate of 60%. Responses were observed in five subjects with acute myeloid leukemia, one with acute lymphoblastic leukemia, one with acute lymphoblastic leukemia, two with Hodgkin lymphoma and one with non-Hodgkin lymphoma (Online Supplementary Table S1).
Table 4.
Response to CD25/Treg-depleted DLI.
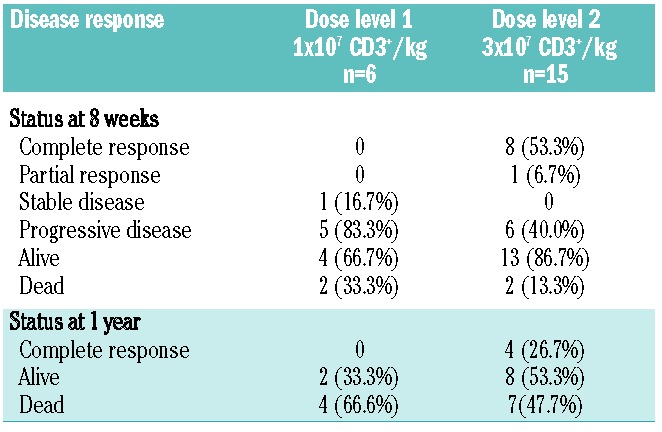
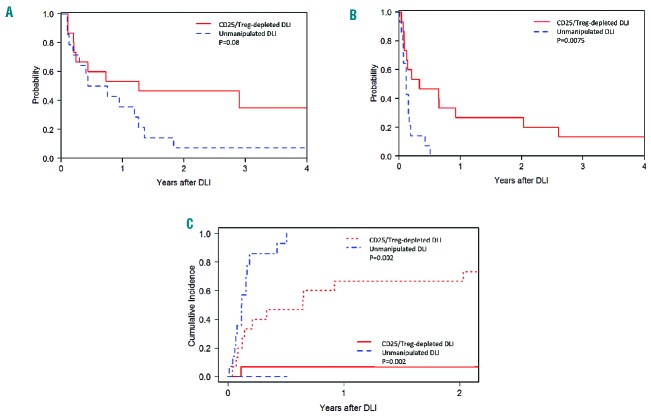
At 1 year, eight (53%) subjects receiving dose level 2 were alive, and four remained in complete remission. At dose level 2, the planned phase II dose, estimated 1-year overall and event-free survival rates were 53% (95% CI, 26–74) and 27% (95% CI, 8–50), respectively. The 1-year cumulative incidences of relapse and non-relapse mortality were 67% (95% CI, 35–86) and 7% (95% CI, 0.4–27), respectively (Figure 2 and Table 5). The median survival time was 8.7 months (range, 1.3 months - not reached) for all subjects and 12.7 months (range, 1.3 months - not reached) at dose level 2. Salvage therapies after CD25/Treg-depleted DLI included second CD25/Treg-depleted DLI outside of the current protocol (n=1), unmanipulated DLI (n=1), subsequent HSCT (n=2), and antibody-drug conjugate therapy.
Figure 2.
Clinical outcomes after CD25/Treg-depleted DLI at dose level 2. (A) Kaplan Meier curves demonstrating overall survival after infusion for recipients of CD25/Treg-depleted DLI at 3×107 CD3+/kg (solid line) versus unmanipulated DLI at cell doses of 2.2–3.7×107 CD3+/kg (dotted line). (B) Kaplan Meier curves demonstrating improved event-free survival after CD25/Treg-depleted DLI (solid line) versus unmanipulated DLI (dotted line). (C) Relapse and non-relapse mortality: plot displaying cumulative incidences of disease relapse and non-relapse mortality after CD25/Treg-depleted DLI (solid and fine dotted lines, respectively) versus unmanipulated DLI (hashed and coarse dotted lines, respectively).
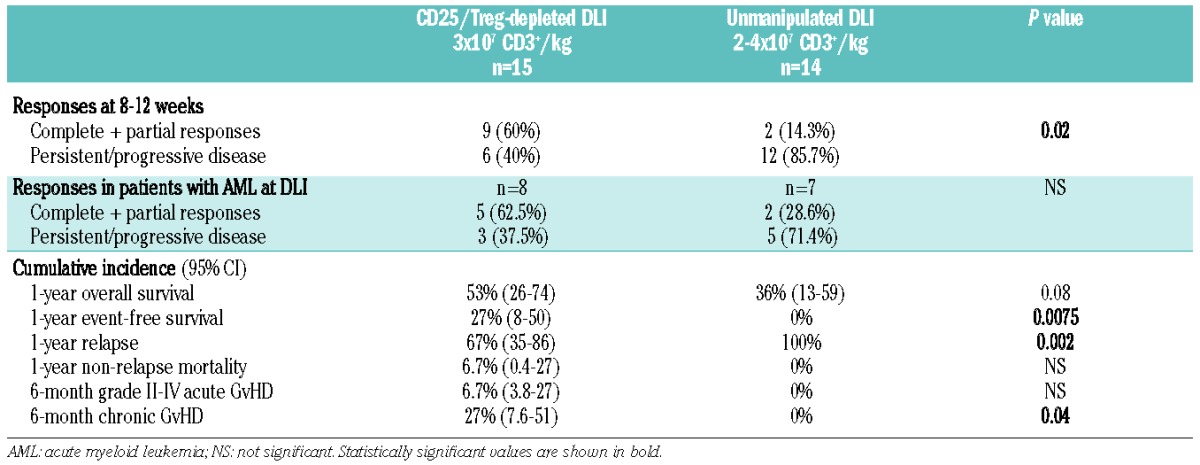
Table 5.
Outcomes of Treg-depleted DLI compared to unmanipulated DLI.
The only clinical characteristic associated with response was time from relapse to DLI, with a median time of 2.7 months (range, 1.4 – 4.7 months) in responders and 5.4 months (range, 1.9 – 14.5 months) in non-responders (P=0.016). Development of GvHD after DLI was not a prerequisite for response; four of nine subjects with an initial response and two of four with sustained complete responses never developed GvHD. Entering DLI in remission was also not necessary: six subjects with disease at the time of DLI achieved a complete response at 8 weeks and three sustained those responses for at least 1 year. All four subjects remaining in complete response at 1 year had prior acute myeloid leukemia. Neither of the two subjects who had undergone prior unmanipulated DLI responded to CD25/Treg-depleted DLI. None of the other clinical factors, including time from HSCT to relapse or DLI, conditioning intensity of prior HSCT, donor chimerism, or receipt of disease-directed chemotherapy prior to DLI, correlated with response.
Comparison to unmanipulated donor lymphocyte infusion
In an exploratory analysis, we collected baseline data on expected toxicity (i.e., subsequent GvHD and non-relapse mortality) and efficacy of unmodified DLI at our center in a contemporaneous cohort of patients. Fifty patients whose donor chimerism was ≥20% received unmanipulated DLI for relapse between November 2006 and May 2011 after undergoing an 8/8 HLA-matched HSCT. Of these 50, 36 received 1×107 CD3+ cells/kg and 14 received 2–4×107 CD3+ cells/kg. To provide an exploratory baseline for subjects receiving dose level 2 of CD25/Treg-depleted DLI, we included the 14 patients receiving 2–4×107 CD3+ cells/kg as a comparator cohort. This cohort included two subjects initially enrolled on study who received unmanipulated DLI given the numbers of donor T cells collected. The age and gender distribution of the comparator cohort was similar to that of the study population, without significant differences in type of malignancy, donor source, prior GvHD incidence, transplant characteristics, time from HSCT to relapse or DLI, receipt of disease-directed therapy prior to DLI, or chimerism at the time of DLI (Online Supplementary Table S2). Five patients (36%) were in complete remission, two in partial remission, and seven (50%) had untreated/progressive disease at the time of the unmanipulated DLI. The median DLI cell dose received was 3×107 CD3+ cells/kg (range, 2.2 × 107 – 3.7 ×107 cells/kg).
Acknowledging caveats of comparing small, heterogeneous cohorts, specifically limited statistical power and selection bias, the response rate after unmanipulated DLI was 14% at 8–12 weeks, lower than the 60% response rate seen after CD25/Treg-depleted DLI at dose level 2 (P=0.02). There were two complete responses, both in recipients with acute myeloid leukemia, which lasted 5.1 and 6.1 months (Online Supplementary Table S3). The 1-year event-free survival rate of this cohort was 0% as compared with 27% in the CD25/Treg-depleted DLI cohort (P=0.0075), the 1-year cumulative incidence rate of relapse was 100% as compared to 67% (P=0.002), and the 1-year overall survival rate was 36% as compared to 53% in the CD25/Treg-depleted DLI cohort (P=0.08). Cumulative incidences of non-relapse mortality at 1 year and acute GvHD at 6 months were not different. Given the early deaths from disease seen after unmanipulated DLI, the difference in incidence of chronic GvHD noted was not deemed clinically relevant (Table 5 and Figure 2).
Immunological effects of CD25/Treg-depleted donor lymphocyte infusions
In the first 2 months after CD25/Treg-depleted DLI, there were no significant changes from prior to DLI in white blood cell count, absolute lymphocyte count, percentages and numbers of circulating peripheral CD3+, CD4+, and CD8+ T cells, Treg and CD20+ B cells, or in Treg to conventional T-cell (Tcon) ratios when analyzing all subjects (Online Supplementary Figure S1 and data not shown). Median CD8:CD4 ratios increased at 1 month (median, 0.74 to 1.06; P=0.013), but this difference was not maintained at 2 months. There were significant drops from baseline in circulating natural killer cells (median, 201.5 to 111.6 to 86.0/μL; P=0.027, 1 month; P=0.002, 2 months) and dendritic cells (median, 301.1 to 99.7 to 17.4/μL; P=0.029, 1 month; P=0.0002, 2 months), which were no different between those who responded to DLI and those who did not. Thus, CD25/Treg-depleted DLI did not notably facilitate numerical expansion of early total lymphocytes, Tcon, B cells, or natural killer cells.
There were qualitative shifts within the naïve and memory compartments of circulating CD4+ T cells after CD25/Treg-depleted DLI (Figure 3A). The percentage of CD4+ naïve T cells increased by 1 month (median, 11.0% to 24.1%; P=0.008) and remained above baseline at 2 months (median, 21.7%; P=0.0005). The percentage of CD4+ terminal effectors decreased by 1 month (median, 12.8% to 8.6%; P=0.053) and remained below baseline at 2 months (median, 2.9%; P=0.003). Increases in CD4+ central memory cells at 2 months (P=0.004) and decreases in CD4+ effector memory cells at 1 and 2 months after DLI (P=0.04 and P=0.0007, respectively) were statisticially significant. Identical shifts were seen in CD8+ T cells: namely, increases in naïve cells (P=0.0006 and P=0.0004), decreases in terminal effectors (P=0.04 and P=0.03), increases in central memory cells (P=0.004 and P=0.004), and decreases in effector memory cells (P=0.01 and P=0.004) at 1 and 2 months after DLI, respectively (Figure 3B). In these analyses after DLI, no differences between responder and non-responder populations were noted, perhaps because of the limited sample size. Similar analyses were not available for bone marrow cell subsets or for patients receiving unmanipulated DLI.
Figure 3.
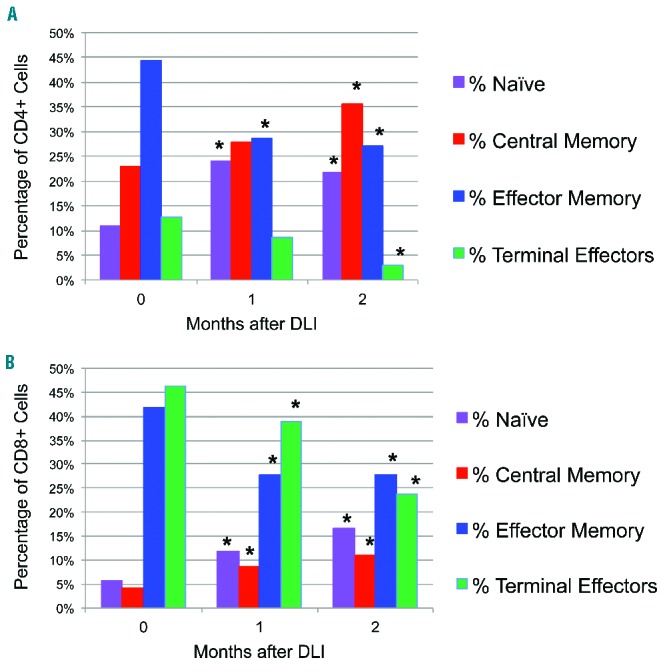
Naïve and central memory cells increase within CD4+ and CD8+ T-cell compartments after CD25/Treg-depleted DLI. Peripheral blood mononuclear cells drawn at indicated times were stained for extracellular markers and analyzed by flow cytometry. (A) Changes in median distribution of CD4+ T cells among all subjects: the percentage of CD3+CD4+ T cells in each subset just prior to (0 months) or in the first 2 months after DLI is indicated. Panels reflect percentage of naïve cells (CD45RO-CD62L+), central memory cells (CD45RO+CD62L+), effector memory cells (CD45RO+CD62L−), and terminal effectors (CD45RO−CD62L−). *Indicates P<0.05 for the comparison between baseline (month 0) and the indicated time point. (B) Changes in median distribution of CD8+ T cells. Individual CD8+ subsets and significant differences among all subjects after CD25/Treg-depleted DLI are displayed as detailed above.
When examining immunological profiles prior to DLI, there were no correlations between response and circulating lymphocyte subset values including CD4+ or CD8+ T-cell counts, CD4+ Tcon or Treg counts, or CD8:CD4 ratios (data not shown). The ratio of peripheral blood Treg to Tcon prior to DLI, the Treg:Tcon ratio in the DLI product itself, and the number of residual Treg infused had no correlation with disease response (P=0.76, P=0.41, and P=0.24, respectively); this was the case regardless of whether all subjects or only those treated with dose level 2 were analyzed.
Discussion
Given the historically poor efficacy of unmanipulated DLI in treating relapse of hematologic malignancies other than chronic myeloid leukemia after HSCT, we investigated whether ex vivo depletion of CD25+ cells including suppressive Treg from DLI products was feasible, safe, and could enhance anti-tumor activity without increasing GvHD. Twenty-one of 23 (91.3%) donors yielded sufficient CD3+ cells for selection and DLI, 20 (87.0%) in one leukapheresis. Given that only two donors did not yield sufficient T cells for CD25 depletion, it is unlikely that the results discussed below are a result of selection bias for “optimal” donors from among the donor pool. The median CD3+ T-cell recovery was 73% after depletion. Thus, it may be possible to target higher doses in future studies, at least for related donors for whom multiple leukaphereses are more practical. This approach proved feasible with a median 2.3 log-depletion of FoxP3+ Treg, which is comparable to efficiencies obtained in prior studies.33
We refer to CD25-depleted products as “Treg-depleted”. CD25 (interleukin-2 receptor-α) is also expressed on activated CD4+ Tcon and CD8+ T cells, so the selection process could affect these populations as well. Depletion of CD25+ donor Tcon cells already activated in vivo at the time of leukapheresis might potentially decrease non-specific alloreactivity but would not be expected to improve graft-versus-tumor effects. We, therefore, postulate that our observed results, particularly as regards efficacy, are primarily a result of Treg depletion, a concept that has support from animal models.23 Extensive phenotyping of the DLI product with characterization of CD25+ cells depleted/retained during processing was not pursued in this phase I study. This limitation could, however, be addressed in future trials which should include characterization of Treg phenotype (e.g., HLA-DR, CTLA-4, Lag-3, CD45RA, Ki-67 expression), suppressive activity, and markers of thymic activity.
Regarding safety, there were no infusion reactions. Subsequent cytopenias and adverse events were no more frequent or severe than expected after unmanipulated DLI. Apart from one case of acute GvHD progressing to death, CD25/Treg-depleted DLI was well-tolerated, and overall GvHD rates were not excessive. Comparison to a limited contemporaneous cohort receiving unmanipulated DLI at a study-equivalent dose at our center showed no significant difference in the incidence of acute GvHD, although small sample sizes limit the statistical robustness of this comparison. However, our 33% observed rate of GvHD requiring systemic therapy after 3×107 CD3+/kg CD25-Treg-depleted DLI compares favorably with other studies of unmanipulated DLI at similar doses, e.g. 45% in a large retrospective analysis by Bar et al., as does our 1-year non-relapse mortality rate of 7%.10 Importantly, prior GvHD does not appear a contraindication to receiving CD25/Treg-depleted DLI, although active GvHD at the time of enrollment was an exclusion criterion.
Regarding efficacy, over 70% of study subjects had acute leukemia or myelodysplastic syndrome, diseases with historically poor response rates and remission durability. The 8-week response rate of 60% at dose level 2 (3×107 CD3+ cells/kg) and the 27% 1-year event-free survival rate, while still leaving much room for improvement, were higher than seen in a contemporaneous group of patients receiving unmanipulated DLI in our exploratory analysis. However, the implications of these comparisons are limited by small sample size, clinical heterogeneity and potential selection bias, and should be confirmed in subsequent prospective trials. The 1-year survival of 53% in the CD25/Treg-depleted study group was in the range of 17–67% survival seen at similar cell doses in other studies, although historical comparisons are also limited by variations in disease distribution, disease status, prior cytoreduction, chimerism, mobilization of the DLI product, and year of DLI.9,10,37
Six of the nine responses were in study subjects with acute leukemia, and four of those six responses were sustained for a year. The observed response rate of 63% and 1-year overall survival of 50% in patients with acute myeloid leukemia/myelodysplastic syndrome at dose level 2 was surprising, particularly as all but one patient had active disease at the time of DLI. After unmanipulated DLI, albeit in a small group, of seven patients with acute myeloid leukemia only two had complete responses, both of whom relapsed within 6 months or less. Findings after CD25/Treg-depleted DLI compare favorably with 2-year survival rates of 56% for DLI in complete response and 15% for DLI with active disease in an European Group for Blood and Marrow Transplantation study devoted to relapse of acute myeloid leukemia after HSCT.7 Responses in our study were not obviously dependent on occurrence of GvHD after DLI, indicating the potential for immunologically separating graft-versus-tumor effects from GvHD, even in subjects with relapsed acute myeloid leukemia, through manipulation of the composition of the DLI.
The numbers and profiles of circulating lymphocyte numbers either before or after CD25/Treg-depleted DLI did not correlate with disease response in this study population. However, significant increases in CD4+ and CD8+ naïve and central memory cells and decreases in CD4+ and CD8+ effector memory and terminal effector cells were seen. Recovery of naïve and central memory cells typically indicates improved thymopoiesis and T-cell diversity and has been associated with decreased relapse and increased survival after HSCT.38,39 Whether this normalization of naïve, memory, and effector-cell distribution is augmented by CD25/Treg depletion could not be determined in this single-arm study. We did not see a correlation between responses and pre-existing CD8+ T-cell counts as previously noted in bone marrow after CD4+ DLI in myeloma and chronic myeloid leukemia, in which donor lymphoid expansion and reversal of residual exhausted T cells were demonstrated, respectively.40,41 However, the current analysis was restricted to early time points only after CD25/Treg-depleted DLI and to peripheral blood samples, not bone marrow. Identifying a cellular mechanism specific to responses after CD25/Treg-depleted or other DLI products will require prospective monitoring of bone marrow and sites of disease rather than just peripheral blood. Characterization of markers of activation and exhaustion such as PD-1 as well as detection of T-cell frequencies against various tumor antigens could be considered.
The impacts of this study are two-fold. First, we confirm that CD25/Treg-depleted DLI is feasible and safe and may be modestly more effective than unmanipulated DLI, particularly in acute myeloid leukemia. Larger numbers of patients will be needed to confirm a disease-specific effect.42 Previously, Maury et al. reported two responses to one infusion of Treg-depleted DLI and four more responses after a second infusion in 17 subjects with relapse after HSCT. In that study, 16 subjects had previously failed standard DLI, and responses were only seen following development of GvHD. Response rates were increased after chemotherapy-induced lymphodepletion was introduced.43 In our study, we targeted a different population, namely subjects early after relapse (19 of 21 without prior DLI), without pre-DLI lymphodepletion, and observed a 60% response rate and responses that lasted at least 1 year in 50% of initial complete responders (n=4). Whether the durability of response can be prolonged by multiple infusions is unknown. The likelihood of clinical response was associated with shorter time from relapse to DLI. Our responses were not stringently linked with GvHD and broaden the application of CD25/Treg-depleted DLI to use earlier in relapse after HSCT.
Secondly, our data support the concept that manipulating the composition of stem cell and other cellular therapies has the potential to improve efficacy and reduce toxicity.44,45 In this vein, trials of ex vivo depletion of naïve T cells from stem cell grafts to reduce GvHD and preserve anti-tumor and anti-infectious activity are underway.46,47 Analogous to the introduction of immune check-point inhibitors to boost activity of otherwise ineffective lymphocytes, modulation of the stimulatory versus suppressive T-cell balance within cellular therapy products and their recipients holds promise.48,49
Significant caveats in interpreting suggestions of efficacy are the small sample size and heterogeneity in this phase I study. Full details for each patient are laid out in the Online Supplementary Tables, but retrospective comparison to a small, diverse comparator group in our secondary analysis cannot take the place of a randomized study in which disease burden, molecular risk subtype, prior therapies, and time from chemotherapy to DLI are rigorously controlled between recipients of unmanipulated and CD25/Treg-depleted DLI. The comparative benefit of CD25-Treg depletion in specific disease groups such as acute myeloid leukemia and the role of lymphodepleting chemotherapy just prior to DLI administration will be addressed in future trials.50
In conclusion, depletion of CD25+ Treg from DLI is feasible, well-tolerated, and may increase efficacy when compared to unmanipulated DLI. Future avenues of research include increasing cell doses or giving multiple infusions of CD25/Treg-depleted donor lymphocytes, prospective randomized evaluation in relapsed acute leukemia versus unmanipulated DLI, and combining CD25/Treg-depleted DLI with novel immunomodulatory agents. Characterization of lymphocytes within active DLI products and at sites of disease response may lead to cellular manipulations capable of dissociating graft-versus-tumor from GvHD activity in the currently bleak situation of relapse after HSCT.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/101/10/1251
References
- 1.Horowitz M, Gale R, Sondel P, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–562. [PubMed] [Google Scholar]
- 2.Kolb H, Schattenberg A, Goldman J, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86(5):2041–2050. [PubMed] [Google Scholar]
- 3.Porter DL, Collins RH, Jr, Shpilberg O, et al. Long-term follow-up of patients who achieved complete remission after donor leukocyte infusions. Biol Blood Marrow Transplant. 1999;5(4):253–261. [DOI] [PubMed] [Google Scholar]
- 4.Collins RH, Shpilberg O, Drobyski WR, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15(2):433–444. [DOI] [PubMed] [Google Scholar]
- 5.Beitinjaneh AM, Saliba R, Bashir Q, et al. Durable responses after donor lymphocyte infusion for patients with residual multiple myeloma following non-myeloablative allogeneic stem cell transplant. Leuk Lymphoma. 2012;53(8):1525–1529. [DOI] [PubMed] [Google Scholar]
- 6.Campregher PV, Gooley T, Scott BL, et al. Results of donor lymphocyte infusions for relapsed myelodysplastic syndrome after hematopoietic cell transplantation. Bone Marrow Transplant. 2007;40(10):965–971. [DOI] [PubMed] [Google Scholar]
- 7.Schmid C, Labopin M, Nagler A, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007;25(31): 4938–4945. [DOI] [PubMed] [Google Scholar]
- 8.El-Jurdi N, Reljic T, Kumar A, et al. Efficacy of adoptive immunotherapy with donor lymphocyte infusion in relapsed lymphoid malignancies. Immunotherapy. 2013;5(5):457–466. [DOI] [PubMed] [Google Scholar]
- 9.Tomblyn M, Lazarus HM. Donor lymphocyte infusions: the long and winding road: how should it be traveled? Bone Marrow Transplant. 2008;42(9):569–579. [DOI] [PubMed] [Google Scholar]
- 10.Bar M, Sandmaier BM, Inamoto Y, et al. Donor lymphocyte infusion for relapsed hmatological malignancies after allogeneic hematopoietic cell transplantation: prognostic relevance of the initial CD3+ T cell dose. Biol Blood Marrow Transplant. 2013;19(6): 949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dazzi F, Szydlo RM, Cross NC, et al. Durability of responses following donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood. 2000;96(8):2712–2716. [PubMed] [Google Scholar]
- 12.Yun HD, Waller EK. Finding the sweet spot for donor lymphocyte infusions. Biol Blood Marrow Transplant. 2013;19(4):507–508. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Y-Z, Barrett J. The Allogeneic CD4+ T-cell-mediated graft-versus-leukemia effect. Leuk Lymphoma. 1997;28(1-2):33–42. [DOI] [PubMed] [Google Scholar]
- 14.Slavin S, Naparstek E, Nagler A, et al. Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse after allogeneic bone marrow transplantation. Blood. 1996;87(6):2195–2204. [PubMed] [Google Scholar]
- 15.Laport GG, Sheehan K, Baker J, et al. Adoptive immunotherapy with cytokine-induced killer cells for patients with relapsed hematologic malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2011;17(11):1679–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar AJ, Hexner EO, Frey NV, et al. Pilot study of prophylactic ex vivo costimulated donor leukocyte infusion after reduced-intensity conditioned allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(7):1094–1101. [DOI] [PubMed] [Google Scholar]
- 17.Fehérvari Z, Sakaguchi S. Development and function of CD25+CD4+ regulatory T cells. Curr Opin Immunol. 2004;16(2):203–208. [DOI] [PubMed] [Google Scholar]
- 18.Piccirillo CA, Shevach EM. Naturally-occurring CD4+CD25+ immunoregulatory T cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16(2):81–88. [DOI] [PubMed] [Google Scholar]
- 19.Thornton AM, Shevach EM. CD4+CD25+ Immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188(2): 287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turk MJ, Guevara-Patiño JA, Rizzuto GA, Engelhorn ME, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200(6):771–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugiyama D, Nishikawa H, Maeda Y, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci USA. 2013;110(44):17945–17950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pastille E, Bardini K, Fleissner D, et al. Transient ablation of regulatory T cells improves antitumor immunity in colitis-associated colon cancer. Cancer Res. 2014; 74(16):4258–4269. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Q, Bucher C, Munger ME, et al. Depletion of endogenous tumor-associated regulatory T cells improves the efficacy of adoptive cytotoxic T-cell immunotherapy in murine acute myeloid leukemia. Blood. 2009;114(18):3793–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106(8):2903–2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rieger K, Loddenkemper C, Maul J, et al. Mucosal FOXP3+ regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107(4):1717–1723. [DOI] [PubMed] [Google Scholar]
- 26.Rezvani K, Mielke S, Ahmadzadeh M, et al. High donor FOXP3-positive regulatory T-cell (Treg) content is associated with a low risk of GVHD following HLA-matched allogeneic SCT. Blood. 2006;108(4):1291–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujioka T, Tamaki H, Ikegame K, et al. Frequency of CD4+FOXP3+ regulatory T-cells at early stages after HLA-mismatched allogeneic hematopoietic SCT predicts the incidence of acute GVHD. Bone Marrow Transplant. 2013;48(6):859–864. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen VH, Shashidhar S, Chang DS, et al. The impact of regulatory T cells on T-cell immunity following hematopoietic cell transplantation. Blood. 2008;111(2):945–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kennedy-Nasser AA, Ku S, Castillo-Caro P, et al. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res. 2014;20(8):2215–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koreth J, Matsuoka K-I, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011; 365(22):2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganguly S, Ross DB, Panoskaltsis-Mortari A, et al. Donor CD4+ Foxp3+ regulatory T cells are necessary for posttransplantation cyclophosphamide-mediated protection against GVHD in mice. Blood. 2014;124(13):2131–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powell DJ, Parker LL, Rosenberg SA. Large-scale depletion of CD25+ regulatory T cells from patient leukapheresis samples. J Immunother. 2005;28(4):403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA-matched sibling donors. Transplantation. 1974;18(4):295–304. [DOI] [PubMed] [Google Scholar]
- 35.Shulman H, Sullivan KM, Weiden P, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. [DOI] [PubMed] [Google Scholar]
- 36.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 37.Chiorean EG, DeFor TE, Weisdorf DJ, et al. Donor chimerism does not predict response to donor lymphocyte infusion for relapsed chronic myelogenous leukemia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10(3): 171–177. [DOI] [PubMed] [Google Scholar]
- 38.Komanduri KV, St John LS, de Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110(13):4543–4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jeljeli M, Guérin-El Khourouj V, Porcher R, et al. Relationship between cytomegalovirus (CMV) reactivation, CMV-driven immunity, overall immune recovery and graft-versus-leukaemia effect in children. Br J Haematol. 2014;166(2):229–239. [DOI] [PubMed] [Google Scholar]
- 40.Bellucci R, Alyea EP, Weller E, et al. Immunologic effects of prophylactic donor lymphocyte infusion after allogeneic marrow transplantation for multiple myeloma. Blood. 2002;99(12):4610–4617. [DOI] [PubMed] [Google Scholar]
- 41.Bachireddy P, Hainz U, Rooney M, et al. Reversal of in situ T-cell exhaustion during effective human antileukemia responses to donor lymphocyte infusion. Blood. 2014;123(9):1412–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ustun C, Miller JS, Munn DH, Weisdorf DJ, Blazar BR. Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation? Blood. 2011;118(19): 5084–5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maury S, Lemoine FM, Hicheri Y, et al. CD4+CD25+ Regulatory T cell depletion improves the graft-versus-tumor effect of donor lymphocytes after allogeneic hematopoietic stem cell transplantation. Sci Transl Med. 2010;2(41):41ra52. [DOI] [PubMed] [Google Scholar]
- 44.Waller EK, Logan BR, Harris WAC, et al. Improved survival after transplantation of more donor plasmacytoid dendritic or naïve T cells from unrelated-donor marrow grafts: results from BMTCTN 0201. J Clin Oncol. 2014;32(22):2365–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reshef R, Huffman AP, Gao A, et al. A survival benefit for reduced intensity allogeneic transplants from young unrelated donors compared to older sibling donors depends on the graft CD8 T-cell Content. Biol Blood Marrow Transplant. 2015;21(2):S42–S43. [Google Scholar]
- 46.Zheng H, Matte-Martone C, Li H, et al. Effector memory CD4+ T cells mediate graft-versus-leukemia without inducing graft-versus-host disease. Blood. 2008;111(4):2476–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bleakley M, Heimfeld S, Loeb KR, et al. Outcomes of acute leukemia patients transplanted with naive T cell–depleted stem cell grafts. J Clin Invest. 2015;125(7):2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frigault MJ, Lee J, Basil M, et al. Identification of chimeric antigen receptors that mediate constitutive or inducible proliferation of T cells. Cancer Immunol Res. 2015;3(4):356–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schroeder T, Frobel J, Cadeddu RP, et al. Salvage therapy with azacitidine increases regulatory T cells in peripheral blood of patients with AML or MDS and early relapse after allogeneic blood stem cell transplantation. Leukemia. 2013;27(9):1910–1913. [DOI] [PubMed] [Google Scholar]